Abstract
Background:
Self-rating of the effects of alcohol (SRE) measures level of response to ethanol in humans. Interestingly, there is a positive relationship between the SRE and risk for abusing alcohol, suggesting mechanistic connections between SRE and alcohol abuse.
Methods:
To identify candidate genes with a role in SRE and alcohol-related behavior more generally, we coupled human genetic analyses with studies in Drosophila melanogaster. We first performed a gene-based analysis of GWAS summary statistics for SRE in the Avon Longitudinal Study of Parents and Children (ALSPAC) sample. Based on prior findings in humans, orthology to fly genes and the availability of genetic reagents, we selected a subset of these genes for studies on ethanol behavior in Drosophila.
Results:
We found 37 genes with nominal associations in our SRE GWAS. We explored the role of 6 orthologous genes in Drosophila ethanol sedation and rapid tolerance. We found that the transcription factor Mef2 is required for normal ethanol sedation in flies. Pan-neuronal expression of two independent Mef2 RNAi transgenes significantly reduced Mef2 expression and made flies resistant to ethanol sedation. Additionally, flies with multiple independent mutant alleles of Mef2 were also resistant to ethanol sedation, confirming a role for Mef2 in this behavior. Altered expression of Mef2 did not change ethanol rapid tolerance or cause a net change in internal ethanol concentrations.
Conclusions:
Our studies indicate that MEF2B influences SRE in humans and that Mef2 impacts ethanol sedation in Drosophila.
Keywords: alcohol, ALSPAC, Drosophila behavior, human, MEF2B/Mef2
Introduction
Alcohol consumption is common in the United States and elsewhere, with rates in 2014 as high as 87.6% of individuals 18 or older reporting lifetime use and 71% reporting use within the last year (SAMHSA, 2015). Although many individuals use alcohol without developing problems, over a quarter of those who abuse alcohol become alcohol dependent (Florez-Salamanca et al., 2013). The World Health Organization estimates that 283 million people over the age of 15 years across the globe have alcohol use disorder (AUD) and that alcohol usage contributes to 3 million premature deaths annually (WHO, 2018) Problematic alcohol consumption also leads to a substantial public health burden in the United States. For example, excessive consumption of alcohol cost the United States an estimated $249 billion in 2010 and caused over 88,000 deaths between 2006 and 2010 (CDC, 2013; Sacks et al., 2015). Despite the substantial, multi-layered consequences of AUD and other forms of problematic alcohol consumption, we currently have a rather incomplete understanding of the mechanisms that drive their genesis.
It is clear, though, that the risk for developing AUD is under genetic influence. Twin studies show that AUD is moderately heritable (h2=0.49, (Verhulst et al., 2015)) indicating genetic factors account for ~50% of the underlying risk. Additionally, outcomes such as frequency or quantity of alcohol use (Dick et al., 2011; Edwards & Kendler, 2013) and intoxication (Wu et al., 2014) exhibit comparable levels of heritability. Genome-wide association studies (GWAS) have been used to identify genetic variance that influences problematic alcohol consumption. Such studies have implicated several genes in alcohol consumption including AUTS2 (Schumann et al., 2011) and KLB (Schumann et al., 2016) (which encode a transcriptional repressor and a receptor for fibroblast growth factor 21 (FGF21), respectively) as well as genes that encode alcohol- and aldehyde dehydrogenases (Clarke et al., 2017; Jorgenson et al., 2017). Other studies have suggested that the gene RYR3 (which encodes a ryanodine receptor) and multiple genes that encode proteins in the SWI/SNF complex influence the development of alcohol dependence (Adkins et al., 2017; Mathies et al., 2015). Despite these and other advances (Ducci & Goldman, 2008; Edenberg & Foroud, 2014; Enoch & Goldman, 2001; Matsushita & Higuchi, 2014; Reilly et al., 2017), our understanding of the genetic etiology of alcohol outcomes is relatively limited.
Genetic model organisms, principally Drosophila melanogaster (fruit flies), Caenorhabditis elegans (worms), and Mus musculus (mice), have been used extensively to identify molecular-genetic mechanisms underlying many different alcohol-related behaviors. These model organisms have conserved behavioral responses to alcohol including (collectively) locomotor activation at low doses, sedation at high doses, tolerance during prolonged or after repeated doses of alcohol, and withdrawal after discontinuation of the drug (Ghezzi et al., 2014; Grotewiel & Bettinger, 2015; Robinson et al., 2012; Scholz et al., 2000; Singh & Heberlein, 2000). Additionally, flies and mice voluntarily consume alcohol and this consumption can escalate with continuing exposure (Crabbe, 2014; Crabbe et al., 2010; Devineni & Heberlein, 2009). Given that humans exhibit comparable behavioral responses to alcohol, it is possible that many of the molecular targets for alcohol or the signaling pathways required for modulating the effects of alcohol on behavior might be conserved across species. There are data that support this possibility. For example, AUTS2, KLB, RYR, RSU1, SWI/SNF and other genes implicated in various alcohol behaviors in humans also influence behavioral responses to acute alcohol in flies, worms, and mice (Adkins et al., 2017; Jorgenson et al., 2017; Mathies et al., 2015; Ojelade et al., 2015; Schumann et al., 2011; Schumann et al., 2016).These findings suggest that the initial response to alcohol rating scale in humans might be driven—at least in part—by changes in behavioral responses to initial alcohol exposure revealed by studies in genetic model organisms.
We previously reported a meta-analysis of GWAS on the Self-Rating of the Effects of Alcohol (SRE) across two adolescent to young adult samples, Avon Longitudinal Study of Parents and Children (ALSPAC) and Spit for Science (S4S) (Edwards et al., 2018). The SRE (Schuckit et al., 1997a) captures the number of alcoholic drinks needed for respondents to experience intoxication when they first began drinking (i.e., initial sensitivity). Higher scores on the SRE have been associated with the development of alcohol use and misuse (Schuckit, 1994; Schuckit et al., 2008), suggesting that it has predictive validity for risk of later problematic alcohol consumption. The meta-analytic SNP-based heritability estimate (h2SNP) in our previous report (Edwards et al., 2018) was modest (h2SNP=0.19, SE=0.10) in the combined ALSPAC and S4S samples. This modest heritability was driven, however, by the ALSPAC sample (h2SNP=0.36, SE=0.14, p=0.04); the heritability estimate did not differ from 0 in S4S. While our previous report focused on primary and secondary analyses of the meta-analytic results, the moderate h2SNP in ALSPAC suggested that loci implicated in that sample might be particularly valuable experimental targets in follow-up studies. We consequently (i) conducted gene-based analyses of SRE in the ALSPAC sample to identify promising loci; (ii) identified, among the loci most strongly implicated in ALSPAC, those genes with orthologues in Drosophila; and (iii) assessed ethanol sedation sensitivity and tolerance in flies expressing RNAi targeting candidate genes.
The studies reported here implicated 37 human genes in SRE responses in the ALSPAC sample. Of these genes, we focused on 6 (APP, ATG5, GPD2, ISL1, MEF2B, and PCDH15) because previous results suggested they might be involved in relevant phenotypes in humans and these 6 genes had orthologues in flies with publicly available RNAi reagents to manipulate them. We found that neuronal expression of RNAi against the fly gene Mef2, a transcription factor involved in numerous processes including myogenesis (Black & Olson, 1998; Taylor & Hughes, 2017), decreased ethanol sedation sensitivity. Subsequently, we found that flies with loss-of-function mutations in Mef2 also had decreased ethanol sedation sensitivity. These alterations in ethanol sedation in flies with decreased Mef2 expression were not accompanied by changes in internal ethanol levels or rapid tolerance to ethanol. Interestingly, another group recently reported that decreased Mef2 function in flies alters rapid tolerance (Adhikari et al., 2018), suggesting that the consequences of Mef2 action in alcohol-related behavior might be context-dependent. Our studies collectively implicate human MEF2B in SRE responses and indicate that flies with altered Mef2 expression have parallel changes in ethanol sedation. Given that MEF2B and Mef2 encode transcription factors, our data further suggest that proteins with MEF2B/Mef2-dependent expression might be targets of ethanol or be involved in the modulation of behavioral responses to the drug.
Materials and Methods
Human sample and phenotype
The Avon Longitudinal Study of Parents and Children (ALSPAC) initially recruited 15,247 pregnant women residing in Avon, UK, with expected dates of delivery April 1, 1991, to December 31, 1992; 14,541 is the initial number of pregnancies for which the mothers enrolled in the ALSPAC study and had either returned at least 1 questionnaire or attended a “Children in Focus” clinic by July 19, 1999. Of these initial pregnancies, there was a total of 14,973 live births and 14,899 children who were alive at 1 year of age. Subsequent phases of enrollment increased the sample size over time. The phases of enrollment are described in more detail elsewhere (Boyd et al., 2013; Fraser et al., 2013). For the current analyses, full or partial offspring phenotypic data were available for 5,626 participants, in part reflecting the need for a subject to have had experience with alcohol to complete items related to alcohol sensitivity. The study website contains details of all the data that is available through a fully searchable data dictionary (http://www.bristol.ac.uk/alspac/researchers/our-data). Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees.
Initial alcohol sensitivity in ALSPAC was assessed using the Self-Rating of the Effects of Alcohol scale (SRE) (Schuckit et al., 1997a). Participants, aged 15.5, 16.5, and 17.5, were asked to consider the first five or so times they consumed alcohol and to report the number of standard drinks (defined in the questionnaire/interview) they consumed before they experienced signs of intoxication. As described previously (Edwards et al., 2018), responses were winsorized to account for outliers and total scores were derived according to recommendations by Schuckit and colleagues (Schuckit et al., 1997b). The total score was used as a continuous outcome in subsequent GWAS, with sex and ancestry-informative principal components as covariates. See Edwards et al. (2018) for additional details.
Human genetic analyses
We used summary statistics from the SRE GWAS in ALSPAC, which contributed to our prior meta-analysis (Edwards et al., 2018) but have not been independently reported, to identify candidate genes of interest for potential follow-up. Summary statistics (the estimated effect, standard error, and p-value for each SNP) were uploaded to FUMA (Watanabe et al., 2017), which utilizes MAGMA (de Leeuw et al., 2015) to conduct gene-based analyses. We selected the 1000 Genomes EUR subset to adjust for linkage disequilibrium [described in (Webb et al., 2017)].
Identification of Drosophila orthologues of candidate SRE genes
Of the human genes that were nominally implicated (pgene<0.001) in SRE variation, we identified genes for investigation in Drosophila based on several criteria: (i) prior evidence of involvement in phenotypes of potential relevance to alcohol response (e.g. substance use, psychopathology, or fatty liver disease) as suggested by searches of the Public Health Genomics Knowledge Base and PubMed; (ii) human-Drosophila orthology as determined using DIOPT (Hu et al., 2011), an online tool available via the DRSC/TRiP Functional Genomics Resources at Harvard University; and (iii) public availability of Drosophila RNAi reagents for manipulating expression of genes in flies.
Drosophila materials
Table sugar (sucrose; multiple brands from Richmond Restaurant Service, Richmond, VA), yellow cornmeal (enriched degerminated, 62–101, Genesee Scientific, San Diego, CA), saf-instant yeast (Lesaffre Yeast Corp., Milwaukee, WI), Drosophila agar type II (66–104, Apex BioResearch Products, Genesee Scientific, San Diego, CA) were used to make fly media along with ampicillin sodium salt (A9518), chloramphenicol (C0378), methyl 4-hydroxybenzoate (tegosept, H5501), and tetracycline (T3383) from Sigma-Aldrich, St. Louis, MO. Polypropylene fly culture bottles (AS-335) and cotton plugs (22–456–882) were from Fisher Scientific, Waltham, MA; polystyrene narrow fly vials (89092–722) were from VWR International, Radnor, PA; fly vial cotton plugs (51–101) were from Genesee Scientific, San Diego, CA. Flugs (49–102) were from Genesee Scientific, San Diego, CA. Ethanol (200 proof, 89125–172) was from VWR International, Radnor, PA. Calbiochem normal goat serum (80000–994) was from VWR International, Radnor, PA; rabbit-anti Mef2 was from Dr. Bruce M. Paterson, NIH; chicken anti-rabbit Alexa 647 (A-21443) was from Thermo Fisher, Waltham, MA; Invitrogen Molecular Probes SlowFade Gold Antifade Mountant (S36936) and Fisherbrand Premium Superfrost Microscope Slides (12–544-7) were from Fisher Scientific, Waltham, MA; paraformaldehyde powder, 95% (158127), sodium chloride (S7653), and Triton X-100 (T8787) were from Sigma Aldrich, St. Louis, MO; and cover glass no.1 (89239–692) was from VWR International, Radnor, PA. Sodium phosphate, dibasic, 7-hydrate, crystal (3824–01), potassium chloride, crystal (3040–01), and potassium phosphate, monobasic, crystal (3246–01) were all J.T.Baker brand from Avantor, Center Valley, PA; Proteinase K (P2308) was from Sigma-Aldrich, St. Louis, MO; DreamTaq Green DNA Polymerase (EP0712) was from Thermo Scientific, Waltham, MA; ExoSAP-IT (782000) Affymetrix, Santa Clara, CA. Plastics for molecular biology and other liquid handling were from Genesee Scientific, San Diego, CA and USA Scientific, Ocala, FL. Oligonucleotides were from Fisher Scientific, Waltham, MA.
Drosophila husbandry and stocks
Flies were cultured in an environmental chamber with a 12-hour light/dark cycle and maintained at 25°C and 60–65% relative humidity. Flies were grown on Drosophila food medium containing 10% sugar, 3.3% cornmeal, 2% yeast, 1% agar, 0.1 g/L ampicillin, 0.125 g/L chloramphenicol, 2 g/L tegosept, 0.02 g/L tetracycline, and live yeast.
w1118 (stock #5905 harboring a w1118 allele in an isogenic background contributed by Michael Ashburner), elavc155 (elav-Gal4), and the Mef2 RNAi line JF03115 were obtained from the Bloomington Drosophila Stock Center (BDSC, Bloomington, IN). A w1118 strain (stock #60000 harboring a w1118 allele in a genetic background used to produce RNAi transgenics) and the Mef2 RNAi stocks v15540 and v15550 (construct ID 5039) were obtained from the Vienna Drosophila RNAi Center (VDRC, Vienna, Austria). The use of w1118 stocks from BDSC or VDRC is defined in the text. All RNAi lines listed in Table 2 for the Drosophila candidate gene studies were obtained from BDSC or VDRC. Mef265, Mef230−5 (from Richard Cripps, The University of New Mexico) and Mef225−34 (from BDSC, stock #9861) were backcrossed for seven generations to Mef2EP−321 (Mef2EP−321, BDSC, stock #43412, an insertion marked with mini-w in the first intron of Mef2) that we previously backcrossed for seven generations to w1118 from BDSC. The Mef2EP−321 insertion did not alter ethanol sedation (not shown). The Mef265, Mef230−5 and Mef225−34 alleles were confirmed by PCR/sequencing using standard methods before and after being backcrossed to Mef2EP-321. Primers for PCR (forward/reverse) and sequencing were as follows: Mef265, 5’-AGATGCTGAATGTCCGAGTGT-3’/5’-GTGATGTGGCTTGTAGTGGC-3’ and 5’-CCTTAATGCAGGTGCGCC-3’; Mef230−5, 5’-CAGTCAGCAGGAATCAGCCA-3’/5’-TTGTTGGTGAGGGACTCGTG-3’ and 5’-TGAGCATGAGCAGTAATTGAAC-3’); Mef225−34, 5’-CAGTCAGCAGGAATCAGCCA-3’/5’-TTGTTGGTGAGGGACTCGTG-3’ and 5’-CCACCATCTCCGTTTCCATC-3’.
Table 2. Ethanol sedation results from top candidates from human GWAS with Drosophila orthologs.
The Drosophila and human genes, the fly RNAi transgenes used, the genotypes tested and the results from analysis of ethanol sedation and rapid tolerance are indicated. ST50 E1 and ST50 E2 are the ST50 values from the first and second exposure to ethanol, respectively. RapTol (E2/E1) is the rapid tolerance ratio of ST50 E2 divided by ST50 E1. S.E.M. is indicated and the number of replicates is shown as N. P values from one-way ANOVA tests (to assess overall effects of genotype) and BMCs (for planned comparisons between genotypes expressing RNAi and their respective controls) are shown.
| Target gene | Gal4 | RNAi | ST50 E1 |
RapTol (E2/E1) |
N | Statistical test | ST50 E1 P value |
RapTol P value |
|---|---|---|---|---|---|---|---|---|
| Appl | elav | JF02878 | 28.16 ± 1.37 | 1.92 ± 0.14 | 8 | ANOVA | 0.0155 | 0.0896 |
| Control | -- | JF02878 | 25.26 ± 1.47 | 2.31 ± 0.14 | 7 | BMC | 0.3304 | 0.1315 |
| Control | elav | -- | 21.90 ± 1.38* | 1.89 ± 0.14 | 8 | BMC | 0.0087 | >0.9999 |
| Atg5 | elav | HMS01244 | 42.19 ± 1.39 | 1.27 ± 0.06 | 8 | ANOVA | <0.0001 | 0.0002 |
| Control | -- | HMS01244 | 29.40 ± 2.74* | 2.33 ± 0.24* | 8 | BMC | 0.0001 | <0.0001 |
| Control | elav | -- | 23.54 ± 0.51* | 1.83 ± 0.07* | 8 | BMC | <0.0001 | 0.0258 |
| Atg5 | elav | JF02661 | 34.90 ± 1.97 | 2.11 ± 0.13 | 8 | ANOVA | 0.0075 | 0.8988 |
| Control | -- | JF02661 | 32.63 ± 1.37 | 2.06 ± 0.11 | 8 | BMC | 0.5776 | >0.9999 |
| Control | elav | -- | 27.69 ± 0.90* | 2.14 ± 0.12 | 8 | BMC | 0.0048 | >0.9999 |
| Atg5 | elav | VSH330300 | 25.39 ± 1.47 | 2.00 ± 0.17 | 8 | ANOVA | 0.0002 | 0.0061 |
| Control | -- | VSH330300 | 19.99 ± 0.69* | 2.52 ± 0.09* | 8 | BMC | 0.0238 | 0.0375 |
| Control | elav | -- | 29.85 ± 1.77 | 1.80 ± 0.16 | 8 | BMC | 0.0670 | 0.6998 |
| Atg5 | elav | KK108904 | 34.90 ± 3.57 | 1.36 ± 0.18 | 8 | ANOVA | 0.0060 | 0.0400 |
| Control | -- | KK108904 | 42.28 ± 2.03 | 1.36 ± 0.09 | 8 | BMC | 0.1041 | >0.9999 |
| Control | elav | -- | 29.31 ± 1.55 | 1.82 ± 0.12 | 8 | BMC | 0.2672 | 0.0520 |
| Cad99c | elav | HMS01451 | 46.44 ± 2.39 | 1.51 ± 0.10 | 8 | ANOVA | 0.0007 | 0.0804 |
| Control | -- | HMS01451 | 33.24 ± 3.45* | 2.41 ± 0.43 | 8 | BMC | 0.0044 | 0.0650 |
| Control | elav | -- | 30.26 ± 1.96* | 1.73 ± 0.18 | 8 | BMC | 0.0007 | >0.9999 |
| Cad99c | elav | JF02660 | 34.51 ± 2.59 | 2.02 ± 0.17 | 8 | ANOVA | 0.0007 | 0.1720 |
| Control | -- | JF02660 | 24.81 ± 0.88* | 2.28 ± 0.14 | 8 | BMC | 0.0012 | 0.3777 |
| Control | elav | -- | 25.26 ± 1.07* | 1.91 ± 0.09 | 8 | BMC | 0.0018 | >0.9999 |
| Cad99c | elav | JF02761 | 27.53 ± 1.00 | 2.02 ± 0.11 | 8 | ANOVA | 0.0073 | 0.0227 |
| Control | -- | JF02761 | 25.84 ± 0.99 | 2.01 ± 0.11 | 8 | BMC | 0.4010 | >0.9999 |
| Control | elav | -- | 23.05 ± 0.68* | 1.67 ± 0.05* | 8 | BMC | 0.0042 | 0.0293 |
| Cad99c | elav | v27215 | 23.74 ± 0.95 | 1.90 ± 0.10 | 8 | ANOVA | 0.9960 | 0.0462 |
| Control | -- | v27215 | 23.85 ± 0.70 | 1.60 ± 0.10 | 8 | BMC | >0.9999 | 0.1393 |
| Control | elav | -- | 23.84 ± 1.2 | 2.00 ± 0.13 | 8 | BMC | >0.9999 | >0.9999 |
| Cad99c | elav | v27216 | 52.88 ± 1.21 | 1.48 ± 0.04 | 8 | ANOVA | <0.0001 | 0.0813 |
| Control | -- | v27216 | 48.45 ± 0.96 | 1.59 ± 0.05 | 8 | BMC | 0.0798 | 0.7097 |
| Control | elav | -- | 40.10 ± 1.94* | 1.76 ± 0.13 | 8 | BMC | <0.0001 | 0.0554 |
| Cad99c | elav | v27212 | 41.54 ± 2.02 | 1.66 ± 0.07 | 8 | ANOVA | 0.0170 | 0.0425 |
| Control | -- | v27212 | 48.96 ± 1.12* | 1.39 ± 0.05 | 8 | BMC | 0.0172 | 0.0562 |
| Control | elav | -- | 42.49 ± 2.12 | 1.44 ± 0.10 | 8 | BMC | >0.9999 | 0.1482 |
| Cad99c | elav | v3739 | 37.50 ± 1.64 | 1.65 ± 0.11 | 8 | ANOVA | 0.0812 | 0.2700 |
| Control | -- | v3739 | 39.30 ± 1.89 | 1.58 ± 0.10 | 8 | BMC | 0.9974 | >0.9999 |
| Control | elav | -- | 43.56 ± 2.00 | 1.34 ± 0.15 | 8 | BMC | 0.0612 | 0.2512 |
| Gpo1 | elav | v19565 | 36.08 ± 1.08 | 1.25 ± 0.05 | 8 | ANOVA | 0.1106 | 0.0080 |
| Control | -- | v19565 | 39.86 ± 1.65 | 1.35 ± 0.06 | 8 | BMC | 0.1711 | 0.5090 |
| Control | elav | -- | 35.64 ± 1.65 | 1.55 ± 0.08* | 8 | BMC | >0.9999 | 0.0049 |
| Gpo1 | elav | KK107425 | 41.30 ± 1.61 | 1.36 ± 0.07 | 8 | ANOVA | <0.0001 | 0.0013 |
| Control | -- | KK107425 | 29.44 ± 1.05* | 1.88 ± 0.07* | 8 | BMC | <0.0001 | 0.0013 |
| Control | elav | -- | 30.24 ± 1.20* | 1.80 ± 0.12* | 8 | BMC | <0.0001 | 0.0054 |
| Gpo1 | elav | HMC04006 | 24.19 ± 0.99 | 2.32 ± 0.18 | 8 | ANOVA | <0.0001 | <0.0001 |
| Control | -- | HMC04006 | 20.36 ± 0.65* | 2.29 ± 0.10 | 8 | BMC | 0.0134 | >0.9999 |
| Control | elav | -- | 39.95 ± 1.01* | 1.38 ± 0.047* | 8 | BMC | <0.0001 | <0.0001 |
| Mef2 | elav | JF03115 | 46.90 ± 2.12 | 1.51 ± 0.07 | 8 | ANOVA | <0.0001 | 0.2099 |
| Control | -- | JF03115 | 37.89 ± 0.96* | 1.63 ± 0.05 | 8 | BMC | 0.0006 | 0.3923 |
| Control | elav | -- | 33.49 ± 1.09* | 1.67 ± 0.07 | 8 | BMC | <0.0001 | 0.1868 |
| Mef2 | elav | v15550 | 57.25 ± 1.80 | 1.39 ± 0.05 | 8 | ANOVA | <0.0001 | 0.6152 |
| Control | -- | v15550 | 44.81 ± 1.31* | 1.49 ± 0.09 | 8 | BMC | <0.0001 | 0.6738 |
| Control | elav | -- | 39.98 ± 1.71* | 1.43 ± 0.06 | 8 | BMC | <0.0001 | >0.9999 |
| Mef2 | elav | v15549 | 48.04 ± 2.53 | - | 8 | ANOVA | <0.0001 | - |
| Control | -- | v15549 | 35.26 ± 1.66* | - | 8 | BMC | 0.0003 | - |
| Control | elav | -- | 33.15 ± 1.58* | - | 8 | BMC | <0.0001 | - |
| tup | elav | HMC03317 | 47.66 ± 0.96 | 1.38 ± 0.06 | 5 | ANOVA | 0.0002 | 0.1069 |
| Control | -- | HMC03317 | 44.64 ± 1.76 | 1.41 ± 0.08 | 5 | BMC | 0.3628 | >0.9999 |
| Control | elav | -- | 34.84 ± 1.66* | 1.62 ± 0.09 | 5 | BMC | 0.0001 | 0.1076 |
| tup | elav | v45859 | 38.51 ± 1.54 | 1.47 ± 0.09 | 8 | ANOVA | 0.0414 | 0.9583 |
| Control | -- | v45859 | 41.13 ± 0.95 | 1.46 ± 0.03 | 8 | BMC | 0.3801 | >0.9999 |
| Control | elav | -- | 35.86 ± 1.52 | 1.49 ± 0.08 | 8 | BMC | 0.3681 | >0.9999 |
| tup | elav | KK101489 | 40.06 ± 2.11 | 1.57 ± 0.12 | 8 | ANOVA | 0.0009 | 0.2162 |
| Control | -- | KK101489 | 44.78 ± 2.40 | 1.47 ± 0.10 | 8 | BMC | 0.3577 | >0.9999 |
| Control | elav | -- | 32.03 ± 1.54* | 1.74 ± 0.088 | 8 | BMC | 0.0344 | 0.7602 |
Drosophila ethanol sedation and rapid tolerance
Flies were collected, stored, and tested for ethanol sedation and rapid tolerance essentially as described (Chan et al., 2014; Sandhu et al., 2015). Sedation time 50 (ST50) values (i.e. the time required for 50% of flies in individual vials to become sedated) were determined by exposing flies to vapor from 85% ethanol in our standard protocol or from other ethanol concentrations as indicated (Figures 2, 3C, 3D and S4). For rapid tolerance, flies were sedated during an initial exposure to ethanol, allowed to recover for 4 hours at 25°C/65% relative humidity, and then sedated during a second exposure to ethanol (Chan et al., 2014). Development of rapid tolerance was quantitated as fold increase in ST50 (i.e. ST50 from sedation 2 ÷ ST50 from sedation 1).
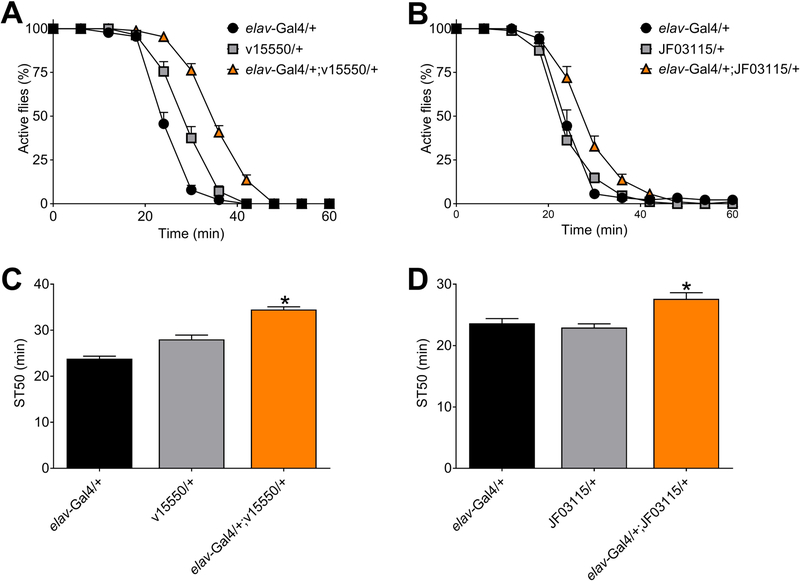
Figure 2. Sedation by high dose ethanol in flies expressing Mef2 RNAi.
(A and B) Sedation time courses in response to vapor from 100% ethanol for flies with pan-neuronal expression of Mef2 RNAi (A, elav-Gal4/+;v15550/+; B, elav-Gal4/+;JF03115/+) and controls (elav-Gal4/+, v15550/+, JF03115/+). (C, D) ST50 values from the data in panels A and B, respectively. (C) Pan-neuronal expression of v15550 (C) and JF03115 (D) increased ST50 values (individual one-way ANOVAs; panel C, p<0.0001; panel D, p=0.0012; panel C, *BMCs vs controls, p<0.0001; panel D, BMCs vs controls, p=0.0012–0.0049; n=8).
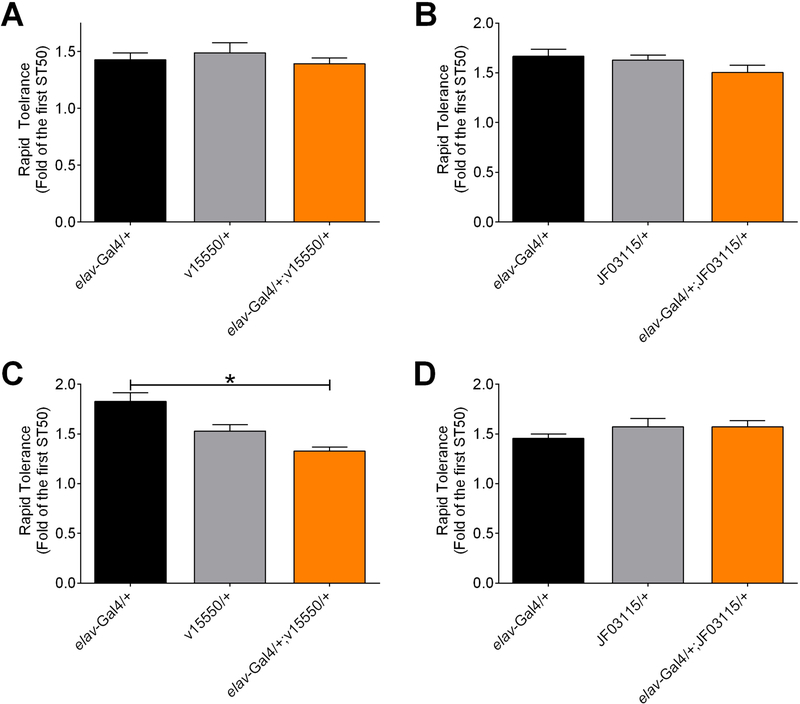
Figure 3. Rapid tolerance and pan-neuronal expression of Mef2 RNAi.
Development of rapid tolerance was not significantly altered by expression of Mef2 RNAi. (A, B) Rapid tolerance in response to exposure of vapor from 85% ethanol. Expression of v15550 (A) or JF03115 (B) in neurons (individual one-way ANOVAs; panel A, p=0.6152; panel B, p=0.2099; n=8). (C, D) Rapid tolerance to sedation from vapor from 100% ethanol. In experiments with v15550, there was a significant overall effect of genotype (one-way ANOVA, p=0.0001, n=8) and elav-Gal4/+;v15550/+ flies were different than elav-Gal4/+ controls (BMC, p<0.0001), but not different than v15550/+ controls (p=0.0860). (D) In studies with JF03115, genotype had no overall effect (one-way ANOVA, p=0.3530, n=8).
Drosophila internal ethanol
Flies were exposed to ethanol as described above for ethanol sedation. After exposure to vapor from 85% ethanol for the durations indicated in figure legends, flies were frozen at −80°C, homogenized in 200 μl of distilled H2O and centrifuged for 20 minutes at 4°C (Chan et al., 2014; Sandhu et al., 2015). Internal ethanol was determined via a spectrophotometric method (Figure 1E as previously described (Chan et al., 2014; Sandhu et al., 2015)) or gas chromatography (GC, Figure S5). For GC, individual 20 μl supernatant sample were pipetted into 20 ml headspace GC vials containing 960 μl deionized water, 500 mg NaCl and 20 μl of 5 mM 1-propanol internal standard. Samples were tested for ethanol concentration using an Agilent model 6890 GC equipped with a flame ionization detector (FID, 0.53 mm ID Rtx BAC-1 capillary column (Restek, Bellefonte, PA) and CTC CombiPal headspace autosampler (Leap Technologies, Carrboro, NC). Samples were incubated and agitated for 10 min at 70°C prior to automated injection. The GC parameters were as follows: 1.5 mL headspace injection volume, 5/1 split ratio, 3 min sample run time, injector temp 200°C, oven temp isothermal 150°C, detector temp 200°C, helium carrier gas flow rate 40 mL/min, nitrogen makeup gas flow rate 18 mL/min, hydrogen flame flow rate 25 mL/min and FID air flow rate 300 mL/min. Data were collected and analyzed by Clarity GC software (Apex Data Systems, Prague, CZ) using a linear regression analysis with no weighting. Ethanol concentrations were calculated by the internal standard method. A seven point calibration curve preceded the analysis of supernatant ethanol concentrations. Quality control ethanol standards at concentrations similar to those found in the test samples were interspersed at regular intervals with test samples. All internal ethanol calculations were corrected for each individual genotype-specific water volume. Water volume was determined as the difference between total wet weight and dry weight (after 24 h at 50°C) of groups of 11 flies in porous 1.7 ml tubes. Each tube of 11 flies was considered one datum.
Figure 1. Ethanol sedation and internal ethanol levels in flies with pan-neuronal expression of Mef2 RNAi.
(A and B) Ethanol sedation time courses of flies with pan-neuronal Mef2 RNAi expression. Expression of Mef2 RNAi transgenes v15550 (A, elav-Gal4/+;v15550/+) and JF03118 (B, elav-Gal4/+;JF03115/+) in neurons extended sedation time-courses compared to controls (elav-Gal4/+, v15550/+, JF03118/+). (C, D) ST50 values derived from the data in panels A and B. Pan-neuronal expression of v15550 (C) and JF03115 (D) increased ST50 values compared to controls (individual one-way ANOVAs, p<0.0001; *Bonferroni’s multiple comparisons (BMCs), p<0.0001 (C), p≤0.006 (D); n=8). (E) Internal ethanol in flies exposed to vapor from 85% ethanol for 30 or 60 min was significantly impacted by genotype and time of ethanol exposure, and there was a significant interaction between these two factors (two-way ANOVA; genotype, p=0.0001; exposure time, p<0.0001; interaction, p=0.0281;, n=8). After 30 min of ethanol exposure, internal ethanol was lower in elav-Gal4;v15550/+ flies compared to elav-Gal4/+ (*BMC p=0.0406) and higher in elav-Gal4/+;J03115/+ flies compared to JF03115/+ controls (*BMC p=0.0361). After 60 min of ethanol exposure, internal ethanol was lower in elav-Gal4/+;v15550/+ flies compared to elav-Gal4/+ controls (*BMC p=0.0432). No other pair-wise differences were found after 30 or 60 min of ethanol exposure (BMC p=0.2935–0.9999).
Drosophila immunohistochemistry
Whole brains from adult female flies were dissected in PBT (100 mM Phosphate buffer, pH 7.2, with 0.03% (v/v) Triton X-100) under a dissecting microscope and fixed in 0.5 mL snap cap tubes containing 4% paraformaldehyde for 20 minutes at room temperature on a tube rotator. Fixed brains were washed with PBT, blocked with 5% normal goat serum (NGS), and incubated in primary antiserum (anti-Mef2, diluted 1:5000 in NGS) on a tube rotator at 4°C for 48 hrs. Brains were washed with 0.3% PBT and exposed to the secondary antibody (chicken anti-rabbit Alexa 647, diluted 1:1000 in NGS) at 4°C for 48 hrs. Brains were then washed with PBT and mounted onto glass slides in SlowFade mounting medium. Images were collected using a Zeiss LSM 700 confocal microscope (Carl Zeiss Microscopy, LLC, Thornwood, NY) housed in the VCU Department of Anatomy and Neurobiology Microscope Facility. Confocal z-stack images using a pin hole of 1 Airy disc unit and Nyquist sampling were collected from each adult brain. Images were captured with a 10X objective using a numerical aperture of 0.3. The microscopy settings were optimized for the mushroom body regions of the brains from RNAi transgene control flies to (i) avoid oversaturation of images and (ii) allow comparisons in pixel intensity between brains expressing the Mef2 RNAi in neurons and controls. The same settings (gain, offset, power) and z-stack slice thickness were used in all images. All images were processed using Image J (National Institutes of Health (Schneider et al., 2012)). A threshold value was individually set for each z-stack so only pixels in the brains were analyzed. Mef2 expression was determined as the mean pixel intensity for each brain (Wu & Luo, 2006) in z-stacks of all images from all brains. The quantitation provided in Figs. 4E and 4F is therefore derived from numerous images representing all portions of 4–5 brains per genotype.
Figure 4. Whole brain Mef2 expression and validation of Mef2 RNAi transgenes.
(A-D) representative (10X) confocal images of whole mount brains immunolabeled with anti-Mef2. In control flies (A, v15550/+; B, JF03115/+), expression of Mef2 was prominent in the calyx of the mushroom bodies (arrowheads) and at lower levels throughout the brain. Detection of Mef2 appeared to be reduced in flies pan-neuronally expressing Mef2 RNAi transgenes (C, elav-Gal4/+;v15550/+; D, elav-Gal4/+;JF03115/+) compared to brains from RNAi transgene control animals (A, B). (E and F) Quantitation of Mef2 immunolabeling. Pixel intensity derived from z-stacks of all images of whole brains was significantly reduced in flies expressing Mef2 RNAi transgenes (E, elav-Gal4/+;v15550/+; F, elav-Gal4/+;JF03115/+) compared to their respective RNAi transgene controls (students t-tests; panel E, p=0.0002; panel F, p<0.0001, n = 4–5). The quantitation provided in Figs. 4E and 4F is derived from numerous images representing all portions of 4–5 brains per genotype.
Statistical analysis of Drosophila results
Statistical analyses (student’s t-test, one- and two-way ANOVAs, Bonferroni’s multiple comparisons (BMC), and normality tests) were performed using Prism 6.04 (GraphPad Software Inc., San Diego, CA). P values < 0.05 were considered statistically significant. All numerical data are reported as mean ± SEM.
Results
Human genetic analyses and identification of loci for potential screening in Drosophila.
As reported previously (Edwards et al., 2018), SRE scores were moderately heritable in the ALSPAC sample (h2SNP=0.36, p=0.04). To explore potential genetic contributions to SRE scores, we used summary statistics from the SRE GWAS in ALSAPC to conduct gene-based analyses in Functional Mapping and Annotation of Genome-Wide Association Studies (FUMA). This yielded results for 18,187 genes (Supplementary Table 1), of which 37 had pgene<0.001 for SRE (Table 1). Of these 37 genes, 29 were orthologous to a total of 52 Drosophila genes (defined as having DIOPT score ≥ 2) (Hu et al., 2011) (Table 1). Additionally, of the 37 genes with pgene<0.001 for SRE, nine had been previously associated with phenotypes (e.g., substance use, psychiatric findings, and others) we postulated might be related to alcohol use or misuse (Table 1). Thus, nine human genes (APP, ATG5, BORCS8, BORCS8-MEF2B, GPD2, ISL1, MEF2B, PCDH15 and SFSWAP) had suggestive associations with SRE, had previously been connected to potentially relevant phenotypes in humans, and were orthologous to at least one gene in flies (Table 1).
Table 1. Candidate loci for follow-up in Drosophila.
37 human genes that reached pgene<0.001 in human gene-based analyses from SRE GWAS study, whether they have any prior association with phenotypes of interest, and their subsequent highest rated fly ortholog(s) and corresponding DIOPT scores (listed orthologs have a DIOPT score ≥2).
| Human Gene Symbol | Pgene | Previously associated human phenotypes of interest1 | Drosophila Gene Symbol | DIOPT Score |
|---|---|---|---|---|
| MEF2B | 0.0007412 | Obsessive compulsive disorder | Mef2 | 4 |
| APP | 0.00050488 | Cognition, neuropsychological tests, psychiatric status rating scales, psychotic disorders, tobacco use disorder | Appl | 13 |
| ATG5 | 0.00058657 | Drug hypersensitivity, tobacco use disorder | Atg5 | 14 |
| BORCS82 | 0.00050716 | Tobacco use disorder, obsessive compulsive disorder | CG32590 | 13 |
| BORCS8-MEF2B3 | 0.0007412 | Tobacco use disorder | Mef2 | 4 |
| GPD2 | 0.00028942 | Fatty liver | Gpo1 | 13 |
| ISL1 | 0.00038948 | ADHD | tup | 11 |
| PCDH15 | 0.00012322 | Alcoholism, tobacco use disorder | Cad99C | 9 |
| SFSWAP | 0.00038044 | Tobacco use disorder | su(w[a]) | 13 |
| ANKRD6 | 0.00035656 | N/A | dgo | 4 |
| ASPG | 0.00081699 | N/A | CG6428, CG8526 | 13 |
| BHLHE40 | 0.00074851 | N/A | Several | 2 |
| BIRC6 | 0.00053743 | N/A | Bruce | 15 |
| C1QTNF5 | 0.00037187 | N/A | N/A | N/A |
| DEPDC7 | 9.33E-05 | N/A | N/A | N/A |
| DSG1 | 1.27E-05 | N/A | N/A | N/A |
| EXOC1 | 2.97E-05 | N/A | Sec3 | 14 |
| IKZF1 | 0.00015571 | N/A | N/A | N/A |
| LGSN | 0.00034442 | N/A | Gs1 | 2 |
| LRRTM1 | 0.00031065 | N/A | caps, trn | 2 |
| MFRP | 0.00037187 | N/A | CG42255 | 2 |
| MMP17 | 0.00013157 | N/A | Mmp2 | 7 |
| PPP2R5E | 0.00059389 | N/A | wdb | 13 |
| PRAMEF11 | 0.00044431 | N/A | N/A | N/A |
| PRB1 | 0.00036335 | N/A | N/A | N/A |
| PSMD6 | 0.00062142 | N/A | Rpn7 | 15 |
| PXMP2 | 0.00076238 | N/A | CG7970 | 7 |
| SCGN | 0.00078013 | N/A | Cbp53E | 8 |
| SCN3B | 0.00026792 | N/A | N/A | N/A |
| SET | 0.00023109 | N/A | Set | 13 |
| STRN3 | 0.00051438 | N/A | Cka | 15 |
| TCP11L1 | 0.00014072 | N/A | CG16721 | 13 |
| THAP2 | 0.00030577 | N/A | CG14860 | 2 |
| TMEM185B | 0.00083227 | N/A | CG14194 | 12 |
| TMEM53 | 0.00069173 | N/A | CG8245 | 14 |
| USP2 | 0.00047617 | N/A | Usp2 | 9 |
| ZBTB44 | 5.54E-06 | N/A | N/A | N/A |
Primary resource was the Public Health Genomics Knowledge Base, supplemented by NCBI literature searches.
This locus is also known as MEF2BNB.
This locus is also known as MEF2BNB-MEF2B
The human genes APP, ATG5, BORCS8, BORCS8-MEF2B, GPD2, ISL1, MEF2B, PCDH15 and SFSWAP are orthologous to a total of 12 Drosophila genes (Table 1). We obtained RNAi reagents from public stock centers to manipulate expression of 6 of the most highly conserved fly genes (Appl, Atg5, Cad99c, Gpo1, Mef2, and tup). We did not explore the 2 remaining fly genes because (i) for CG32590 no function has been ascribed to its gene product and (ii) for su(w[a]) only a single RNAi reagent was available to manipulate it and it is a rather complex locus with several additional genes residing within its transcription unit (Gramates et al., 2017), greatly complicating genetic analyses.
Initial screening of Drosophila orthologues in ethanol sedation and rapid tolerance
To explore the role of the 6 Drosophila genes in ethanol-related behavior, we constitutively expressed RNAi targeting each gene (22 RNAi transgenes total) in neurons using elav-Gal4 and then assessed ethanol sedation and ethanol rapid tolerance in adults. Expression of 3 different RNAi transgenes targeting Mef2, two of which have independent target sequences (Figure S1), increased ST50 values relative to both genetic controls (Table 2 and see below). In contrast, expression of Mef2 RNAi transgenes in neurons did not alter the development of rapid tolerance (Table 2). Thus, we focused our subsequent fly studies on the role of Mef2 in ethanol sedation. Expression of RNAi targeting the other 5 Drosophila genes did not consistently alter either ethanol sedation or rapid tolerance compared to controls (Table 2). We note, however, that neuronal expression of 2 RNAi transgenes against Cad99C with unique target sequences (HMS01451 and JF02660) made flies resistant to ethanol sedation (Table 2), suggesting that this gene might also warrant further study.
Mef2 in Drosophila ethanol sedation
Human MEF2B is orthologous to Drosophila Mef2 (Table 1). Both genes encode transcription factors with three domains: MADS (MCM1, agamous, deficiens, SRF), MEF2, and transcriptional activation (Figure S2) (Chen et al., 2017; Potthoff & Olson, 2007). Using the longest isoforms for both human MEF2B (isoform 1) and fly Mef2 (isoform H), we found that the proteins are 33% similar/26% identical/39% gaps overall at the amino acid level (Altschul et al., 2005). Importantly, the MADS and MEF2 domains in the human and Drosophila gene products are 85.5% identical/96.4% similar and 82.8% identical/93.1% similar, respectively (Figure S2) (Altschul et al., 2005; Potthoff & Olson, 2007). The transcriptional activation domain at the amino acid level is not as well conserved as the other two domains with only 16.5% identity/23% similarity (Altschul et al., 2005; Potthoff & Olson, 2007) (Figure S2). The overall conservation in domain structure of the proteins, along with the conservation of the primary amino acid sequences of these domains, suggests that MEF2B and Mef2 might have conserved functions in humans and Drosophila. We note that the MEF2B chromosomal region in humans has two non-overlapping genes (MEF2B and BORCS8), each of which produces its own transcript. This region also produces a read-through transcript, BORCS8-MEF2B (a.k.a. MEF2BNB-MEF2B), but the fly orthologs of BORCS8 and MEF2B are on different chromosomes (Gramates et al., 2017), precluding a BORCS8-MEF2 read-through product in Drosophila. Given that BORCS8 has no known function and that no BORCS8-MEF2 product exists in flies, BORCS8 and BORSCS8-MEF2B were not considered further in the current study.
As noted above, pan-neuronal expression of the Mef2 RNAi v15550 increased sedation time 50 (ST50) values compared to controls (Figure 1A, ethanol sedation time-course; Figure 1C, ST50 values). Similarly, pan-neuronal expression of a second Mef2 RNAi (v15549) with the same target sequence as v15550 also increased ST50 values compared to controls (Figure S3). More importantly, flies with pan-neuronal expression of another RNAi transgene with a distinct target sequence in Mef2 (JF03115) also had increased ST50 values (Figure 1B, ethanol sedation time-course; Figure 1D, ST50 values). Therefore, pan-neuronal expression of Mef2 RNAi transgenes impacts ethanol sedation in flies.
To determine if the altered ethanol sedation in flies expressing Mef2 RNAi could be explained by altered internal ethanol levels, we measured internal ethanol concentrations in the same genotypes assessed in ethanol sedation. We found that expression of Mef2 in neurons had no significant effect on internal ethanol in flies (Figures 1E and S5). Expression of Mef2 RNAi in neurons therefore affects ethanol sedation without having a measurable impact on internal ethanol concentrations.
While completing our studies on human MEF2B and fly Mef2, Adhikari and colleagues (Adhikari et al., 2018) reported that flies with pan-neuronal expression of Mef2 RNAi had increased sensitivity to ethanol sedation (evidenced by decreased ST50 values), although pan-neuronal expression of a Mef2 dominant-negative protein did not impact ethanol sedation in this study. The ST50 values from control flies in the experiments conducted by Adhikari et. al were ~15 minutes, which is shorter than ST50 values from our studies (30–40 minutes when using vapor from 85% ethanol). This difference in ST50 values raised the possibility that knocking down Mef2 might decrease ST50 values when they are relatively short (as in Adhikari et. al), while knocking down Mef2 might increase ST50 values when they are relatively long (as in our studies). To explore this possibility, we expressed Mef2 RNAi transgenes pan-neuronally in flies and then assessed their sedation in response to vapor from 100% ethanol. Exposure of flies to vapor from 100% ethanol appeared to decrease overall ST50 values as expected (Figure 2). More importantly, though, flies with pan-neuronal expression of Mef2 RNAi transgenes v15550 (Figure 2A and 2C) and JF03115 (Figure 2B and 2D) continued to have significantly increased ST50 values compared to controls even when exposed to vapor from the highest possible concentration of ethanol in our behavioral paradigm. Additionally, we found that pan-neuronal expression of Mef2 RNAi transgene v15550 increased ST50 values in flies sedated by vapor from 55% ethanol (the same concentration used to produce vapor in Adhikari and colleagues; Figure S4). Although additional studies would be required to fully explore the differences between the phenotypes seen in flies with altered Mef2 reported here and by Adhikari and colleagues (Adhikari et al., 2018), our data suggest that these differences are not due to the overall magnitude of ST50 values obtained from the behavioral paradigms used in the respective studies.
Adhikari and colleagues also reported that pan-neuronal expression of Mef2 RNAi or a Mef2 dominant negative protein decreased the development of rapid tolerance to ethanol. We therefore also assessed ethanol rapid tolerance, calculated as either the relative (Figure 3) or absolute (Figure S6) change in ST50 using vapor from 85% (Figure 3A, 3B, S6A, S6B) or 100% (Figures 3C, 3D, S6C, S6D) ethanol. In contrast to Adhikari and colleagues, expression of Mef2 RNAi in neurons did not alter rapid tolerance under the conditions of our experiments (Figures 3 and S6). Additional studies would again be required to fully explore the differences between our data and the data from Adhikari et. al (Adhikari et al., 2018), but as we found for sedation (see above) these differences were not readily resolved by increasing the ethanol concentration used for exposure.
We used immunofluorescence to assess whether pan-neuronal expression of v15550 and JF03115 decreased Mef2 levels in whole fly brains (Figure 4). Control flies harboring the Mef2 RNAi transgenes without a Gal4 driver (Figure 4A, v15550/+; Figure 4B, JF30015/+) had strong expression of Mef2 in the mushroom bodies (arrowheads) as previously reported (e.g. Crittenden et al., 2018). Visual inspection of representative images at the level of the mushroom bodies suggested that this Mef2 signal was decreased in flies with pan-neuronal expression of Mef2 RNAi transgenes (Figure 4C: elav-Gal4/+;v15550/+; Figure 4D: elav-Gal4/+;JF30115/+). Quantitation of the Mef2 signal from all optical sections from all anatomical levels representing 4–5 whole brains per genotype confirmed that pan-neuronal expression of Mef2 RNAi transgenes significantly decreased Mef2 expression (Figure 4E and 4F). Considering that our quantitation of Mef2 signal was derived from all available optical images of several brains per genotype, that our staining in control brains was similar to previously reported patterns of Mef2 expression (e.g. Crittenden et al., 2018), and that our quantitation Mef2 expression is based on detection of Mef2 protein (the presumed active product of the Mef2 gene), our results indicate that both of the Mef2 RNAi transgenes used throughout our studies knockdown expression of Mef2 in neurons.
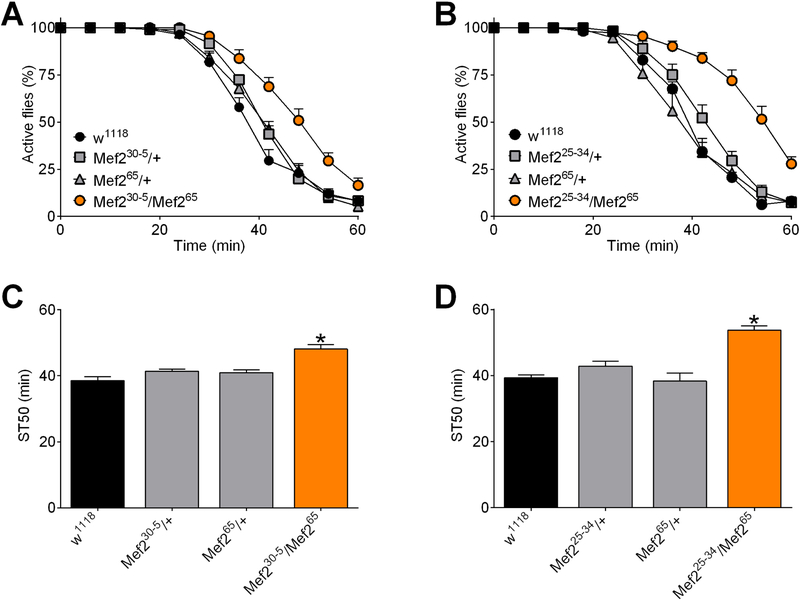
To further explore the possibility that Mef2 plays a role in ethanol sedation, we obtained several previously characterized loss-of-function Mef2 mutant alleles (Mef225−34 (Nguyen et al., 2002), Mef230−5 (Baker et al., 2005; Lovato et al., 2009), and Mef265 (Ranganayakulu et al., 1995)) (Figure S1), backcrossed them to a control stock (see Methods), and then performed genetic complementation analyses. Flies heterozygous for Mef230−5, Mef265, and Mef225−34, had no detectable change in ST50 values (Figure 5). Importantly, Mef230−5/Mef265 (Figure 5A, time-courses; Figure 5C, ST50 values) and Mef225−34/Mef265 (Figure 5B, time-courses; Figure 5D, ST50 values) transheterozygous flies had increased ST50 values compared to all other groups tested. These data confirm that flies with reduced Mef2 function have increased ST50 values under the conditions used in our experiments.
Figure 5. Mef2 mutants are resistant to ethanol sedation.
(A and B) Ethanol sedation time courses of control flies (w1118 from BDSC) and the indicated Mef2 genotypes. Compound heterozygous Mef2 mutants Mef230−5/Mef265 (A) and Mef225−34/Mef265 (B) took longer to become sedated than flies heterozygous for these alleles. (C, D) ST50 values, derived from the data in panels A and B, respectively. There was a significant overall effect of Mef2 genotype on ST50 values (individual one-way ANOVAs; panel C, p<0.0001; panel D, p<0.0001; n=10). ST50 values were greater in Mef230−5/Mef265 (C) and Mef225−34/Mef265 (D) flies than in all other genotypes (*BMCs; panel C, p≤0.0003; panel D, p<0.0001).
Discussion
The overarching goal of this study was to explore molecular-genetic underpinnings of behavioral responses to alcohol by (i) using human genetic analyses to identify candidate genes that might influence SRE and then (ii) testing the role of Drosophila orthologues of those human candidate genes in fly ethanol sedation and rapid tolerance. We chose to focus our studies on human SRE scores and fly ethanol sedation/rapid tolerance in part because these measures are well established responses to ethanol exposure in the respective species (Scholz et al., 2000; Schuckit et al., 1997b; Schuckit et al., 1996; Singh & Heberlein, 2000) and both measures have demonstrated value for identifying genes that influence alcohol abuse, dependence or other aspects of alcohol abuse (Adkins et al., 2017; Juraeva et al., 2015; Lasek et al., 2011; Morozova et al., 2009; Schuckit, 1994; Schuckit et al., 2005; Schuckit et al., 2003; Schuckit et al., 1997b; Schuckit et al., 1996; Schumann et al., 2011). Additionally, both the SRE in humans and ethanol sedation/rapid tolerance in flies are behavioral responses to acute ethanol exposure, raising the possibility that our approach could uncover evolutionarily conserved mechanisms driving the behaviors in humans and flies.
Our SRE GWAS in the ALSPAC sample found 37 candidate genes with pgene < 0.001. We prioritized our examination of fly orthologues of these genes based on 3 criteria: reported roles of the candidate genes in human phenotypes that might predict roles in ethanol behavior, presence of obvious orthologous genes in the fly genome, and availability of Drosophila RNAi reagents to manipulate gene expression. A total of 6 human and 6 orthologous fly genes met these criteria: APP/Appl, ATG5/Atg5, GPD2/Gpo1, ISL1/tup, MEF2B/Mef2 and PCDH15/Cad99C. We consequently explored the role of the fly orthologues in ethanol sedation and rapid tolerance.
Flies with constitutive expression of RNAi targeting Mef2 in neurons, as well as flies with loss of function mutations in Mef2, were resistant to ethanol sedation in our experiments. Decreased function of Mef2 did not impact internal ethanol levels, indicating that Mef2 influences the pharmacodynamic response to ethanol. Interestingly, decreased function of Mef2 did not alter rapid tolerance to ethanol, suggesting that Mef2 might influence acute sedation, but not tolerance, under the conditions used in our studies. Importantly, our studies on Mef2 used 3 different RNAi transgenes with 2 different target sequences and 3 independent loss-of-function alleles, all confirmed at the protein or DNA level, collectively demonstrating a role for Mef2 in fly ethanol sedation.
Another group also recently reported that Mef2 plays a role in Drosophila alcohol-related behavior. In contrast to our results, their studies indicate that constitutive neuronal expression of RNAi against Mef2 or expression of a Mef2 dominant negative alters rapid tolerance to ethanol, but not necessarily ethanol sedation (Adhikari et al., 2018). We noted that the ST50 values were somewhat shorter in the previous report when compared to our results, but increasing the ethanol concentration to the maximum possible in our studies (thereby shortening the ST50) did not meaningfully alter our conclusions. Additional experiments will therefore be required to address whether the differences between the previously published and our results might be explained by differences in behavioral paradigms, genetic backgrounds, or environmental conditions. Importantly, though, both studies demonstrate that Mef2 influences behavioral responses to acute ethanol exposure in flies, thereby mutually reinforcing the other.
The human MEF2 family of genes has 4 members (MEF2A, MEF2B, MEF2C and MEF2D) whereas the fly genome contains only a single orthologue (Mef2). The primary amino acid sequence of fly Mef2 protein has similar overall levels of conservation (32 to 43 percent identical, 43 to 56 percent similar (Hu et al., 2011)) relative to all of the human MEF2 proteins. All gene products are known or predicted to be transcription factors (Brand, 1997; Potthoff & Olson, 2007) and are involved in numerous biological processes (e.g., muscle differentiation (Black & Olson, 1998; Taylor & Hughes, 2017), human disease/cancer (Chen et al., 2017; Pon & Marra, 2016), neuronal differentiation (Li et al., 2008; Lyons et al., 1995; Shalizi & Bonni, 2005), and others. Of the MEF2 family members within our ALSPAC SRE gene-based analyses, MEF2B was the most robustly implicated (pgene=0.0007). Additionally, though, our analyses suggested that MEF2A might also be involved (pgene=0.02), whereas MEF2C and MEF2D were not associated with SRE scores in our studies (p>0.05). None of the MEF2 family members have, to our knowledge, met stringent genome-wide significance criteria for associations with alcohol-related outcomes in other human GWAS. Interestingly, though, using the recently developed online tool GWAS ATLAS (atlas.ctglab.nl (Watanabe et al., 2018)), which processes GWAS results through MAGMA to derive gene-based summary statistics, we identified nominal associations (p<0.05) between all four human MEF2 genes and alcohol outcomes. Furthermore, MEF2B is associated with women’s alcohol consumption in a GWAS study (Schumann et al., 2016) based on a secondary analysis of that study’s GWAS summary statistics using the PheWAS option in the online GWAS ATLAS (http://atlas.ctglab.nl/PheWAS). PheWAS also reveals that aggregate variation in MEF2B is associated with changes in alcohol use from 10 years prior in UK Biobank data. The PheWAS tool and literature searches reveal associations between alcohol-related outcomes and other human MEF2 genes as well: MEF2A was associated with alcohol intake frequency in UK Biobank, MEF2C was associated with alcohol dependence symptom counts in COGA (Wang et al., 2013), and both MEF2C and MEF2D were associated with a range of alcohol-related outcomes in UK Biobank including alcohol intake frequency, drinking status, and amount of alcohol consumed on drinking days. These diverse associations—in conjunction with the results of the current study—suggest that multiple or perhaps even all members of the MEF2 family might impact variation in alcohol outcomes. Together, our studies in humans and flies, in combination with previous studies in humans, suggest that MEF2 family members might impact initial sensitivity to ethanol, thereby influencing the risk for abusing the drug.
As transcription factors, MEF2 family members presumably influence alcohol-related behaviors principally by regulating expression of other genes. Adhikari and colleagues recently reported that Mef2 induces the expression of the immediate early gene Hr38, thereby regulating rapid tolerance in flies (Adhikari et al., 2018). Additional Mef2 target genes might also be important for ethanol behaviors. For example, Sivachenko and colleagues identified 342 genes with Mef2-dependent expression in flies (Sivachenko et al., 2013). Fifteen of these 342 genes (~3.5-fold more than expected by chance) are known to influence behavioral responses to ethanol in Drosophila, suggesting that genes regulated by Mef2 family members might be enriched for genes that influence alcohol-related behavior. A more comprehensive characterization of Mef2-regulated gene expression could therefore be a gateway for better understanding mechanisms underlying a variety of behaviors in response to alcohol exposure.
Beyond studies on alcohol, Mef2 genes are important for the effects of cocaine on neuronal morphology and behavioral responses. Cocaine increases dendritic spine density in the mouse nucleus acumbens via a process that requires reductions in Mef2 protein activity, and behavioral sensitization to cocaine requires an increase in Mef2 protein activity (Pulipparacharuvil et al., 2008). Combined with our findings, these results raise the possibility that Mef2 proteins and their target genes might have conserved roles in behavioral and other types of responses to multiple drugs of abuse. A more comprehensive understanding of Mef2 in alcohol behavior could therefore have important implications for risk assessment for—and potentially treatment of—substance abuse more broadly.
Supplementary Material
Acknowledgements
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. The UK Medical Research Council and Wellcome (Grant ref: 102215/2/13/2) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors and A.C.E. and M.G. will serve as guarantors for the contents of this paper. A comprehensive list of grants funding is available on the ALSPAC website (http://www.bristol.ac.uk/alspac/external/documents/grant-acknowledgements.pdf); This research was specifically funded by NIH AA018333. GWAS data was generated by Sample Logistics and Genotyping Facilities at Wellcome Sanger Institute and LabCorp (Laboratory Corporation of America) using support from 23andMe. Fly stocks were obtained from the Vienna Drosophila Resource Center and the Bloomington Drosophila Stock Center (NIH P40OD018537) for use in this study. The authors thank Dr. Bruce M. Paterson for the Mef2 antibody and Lara Lewellyn, Jena Butler, Ian Hines, Charlotte Collier, and Matt Hewitt for technical assistance. The work was supported by grants to A.C.E. (AA022537), L.D.M. (AA022537, AA024482) both from the US National Institutes of Health, and M.G. (AA022537, AA020634) from the US National Institutes of Health, National Institute of Alcohol Abuse and Alcoholism and the VCU Presidential Research Quest Fund. Microscopy was performed at the VCU Microscopy Facility, supported, in part, by funding from NIH-NCI Cancer Center Support Grant P30 CA016059.
Footnotes
Conflict of Interest
The authors have no conflict of interests to declare.
References
- Adhikari P, Orozco D, Randhawa H, Wolf FW (2018) Mef2 induction of the immediate early gene Hr38/Nr4a is terminated by Sirt1 to promote ethanol tolerance. Genes Brain Behav:e12486. doi: 10.1111/gbb.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkins AE, Hack LM, Bigdeli TB, Williamson VS, McMichael GO, Mamdani M, Edwards AC, Aliev F, Chan RF, Bhandari P, Raabe RC, Alaimo JT, Blackwell GG, Moscati A, Poland RS, Rood B, Patterson DG, Walsh D, Collaborative Study of the Genetics of Alcoholism C, Whitfield JB, Zhu G, Montgomery GW, Henders AK, Martin NG, Heath AC, Madden PAF, Frank J, Ridinger M, Wodarz N, Soyka M, Zill P, Ising M, Nothen MM, Kiefer F, Rietschel M, German Study of the Genetics of Addiction C, Gelernter J, Sherva R, Koesterer R, Almasy L, Zhao H, Kranzler HR, Farrer LA, Maher BS, Prescott CA, Dick DM, Bacanu SA, Mathies LD, Davies AG, Vladimirov VI, Grotewiel M, Bowers MS, Bettinger JC, Webb BT, Miles MF, Kendler KS, Riley BP (2017) Genomewide Association Study of Alcohol Dependence Identifies Risk Loci Altering Ethanol-Response Behaviors in Model Organisms. Alcohol Clin Exp Res 41:911–928. doi: 10.1111/acer.13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Wootton JC, Gertz EM, Agarwala R, Morgulis A, Schaffer AA, Yu YK (2005) Protein database searches using compositionally adjusted substitution matrices. FEBS J 272:5101–5109. doi: 10.1111/j.1742-4658.2005.04945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PW, Tanaka KK, Klitgord N, Cripps RM (2005) Adult myogenesis in Drosophila melanogaster can proceed independently of myocyte enhancer factor-2. Genetics 170:1747–1759. doi: 10.1534/genetics.105.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black BL, Olson EN (1998) Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu Rev Cell Dev Biol 14:167–196. doi: 10.1146/annurev.cellbio.14.1.167. [DOI] [PubMed] [Google Scholar]
- Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, Molloy L, Ness A, Ring S, Davey Smith G (2013) Cohort Profile: the ‘children of the 90s’--the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 42:111–127. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand NJ (1997) Myocyte enhancer factor 2 (MEF2). Int J Biochem Cell Biol 29:1467–1470. doi: 10.1016/S1357-2725(97)00084-8. [DOI] [PubMed] [Google Scholar]
- CDC (2013) Alcohol and Public Health: Alcohol-Related Disease Impact (ARDI). Average for United States 2006–2010 Alcohol-Attributable Deaths Due to Excessive Alcohol Use. Centers for Disease Control and Prevention. https://nccd.cdc.gov/DPH_ARDI/Default/Report.aspx?T=AAM&P=f6d7eda7-036e-4553-9968-9b17ffad620e&R=d7a9b303-48e9-4440-bf47-070a4827e1fd&M=8E1C5233-5640-4EE8-9247-1ECA7DA325B9&F=&D [Google Scholar]
- Chan RF, Lewellyn L, DeLoyht JM, Sennett K, Coffman S, Hewitt M, Bettinger JC, Warrick JM, Grotewiel M (2014) Contrasting influences of Drosophila white/mini-white on ethanol sensitivity in two different behavioral assays. Alcoholism, Clinical and Experimental Research 38:1582–1593. doi: 10.1111/acer.12421 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Gao B, Ponnusamy M, Lin Z, Liu J (2017) MEF2 signaling and human diseases. Oncotarget 8:112152–112165. doi: 10.18632/oncotarget.22899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke TK, Adams MJ, Davies G, Howard DM, Hall LS, Padmanabhan S, Murray AD, Smith BH, Campbell A, Hayward C, Porteous DJ, Deary IJ, McIntosh AM (2017) Genome-wide association study of alcohol consumption and genetic overlap with other health-related traits in UK Biobank (N=112 117). Mol Psychiatry 22:1376–1384. doi: 10.1038/mp.2017.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC (2014) Rodent models of genetic contributions to motivation to abuse alcohol. Nebr Symp Motiv 61:5–29. https://www.ncbi.nlm.nih.gov/pubmed/25306777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Belknap JK (2010) The complexity of alcohol drinking: studies in rodent genetic models. Behav Genet 40:737–750. doi: 10.1007/s10519-010-9371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden JR, Skoulakis EMC, Goldstein ES, Davis RL (2018) Drosophila mef2 is essential for normal mushroom body and wing development. Biol Open 7. doi: 10.1242/bio.035618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw CA, Mooij JM, Heskes T, Posthuma D (2015) MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput Biol 11:e1004219. doi: 10.1371/journal.pcbi.1004219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devineni AV, Heberlein U (2009) Preferential ethanol consumption in Drosophila models features of addiction. Curr Biol 19:2126–2132. doi: 10.1016/j.cub.2009.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Meyers JL, Rose RJ, Kaprio J, Kendler KS (2011) Measures of Current Alcohol Consumption and Problems: Two Independent Twin Studies Suggest a Complex Genetic Architecture. Alcohol Clin Exp Res 35:2152–2161. doi: 10.1111/j.1530-0277.2011.01564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducci F, Goldman D (2008) Genetic approaches to addiction: genes and alcohol. Addiction 103:1414–1428. doi: 10.1111/j.1360-0443.2008.02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Foroud T (2014) Genetics of alcoholism. Handbook of clinical neurology 125:561–571. doi: 10.1016/B978-0-444-62619-6.00032-X [doi]. [DOI] [PubMed] [Google Scholar]
- Edwards AC, Deak JD, Gizer IR, Lai D, Chatzinakos C, Wilhelmsen KP, Lindsay J, Heron J, Hickman M, Webb BT, Bacanu SA, Foroud TM, Kendler KS, Dick DM, Schuckit MA (2018) Meta-Analysis of Genetic Influences on Initial Alcohol Sensitivity. Alcohol Clin Exp Res 42:2349–2359. doi: 10.1111/acer.13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AC, Kendler KS (2013) Alcohol consumption in men is influenced by qualitatively different genetic factors in adolescence and adulthood. Psychol Med 43:1857–1868. doi: 10.1017/S0033291712002917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Goldman D (2001) The genetics of alcoholism and alcohol abuse. Curr Psychiatry Rep 3:144–151. https://www.ncbi.nlm.nih.gov/pubmed/11276410 [DOI] [PubMed] [Google Scholar]
- Florez-Salamanca L, Secades-Villa R, Hasin DS, Cottler L, Wang S, Grant BF, Blanco C (2013) Probability and predictors of transition from abuse to dependence on alcohol, cannabis, and cocaine: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Am J Drug Alcohol Abuse 39:168–179. doi: 10.3109/00952990.2013.772618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, Davey Smith G, Henderson J, Macleod J, Molloy L, Ness A, Ring S, Nelson SM, Lawlor DA (2013) Cohort Profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol 42:97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi A, Krishnan HR, Atkinson NS (2014) Susceptibility to ethanol withdrawal seizures is produced by BK channel gene expression. Addict Biol 19:332–337. doi: 10.1111/j.1369-1600.2012.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramates LS, Marygold SJ, Santos GD, Urbano JM, Antonazzo G, Matthews BB, Rey AJ, Tabone CJ, Crosby MA, Emmert DB, Falls K, Goodman JL, Hu Y, Ponting L, Schroeder AJ, Strelets VB, Thurmond J, Zhou P, the FlyBase C (2017) FlyBase at 25: looking to the future. Nucleic Acids Res 45:D663–D671. doi: 10.1093/nar/gkw1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewiel M, Bettinger JC (2015) Drosophila and Caenorhabditis elegans as Discovery Platforms for Genes Involved in Human Alcohol Use Disorder. Alcoholism, Clinical and Experimental Research 39:1292–1311. doi: 10.1111/acer.12785 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Flockhart I, Vinayagam A, Bergwitz C, Berger B, Perrimon N, Mohr SE (2011) An integrative approach to ortholog prediction for disease-focused and other functional studies. BMC Bioinformatics 12:357. doi: 10.1186/1471-2105-12-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgenson E, Thai KK, Hoffmann TJ, Sakoda LC, Kvale MN, Banda Y, Schaefer C, Risch N, Mertens J, Weisner C, Choquet H (2017) Genetic contributors to variation in alcohol consumption vary by race/ethnicity in a large multi-ethnic genome-wide association study. Mol Psychiatry 22:1359–1367. doi: 10.1038/mp.2017.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juraeva D, Treutlein J, Scholz H, Frank J, Degenhardt F, Cichon S, Ridinger M, Mattheisen M, Witt SH, Lang M, Sommer WH, Hoffmann P, Herms S, Wodarz N, Soyka M, Zill P, Maier W, Junger E, Gaebel W, Dahmen N, Scherbaum N, Schmal C, Steffens M, Lucae S, Ising M, Smolka MN, Zimmermann US, Muller-Myhsok B, Nothen MM, Mann K, Kiefer F, Spanagel R, Brors B, Rietschel M (2015) XRCC5 as a risk gene for alcohol dependence: evidence from a genome-wide gene-set-based analysis and follow-up studies in Drosophila and humans. Neuropsychopharmacology 40:361–371. doi: 10.1038/npp.2014.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasek AW, Lim J, Kliethermes CL, Berger KH, Joslyn G, Brush G, Xue LQ, Robertson M, Moore MS, Vranizan K, Morris SW, Schuckit MA, White RL, Heberlein U (2011) An Evolutionary Conserved Role for Anaplastic Lymphoma Kinase in Behavioral Responses to Ethanol. Plos One 6. doi: 10.1371/journal.pone.0022636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Radford JC, Ragusa MJ, Shea KL, McKercher SR, Zaremba JD, Soussou W, Nie Z, Kang YJ, Nakanishi N, Okamoto S, Roberts AJ, Schwarz JJ, Lipton SA (2008) Transcription factor MEF2C influences neural stem/progenitor cell differentiation and maturation in vivo. Proc Natl Acad Sci U S A 105:9397–9402. doi: 10.1073/pnas.0802876105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovato TL, Adams MM, Baker PW, Cripps RM (2009) A molecular mechanism of temperature sensitivity for mutations affecting the Drosophila muscle regulator Myocyte enhancer factor-2. Genetics 183:107–117. doi: 10.1534/genetics.109.105056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons GE, Micales BK, Schwarz J, Martin JF, Olson EN (1995) Expression of mef2 genes in the mouse central nervous system suggests a role in neuronal maturation. J Neurosci 15:5727–5738. doi: 10.1523/JNEUROSCI.15-08-05727.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathies LD, Blackwell GG, Austin MK, Edwards AC, Riley BP, Davies AG, Bettinger JC (2015) SWI/SNF chromatin remodeling regulates alcohol response behaviors in Caenorhabditis elegans and is associated with alcohol dependence in humans. Proc Natl Acad Sci U S A 112:3032–3037. doi: 10.1073/pnas.1413451112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita S, Higuchi S (2014) Genetic differences in response to alcohol. Handb Clin Neurol 125:617–627. doi: 10.1016/B978-0-444-62619-6.00036-7. [DOI] [PubMed] [Google Scholar]
- Morozova TV, Ayroles JF, Jordan KW, Duncan LH, Carbone MA, Lyman RF, Stone EA, Govindaraju DR, Ellison RC, Mackay TF, Anholt RR (2009) Alcohol sensitivity in Drosophila: translational potential of systems genetics. Genetics 183:733–745, 731SI-712SI. doi: 10.1534/genetics.109.107490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T, Wang J, Schulz RA (2002) Mutations within the conserved MADS box of the D-MEF2 muscle differentiation factor result in a loss of DNA binding ability and lethality in Drosophila. Differentiation 70:438–446. doi: 10.1046/j.1432-0436.2002.700806.x. [DOI] [PubMed] [Google Scholar]
- Ojelade SA, Jia T, Rodan AR, Chenyang T, Kadrmas JL, Cattrell A, Ruggeri B, Charoen P, Lemaitre H, Banaschewski T, Buchel C, Bokde AL, Carvalho F, Conrod PJ, Flor H, Frouin V, Gallinat J, Garavan H, Gowland PA, Heinz A, Ittermann B, Lathrop M, Lubbe S, Martinot JL, Paus T, Smolka MN, Spanagel R, O’Reilly PF, Laitinen J, Veijola JM, Feng J, Desrivieres S, Jarvelin MR, Consortium I, Schumann G, Rothenfluh A (2015) Rsu1 regulates ethanol consumption in Drosophila and humans. Proc Natl Acad Sci U S A 112:E4085–4093. doi: 10.1073/pnas.1417222112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pon JR, Marra MA (2016) MEF2 transcription factors: developmental regulators and emerging cancer genes. Oncotarget 7:2297–2312. doi: 10.18632/oncotarget.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff MJ, Olson EN (2007) MEF2: a central regulator of diverse developmental programs. Development 134:4131–4140. doi: 10.1242/dev.008367. [DOI] [PubMed] [Google Scholar]
- Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, Russo SJ, Sikder D, Dewey CM, Davis MM, Greengard P, Nairn AC, Nestler EJ, Cowan CW (2008) Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron 59:621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganayakulu G, Zhao B, Dokidis A, Molkentin JD, Olson EN, Schulz RA (1995) A series of mutations in the D-MEF2 transcription factor reveal multiple functions in larval and adult myogenesis in Drosophila. Dev Biol 171:169–181. doi: 10.1006/dbio.1995.1269. [DOI] [PubMed] [Google Scholar]
- Reilly MT, Noronha A, Goldman D, Koob GF (2017) Genetic studies of alcohol dependence in the context of the addiction cycle. Neuropharmacology 122:3–21. doi: 10.1016/j.neuropharm.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BG, Khurana S, Kuperman A, Atkinson NS (2012) Neural adaptation leads to cognitive ethanol dependence. Curr Biol 22:2338–2341. doi: 10.1016/j.cub.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD (2015) 2010 National and State Costs of Excessive Alcohol Consumption. Am J Prev Med 49:e73–79. doi: 10.1016/j.amepre.2015.05.031. [DOI] [PubMed] [Google Scholar]
- SAMHSA (2015) 2015 National Survey on Drug Use and Health: Detailed Tables. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.htm#tab2-6b
- Sandhu S, Kollah AP, Lewellyn L, Chan RF, Grotewiel M (2015) An inexpensive, scalable behavioral assay for measuring ethanol sedation sensitivity and rapid tolerance in Drosophila. J Vis Exp. doi: 10.3791/52676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz H, Ramond J, Singh CM, Heberlein U (2000) Functional ethanol tolerance in Drosophila. Neuron 28:261–271. doi: 10.1016/S0896-6273(00)00101-X. [DOI] [PubMed] [Google Scholar]
- Schuckit MA (1994) Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry 151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Beltran I, Waylen A, Horwood J, Davis JM, Team AS (2005) Performance of a self-report measure of the level of response to alcohol in 12- to 13-year-old adolescents. J Stud Alcohol 66:452–458. doi: 10.15288/jsa.2005.66.452. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, Isacescu V (2003) Level of response to alcohol measured on the self-rating of the effects of alcohol questionnaire in a group of 40-year-old women. Am J Drug Alcohol Abuse 29:191–201. doi: 10.1081/ADA-120018846. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Tipp JE (1997a) The Self-Rating of the Effects of alcohol (SRE) form as a retrospective measure of the risk for alcoholism. Addiction 92:979–988. doi: 10.1111/j.1360-0443.1997.tb02977.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Trim R, Heron J, Horwood J, Davis JM, Hibbeln JR, Team AS (2008) The performance of elements of a ‘level of response to alcohol’-based model of drinking behaviors in 13-year-olds. Addiction 103:1786–1792. doi: 10.1111/j.1360-0443.2008.02325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Smith TL, Wiesbeck GA, Kalmijn J (1997b) The relationship between Self-Rating of the Effects of alcohol and alcohol challenge results in ninety-eight young men. J Stud Alcohol 58:397–404. doi: 10.15288/jsa.1997.58.397. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tsuang JW, Anthenelli RM, Tipp JE, Nurnberger JI Jr., (1996) Alcohol challenges in young men from alcoholic pedigrees and control families: a report from the COGA project. J Stud Alcohol 57:368–377. doi: 10.15288/jsa.1996.57.368. [DOI] [PubMed] [Google Scholar]
- Schumann G, Coin LJ, Lourdusamy A, Charoen P, Berger KH, Stacey D, Desrivieres S, Aliev FA, Khan AA, Amin N, Aulchenko YS, Bakalkin G, Bakker SJ, Balkau B, Beulens JW, Bilbao A, de Boer RA, Beury D, Bots ML, Breetvelt EJ, Cauchi S, Cavalcanti-Proenca C, Chambers JC, Clarke TK, Dahmen N, de Geus EJ, Dick D, Ducci F, Easton A, Edenberg HJ, Esko T, Fernandez-Medarde A, Foroud T, Freimer NB, Girault JA, Grobbee DE, Guarrera S, Gudbjartsson DF, Hartikainen AL, Heath AC, Hesselbrock V, Hofman A, Hottenga JJ, Isohanni MK, Kaprio J, Khaw KT, Kuehnel B, Laitinen J, Lobbens S, Luan J, Mangino M, Maroteaux M, Matullo G, McCarthy MI, Mueller C, Navis G, Numans ME, Nunez A, Nyholt DR, Onland-Moret CN, Oostra BA, O’Reilly PF, Palkovits M, Penninx BW, Polidoro S, Pouta A, Prokopenko I, Ricceri F, Santos E, Smit JH, Soranzo N, Song K, Sovio U, Stumvoll M, Surakk I, Thorgeirsson TE, Thorsteinsdottir U, Troakes C, Tyrfingsson T, Tonjes A, Uiterwaal CS, Uitterlinden AG, van der Harst P, van der Schouw YT, Staehlin O, Vogelzangs N, Vollenweider P, Waeber G, Wareham NJ, Waterworth DM, Whitfield JB, Wichmann EH, Willemsen G, Witteman JC, Yuan X, Zhai G, Zhao JH, Zhang W, Martin NG, Metspalu A, Doering A, Scott J, Spector TD, Loos RJ, Boomsma DI, Mooser V, Peltonen L, Stefansson K, van Duijn CM, Vineis P, Sommer WH, Kooner JS, Spanagel R, Heberlein UA, Jarvelin MR, Elliott P (2011) Genome-wide association and genetic functional studies identify autism susceptibility candidate 2 gene (AUTS2) in the regulation of alcohol consumption. Proc Natl Acad Sci U S A 108:7119–7124. doi: 10.1073/pnas.1017288108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann G, Liu C, O’Reilly P, Gao H, Song P, Xu B, Ruggeri B, Amin N, Jia T, Preis S, Segura Lepe M, Akira S, Barbieri C, Baumeister S, Cauchi S, Clarke TK, Enroth S, Fischer K, Hallfors J, Harris SE, Hieber S, Hofer E, Hottenga JJ, Johansson A, Joshi PK, Kaartinen N, Laitinen J, Lemaitre R, Loukola A, Luan J, Lyytikainen LP, Mangino M, Manichaikul A, Mbarek H, Milaneschi Y, Moayyeri A, Mukamal K, Nelson C, Nettleton J, Partinen E, Rawal R, Robino A, Rose L, Sala C, Satoh T, Schmidt R, Schraut K, Scott R, Smith AV, Starr JM, Teumer A, Trompet S, Uitterlinden AG, Venturini C, Vergnaud AC, Verweij N, Vitart V, Vuckovic D, Wedenoja J, Yengo L, Yu B, Zhang W, Zhao JH, Boomsma DI, Chambers J, Chasman DI, Daniela T, de Geus E, Deary I, Eriksson JG, Esko T, Eulenburg V, Franco OH, Froguel P, Gieger C, Grabe HJ, Gudnason V, Gyllensten U, Harris TB, Hartikainen AL, Heath AC, Hocking L, Hofman A, Huth C, Jarvelin MR, Jukema JW, Kaprio J, Kooner JS, Kutalik Z, Lahti J, Langenberg C, Lehtimaki T, Liu Y, Madden PA, Martin N, Morrison A, Penninx B, Pirastu N, Psaty B, Raitakari O, Ridker P, Rose R, Rotter JI, Samani NJ, Schmidt H, Spector TD, Stott D, Strachan D, Tzoulaki I, van der Harst P, van Duijn CM, Marques-Vidal P, Vollenweider P, Wareham NJ, Whitfield JB, Wilson J, Wolffenbuttel B, Bakalkin G, Evangelou E, Liu Y, Rice KM, Desrivieres S, Kliewer SA, Mangelsdorf DJ, Muller CP, Levy D, Elliott P (2016) KLB is associated with alcohol drinking, and its gene product beta-Klotho is necessary for FGF21 regulation of alcohol preference. Proc Natl Acad Sci U S A 113:14372–14377. doi: 10.1073/pnas.1611243113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalizi AK, Bonni A (2005) brawn for brains: the role of MEF2 proteins in the developing nervous system. Curr Top Dev Biol 69:239–266. doi: 10.1016/S0070-2153(05)69009-6. [DOI] [PubMed] [Google Scholar]
- Singh CM, Heberlein U (2000) Genetic control of acute ethanol-induced behaviors in Drosophila. Alcohol Clin Exp Res 24:1127–1136. doi: 10.1111/j.1530-0277.2000.tb02075.x. [DOI] [PubMed] [Google Scholar]
- Sivachenko A, Li Y, Abruzzi KC, Rosbash M (2013) The transcription factor Mef2 links the Drosophila core clock to Fas2, neuronal morphology, and circadian behavior. Neuron 79:281–292. doi: 10.1016/j.neuron.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MV, Hughes SM (2017) Mef2 and the skeletal muscle differentiation program. Semin Cell Dev Biol 72:33–44. doi: 10.1016/j.semcdb.2017.11.020. [DOI] [PubMed] [Google Scholar]
- Verhulst B, Neale MC, Kendler KS (2015) The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychol Med 45:1061–1072. doi: 10.1017/S0033291714002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JC, Foroud T, Hinrichs AL, Le NX, Bertelsen S, Budde JP, Harari O, Koller DL, Wetherill L, Agrawal A, Almasy L, Brooks AI, Bucholz K, Dick D, Hesselbrock V, Johnson EO, Kang S, Kapoor M, Kramer J, Kuperman S, Madden PA, Manz N, Martin NG, McClintick JN, Montgomery GW, Nurnberger JI Jr., Rangaswamy M, Rice J, Schuckit M, Tischfield JA, Whitfield JB, Xuei X, Porjesz B, Heath AC, Edenberg HJ, Bierut LJ, Goate AM (2013) A genome-wide association study of alcohol-dependence symptom counts in extended pedigrees identifies C15orf53. Mol Psychiatry 18:1218–1224. doi: 10.1038/mp.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Stringer S, Frei O, Umićević Mirkov M, Polderman TJC, van der Sluis S, Andreassen OA, Neale BM, Posthuma D (2018) A global overview of pleiotropy and genetic architecture in complex traits. bioRxiv 500090. doi: 10.1101/500090. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Taskesen E, van Bochoven A, Posthuma D (2017) Functional mapping and annotation of genetic associations with FUMA. Nat Commun 8:1826. doi: 10.1038/s41467-017-01261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb BT, Edwards AC, Wolen AR, Salvatore JE, Aliev F, Riley BP, Sun C, Williamson VS, Kitchens JN, Pedersen K, Adkins A, Cooke ME, Savage JE, Neale Z, Cho SB, Dick DM, Kendler KS (2017) Molecular Genetic Influences on Normative and Problematic Alcohol Use in a Population-Based Sample of College Students. Frontiers in Genetics 8. doi: 10.3389/fgene.2017.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2018) Global status report on alcohol and health 2018. Geneva: World Health Organization; 2018 Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Wu JS, Luo L (2006) A protocol for dissecting Drosophila melanogaster brains for live imaging or immunostaining. Nat Protoc 1:2110–2115. doi: 10.1038/nprot.2006.336. [DOI] [PubMed] [Google Scholar]
- Wu SH, Guo Q, Viken RJ, Reed T, Dai J (2014) Heritability of usual alcohol intoxication and hangover in male twins: the NAS-NRC Twin Registry. Alcohol Clin Exp Res 38:2307–2313. doi: 10.1111/acer.12487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.