Abstract
Background.
Previous neuroimaging studies examining relations between alcohol misuse and cortical thickness have revealed that increased drinking quantity and alcohol-related problems are associated with thinner cortex. Although conflicting regional effects are often observed, associations are generally localized to frontal regions (e.g., dorsolateral prefrontal cortex (DLPFC), inferior frontal gyrus (IFG), and anterior cingulate cortex). Inconsistent findings may be attributed to methodological differences, modest sample sizes, and limited consideration of sex differences.
Method.
This study examined neuroanatomical correlates of drinking quantity and heavy episodic drinking in a large sample of younger adults (N=706; M age = 28.8; 51% female) using magnetic resonance imaging data from the Human Connectome Project. Exploratory analyses examined neuroanatomical correlates of executive function (flanker task) and working memory (list sorting).
Results.
Hierarchical linear regression models (controlling for age, sex, education, income, smoking, drug use, twin status, and intracranial volume) revealed significant inverse associations between drinks in past week and frequency of heavy drinking and cortical thickness in a majority of regions examined. The largest effect sizes were found for frontal regions (DLPFC, IFG, and the precentral gyrus). Follow-up regression models revealed that the left DLPFC was uniquely associated with both drinking variables. Sex differences were also observed, with significant effects largely specific to men.
Conclusions.
This study adds to the understanding of brain correlates of alcohol use in a large, gender-balanced sample of younger adults. Although the cross-sectional methodology precludes causal inferences, these findings provide a foundation for rigorous hypothesis testing in future longitudinal investigations.
Keywords: Alcohol, cortical thickness, dorsolateral prefrontal cortex, heavy drinking, human connectome project, neuroanatomy
Introduction
Detrimental effects of alcohol on brain structure have been widely reported in the literature (Oscar-Berman & Marinković, 2007; Pfefferbaum, Rosenbloom, Deshmukh, & Sullivan, 2001). These morphological effects include reductions in grey matter volume of cortical and subcortical structures (e.g., Fein et al., 2002; Grodin, Lin, Durkee, Hommer, & Momenan, 2013) and various abnormalities in brain activation patterns revealed via functional neuroimaging (e.g., Chanraud, Pitel, Pfefferbaum, & Sullivan, 2011; Shokri-Kojori, Tomasi, Wiers, Wang, & Volkow, 2017; Zheng, Kong, Chen, Zhang, & Zheng, 2015). In particular, abnormalities in areas of the frontal lobes, including the dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortex (ACC) and orbitofrontal cortex (OFC), have been emphasized as neuroanatomical hallmarks of alcohol use disorder (AUD) (Mechtcheriakov et al., 2007; Moselhy, Georgiou, & Kahn, 2001). Functional consequences of alcohol-related decreases in DLPFC volume include impairments in executive functions, behavioral control, and decision-making, among other higher-level neurocognitive processes (Crews & Boettiger, 2009).
Previous research has also shown that lower levels of cortical thickness, most prominently in the frontal regions of the brain, are correlated with greater levels of alcohol use and misuse. However, these findings have not been consistent across all studies. A magnetic resonance imaging (MRI) by Durazzo et al., (2011) compared individuals with AUD (N = 75) to healthy controls (N = 43) and found that the predominately male (96%) AUD group exhibited significantly thinner frontal cortices, including the DLPFC, ACC, anterior insula, and medial and lateral OFC (see also Durazzo, Mon, Gazdzinski & Meyerhoff 2013). Fortier et al., (2011) compared abstinent AUD participants to controls using a more sex balanced sample (38% female). The AUD group exhibited thinner cortex in a variety of frontal regions largely corresponding to the DLPFC, but there were no significant differences in the additional frontal regions reported by Durazzo et al. (2011; 2013). Momenan et al. (2012) compared cortical gray matter thickness in a large sample of individuals with AUD (N = 130) and controls (N = 69). The AUD group exhibited significantly thinner cortex in the bilateral DLPFC, right insula, precentral gyrus, and precuneus. Significant sex differences were also found with the AUD women exhibiting differences from control women in precentral and postcentral gyri, but none of the classic prefrontal regions. Men with AUD, however, exhibited differences from control men in frontal regions (anterior insula, DLPFC). Pennington et al. (2015) reported that male participants with AUD exhibited less bilateral ACC thickness compared to male control participants. Most recently, a ‘mega-analysis’ of structural MRI data pooled from 23 research laboratories (Mackey et al. 2018) found that individuals with AUD (N = 898) had lower levels of cortical thickness compared to controls (N = 292) in many of the same regions identified in prior studies, including DLPFC, ACC, insula, OFC, precentral gyrus, precuneus, and posterior cingulate, among others. Sex differences were not reported in this study.
Studies have also investigated sub-clinical samples of drinkers. For example, Mashhoon et al., (2014) examined cortical thickness in a sample of 23 (48% female) emerging adult binge drinkers vs. 31 (48% female) emerging adult light drinkers. In a priori region of interest analyses, they found that the rostral ACC and left dorsal posterior cingulate cortex were significantly thinner in the binge drinking group than the light drinking group. Within the binge drinking group, thickness of the rostral ACC was negatively correlated with alcohol consumption over the last three months, the average number of drinks consumed per drinking period, and the number of drinks consumed per day.
Taken together these studies generally suggest that alcohol misuse, possibly even at levels below clinical threshold for AUD, is associated with less cortical thickness than is typical; however, the specific regions implicated differ widely across studies (see summary of significant findings in Table 1). A number of methodological differences across previous studies may explain these inconsistent results. First, studies have not consistently considered sex effects or included samples that were highly unbalanced between male and female participants (e.g., <10% females; (Durazzo et al., 2011; Pennington et al., 2015). Second, previous research has varied widely in level of alcohol misuse, presence of AUD, and whether participants were currently or in treatment or recovery from AUD. Third, previous studies included relatively small sample sizes, with a few notable exceptions (Mackey et al., 2018; Momenan et al., 2012). Fourth, ages of participants varied widely across studies, from young adults ages 18–24 (e.g., Mashhoon et al., 2014) to adults in their 40–50s (e.g., Durazzo et al., 2011; Pennington et al., 2015). Finally, studies differed widely in the analytic approach used, including conducting whole-brain analyses (Durazzo et al., 2011; Fortier et al., 2011; Momenan et al., 2012) or restricting to a small number of ROIs (Bae et al., 2016; Durazzo et al., 2011; Fortier et al., 2011; Momenan et al., 2012; Pennington et al., 2015). Even within latter group, the specific ROIs examined varied widely (see Table 1). This variation across sample characteristics and analytic methods leads to a lack of consensus and limits direct comparison across studies.
Table 1.
Region of Interest Selection Based on Previous Findings
| Region | Durazzo (2011) |
Durazzo (2013) |
Fortier (2011) |
Mashhoon (2014) | Momenan (2012) | Pennington (2015) | Bae (2016) |
|---|---|---|---|---|---|---|---|
| DLPFC | X | X | X | X | X | X | |
| ACC | X | X | X | X | |||
| Anterior insula | X | X | X | X | |||
| OFC | X | X | |||||
| IFG | X | ||||||
| Precentral gyrus | X | X | |||||
| Postcentral gyrus | X | X | |||||
| Precuneus | X | ||||||
| Posterior Cingulate | X | ||||||
| Middle Temporal | X | ||||||
| Superior Temporal | X | ||||||
| Lateral Occipital | X |
Note. DLPFC = Dorsolateral prefrontal cortex; ACC = Anterior cingulate cortex; OFC = Orbitofrontal cortex;
IFG = Inferior frontal gyrus; Regions marked with X indicate statistically significant findings in the respective papers
As a result of inconsistent findings and limitations in the current literature, the aims of the current study were two-fold. First, to clarify the inconsistency across studies, we sought to examine cortical thickness in a comprehensive list of ROIs to determine which regions were associated with drinking quantity and heavy drinking. Second, we sought to explore these variables within a sample that was significantly larger, and more sex balanced than samples in previously published work. The current study examined T1-weighted structural MRI data from active drinkers in the open-source Human Connectome Project (HCP) dataset. The HCP is a multi-site neuroimaging research study that is systematically mapping the structure and function of the human brain and its clinical and neurocognitive correlates (see Van Essen et al. 2013 for an overview of the HCP). We capitalized on this unique resource to examine associations between cortical thickness and two indices of alcohol use—drinking quantity in the past week and frequency of heavy episodic drinking—in the largest sample of participants in a single study to date. Two exploratory aims were also investigated. First, we also examined sex differences in the patterns of association between cortical thickness and the alcohol variables. Second, we explored neuroanatomical correlates of two domains of cognitive functioning from the HCP behavioral assessment that are consistently linked to frontal cortical structures: executive function and working memory. Based on the general trends in previous studies, we hypothesized that cortical thickness would be negatively associated with alcohol consumption and frequency of heavy drinking, particularly in frontal regions (e.g., DLPFC, OFC, ACC, and anterior insula). Given the limited research examining sex differences in the associations between cortical thickness and alcohol use, no specific hypotheses regarding sex effects were made. For the analyses of cognitive processing, we predicted positive associations between cortical thickness and executive function and working memory.
Materials and Methods
Participants
Structural MRI brain scans were drawn from the 1200 Subjects HCP dataset (released March 1, 2017; available at http://www.humanconnectome.org/). The primary HCP participant pool consisted of community adults between the ages of 22–37. Participants were excluded from the HCP study if they had a history of neurodevelopmental, neuropsychiatric, or neurological disorders, as well as significant medical conditions such as diabetes or high blood pressure. The use of drugs or tobacco were not exclusionary and these variables were included as covariates in all analyses (see below). The current study sample consisted of HCP participants who reported consuming at least one alcoholic drink in the past-week retrospective alcohol assessment (details below), resulting in a sample of 711 participants. An additional 5 participants were excluded due to incomplete data (3 did not complete the drinking assessments, and 2 did not provide full demographic information). The final sample consisted of 706 participants.
Assessments
Access to restricted participant demographic and clinical data was granted via written authorization from the HCP Connectome Coordination Facility. Participants completed the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA, Bucholz et al., 1994). The SSAGA assesses physical, psychological, and social symptoms of alcohol abuse and dependence as well as other psychiatric disorders using DSM-III-R and DSM-IV criteria. The SSAGA also covers general demographic information, medical history, information about tobacco and drug use, and other mental health variables. Participants also completed a seven-day retrospective report of alcohol and tobacco use. Participants provided a commercially available urine drug screen for biochemical testing of recent drug use. Two neurocognitive measures from the HCP behavioral assessment battery were assessed here. These measures were drawn from the NIH Toolbox (http://www.healthmeasures.net/exploremeasurement-systems/nih-toolbox), including a measure of executive function/inhibition (flanker task) and a measure of working memory (list sorting). Full details of the HCP behavioral measures are provided elsewhere (Barch et al., 2013).
Alcohol Variables
Two alcohol variables from the HCP dataset were examined in the current study. The first, “Total drinks in past 7 days”, was taken from the retrospective alcohol use assessment. The second, “Frequency of drinking 5+ drinks in past 12 months”, was taken from the SSAGA assessment. This variable was coded categorically in the HCP dataset, but the coding for the highest frequency differed for males and females (i.e., the maximum response of 3+ days/week was coded as 1 for males, but 2 for females). The next lowest value (1–2 days/week) was coded as 2 for both males and females. Therefore, no distinction could be made between 1–2 vs. 3+ days/week for females. To standardize the maximum response, the data were recoded as follows (and reverse-scored such that higher values reflect greater frequency): 1 = never; 1–11 days/year = 2; 13 days/month = 3; weekly or greater = 4. Selection of these two alcohol variables was based on previous research examining continuous associations between recent drinking quantity and cortical thickness (e.g., Mashhoon et al. 2014) and prior research that focused specifically on structural brain correlates of binge drinking as a unique predictor of cortical thickness differences (e.g., Mashhoon et al. 2014).
MRI Data Acquisition and Data Quality Control
High-resolution T1-weighted structural images were collected on a 3T Siemens Skyra scanner (Siemens AG, Erlanger, Germany) with a 32-channel head coil (for full acquisition protocol, see Van Essen et al. 2013). Briefly, images were acquired with a 0.7 mm3 isotropic resolution (FOV = 224 × 240, matrix = 320 × 320, 256 sagittal slices; TR = 2400 ms and TE = 2.14 ms). Following MRI, each structural scan was examined by a trained rater to assess the overall quality of the scan’s contrast, blurring, ringing, and other possible artifacts. Only the scans rated as excellent were released as part of the HCP dataset. For full explanation of HCP quality control procedures, see Marcus et al., 2013.
FreeSurfer Processing Pipelines
Cortical thickness data were generated using the HCP minimal preprocessing pipeline described in Glasser et al., 2013. In the first part of the pipeline, PreFreeSurfer is used to produce a clear structural volume space for each subject, align the images, perform a B1 correction, and register the subject’s structural volume space to the MNI space. The second part of the pipeline is based on FreeSurfer version 5.2 (http://surfer.nmr.mgh.harvard.edu) (Fischl, 2012). This pipeline ensures that the volume is segmented into predefined structures, that the white and pial surfaces are reconstructed, and that FreeSurfer’s folding based surface registration (to the surface atlas fsaverage) is performed. Structural data analyzed for the current study came from this second step. This pipeline generates 34 anatomical regions of interest (ROIs) per cortical hemisphere. The mean cortical thickness in each of the 68 ROIs was provided per person in the HCP public dataset.
Selection of Regions of Interest
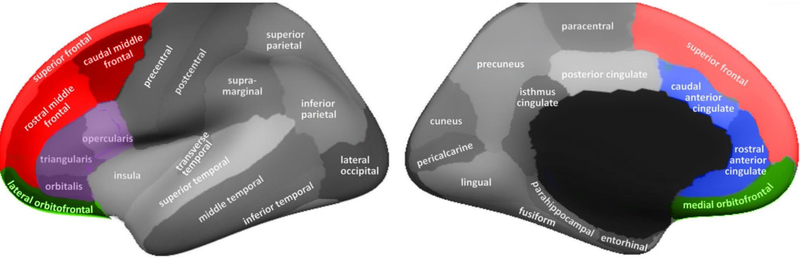
We examined cortical thickness in a series of a priori regions that were chosen based on previous studies on cortical thickness in alcohol use samples (Bae et al., 2016; Durazzo et al., 2013, 2011; Fortier et al., 2011; Mashhoon et al., 2014; Momenan et al., 2012; Pennington et al., 2015). Specifically, we identified 18 bilateral ROIs that have shown significant differences in at least one of these prior studies. Following conventions used in prior studies (e.g., Durazzo et al., 2013, 2011; Pennington et al., 2015), a subset of these regions were combined into composite regions corresponding to the ACC, OFC, IFG, and DLPFC, reducing the number of regions from 18 to 12. The ACC composite region was created by combining the caudal and rostral ACC segments. The DLPFC composite was created by combining the caudal MFG, rostral MFG, and the SFG segments. Lastly, the OFC composite was created by combining the lateral and medial OFC segments. The only previous study to observe significant results for the IFG (Momenan et al., 2012) did not indicate a specific sub-component of the IFG (pars opercularis, pars orbitalis, pars triangularis), so these three regions were combined into a single IFG composite region. The final list of 12 a priori ROIs and the studies reporting differences in these areas is provided in Table 1. The anatomical locations of individual ROIs and composite regions are depicted in Figure 1. These ROIs were examined separately for the left and right hemispheres using the average cortical thickness values for each region provided in the HCP dataset.
Figure 1. Anatomical locations of FreeSurfer regions of interest.
Lateral (top) and medial (bottom) views of inflated brain surface showing locations of regions of interest produced by cortical segmentation in FreeSurfer. The four composite regions are depicted in color: DLPFC is shown in red (consisting of superior frontal gyrus, and caudal and rostral middle frontal gyrus); ACC is shown in blue (consisting of caudal and rostral anterior cingulate); OFC is shown in green (comprised of lateral and medial OFC); IFG is shown in purple (comprised on pars opercularis, pars orbitalis, and pars triangularis sub-regions).
Data Analytic Plan
Selection of the covariates was based on previous work that has shown that age (e.g., Peters, 2006), sex (e.g., Taki et al., 2011), socioeconomic status (e.g., Piccolo, Merz, He, Sowell, & Noble, 2016), education (e.g., Boller, Mellah, Ducharme-Laliberté, & Belleville, 2017), use of tobacco (e.g., Durazzo et al., 2013) and cannabis and other drug use (e.g., Li et al., 2014; Lopez-Larson et al., 2011) are significantly associated with variation in cortical thickness. Twin status (i.e., monozygotic or not; dizygotic or not) was included given the twin enrichment of the HCP dataset. Estimated total intracranial volume was included to control for differences in overall head size.
Hierarchical multiple linear regression models were conducted to examine associations between cortical thickness indices and alcohol variables. Separate regression models were calculated for each of the ROIs. Step one of the models included age, sex, education, income, twin status, total intracranial volume, tobacco use, cannabis use, and other drug use as covariates. On the SSAGA, tobacco use was defined as total tobacco use in the past 7 days, cannabis use was defined as the total times participants reported using marijuana on the SSAGA; and other illicit drug use was defined as the total times participants reporting cocaine, hallucinogens, opiates, sedatives or other drugs on the SSAGA. In addition to the selfreport drug use variables, a binary variable was calculated based on any positive screen on the urine drug test (1 = any positive; 0 = all negative). Each cortical thickness ROI was added in the second step of the model and change in R2 was examined. Separate regression models were calculated for drinks in past week and frequency of heavy drinking. Given the relatively large number of ROIs, we applied a two-tailed False Discovery Rate (FDR; Benjamini & Hochberg, 1995) correction of q < .05 to reduce inflation of type I error rate in the individual regression analyses.
We then conducted an iterative regression analysis to determine the unique contributions of the ROIs that were significantly associated with the drinking variables in the individual regression models, after correcting for multiple comparisons (similar to the approach used in (Owens, Duda, Sweet, & MacKillop, 2018). In these models, individual ROIs that were found to be associated with alcohol use at FDR q < .05 in the previous step were entered sequentially starting with the largest effect size and proceeding to the smallest effect size. At each iteration, ROIs that significantly increased the variance explained by the model (i.e., had a significant ΔR2) were retained in the model. The iterative regression analysis used a conventional significance level of p < .05 and 95% confidence intervals are reported for regression coefficients.
Finally, we conducted two exploratory analyses. First, we examined potential sex difference associations between cortical thickness and the alcohol variables by repeating the primary analyses (i.e., the hierarchical multiple linear regression models) including an interaction term that was calculated by standardizing each region and sex, then multiplying each region by sex. All 24 ROIs were examined and an FDR of q < .05 was used. Continuous associations between cortical thickness in the 24 ROIs and cognitive performance on the flanker and list sorting tasks were explored using Pearson correlations with an FDR of q < .05 applied.
Results
Preliminary Analyses
Complete sample characteristics are provided in Table 2. Participants consumed an average of 7.2 drinks in the past week, and 44.5% engaged in heavy drinking (5+ drinks on an occasion) on a monthly or greater basis. Compared to females, males consumed significantly more drinks in the past week, F(1,705) = 86.11, p < .0001, η2 = .11, and reported significantly higher frequency of heavy drinking, F(1, 705) = 92.60, p < .0001, η2 = .12. See Table 2 for full comparisons between male and female participants.
Table 2.
Sample Characteristics
|
Variable |
Overall Mean(SD); %; Median |
Males Mean(SD); %; Median |
Females Mean(SD); %; Median |
Sig. |
|---|---|---|---|---|
| Sex | 51% Female | --- | --- | |
| Age | 28.77 (3.60) Range 22–37 | 28.10 (3.58) Range 22–37 | 29.40 (3.51) Range 22–36 | p < .001 |
| Education | 15.08 (1.74) Range 11–17 | 15.01 (1.72) Range 11–17 | 15.08 (1.74) Range 11–17 | p = .37 |
| Income (median) | $50,000–74,999 | $50,000–74,999 | $50,000–74,999 | p = .47 |
| Drinks Last 7 Days | 7.22 (6.63); Range 1–28 | 9.46 (7.75) Range 1–28 | 5.08 (4.42) Range 1–26 | p < .001 |
| Heavy Drinking Freq. | p < .001 | |||
| Never | 23.4% | 13.1% | 33.1% | |
| 1–11 days/year | 32.2% | 25.9% | 38.1% | |
| 1–3 days/month | 20.0% | 25.0% | 15.2% | |
| Weekly+ | 24.5% | 36.0% | 13.5% | |
| Drinks / Drinking Day | 1 (30%); 2 (28%); 3 (17%); 4 (8%); 5–6 (13%); 7+ (4%) | 1 (15%); 2 (26%); 3 (21%); 4 (12%); 5–6 (18%); 7+ (8%) | 1 (45%); 2 (30%); 3 (13%); 4 (4%); 5–6 (8%); 7+ (0%) | p < .001 |
| Tobacco Use (7-day) | 8.1 (25.60) Range 0 –195 | 10.78 (27.84) Range 0 –175 | 5.57 (20.69) Range 0 –195 | p = .005 |
| Cannabis Use | None (35.7%); 1–5x (22.1%); 6–10x (9.2%); 11–100x (14.4%); 101–999x (7.6%); 1000+ (10.9%) | None (31.1%); 1–5x (18.9%); 6–10x (8.7%); 11–100x (15.7%); 101–999x (10.2%); 1000+ (15.4%) | None (40.1%); 1–5x (25.1%); 6–10x (9.7%); 11–100x (13.3%); 101–999x (5.2%); 1000+ (6.6%) | p < .001 |
| Illicit Drug Use | None (73.4%); 1–2x (6.2%); 3–10x (9.3%); 11–25x (4.1%); 25+ (6.9%) | None (66.3%); 1–2x (7.0%); 3–10x (11.0%); 11–25x (5.2%); 25+ (10.5%) | None (80.1%); 1–2x (5.5%); 3–10x (7.7%); 11–25x (3.0%); 25+ (3.6%) | p < .001 |
| SSAGA AUD | 27.0% Positive | 36.3% Positive | 18.0% Positive | p < .001 |
| SSAGA CUD | 11.0% Positive | 17.7% Positive | 5.8% Positive | p < .001 |
Note: N = 706; Illicit drug use included cocaine, hallucinogens, opiates, sedatives, stimulants; SSAGA = Semi-structured Assessment for the Genetics of Alcoholism; AUD = Alcohol use disorder; CUD = Cannabis use disorder
We examined bivariate correlations among mean cortical thickness values for the 24 ROIs included in the primary analyses. The complete correlation matrix is provided in Supplementary Table 2. The magnitude of the associations between ROIs within the same hemisphere varied widely, with slightly smaller magnitude correlations in the right (rs = .15 - .79) compared to left (rs = .30 - .79) hemisphere. Magnitude of correlations between the hemispheric homologues of bilateral ROIs also varied (rs = .37 - .85), with the largest magnitude associations between left and right DLPFC (r = .85), IFG (r = .73), precentral gyrus (r = .77), postcentral gyrus (r = .77), precuneus (r = .75), and superior temporal gyrus (r = .75).
Cortical thickness and drinking quantity
Multiple regression analyses examining drinking quantity (drinks in past week) are presented in Table 3 (the covariate-only model is presented in Supplementary Table 2). After applying FDR correction, these analyses revealed statistically significant effects for 18 out of 24 ROIs. In each case, lower cortical thickness was associated with greater drinking quantity. Among the significant ROIs, effect sizes were generally small in magnitude, with the largest found for left DLPFC, the left precentral gyrus, and the right superior temporal gyrus. The iterative regression to determine incremental associations of each ROI with alcohol use began with the ROI that had the largest effect size (left DLPFC). Subsequently, we added the remaining significant ROIs individually in order of effect size to determine which ROIs would account for significant variance in drinking quantity. This analysis revealed that after including left DLPFC, no additional ROIs were associated with a statistically significant change in R2 (ps > .05). To explore whether the observed significant associations were driven by negative impact of other substance use on cortical thickness (e.g., Battistella et al., 2014; Karama et al., 2015; Mackey et al., 2018), we conducted a follow-up regression analysis with cortical thickness in each ROI as the dependent variable and alcohol, cannabis, nicotine, and illicit drug use variables as predictors (along with the same covariates as above). The same regions had significant R2 change as the original analysis that examined alcohol alone (data not shown), suggesting that the primary results are not solely attributed to effects of other substance use.
Table 3.
Cortical Thickness in Individual Regions of Interest Predicting Drinks in Past Week
| Region / Composite | L/R | B | SE | β | t | p | 95% CI | ΔR2 | FDR |
|---|---|---|---|---|---|---|---|---|---|
| DLPFC | L | −6.66 | 1.97 | −0.12 | −3.37 | <.001 | −10.53, –2.78 | .011 | sig. |
| R | −5.71 | 2.09 | −0.10 | −2.73 | .006 | −9.81, –1.61 | .007 | sig. | |
| ACC | L | −4.26 | 1.54 | −0.09 | −2.76 | .006 | −7.29, −1.24 | .007 | sig. |
| R | −1.63 | 1.27 | −0.04 | −1.29 | .199 | −4.13, 0.86 | .000 | n.s. | |
| Insula | L | −4.53 | 1.57 | −0.10 | −2.88 | .004 | −7.61, –1.44 | .007 | sig. |
| R | −2.70 | 1.72 | −0.06 | −1.57 | .117 | −6.09, 0.68 | .001 | n.s. | |
| OFC | L | −4.27 | 1.85 | −0.08 | −2.30 | .022 | −7.90, −0.63 | .004 | sig. |
| R | −4.04 | 2.01 | −0.07 | −2.01 | .045 | −7.99, −0.09 | .003 | n.s. | |
| IFG | L | −5.83 | 1.91 | −0.11 | −3.06 | .002 | −9.57, −2.09 | .009 | sig. |
| R | −6.41 | 1.97 | −0.11 | −3.25 | .001 | −10.28, −2.54 | .010 | sig. | |
| Precentral gyrus | L | −6.45 | 1.88 | −0.12 | −3.42 | <.001 | −10.15, −2.75 | .011 | sig. |
| R | −6.25 | 2.06 | −0.11 | −3.03 | .003 | −10.30, −2.20 | .008 | sig. | |
| Postcentral gyrus | L | −5.83 | 2.25 | −0.09 | −2.59 | .010 | −10.24, −1.41 | .006 | sig. |
| R | −5.58 | 2.27 | −0.09 | −2.45 | .014 | −10.04, −1.11 | .005 | sig. | |
| Precuneus | L | −4.84 | 1.95 | −0.09 | −2.49 | .013 | −8.66, −1.02 | .005 | sig. |
| R | −3.63 | 2.03 | −0.06 | −1.79 | .074 | −7.62, 0.35 | .002 | n.s. | |
| Posterior Cingulate | L | −4.46 | 1.65 | −0.09 | −2.70 | .007 | −7.70, −1.22 | .006 | sig. |
| R | −2.22 | 1.48 | −0.05 | −1.50 | .134 | −5.13, 0.69 | .001 | n.s. | |
| Middle Temporal | L | −4.65 | 1.73 | −0.09 | −2.68 | .007 | −8.05, −1.25 | .006 | sig. |
| R | −4.68 | 1.81 | −0.09 | −2.58 | .010 | −8.25, −1.12 | .006 | sig. | |
| Superior Temporal | L | −4.74 | 1.73 | −0.10 | −2.74 | .006 | −8.14, −1.34 | .007 | sig. |
| R | −6.73 | 1.81 | −0.13 | −3.71 | <.001 | −10.29, −3.17 | .014 | sig. | |
| Lateral Occipital | L | −4.83 | 2.10 | −0.08 | −2.30 | .021 | −8.95, −0.72 | .004 | sig. |
| R | −2.22 | 2.17 | −0.04 | −1.02 | .307 | −6.48, 2.04 | −.001 | n.s. |
Note: L=left; R=Right; FDR=False discovery rate (sig. indicates q < .05); DLPFC = Dorsolateral prefrontal cortex; ACC = Anterior cingulate cortex; OFC = Orbitofrontal cortex; IFG = Inferior frontal gyrus; ΔR2 = change in R2 from covariate model (R2=.207)
Cortical thickness and heavy drinking frequency
Regression results for the heavy drinking frequency variable are presented in Table 4. Compared to the drinking quantity results above, these analyses generally revealed smaller magnitude effects in fewer ROIs following FDR correction (14 out of 24 ROIs). In each case, higher frequency of heavy drinking was associated with lower cortical thickness. The largest effect sizes were found for the left DLPFC and the left precentral gyrus. Results of the iterative regression analysis were similar to drinking quantity. Beyond left DLPFC, no other ROIs accounted for a significant change in R2 (ps > .05). Similar to the drinking quantity analyses, we conducted a follow-up analysis with cortical thickness as the dependent variable. Once again, the results were identical with the exception of left IFG which was no longer significant in the follow-up model (data not shown).
Table 4.
Cortical Thickness in Individual Regions of Interest Predicting Heavy Drinking Frequency
| Region / Composite | L/R | B | SE | β | t | p | 95% CI | ΔR2 | FDR |
|---|---|---|---|---|---|---|---|---|---|
| DLPFC | L | −1.08 | 0.33 | −0.12 | −3.28 | .001 | −1.73, −0.43 | .010 | sig. |
| R | −1.08 | 0.35 | −0.11 | −3.11 | .002 | −1.77, −0.40 | .009 | sig. | |
| ACC | L | −0.79 | 0.26 | −0.11 | −3.07 | .002 | −1.29, −0.28 | .009 | sig. |
| R | −0.27 | 0.21 | −0.04 | −1.25 | .210 | −0.68, 0.15 | .000 | n.s. | |
| Insula | L | −0.65 | 0.26 | −0.09 | −2.48 | .014 | −1.17, −0.13 | .005 | sig. |
| R | −0.35 | 0.29 | −0.04 | −1.20 | .230 | −0.91, 0.22 | .000 | n.s. | |
| OFC | L | −0.30 | 0.31 | −0.03 | −0.96 | .339 | −0.91, 0.31 | −.001 | n.s. |
| R | −0.50 | 0.34 | −0.05 | −1.49 | .137 | −1.16, 0.16 | .001 | n.s. | |
| IFG | L | −0.92 | 0.32 | −0.10 | −2.90 | .004 | −1.55, −0.30 | .008 | sig. |
| R | −0.70 | 0.33 | −0.07 | −2.13 | .034 | −1.35, −0.05 | .003 | n.s. | |
| Precentral gyrus | L | −1.03 | 0.31 | −0.11 | −3.27 | .001 | −1.65, −0.41 | .010 | sig. |
| R | −1.04 | 0.34 | −0.11 | −3.01 | .003 | −1.71, −0.36 | .009 | sig. | |
| Postcentral gyrus | L | −1.00 | 0.38 | −0.09 | −2.67 | .008 | −1.74, −0.27 | .006 | sig. |
| R | −1.07 | 0.38 | −0.10 | −2.82 | .005 | −1.81, −0.32 | .007 | sig. | |
| Precuneus | L | −0.60 | 0.33 | −0.06 | −1.84 | .066 | −1.24, 0.04 | .002 | n.s. |
| R | −0.60 | 0.34 | −0.06 | −1.78 | .075 | −1.27, 0.06 | .002 | n.s. | |
| Posterior Cingulate | L | −0.66 | 0.28 | −0.08 | −2.40 | .016 | −1.20, −0.12 | .005 | sig. |
| R | −0.70 | 0.25 | −0.10 | −2.86 | .004 | −1.19, −0.22 | .008 | sig. | |
| Middle Temporal | L | −0.69 | 0.29 | −0.08 | −2.37 | .018 | −1.26, −0.12 | .005 | sig. |
| R | −0.70 | 0.30 | −0.08 | −2.32 | .021 | −1.30, −0.11 | .004 | sig. | |
| Superior Temporal | L | −0.33 | 0.29 | −0.04 | −1.15 | .252 | −0.90, 0.24 | .000 | n.s. |
| R | −0.89 | 0.30 | −0.10 | −2.92 | .004 | −1.49, −0.29 | .008 | sig. | |
| Lateral Occipital | L | −0.66 | 0.35 | −0.07 | −1.87 | .061 | −1.34, 0.03 | .002 | n.s. |
| R | −0.29 | 0.36 | −0.03 | −0.80 | .423 | −1.00, 0.42 | −.001 | n.s. |
Note: L=left; R=Right; FDR=False discovery rate (sig. indicates q < .05); DLPFC = Dorsolateral prefrontal cortex; ACC = Anterior cingulate cortex; OFC = Orbitofrontal cortex; IFG = Inferior frontal gyrus; ΔR2 = change in R2 from covariate model (R2=.195)
Exploratory Analyses of Sex Effects
We explored potential sex differences in the relationship between cortical thickness and alcohol variables. In the case of drinks in past week, we found significant region × sex interactions (after FDR correction) in 10 of the 24 ROIs examined (Table 5), including left ACC, left DLPFC, left insula, left OFC, left posterior cingulate, left MTG, and bilateral IFG and STG. In each case, male participants showed a significant negative association between cortical thickness and drinks in the past week which was not observed in female participants. None of region × sex interactions were significant for heavy drinking frequency.
Table 5.
Sex × Region of Interest Interactions in Regression Models Predicting Drinks in Past Week
| Region / Composite | L/R | B | SE | β | t | p | 95% CI | ΔR2 | FDR |
|---|---|---|---|---|---|---|---|---|---|
| DLPFC | L | −1.17 | 0.45 | −0.13 | −2.58 | .010 | −2.06, −0.28 | .003 | sig. |
| R | −0.90 | 0.45 | −0.10 | −1.99 | .047 | −1.79, −0.01 | .009 | n.s. | |
| ACC | L | −1.16 | 0.45 | −0.13 | −2.58 | .010 | −2.04, −0.28 | .016 | sig. |
| R | −0.54 | 0.45 | −0.05 | −1.19 | .234 | −1.43, 0.35 | .009 | n.s. | |
| Insula | L | −1.31 | 0.46 | −0.14 | −2.85 | .005 | −2.22, −0.41 | .013 | sig. |
| R | −0.73 | 0.47 | −0.08 | −1.56 | .118 | −1.65, 0.19 | .004 | n.s. | |
| OFC | L | −1.36 | 0.45 | −0.15 | −2.99 | .003 | −2.25, −0.47 | .016 | sig. |
| R | −0.62 | 0.45 | −0.07 | −1.37 | .170 | −1.51, 0.27 | .015 | n.s. | |
| IFG | L | −1.35 | 0.45 | −0.15 | −3.00 | .003 | −2.23, −0.47 | .020 | sig. |
| R | −1.16 | 0.45 | −0.13 | −2.56 | .011 | −2.04, −0.27 | .008 | sig. | |
| Precentral gyrus | L | −0.87 | 0.45 | −0.10 | −1.92 | .056 | −1.76, 0.02 | .019 | n.s. |
| R | −0.44 | 0.46 | −0.05 | −0.96 | .336 | −1.33, 0.46 | .023 | n.s. | |
| Postcentral gyrus | L | −0.50 | 0.45 | −0.05 | −1.10 | .273 | −1.38, 0.39 | .007 | n.s. |
| R | −0.57 | 0.45 | −0.06 | −1.28 | .202 | −1.46, 0.31 | .009 | n.s. | |
| Precuneus | L | −0.36 | 0.45 | −0.04 | −0.78 | .435 | −1.25, 0.54 | .017 | n.s. |
| R | 0.29 | 0.45 | 0.03 | 0.64 | .519 | −0.60, 1.18 | .010 | n.s. | |
| Posterior Cingulate | L | −1.08 | 0.45 | −0.12 | −2.41 | .016 | −1.96, −0.20 | .021 | sig. |
| R | 0.05 | 0.45 | 0.01 | 0.10 | .918 | −0.84, 0.93 | .018 | n.s. | |
| Middle Temporal | L | −1.09 | 0.45 | −0.12 | −2.40 | .016 | −1.97, −0.20 | .005 | sig. |
| R | −0.58 | 0.46 | −0.07 | −1.28 | .203 | −1.48, 0.31 | .002 | n.s. | |
| Superior Temporal | L | −1.35 | 0.45 | −0.15 | −2.99 | .003 | −2.23, −0.46 | .011 | sig. |
| R | −1.15 | 0.45 | −0.13 | −2.54 | .011 | −2.04, −0.26 | .003 | sig. | |
| Lateral Occipital | L | −0.86 | 0.45 | −0.09 | −1.90 | .058 | −1.74, 0.03 | .018 | n.s. |
| R | −0.58 | 0.46 | −0.06 | −1.27 | .203 | −1.48, 0.31 | .015 | n.s. |
Note: L=left; R=Right; FDR=False discovery rate (sig. indicates q < .05); DLPFC = Dorsolateral prefrontal cortex; ACC = Anterior cingulate cortex; OFC = Orbitofrontal cortex; IFG = Inferior frontal gyrus; ΔR2 = change in R2 from covariate model (R2=.176).
Exploratory Analyses of Cognitive Performance
Results of the exploratory correlations between cognitive performance on flanker and list sorting tasks and the alcohol and cortical thickness variables are presented in Supplementary Table 2. Neither drinks in the past week nor heavy drinking frequency were correlated with flanker or list sorting performance (ps > .17). Flanker task performance was not significantly correlated with cortical thickness in any of the 24 ROIs. Working memory performance on the list sorting task was significantly (after FDR correction) positively correlated with cortical thickness in 9 ROIs, including bilateral middle temporal cortex and posterior cingulate and right precentral gyrus, insula, precuneus, superior temporal gyrus, and lateral occipital gyrus. However, the magnitude of the correlations was generally small (rs .09 - .12).
Discussion
The current study examined neuroanatomical correlates of alcohol use and heavy episodic drinking in one of the largest sex-balanced samples to date. Consistent with previous studies, we found that thinner cortex in a number of regions was associated with greater alcohol consumption and more frequent heavy drinking. These findings make three important contributions to the literature: 1) they empirically demonstrate the unique association of the left DLPFC with alcohol use compared to other regions, 2) they demonstrate sex differences in associations of cortical thickness with alcohol use, and 3) they indicate significant associations between cortical morphometry and drinking in a relatively young and typical sample of drinkers. These contributions are discussed below.
The first main contribution of these findings is the robust association between the left DLPFC and drinking quantity and frequency of heavy drinking. This finding is consistent with several prior cortical thickness studies showing significantly thinner cortex in DLPFC among individuals with AUD or those who report binge drinking (Bae et al., 2016; Durazzo et al., 2013, 2011; Fortier et al., 2011; Mackey et al., 2018; Pennington et al., 2015). The DLPFC findings are particularly important given the role of this region in cognitive control and other executive functions that contribute to drinking decisions (Niendam et al., 2012). The DLPFC is also commonly targeted via neuromodulation interventions using non-invasive brain stimulation techniques (e.g., Boggio et al., 2008; Coles, Kozak, & George, 2018; Lupi et al., 2017; Mishra, Nizamie, Das, & Praharaj, 2010) and cognitive interventions such as executive function or working memory training (Duda and Sweet, In Press; Olesen, Westerberg and Klingberg, 2004; Takeuchi et al., 2011). Furthermore, by demonstrating that no regions are associated with alcohol use beyond their shared correlations with the DLPFC, the current study highlights the importance of DLPFC in understanding how cortical thickness is linked with alcohol use. There are several possible interpretations of this finding. One interpretation is that reduced DLFPC thickness is causing more problematic alcohol use (perhaps through impaired executive control or other DLPFC-mediated cognitive mechanisms) and all other regional cortical thickness associations with alcohol are solely the result of their association with DLFPC thickness. This would suggest cortical thickness in the DLFPC is a key driver of alcohol use. Another interpretation is that alcohol use is causing cortical thinning across the whole brain through a single mechanism, with the most severe atrophy occurring in the DLPFC. This would suggest that the hierarchical regression results are indicative only of the high magnitude of the correlations between the DLFPC and alcohol. However, the cross-sectional nature of the HCP data does not permit conclusions about causality, and future longitudinal research is needed to tease apart such interpretations.
The significant sex differences are also notable. Studies examining differences in the influence of alcohol on the brain between men and women are sparse and discrepant (Hommer, 2003). Some studies of AUD samples have reported greater brain shrinkage in women compared to men (e.g., Hommer, Momenan, Kaiser, & Rawlings, 2001), others have reported effects in the reverse direction (Pfefferbaum et al., 2001) or no sex differences (Gescuk, Woods, Mello, Weiss & Mendelson; Pfefferbaum & Sullivan 2002). Although several previous studies examined cortical thickness in relation to alcohol misuse, only one study by Momenan et al. (2012) reported sex effects. The large sample size and relatively equal sex distribution of the HCP dataset provided us the opportunity to test sex effects sufficient statistical power. Our results suggest that the associations between reduced cortical thickness and drinking in this sample of younger adult drinkers may be driven by effects in the men which differs somewhat from findings in the previous AUD studies (with the exception of Pfefferbaum et al., 2001). The regions implicated were predominately localized in the DLPFC and other frontal regions (e.g, OFC, IFG, ACC), again supporting the important role of frontal regions in drinking behavior. However, associations between drinking quantity and regions of temporal cortex (e.g., MTG, STG) and the posterior cingulate also differed between males and females.
A final important contribution of the current study relates to the nature of the HCP sample, which was notable in its relatively younger age and lack of neurodevelopmental disorders, neuropsychiatric disorders, and neurologic disorders (Van Essen et al., 2013) which may negatively impact cortical thickness. Significant negative associations between reduced cortical thickness and alcohol variables in a sample of young adults without an extensive life-long history of alcohol misuse suggests that low levels of cortical thickness may be a risk factor for engagement in alcohol misuse. However, we emphasize that conclusions regarding causation are outside the scope of a cross-sectional study.
These findings should be considered within the context of several potential limitations. One notable limitation concerns the alcohol measures, which were based on retrospective self-report and, as such, may have been subject to recall bias or demand characteristics. Of note, since the HCP was not primarily focused on alcohol use, the measurement resolution for some of the alcohol variables was somewhat coarse. This is particularly true in the case of lifetime alcohol use and AUD severity which are important variables to consider. Unfortunately, the variables provided in the HCP dataset do not permit accurate calculation of a continuous measure of AUD severity or an accurate index of lifetime alcohol exposure. Another consideration is that the sample was comprised of adults between the ages of 22–35. Although the younger age range allowed us to infer relationships with brain structure that are presumably independent of extensive neurotoxicity from chronic alcohol misuse, these findings may not generalize to other age groups or individuals with comorbid psychiatric or other health conditions. Additionally, it is possible that effect sizes were smaller in the current results than would be the case if a clinical sample were used with more severe AUD (e.g., Mackey et al., 2018).
In summary, this study further clarifies the structural brain correlates of alcohol use in a large sample of drinkers. The large HCP cohort provided high statistical power and we observed significant effects in a majority of regions reported in prior studies. When considered together, however, the DLPFC was the region most robustly and uniquely associated with the alcohol variables. Our findings are also consistent with the recent ‘mega-analysis’ of cortical thickness deficits in people with AUD compared to healthy controls (Mackey et al., 2018), but also provide an important extension by demonstrating significant associations in a comparatively younger sample of drinkers. Moreover, our sex balanced sample allowed for the investigation of sex differences in relation to cortical thickness and alcohol misuse; findings demonstrated that male participants exhibited significant negative associations between cortical thickness and drinking quantity in several regions which were not observed in female participants. The consistency between our results and prior studies is an important finding in the context of prominent concerns about reproducibility in addictions (Munafò, 2017) and neuroimaging (Gorgolewski & Poldrack, 2016) research. Although the current study cannot speak to disentangling cause from consequence, these findings provide a foundation for rigorous hypothesis testing in future longitudinal investigations. Another potentially important future direction is to examine the cognitive and neuropsychological correlates of these associations between cortical thickness and alcohol use. The HCP dataset includes a wide array of cognitive, emotional, and neuropsychological measures that would permit such analyses in future studies.
Supplementary Material
Funding Support and Acknowledgements:
Contributions to the work was partially supported by NIH grant AA026392 (MA, JM) and AA025911 (LHS, JM, MA), the Peter Boris Chair in Addictions Research (JM), and the Gary Sperduto Endowed Professorship in Clinical Psychology (LHS). JM is a principal in BEAM Diagnostics, Inc.. The data used in this project are from the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University in St. Louis. The authors are deeply appreciative to the Human Connectome Project for open access to its data. No funding sources were involved in study design or collection, analysis, and interpretation of the data. These findings do not reflect the official position of the National Institutes of Health
References
- Bae S, Kang I, Lee BC, Jeon Y, Cho HB, Yoon S, Lim SM, Kim J, Lyoo IK, Kim JE, & Choi I-G (2016). Prefrontal Cortical Thickness Deficit in Detoxified Alcohol-dependent Patients. Experimental Neurobiology, 25(6), 333–341. 10.5607/en.2016.25.6.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Burgess GC, Harms MP, Petersen SE, Schlaggar BL, Corbetta M, Glasser MF, Curtiss S, Dixit S, Feldt C, Nolan D, Bryant E, Hartley T, Footer O, Bjork JM, Poldrack R, Smith S, Johansen-Berg H, Snyder AZ, Van Essen DC, & WU-Minn HCP Consortium. (2013). Function in the human connectome: Task-fMRI and individual differences in behavior. NeuroImage, 80, 169–189. 10.1016/j.neuroimage.2013.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistella G, Fornari E, Annoni J-M, Chtioui H, Dao K, Fabritius M, Favrat B, Mall J-F, Maeder P, & Giroud C (2014). Long-Term Effects of Cannabis on Brain Structure. Neuropsychopharmacology, 39(9), 2041–2048. 10.1038/npp.2014.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 57(1), 289–300. [Google Scholar]
- Boggio PS, Sultani N, Fecteau S, Merabet L, Mecca T, Pascual-Leone A, Basaglia A, & Fregni F (2008). Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: A double-blind, sham-controlled study. Drug and Alcohol Dependence, 92(1–3), 55–60. 10.1016/j.drugalcdep.2007.06.011 [DOI] [PubMed] [Google Scholar]
- Boller B, Mellah S, Ducharme-Laliberté G, & Belleville S (2017). Relationships between years of education, regional grey matter volumes, and working memory-related brain activity in healthy older adults. Brain Imaging and Behavior, 11(2), 304–317. 10.1007/s11682-016-9621-7 [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Reich T, Schmidt I, & Schuckit MA (1994). A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. Journal of Studies on Alcohol, 55(2), 149–158. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Pfefferbaum A, & Sullivan EV (2011). Disruption of functional connectivity of the default-mode network in alcoholism. Cerebral Cortex, 21(10), 2272–2281. 10.1093/cercor/bhq297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles AS, Kozak K, & George TP (2018). A review of brain stimulation methods to treat substance use disorders. The American Journal on Addictions, 27(2), 71–91. 10.1111/ajad.12674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, & Boettiger CA (2009). Impulsivity, frontal lobes and risk for addiction. Pharmacology, Biochemistry, and Behavior, 93(3), 237–247. 10.1016/j.pbb.2009.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda B, & Sweet LH (n.d.). Functional Brain Changes Associated with Cognitive Training in Healthy Older Adults: A Preliminary ALE Meta-Analysis. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Mon A, Gazdzinski S, & Meyerhoff DJ (2013). Chronic cigarette smoking in alcohol dependence: Associations with cortical thickness and N-acetylaspartate levels in the extended brain reward system . Addiction Biology. 10.1111/j.1369-1600.2011.00407.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Tosun D, Buckley S, Gazdzinski S, Mon A, Fryer SL, & Meyerhoff DJ (2011). Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcoholism, Clinical and Experimental Research, 35(6), 1187–1200. 10.1111/j.1530-0277.2011.01452.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, & Meyerhoff DJ (2002). Cortical Gray Matter Loss in Treatment-Naive Alcohol Dependent Individuals. Alcoholism: Clinical and Experimental Research, 26(4), 558–564. 10.1111/j.1530-0277.2002.tb02574.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B (2012). FreeSurfer. NeuroImage, 62(2), 774–781. 10.1016/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier CB, Leritz EC, Salat DH, Venne JR, Maksimovskiy AL, Williams V, Milberg WP, & Mcglinchey RE (2011). Reduced cortical thickness in abstinent alcoholics and association with alcoholic behavior. Alcoholism, Clinical and Experimental Research, 35(12), 2193–2201. 10.1111/j.1530-0277.2011.01576.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JR, Van Essen DC, Jenkinson M, & WU-Minn HCP Consortium, for the W.-M. H. (2013). The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage, 80, 105–124. 10.1016/j.neuroimage.2013.04.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski KJ, & Poldrack RA (2016). A Practical Guide for Improving Transparency and Reproducibility in Neuroimaging Research. PLOS Biology, 14(7), e1002506 10.1371/journal.pbio.1002506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodin EN, Lin H, Durkee CA, Hommer DW, & Momenan R (2013). Deficits in cortical, diencephalic and midbrain gray matter in alcoholism measured by VBM: Effects of co-morbid substance abuse. NeuroImage. Clinical, 2, 469–476. 10.1016/j.nicl.2013.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer D (2003). Male and female sensitivity to alcohol-induced brain damage. Alcohol Research & Health : The Journal of the National Institute on Alcohol Abuse and Alcoholism, 27(2), 181–185. [PMC free article] [PubMed] [Google Scholar]
- Hommer D, Momenan R, Kaiser E, & Rawlings RR (2001). Evidence for a Gender-Related Effect of Alcoholism on Brain Volumes. American Journal of Psychiatry, 158(2), 198–204. 10.1176/appi.ajp.158.2.198 [DOI] [PubMed] [Google Scholar]
- Karama S, Ducharme S, Corley J, Chouinard-Decorte F, Starr JM, Wardlaw JM, Bastin ME, & Deary IJ (2015). Cigarette smoking and thinning of the brain’s cortex. Molecular Psychiatry, 20(6), 778–785. 10.1038/mp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroft CL, Gescuk B, Woods BT, Mello NK, Weiss RD, & Mendelson JH (n.d.) Brain ventricular size in female alcoholics: an MRI study. Alcohol (Fayetteville, N.Y.), 8(1), 31–34. [DOI] [PubMed] [Google Scholar]
- Li M, Tian J, Zhang R, Qiu Y, Wen X, Ma X, Wang J, Xu Y, Jiang G, & Huang R (2014). Abnormal cortical thickness in heroin-dependent individuals. NeuroImage, 88, 295–307. 10.1016/J.NEUROIMAGE.2013.10.021 [DOI] [PubMed] [Google Scholar]
- Lopez-Larson MP, Bogorodzki P, Rogowska J, McGlade E, King JB, Terry J, & Yurgelun-Todd D (2011). Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behavioural Brain Research, 220(1), 164–172. Retrieved from https://www.sciencedirect.com/science/article/pii/S0166432811000969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupi M, Martinotti G, Santacroce R, Cinosi E, Carlucci M, Marini S, Acciavatti T, & di Giannantonio M (2017). Transcranial Direct Current Stimulation in Substance Use Disorders. The Journal of ECT, 33(3), 203–209. 10.1097/YCT.0000000000000401 [DOI] [PubMed] [Google Scholar]
- Mackey S, Allgaier N, Chaarani B, Spechler P, Orr C, Bunn J, Allen NB, Alia-Klein N, Batalla A, Blaine S, Brooks S, Caparelli E, Chye YY, Cousijn J, Dagher A, Desrivieres S, Feldstein-Ewing S, Foxe JJ, Goldstein RZ, Goudriaan AE, Heitzeg MM, Hester R, Hutchison K, Korucuoglu O, Li C-SR, London E, Lorenzetti V, Luijten M, Martin-Santos R, May A, Momenan R, Morales A, Paulus MP, Pearlson G, Rousseau M-E, Salmeron BJ, Schluter R, Schmaal L, Schumann G, Sjoerds Z, Stein DJ, Stein EA, Sinha R, Solowij N, Tapert S, Uhlmann A, Veltman D, van Holst R, Whittle S, Wright MJ, Yücel M, Zhang S, Yurgelun-Todd D, Hibar DP, Jahanshad N, Evans A, Thompson PM, Glahn DC, Conrod P, Garavan H, & ENIGMA Addiction Working Group. (2018). Mega-Analysis of Gray Matter Volume in Substance Dependence: General and Substance-Specific Regional Effects. American Journal of Psychiatry, appi.ajp.2018.1. 10.1176/appi.ajp.2018.17040415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DS, Harms MP, Snyder AZ, Jenkinson M, Wilson JA, Glasser MF, Barch DM, Archie KA, Burgess GC, Ramaratnam M, Hodge M, Horton W, Herrick R, Olsen T, McKay M, House M, Hileman M, Reid E, Harwell J, Coalson T, Schindler J, Elam JS, Curtiss SW, Van Essen DC, & WU-Minn HCP Consortium. (2013). Human Connectome Project informatics: Quality control, database services, and data visualization. NeuroImage, 80, 202–219. 10.1016/j.neuroimage.2013.05.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashhoon Y, Czerkawski C, Crowley DJ, Cohen-Gilbert JE, Sneider JT, & Silveri MM (2014). Binge Alcohol Consumption in Emerging Adults: Anterior Cingulate Cortical “Thinness” Is Associated with Alcohol Use Patterns. Alcoholism: Clinical and Experimental Research. 10.1111/acer.12475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechtcheriakov S, Brenneis C, Egger K, Koppelstaetter F, Schocke M, & Marksteiner J (2007). A widespread distinct pattern of cerebral atrophy in patients with alcohol addiction revealed by voxel-based morphometry. Journal of Neurology, Neurosurgery, and Psychiatry. 10.1136/jnnp.2006.095869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra BR, Nizamie SH, Das B, & Praharaj SK (2010). Efficacy of repetitive transcranial magnetic stimulation in alcohol dependence: A sham-controlled study. Addiction, 105(1), 49–55. 10.1111/j.1360-0443.2009.02777.x [DOI] [PubMed] [Google Scholar]
- Momenan R, Steckler LE, Saad ZS, van Rafelghem S, Kerich MJ, & Hommer DW (2012). Effects of alcohol dependence on cortical thickness as determined by magnetic resonance imaging. Psychiatry Research. 10.1016/j.pscychresns.2012.05.003 [DOI] [PubMed] [Google Scholar]
- Moselhy HF, Georgiou G, & Kahn A (2001). Frontal lobe changes in alcohoslism: a review of the literature. Alcohol and Alcoholism, 36(5), 357–368. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11524299 [DOI] [PubMed] [Google Scholar]
- Munafò MR (2017). Promoting reproducibility in addiction research. Addiction, 112(9), 1519–1520. 10.1111/add.13853 [DOI] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, & Carter CS (2012). Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive, Affective, & Behavioral Neuroscience, 12(2), 241–268. 10.3758/s13415-011-0083-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen PJ, Westerberg H, & Klingberg T (2004). Increased prefrontal and parietal activity after training of working memory. Nature Neuroscience, 7(1), 75–79. 10.1038/nn1165 [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, & Marinković K (2007). Alcohol: effects on neurobehavioral functions and the brain. Neuropsychology Review, 17(3), 239–257. 10.1007/s11065-007-9038-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens MM, Duda B, Sweet LH, & MacKillop J (2018). Distinct functional and structural neural underpinnings of working memory. NeuroImage, 174, 463–471. 10.1016/j.neuroimage.2018.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington DL, Durazzo TC, Schmidt TP, Abé C, Mon A, & Meyerhoff DJ (2015). Alcohol use disorder with and without stimulant use: brain morphometry and its associations with cigarette smoking, cognition, and inhibitory control. PloS One, 10(3), e0122505 10.1371/journal.pone.0122505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R (2006). Ageing and the brain. Postgraduate Medical Journal, 82(964), 84–88. 10.1136/pgmj.2005.036665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Deshmukh A, & Sullivan EV (2001). Sex Differences in the Effects of Alcohol on Brain Structure. American Journal of Psychiatry, 158(2), 188–197. 10.1176/appi.ajp.158.2.188 [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, & Sullivan EV (2002). Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. NeuroImage, 15(3), 708–718. 10.1006/nimg.2001.1018 [DOI] [PubMed] [Google Scholar]
- Piccolo LR, Merz EC, He X, Sowell ER, Noble KG, & Pediatric Imaging NGS (2016). Age-Related Differences in Cortical Thickness Vary by Socioeconomic Status. PLOS ONE, 11(9), e0162511 10.1371/journal.pone.0162511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokri-Kojori E, Tomasi D, Wiers CE, Wang G-J, & Volkow ND (2017). Alcohol affects brain functional connectivity and its coupling with behavior: greater effects in male heavy drinkers. Molecular Psychiatry, 22(8), 1185–1195. 10.1038/mp.2016.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Hashizume H, Sassa Y, Nagase T, Nouchi R, & Kawashima R (2011). Failing to deactivate: The association between brain activity during a working memory task and creativity. NeuroImage, 55(2), 681–687. 10.1016/j.neuroimage.2010.11.052 [DOI] [PubMed] [Google Scholar]
- Taki Y, Thyreau B, Kinomura S, Sato K, Goto R, Kawashima R, & Fukuda H (2011). Correlations among brain gray matter volumes, age, gender, and hemisphere in healthy individuals. PloS One, 6(7), e22734 10.1371/journal.pone.0022734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Smith SM, Barch DM, Behrens TEJ, Yacoub E, & Ugurbil K (2013). The WU-Minn Human Connectome Project: An Overview David. Neuroimage., 62–79. 10.1016/j.neuroimage.2013.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Kong L, Chen L, Zhang H, & Zheng W (2015). Acute Effects of Alcohol on the Human Brain: A Resting-State fMRI Study. BioMed Research International, 2015, 1–10. 10.1155/2015/947529 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.