Abstract
Inhibitory control matures through adolescence and into early adulthood, impacting decision making. Impairments in inhibitory control are associated with various psychopathologies, many of which emerge during adolescence. In this review, we examine the neural basis of developmental improvements in inhibitory control by integrating findings from humans and non-human primates identifying the structural and functional specialization of executive brain systems that mediates cognitive maturation. Behavioral manifestations of response inhibition suggest that adolescents are capable of producing adult level responses on occasion, but lack the ability to engage systems mediating response inhibition in a consistent fashion. Maturation is associated with changes in structural anatomy, as well as local and systems-level connectivity. Functional changes revealed by neuroimaging and neurophysiology, indicate that maturation of inhibitory control is achieved through improvements in response preparation, error processing, and planned responses.
Keywords: Response inhibition, development, executive function, neurophysiology, fMRI, antisaccade, prefrontal
Maturation of neural systems supporting inhibitory control
Adolescence is a unique time in development, demarcated by puberty and characterized by an increase in impulsive sensation-seeking behaviors. In some teenagers, these changes elevate to extreme risk-taking and counter-productive behaviors, in some cases even undermining survival (e.g., careless driving, extreme sports) [1]. This adolescent phenotype has been traditionally attributed to limitations in engaging inhibitory control: the ability to voluntarily suppress a reactive response in order to generate a planned executive goal driven response [2–4]. In recent years, a more nuanced picture has emerged. Inhibitory control is evident early in development [5] with incremental improvements that continue into adulthood [6–8]. By adolescence, cognitive control has improved significantly with evidence for the ability to engage cognitive control systems at adult levels; however, the ability to engage these processes in a flexible and consistent fashion continues to be limited until adulthood [4].
Significant advances in our understanding of the specialization of the neurocognitive systems needed for optimal and reliable adult level inhibitory control have been made in recent years. Human brain imaging studies relying on functional Magnetic Resonance (fMRI) and Magnetoencephalography (MEG) have identified age-related changes in brain areas engaged during inhibitory control, most importantly in the prefrontal (PFC) and anterior cingulate cortex (ACC). Neurophysiological studies in non-human primates have also begun to reveal the neural mechanisms underlying the ability to prepare and effectively inhibit prepotent responses. In this review, we integrate findings about age-related, systems level changes affecting inhibitory control in humans, with ones about the underlying neurophysiology afforded by non-human primate work, to elucidate the neural mechanisms that support the transition from adolescence to adult-level optimal inhibitory control. Understanding the neural basis of the maturation of cognitive control in normative development can also offer insights into impaired development in psychopathologies, which are in general demarcated by disrupted inhibitory control (see Box 1) [9].
Text Box 1: Clinical Manifestations of Impaired Inhibitory Control.
Inhibitory control matures through adolescence, a time when many psychopathologies emerge [82]. Increased AS errors compared to controls is evident across mental illnesses, neurodevelopmental disorders, and other conditions including ADHD [1, 2], autism [83], depression [84], substance use [85], bipolar disorder [86] and predominantly – schizophrenia [37]. In schizophrenia, which typically emerges in late adolescence to early adulthood, AS impairment has been associated with prefrontal abnormality [87] reflected in impaired recruitment of prefrontal regions and their connectivity in AS tasks [88,89]. As such, AS impairment is deemed a possible biomarker of the disease. AS associations with executive dysfunction in patients [90] underscore the role of inhibitory control in cognition and its sensitivity to psychopathology. Thus, inhibitory control may serve as a marker of the integrity of the maturation of systems-level brain processes that are impaired in psychopathology.
We first review the behavioral phenotype of limited inhibitory control during adolescence in humans and non-human primates. Secondly, we review the evidence for brain structural changes in relevant brain systems and their connectivity. Next, we present evidence for age related changes in brain functional processing based on human fMRI and MEG studies, as well as non-human primate neurophysiology. We end by proposing a model of the maturation of inhibitory control that encompasses the lines of evidence accrued through studies in humans and non-human primates.
Behavioral manifestations of inhibitory control
Inhibitory control is measured using tasks that require voluntary cessation of a reflexive response, or suppression of interference from an established response set, to generate a planned goal-driven response determined by an instruction. Examples of paradigms involving stopping a reflexive response include antisaccade (see Glossary), flanker, and stop-signal tasks; and examples of tasks involving suppression of interference include go/no-go and Stroop. From the neural perspective, inhibitory performance requires a number of processes: engaging neural systems that pre-emptively dampen activity of reflexive responses (e.g., premotor regions); increased engagement of executive systems supporting generating a goal driven response (e.g., prefrontal regions); and recruitment of mechanisms that support engaging the abovementioned systems in a ready manner (systems functional integration).
In terms of developmental trajectory, inhibitory control is evident relatively early on, in infancy, as evidenced by the ability of infants to voluntary inhibit responses as required by the abovementioned tasks, albeit inconsistently [5,10]. Performance rapidly improves through childhood [11,12] subsequently showing more subtle yet continued improvement through adolescence [6,13–15]. Importantly, while the ability to generate a single correct inhibitory response is evident early in development, what improves through adolescence is the proportion of trials with correct inhibitory responses [16]. From a neurobiological perspective, this implies that the circuitry that can dampen reflexive sensorimotor responses and select an appropriate action among alternatives is available early in development. Yet the ability to engage systems-level processes that recruit inhibitory and executive circuits in a flexible, controlled, and ready manner, continues to substantially improve through adolescence. As described below, these improvements proceed in parallel with maturation of relevant brain regions.
The antisaccade task [17] (AS) is a particularly useful probe of neurocognitive maturation of inhibitory control both in humans and in non-human primates. In the AS task, subjects are instructed to suppress the prepotent tendency to look toward a salient visual stimulus that appears in an unexpected location, and instead make a voluntary eye movement to its mirror location (Fig. 1A–B). Thus, the AS requires that: (1) a rule be kept on-line (“do not look at an upcoming visual stimulus”); (2) reflexive sensorimotor preparatory processes be dampened; and (3) executive processes for voluntary responses be engaged by a goal (“look in the mirror location”), rather than a sensory stimulus. It is important to note that in addition to inhibitory control, cognitive processes including working memory and attention mechanisms are also engaged, as the task instruction to suppress interference by the distractor needs to be kept on line [18]. As reviewed below, the AS task has been used extensively to understand inhibitory control and its development in humans and non-human primates, providing a wealth of information regarding the behavior, as well as the brain mechanisms behind its late maturation.
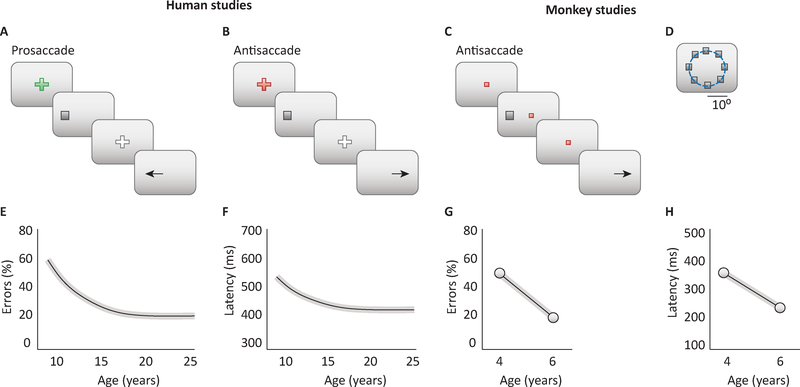
Figure 1. Antisaccade behavior in humans and nonhuman primates.
A-B. Trial structure of the prosaccade (A) and antisaccade task (B) in human studies. C. Task trial structure in the antisaccade task in monkey studies. D. Possible locations of the stimulus in the monkey AS task. E. Percentage of errors in antisaccade task is illustrated schematically as a function of age for humans tested across a range of ages. F. Response latencies in antisaccade trials as a function of age. G. Percentage of errors in AS task as a function of age, for monkeys tested at two time points of maturation, in adolescence and adulthood. H. Response latencies in monkeys as a function of age. Panels of human studies based on results from [6]; monkey studies from [32].
Given debates about sex differences in spatial abilities and known sex differences in the timing of puberty, the question arises whether inhibitory control, for instance as assessed via the AS task, displays differences between males and females. To date, the AS task has not revealed any noticeable sex differences. This is perhaps somewhat expected, given that the set of cognitive mechanisms this paradigm engages, in particular core top-down modulation of behavior, are foundational cognitive process across the sexes and do not tend to show marked sex-difference [6]. Of note, however, white matter does mature earlier in females [19,20] thus the developmental trajectory and time of maturation of adult level inhibitory control may differ by sex in more subtle ways that have not yet been identified.
Maturation of inhibitory control performance
Human Adolescence
As mentioned earlier, the ability to generate an inhibitory response is evident in infancy. Specifically, nine-month old infants can select between simultaneously presented locations by inhibiting response to the distractor location, similarly to adults [5,10]. Subsequently, in childhood, there is a steep increase in inhibitory abilities, across a range of inhibitory tasks (see Glossary for definitions of tasks). Flanker tasks, which require suppressing distracting peripheral stimuli, show that interference control significantly improves through childhood with little improvement after adolescence [11]. Similarly, the ability to stop an initiated response in the stop-signal task [13], to change an established response in the go/no-go task [21], and to suppress a learned response in the Stroop task [22], improve significantly into childhood but are mostly adult-like by adolescence. Together, these studies provide compelling evidence regarding the acquisition through childhood of the ability to incrementally apply executive systems to suppress responses.
The antisaccade task is particularly well-suited to probe core inhibitory processes that continue to mature through adolescence. Unlike other inhibitory tasks that require a button press and may be prone to various strategies, the AS requires a saccade response, the fastest movement the body can make. Critical to performing a correct AS is the preparatory period [23,24], which provides a useful quantifiable variable – latency to initiate a correct response – in addition to proportion of correct responses. Several studies have assessed developmental improvements in AS performance, and similar to the abovementioned inhibitory tasks, the most significant improvement occurs through childhood but there is continued significant development through adolescence [14,25–27]. The developmental trajectory is curvilinear [14,25–27], and an inverse function provides best fits to it [6,14,26]. This trajectory depicts steep decreases in error rates (Fig. 1E) and latency of correct responses (Fig. 1F) through childhood, followed by a much lower but still significant slope through adolescence, reaching stability in adulthood (Fig 1B–D). These findings have been confirmed in a longitudinal study assessing participants 8 to 30 years of age [6]. In addition, intra-subject variability in AS latency decreases with age [26,27]. It is typical for subjects, in some of the trials, to display saccadic deviations during the eye movement towards the stimulus, before correcting away the eye movement to the correct location; intra-subject variability in these saccadic deviations, similarly to the AS latency, decrease with age [28]. Interestingly, developmental improvements do not appear to be affected by IQ or sex, indicating that more general components of inhibitory control are being assessed [6]. It is important to note that in childhood 30–50% of trials are correct [14,25,27] underscoring that the ability to perform an AS is available early in development and what improves is the ability to effectively recruit executive processes in a sustained fashion. Also of note, in the presence of impending rewards AS performance in adolescence improves, approximating adult levels reflecting the ability to push the engagement of executive processes for the purpose of reward receipt [29]. Together, these findings establish that the ability to access inhibitory processes in a ready and controlled fashion underlies the transition to mature adult level cognitive control.
Non-human primates
The hallmarks of behavioral maturation in adolescence are observed not only in humans, but also in other primate species. Macaque monkeys age approximately 3 times faster than humans, entering puberty at ~3.5 years of age and reaching full sexual maturity at 5 years [30,31]. A number of physiological markers can be used to ascertain puberty, such as growth in body mass, femur length, testicular size, closure of epiphyseal plates in the bones of the extremities, and serum testosterone concentration [32]. Monkeys can be trained in the AS task (Fig. 1C–D) and tested in adolescence and in adulthood. In a longitudinal experimental study, monkeys were originally trained in the AS task at the time of puberty, allowed to reach asymptotic performance, and behavioral results were obtained through this training period [32]. The animals were then returned to their colony and were not exposed to any behavioral task. A second round of data was then obtained in adulthood, approximately 2 years after the original tests. The monkeys’ performance in the AS task greatly improved between adolescence and adulthood (Fig. 1G). Intra-subject (session-to-session) variability in the proportion of correct trials from session to session is also much greater in the adolescent than in the adult animals. One can wonder whether the improvement in overall performance is the result of slower reaction times in adult animals, which could afford them more time to process and resist the prepotent effect of the visual stimulus. In fact, the picture was quite the opposite, and consistent with results from humans: reaction times in monkeys were faster in adulthood than in adolescence (Fig. 1H). Furthermore, improvements in performance in adulthood were generally greater in more demanding versions of the AS task, e.g. the “gap” version, which involves turning off the fixation point before the appearance of the visual stimulus and thus making it more difficult to resist an eye movement at the stimulus onset [32].
Neural systems maturation
Human Structural and Functional Developmental Changes
The adolescent brain is undergoing maturation in parallel to improvements in inhibitory control. Magnetic Resonance Imaging (MRI) studies in humans show that gray matter volume decreases into adulthood, with areas of the association cortex including the prefrontal cortex continuing to mature in adolescence [33]. The striatum and thalamus, both of which are connected to fronto-parietal cortical regions, also continue to mature through adolescence [34]. Decreases in gray matter thickness are thought to be associated with synaptic pruning, which postmortem studies show proceeds from childhood into the third decade of life in prefrontal cortex [35].
White matter connectivity also shows significant changes through childhood and adolescence. Diffusion Tensor Imaging (DTI) studies show that the integrity of white matter tracts predominantly increases through childhood reaching adult levels by mid-adolescence, including the superior longitudinal fasciculus which provides connectivity between dorsal prefrontal and parietal regions [20,36]. However, within the basal ganglia integrative zones that determine action the relative extent of affective projections in relation to projections from cognitive control systems decreases with age. This age-related change was attributable solely to age-related decreases in affective inputs as cognitive inputs were stable by adolescence. Importantly, these changes were found to mediate the abovementioned greater sensitivity in adolescence to rewards when performing the AS [37]. White matter specialization through development is believed to be subserved, in part, by myelination of tracts proceeding in a Hebbian ‘use it or lose it’ fashion, predominantly fortifying the tracts that are used more frequently.
Resting state fMRI has also shown developmental progressions through adolescence. In particular, there is evidence for decreases in functional connectivity between prefrontal and subcortical regions, including frontostriatal [38,39] and frontoamygdalar [40] connectivity. These changes are thought to reflect, in part, the decreasing influence of affective processes through adolescence. At the network level, the overall network organization is established by childhood, but internetwork connectivity that engages prefrontal systems (cingulo- opercular/salience networks) with other networks, including sensory ones, strengthens through childhood into adolescence [41,42]. Taken together, evidence indicates that prefrontal connectivity with brain regions that require top-down processes for optimal and sustained inhibitory control, continues to mature through adolescence.
Maturation of Non-Human Primate Frontal Connectivity
Anatomical studies in monkeys demonstrate that inhibitory synapses of interneurons onto pyramidal neurons in the prefrontal cortex strengthen in adolescence [43]. The connections of parvalbumin interneurons, in particular, have been found to continue to mature into adulthood [44]. It may be tempting to link stronger inhibitory synaptic connections with improved inhibitory control at the behavioral level, but the effects of synaptic maturation are likely to be more indirect, in concert with the idea that cognitive mechanisms of inhibition may not be equated with neural ones [18]. Analysis of simultaneously recorded spike trains with extracellular electrodes also confirms that the overall strength of intrinsic effective prefrontal connectivity increases between the time of adolescence and adulthood [45]. Synaptic projections of neurons releasing neuromodulators, most importantly dopamine, continue to mature in adolescence [46], and computational models suggest that optimal dopamine levels may be linked to improved signal-to-noise stimulus representations [47]. Collectively, these findings suggest that adolescence is characterized by a reorganization of prefrontal circuits at the macro- (between areas) and microscopic (between neurons) levels. The functional consequences of this circuit maturation are readily observable in imaging and neurophysiological experiments.
Neurofunctional changes in adolescence
Human fMRI
fMRI studies of the development of inhibitory control show both age-related increases and decreases in prefrontal engagement [48–50]. These results, however, are not necessarily contradictory. Working on a model where adult brain function is considered the optimal mature processing, increased prefrontal activity in youth reflects greater effort to generate an inhibitory response similar to increased function with more cognitive load in adults [51]. Decreased activity would reflect inability to engage systems at optimal levels [52], as decreased blood-oxygen-level-dependent (BOLD) responses are known to result from greater synchronization of relevant prefrontal systems [53]. Accumulating evidence indicates that when considering correct trials, children show greater engagement of prefrontal systems [6,54] that is associated with better performance [6,55].
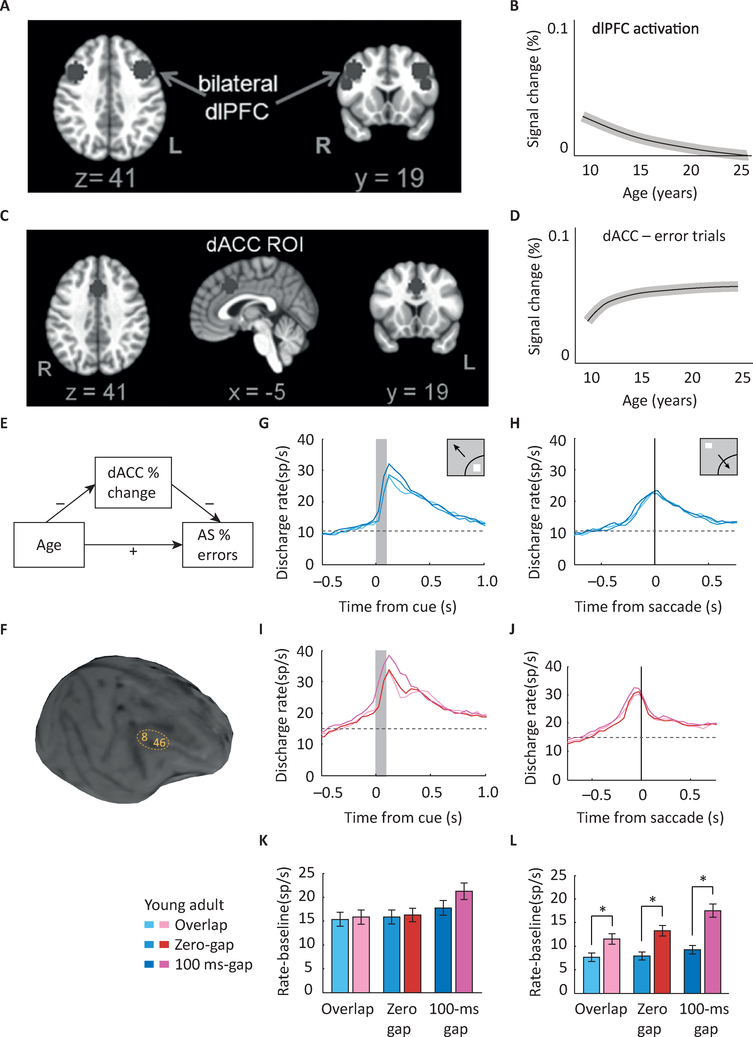
Most studies have been cross-sectional, which can be challenged by cohort effects. The one longitudinal fMRI that investigated inhibitory control found that prefrontal engagement when generating correct antisaccades was greatest during childhood, but by adolescence dorsolateral prefrontal cortex (dlPFC) was engaged at adult levels (Fig. 2A–B) [6]. The dorsal anterior cingulate cortex (dACC), which was correlated with performance, showed increasing engagement through adolescence mediating behavioral developmental improvements in AS performance (Fig. 2C–E). Age related increases in anterior cingulate function during inhibitory control have been found with fMRI in studies using Stroop, AS, and stop signal tasks [22] as well as with EEG [56,57]. The dACC is known to support performance monitoring [58] and works as an alerting system to engage cognitive control systems [59]. Thus, during adolescence immaturities in error processing may play a critical role in limitations in inhibitory control.
Figure 2. Maturation of response inhibition in imaging and physiological studies.
A. image of human brain indicating areas in the dorsolateral prefrontal cortex (dlPFC) that undergo changes in AS task activation during adolescence. B. Schematic diagram of mean growth curve indicating dlPFC signal changes with age. Of executive control regions, only the right dlPFC demonstrates developmental changes in activation. C. Image of human brain indicating areas in the dorsal Anterior Cingulate Cortex (dACC) that undergo changes in activation during adolescence. D. Schematic diagram of signal change in dACC with age. dACC activation is associated with better overall task performance, as indicated by lower AS error rates. E. Signal change in the dACC during corrected error trials mediates the effect of age on AS error rates. F. MRI image of an adolescent monkey with areas 8 and 46 of the dlPFC indicated. Arcuate Sulcus (AS) and Principal Sulcus (PS) are also shown in the figure. G-J. Mean firing rate in the young (G-H) and adult (I-J) stage for monkeys, in three variants of the antisaccade task (overlap, zero-gap, gap). Responses are shown for stimulus in the receptive field (G,I) and for saccade in the receptive field (H,J). K-L. Histograms average firing rates relative to baseline in a 200 ms window, for the stimulus in the receptive field (K) and saccade in the receptive field condition (L). No decrease was observed in activation elicited by the visual stimulus in adulthood (G,I,K). A significant increase in activity was present in adulthood for the representation of the goal of the saccade (H,J,L). Panels A-E adapted with permission from [6]; Panels F-L from [32].
As discussed above, potential rewards have an impact on AS performance enhancing the ability to suppress saccades particularly in adolescence. Studies have found that during rewarded AS trials in adolescence, there is greater activation in the nucleus accumbens (NAcc) [60,61], a region rich in dopaminergic inputs and known to underlie reward processing [62,63]. Adolescents also showed greater activity in the Frontal Eye Fields (FEF), a primary region supporting AS performance, compared to adults during correct rewarded AS responses suggesting that adolescents may be exerting greater effort to perform a task that is difficult in order to obtain a reward. Thus, increased engagement of the NAcc may impact the degree that systems underlying the ability to obtain reward receipt are engaged in adolescence. When probing associations with the Zuckerman Sensation Seeking Scale, which characterizes sensation seeking traits and predispositions for thrill and risk-taking behaviors, there is no clear association with AS performance [64]. However, activation of the NAcc during the rewarded AS was found to have unique associations with sensation seeking through development. Greater NAcc activity in childhood and early adolescence was linked to reduced sensation seeking, whereas subsequently in later adolescence, greater NAcc activation was associated with greater sensation seeking [64]. These results suggest that as the system is maturing in later adolescence, inhibitory control in the presence of rewards reflects increased sensation seeking.
fMRI studies have also found that the ability of the PFC to engage other brain systems in a top-down fashion plays an important role in developmental improvements in AS performance. Granger causality analyses found a significant age related increase in the number of significant connections and the strength of effective functional connectivity from PFC to other cortical and subcortical regions supporting the integration of frontal, oculomotor, and subcortical systems [65]. These results suggest that core local processing of prefrontal systems undergo significant maturation in childhood and are available at adult levels by adolescence; however, prefrontal engagement with other cortical and subcortical regions continues to strengthen into adolescence, which may underlie the lack of reliable and ready adolescent engagement of executive systems.
Human MEG
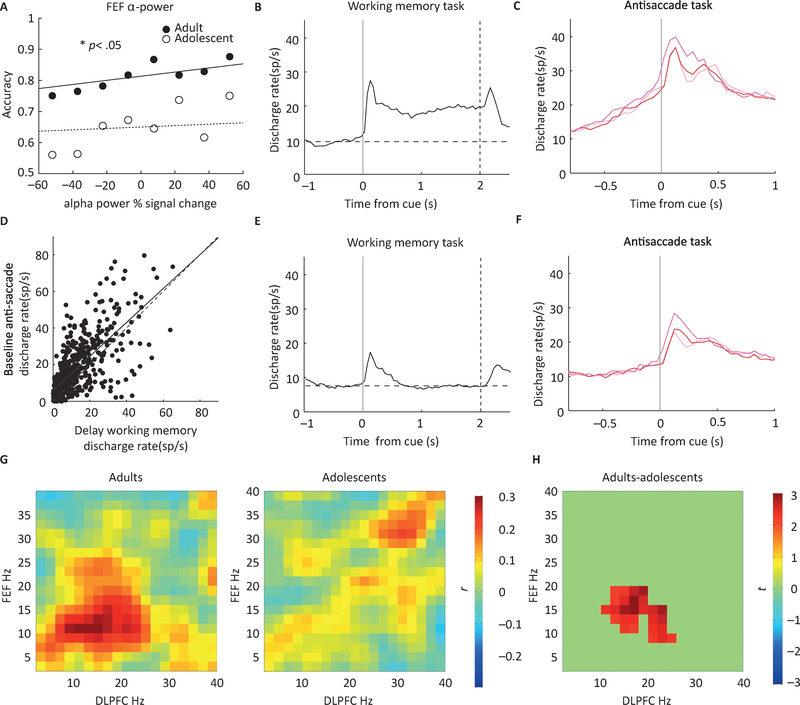
Magnetoencephalography (MEG) affords a temporal resolution at the millisecond level, allowing characterization of electrophysiological activities generated by neuronal dynamics throughout different phases of cognitive processing. MEG has indicated that Global Field Power has a delayed progression in adolescents compared to adults when performing a go/no-go task, suggesting immaturities in the ability to generate voluntary inhibitory responses [66]. Similarly, EEG studies show the importance of oscillatory activity in the preparatory period of the AS where increases in medial frontal theta and suppression of posterior alpha precede correct responses [24]. A developmental MEG study, focused on the preparatory period while performing antisaccades and prosaccades, showed marked differences in oscillatory activity in adolescents compared to adults that corresponded with performance [23], [67]. Adolescents showed adult level beta-band power in dlPFC but lower Frontal Eye Field (FEF) alpha-band power (Fig. 3A) and beta/alpha dlPFC/FEF cross-frequency coupling (Fig. 3G). Beta rhythms have been associated with cortical glutamatergic function in deep layers [68], through top down inputs activating pyramidal neurons. Alpha rhythms have been associated with dampening of neuronal activities through inhibitory processes [69] supporting inhibitory mechanisms in the AS task [70]. These results, consistent with ideas discussed earlier, indicate that dlPFC executive systems are on-line by adolescence, but prefrontal systems are not readily engaged during executive responses, undermining optimal inhibitory control.
Figure 3. Time course of Response Inhibition.
A. Accuracy in AS task as a function of alpha power signal change in Frontal Eye Fields (FEF) observed in human MEG studies, in adults and adolescents. B. Mean firing rate of neurons with significant delay-period activity in the working memory, Oculomotor Delayed Response (ODR) task in monkey studies. C. Firing rate of the same group of neurons in the anti-saccade task, selected based their responses in the ODR task. Ramping of activity is present prior to the onset of the stimulus in the AS task. D. Regression across all neurons. Each dot represents a single neuron tested in the working memory and AS Task. Solid line represents linear regression. Firing rate in the delay period activity of the working memory task is predictive of preparatory activity in the AS task. E, F: Mean firing rate as in B and C, for neurons that did not display significant delay period activity in the working memory task. Much less ramping activity is present for these neurons in the AS task. G. Functional coupling between dlPFC beta-band activity and FEF alpha-band activity associated with the AS task for both adults and adolescents in human MEG studies. Colorbar indicates the strength of functional connectivity. H. Spectral cluster that showed significant age differences. Stronger beta-alpha amplitude coupling between the dlPFC and the FEF for the AS task was found in adults Colorbar indicates the test statistic t. Panels A,G-H reproduced with permission from [67]; panels B-F adapted from [71].
Developmental Neurophysiology in Non-human Primates
Compared to analyses using non-invasive approaches, neurophysiological studies in monkeys have provided a more granular picture of developmental changes that occur at the level of single neurons in the context of inhibitory control maturation. As mentioned, the AS task proved particularly productive in this context. In principle, the developmental improvements in AS performance could be associated with lower neuronal activity elicited by the stimulus (i.e. more effective filtering of the prepotent stimulus that needs to be resisted) or by more effective representation of the correct target of the eye movement (i.e. enhancement of the goal). Neurophysiological studies reveal that the latter is the case (Fig. 2F–K). The activity of neurons representing the location of where the eventual eye movement needs to be directed is greater in the PFC of adult animals [32]. What is most enhanced is the activity representing the vector inversion (see Glossary), the shift of attention and preparation of a response away from the stimulus. The change was primarily localized in the prefrontal cortex, compared to the posterior parietal cortex [71]. In contrast, neurons with purely eye-movement driven responses exhibited similar activity at the two developmental stages reflecting similar processing of the presence of a target. Of note, the total level of activity, integrated across the entire period of the task and across all populations of neurons, exhibited only subtle differences between adolescence and adulthood; it was the precise timing of activation for different stimulus conditions that revealed the functional maturation. These results provide evidence that there are important developmental improvements in engaging neural systems that support generating an executive planned response in the AS task that may contribute to the ability to suppress the reflexive response.
Neurophysiological studies have also confirmed that the preparatory period of the AS task is critical for a successful inhibitory response [23]. As in humans, alpha oscillations modulate preparatory activity in non-human primates; alpha power in deep cortical layers provides a pulsed inhibition signal to deeper and upper cortical layers. [72]. Accordingly, changes in neuronal activity between non-human primate adolescence and adulthood have been found in neurophysiological recordings from the preparatory fixation period [71]. Baseline activity was higher overall in the adult group and increased (“ramped”) with a faster rate prior to the onset of the stimulus (Fig. 3C). The level of this activity was also predictive of behavior, as correct trials were characterized by a higher firing rate prior to the onset of the stimulus. Neuronal firing in the fixation period is likely driven by the activity encoding task and rule contingencies necessary to perform the AS task that engage preparatory inhibitory processes. As mentioned above, activity preceding the onset of the stimulus in saccadic tasks is predictive of errors in human EEG and fMRI experiments [73–75]. Additionally, examining responses on a neuron-by-neuron basis (Fig. 3B–F) determined that neurons that exhibited the highest levels of baseline activity in the AS task also exhibited higher levels of delay period activation in the working memory tasks, performance which also improved between adolescence and adulthood [76]. These results demonstrate that preparatory processes are critical for response inhibition and represent a neural resource shared between multiple cognitive functions, which mature in tandem between adolescence and adulthood.
Concluding Remarks
Taken together, human neuroimaging studies and animal neurophysiology delineate the specific processes that underlie the maturation of inhibitory control. Based on these studies, a primary way in which inhibitory control matures is the emergence of neural activity patterns that establish a ready preparatory state to inhibit a response. The ability to engage these preparatory systems may be mediated by improved computations afforded by increases in inhibitory circuitry and the ability to integrate systems level processes. These developmental changes support PFC integration, including error processing and planning an effective response. Importantly, adolescent immaturities in these brain processes do not undermine the ability to generate inhibitory responses but limit the ability to engage cognitive control in a sustained and controlled fashion.
The AS task involves, in addition to processes that are specific to inhibitory control, other cognitive processes that are also actively maturing in adolescence, such as working memory and attention, and these may contribute to improvement in task performance over adolescence. Working memory systems are known to undergo a protracted maturation through adolescence in humans and monkeys [76,77]. Working memory is engaged in AS, as the task instruction to inhibit a saccade is maintained online to guide behavior. Working memory development through adolescence, similarly to the maturation of other cognitive functions as discussed earlier, has also been found to rely on systems other than PFC such as visual association cortex. Attention and eye movement circuitries are greatly overlapping [78] as these systems are largely dependent on each other. It is the effective top-down modulation of these neural systems that is core to AS performance and the primary substrate of inhibitory control development during adolescence.
This neural profile of limitations in readily engaging inhibitory control in adolescence may be associated with the recognized phenotype of impulsivity in adolescence and sensation seeking. Impulsivity is characterized by reactive actions directed towards immediate rewards, and that are lacking in forethought [79]. Relatedly, sensation seeking is characterized by the drive to obtain novel experiences that result in increased reward sensations and motivate exploration often involving impulsivity [80]. As such, sensation seeking in adolescence is an adaptive process present across species and societies, believed to motivate exploration and information seeking needed to specialize systems defining adult modes of operation [1]. Sensation seeking however can sometimes lead to risk-taking undermining survival. The AS task is not a direct measure of these behavioral traits and, as discussed above, a link between sensation seeking and AS performance is not readily found except in the engagement of the NAcc in later adolescence. Nevertheless, we would argue, the AS task probes core neural processing that underlie the top-down executive systems inherent in these constructs. That is, the ability to suppress the prepotent reactive saccade to a visual stimulus in the AS requires that neural circuitry that engages executive prefrontal systems to inhibit reflexive subcortical systems be effectively recruited in a timely fashion. Impulsivity and sensation seeking are similarly subserved by a failure in engaging executive prefrontal systems to stop a subcortical reactive reward driven response. Thus, immaturities evident in the systems underlying AS performance reflect a state of development of predisposition for reactive behaviors. Further evidence however is needed to establish a more definitive link between core AS inhibitory control and the more complex motivational processes involved in sensation seeking. Delayed discounting tasks that require choosing a waiting period before receiving a large reward rather than an immediate smaller reward are valuable in characterizing the ability to contextualize suppression of a reactive response. Delayed discounting improves into adolescence [81] (i.e., subjects are able to wait longer for large rewards) adding to the profile of sensation seeking. Associations between AS performance and delayed discounting could further the ability to link maturity of executive top down circuitry and sensation seeking.
Together, human and non-human primate studies show that in adolescence – a time of increased reactive behavior including sensation seeking – inhibitory control is limited, and its neurobiological underpinnings are immature, particularly in the ability to engage top-down executive systems in an effective manner (see Outstanding Questions). Importantly, a failure to normatively strengthen inhibitory systems may play an important role in psychopathology, which across illnesses is associated with impaired response inhibition.
Outstanding Questions Box.
fMRI results suggest that the anterior cingulate cortex exhibits the most prolonged pattern of maturation and is differentially activated between adolescence and adulthood, in a manner that predicts performance in the AS task. What is the nature of neurophysiological changes that characterize this maturation?
What are the sources of variability in inhibitory control performance, and what mechanisms drive this variability down during development? Studies of working memory maturation hint that the amplitude of large-scale task-related brain activity stabilizes during adolescence, and that behaviorally, trial-to-trial variability in reaction time and accuracy of eye movements are related to fluctuations in the amplitude of task- related brain activations. It is yet to be determined whether similar mechanisms are in place for response inhibition.
How do changes in neurotransmitter concentration (dopamine, GABA, glutamate) in association cortices through adolescence relate to normative and impaired development of cognitive control?
Variability in the environment plays a critical role in the development of inhibitory control in humans. It affects neurophysiological maturation and is a source of inter-individual differences in inhibitory control. What are the effects of environmental factors such as education, parenting styles, social dynamics, or trauma, on the development of inhibitory control and its neural underpinnings? Does task training in monkeys affect the development of neural circuits?
Adolescence is a time of increased reward reactivity, particularly in response to peer influence as the drive to establish social circles predominates. How do social influences and associated reward mechanisms affect inhibitory control and its neurophysiological maturation? And how do these influences inform risk-taking in adolescence?
What are the links between limitations in inhibitory control and the emergence of psychopathology in adolescence? Can impaired inhibitory control in adolescence serve as a biomarker for a trajectory of behavioral abnormality that can lead to psychopathology? Better understanding of these links can inform etiology, predictive models, and interventions that build upon the plasticity of adolescent development.
Highlights.
The neural circuits necessary for inhibitory control are mostly present in adolescence. Lower overall performance than in adults, however, and higher variability, as exemplified in the antisaccade task, suggests that adolescents are unable to engage these circuits in a controlled and sustained manner.
Maturation of inhibitory control relies on changes at the neuron-circuit level as well as between-area connectivity, particularly in the prefrontal and anterior cingulate cortex, and their downstream targets.
Transition from adolescence to adulthood in the antisaccade task is characterized by changes in activation of the dlPFC and dACC, as well as enhancement of neural activity representing the correct target of the eye movement.
Neural activity during the preparation phase in the antisaccade task, before the onset of the stimulus that needs to be avoided, is also a critical predictor of inhibitory control and underlies developmental improvements.
Acknowledgments
This work was supported by the National Institute of Mental Health of the National Institutes of Health under award number R01 MH117996 to CC and BL, MH080243, MH067924, and the Staunton Foundation to BL. We wish to thank Terrence Stanford and Emilio Salinas for valuable contributions to the manuscript.
Glossary
- Antisaccade
A behavioral task that requires subjects to make a fast eye movement at a direction opposite to a visual stimulus. This task is used widely to probe inhibitory control
- Flanker
A behavioral task involving a central target stimulus (e.g. a rightward arrow) flanked by non-target stimuli of three possible types: ones that signify the same response as the target (congruent flankers e.g. rightward arrows), the opposite response (incongruent flankers, e.g. leftward arrows), or a neutral response. The task requires suppression of incongruent flankers
- Go/No-Go
A behavioral task that requires responses to frequently presented stimuli (go responses) while refraining from responding to infrequently occurring targets (no-go responses). The task requires suppression of an established response
- Stop Signal
A task involving presentation of a target e.g. requiring an eye or hand movement towards it, followed by a signal to abort the response. The task becomes more difficult the closer the stop signal (also known as countermanding) is presented to response initiation. The task requires termination of an initiated response
- Stroop
A task requiring subjects to name the color of a word, which itself spells the name of a different color. The task requires suppression of an established learned association
- Vector Inversion
The transformation of neural activity to represent the location of the eye movement, which is opposite to the location of the stimulus in the antisaccade task
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spear LP (2000) The adolescent brain and age-related behavioral manifestations. Neurosci.Biobehav.Rev 24, 417–463 [DOI] [PubMed] [Google Scholar]
- 2.Bari A and Robbins TW (2013) Inhibition and impulsivity: behavioral and neural basis of response control. Prog. Neurobiol 108, 44–79 [DOI] [PubMed] [Google Scholar]
- 3.Nigg JT (2000) On inhibition/disinhibition in developmental psychopathology: views from cognitive and personality psychology and a working inhibition taxonomy. Psychol. Bull 126, 220–246 [DOI] [PubMed] [Google Scholar]
- 4.Luna B et al. (2015) An integrative model of the maturation of cognitive control. Annu. Rev. Neurosci 38, 151–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amso D and Johnson SP (2005) Selection and inhibition in infancy: evidence from the spatial negative priming paradigm. Cognition 95, B27–B36 [DOI] [PubMed] [Google Scholar]
- 6.Ordaz SJ et al. (2013) Longitudinal growth curves of brain function underlying inhibitory control through adolescence. J. Neurosci 33, 18109–18124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dempster FN (1992) The rise and fall of the inhibitory mechanism: toward a unified theory of cognitive development and aging. Dev. Rev 12, 45–75 [Google Scholar]
- 8.Bjorklund DF and Harnishfeger KK (1995) The evolution of inhibition mechanisms and their role in human cognition and behavior In Interference and inhibition in cognition (Dempster FN and Brainerd CJ, eds), pp. 141–173, Academic Press [Google Scholar]
- 9.Nigg JT (2017) Annual Research Review: On the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking, and inhibition for developmental psychopathology. J. Child Psychol. Psychiatry 58, 361–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson MH (1995) The inhibition of automatic saccades in early infancy. Dev. Psychobiol 28, 281–291 [DOI] [PubMed] [Google Scholar]
- 11.Cragg L (2016) The development of stimulus and response interference control in midchildhood. Dev. Psychol 52, 242–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van de Laar MC et al. (2014) Development of response activation and inhibition in a selective stop-signal task. Biol. Psychol 102, 54–67 [DOI] [PubMed] [Google Scholar]
- 13.Huizinga M et al. (2006) Age-related change in executive function: Developmental trends and a latent variable analysis. Neuropsychologia 44, 2017–2036 [DOI] [PubMed] [Google Scholar]
- 14.Luna B et al. (2004) Maturation of cognitive processes from late childhood to adulthood. Child Dev. 75, 1357–1372 [DOI] [PubMed] [Google Scholar]
- 15.Bedard AC et al. (2002) The development of selective inhibitory control across the life span. Dev. Neuropsychol 21, 93–111 [DOI] [PubMed] [Google Scholar]
- 16.Klenberg L et al. (2015) Examining methodological variation in response inhibition: The effects of outcome measures and task characteristics on age-related differences. Child Neuropsychol. J. Norm. Abnorm. Dev. Child. Adolesc 21, 586–602 [DOI] [PubMed] [Google Scholar]
- 17.Hallett PE (1978) Primary and secondary saccades to goals defined by instructions. Vision Res. 18, 1279–1296 [DOI] [PubMed] [Google Scholar]
- 18.MacLeod CM et al. (2003) In opposition to inhibition. Psychol. Learn. Motiv. Adv. Res. Theory Vol 43 43, 163–214 [Google Scholar]
- 19.Giedd JN et al. (1997) Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry 21, 1185–201 [DOI] [PubMed] [Google Scholar]
- 20.Simmonds D et al. (2014) Developmental stages and sex differences of white matter and behavioral development through adolescence: a longitudinal diffusion tensor imaging (DTI) study. NeuroImage 92, 356–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis FC et al. (2017) Evidence of substantial development of inhibitory control and sustained attention between 6 and 8years of age on an unpredictable Go/No-Go task. J. Exp. Child Psychol 157, 66–80 [DOI] [PubMed] [Google Scholar]
- 22.Adleman NE et al. (2002) A developmental fMRI study of the Stroop Color-Word task. NeuroImage 16, 61–75 [DOI] [PubMed] [Google Scholar]
- 23.Everling S and Munoz DP (2000) Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J. Neurosci 20, 387–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Noordt SJ et al. (2017) Cognitive control in the eye of the beholder: Electrocortical theta and alpha modulation during response preparation in a cued saccade task. Neuroimage 145, 82–95 [DOI] [PubMed] [Google Scholar]
- 25.Fischer B et al. (1997) On the development of voluntary and reflexive components in human saccade generation. Brain Res. 754, 285–297 [DOI] [PubMed] [Google Scholar]
- 26.Klein C et al. (2005) Lifespan development of pro- and anti-saccades: multiple regression models for point estimates. Dev. Brain Res 160, 113–123 [DOI] [PubMed] [Google Scholar]
- 27.Munoz DP et al. (1998) Age-related performance of human subjects on saccadic eye movement tasks. Exp. Brain Res 121, 391–400 [DOI] [PubMed] [Google Scholar]
- 28.West GL and Lippé S (2016) The development of inhibitory saccadic trajectory deviations correlates with measures of antisaccadic inhibition. Neuroreport 27, 1196–1201 [DOI] [PubMed] [Google Scholar]
- 29.Geier CF and Luna B (2012) Developmental effects of incentives on response inhibition. Child Dev. 83, 1262–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plant TM et al. (2005) Postnatal and pubertal development of the rhesus monkey (Macaca mulatta) testis. Ann N Acad Sci 1061, 149–62 [DOI] [PubMed] [Google Scholar]
- 31.Herman RA et al. (2006) Prenatal androgen blockade accelerates pubertal development in male rhesus monkeys. Psychoneuroendocrinology 31, 118–30 [DOI] [PubMed] [Google Scholar]
- 32.Zhou X et al. (2016) Behavioral response inhibition and maturation of goal representation in prefrontal cortex after puberty. Proc Natl Acad Sci U A 113, 3353–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gogtay N et al. (2004) Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U A 101, 8174–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raznahan A et al. (2014) Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proc. Natl. Acad. Sci. 111, 1592–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petanjek Z et al. (2011) Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc. Natl. Acad. Sci. U. S. A 108, 13281–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lebel C et al. (2012) Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage 60, 340–52 [DOI] [PubMed] [Google Scholar]
- 37.Larsen B et al. (2018) Developmental Changes in the Integration of Affective and Cognitive Corticostriatal Pathways are Associated with Reward-Driven Behavior. Cereb. Cortex N. Y. N 1991 28, 2834–2845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Supekar K et al. (2009) Development of large-scale functional brain networks in children. PLoS Biol 7, e1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porter JN et al. (2015) Age-related changes in the intrinsic functional connectivity of the human ventral vs. dorsal striatum from childhood to middle age. Dev Cogn Neurosci 11, 83–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jalbrzikowski M et al. (2017) Development of white matter microstructure and intrinsic functional connectivity between the amygdala and ventromedial prefrontal cortex: associations with anxiety and depression. Biol. Psychiatry 82, 511–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Martino A et al. (2014) Unraveling the miswired connectome: a developmental perspective. Neuron 83, 1335–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marek S et al. (2015) The Contribution of Network Organization and Integration to the Development of Cognitive Control. PLoS Biol 13, e1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzalez-Burgos G et al. (2015) Functional Maturation of GABA Synapses During Postnatal Development of the Monkey Dorsolateral Prefrontal Cortex. Cereb Cortex 25, 4076–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erickson SL and Lewis DA (2002) Postnatal development of parvalbumin- and GABA transporter-immunoreactive axon terminals in monkey prefrontal cortex. J Comp Neurol 448, 186–202. [DOI] [PubMed] [Google Scholar]
- 45.Zhou X et al. (2014) Age-dependent changes in prefrontal intrinsic connectivity. Proc Natl Acad Sci U A 111, 3853–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larsen B and Luna B (2018) Adolescence as a neurobiological critical period for the development of higher-order cognition. Neurosci Biobehav Rev 94, 179–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Durstewitz D et al. (1999) A neurocomputational theory of the dopaminergic modulation of working memory functions. J Neurosci 19, 2807–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luna B et al. (2001) Maturation of widely distributed brain function subserves cognitive development. NeuroImage 13, 786–793 [DOI] [PubMed] [Google Scholar]
- 49.Marsh R et al. (2006) A developmental fMRI study of self-regulatory control. Hum. Brain Mapp 27, 848–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tamm L et al. (2002) Maturation of brain function associated with response inhibition. J. Am. Acad. Child Adolesc. Psychiatry 41, 1231–1238 [DOI] [PubMed] [Google Scholar]
- 51.Carpenter PA et al. (1999) Graded functional activation in the visuospatial system with the amount of task demand. J. Cogn. Neurosci 11, 9–24 [DOI] [PubMed] [Google Scholar]
- 52.Luna B et al. (2010) What has fMRI told us about the Development of Cognitive Control through Adolescence? Brain Cogn. 72, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghuman AS et al. (2008) The effects of priming on frontal-temporal communication. Proc. Natl. Acad. Sci. U. S. A 105, 8405–8409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alahyane N et al. (2014) Developmental improvements in voluntary control of behavior: Effect of preparation in the fronto-parietal network? NeuroImage 98, 103–117 [DOI] [PubMed] [Google Scholar]
- 55.Dwyer DB et al. (2014) Large-scale brain network dynamics supporting adolescent cognitive control. J. Neurosci 34, 14096–14107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferdinand NK and Kray J (2014) Developmental changes in performance monitoring: How electrophysiological data can enhance our understanding of error and feedback processing in childhood and adolescence. Behav. Brain Res 263, 122–132 [DOI] [PubMed] [Google Scholar]
- 57.Segalowitz SJ et al. (2010) Electrophysiological changes during adolescence: a review. Brain Cogn. 72, 86–100 [DOI] [PubMed] [Google Scholar]
- 58.Braver TS et al. (2001) Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb. Cortex 11, 825–836 [DOI] [PubMed] [Google Scholar]
- 59.Cavanagh JF and Frank MJ (2014) Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci 18, 414–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geier CF et al. (2010) Immaturities in reward processing and its influence on inhibitory control in adolescence. Cereb. Cortex 20, 1613–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Padmanabhan A et al. (2011) Developmental changes in brain function underlying the influence of reward processing on inhibitory control. Dev Cogn Neurosci 1, 517–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schultz W et al. (2000) Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb. Cortex 10, 272–84 [DOI] [PubMed] [Google Scholar]
- 63.Cooper JC and Knutson B (2008) Valence and salience contribute to nucleus accumbens activation. Neuroimage 39, 538–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hawes SW et al. (2017) Modulation of reward-related neural activation on sensation seeking across development. Neuroimage 147, 763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hwang K et al. (2010) Strengthening of top-down frontal cognitive control networks underlying the development of Inhibitory control: a functional magnetic resonance imaging effective connectivity study. J. Neurosci 30, 15535–15545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vara AS et al. (2014) Neural mechanisms of inhibitory control continue to mature in adolescence. Dev. Cogn. Neurosci 10C, 129–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hwang K et al. (2016) Frontal preparatory neural oscillations associated with cognitive control: A developmental study comparing young adults and adolescents. NeuroImage 136, 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roopun AK et al. (2010) Cholinergic neuromodulation controls directed temporal communication in neocortex in vitro. Front. Neural Circuits 4, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jensen O and Mazaheri A (2010) Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front. Hum. Neurosci 4, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Belyusar D et al. (2013) Oscillatory alpha-band suppression mechanisms during the rapid attentional shifts required to perform an anti-saccade task. NeuroImage 65, 395–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou X et al. (2016) Distinct Roles of the Prefrontal and Posterior Parietal Cortices in Response Inhibition. Cell Rep 14, 2765–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnston K et al. (2019) Alpha Oscillations Modulate Preparatory Activity in Marmoset Area 8Ad. J Neurosci 39, 1855–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bender J et al. (2013) Differential roles of the frontal and parietal cortices in the control of saccades. Brain Cogn 83, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Curtis CE and D’Esposito M (2003) Success and failure suppressing reflexive behavior. J Cogn Neurosci 15, 409–18 [DOI] [PubMed] [Google Scholar]
- 75.Miller LM et al. (2005) Functional interactions between oculomotor regions during prosaccades and antisaccades. Hum Brain Mapp 26, 119–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou X et al. (2016) Neural correlates of working memory development in adolescent primates. Nat Commun 7, 13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simmonds DJ et al. (2017) Protracted development of executive and mnemonic brain systems underlying working memory in adolescence: a longitudinal fMRI study. Neuroimage DOI: 10.1016/j.neuroimage.2017.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Corbetta M et al. (2008) The reorienting system of the human brain: from environment to theory of mind. Neuron 58, 306–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Evenden JL (1999) Varieties of impulsivity. Psychopharmacology (Berl.) 146, 348–61 [DOI] [PubMed] [Google Scholar]
- 80.Zuckerman M (2009) Sensation Seeking In Handbook of Individual Differences in Social Behavior (Leary M and Hoyle R, eds), pp. 455–465, The Guilford Press [Google Scholar]
- 81.Stanger C et al. (2013) A developmental perspective on neuroeconomic mechanisms of contingency management. Psychol Addict Behav 27, 403–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paus T et al. (2008) Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci 9, 947–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luna B et al. (2007) Maturation of executive function in autism. Biol. Psychiatry 61, 474–481 [DOI] [PubMed] [Google Scholar]
- 84.Hoffmann A et al. (2018) Cerebral blood flow responses during prosaccade and antisaccade preparation in major depression. Eur. Arch. Psychiatry Clin. Neurosci DOI: 10.1007/s00406-018-0956-5 [DOI] [PubMed] [Google Scholar]
- 85.Chung T et al. (2011) Enhancing response inhibition by incentive: comparison of adolescents with and without substance use disorder. Drug Alcohol Depend. 115, 43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gooding DC and Tallent KA (2001) The association between antisaccade task and working memory task performance in schizophrenia and bipolar disorder. J. Nerv. Ment. Dis 189, 8–16 [DOI] [PubMed] [Google Scholar]
- 87.Clementz BA (1998) Psychophysiological measures of (dis)inhibition as liability indicators for schizophrenia. Psychophysiology 35, 648–68 [PubMed] [Google Scholar]
- 88.Tu P et al. (2010) Reduced functional connectivity in a right-hemisphere network for volitional ocular motor control in schizophrenia. Brain 133, 625–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lencer R et al. (2019) Alterations in intrinsic fronto-thalamo-parietal connectivity are associated with cognitive control deficits in psychotic disorders. Hum. Brain Mapp 40, 163–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hutton SB et al. (2004) The relationship between antisaccades, smooth pursuit, and executive dysfunction in first-episode schizophrenia. Biol. Psychiatry 56, 553–9 [DOI] [PubMed] [Google Scholar]