Abstract
Alcohol problems are influenced by both genetic and environmental factors. Evidence from twin models and measured gene-environment interaction studies has demonstrated that the importance of genetic influences changes as a function of the environment. Research has also shown that family-centered interventions may protect genetically susceptible youth from developing substance use problems. In this study, we brought large-scale gene identification findings into an intervention study to examine gene-by-intervention effects. Using genome-wide polygenic scores derived from an independent genome-wide association study of adult alcohol dependence, we examined whether an adolescent family-centered intervention would moderate the effect of genetic risk for alcohol dependence on lifetime alcohol dependence in young adulthood, approximately 15 years after the start of intervention, among European American (N = 271; 48.3% in the intervention condition) and African American individuals (N = 192; 51.6% in the intervention condition). We found that among European American individuals, the intervention moderated the association between alcohol dependence polygenic scores and lifetime alcohol dependence diagnosis in young adulthood. Among participants in the control condition, higher alcohol dependence polygenic scores were associated with a greater likelihood of receiving an alcohol dependence diagnosis; in contrast, among participants in the intervention condition, there was no association between alcohol dependence polygenic scores and alcohol dependence diagnosis. No moderation effect was found among African Americans. These results demonstrate that modifying environments of genetically vulnerable youth could reduce the likelihood of developing alcohol dependence and underscore the significance of environmentally focused prevention and intervention efforts.
Keywords: alcohol problems, gene-by-intervention, gene-by-environment, polygenic, family-centered intervention
Alcohol use disorder is a serious and common psychiatric condition that affects 15 million individuals in the United States (SAMSHA, 2017). It is associated with a significant public health, social, and economic burden in terms of health care expenses, lost productivity, crime, and accidents (Sacks, Gonzales, Bouchery, Tomedi, & Brewer, 2015). The heritability estimate for alcohol use disorders is about 50% (Verhulst, Neale, & Kendler, 2015), indicating that genetic influences account for a large portion of the observed variation between individuals in alcohol use disorders. However, genetic predispositions for complex behavioral outcomes like alcohol dependence are not deterministic. Research has shown that the importance of genetic influences changes as a function of the environment (Dick & Kendler, 2012; Young-Wolff, Enoch, & Prescott, 2011). Twin studies and measured gene-environment interaction (G×E) studies indicate that environmental factors (e.g., parental monitoring, peer deviance) moderate genetic influences on alcohol problems. This line of work is rooted in the theoretical literature on mechanisms of G×E by demonstrating that protective environments (greater social control/protective contexts) may attenuate genetic predispositions (Shanahan & Hofer, 2005), which shows that environments that exert more social controls (e.g., greater parental knowledge of one’s whereabouts; greater parental involvement) reduce the opportunity to express a genetic predisposition toward substance use. In contrast, environments that provide greater opportunities via increased access to substances or acceptance of substance use (e.g., affiliation with deviant peers) allow for enhanced expression of genetic predispositions (Dick & Kendler, 2012). Recent research also provided some evidence that family-centered interventions—a protective context where the environment has been directly modified and presumably exerts more social control—may be moderators of genetic influences (Brody, Chen, Beach, Philibert, & Kogan, 2009; Brody et al., 2014).
With widespread recognition that both genetic and environmental influences shape complex behaviors, a growing number of prevention and intervention studies are incorporating genetic components. Gene by intervention (G×I) research examines whether intervention changes the association between genetic predispositions and deleterious outcomes, or alternatively, can be framed as whether the effectiveness of the intervention varies as a function of genotype (these are statistically equivalent). Initial evidence from the G×I literature indicate that interventions may protect genetically susceptible youth from developing substance use problems and related behaviors. For example, data from the Strong African American Families preventive intervention study showed that youth with genetic risk for risky behavior (as indexed by carrying the 5HTTLPR short allele) who were assigned to the control condition showed greater rates of risky substance behaviors in adolescence than those with genetic risk in the intervention condition (Brody et al., 2009). A similar pattern of results for externalizing psychopathology in young adulthood was found from the school-based Fast Track intervention project (Albert et al., 2015). Children who were more biologically sensitive to stress (as indexed by genetic variation in NR3C1, a glucocorticoid receptor gene) had higher rates of externalizing behaviors at age 25 in the control condition and lower rates of externalizing behavior when they were enrolled in the Fast Track intervention.
Despite a growing interest in integrating genetics into prevention/intervention studies, most of the previous studies have focused on candidate genes. Although these are useful as a proof of principle, using candidate genes in association and G×E analyses has generated a great deal of inconsistent results (Dick et al., 2015; Duncan & Keller, 2011). Candidate gene research does not represent the current state of the science in genetics. Very few well-replicated associations have emerged from studying pre-specified genes of interest (Bosker et al., 2011; Collins, Kim, Sklar, O’Donovan, & Sullivan, 2012). Large genome-wide association studies (GWAS) that systematically scan genetic variants across the genome without any a priori evidence have largely failed to find support for the candidate genes that were previously believed to be involved in psychiatric and substance use outcomes (Bosker et al., 2011; Collins et al., 2012; Hart & Kranzler, 2015). It has become clear that complex behavioral outcomes have a polygenic architecture, influenced by many genes of small effect (Plomin, Haworth, & Davis, 2009). Because of the technological advances in genotyping and the relatively inexpensive methods now available for genotyping hundreds of thousands of genetic variants across the genome (Levinson et al., 2014), candidate gene approaches are no longer considered state of the science.
The present study addressed a critical gap in the area of gene-by-intervention research by integrating best practices in genetics with intervention science. We moved beyond the traditional candidate gene approach in studying G×I effects and instead incorporated polygenic risk scores. In recent years, polygenic scoring has developed as a way to characterize aggregate genetic risk for complex behavioral outcomes like alcohol dependence. This approach considers the contributions of many common genetic variants of small effects across the genome (Wray et al., 2014). Polygenic scores are typically calculated for each individual by summing the number of alleles for each single nucleotide polymorphism (SNP) weighted by the effect size (e.g., odds ratios for case-control) drawn from results of an independent discovery GWAS study. The scores then represent aggregate additive effects of the many variants that showed varying levels of association with the outcome. As a hypothetical example, consider two SNPs with the following alleles: SNP 1 (A/G) and SNP 2 (C/T). If a GWAS indicates that G and T alleles are associated with more alcohol dependence symptoms (beta weights = 0.03 and 0.05), an individual who carries one copy for the G and T alleles will have a polygenic score equivalent to 1*(0.03) + 1*(0.05) = 0.08. An individual who carries two copies for the G and T alleles will have a polygenic score equivalent to 2*(0.03) + 2*(0.05) = 0.16.
We capitalized on a randomized, early adolescent intervention study, the Family Check-Up (FCU; Dishion & Kavanagh, 2003), to examine the interactive effects between a genetic predisposition for alcohol dependence and the intervention on long-term alcohol outcome in young adulthood (approximately 15 years after the start of the intervention). The FCU is a family-centered intervention delivered in public middle school settings and to families. The FCU is designed to promote family management practices by supporting parents’ accurate appraisal of the child’s adjustment and their own parenting, and then helping them identify appropriate resources and change strategies (Dishion, Stormshak, & Siler, 2010). Evaluations of the randomized experimental study have documented the effectiveness of the FCU in reducing levels of involvement with deviant peers (van Ryzin, Stormshak, & Dishion, 2012) and levels of youth substance use (e.g., Dishion, Nelson, & Kavanagh, 2003; Véronneau, Connell, Dishion, Kavanagh, 2016), with changes associated with improvements in parent monitoring practices (Dishion et al., 2003). Previous results also indicated that the FCU was associated with less growth in alcohol use and related problem behaviors across adolescence, as well as a decreased likelihood of alcohol dependence/abuse diagnoses by age 18 (Connell, Dishion, Yasui, & Kavanagh, 2007). In this study, we examined whether the FCU would alter associations between genotypic risk scores and alcohol dependence.
We used results from one of the largest published large-scale GWAS studies on adult alcohol dependence (Gelernter et al., 2014), where independent, significant effects of different genetic variants were identified in European American and African American populations, to calculate genome-wide polygenic scores (GPS), which capture aggregate genetic risk, in our sample. This allowed us to test whether the FCU adolescent family-centered intervention moderated the association between alcohol dependence polygenic scores and alcohol dependence in young adulthood. We focused on the follow-up assessment in young adulthood because it represents an important period for the development of alcohol use patterns and problems (SAMHSA, 2017), and a large proportions of alcohol use disorders begin in young adulthood (Hasin, Stinson, Ogburn, & Grant, 2007). We examined alcohol outcomes as measured by lifetime alcohol dependence diagnosis. Given that rates of alcohol consumption and alcohol dependence vary across racial/ethnic groups (Grant et al., 2015) and there are important differences in genetic diversity, allele frequencies, and linkage disequilibrium patterns between European Americans and African Americans (Campbell & Tishkoff, 2008; Gabriel et al., 2002), we stratified the sample by analyzing separately within European American and African American groups in our sample. Accordingly, the purpose of the present study was to examine the extent to which the association between alcohol dependence genome-wide polygenic scores (AD-GPS) and the likelihood of alcohol dependence in young adulthood was moderated by intervention. We hypothesized that the association between AD-GPS and alcohol dependence would be reduced among the individuals in the intervention group within European Americans and African Americans.
Methods
Participants
This project was part of the larger Project Alliance 1 (PAL1) study that implemented a randomized experimental trial of the Family Check-Up (FCU), a family-centered intervention starting in middle school (Dishion & Kavanagh, 2003). The goal of the intervention was to reduce adolescent problem behavior and improve mental health by promoting family management skills and improving parenting practices in the areas of supervision, involvement, and monitoring of the child’s behavior (Dishion & Stormshak, 2007). Participating youth (N = 998) were followed from Grade 6 to young adulthood (Dishion & Kavanagh, 2003). Participants and their families were recruited in sixth grade from three public middle schools in an ethnically diverse metropolitan area in the Pacific Northwest region of the United States. All youth and families who were enrolled in the three participating schools were recruited to participate in the experimental prevention study. Fifty percent of the PAL1 youth and their families were randomized at the individual level to the intervention condition in Grade 6 (the first year of middle school) and to specific home room teachers in Grade 7 (n = 500 in the intervention group and n = 498 in the control group). Family resource centers were established in each of the three participating public middle schools as part of the intervention. The entire intervention group had access to parent-centered services of the family resource center. These services included brief consultations with parents, such as telephone consultations, in person contact, and access to videotapes and books relevant to parents’ concerns. In addition, all families in the intervention group could request a brief three-session intervention, based on motivational interviewing, that consists of an initial interview, an assessment session, and a feedback session by a trained parent consultant. 115 families (23%) in the intervention group elected to receive this. At baseline (Wave 1), participants averaged 12.22 years old (SD = 0.48), and 52.7% of youth were male, 42.4% self-identified as European American, 29.2% as African American, and 28.4% as other ethic/racial groups or mixed race. The University of Oregon’s Institutional Review Board approved this study. All parents (or guardians) provided written consent, and adolescents provided assent for participation in the study.
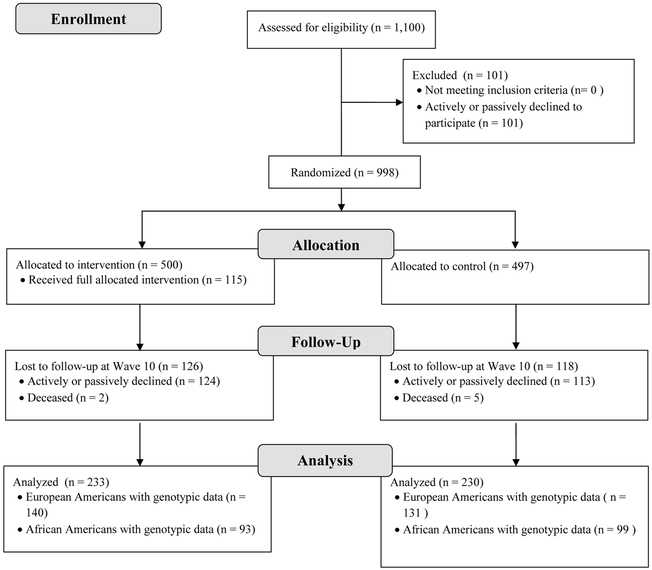
The current analyses focused on a subset of target participants who completed the Wave 10 assessment, approximately 15 years after baseline, and for whom genomic data were also available (Mage = 27.59 years, SD = 0.49; 48.6% male). One critical factor for deriving non-biased polygenic scores is matching ancestry between the discovery GWAS and the independent target sample (Martin et al., 2017). In order to create AD-GPS that were matched between the discovery GWAS (Gelernter et al., 2014) and PAL1 samples, we limited analyses to the European American (EA; n = 140 intervention; n = 131 control) and African American (AA; n = 93 intervention; n = 99 control) individuals, two of the largest racial/ethnic groups in PAL1 (See Figure 1 for a participant flow diagram). Another reason that we limited analyses to EA and AA participants is because the structure of the human genome differs across ancestral background such that there are differences in genetic diversity, linkage disequilibrium, and allele frequencies across groups (Campbell & Tishkoff, 2008; Gabriel et al., 2002). In the context of genetic associations studies, population stratification (systematic differences in allele frequencies between populations of different ancestry) can lead to spurious genetic associations, and effects detected will not necessarily have the same impact across different ancestry groups (see Cardon & Palmer, 2003 for a review). Accordingly, in order to minimize the issue of population stratification, we analyzed data separately within European American (EA) and African American (AA) groups in our sample.
Figure 1.
The flow diagram.
Measures
Lifetime Alcohol Dependence Diagnosis.
At Wave 10 (age 26-27), participants completed the comprehensive and standardized Composite International Diagnostic Interview (CIDI; Kessler & Üstün, 2004; World Health Organization, 1997) modules. Lifetime alcohol dependence (AD) diagnoses were assessed using CIDI. AD diagnoses were made based on DSM-IV criteria—that is, meeting 3 or more AD criteria in a single year (American Psychiatric Association, 1994). It is important to note that at Wave 10, participants had passed through the period of highest risk for onset of alcohol dependence. According to the National Epidemiologic Survey on Alcohol and Related Conditions, the average age at onset for DSM-IV alcohol dependence is 21.9 years (Hasin, Stinson, Ogburn, & Grant, 2007). Lifetime AD diagnosis was coded as 1 = affected and 0 = not affected.
Intervention Status.
Random assignment was coded as 0 = control condition and 1 = intervention group.
Genotyping.
DNA was collected via saliva sample using the Oragene data collection kits in young adulthood (Wave 10) and extracted according to Oragene’s recommended procedures. Genotyping was performed at Rutgers University Cell and DNA Repository (RUCDR) using the Affymetrix BioBank Array. Imputation was conducted to 1000 Genomes (Phase 3 reference panel; 1000 Genomes Project Consortium, 2015) using SHAPEIT2 (Delaneau, Zagury, & Marchini, 2013) and then IMPUTE2 (Howie, Donnelly, & Marchini, 2009). Single nucleotide polymorphisms (SNPs) that are palindromic with ambiguous effect directions (A/T or C/G), SNPs with a genotyping rate of < 0.95, SNPs that did not pass Hardy-Weinberg equilibrium (HWE; p < 10−6), or SNPs with a minor allele frequency (MAF) < 0.01 were excluded. In total, 2,067,148 SNPs passed quality control and data cleaning thresholds and were available for analysis.
Alcohol Dependence Genome-Wide Polygenic Scores (AD-GPS).
A typical approach to construct polygenic scores uses genome-wide association analysis (GWAS) summary statistics from a discovery sample to calculate polygenic scores in an independent target sample (Wray et al., 2014). In this study, we used genome-wide association estimates from the largest published GWAS of DSM-IV alcohol dependence to date (Gelernter et al., 2014) as the discovery GWAS to construct alcohol dependence polygenic scores in the PAL1 sample using the --score procedure in PLINK (Purcell et al., 2007). The Gelernter et al. discovery sample included 5,131 European American and 4,629 African American participants. The --score procedure calculates a linear function of the number of alleles an individual possessed from the score SNPs, with each SNP weighted by the negative log of the GWAS association p value and sign of the association (odds ratio – 1) statistic. Polygenic scores represent weighted sum of the number of risk alleles. We used separate estimates for European American and African American from the discovery GWAS to calculate polygenic scores in European American and African American in PAL1, respectively. Matching SNPs were clumped for linkage disequilibrium (LD) based on 1000 Genome phase 3 reference panel genotype data of individuals with the same ancestry as individuals from the discovery GWAS using discovery GWAS p-values for clumping. Given that there are no set criteria for establishing a threshold to create maximally informative scores (Evans, Visscher, & Wray, 2009), we calculated a series of polygenic scores in PAL1 that included SNPs meeting increasingly stringent p-value thresholds (p<0.50, p<0.40, p<0.30, p<0.20, p<0.10, p<0.05, p<0.01, p<0.001, p<0.0001) in the discovery GWAS (Gelernter et al., 2014).
Covariates and Measures for Sensitivity Analyses.
We included sex (male = 1, female = 0), age at Wave 10 (assessment of long-term outcome measures), and first three principal components for genetic ancestry in all analyses to make further adjustment for population stratification.
In sensitivity analyses, we used baseline (Wave 1) alcohol consumption and teacher-report of target participants’ risk behavior in Grade 6 at baseline (Wave 1) to evaluate whether the pattern of effects changed when the analyses included these variables as covariates in association and moderation analyses. Wave 1 alcohol consumption was a self-report measure of frequency of alcohol use during the past month. Teachers were asked to rate their full roster of Grade 6 students using a revised version of the TRISK measure developed by Soberman (1994). The measure included 16 items that assess the frequency of youth engagement in various risk behaviors (e.g., aggression, disliking school). All items were rated on a 5-point scale from 1 (never almost never) to 5 (always almost always). Items were averaged, and the scores were then standardized within classroom.
Analytic Strategy
We conducted preliminary analyses to determine which p-value threshold polygenic scores maximized the variance accounted for (R2) in alcohol dependence symptoms in the PAL1 sample. The percent variance accounted for (above and beyond participants’ age and sex) ranged between <0.01% to 2.40% for European American and <0.01% to 0.70% for African American participants. We carried forward polygenic scores constructed with p < 0.0001 (189 SNPs) and p < 0.001(2572 SNPs) for European American and African American, respectively, for subsequent association and moderation analyses, as they maximized R2.
To test whether intervention moderated the effects of AD-GPS on long term alcohol related outcomes (at Wave 10), we conducted a series of regression models predicting lifetime alcohol dependence diagnosis as a function of AD-GPS, intervention status, a product term (AD-GPS × Intervention status) measuring the interactive effect between mean-centered AD-GPS and intervention, controlling for participants’ age, sex, and first three principal components of genetic ancestry (PC1-PC3). We interpreted a statistically significant product term (p < .05) as indicative of moderation. We conducted follow-up analyses, as outlined by Aiken and West (1991), to interpret significant moderation by testing for significant simple slopes by groups.
In sensitivity analyses, we included baseline alcohol consumption and baseline severity of risk behavior as perceived by teachers as additional covariates in all models to test whether the pattern of observed effects changed. We included these additional covariates to examine whether G×I effects were robust controlling for behavioral risk and drinking in adolescence given prior evidence that externalizing problems and drinking in adolescence are predictors of subsequent alcohol problems, including alcohol dependence, in young adulthood (e.g., Englund, Egeland, Oliva, & Collins, 2008; Guo, Hawkins, Hill, & Abbott, 2001).
Results
Sample Representativeness Analyses
To determine whether this subsample of EAs and AAs (those completed Wave 10 assessment, approximately 15 years after baseline, and for whom genomic data were also available) was different from the EAs and AAs excluded in current analyses (lost to attrition or without genotypic data), a series of comparison tests were run on demographic and other relevant variables. Among European Americans, no differences between this analytic subset and those EAs excluded were detected on age and baseline alcohol consumption. However, this analytic subset had lower teacher-rated behavioral risk at baseline (t(421) = −2.16, p = .03) and was more likely to be female than was the group without Wave 10 outcome measures and genotypic data (χ2 = 5.40, df= 1, p = .02). Similarly, among African Americans, no differences between this analytic subset and those AAs excluded were detected on age and baseline alcohol consumption. However, this analytic subset had lower teacher-rated behavioral risk at baseline (t(289) = −2.01, p = .04) and was more likely to be female than was the group without Wave 10 outcome measures and genotypic data (χ2 = 9.76, df= 1, p < .01).
Descriptive Statistics
Descriptive statistics for study’s variables for the European American and African American samples are summarized in Table 1.
Table 1.
Descriptive Statistics for Control and Intervention Group Participants
| European American |
African American |
|||
|---|---|---|---|---|
| Variable | Control (n = 140) |
Intervention (n = 131) |
Control (n = 93) |
Intervention (n = 99) |
| M (SD) | M (SD) | M (SD) | M (SD) | |
| Age | 27.56 (0.51) | 27.55 (0.51) | 27.62 (0.52) | 27.64 (0.40) |
| W1 Alcohol Consumption | 0.42 (1.39) | 0.40 (1.15) | 0.35 (0.85) | 0.84 (2.47) |
| W1 TRISK | −0.30 (0.85) | −0.25 (0.81) | 0.31 (0.99) | 0.45 (1.02) |
| AD-GPS | 0.80 (0.16) | 0.80 (0.15) | 0.34 (0.02) | 0.34 (0.02) |
| n (%) | n (%) | n (%) | n (%) | |
| Malea | 66 (50.4) | 68 (48.6) | 43 (43.4) | 48 (51.6) |
| Alcohol Dependence Dxa | 32 (24.4) | 33 (23.7) | 12 (12.1) | 19 (20.4) |
Note. Age is calculated in years at Wave 10 outcome assessment. Sex coded as: 0 = females, 1 = males. Alcohol dependence diagnosis: 0 = unaffected, 1 = affected. W1 = Wave 1 baseline (Grade 6). Alcohol consumption = frequency of past-month alcohol consumption. TRISK = teacher-rated risk behavior (standardized within classroom) at baseline. AD-GPS = alcohol dependence genome-wide polygenic scores. Alcohol Dependence Dx = lifetime alcohol dependence diagnosis assessed at Wave 10 (~Age 27).
For binary variables, n and proportion for response category = 1 were reported.
Polygenic Association and G×I Effect
European American.
The AD-GPS was not associated with alcohol dependence diagnoses among European American participants. However, there was a significant AD-GPS by intervention status effect (b = −4.29, SE = 1.95, p = .03; Table 2). Follow-up analyses revealed that among participants randomized to the control condition, those with a higher AD-GPS were more likely to receive an alcohol dependence diagnosis (b = 2.58, SE = 1.32, p = .05). In contrast, among participants in the intervention condition, there was no association between the AD-GPS and alcohol dependence diagnosis (b = −1.71, SE = 1.44, p = .24). In other words, the previously observed association between genetic risk and adult alcohol dependence (Gelernter et al., 2014) was present only among individuals in the control condition; genotype was not associated with alcohol dependence among individuals who received the intervention. For illustrative purpose, we created a low AD-GPS versus high AD-GPS based on a median split. Among the control group participants, prevalence of lifetime alcohol dependence was 18% in the low AD-GPS groups as compared to 31% in the high AD-GPS group. In contrast, among the intervention group participants, prevalence of lifetime alcohol dependence was 26% in the low AD-GPS group as compared to 21% in the high AD-GPS group, a nonsignificant difference.
Table 2.
Logistic Regression Models Predicting Lifetime Alcohol Dependence Diagnosis from Alcohol Dependence Polygenic Scores, Intervention Status, and their Interaction among European Americans
| Main Effect Model |
G × I Model |
|||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | b | SE | P | 95% CI | b | SE | P | 95% CI |
| Intercept | −14.81 | 7.89 | 0.06 | [−30.27, 0.65] | −14.52 | 7.98 | 0.07 | [−30.17, 1.13] |
| PC1 | 80.09 | 81.48 | 0.33 | [−79.62, 239.79] | 64.61 | 82.42 | 0.43 | [−96.93, 226.15] |
| PC2 | 243.39 | 165.88 | 0.14 | [−568.52, 81.74] | 230.88 | 162.02 | 0.15 | [−548.45, 86.68] |
| PC3 | −60.96 | 62.70 | 0.33 | [−183.85, 61.94] | −53.94 | 62.39 | 0.39 | [−176.22, 68.33] |
| Age | 0.04 | 0.02 | 0.12 | [−0.01, 0.08] | 0.04 | 0.02 | 0.12 | [−0.01, 0.08] |
| Sex | −0.16 | 0.29 | 0.59 | [−0.73, 0.42] | −0.14 | 0.30 | 0.63 | [−0.73, 0.44] |
| Intervention | −0.04 | 0.29 | 0.88 | [−0.61, 0.53] | 0.01 | 0.30 | 0.98 | [−0.58, 0.59] |
| AD-GPS | 0.65 | 0.95 | 0.50 | [−1.22, 2.52] | 2.58 | 1.32 | 0.05 | [0.00, 5.16] |
| AD-GPS × I | -- | -- | -- | -- | −4.29 | 1.95 | 0.03 | [−8.11, −0.47] |
Note. Boldface indicates estimates P < 0.05. PC = principal component for genetic ancestry. Age at Wave 10 assessment. Sex coded as 1 = male, and 0 = female. AD-GPS = alcohol dependence genome-wide polygenic scores. Intervention coded as 1 = intervention, and 0 = control. I = intervention. N = 271.
African American.
There was no association between AD-GPS and the likelihood of alcohol dependence among African American participants. Further, there was no evidence of a moderation effect between AD-GPS and intervention status in predicting lifetime alcohol dependence (Table 3).
Table 3.
Logistic Regression Models Predicting Lifetime Alcohol Dependence Diagnosis from Alcohol Dependence Polygenic Scores, Intervention Status, and their Interaction among African Americans
| Main Effect Model |
G × I Model |
|||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | b | SE | P | 95% CI | b | SE | P | 95% CI |
| Intercept | −8.23 | 12.32 | 0.50 | [−32.38, 15.92] | −5.02 | 12.09 | 0.68 | [−28.72, 18.69] |
| PC1 | 40.39 | 48.92 | 0.41 | [−55.50, 136.27] | 41.71 | 49.33 | 0.40 | [−54.98, 138.41] |
| PC2 | 207.19 | 261.62 | 0.43 | [−719.96, 305.58] | 209.91 | 262.89 | 0.42 | [−725.16, 305.35] |
| PC3 | 115.96 | 110.28 | 0.29 | [−332.09, 100.18] | 116.36 | 111.16 | 0.30 | [−334.22, 101.50] |
| Age | 0.00 | 0.04 | 0.92 | [−0.07, 0.08] | 0.00 | 0.04 | 0.92 | [−0.07, 0.08] |
| Sex | 0.40 | 0.41 | 0.33 | [−0.41, 1.21] | 0.41 | 0.41 | 0.32 | [−0.40, 1.22] |
| Intervention | 0.59 | 0.41 | 0.15 | [−0.21, 1.38] | 0.60 | 0.41 | 0.14 | [−0.20, 1.41] |
| AD-GPS | 9.47 | 9.57 | 0.32 | [−9.29, 28.23] | 13.12 | 14.19 | 0.36 | [−14.69, 40.92] |
| AD-GPS × I | -- | -- | -- | -- | −6.45 | 18.50 | 0.73 | [−42.71, 29.82] |
Note. PC = principal component for genetic ancestry. Age at Wave 10 assessment. Sex coded as 1 = male, and 0 = female. AD-GPS = alcohol dependence genome-wide polygenic scores. Intervention coded as 1 = intervention, and 0 = control. I = intervention. N = 192.
Sensitivity Analyses
We conducted additional analyses to evaluate whether the pattern of observed effects among European American individuals were robust when we included two additional covariates from baseline (Grade 6; Wave 1): alcohol consumption and teacher-report of target participants’ risk behavior. At Wave 1, 83% of the youth reported no alcohol consumption in the past month. For predicting lifetime alcohol dependence, the pattern of effects was consistent after controlling for these additional covariates from baseline. The interaction between AD-GPS and intervention remained statistically significant (b = −4.07, SE = 1.99, p = .04). Follow-up analysis showed that higher AD-GPS was associated at trend level with a greater likelihood of alcohol dependence for those in the control condition (b = 2.47, SE = 1.33, p = .06), but not those in the intervention condition (b = −1.60, SE = 1.51, p = .29).
Discussion
In this study, we took a polygenic approach to examine the interactive effect between genetic risk for alcohol dependence and a randomized family-centered intervention, delivered in public school setting and to families, on long-term alcohol outcomes in young adulthood, approximately 15 years after the start of intervention. We found that among European American individuals, the intervention moderated the association between alcohol dependence polygenic scores and lifetime alcohol dependence diagnosis in young adulthood. Specifically, among participants in the control condition, higher AD-GPS was associated with a greater likelihood of receiving an alcohol dependence diagnosis. In contrast, among participants in the intervention condition, there was no association between AD-GPS and alcohol dependence diagnosis. Among African American participants, there was no evidence of AD-GPS by intervention status on alcohol dependence diagnosis.
Almost all of the G×I studies to date have focused on classic “usual suspect” candidate genes. However, the landscape of molecular genetics has evolved, and the use of a polygenic approach to index aggregate genetic risk better reflects our understanding of the polygenic architecture of complex behaviors, such as alcohol dependence (Plomin et al., 2009; Salvatore et al., 2014). In this study, we expanded upon previous evidence of candidate gene-by-intervention studies (Albert et al., 2015; Brody et al., 2009) to test for a moderating role of intervention in altering the association between genome-wide polygenic scores and long term alcohol outcomes. Our findings indicated a significant G×I effect among European American participants such that the association between alcohol dependence polygenic scores and clinical alcohol dependence diagnosis in young adulthood was only evident in the control group. In contrast, there was no association between polygenic scores and alcohol dependence diagnosis in the intervention group, indicating that intervention may protect genetically susceptible youth from developing alcohol related problems.
Consistent with the broader twin studies and measured G×E literature (Dick & Kendler, 2012, Shanahan & Hofer, 2005; Young-Wolff et al., 2011), our results demonstrate that a protective environment (as characterized by the intervention condition with emphasis on family and parenting processes) could attenuate the influence of genetic predispositions. Individuals with higher genetic vulnerability toward alcohol dependence may particularly benefit from preventive interventions.
Contrary to the G×I effect observed among European American individuals, there was no statistically significant interactive effect in predicting the likelihood of alcohol dependence among African American individuals. Notably, the confidence intervals around the estimate for G×I effect is large, indicative of a potential problem of low power. Although we used weights from the largest published GWAS to date on alcohol dependence for AA individuals (Gelernter et al., 2014), we note that the sample size for this discovery sample was still relatively small, and the predictive power polygenic scores is a function of the power of the GWAS in the discovery sample (Dudbridge, 2013; Martin et al., 2017). The sample size in the discovery GWAS for AAs was smaller in comparison to the sample of EAs included in the discovery, and also far from the large sample sizes that are typically required for a better-powered GWAS of complex behaviors (e.g., UK Biobank; Clarke et al., 2017). Thus, it is possible that our null finding is a reflection of a low predictive power of the alcohol dependence polygenic scores constructed and used in the current study. Populations of non-European descent, particularly those of African ancestry, have been historically underrepresented in gene identification efforts as a whole across fields (Dick, Barr, Guy, Nasim, & Scott, 2017; Popejoy & Fullerton, 2016). Large-scale consortium efforts, such as the Psychiatric Genomics Consortium, are slowly making attempts to increase the inclusion of individuals of African descent (Agrawal, Edenberg, & Gelernter, 2016) to conduct well-powered GWAS. Extremely large meta-analyses are needed to detect robust genetic main effects in complex behavioral outcomes such as alcohol dependence (Clarke et al., 2017; Hart & Kranzier, 2015; Walters et al., 2018). Predictive power of polygenic scores in non-European ancestry groups will improve as GWAS of larger sample sizes with diverse populations becomes available.
Our results should be interpreted within the context of the following limitations. First, we focused only on European American and African American individuals in order to minimize concerns regarding population stratification and the availability of ancestry-specific GWAS results from the Gelernter et al. (2014) alcohol dependence GWAS. Thus, findings may not be generalizable to samples of other ancestral background. In addition, there is more genetic diversity in African ancestry samples. Although most genotyping arrays enable a comprehensive capture of genetic diversity across diverse populations, more SNPs are needed to capture the variation across the genome in African ancestry samples (Campbell & Tishkoff, 2008; Johnston et al., 2017). Thus, caution should be taken regarding the interpretation of the our results among African American participants.
Second, randomized intervention studies represent a unique opportunity to examine gene-environment interaction because the environment in this case is randomly assigned and directly manipulated, with less room for error in measurement of the environmental condition under consideration. In order to preserve the randomization of intervention assignment, we followed the intent-to-treat strategy where all individuals who were randomly assigned to the intervention group were included in the analyses regardless of the level of engagement and participation in various components of the intervention. Although the intervention status in this study was randomly assigned, the issue of gene-environment correlation could still be at play because of varying levels of engagement and participation in different aspects of intervention.
Third, a polygenic approach is a useful way to bring large-scale gene identification findings forward to smaller developmental and prevention intervention studies. Although we found evidence for our hypothesized G×I effects, polygenic scores only account for a small amount of the variance. This is consistent with the literature that shows polygenic scores generally only account for a small amount of variance in alcohol-related phenotypes (e.g., Clarke et al., 2017). Our results caution against using polygenic scores in a clinical setting for complex behavioral outcomes such as alcohol dependence to identify for whom intervention is likely to be effective. However, polygenic scores can be carried forward into prevention studies to characterize a more global index of genetic risk, allowing us to better characterize mechanisms of risk and resilience. In addition, genome-wide polygenic scores, by design, are not necessarily biologically meaningful on their own. These scores represent an weighted linear combination of disease-associated alleles without any explicit insights into biological processes. Finally, we found evidence for the hypothesized interactive effect between polygenic scores and intervention status. However, understanding the mechanisms of gene-by-intervention effects at multiple levels (e.g., behavioral, neurobiological) is an important next step.
In conclusion, the present study adds to the literature by bringing large-scale gene identification efforts into a randomized adolescent intervention study with long-term longitudinal data that followed youth through the peak period for alcohol use and the development of alcohol problems in young adulthood to understand gene-by-intervention effects. Using GWAS results for alcohol dependence in a discovery sample to calculate polygenic scores in an independent preventive intervention sample represents a coordinated approach that capitalizes the current state of the art in genetics research to address important questions that are of great interest to prevention scientists. Our results illustrate that modifying environments of genetically vulnerable youth could reduce the likelihood of developing alcohol dependence. This highlights that the importance of genetic influences changes as a function of the environment and underscores the significance of environmentally focused prevention and intervention efforts. Future research on incorporating genetics into prevention studies will benefit from a close interdisciplinary collaboration between the fields of prevention science and genetics and identification of mechanisms underlying the interactive effect between genetic predispositions and intervention on outcomes, with important implications for strategic targets for prevention and intervention.
Acknowledgements:
This project was supported by the National Institutes of Health (NIH) Grants R01AA022071 (PI: Dishion) from NIAAA and R01DA07031 (PI: Dishion) from NIDA. This research was also supported in part by NIH grants K02AA018755 (PI: Dick) and K01AA024152 (PI: Salvatore). We gratefully acknowledge the contribution of the Project Alliance staff, Portland Public Schools, and the participating youth and families.
Funding: This project was supported by the National Institutes of Health (NIH) Grants R01AA022071 (PI: Dishion) from NIAAA and R01DA07031 (PI: Dishion) from NIDA. This research was also supported in part by NIH grants K02AA018755 (PI: Dick) and K01AA024152 (PI: Salvatore).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest: Dr. Thomas Dishion was the developer of the Family Check-Up preventive intervention. The authors declare that they have no conflict of interest.
Ethical Approval: The University of Oregon’s Institutional Review Board approved all of the study procedures. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- Agrawal A, Edenberg HJ, & Gelernter J (2016). Meta-analyses of genome-wide association data hold new promise for addiction genetics. Journal of Studies on Alcohol and Drugs, 77, 676–680. doi: 10.15288/jsad.2016.77.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken LS, & West SG (1991). Multiple regression: Testing and interpreting interactions. Newbury Park, CA: SAGE. [Google Scholar]
- Albert D, Belsky DW, Crowley DM, Latendresse SJ, Aliev F, Riley B, … & Dodge KA (2015). Can genetics predict response to complex behavioral interventions? Evidence from a genetic analysis of the Fast Track Randomized Control Trial. Journal of Policy Analysis and Management, 34(3), 497–518. doi: 10.1002/pam.21811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2000). Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.). Washington DC: Author. [Google Scholar]
- Bosker FJ, Hartman CA, Nolte IM, Prins BP, Terpstra P, Posthuma D, … & Hoogendijk WJ (2011). Poor replication of candidate genes for major depressive disorder using genome-wide association data. Molecular Psychiatry, 16(5), 516–532. doi: 10.1038/mp.2010.38 [DOI] [PubMed] [Google Scholar]
- Brody GH, Chen YF, Beach SR, Kogan SM, Yu T, DiClemente RJ, … & Philibert RA (2014). Differential sensitivity to prevention programming: A dopaminergic polymorphism-enhanced prevention effect on protective parenting and adolescent substance use. Health Psychology, 33(2), 182–191. doi: 10.1037/a0031253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Chen YF, Beach SR, Philibert RA, & Kogan SM (2009). Participation in a family-centered prevention program decreases genetic risk for adolescents’ risky behaviors. Pediatrics, 124(3), 911–917. doi: 10.1542/peds.2008-3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MC, & Tishkoff SA (2008). African genetic diversity: implications for human demographic history, modern human origins, and complex disease mapping. Annual Review Genomics Human Genetics., 9, 403–433. doi: 10.1146/annurev.genom.9.081307.164258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardon LR, & Palmer LJ (2003). Population stratification and spurious allelic association. The Lancet, 361(9357), 598–604. doi: 10.1016/S0140-6736(03)12520-2 [DOI] [PubMed] [Google Scholar]
- Clarke T, Adams M, Davies G, Howard D, Hall L, Padmanabhan S, … McIntosh A (2017). Genome-wide association study of alcohol consumption and genetic overlap with other health-related traits in UK Biobank (N=112 117). Molecular Psychiatry, 22(10), 1376–1384. doi: 10.1038/mp.2017.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AL, Kim Y, Sklar P, O'Donovan MC, Sullivan PF, & International Schizophrenia Consortium. (2012). Hypothesis-driven candidate genes for schizophrenia compared to genome-wide association results. Psychological Medicine, 42(3), 607–616. doi: 10.1017/S0033291711001607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell AM, Dishion TJ, Yasui M, & Kavanagh K (2007). An adaptive approach to family intervention: Linking engagement in family-centered intervention to reductions in adolescent problem behavior. Journal of Consulting and Clinical Psychology, 75, 568–579. doi: 10.1037/0022-006X.75.4.568 [DOI] [PubMed] [Google Scholar]
- Delaneau O, Zagury J, & Marchini J (2013). Improved whole-chromosome phasing for disease and population genetic studies. Nature Methods, 10(1), 5–6. doi: 10.1038/nmeth.2307 [DOI] [PubMed] [Google Scholar]
- Dick DM, Agrawal A, Keller MC, Adkins A, Aliev F, Monroe S, … & Sher KJ (2015). Candidate gene–environment interaction research: Reflections and recommendations. Perspectives on Psychological Science, 10(1), 37–59. doi: 10.1177/1745691614556682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick D, Barr P, Guy M, Nasim A, & Scott D (2017). Genetic research on alcohol use outcomes in African American populations: A review of the literature, associated challenges, and implications. American Journal on Addictions., 26(5), 486–493. doi: 10.1111/ajad.12495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, & Kendler KS (2012). The impact of gene–environment interaction on alcohol use disorders. Alcohol Research Current Reviews, 34(3), 318–324. [PMC free article] [PubMed] [Google Scholar]
- Dishion TJ, & Kavanagh K (2003). Intervening in adolescent problem behavior: A family-centered approach. New York, NY: Guilford Press. [Google Scholar]
- Dishion TJ, Nelson SE, & Kavanagh K (2003). The family check-up with high-risk young adolescents: Preventing early-onset substance use by parent monitoring. Behavior Therapy, 34(4), 553–571. doi: 10.1016/S0005-7894(03)80035-7 [DOI] [Google Scholar]
- Dishion TJ, & Stormshak EA (2007). Intervening in children’s lives: An ecological, family-centered approach to mental health care. Washington, DC: American Psychological Association. [Google Scholar]
- Dishion TJ, Stormshak EA, & Siler C (2010). An ecological approach to intervention with high-risk students in schools: Using the Family Check-Up to motivate parents’ positive behavior support In. Shinn M & Walker H (Eds.), Intervention for achievement and behavior problems in a three-tier model including RTI (pp. 101–123). Bethesda, MD: National Association of School Psychologists. [Google Scholar]
- Dudbridge F (2013). Power and predictive accuracy of polygenic risk scores. PLoS Genetics, 9(3), 1–17. doi: 10.1371/journal.pgen.1003348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LE, & Keller MC (2011). A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. American Journal of Psychiatry, 168(10), 1041–1049. doi: 10.1176/appi.ajp.2011.11020191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund MM, Egeland B, Oliva EM, & Collins WA (2008). Childhood and adolescent predictors of heavy drinking and alcohol use disorders in early adulthood: a longitudinal developmental analysis. Addiction, 103, 23–35. doi: 10.1111/j.1360-0443.2008.02174.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DM, Visscher PM, & Wray NR (2009). Harnessing the information contained within genome-wide association studies to improve individual prediction of complex disease risk. Human Molecular Genetics, 18, 3525–3531. doi: 10.1093/hmg/ddp295 [DOI] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, … & Liu-Cordero SN (2002). The structure of haplotype blocks in the human genome. Science, 296(5576), 2225–2229. doi: 10.1126/science.1069424 [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler HR, Sherva R, Almasy L, Koesterer R, Smith AH, … & Wodarz N (2014). Genome-wide association study of alcohol dependence: significant findings in African-and European-Americans including novel risk loci. Molecular Psychiatry, 19(1), 41–49. doi: 10.1038/mp.2013.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, … & Hasin DS (2015). Epidemiology of DSM-5 alcohol use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry, 72, 757–766. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Hawkins J, Hill K, & Abbott R (2001). Childhood and adolescent predictors of alcohol abuse and dependence in young adulthood. Journal of Studies on Alcohol., 62, 754–762. doi: 10.15288/jsa.2001.62.754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AB, & Kranzler HR (2015). Alcohol dependence genetics: Lessons learned from Genome-Wide Association Studies (GWAS) and Post-GWAS analyses. Alcoholism: Clinical and Experimental Research, 39(8), 1312–1327. doi: 10.1111/acer.12792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, & Grant BF (2007). Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry, 64(7), 830–842. doi: 10.1001/archpsyc.64.7.830 [DOI] [PubMed] [Google Scholar]
- Howie BN, Donnelly P, & Marchini J (2009). A Flexible and Accurate Genotype Imputation Method for the Next Generation of Genome-Wide Association Studies. PLoS Genetics, 5(6), e1000529. doi: 10.1371/journal.pgen.1000529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston HR, Hu Y-J, Gao J, O’Connor TD, Abecasis GR, Wojcik GL, … & CAAPA Consortium. (2017). Identifying tagging SNPs for African specific genetic variation from the African Diaspora Genome. Scientific Reports, 7, 46398. doi: 10.1038/srep46398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R, & Üstün T (2004). The World Mental Health (WMH) Survey Initiative version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI). International Journal of Methods in Psychiatric Research, 13(2), 93–121. doi: 10.1002/mpr.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson DF, Mostafavi S, Milaneschi Y, Rivera M, Ripke S, Wray NR, & Sullivan PF (2014). Genetic studies of major depressive disorder: why are there no genome-wide association study findings and what can we do about it?. Biological Psychiatry, 76(7), 510–512. doi: 10.1016/j.biopsych.2014.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AR, Gignoux CR, Walters RK, Wojcik GL, Neale BM, Gravel S, … Kenny EE (2017). Human demographic history impacts genetic risk prediction across diverse populations. American Journal of Human Genetics, 100, 635–649. doi: 10.1016/j.ajhg.2017.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, Haworth CM, & Davis OS (2009). Common disorders are quantitative traits. Nature Reviews Genetics, 10(12), 872–878. doi: 10.1038/nrg2670 [DOI] [PubMed] [Google Scholar]
- Popejoy A, & Fullerton S (2016). Genomics is failing on diversity. Nature, 538(7624), 161–164. doi: 10.1038/538161a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira M, Bender D, … Sham P (2007). PLINK: A tool set for whole-genome association and population-based linkage analyses. American Journal of Human Genetics, 81(3), 559–575. doi: 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks J, Gonzales K, Bouchery E, Tomedi L, & Brewer R (2015). 2010 National and State Costs of Excessive Alcohol Consumption. American Journal of Preventive Medicine, 49(5), E73–E79. doi: 10.1016/j.amepre.2015.05.031 [DOI] [PubMed] [Google Scholar]
- Salvatore JE, Aliev F, Edwards AC, Evans DM, Macleod J, Hickman M, … & Latvala A (2014). Polygenic scores predict alcohol problems in an independent sample and show moderation by the environment. Genes, 5(2), 330–346. doi: 10.3390/genes5020330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan MJ, & Hofer SM (2005). Social context in gene–environment interactions: Retrospect and prospect. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 60(Special_Issue_1), 65–76. doi: 10.1093/geronb/60.Special_Issue_1.65 [DOI] [PubMed] [Google Scholar]
- Soberman L (1994). Psychometric validation of a brief teacher screening instrument (TRISK) [Unpublished doctoral dissertation]. University of Oregon, Eugene, OR. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2017). Results from the 2016 National Survey on Drug Use and Health: Detailed Tables. Rockville, MD: SAMHSA. [Google Scholar]
- Van Ryzin MJ, Stormshak EA, & Dishion TJ (2012). Engaging parents in the Family Check-Up in middle school: Longitudinal effects on family conflict and problem behavior through the high school transition. Journal of Adolescent Health, 50, 627–633. doi: 10.1016/j.jadohealth.2011.10.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst B, Neale MC, & Kendler KS (2015). The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychological Medicine, 45(5), 1061–1072. doi: 10.1017/S0033291714002165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronneau M, Dishion TJ, Connell AM, & Kavanagh K (2016). A randomized, controlled trial of the family check-up model in public secondary schools: Examining links between parent engagement and substance use progressions from early adolescence to adulthood. Journal of Consulting and Clinical Psychology, 84(6), 526–543. doi: 10.1037/a0040248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters RK, Polimanti R, Johnson EC, McClintick JN, Adams MJ, Adkins AE, … & Agrawal A (2018). Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nature Neuroscience, 21, 1656–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (1997). Composite International Diagnostic Interview (CIDI, ver. 2.1). Geneva, Switzerland: World Health Organization. [Google Scholar]
- Wray NR, Lee SH, Mehta D, Vinkhuyzen AA, Dudbridge F, & Middeldorp CM (2014). Research review: polygenic methods and their application to psychiatric traits. Journal of Child Psychology and Psychiatry, 55(10), 1068–1087. doi: 10.1111/jcpp.12295 [DOI] [PubMed] [Google Scholar]
- Young-Wolff KC, Enoch M-A, & Prescott CA (2011). The Influence of Gene-Environment Interactions on Alcohol Consumption and Alcohol Use Disorders: A Comprehensive Review. Clinical Psychology Review, 31(5), 800–816. doi: 10.1016/j.cpr.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]