Abstract
Introduction:
Impairment of monocarboxylate transporter- (MCT) dependent astrocyte-neuron lactate transfer disrupts long-term memory and erases drug-associated memories in mice. However, few studies have examined how drugs of abuse alter astrocyte-neuron lactate transfer in neurocircuits related to addiction. This is particularly pertinent for ethanol, which has been demonstrated to impair CNS glucose uptake and significantly alter peripheral levels of glucose, lactate, acetate, and ketones.
Methods:
We subjected C57BL/6J mice to a chronic intermittent ethanol (CIE) exposure paradigm to investigate how chronic ethanol exposure alters the concentration of glucose and lactate within the serum and CNS during withdrawal. Next, we determine how chronic injections of lactate (1g/kg, twice daily for two weeks) influence central and peripheral glucose and lactate concentrations. Finally, we determine how CIE and chronic lactate injection affect astrocyte-neuron lactate transfer by analyzing the expression of MCTs.
Results:
Our results show that CIE induces lasting changes in CNS glucose and lactate concentrations, accompanied by increased expression of MCTs. Interestingly, although chronic lactate injection mimics the effect of ethanol on CNS metabolites, chronic lactate injection is not associated with increased expression of MCTs.
Conclusion:
CIE increases CNS concentrations of glucose and lactate and augments the expression of MCTs. Although we found that chronic lactate injection mimics ethanol-induced increases in CNS lactate and glucose, lactate failed to alter the expression of MCTs. This suggests that although lactate may influence the homeostasis of bioenergetic molecules in the CNS, ethanol-associated increases in lactate are not responsible for increased MCT expression.
Introduction
Drug addiction has conventionally been attributed to stereotypic changes in glutamatergic, GABAergic, and dopaminergic signaling, resulting in behavioral manifestations such as tolerance, impulsive drug seeking, craving, and withdrawal (Ayers-Ringler et al., 2016; D’Souza, 2015; Jiao et al., 2015; Koob and Volkow, 2016). However, no single neurotransmitter system seems to fully account for the neurobiological and behavioral manifestations of addiction. Interestingly, numerous investigations have revealed that drugs of abuse are associated with altered handling of bioenergetic compounds, including glucose and lactate. For instance, ex vivo NMR has demonstrated that opioids and barbiturates alter the concentration of glucose and lactate within the CNS (Serres et al., 2004) , and lactate transport has been demonstrated to be necessary for the maintenance of long-term memory and cocaine-associated drug memories prompting relapse (Boury-Jamot et al., 2016a; Boury-Jamot et al., 2016b; Suzuki et al., 2011; Zhang et al., 2016).
Perhaps most intriguingly, ethanol is a known metabolic toxin that impairs glucose uptake in astrocytes and neurons, impairs mitochondrial function, and results in hypoglycemia as well as increased serum concentrations of lactate, acetate, and ketones (Abdul Muneer et al., 2011; Eravci et al., 1999; Haorah et al., 2013; Jung, 2015; Kreisberg, 1967; Ma et al., 2017; Rawat and Kuriyama, 1972). The CNS is remarkable in its ability to utilize these alternative energy sources during times of glucose starvation (Hertz and Rothman, 2016). This is at least partially due to the unique energetic relationship that exists between neurons and astrocytes.
Neurons are perhaps the most energetically demanding cells of the body, requiring vast quantities of ATP to maintain their excitability via the Na+/K+-ATPase, power the molecular motors of axonal transport, and provide the energetic substrate required for the extensive structural remodeling associated with synaptic plasticity. In spite of this, neurons are heavily reliant on astrocytes for energetic compounds (Mason, 2017; Simpson et al., 2007). Although neurons express the high affinity glucose transporter GLUT3, which is induced during periods of increased cerebral glucose utilization (Ferreira et al., 2011), the perivascular astroglial sheath provides a complete covering of the brain microvessels, limiting neuronal access to glucose and necessitating the indirect acquisition of bioenergetic compounds via astrocytes (Mathiisen et al., 2010). This is hypothesized to occur via a process known as the astrocyte-neuron lactate shuttle (ANLS).
According to this hypothesis, glucose is concentrated in astrocytes by the action of the GLUT1 transporter located on the perivascular astroglial sheath and astrocytic endfeet (Mason, 2017). Astrocytes are heavily glycolytic, and subsequently metabolize glucose to pyruvate, which may either enter the TCA cycle via the action of pyruvate dehydrogenase or undergo conversion to lactate by lactate dehydrogenase (LDH) (Pellerin and Magistretti, 2012). Astrocyte-derived lactate is then shuttled to neurons by monocarboxylate transporters (MCTs), exiting astrocytes via MCT1 or MCT4 and entering neurons via MCT2 (Mason, 2017; Pellerin and Magistretti, 2012). Here, lactate is converted back to pyruvate, which is used to fuel the TCA cycle and oxidative metabolism (Mason, 2017). Interestingly, astrocytes also store abundant supplies of glycogen, which is broken down by glycogen phosphorylase (GP) during periods of increased neuronal activity to provide the active neurons with additional lactate as bioenergetic fodder (Brown and Ransom, 2015; Pellerin and Magistretti, 2012). Furthermore, astrocytes may take up and utilize alternative metabolites such as ketone bodies, lactate, and acetate, which modulate the bioenergetic processes of astrocytes, and likely exert a downstream effect on neuronal activity. For example, the ketone body beta-hydroxybutyrate strongly reduces glucose consumption in mouse astrocytes and hippocampal slices by inhibiting glycolysis and stimulating pyruvate breakdown via the TCA cycle (Valdebenito et al., 2016). Conversely, episodes of hypoglycemia have been demonstrated to result in increased neuronal and astrocytic glucose utilization during subsequent euglycemic periods (Jiang et al., 2009). Thus, modulation of circulating levels of metabolic substrates as well as ethanol-induced inhibition of glucose uptake may alter the handling of glucose and lactate within astrocytes and neurons of the CNS. If maintained, these changes may preclude the normal utilization of serum metabolites upon return to baseline conditions.
In this study, we examine the effects of chronic ethanol exposure on CNS glucose and lactate concentrations during withdrawal. Furthermore, we investigate whether chronic ethanol exposure alters lactate handling within the CNS by examining the expression of MCT transporters in neurocircuits implicated in addiction such as the fronto-striatal and hippocampal-striatal circuits encompassing the hippocampus (HCP) and medial prefrontal cortex (mPFC). Our study demonstrates that chronic ethanol exposure induces sustained changes in lactate and glucose handling as well as changes in the ANLS. These bioenergetic modifications may play a role in ethanol sensitivity or withdrawal, and may be tenable targets for pharmacotherapeutic treatment of alcohol use disorder (AUD).
MATERIALS AND METHODS
Animals
Young adult male C57BL/6J mice (7 weeks old, Jackson Laboratories, Bar Harbor, ME) were group housed (4–5 mice per group) in standard Plexiglas cages and were given one week to acclimatize to a reverse-phase sleep-wake cycle with lights on at 0200 and lights off at 1400 prior to commencing chronic intermittent ethanol exposure. Food and water were provided ad libitum. All animal care and handling procedures were approved by the Mayo Clinic Institutional Care and Use Committee in accordance with National Institute of Health guidelines.
Chronic Intermittent Ethanol Exposure
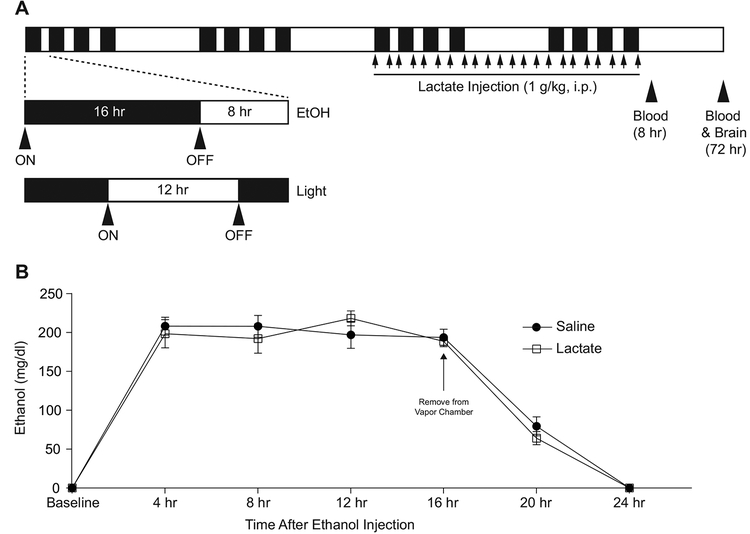
Young adult male mice, aged 8 weeks, were subjected to a chronic intermittent ethanol exposure (CIE) paradigm formerly demonstrated to reliably induce withdrawal seizures as well as neural adaptations associated with addiction and withdrawal (Becker et al., 1997; DePoy et al., 2013; Metten et al., 2010) (Figure 1). Briefly, mice underwent 4 one-week cycles, each consisting of 4 consecutive days of ethanol vapor/room air exposure followed by a 3 day interlude (withdrawal period), during which animals were left in their home cage. Ethanol vapor/room air was administered for 16 hr/day during a reverse-phase sleep/wake cycle using experimental vapor administration chambers (Morton et al., 2014). This period began during the dark phase at 1800 and encompassed 8 hr of the dark phase and 8 hr of the light phase, at which point mice were returned to their home cage for the remaining 8 hr of the 24-hr day. Vapor administration chambers vaporize alcohol by pumping 95% ethanol into a flask to mix the vaporized ethanol with room air, and continuously pump the mixture into a sealed chamber to maintain an ethanol concentration of 12–14 mg/L. Air control chambers received identical airflow rates from room air. Prior to each vapor exposure, mice undergoing ethanol treatment were administered a loading dose of 1.5 g/kg ethanol (20% v/v in 0.9% saline) and 68.1 mg/kg pyrazole (Sigma Aldrich, St. Louis, MO) in a single intraperitoneal (i.p.) injection. Pyrazole is a small-molecule inhibitor of alcohol dehydrogenase, which prevents ethanol metabolism and helps to maintain a stable blood alcohol concentration (BAC) throughout the entirety of vapor exposure (Becker and Hale, 1993; Becker et al., 1997). Saline/air control mice received equal volume i.p. injections of 68.1 mg/kg pyrazole dissolved in 0.9% saline. After injections, mice were immediately placed inside the vapor chambers, where they retained ad libitum access to food and water. Our previous experiments have demonstrated that these procedures yield consistent blood alcohol concentrations (BACs) of approximately 200 mg/dL for the duration of the ethanol exposure period.
Figure 1:
A: Schematic representation of chronic intermittent ethanol (CIE) exposure paradigm. Mice were exposed to ethanol for 16 hr each day for 4 consecutive days followed by a 3-day withdrawal period. This 7-day protocol was repeated for 4 weeks. During the latter 2 weeks, mice were injected twice daily with either 1g/kg lactate or saline vehicle upon entering and removal from the vapor chambers. Blood was drawn 8 hr following the final ethanol exposure, and brains were removed for metabolic analysis 72 hr after the final vapor exposure. B: Time course of blood ethanol concentration during and after the final vapor exposure of the CIE exposure paradigm shown in (A). Blood ethanol concentrations were maintained at approximately 200 mg/dl for the duration of ethanol exposure and returned to 0 mg/dl prior to 8 hr after removal from the vapor chamber. n=5.
Chronic Lactate Exposure
Mice were subjected to chronic lactate exposure using twice-daily i.p. injections of 1.0 g/kg lactate in 0.9% saline. This dose has formerly been demonstrated to produce antidepressant-like effects in various murine models of depression (Carrard et al., 2018), and thus was hypothesized to be sufficient to influence neural pathways or signaling processes involved in drinking behavior or ethanol sensitivity. Lactate injections began two weeks after initiation of CIE and continued until the end of the 4-week ethanol exposure period (2 weeks of lactate injection). Injections were administered at 1000 hrs and 1800 hrs daily, concordant with placement in and removal from the ethanol chambers on days when ethanol vapor/air was administered. In order to minimize the number of injections given to each animal, lactate administered prior to vapor exposure was dissolved within the previously described solutions. Thus, animals exposed to ethanol were administered a priming dose of 1.5 g/kg ethanol, 68.1 mg/kg pyrazole, and 1.0 g/kg lactate dissolved in 0.9% saline; mice placed in the air chambers were administered 1.0 g/kg lactate and 68.1 mg/kg pyrazole dissolved in 0.9% saline.
Tissue Processing and Western Blot
Adult mice (aged 12 weeks) were sacrificed 72 hr following CIE or chronic lactate exposure. Mice (n=6) were anesthetized with carbon dioxide and were rapidly decapitated. Blood was collected upon decapitation, and brains were rapidly dissected in order to isolate the medial prefrontal cortex and the hippocampus. Brain tissue was immediately immersed in cold extraction buffer containing 50 mM Tris buffer (pH 7.4), 2 mM EDTA, 5 mM EGTA, 0.1% SDS, protease inhibitor cocktail (Roche, Basel, Switzerland), and phosphatase inhibitor cocktail types I and II (Sigma, St. Louis, MO). Tissue was homogenized using 1.0 mm diameter ceria stabilized zirconium oxide beads in a Bullet Blender Storm 24 with air cooling (Next Advance, Inc., Averill Park, NY) for 3 minutes. Homogenates were centrifuged at 500 g at 4 °C for 15 min, and the supernatants were collected. Proteins were analyzed using the Bradford protein assay (Bio-Rad, Hercules, CA), and equal amounts of protein (20 ug) were separated on 4–12% NuPAGE Bis-Tris gels at 130 V for 2 hr, transferred onto PVDF membranes at 30 V for 1 hr, and analyzed using antibodies against MCT1 (1:500, Santa Cruz, Dallas, TX), MCT2 (1:500, Santa Cruz, Dallas, TX), MCT4 (1:500, Santa Cruz, Dallas, TX), and GAPDH (1:1000, Millipore, Burlington, MA). Blots were developed using SuperSignal West Dura Chemiluminescent Substrate (Thermo Scientific, Rockford, IL), and chemilumiscent bands were detected on a Kodak Image Station 4000R scanner (New Haven, CT). All images were quantified using NIH Image J software.
Lactate and Glucose Measurements
Lactate and glucose concentrations were measured in brain homogenates and serum of 12-week old animals using an Analox GL5 multi-metabolite analyzer (Analox Instruments, United Kingdom) with the accompanying kits. Brain homogenates were obtained as previously described (See Tissue Processing and Western Blot). Cell-free serum was isolated from blood by spinning samples at 15,000 g for 20 minutes and then collecting the translucent supernatant. Lactate and glucose concentrations were measured in serum at and brain homogenate at time points 8 and 72 hr following chronic ethanol and chronic lactate exposure, as well as timepoints 10 min, 30 min, 3 hr, 24 hr, and 72 hr following acute administration of 1.0 g/kg lactate (n=5). Lactate and glucose concentrations were analyzed independently using unique Analox settings and were standardized to known injections of 144.1 mg/dl glucose and 72.1 mg/dl lactate.
Statistical Analysis
All data are expressed as mean ± SEM and were analyzed by two-way ANOVA (lactate and glucose analyses) using Bonferroni post-hoc analysis or unpaired two-tailed Student’s t-tests (Western Blot). Bonferroni post-hoc analysis was utilized only when a significant interaction between variables was observed. Importantly, all time-dependent experiments were carried out using 5–8 mice per time point, and were analyzed using unmatched two-way ANOVA. Results of comparisons were considered statistically different if the p-value was < 0.05.
RESULTS
Chronic ethanol exposure produces prolonged peripheral hypoglycemia and lactic acidosis but persistently elevates glucose and lactate within the mPFC and HPC
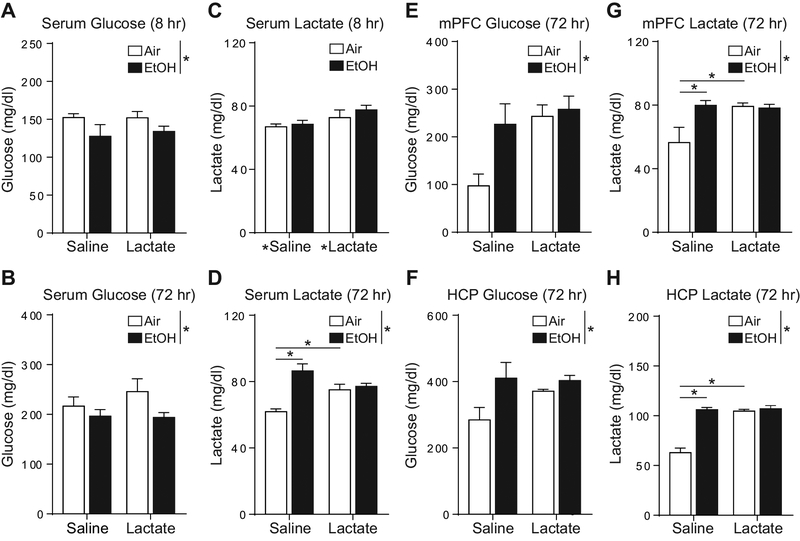
In order to examine the metabolic effects of chronic ethanol exposure within the periphery and CNS, mice were subjected to a chronic intermittent ethanol exposure paradigm formerly demonstrated to reliably reproduce the behavioral manifestations of addiction and withdrawal (Becker et al., 1997; DePoy et al., 2013; Metten et al., 2010) (Figure 1A). Using this protocol, we were able to maintain a blood ethanol content of approximately 200 mg/dl during periods of vapor exposure (Figure 1B). During the final two weeks of CIE, mice were subjected to twice daily i.p. injections of either 1.0 g/kg lactate or saline control. Given that chronic ethanol exposure is associated with lactic acidosis, we hypothesized that these chronic lactate injections may mimic the effects of CIE in the brain of air-exposed animals or produce additive or synergistic effects on the metabolic changes observed within the brains of ethanol-exposed mice (Abdul Muneer et al., 2011; Eravci et al., 1999; Haorah et al., 2013; Jung, 2015; Kreisberg, 1967; Ma et al., 2017; Rawat and Kuriyama, 1972). Following four weeks of CIE, we analyzed blood and brain tissue for levels of lactate and glucose in mice injected with saline or lactate during the withdrawal period 8 and 72 hr following removal from the vapor chamber. Blood metabolites were measured at both 8 and 72 hr, while brain tissue was analyzed only at 72 hr. These time points have formerly been demonstrated to be associated with early and late symptoms of withdrawal (Becker and Hale, 1993; Becker and Veatch, 2002; Rodd-Henricks et al., 2000), and were marked by a return of blood ethanol content to 0 mg/dl (Figure 1B), thus allowing us to distinguish the effects of chronic ethanol exposure from those of acute intoxication.
Analysis of serum glucose concentrations 8 hr after CIE using two-way ANOVA revealed a main effect of ethanol exposure in significantly reducing serum glucose (F1,18=5.71; p<0.05; Figure 2A) without a main effect of lactate injection (F1,18=0.11; p=0.74; Figure 2A) and no group (ethanol) x treatment (lactate) interaction. Interestingly, this main effect of ethanol exposure on serum glucose concentration was maintained 72 hr after removing mice from the vapor chamber (F1,18=4.49; p<0.05; Figure 2B), while lactate injection continued to have no significant effect on serum glucose concentrations (F1,18=0.611; p=0.4446; Figure 2B) without interaction between the effects of ethanol and lactate exposure. In contrast, for serum lactate concentrations 8 hr after CIE, two-way ANOVA revealed a significant main effect of lactate injection in increasing serum lactate concentrations (F1,18=4.73; p<0.05; Figure 2C), while ethanol exposure exerted no significant effect on measured levels of lactate (F1,18=0.91; p=0.35; Figure 2C), and no group x treatment interaction. Conversely, 72 hr following removal from the vapor chamber, we found a significant main effect of ethanol exposure in increasing serum lactate (F1,18=23.82; p<0.001; Figure 2D) with no main effect of lactate injections (F1,18=0.49; p=0.49; Figure 2D) in two-way ANOVA. Interestingly, two-way ANOVA revealed significant interaction between the effects of lactate injection and ethanol exposure on serum lactate concentrations 72 hr after CIE (F1,18=17.08; p<0.001; Figure 2D). Bonferroni post-hoc statistical analysis showed that although CIE significantly increased serum lactate 72 hr after ethanol exposure in saline-injected animals (p<0.001; Figure 2D), CIE did not significantly affect serum lactate in lactate-injected animals (p>0.99). Furthermore, lactate injections were found to significantly increase serum lactate concentrations in air-exposed animals (p<0.05; Figure 2D), but not in mice subjected to CIE (p=0.85; Figure 2D).
Figure 2:
The effects of ethanol and chronic lactate injections on serum and brain concentrations of glucose and lactate. A, B: CIE significantly decreased serum concentrations of glucose at both 8 and 72 hr after vapor exposure (n=5), but C, D: resulted increased serum concentrations of lactate at both time points (n=5). Within the mPFC and HCP (E-H), CIE increased concentrations of glucose (E, F) and lactate (G, H) 72 hr after vapor exposure. Chronic lactate injection (1g/kg) had no effect on serum concentrations of glucose or lactate (A-D), but mimicked the effects of CIE in increasing concentrations of glucose and lactate within both brain regions examined (E-H) (n=5). * Indicate main effect or post-hoc analysis of p<0.05.
Lactate and glucose concentrations within the HCP and mPFC responded similarly to CIE and repeated lactate injections. For example, two-way ANOVA analyzing glucose concentrations within the mPFC revealed a significant main effect of ethanol exposure in increasing glucose concentrations within the mPFC (F1,18=5.42; p<0.05; Figure 2E). In spite of this, there was no main effect of lactate injection or significant interaction between the effects of lactate injection and ethanol exposure on concentrations of glucose within the mPFC (F1,18=3.42; p=0.08; Figure 2E). This pattern of metabolite response to CIE and chronic lactate injection was maintained for both lactate and glucose concentrations within both the mPFC and HCP. Accordingly, two-way ANOVA showed that chronic ethanol exposure exerted a main effect in significantly increasing concentrations of glucose within the HCP (F1,18=8.23; p<0.01; Figure 2F), as well as increasing lactate concentrations within both the mPFC (F1,18=4.94; p<0.05; Figure 2G) and the HCP (F1,18=43.82; p<0.001; Figure 2H). There was no main effect of lactate injection on levels of glucose within the HCP or lactate within the mPFC or HCP. However, there was a significant interaction between the effects of lactate injection and ethanol exposure on levels of lactate within the mPFC (F1,18=5.88; p<0.05; Figure 2G) and HCP (F1,18=35.19; p<0.0001; Figure 2H). However, there was no significant interaction between the effects of lactate injection and ethanol exposure on concentrations of glucose within the HCP (F1,18=2.92; p=0.10; Figure 2F). Bonferroni post-hoc analysis of lactate x ethanol interactions on the concentrations of lactate within the mPFC and HCP revealed that CIE-induced increases in mPFC (p<0.05; Figure 2G) and HCP lactate (p<0.0001; Figure 2H), occurred in saline-injected animals but not in mice injected with lactate. Similarly, repeated lactate injections were found to significantly increase lactate concentrations within both the mPFC (p<0.05; Figure 2G) and the HCP (p<0.001; Figure 2H) of air-exposed animals but not in mice subjected to CIE. Therefore, these results suggest that within the CNS, the effects of chronic lactate injection mimicked the effect of ethanol exposure.
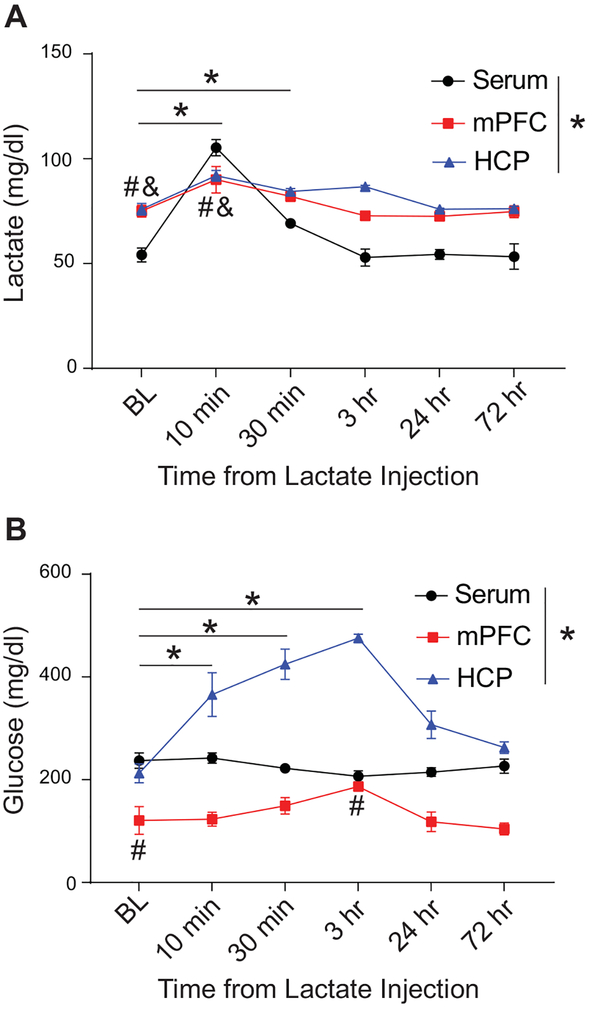
Lactate and glucose may be transported into the CNS from the blood or may arise from glycogenolysis within astrocytes. In order to examine the likely source of elevated glucose and lactate following chronic ethanol exposure or chronic lactate injection, we examined the time course of glucose and lactate changes within the periphery and CNS following acute injection of 1.0 g/kg lactate (Figure 3A-B). Two-way ANOVA analyzing the effect of acute lactate injection on lactate and glucose concentrations in the serum, mPFC, and HCP across time revealed that acute lactate injection exerted a significant main effect on both lactate (F5,54=37.82; p<0.001; Figure 3A) and glucose (F5,54=13.18; p<0.001; Figure 3B) concentrations across time. Additionally, we found that there was a significant main effect of tissue type on the concentration of lactate (F2,54=48.91; p<0.0001; Figure 3A) and glucose (F2,54=182.70; p<0.0001; Figure 3B) across time. Importantly, we found that there was a significant interaction between the effects of the tissue (serum, mPFC, or HCP) and the time after lactate injection on the measured levels of lactate (F10,54=8.37; p<0.0001; Figure 3A) and glucose (F10,54=8.82; p<0.0001; Figure 3B). Bonferroni post-hoc analysis revealed that lactate injection resulted in immediate significant elevation of serum lactate 10 min (p<0.001) and 30 min (p<0.05) following injection as well as transient immediate increases in lactate within the mPFC (p<0.05) and HCP (p<0.01) 10 min following injection (Figure 3A) compared to baseline concentrations within each tissue. Interestingly, this brief elevation of serum and CNS lactate was accompanied by a slightly delayed and comparatively sustained increase in glucose concentration within the mPFC and HCP (Figure 3B). In the HCP, acute lactate injection significantly increased glucose concentrations 10 min (p<0.001), 30 min (p<0.001), and 3 hr (p<0.001) following injection compared to baseline (Figure 3B). Glucose levels within the mPFC rose comparatively slowly, and were not significantly elevated compared to baseline until 3 hr (p<0.05) following acute lactate injection (Figure 3B). Perhaps most importantly, both the serum and CNS levels of glucose and lactate returned to baseline by 24 hr following acute lactate injection (Figure 3A-B), suggesting that the observed increase in lactate and glucose concentrations within the CNS 72 hr after CIE or chronic lactate injection may be due to modulation of homeostatic processes controlling the balance of glucose and lactate within the periphery and CNS.
Figure 3:
The effects of acute lactate injection on the time course of lactate and glucose concentration within the serum, mPFC, and HCP compared to baseline. Acute lactate injection (1g/kg) resulted in an immediate increase in serum and CNS lactate (A) followed by a delayed increase in CNS glucose, peaking approximately 3 hr after administration (B). Brain and blood levels of glucose and lactate returned to baseline by 24 hr following lactate injection (A, B). In (A), * indicate p<0.05 for post-hoc analysis of serum lactate at time points compared to baseline. # indicate p<0.05 for post-hoc analysis of HCP at time points compared to baseline. & indicate p<0.05 for post-hoc analysis of mPFC at time points compared to baseline. n=4. In (B), * indicates p<0.05 for post-hoc analysis of HCP at time points compared to baseline. # indicates p<0.05 for post-hoc analysis of mPFC at time points compared to baseline. n=4. BL = baseline.
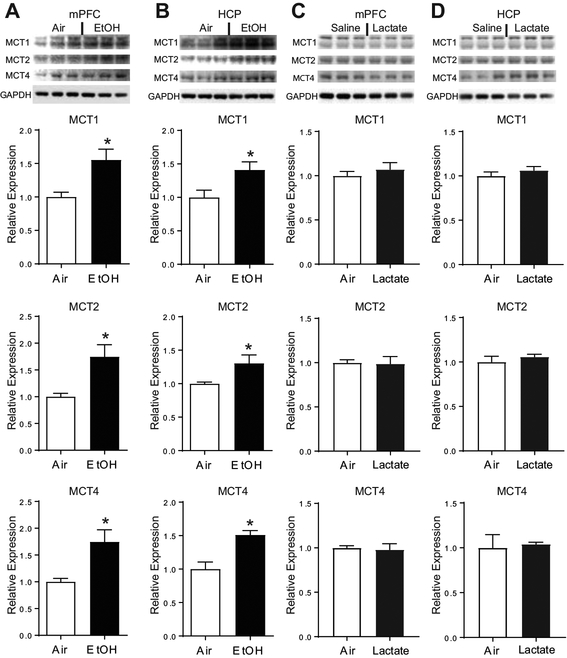
Chronic ethanol exposure, but not chronic lactate injection, induces monocarboxylate transporters of the astrocyte-neuron lactate shuttle
Next, we aimed to determine whether CIE-induced increases in CNS lactate concentrations were associated with altered expression of monocarboxylate transporters (MCTs) of the astrocyte-neuron lactate shuttle (ANLS). These transporters are instrumental in mediating lactate transfer from astrocytes to neurons and therefore are required for effective neuronal utilization of lactate (Perez-Escuredo et al., 2016; Simpson et al., 2007). Importantly, we found that CIE is associated with increased expression of all three MCTs within both the mPFC and HCP, including MCT1 (mPFC, t(10)=3.19, p<0.01; HCP, t(10)=2.55, p<0.05) and MCT4 (mPFC, t(10)=2.03, p<0.05; HCP, t(10)=4.11, p<0.05), which transport lactate from astrocytes into the extracellular space, as well as MCT2 (mPFC, t(10)=3.20, p<0.01; HCP, t(10)=2.41, p<0.05), which is primarily expressed by neurons and mediates the neuronal import of lactate (Figure 4A-B).
Figure 4:
CIE induces the expression of MCTs independently of increased lactate concentrations. CIE increased the expression of MCT1, MCT2, and MCT4 within the mPFC (A) as well as within the HCP (B) (n=6). In contrast, chronic lactate administration did not alter the expression of MCT proteins within either brain region (C, D) (n=3). * indicates p<0.05.
In order to determine whether induction of MCTs was directly related to the chronic elevation in lactate associated with CIE, we injected air exposed animals with 1g/kg lactate twice daily for two weeks. In contrast to chronic ethanol exposure, repeated lactate injections did not alter the expression of MCT transporters within the mPFC (MCT1, t(4)=0.79, p=0.47; MCT2, t(4)= 0.15, p=0.89; MCT4, t(4)=0.28, p=0.79; Figure 4C) or HCP (MCT1, t(4)=0.98, p=0.38; MCT2, t(4)=0.83, p=0.45; MCT4, t(4)=0.27, p=0.80; Figure 4D). Therefore, although both CIE and chronic lactate exposure caused sustained elevations of CNS lactate (Figure 2E-H), only chronic ethanol exposure enhanced the expression of MCTs of the ANLS (Figure 4).
DISCUSSION
Here, we demonstrate that chronic ethanol exposure induces sustained changes in the balance of lactate and glucose within both the CNS and periphery during the early and late phases of ethanol withdrawal. More specifically, we show that CIE causes peripheral hypoglycemia and lactic acidosis while increasing the concentration of both glucose and lactate within the CNS. Although the peripheral effects are sometimes modest (but statistically significant), the observed 15–20% reduction in blood glucose concentration and 10–30% increase in serum lactate have been demonstrated to produce significant effects on striated muscle, cardiac muscle, and neurons, and result in exaggerated metabolic changes within the CNS (Mizock 1989; Morales and Schneider, 2014). Interestingly, chronic intraperitoneal administration of lactate mimics these changes within the brain but not within the periphery. This suggests that ethanol-induced increases in peripheral lactate may account for the observed increase in both lactate and glucose concentrations within the CNS. This is supported by the observation that peripheral lactate concentrations remain elevated for at least 72 hr in mice subjected to CIE. Alternatively, chronic lactate injection fails to significantly alter serum glucose or lactate concentrations. Moreover, our observed time-course of lactate and glucose concentrations within the CNS following acute lactate injection demonstrates that acute lactate exposure fails to produce any sustained significant increase in brain lactate concentrations. Although acute lactate injection does result in a delayed and comparatively prolonged increase in CNS glucose concentrations, these glucose concentrations return to baseline by 24-hours following lactate injection and are not sustained to 72-hours, as in the case of chronic lactate injection. Together, these results demonstrate that CIE results in long-lasting elevations in peripheral lactate concentrations as well as reduced peripheral glucose levels that are not reproduced by either acute or chronic lactate administration. In contrast CIE-induced increases in CNS lactate and glucose are mimicked by chronic lactate administration, and are transiently mirrored by acute lactate injection. Combined, this suggests that CIE exerts unique effects on homeostatic systems controlling central and peripheral glucose and lactate concentrations, which are not precisely imitated by either acute or chronic lactate injection.
Importantly, we found that chronic ethanol exposure increases the expression of a family of monocarboxylate transporters (MCT1, MCT2, and MCT4) involved in astrocyte-neuron lactate transfer. In contrast, chronic lactate injection did not increase the expression of these transporters. This suggests that chronic ethanol exposure may augment astrocyte-neuron lactate transfer. Furthermore, this suggests that increased concentrations of peripheral or CNS lactate are not directly responsible for the observed upregulation of the astrocyte-neuron lactate shuttle, and that CIE induces expression of MCTs in a lactate-independent manner.
In addition to lactate, MCTs transport other monocarboxylate compounds including pyruvate and the ketone bodies acetone, acetoacetate, and beta-hydroxybutyrate (Halestrap, 2013; Perez-Escuredo et al., 2016). Previous studies have demonstrated that in addition to lactate, chronic ethanol exposure increases the production of ketone bodies (Kreisberg, 1967). Ketogenic diets and ketone supplementation have been demonstrated to improve memory, enhance hippocampal long-term potentiation, and reduce the severity of ethanol withdrawal syndrome (Dencker et al., 2018; Hernandez et al., 2018; Newman et al., 2017). Similarly, astrocyte-neuron lactate transfer is necessary for long-term memory formation and long-term potentiation (Suzuki et al., 2011). AUD and addiction have been characterized as pathologic manifestations of memory formation. Appropriately, previous studies have demonstrated that astrocyte-neuron lactate transfer is required for maintenance of drug memories and promotes relapse (Boury-Jamot et al., 2016a; Boury-Jamot et al., 2016b). Combined, these results suggest that chronic ethanol exposure may induce maladaptive drug memories and synaptic potentiation by increasing the expression of MCTs and thus augmenting astrocyte-neuron lactate transfer. Furthermore, our results suggest that CIE-induced increases in peripheral lactate are not directly responsible for MCT induction. Instead, it is likely that other metabolites that increase in concentration as a result of chronic ethanol exposure and are also transported by MCTs, such as ketone bodies, induce the expression of MCTs and enhance astrocyte-neuron lactate transfer. This strengthened astrocyte-neuron lactate shuttle may be further augmented by ethanol-induced increases in lactate, thus promoting long-term potentiation and strengthening drug-associated memories.
The bioenergetic systems of the tricarboxylic acid (TCA) cycle and oxidative phosphorylation are intricately related to synaptic transmission in the CNS. Glutamate is a negatively charged amino acid with limited transport and permeability across the blood-brain barrier (Hertz et al., 1999; Hertz et al., 2000; Hertz and Rothman, 2016). As such, the majority of CNS glutamate must be synthesized de novo (Hertz et al., 1999; Hertz and Rothman, 2016). Accordingly, neurons and astrocytes interact metabolically to convert bioenergetic molecules such as glucose, lactate, acetate, and beta-hydroxybutyrate to glutamate, which may subsequently be utilized as a neurotransmitter or fully metabolized to CO2 and water after entering the TCA cycle in the form of alpha-ketoglutarate (Hertz et al., 1999; Hertz and Rothman, 2016). This is supported by the finding that excitatory amino acid transporters (EAATs) colocalize with mitochondria to a macromolecular complex that supports the oxidative metabolism of glutamate (Bauer et al., 2012). Thus, the metabolic degradation of glucose and lactate via glutamate serves the purpose of adjusting transmitter pools of glutamate to meet the demands of glutamatergic transmission, while the oxidative metabolism of glutamate may also be utilized to accommodate the varying energy demands of the CNS. This suggests that modulation of important metabolic processes such as the ANLS may alter glutamate processing and glutamatergic neurotransmission by altering the uptake and processing of synaptic glutamate as a neurotransmitter or bioenergetic substrate. This is supported by the observation that ethanol withdrawal is associated with reduced expression of the glutamate uptake transporter EAAT2 (Abulseoud et al., 2014; Ayers-Ringler et al., 2016), as well as increased expression of MCTs. Thus, it is possible that disruption of the ANLS or other metabolic processes contribute to the glutamatergic dysfunction classically associated with ethanol intoxication and withdrawal.
Importantly, both pyrazole and CO2-mediated euthanasia may induce metabolic changes, potentially resulting in changes in lactate or glucose concentrations within the serum or CSF. For example, pyrazole has been demonstrated to inhibit glutamatergic signaling by metabotropic glutamate receptors and impair mitochondrial oxidative phosphorylation and calcium uptake (Cederbaum and Rubin, 1974; Conti et al., 2005; Chae et al., 2013). Similarly, CO2 exposure prior to death may result in transient respiratory acidosis (Traslavina et al., 2010). In spite of this, all air- and ethanol-exposed animals subjected to CIE were injected with pyrazole and underwent identical euthanasia protocols, effectively controlling for CO2- or pyrazole-induced changes in serum and brain lactate and glucose. Furthermore, respiratory acidosis primarily results in production of carbonic acid with only minimal effects on lactate levels (Traslavina et al., 2010). Finally, although it is possible that pyrazole or CO2 may have minimally affected serum or CNS concentrations of glucose and lactate, a clear effect of ethanol was distinguishable from any minimal effects of these interventions.
Future studies should more closely investigate the molecular mechanisms underlying ethanol-induced augmentation of the ANLS and scrutinize the molecular underpinnings connecting glutamatergic signaling and astrocyte-neuron lactate transfer in the setting of ethanol intoxication and withdrawal. More specifically, the effects of chronic ethanol on astrocytic glycogen storage and breakdown should be investigated, as should the effects of CIE on lactate dehydrogenase (LDH) expression and activity in both neurons and astrocytes. Similarly, chronic lactate injection and long-term exposure to ketone bodies should be investigated for their effects on astrocytic glycogenolysis as well as astrocytic and neuronal LDH expression and activity. The effect of ketones on CNS MCT expression should also be explored. Finally, linking metabolic dysfunction in the form of ANLS perturbation or mitochondrial dysfunction with ethanol-induced changes in glutamatergic signaling may help to provide a more complete understanding of the effects of ethanol on CNS function and offer unique targets such as LDH, GP, or MCTs, which may be targeted for the treatment or prevention of AUD.
ACKNOWLEDGEMENTS
We thank all members of the DSC.’s laboratory for interest, help and comments.
FUNDING
This work was supported by the Samuel C. Johnson Genomics of Addiction Program at Mayo Clinic, the Ulm Foundation, the Godby Foundation, and the National Institute on Alcohol Abuse and Alcoholism (AA018779).
Footnotes
CONFLICT OF INTEREST
D-S Choi is a scientific advisory board member to Peptron Inc. Peptron had no role in preparation, review, or approval of the manuscript. All the other authors declare no biomedical financial interests or potential conflicts of interest.
REFERENCES
- Abdul Muneer PM, Alikunju S, Szlachetka AM, Mercer AJ, and Haorah J (2011). Ethanol impairs glucose uptake by human astrocytes and neurons: protective effects of acetyl-L-carnitine. Int J Physiol Pathophysiol Pharmacol 3, 48–56. [PMC free article] [PubMed] [Google Scholar]
- Abulseoud OA, Camsari UM, Ruby CL, Kasasbeh A, Choi S, and Choi DS (2014). Attenuation of ethanol withdrawal by ceftriaxone-induced upregulation of glutamate transporter EAAT2. Neuropsychopharmacology 39, 1674–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers-Ringler JR, Jia YF, Qiu YY, and Choi DS (2016). Role of astrocytic glutamate transporter in alcohol use disorder. World J Psychiatry 6, 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DE, Jackson JG, Genda EN, Montoya MM, Yudkoff M, and Robinson MB (2012). The glutamate transporter, GLAST, participates in a macromolecular complex that supports glutamate metabolism. Neurochem Int 61, 566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Diaz-Granados JL, and Weathersby RT (1997). Repeated ethanol withdrawal experience increases the severity and duration of subsequent withdrawal seizures in mice. Alcohol 14, 319–326. [DOI] [PubMed] [Google Scholar]
- Becker HC, and Hale RL (1993). Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal “kindling”. Alcohol Clin Exp Res 17, 94–98. [DOI] [PubMed] [Google Scholar]
- Becker HC, and Veatch LM (2002). Effects of lorazepam treatment for multiple ethanol withdrawals in mice. Alcohol Clin Exp Res 26, 371–380. [PubMed] [Google Scholar]
- Bliss TM, and Sapolsky RM (2001). Interactions among glucose, lactate and adenosine regulate energy substrate utilization in hippocampal cultures. Brain Res 899, 134–141. [DOI] [PubMed] [Google Scholar]
- Boury-Jamot B, Carrard A, Martin JL, Halfon O, Magistretti PJ, and Boutrel B (2016a). Disrupting astrocyte-neuron lactate transfer persistently reduces conditioned responses to cocaine. Mol Psychiatry 21, 1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boury-Jamot B, Halfon O, Magistretti PJ, and Boutrel B (2016b). Lactate release from astrocytes to neurons contributes to cocaine memory formation. Bioessays 38, 1266–1273. [DOI] [PubMed] [Google Scholar]
- Brown AM, and Ransom BR (2015). Astrocyte glycogen as an emergency fuel under conditions of glucose deprivation or intense neural activity. Metab Brain Dis 30, 233–239. [DOI] [PubMed] [Google Scholar]
- Carrard A, Elsayed M, Margineanu M, Boury-Jamot B, Fragniere L, Meylan EM, Petit JM, Fiumelli H, Magistretti PJ, and Martin JL (2018). Peripheral administration of lactate produces antidepressant-like effects. Mol Psychiatry 23, 392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederbaum AI and Rubin E (1974). Effects of pyrazole, 4-bromopyrazole and 4-methylpyrazole on mitochondrial function. Biochem Pharmacol. 23, 203–13. [DOI] [PubMed] [Google Scholar]
- Chae E, Shin YJ, Ryu EJ, Ji MK, Ryune Cho N, Lee KH, Jeong HJ, Kim SJ, Choi Y, Seok Oh K, Park CE, and Soo Yoon Y (2013). Discovery of biological evaluation of pyrazole/imidazole amides as mGlu5 receptor negative allosteric modulators. Bioorg Med Chem Lett. 23, 2134–9. [DOI] [PubMed] [Google Scholar]
- Conti P, Grazioso G, di Ventimiglia SJ, Pinto A, Roda G, Madsen U, Bräuner-Osborne H, Nielsen B, Costagli C, and Galli A (2005). Synthesis of novel N1-substituted bicyclic pyrazole amino acids and evaluation of their interaction with glutamate receptors. Chem Biodivers. 2, 748–57. [DOI] [PubMed] [Google Scholar]
- D’Souza MS (2015). Glutamatergic transmission in drug reward: implications for drug addiction. Front Neurosci 9, 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dencker D, Molander A, Thomsen M, Schlumberger C, Wortwein G, Weikop P, Benveniste H, Volkow ND, and Fink-Jensen A (2018). Ketogenic Diet Suppresses Alcohol Withdrawal Syndrome in Rats. Alcohol Clin Exp Res 42, 270–277. [DOI] [PubMed] [Google Scholar]
- DePoy L, Daut R, Brigman JL, MacPherson K, Crowley N, Gunduz-Cinar O, Pickens CL, Cinar R, Saksida LM, Kunos G, et al. (2013). Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proc Natl Acad Sci U S A 110, 14783–14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eravci M, Kley S, Pinna G, Prengel H, Brodel O, Hiedra L, Meinhold H, and Baumgartner A (1999). Gene expression of glucose transporters and glycolytic enzymes in the CNS of rats behaviorally dependent on ethanol. Brain Res Mol Brain Res 65, 103–111. [DOI] [PubMed] [Google Scholar]
- Ferreira JM, Burnett AL, and Rameau GA (2011). Activity-dependent regulation of surface glucose transporter-3. J Neurosci 31, 1991–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP (2013). Monocarboxylic acid transport. Compr Physiol 3, 1611–1643. [DOI] [PubMed] [Google Scholar]
- Haorah J, Rump TJ, and Xiong H (2013). Reduction of brain mitochondrial beta-oxidation impairs complex I and V in chronic alcohol intake: the underlying mechanism for neurodegeneration. PLoS One 8, e70833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AR, Hernandez CM, Campos K, Truckenbrod L, Federico Q, Moon B, McQuail JA, Maurer AP, Bizon JL, and Burke SN (2018). A Ketogenic Diet Improves Cognition and Has Biochemical Effects in Prefrontal Cortex That Are Dissociable From Hippocampus. Front Aging Neurosci 10, 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Dringen R, Schousboe A, and Robinson SR (1999). Astrocytes: glutamate producers for neurons. J Neurosci Res 57, 417–428. [PubMed] [Google Scholar]
- Hertz L, and Rothman DL (2016). Glucose, Lactate, beta-Hydroxybutyrate, Acetate, GABA, and Succinate as Substrates for Synthesis of Glutamate and GABA in the Glutamine-Glutamate/GABA Cycle. Adv Neurobiol 13, 9–42. [DOI] [PubMed] [Google Scholar]
- Hertz L, Yu AC, Kala G, and Schousboe A (2000). Neuronal-astrocytic and cytosolic-mitochondrial metabolite trafficking during brain activation, hyperammonemia and energy deprivation. Neurochem Int 37, 83–102. [DOI] [PubMed] [Google Scholar]
- Jiang L, Herzog RI, Mason GF, de Graaf RA, Rothman DL, Sherwin RS, and Behar KL (2009). Recurrent antecedent hypoglycemia alters neuronal oxidative metabolism in vivo. Diabetes 58, 1266–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao D, Liu Y, Li X, Liu J, and Zhao M (2015). The role of the GABA system in amphetamine-type stimulant use disorders. Front Cell Neurosci 9, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung ME (2015). Alcohol Withdrawal and Cerebellar Mitochondria. Cerebellum 14, 421–437. [DOI] [PubMed] [Google Scholar]
- Koob GF, and Volkow ND (2016). Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3, 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisberg RA (1967). Effect of alcohol on glucose production and lactate, pyruvate and ketone body metabolism by the isolated perfused rat liver. Diabetes 16, 784–790. [DOI] [PubMed] [Google Scholar]
- Ma L, Dong JX, Wu C, Li XY, Chen J, Zhang H, and Liu Y (2017). Spectroscopic, Polarographic, and Microcalorimetric Studies on Mitochondrial Dysfunction Induced by Ethanol. J Membr Biol 250, 195–204. [DOI] [PubMed] [Google Scholar]
- Mason S (2017). Lactate Shuttles in Neuroenergetics-Homeostasis, Allostasis and Beyond. Front Neurosci 11, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiisen TM, Lehre KP, Danbolt NC, and Ottersen OP (2010). The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 58, 1094–1103. [DOI] [PubMed] [Google Scholar]
- Metten P, Sorensen ML, Cameron AJ, Yu CH, and Crabbe JC (2010). Withdrawal severity after chronic intermittent ethanol in inbred mouse strains. Alcohol Clin Exp Res 34, 1552–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizock BA (1989). Lactic Acidosis. Dis Mon 35, 233–300. [DOI] [PubMed] [Google Scholar]
- Moralez J and Schneider D (2014). Hypoglycemia. Am J Med 127, 17–24. [DOI] [PubMed] [Google Scholar]
- Morton RA, Diaz MR, Topper LA, and Valenzuela CF (2014). Construction of Vapor Chambers Used to Expose Mice to Alcohol During the Equivalent of all Three Trimesters of Human Development. J. Vis. Exp. 89, e51835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JC, Covarrubias AJ, Zhao M, Yu X, Gut P, Ng CP, Huang Y, Haldar S, and Verdin E (2017). Ketogenic Diet Reduces Midlife Mortality and Improves Memory in Aging Mice. Cell Metab 26, 547–557 e548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, and Magistretti PJ (2012). Sweet sixteen for ANLS. J Cereb Blood Flow Metab 32, 1152–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Escuredo J, Van Hee VF, Sboarina M, Falces J, Payen VL, Pellerin L, and Sonveaux P (2016). Monocarboxylate transporters in the brain and in cancer. Biochim Biophys Acta 1863, 2481–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawat AK, and Kuriyama K (1972). Ethanol oxidation: effect on the redox state of brain in mouse. Science 176, 1133–1135. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Shaikh SR, Murphy JM, McBride WJ, Lumeng L, and Li TK (2000). Alcohol deprivation effect is prolonged in the alcohol preferring (P) rat after repeated deprivations. Alcohol Clin Exp Res 24, 8–16. [PubMed] [Google Scholar]
- Serres S, Bezancon E, Franconi JM, and Merle M (2004). Ex vivo analysis of lactate and glucose metabolism in the rat brain under different states of depressed activity. J Biol Chem 279, 47881–47889. [DOI] [PubMed] [Google Scholar]
- Simpson IA, Carruthers A, and Vannucci SJ (2007). Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab 27, 1766–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, and Alberini CM (2011). Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144, 810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traslavina RP, King EJ, Loar AS, Riedel ER, Garvey MS, Ricart-Arbona R, Wolf FR, and Couto SS (2010). Euthanasia by CO₂ inhalation affects potassium levels in mice. J Am Assoc Lab Anim Sci. 49, 316–22. [PMC free article] [PubMed] [Google Scholar]
- Valdebenito R, Ruminot I, Garrido-Gerter P, Fernandez-Moncada I, Forero-Quintero L, Alegria K, Becker HM, Deitmer JW, and Barros LF (2016). Targeting of astrocytic glucose metabolism by beta-hydroxybutyrate. J Cereb Blood Flow Metab 36, 1813–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xue Y, Meng S, Luo Y, Liang J, Li J, Ai S, Sun C, Shen H, Zhu W, et al. (2016). Inhibition of Lactate Transport Erases Drug Memory and Prevents Drug Relapse. Biol Psychiatry 79, 928–939. [DOI] [PubMed] [Google Scholar]