Abstract
Boron neutron capture therapy (BNCT) is a therapeutic modality which has been used for the treatment of cancers, including brain and head and neck tumors. For effective treatment via BNCT, efficient and selective delivery of a high boron dose to cancer cells is needed. Prostate specific membrane antigen (PSMA) is a target for prostate cancer imaging and drug delivery. In this study, we conjugated boronic acid or carborane functional groups to a well-established PSMA inhibitor scaffold to deliver boron to prostate cancer cells and prostate tumor xenograft models. Eight boron-containing PSMA inhibitors were synthesized. All of these compounds showed strong binding affinity to PSMA in a competition radioligand binding assay (IC50 from 555.7 nM-20.3 nM). Three selected compounds 1a, 1d and 1f were administered to mice, and their in vivo blocking of 68Ga-PSMA-11 uptake was demonstrated through a positron emission tomography (PET) imaging and biodistribution experiment. Biodistribution analysis demonstrated boron uptake of 4–7 μg/gram in 22Rv1 prostate xenograft tumors and similar tumor/muscle ratios compared to the ratio for the most commonly used BNCT compound, 4-borono-L-phenylalanine (BPA). Taken together, this data suggest a potential role for PSMA targeted BNCT agents in prostate cancer therapy following suitable optimization.
Keywords: Boron neutron capture therapy (BNCT), prostate cancer, prostate specific membrane antigen (PSMA) inhibitor, carborane, boron uptake
Graphical Abstract
INTRODUCTION
Boron neutron capture therapy is a cancer treatment method based on the selective uptake of boron-containing drugs into cancer cells over normal cells, followed by selective neutron beam exposure to the cancerous regions.1,2,3 This irradiation initiates the emission of cytotoxic alpha particles and lithium ions. The released high linear energy transfer (LET) α-particles can travel a distance of only around 9 μm in tissue, which is approximately the diameter of a cell. Therefore, their short-range cytotoxicity can significantly improve tumor control with concomitant sparing of the surrounding normal tissues.4,5 This therapeutic modality has been successfully used for treatment of patients with primary brain and head and neck tumors.6,7,8 Clinically used BNCT reagents include 4-borono-L-phenylalanine (BPA) and sodium borocaptate (BSH).9,10,11 However, due to various limitations including relatively low target to background ratio binding of boron-labeled therapeutic agents, BNCT has yet to emerge as a standard of care treatment modality for most cancer types.
Prostate cancer (PCa) is one of the most prevalent noncutaneous cancer in men.12,13 Prostate specific membrane antigen, also known as glutamate carboxypeptidase II (GCPII) or N-acetyl-L-aspartyl-L-glutamate peptidase, is a cell surface enzyme which is highly expressed on prostate cancer cells.14 Recently, urea based inhibitor agents targeting the enzymatic domain of PSMA have been developed for both imaging and therapy of prostate cancer.15,16,17,18 Therapy with radionuclides has been demonstrated using 177Lu and 225Ac, which exhibit successful tumor control but also have side effects including xerostomia and bone marrow suppression.19,20 PSMA targeted therapeutics and imaging agents have been extended beyond radionuclides, including nanoparticles and antibody drug conjugates.21,22,23
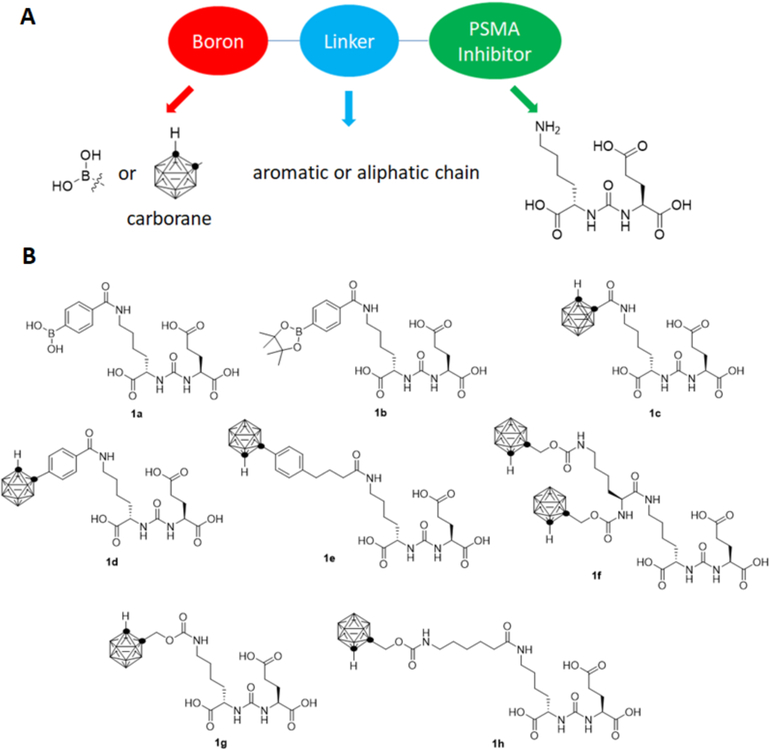
The high target to background ratio of PSMA targeted imaging and therapeutic agents, coupled with the high efficacy of BNCT, suggest the potential for boron labeled PSMA targeting agents as effective BNCT agents. In principle, this approach could combine the benefit of high target to background ratio of PSMA directed therapeutics with the spatial localization offered by a neutron beam. Therefore, this approach could be used for treatment of oligometastatic prostate cancer, potentially the minimizing off target side effects of radionuclides including xerostomia and bone marrow suppression. Boron labeled PSMA targeting agents have been reported, their binding to the enzyme characterized, and initial biodistribution studied using radioiodinated derivatives.24,25 However, these studies did not determine the degree of boron uptake in tumors, which is necessary to predict therapeutic efficacy. We hypothesize that by using boron-containing target-specific molecules, the localized and enhanced expression of PSMA on PCa cells in comparison to normal tissue could enable preferential and adequate delivery of boron to PCa for BNCT. We designed a series of boron labeled agents targeting PSMA including three components – a urea-based PSMA binding group, a linker moiety, and a substituent containing one, ten, or twenty boron atoms (Figure 1). In this study, we report the synthesis of a series of boron-labeled agents targeting PSMA, and their initial in vitro and in vivo evaluation in biodistribution assays.
Figure 1.
(A) The design of boron labeled PSMA targeted agents includes a urea based inhibitory element, connected to a boron containing substituent via a linker moiety. (B) Structures of boron labeled PSMA agents evaluated in this study.
EXPERIMENTAL SECTION
2.1. Materials
o-Carborane was purchased from Boron Specialties (PA, U.S.A.). Decaborane and all other chemicals were purchased from Sigma-Aldrich (MO, U.S.A.). RPMI-1640 medium, Fetal bovine serum (FBS) and penicillin-streptomycin solutions were purchased from Life Technologies (NY, U.S.A.).
2.2. Chemistry
Details for the synthesis and characterizations of all compounds are provided in the supplementary information.
2.3. Cell Culture
The PSMA-expressing human prostate cancer cell line 22Rv1 was obtained from ATCC. 22Rv1 cells were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 100 units of penicillin and 100 μg/mL streptomycin in a humidified incubator at 37 °C and 5% CO2. Cells were removed from flasks for passage or for transfer to 48-well assay plates by incubating them with 0.25% trypsin.
2.4. IC50 Measurement
IC50 values of the boron-containing compounds 1a-h were determined by screening in a competition radioligand binding assay against 68Ga-PSMA-11. 68Ga-PSMA-11 was synthesized as previously reported using at 68Ge/68Ga generator and a manual synthesis model, using 5 μg precursor yielding approximately 10–35 mCi per batch.26 2-(phosphonomethyl) pentanedioic acid (PMPA) is a known PSMA inhibitor and has been chosen as a positive control.27 22Rv1 cells were plated in a 48-well plates (250 μL/well) 48 h before testing (triplet) in RPMI1640 medium supplemented with 10% fetal bovine serum. The cell number was about 250,000 per well when the assay was performed. The growth medium was removed and washed with PBS for three times. Various concentrations (0.01–100000 nM) of the tested compounds in serum free RPMI1640, together with 10 μCi (5 ng) of 68Ga-PSMA-11, were added to cells. The cells were incubated in this buffer for 1 h at 37° C. Then the radioactive medium was removed by pipette, and cells were washed by PBS twice, and 250 μL of 5N NaOH was added to lyse the cells. The lysate was transferred to small vials, and the bound radioactivity was counted using a Hidex gamma counter. IC50 values were determined by nonlinear regression using GraphPad Prism software (GraphPad Software).
2.5. Log P Measurement
Log P measurements were made using the “shake-flask” method25,28. The ICP matrix was prepared using 2% H2SO4, 2% HNO3 and 0.3% triton in Millipore water. 50 μL of 1 mM compound in PBS (pH = 7.4) was added to each of three vials containing 50 μL of 1-octanol. The vials were shaken on a vortex mixer for 10 min, followed by centrifugation for a further 10 min. Aliquot (30 μL) of each PBS and octanol layer were transferred to 15 mL Falcon tubes and diluted to 10 mL with ICP matrix buffer or 0.3 mL of EtOH and 9.7 mL of ICP matrix buffer respectively. The boron content was measured by Inductively Coupled Plasma Optical-Emission Spectrometry (ICP-OES, Thermo Scientific iCAP 7000 series). Log P values were calculated as Log{[boron content per mL(1-octanol)]/[ boron content per mL(buffer)]}.
2.6. Internalization Studies
When cells reached 70% confluency in a T75 flask, medium was removed, and the cells were washed twice with PBS. 20 mL 100 μM compound in serum free RPMI 1640 was added and the cells were incubated at 37 °C for 1 hour. The medium was removed and the cells were washed with PBS twice. The cells were detached with 0.25% trypsin solution. The cells were washed three times with PBS and then treated with a solution of 50 mM glycine and 100 mM NaCl at pH 3 for 2 min at 37 °C and 5% CO2. Cells were then centrifuged (3 min at 12,000 rpm), and the supernatant was collected. This treatment was repeated two additional times, and the combined supernatants were diluted to 10 mL with ICP matrix. The pellet was digested by 400 μL 1:1 H2SO4/HNO3 mixture overnight, then diluted to 10 mL by ICP matrix. The boron content was measured by Inductively Coupled Plasma Optical-Emission Spectrometry (ICP-OES, Thermo Scientific iCAP 7000).
2.7. Inoculation of Mice with Xenograft
All animal studies were conducted according to an Institutional Animal Care & Use Program (IACUC) approved protocol. Five to six weeks old, male athymic mice (nu/nu, homozygous; Jackson Laboratories), housed in aseptic conditions, received subcutaneous tumor cell inoculation, as approved by the University of California, San Francisco Institutional Animal Care and Use Committee. In brief, 5 million 22Rv1 cells in a 200 μL 1:1 mixture of complete medium and matrigel (Fisher Scientific, IL) was injected in the left thigh of the animals. All mice were subjected to undergo PET imaging as well as biodistribution analysis when the tumor reached a size of 300 – 500 mm3.
2.8. Serum protein binding assay
25 μl of 1 mM compound in PBS was added to 0.5 ml mouse serum. The mixture was vortexed for 5 mins and incubated at 37 °C for 30 mins. Then 0.5 ml acetonitrile was added to precipitate the protein. The mixture was then centrifuged (3 min at 12,000g), and the supernatants and pellet were collected respectively. ICP matrix was prepared by 2% H2SO4, 2% HNO3, 0.3% triton in millipore water. The supernatants were diluted to 10 ml by ICP matrix. The pellet was digested by 400 μl 1:1 H2SO4/HNO3 mixture overnight, then diluted to 10 ml by ICP matrix. The boron content was measured by ICP-OES.
2.9. Toxicity Test
Cell toxicity assay was performed using a CellTiter-Glo kit to measure the viability of cells after incubating with various concentrations of compounds. 22Rv1 cells were plated in a 96-well plates (100 μL/well) 48 h before testing (triplet) in RPMI1640 medium supplemented with 10% fetal bovine serum. The growth medium was removed and washed with PBS for three times. Various concentrations (0.01–100000 nM) of the tested compounds in PBS and blank were added to cells. The cells were incubated in this buffer for 4 h at 37° C. Then 100 μL of CellTiter-Glo buffer was added to each well. The mixture was incubated at room temperature for 15 minutes and the luminescence was measured using a luminometer.
For animal toxicity experiments, non-tumor bearing male athymic mice were administered graded intraperitoneal doses of the selected boronated compounds to determine the maximum tolerated dose (5 mg/mouse, 7.5 mg/mouse, 15 mg/mouse or 30mg/mouse), and were monitored for 24 hours. Each testing group contained two mice. If animals showed physical symptoms as outlined by our IACUC guidelines such as loss of activity, animals were euthanized.
2.10. In Vivo PET Imaging Studies
Approximately three weeks after implantation, animals with tumors reaching 300–500 mm3 were anesthetized by isoflurane inhalation and were administered 200 μL of PBS, compound 1a (30 mg/mouse in 200 μL of PBS), 1d (5 mg/mouse in a mixture of 40 μL of DMSO and 160 μL of PBS) or compound 1f (7.5 mg/mouse in a mixture of 40 μL of DMSO and 160 μL of PBS) through a single intraperitoneal injection. BPA (5 mg/mouse) was administered to mice through oral gavage due to its low solubility in buffer. Approximately 15 minutes after the administration of these compounds, 100–150 μCi (3.70–5.55 MBq, 50 – 75 ng) of 68Ga-PSMA-11 was administered through tail vein injection. The animals were imaged with 10 min acquisition by a microPET/CT imaging system (Inveon, Siemens, Germany) at 1 h post injection of the first administration. PET imaging data were acquired in list mode and reconstructed with the iterative OSEM 2-D reconstruction algorithm provided by the manufacturer. Imaging data were viewed and processed using open source Amide software.
2.11. Biodistribution Studies
The 22Rv1 tumor bearing mice were sacrificed at the 1 hour or 4 hour time points post injection of 68Ga-PSMA-11. Blood was collected by cardiac puncture. Major organs (brain, bone, heart, kidney, liver, lung, muscle, pancreas, salivary, skin, spleen and subcutaneous tumor) were harvested, weighed, and counted in an automated gamma counter (Hidex). The percent injected dose per gram of tissue (% ID/g) was calculated by comparison with standards of known radioactivity.
2.12. Organ Digestion and ICP Mass
Tissue samples were digested for 2 days at room temperature in 1 mL of a 1:1 mixture of concentrated sulfuric and nitric acids. After digestion, 1.5 mL of a 5 % Triton X-100 solution in water was added to each sample. The samples were then sonicated for 90 min. 1 mL of the sample solution was transferred to a 15 mL centrifuge tube and diluted to 10 mL with ICP matrix. The samples were processed for boron analysis by ICP-OES.
RESULTS
3.1. Synthesis of Compound 1a-1f
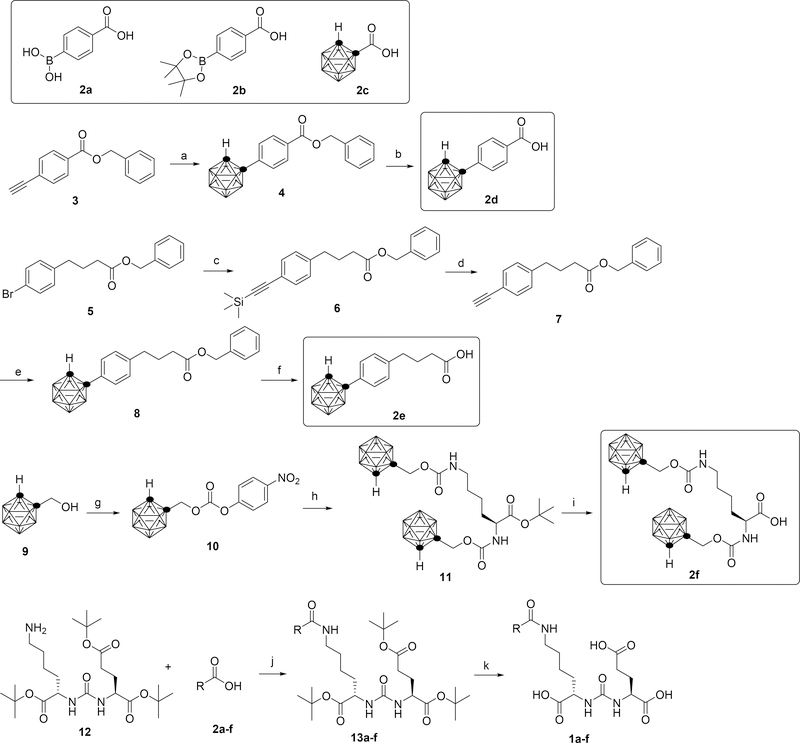
The syntheses of compounds 1a-f are outlined in scheme 1. Various boron-containing activated esters were coupled to a common synthetic intermediate 12 to yield the final products. Starting materials 2a and 2b are commercially available. Starting materials 2c24, 329, 530, 931, 1232 and reagent B10H12(MeCN)231 were obtained following known literature procedures. Briefly, compound 3 was reacted with B10H12(MeCN)2 followed by deprotection to generate intermediate 2d. Sonogashira coupling of compound 5 with trimethylsilyl acetylene followed by a deprotection formed intermediate 7. Reacting 7 with B10H12(MeCN)2 followed by deprotection gave carborane containing intermediate 2e. Mixing compound 9 with 4-nitrophenyl chloroformate produced carbonate 10. Then 10 was reacted with L-lysine tert-butyl ester hydrochloride followed by deprotection to give di-carborane containing intermediate 2f. Each of the obtained boron containing intermediates 2a-f was mixed with N,N ′ -Dicyclohexylcarbodiimide (DCC) and N-Hydroxysuccinimide (NHS) in THF and stirred overnight, the precipitation was removed by filtration. Then, compound 12 was added to the solution, the mixture was stirred for 24 hours. After purification by chromatography, each of compound 13a-f was reacted with TFA in DCM to remove the protecting groups, yielding the final products 1a-f.
Scheme 1. Reagents and conditions for the synthesis of compounds 1a-f:
Key synthetic intermediates are outlined. (a) B10H12(MeCN)2, AgNO3, PhMe, 100 °C. (b) Pd/C, H2, MeOH, rt. (c) trimethylsilyl acetylene, PdCl2(PPh3)2, Et3N, 70 °C. (d) Bu4NF, THF, rt. (e) B10H12(MeCN)2, AgNO3, PhMe, 100 °C. (f) Pd/C, H2, MeOH, rt. (g) 4-nitrophenyl chloroformate, Et3N, PhMe, 70 °C. (h) L-lysine tert-butyl ester, Et3N, THF, rt. (i) TFA, DCM, rt. (j) DCC, NHS, THF, rt. (k) TFA, DCM.
3.2. Synthesis of Compounds 1g and 1h
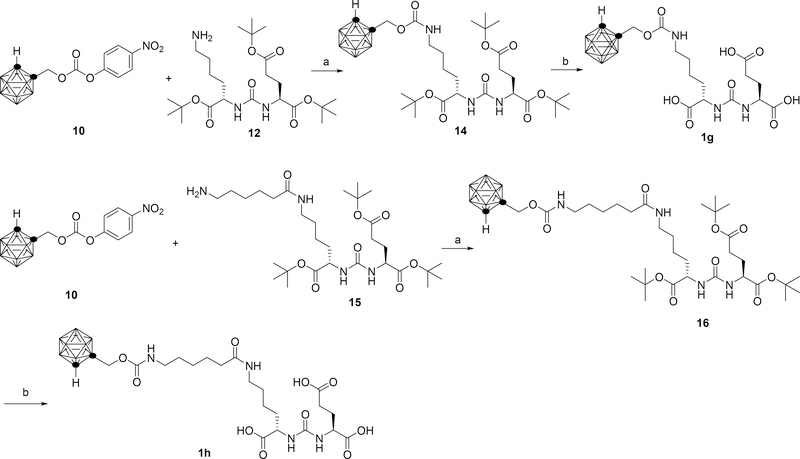
The synthesis of compound 1g and 1h is outlined in scheme 2. The starting material 15 was obtained following a known literature procedure.33 Intermediate 10 was reacted with compound 12 or 15 followed by trifluoroacetic acid (TFA) deprotection to generate final compounds 1g or 1h respectively.
Scheme 2. Reagents and conditions for the synthesis of compounds 1g, 1h.
(a) Et3N, THF, rt. (b) TFA, DCM, rt.
3.3. In Vitro Binding Studies
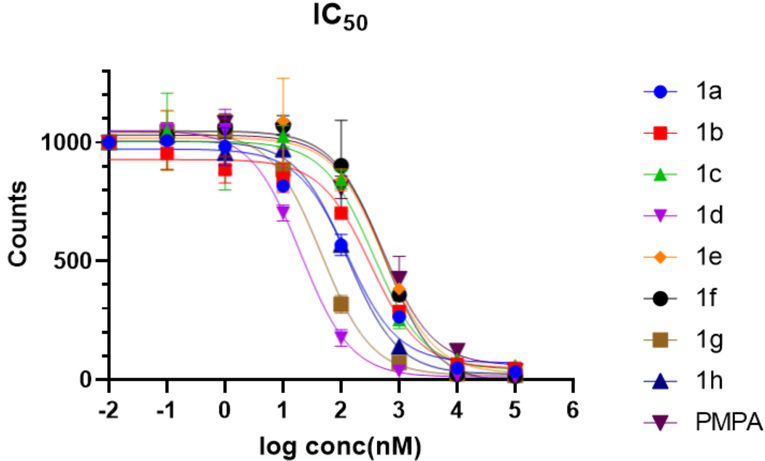
The relative binding affinities of compounds 1a-h were determined using a 68Ga-PSMA-11 competitive radioligand binding assay (Figure 2). The compounds that have been prepared are shown in Table 1. PMPA was selected for comparison and as a positive control.27 In general, most of the compounds had a very high binding affinity, improved compared to PMPA, ranging from approximately 20 – 550 nM. The relatively high IC50 for PMPA observed compared against a prior study may be due in part to the high affinity of the 68Ga-PSMA-11 ligand used and/or differences in cell type.25 Based on the IC50 values of compounds 1d/1e and 1g/1h, compounds with shorter linkers had higher binding affinities than compounds with longer linkers. The dicarborane compound 1f had an increased IC50, possibly due to its branched structure and bulky size.
Figure 2.
IC50 of compounds 1a-1h determined by a competitive binding assay against 68Ga-PSMA-11 on PSMA expressing 22Rv1 cells.
Table 1:
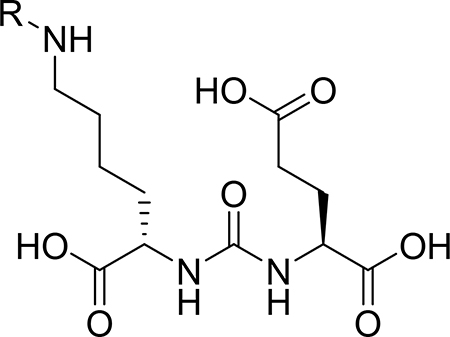 | |||||
|---|---|---|---|---|---|
| Entry | R | IC50 (nM) | Entry | R | IC50 (nM) |
| 1a | 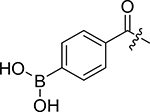 |
130.3±28.9 | 1f | 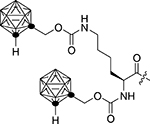 |
555.5±79.3 |
| 1b | 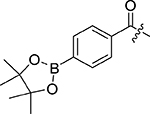 |
318.4±21.1 | 1g | 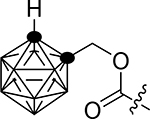 |
45.7±6.7 |
| 1c | 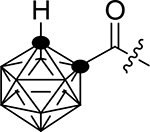 |
363.8±8.7 | 1h | 128.0±8.4 | |
| 1d | 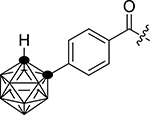 |
20.3±2.4 | control | 2-PMPA | 523.5±84.0 |
| 1e | 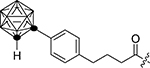 |
536.8±58.7 | |||
Cell line in all cases is 22Rv1.
The competitive binding compound is 68Ga-PSMA-11.
Cells were plated 48 h before testing (in triplicate). Various concentration (0.01–100000 nM) of compounds with 10 μCi of 68Ga-PSMA-11 were added to cells and incubated for 1h. Cells were washed and lysed. A gamma counter was used to measure the bound fraction to the lysed cells.
Based on these encouraging binding assays, three compounds were advanced for in vivo evaluation. Compound 1a was selected for further study due to its good binding affinity, excellent water solubility and as a representative for the boronic acid PSMA inhibitor series, as a complement to the more hydrophobic carborane series. Carborane containing compound 1d was selected since it showed the highest binding affinity. Compound 1f was selected due to its highest boron content among the compounds (20 boron atoms per molecule).
3.4. LogP Hydrophobicity Assay, Internalization Assay and Serum Protein Binding Assay
The logP values of compound 1a, 1d and 1f were measured to assess their hydrophilicity using the shake flask method and analysis of the resulting fractions via ICP-OES. Compared against the carborane containing compounds 1d and 1f, the boronic acid compound 1a showed increased hydrophilicity. A cellular internalization assay was performed in 22Rv1 cells. In the internalization assay, we found that a large portion of compound 1a was bound at the membrane instead of being internalized into the cells. For compound 1d and 1f, most of the compounds were internalized into the cells (Table 2). The distribution of compounds 1a, 1d and 1f in rat serum protein was measured. We found that only a small percentage of the tested compounds are bound to serum protein (supplemental table S2).
Table 2.
Property of selected compounds
| Compound | LogP | Internalization (×109 boron/cell)α |
|
|---|---|---|---|
| pellet | membrane | ||
| 1a | −1.8 ± 0.2 | 0.14 ± 0.08 | 0.093 ± 0.053 |
| 1d | −0.2 ± 0.2 | 3.3 ± 0.62 | 0.22 ± 0.04 |
| 1f | 0.2 ± 0.2 | 4.2 ± 1.9 | 0.14 ± 0.063 |
100 μM selected compounds were incubated with cells for 1 h.
3.5. Cell Toxicity Assay and Acute Toxicity Assays
Compounds 1a, 1d and 1f were selected for cell toxicity assay using the CellTiter-Glo assay. After incubating 22Rv1 with these three tested compounds for 4 hours, no obvious toxicity was observed for all of the three compounds even up to 100 μM, suggesting a low cell toxicity of these compounds (supplemental table S3 and figure S1).
A preliminary acute toxicity assay was performed prior to biodistribution and imaging studies. Compound 1a was dissolved in 200 μL of PBS. Compound 1d or 1f was dissolved in a mixture of 40 μL of DMSO and 160 μL of PBS. Non-tumor bearing male athymic mice were administered graded doses of the selected boronated compounds via intraperitoneal injection (5 mg/mouse, 7.5 mg/mouse, 15 mg/mouse or 30mg/mouse), and were monitored closely. Compound 1a demonstrated no acute toxicity up to 30 mg per mouse. For compound 1d and 1f, the maximum tolerated dose was 5 mg per mouse and 7.5 mg per mouse, respectively. When 7.5 mg 1d/mouse or 15 mg 1f/mouse was administered, one mouse in each group demonstrated acute toxicity requiring euthanasia (supplemental table S4). No obvious hemorrhages or damaged organs were observed on gross dissection.
3.6. Outline of In Vivo PET Imaging and Biodistribution Assays
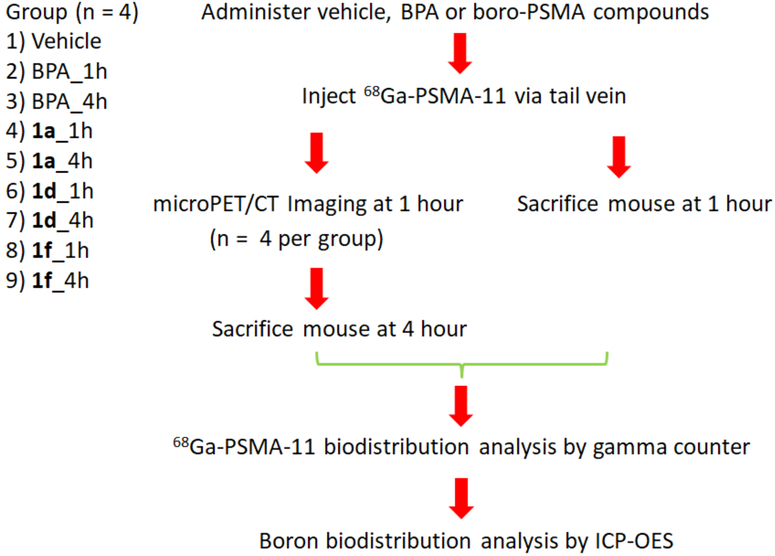
Next, we determined the ability of the boron labeled PSMA agents to bind to PSMA in vivo using a PET imaging assay, and determined the boron biodistribution by harvesting the organs and recording ICP-OES of the resulting tissues (Figure 3). The in vivo 68Ga-PSMA-11 blocking assay and boron biodistribution studies were performed in athymic mice bearing 22Rv1 tumor xenografts three weeks post inoculation. The study population consisted of nine groups (n = 4 mice for each group). Vehicle (PBS) was used as a negative control. Boronophenylalanine (BPA) is the most widely used compound in BNCT clinical trials, and was included for comparison.34 Tested compounds were administered followed by the injection of approximately 100 μCi (3.7 MBq) of 68Ga-PSMA-11. For the vehicle group, mice were imaged after 1 hour post injection and were immediately sacrificed for organ collection after imaging. For each of BPA and candidate compounds 1a, 1d and 1f, mice were split to either the 1 hour group or the 4 hour group. For the 1 hour group, mice were sacrificed and their organs were collected at 1 hour post injection. For the 4 hour group, mice were imaged at 1 hour post injection and were subsequently sacrificed at 4 hour post injection for organ collection.
Figure 3.
Flow chart of PET imaging, 68Ga-PSMA-11 biodistribution and boron biodistribution assay performed in mice bearing 22Rv1 xenograft tumors.
3.7. In Vivo PET Imaging and 68Ga-PSMA-11 Biodistribution Analysis
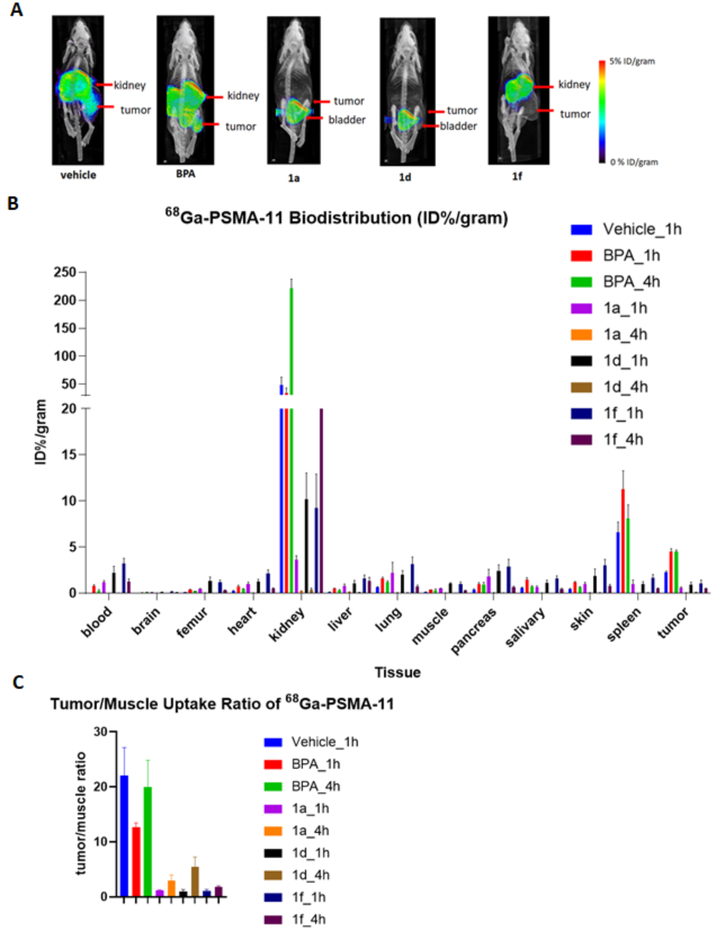
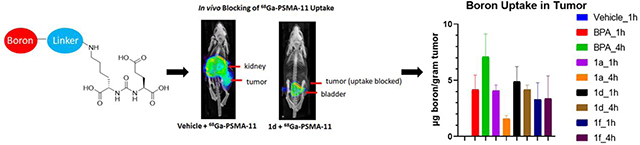
Analysis of the micro PET/CT images demonstrated that for the vehicle and BPA group, there was high 68Ga-PSMA-11 uptake in the tumor, as expected based on the known expression of PSMA in 22Rv1 xenografts.35,36 On the other hand, boron-containing PSMA inhibitors 1a, 1d, and 1f blocked 68Ga-PSMA-11 uptake in the tumors, demonstrating the strong in vivo binding between boron-containing PSMA inhibitors and PSMA (Figure 4A). The 68Ga-PSMA-11 biodistribution showed high uptake in kidney, spleen and tumor, similar with previously published results.36 As expected, the addition of BPA did not significantly block uptake in target organs including kidney, spleen and tumor. Unexpectedly, there was an apparent increase in the blood and tumor concentration of 68Ga-PSMA-11 with BPA treatment. The reasons for this are unclear, but may relate to changes in metabolism and/or clearance pathways induced by BPA treatment. In contrast, 1a and 1d significantly reduced the uptake of 68Ga-PSMA-11 in kidney, spleen and tumor. Compound 1f reduced uptake of 68Ga-PSMA-11 in spleen and tumor, but there was still moderate amount of uptake at kidney (Figure 4B). The tumor/blood ratio and tumor/muscle ratio showed a decrease when inhibitors were administered (Figure 4C). For compound 1a and 1d, the ratio markedly increased after 4 hour compared to 1 hour, while for compound 1f, the ratio only slightly increased, which indicates a longer circulation life time of 1f. Similar results were obtained through region of interest analysis of the micro PET/CT images (supplemental table S5 and S6). Taken together, these data indicate that boron labeled PSMA inhibitors 1a, 1d, and 1f are able to block 68Ga-PSMA-11 uptake in target tissues including tumor and kidney, suggesting a high degree of receptor occupancy in vivo.
Figure 4.
(A) PET imaging results showed efficient blocking of 68Ga-PSMA-11 uptake by boro-PSMA compounds, but not by BPA. (B) 68Ga-PSMA-11 biodistribution as determined by collection of organs and gamma counting. (C) Tumor/muscle uptake ratio of 68Ga-PSMA-11.
3.8. In Vivo Boron Biodistribution Analysis
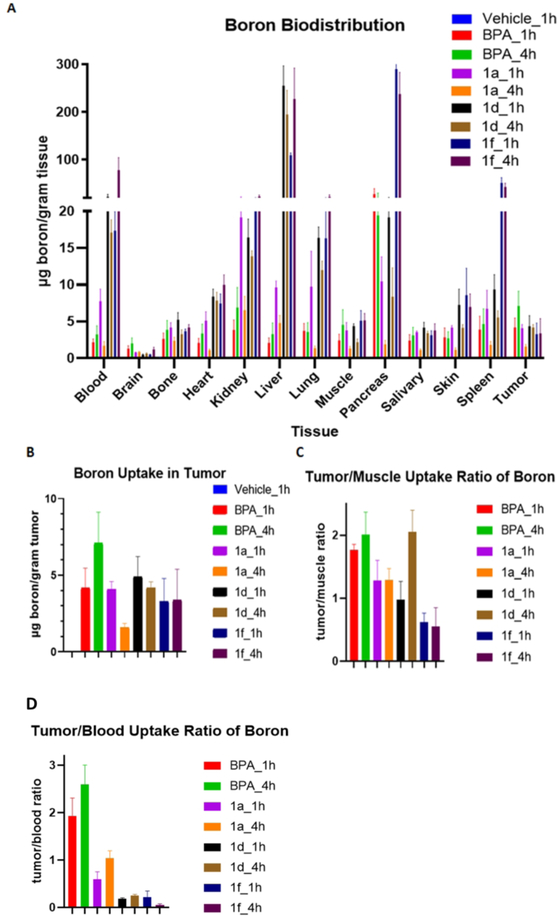
The organs collected were also subjected to ICP-OES to assay total boron content (Figure 5A and Figure S2 in Supplementary). As expected, the vehicle group resulted in minimal boron uptake in tissue when no boron compounds were administered. The known BNCT compound BPA demonstrated accumulation at blood, kidney, pancreas and especially liver. For 1a, the blood boron content significantly decreased at 4 hour compared to 1 hour from 7.8 μg/gram tissue to 1.7 μg/gram, suggesting quick clearance of this compound. There was more uptake in kidney than in liver, suggesting primarily renal elimination, as expected based on its hydrophilic character. The tumor boron uptake was 4.1 μg/gram tissue, which is similar to boron uptake in muscle (3.8 μg/gram). For compound 1d, the blood boron content slightly decreased at 4 hour compared to 1 hour from 22.5 μg/gram tissue to 17.1 μg/gram tissue, indicating a longer circulation time in vivo. More uptake was observed in liver than in kidney, suggesting primarily hepatobiliary clearance. The boron uptake in tumor was 4.4 μg/gram tissue at 1 hour, similar to the boron uptake in muscle (4.4 μg/gram). However, the boron uptake in tumor was 4.2 μg/gram tissue at 4 hour, two fold higher than the boron uptake in muscle (2.2 μg/gram tissue) (Figure 5C). For compound 1f, the blood boron content significantly increased at 4 hour compared to 1 hour from 17.4 μg/gram tissue to 78.3 μg/gram tissue, suggesting a slow absorption and long circulation time for the compound in mice. The liver had greater uptake than kidney, again suggesting hepatobiliary clearance. The tumor boron uptake is 3.3 μg/gram tissue at 1 hour and 3.4 μg/gram tissue at 4 hour, which was lower than the boron uptake in muscle (5.1 μg/gram tissue and 5.1 μg/gram tissue, respectively). Overall, the more hydrophobic compounds 1d and 1f had a longer circulation time in blood. Similar total tumor uptake, ranging from 3.3 – 7.1 μg/gram was seen between BPA, compounds 1d, and 1f, while compound 1a demonstrated decreased tumor uptake at four hours.
Figure 5.
(A) Boron biodistribution. Tissues were harvested as indicated in figure 3, homogenized and boron content was determined by ICP-OES. (B) Boron uptake in tumor (C) Tumor/muscle uptake ratio of boron. (D) Tumor/blood uptake ratio of boron.
DISCUSSION
In this report, we demonstrate the synthesis and initial in vitro and in vivo evaluation of a series of boron-containing PSMA inhibitors based around a common urea skeleton. Although all these eight Boro-PSMA compounds share the same urea-based PSMA binding skeleton, the alteration of the side chain significantly influenced their binding affinity, cell internalization ability and pharmacokinetics. Overall, all compounds demonstrated equal to or improved ability to bind PSMA based on a competition radioligand binding assay, when compared against known high affinity PSMA inhibitor, PMPA. The very high binding affinity of these compounds is not surprising given the well-known versatility of the urea-based PSMA binding scaffold.37,38,39
Based on these encouraging initial results, three compounds were advanced to initial in vivo testing using imaging and boron biodistribution assays. Compound 1a was selected as a hydrophilic, single boron containing agent. Compound 1d was selected as a representative of the carborane group, with the highest overall IC50 of 20.3 nM. Compound 1f was selected as it had two carborane substituents and the largest number of boron atoms (20) as well as reasonable binding affinity. All three compounds demonstrated high PSMA binding in vivo as demonstrated by PET imaging and 68Ga-PSMA-11 biodistribution analysis, while BPA did not demonstrate in vivo PSMA binding. However, the boro-PSMA compounds demonstrated differing pharmacokinetics and overall tumor boron uptake. The hydrophilic compound 1a demonstrated rapid renal clearance, and efficient binding of PSMA in vivo as judged by the blocking of 68Ga-PSMA-11 uptake in tumor. However, the overall tumor boron uptake was the lowest of all compounds tested at four hours, despite the highest administered dose, possibly due to only a single boron atom per molecule. Overall, compound 1a is a very hydrophilic molecule due to its three carboxylic acids and the boronic acid group. In contrast, the more hydrophobic carborane compounds 1d and 1f demonstrated primary hepatobiliary clearance, while still demonstrating efficient 68Ga-PSMA-11 blocking in vivo. The in vivo half life of these compounds appeared to be significantly greater than 1a as judged by the high residual boron content in the blood at four hours after administration. Compounds 1d and 1f demonstrated similar overall boron uptake in tumor when compared against BPA, the most commonly used agent for BNCT. However, an unexpected result is that the increased boron content per molecule did not increase the uptake of 1f in tumor when compared against 1d, possibly because of slow absorption. For compound 1d and 1f, the boron concentration in the blood was still high after 4 hours, indicating an increased boron uptake at tumor after 4 hours, but also a higher risk of toxicity.
In this study, we assayed the receptor engagement through competition binding assays with 68Ga-PSMA-11 PET, and direct tumoral uptake through biodstribution assays and ICP-MS of total tissue boron content. Another alternative way to determine biodistribution of boron containing compounds could be considered for future studies using direct labeling of the Boro-PSMA derivatives with chelators (DOTA, DTPA), that can coordinate metal imaging probes such as 68Ga for PET or Gd for MRI.40,41
To date, there have been two reports of boro-PSMA inhibitors. Byun’s group reported the synthesis of carborane containing PSMA inhibitors and a PSMA-inhibitor X-ray structural characterization.24 However, the compounds were not further evaluated in animal models. Valliant’s group also produced a series of carborane containing PSMA inhibitors, but the IC50 were not improved compared to PMPA.25 In contrast, the boro-PSMA inhibitors in our study exhibited a significantly enhanced IC50 up to 26-fold reduced compared to PMPA. We also further revealed their structure activity relationship, proved their in vivo binding of PSMA, determined their boron biodistribution and tumor uptake in a 22Rv1 xenograft mice model. Taken together, these experiments demonstrate similar overall uptake of Boro-PSMA compounds 1d and 1f when compared against BPA, the most commonly used BNCT agent. When compared against the previously reported studies evaluating BPA uptake in tumor, our BPA results demonstrate a tumor/muscle uptake ratio of 2.0 at 4 hour time point, consistent with other prior reports ranging between 1.5 and 4.4 42 In our study, the BPA uptake at tumor is 7.1±4.0 μg/gram at 4 hour. Overall, the BPA biodistribution results are similar to prior reports conducted under similar conditions.43,44
A tumor uptake of at least 20–50 μg/gram boron is typically thought to be necessary for effective tumor therapy. The boron ratio in tumor/normal tissue needs to be greater than 3:1 for selectivity.3 However, the boron uptake did not reach the requirements, suggesting that the current compounds and/or dosing regimens are not sufficient for therapy. In order to deliver sufficient boron to the tumor, both high affinity binding and high abundance targets are required. In this study, a large amount of boron compound was administered, saturating PSMA binding sites in vivo as verified using the 68Ga-PSMA-11 binding and blocking assay. Several approaches could be used to improve boron delivery to tumor. Firstly, the timing and route of administration of the compounds could be varied. The very high blood content of compounds 1d and 1f at the latest time point tested, as well as the increased tumor to muscle ratio at four hours compared against one hour, suggests that a longer incubation time could improve the overall boron delivery as well as tumor to blood and tumor to muscle ratios. The second potential route for improvement would be to include larger numbers of boron atoms per PSMA-inhibitor. While compound 1f contains 20 atoms, larger substitutents such as dendrimers and nanoparticles have been reported.45,46,47,48,49 In principle, these substituents could be appended to the urea-based PSMA agent to achieve higher boron delivery. Finally, the use of animal models with increased PSMA expression would likely yield improved tumor boron uptake. While the 22Rv1 model was selected due to convenient, rapid and predictable tumor growth, its overall PSMA expression is low compared against other cell lines such as LNCaP or PC3-pip.50 These improvements to the method represent important areas for future investigation. Following optimization of the pharmacokinetics, and dosing regimens, initial treatment studies could be performed in mice bearing appropriate tumor xenografts.
CONCLUSION
In this study, a series of boronic acid- and carborane-containing PSMA inhibitors were successfully synthesized. The in vitro binding data demonstrated high binding affinities to PSMA for all the boron-containing inhibitors. In particular, compound 1d showed a 26 fold lower IC50 value that the well-known PSMA inhibitor PMPA. PET imaging data demonstrated blocking of 68Ga-PSMA-11 uptake by boron containing PSMA compounds, demonstrating the in vivo binding of the PSMA inhibitors to their target. The boron uptake and distribution assay showed around 4 μg boron/gram tumor uptake and similar tumor/muscle ratios compared to the ratio for the standard compound BPA. Although the uptake is below the required 20 μg boron/gram tumor threshold, these data suggest that with further optimization, treatment of oligometastatic prostate cancer with Boro-PSMA is feasible.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by an American Cancer Society Individual Research Grant (IRG-97–150-13), and the New Directions in Prostate Cancer Research Award of the University of California, San Francisco Prostate Cancer Research Program. This publication was supported by the Helen Diller Family Comprehensive Cancer Center Support Grant of the National institutes of Health under P30 CA 82103. Dr. Flavell was additionally supported by the David Blitzer Young Investigator Award of the Prostate Cancer Foundation and a Physician Research Training Grant from the Department of Defense (PC 150932). We thank Dr. Rob Franks and Dr. Brian Dreyer of the Marine Analytical Laboratory in the University of California, Santa Cruz for assistance with ICP measurements, Kyoughee Seo in the University of California, San Francisco for help with mice dissection.
ABBREVIATIONS
- BNCT
boron neutron capture therapy
- PSMA
prostate specific membrane antigen
- PET
positron emission tomography
- LET
linear energy transfer
- BPA
4-borono-L-phenylalanine
- BSH
sodium borocaptate
- PCa
prostate cancer
- GCPII
glutamate carboxypeptidase II
- FBS
fetal bovine serum
- PMPA
2-(phosphonomethyl) pentanedioic acid
- ICP-OES
inductively coupled plasma optical-emission spectrometry
- IACUC
Institutional Animal Care & Use Program
- DCC
N,N′-Dicyclohexylcarbodiimide
- NHS
N-Hydroxysuccinimide
- TFA
trifluoroacetic acid
Footnotes
The authors have declared that no competing interest exists
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: Synthesis procedure, 1H and 13C NMR spectra of compounds 1a-h, supplementary figures and tables.
REFERENCES
- (1).Heber EM; Hawthorne MF; Kueffer PJ; Garabalino MA; Thorp SI; Pozzi ECC; Hughes AM; Maitz CA; Jalisatgi SS; Nigg DW; Curotto P, Trivillin VA; Schwint AE Therapeutic efficacy of boron neutron capture therapy mediated by boron-rich liposomes for oral cancer in the hamster cheek pouch model. Proc. Natl. Acad. Sci 2014, 111, 16077–16081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Nedunchezhian K; Aswath N; Thiruppathy M; Thirugnanamurthy S Boron neutron capture therapy - a literature review. J. Clin. Diagnostic Res 2016, 10, ZE01–ZE04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Barth RF; Mi P; Yang W Boron delivery agents for neutron capture therapy of cancer. Cancer Commun. 2018, 38, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Pozzi ECC; Cardoso JE; Colombo LL; Thorp S; Hughes AM; Molinari AJ; Garabalino MA; Heber EM; Miller M; Itoiz ME; Aromando RF; Nigg DW; Quintana J; Trivillin VA Schwint AE Boron neutron capture therapy (BNCT) for liver metastasis: therapeutic efficacy in an experimental model. Radiat. Environ. Biophys 2012, 51, 331–339. [DOI] [PubMed] [Google Scholar]
- (5).Haapaniemi A; Kankaanranta L; Saat R; Koivunoro H; Saarilahti K; Makitie A; Atula T; Joensuu H Boron neutron capture therapy in the treatment of recurrent laryngeal cancer. Int. J. Radiat. Oncol. Biol. Phys 2016, 95, 404–410. [DOI] [PubMed] [Google Scholar]
- (6).Chanana AD; Capala J; Chadha M; Coderre JA; Diaz AZ; Elowitz EH; Iwai J; Joel DD; Liu HB; Ma R; Pendzick N; Peress NS; Shady MS; Slatkin DN; Tyson GW; Wielopolski L Boron neutron capture therapy for glioblastoma multiforme: interim results from the phase I/II dose-escalation studies. Neurosurgery 1999, 44, 1182–1193. [DOI] [PubMed] [Google Scholar]
- (7).Barth RF; Coderre JA; Vicente MGH; Blue TE Boron neutron capture therapy of cancer: current status and future prospects. Clinical Cancer Research. 2005, 11, 3987–4002. [DOI] [PubMed] [Google Scholar]
- (8).Barth RF; Vicente MGH; Harling OK; Kiger WS; Riley KJ; Binns PJ; Wagner FM; Suzuki M; Aihara T; Kato I; Kawabata S Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiation Oncology. 2012, 7, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Henriksson R; Capala J; Michanek A; Lindahl SÂ; Salford LG; Franzén L; Blomquist E; Westlin JE; Bergenheim AT Boron neutron capture therapy (BNCT) for glioblastoma multiforme: a phase II study evaluating a prolonged high-dose of boronophenylalanine (BPA). Radiother. Oncol 2008, 88, 183–191. [DOI] [PubMed] [Google Scholar]
- (10).Takagaki M; Oda Y; Miyatake SI; Kikuchi H; Kobayashi T; Sakurai Y; Osawa M; Mori K; Ono K Boron neutron capture therapy: preliminary study of BNCT with sodium borocaptate (Na2B12H11SH) on glioblastoma. J. Neurooncol 1997, 35, 177–185. [DOI] [PubMed] [Google Scholar]
- (11).Haritz D; Gabel D; Huiskamp R Clinical phase-I study of Na2B12H11SH (BSH) in patients with malignant glioma as precondition for boron neutron capture therapy (BNCT). Int. J. Radiat. Oncol. Biol. Phys 1994, 28, 1175–1181. [DOI] [PubMed] [Google Scholar]
- (12).Johansson JE; Andrén O; Andersson SO; Dickman PW; Holmberg L; Magnuson A; Adami HO Natural history of early, localized prostate cancer. J. Am. Med. Assoc. 2004, 291, 2713–2719. [DOI] [PubMed] [Google Scholar]
- (13).Bray F; Ferlay J; Soerjomataram I; Siegel RL; Torre LA; Jemal A Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin 2018, 68, 394–424. [DOI] [PubMed] [Google Scholar]
- (14).Perner S; Hofer MD; Kim R; Shah RB; Li H; Möller P; Hautmann RE; Gschwend JE; Kuefer R; Rubin MA Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum. Pathol 2007, 38, 696–701. [DOI] [PubMed] [Google Scholar]
- (15).Maurer T; Eiber M; Schwaiger M; Gschwend JE Current use of PSMA-PET in prostate cancer management. Nature Reviews 2016, 13, 226–235. [DOI] [PubMed] [Google Scholar]
- (16).Eder M; Schäfer M; Bauder-Wüst U; Hull WE; Wängler C; Mier W; Haberkorn U; Eisenhut M 68Ga-Complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug. Chem 2012, 23, 688–697. [DOI] [PubMed] [Google Scholar]
- (17).Hillier SM; Maresca KP; Femia FJ; Marquis JC; Foss CA; Nguyen N; Zimmerman CN; Barrett JA; Eckelman WC; Pomper MG; Joyal JL; Babich JW Preclinical evaluation of novel glutamate-urea-lysine analogues that target prostate-specific membrane antigen as molecular imaging pharmaceuticals for prostate cancer. Cancer Res. 2009, 69, 6932–6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Barinka C; Byun Y; Dusich CL; Banerjee SR; Chen Y; Castanares M; Kozikowski AP; Mease RC; Pomper MG; Lubkowski J Interactions between Human Glutamate Carboxypeptidase II and Urea-Based Inhibitors: Structural Characterization. J. Med. Chem 2008, 51, 7737–7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Fendler WP; Rahbar K; Herrmann K; Kratochwil C; Eiber M 177Lu-PSMA radioligand therapy for prostate cancer. J. Nucl. Med 2017, 58, 1196–1200. [DOI] [PubMed] [Google Scholar]
- (20).Kratochwil C; Bruchertseifer F; Giesel FL; Weis M; Verburg FA; Mottaghy F; Kopka K; Apostolidis C; Haberkorn U; Morgenstern A 225Ac-PSMA-617 for PSMA-targeted radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med 2016, 57, 1941–1944. [DOI] [PubMed] [Google Scholar]
- (21).Hrkach J; Von Hoff D; Ali MM; Andrianova E; Auer J; Campbell T; De Witt D; Figa M; Figueiredo M; Horhota A; Low Susan; McDonnell K; Peeke E; Retnarajan B; Sabnis A; Schnipper E; Song JJ; Song YH; Summa J; Tompsett D; Troiano G; Van Geen Hoven T; Wright J; LoRusso P; Kantoff PW; Bander NH; Sweeney C; Farokhzad OC; Langer R; Zale S Preclinical development and clinical translation of a PSMA-targeted Docetaxel nanoparticle with a differentiated pharmacological profile. Sci. Transl. Med 2012, 4, 128–139. [DOI] [PubMed] [Google Scholar]
- (22).Wang X; Ma D; Olson WC; Heston WDW In Vitro and In Vivo Responses of advanced prostate tumors to PSMA ADC, an Auristatin-conjugated antibody to prostate-specific membrane antigen. Mol. Cancer Ther 2011, 10, 1728–1739. [DOI] [PubMed] [Google Scholar]
- (23).Xiang B; Dong DW; Shi NQ; Gao W; Yang ZZ; Cui Y; Cao DY; Qi XR PSA-responsive and PSMA-mediated multifunctional liposomes for targeted therapy of prostate cancer. Biomaterials 2013, 34, 6976–6991. [DOI] [PubMed] [Google Scholar]
- (24).Youn S; Kim KI; Ptacek J; Ok K; Novakova Z; Kim YH; Koo JH; Barinka C; Byun Y Carborane-containing urea-based inhibitors of glutamate carboxypeptidase II: synthesis and structural characterization. BioorganicMed. Chem. Lett 2015, 25, 5232–5236. [DOI] [PubMed] [Google Scholar]
- (25).El-Zaria ME; Genady AR; Janzen N; Petlura CI; Beckford Vera DR; Valliant JF Preparation and evaluation of carborane-derived inhibitors of prostate specific membrane antigen (PSMA). Dalt. Trans 2014, 43, 4950–4961. [DOI] [PubMed] [Google Scholar]
- (26).Nanabala R; Anees MK; Sasikumar A; Joy A; Pillai MRA Preparation of [68Ga]PSMA-11 for PET-CT imaging using a manual synthesis module and organic matrix based 68Ge/68Ga generator. Nucl. Med. Biol 2016, 43, 463–469. [DOI] [PubMed] [Google Scholar]
- (27).Mesters JR; Henning K; Hilgenfeld R Human glutamate carboxypeptidase II inhibition: structures of GCPII in complex with two potent inhibitors, Quisqualate and 2-PMPA. Acta Crystallogr. Sect. D Biol. Crystallogr 2007, 63, 508–513. [DOI] [PubMed] [Google Scholar]
- (28).Leo A; Hansch C; Elkins D Partition coefficients and their uses. Chem. Rev 1971, 71, 525–616. [Google Scholar]
- (29).García LA; Arias E; Moggio I; Romero J; Ledezma A; Ponce A; Perez O Fluorescent core-sheath fibers by electrospinning of a phenyleneethynylene/ poly(styrene-co-maleimide) blend. Polymer 2011, 52, 5326–5334. [Google Scholar]
- (30).Wakasugi K; Iida A; Misaki T; Nishii Y; Tanabe Y Simple, mild, and practical esterification, thioesterification, and amide Formation utilizing p-toluenesulfonyl chloride and N-methylimidazole. Adv. Synth. Catal. 2003, 345, 1209–1214. [Google Scholar]
- (31).Toppino A; Genady AR; El-Zaria ME; Reeve J; Mostofian F; Kent J; Valliant JF High yielding preparation of dicarba-closo-dodecaboranes using a silver(I) mediated dehydrogenative alkyne-insertion reaction. Inorg. Chem 2013, 52, 8743–8749. [DOI] [PubMed] [Google Scholar]
- (32).Wang M; McNitt CD; Wang H; Ma X; Scarry SM; Wu Z; Popik VV; Li Z The efficiency of 18F labelling of a prostate specific membrane antigen ligand via strain-promoted azide-alkyne reaction: reaction speed versus hydrophilicity. Chem. Commun 2018, 54, 7810–7813. [DOI] [PubMed] [Google Scholar]
- (33).Cleeren F; Lecina J; Billaud EMF; Ahamed M; Verbruggen A; Bormans GM New chelators for low temperature Al18F-labeling of biomolecules. Bioconjug. Chem 2016. 27, 790–798. [DOI] [PubMed] [Google Scholar]
- (34).Capala J; Britta H; Skold K Boron neutron capture therapy for glioblastoma multiforme: clinical studies in Sweden. J. neuro-oncology 2003, 62, 135–144. [DOI] [PubMed] [Google Scholar]
- (35).Wang Y; Shao G; Wu J; Cui C; Zang S; Qiu F; Jia R; Wang Z; Wang F Preparation of 68Ga-PSMA-11 with a synthesis module for micro PET-CT imaging of PSMA expression during prostate cancer progression. Contrast Media Mol. Imaging 2018, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Moon SH; Hong MK; Kim YJ; Lee YS; Lee DS; Chung JK; Jeong JM Development of a Ga-68 labeled PET tracer with short linker for prostate-specific membrane antigen (PSMA) targeting. Bioorganic Med. Chem 2018, 26, 2501–2507. [DOI] [PubMed] [Google Scholar]
- (37).Kozikowski AP; Nan F; Conti P; Zhang J; Ramadan E; Bzdega T; Wroblewska B; Neale JH; Pshenichkin S; Wroblewski JT Design of remarkably simple, yet potent urea-based inhibitors of glutamate carboxypeptidase II (NAALADase). J. Med. Chem. 2001, 44, 298–301. [DOI] [PubMed] [Google Scholar]
- (38).Banerjee SR; Foss CA; Castanares M; Mease RC; Byun Y; Fox JJ; Hilton J; Lupold SE; Kozikowski AP; Pomper MG Synthesis and evaluation of technetium-99m- and rhenium-labeled inhibitors of the prostate-specific membrane antigen (PSMA). J. Med. Chem. 2008, 51, 4504–4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Barrett JA; Babich JW; Zimmerman CN; Maresca KP; Hillier SM; Eckelman WC; Barone C; Femia FJ; Keith D; Joyal JL; Zimmerman CN; Kozikowski AP; Barrett JA; Eckelman WC; Babich JW A series of halogenated heterodimeric inhibitors of prostate specific membrane antigen (PSMA) as radiolabeled probes for targeting prostate cancer. J. Med. Chem. 2008, 52, 347–357. [DOI] [PubMed] [Google Scholar]
- (40).Iguchi Y; Michiue H; Kitamatsu M; Hayashi Y; Takenaka F; Nishiki T; Matsui H Tumor-specific delivery of BSH-3R for boron neutron capture therapy and positron emission tomography imaging in a mouse brain tumor model. Biomaterials 2015, 56, 10–17. [DOI] [PubMed] [Google Scholar]
- (41).Geninatti-Crich S; Alberti D; Szabo I; Deagostino A; Toppino A; Barge A; Ballarini F; Bortolussi S; Bruschi P; Protti N; Stella S; Altieri S; Venturello P; Aime S MRI-guided neutron capture therapy by use of a dual gadolinium/boron agent targeted at tumour cells through upregulated low-density lipoprotein transporters. Chem. - A Eur. J 2011, 17, 8479–8486. [DOI] [PubMed] [Google Scholar]
- (42).Evangelista L; Jori G; Martini D; Sotti G Boron neutron capture therapy and 18F-labelled borophenylalanine positron emission tomography: A critical and clinical overview of the literature. Applied Radiation and Isotopes. 2013, 91–101. [DOI] [PubMed] [Google Scholar]
- (43).Carpano M; Perona M; Rodriguez C; Nievas S; Olivera M; Santa Cruz GA; Brandizzi D; Cabrini R; Pisarev M; Juvenal GJ; Dagrosa MA Experimental studies of boronophenylalanine (10BPA) biodistribution for the individual application of boron neutron capture therapy (BNCT) for malignant melanoma treatment. Int. J. Radiat. Oncol. Biol. Phys. 2015. 93, 344–352. [DOI] [PubMed] [Google Scholar]
- (44).Coderre J. a; Glass JD; Fairchild RG; Micca PL; Fand I; Joel DD Selective delivery of boron by the melanin precursor analogue p-boronophenylalanine to tumors other than melanoma. Cancer Res. 1990, 50, 138–141. [PubMed] [Google Scholar]
- (45).Wu G; Yang W; Barth RF; Kawabata S; Swindall M; Bandyopadhyaya AK; Tjarks W; Khorsandi B; Blue TE; Ferketich AK; Yang M; Christoforidis GA; Sferra TJ; Binns PJ; Riley KJ; Ciesielski MJ; Fenstermaker RA Molecular targeting and treatment of an epidermal growth factor receptor-positive glioma using boronated Cetuximab. Clin. Cancer Res 2007, 13, 1260–1268. [DOI] [PubMed] [Google Scholar]
- (46).Takeuchi I; Nomura K; Makino K Hydrophobic boron compound-loaded poly(L-lactide-co-glycolide) nanoparticles for boron neutron capture therapy. Colloids Surfaces B Biointerfaces 2017, 159, 360–365. [DOI] [PubMed] [Google Scholar]
- (47).Sumitani S; Nagasaki Y Boron neutron capture therapy assisted by boron-conjugated nanoparticles. Polymer Journal. 2012, 44, 522–530. [Google Scholar]
- (48).Oleshkevich E; Morancho A; Saha A; Galenkamp KMO; Grayston A; Crich SG; Alberti D; Protti N; Comella JX; Teixidor F; Rosell A; Viñas C Combining magnetic nanoparticles and icosahedral boron clusters in biocompatible inorganic nanohybrids for cancer therapy. Nanomedicine Nanotechnology, Biol. Med 2019, 20, 101986. [DOI] [PubMed] [Google Scholar]
- (49).Hey-Hawkins E; Viñas-Teixidor C Boron-based compounds: potential and emerging applications in medicine. John Wiley & Sons, Ltd, Hoboken, NJ, USA, 2018. [Google Scholar]
- (50).Taylor RM; Severns V; Brown DC; Bisoffi M; Sillerud LO Prostate cancer targeting motifs: expression of αvβ3, neurotensin receptor 1, prostate specific membrane antigen, and prostate stem cell antigen in human prostate cancer cell lines and xenografts. Prostate 2012, 72, 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.