Abstract
Study Design
A rat puncture injury intervertebral disc (IVD) degeneration model with structural, biomechanical, and histological analyses.
Objective
To determine if males and females have distinct responses in the IVD after injury.
Summary of Background Data
Low back pain (LBP) and spinal impairments are more common in women than men. However, sex differences in IVD response to injury have been underexplored, particularly in animal models where sex differences can be measured without gender confounds.
Methods
Forty-eight male and female Sprague Dawley rats underwent sham, single annular puncture with tumor necrosis factor α (TNFα) injection (1x), or triple annular puncture with TNFα injection (3x) surgery. Six weeks after surgery, lumbar IVDs were assessed by radiologic IVD height, spinal motion segment biomechanical testing, histological degeneration grading, second harmonic generation (SHG) imaging, and immunofluorescence for fibronectin and α-smooth muscle actin.
Results
Annular puncture injuries significantly increased degenerative grade and IVD height loss for males and females, but females had increased degeneration grade particularly in the annulus fibrosus (AF). Despite IVD height loss, biomechanical properties were largely unaffected by injury at six weeks. However, biomechanical measures sensitive to outer AF differed by sex after 3x injury--male IVDs had greater torsional stiffness, torque range, and viscoelastic creep responses. SHG intensity of outer AF was reduced after injury only in female IVDs, suggesting sex differences in collagen remodeling. Both males and females exhibited decreased cellularity and increased fibronectin expression at injury sites.
Conclusions
IVD injury results in distinct degeneration and functional healing responses between males and females. The subtle sex differences identified in this animal model suggest differences in response to IVD injury that might explain some of the variance observed in human LBP, and demonstrate the need to better understand differences in male and female IVD degeneration patterns and pain pathogenesis.
Level of Evidence
N/A
Keywords: intervertebral disc, sex differences, fibrosis, degeneration, rodent model, biomechanics, second-harmonic generation imaging, immunofluorescence
Introduction
Low back pain (LBP) is the most prevalent musculoskeletal disease, and responsible for the most years lived with disability of any disease (1–3). 50–80% of the US population experiences LBP, and prevalence is increasing (4–6). However, LBP remains difficult to treat, as both surgical and non-surgical interventions produce inconsistent results (7, 8).
Chronic pain is more prevalent in women than men (9). Findings for LBP specifically are more mixed, but many studies find greater prevalence (10) and severity (11) of LBP in women. While pain differences are sometimes attributed to cultural/gender differences, such as who is more likely to work a manual labor job or who is more likely to visit a physician for a pain complaint, rather than biological/sex differences, animal studies have demonstrated clear nervous system sex differences in chronic pain (12–16). These animal study findings have no gender confound, for animals do not have a gender. Therefore, differences between men and women in LBP cannot be contributed exclusively to gender/cultural effects, warranting investigation of sex differences in LBP animal models.
IVD degeneration is strongly associated with LBP, although pain origin is often multifactorial (17–21). Due to its significant role in LBP, in vivo animal models of IVD degeneration have been developed across multiple species. Annular puncture injury models are widely used (22–26), and can assess sex differences in IVD degeneration without gender-related confounds.
In humans, spinal anatomy and biomechanical properties differ between males and females. Females have greater lordotic angle and lordotic wedging of lumbar vertebrae (27–30), and there are sex differences in spino-pelvic alignment (30–32). Biomechanically, female spines exhibit greater flexibility and range of motion (33, 34). Furthermore, IVD degeneration causes more biomechanical property changes in male IVDs (33), suggesting sex-specific IVD degeneration. Similar anatomical differences have not been well described in standard rodent models (Rattus norvegicus, Mus musculus), but sex differences in radiologic disc space narrowing and wedging that varied with animal age have been seen in the sand rat (Psammomys obesus)(35).
How males and females differ in response to IVD injury remains a critical question that is poorly understood and likely to inform more precise IVD degeneration treatments. Almost no previous work in animal models of IVD degeneration have compared males and females as separate cohorts. In this work, we apply a rat IVD degeneration model to male and female rats to determine if IVDs exhibit sex-specific injury responses. More specifically, we determined the effects of sex and injury severity on the progression of IVD degeneration that was quantified using IVD height, degeneration grade, functional biomechanical properties, AF collagen structure, and fibrosis.
Material and Methods
Study Design
IVD degeneration was induced using a rat annular puncture model. A rat model was used because their IVDs are commonly used and known to have many similarities in structure and anatomy to human IVDs, it is a small animal model that is sufficiently large to produce precise surgical injuries, and rats are amenable to future pain behavioral testing (22, 25, 36, 37).
Animals and surgical intervention
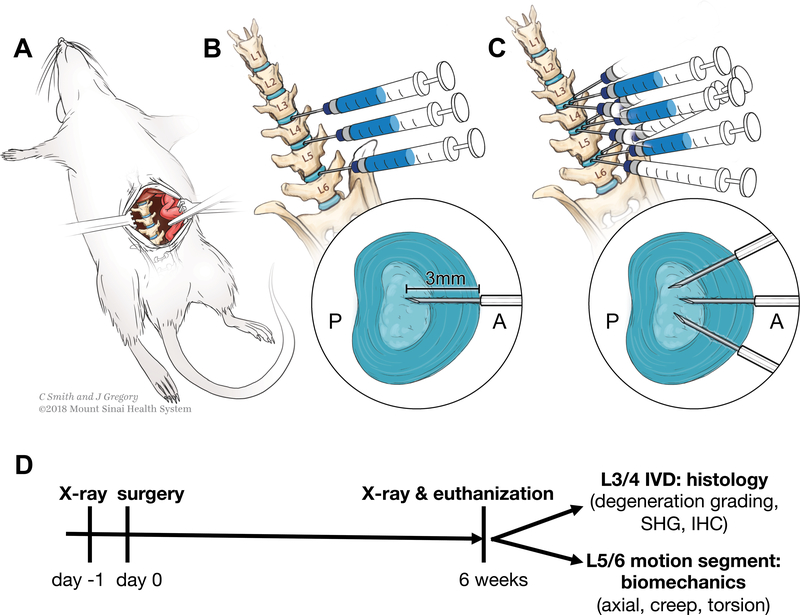
Procedures were approved by Icahn School of Medicine Institutional Animal Care and Use Committee. 48 skeletally-mature, 4-month old Sprague-Dawley rats were used (24 male, 24 female) (Charles River Laboratory, Wilmington, MA). Rats were randomly divided into three groups: sham, single (1x), or triple puncture injury (3x). Anterior puncture with TNFα injection was used with full-depth/complete AF injuries since this was a previously validated to induce repeatable and severe IVD degeneration (25). Single and triple puncture injures were applied to induce varying degeneration levels. Procedures were performed under 2–3% isofluorane (Baxter, Deerfield, IL). Sham surgery was an anterior abdominal incision with exposure of L3/4, L4/5, and L5/6 IVDs (Figure 1A). 1x surgery used the same approach, but exposed IVDs were punctured with a 26G needle midline anteriorly, and injected with 2.5μL of 0.1 ng/μL tumor necrosis factor alpha (TNFα) (80045RNAE50; Sino Biological Inc., Beijing, China) (Figure 1B). 3x surgery had additional punctures left and right anterolaterally (Figure 1C). One animal died during surgery. Animals maintained Body Conditioning Score > 2. Rats were euthanized six weeks after surgery for tissue collection (Figure 1D).
Figure 1. Surgical approach for sham and 1x and 3x annular puncture surgeries.
(A) Sham procedure with lumbar IVD exposure. (B) 1x annular puncture surgery with TNFα injection. (C) 3x annular puncture surgery with three punctures and a single TNFα injection. (D) Study design timeline.
Intervertebral disc height
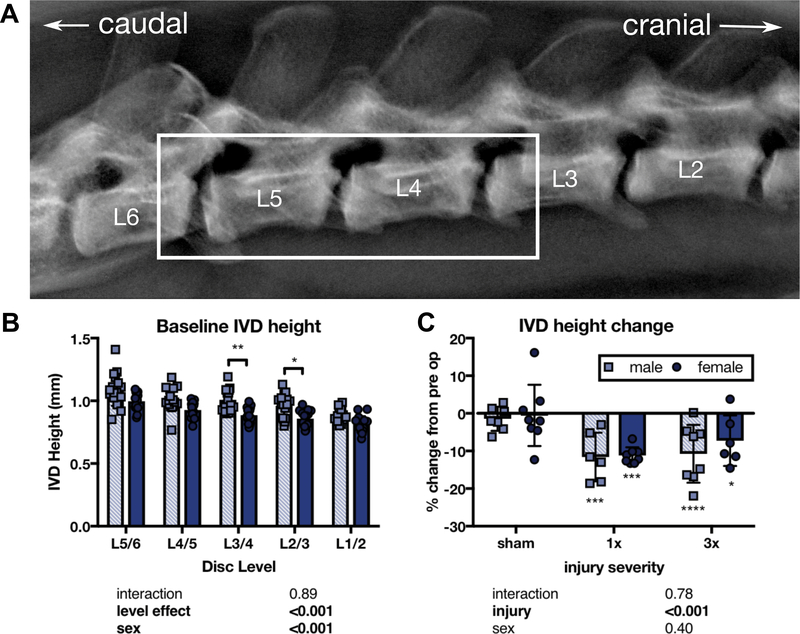
IVD structural change was assessed via in vivo radiologic measurements of IVD height, which has been shown to be sensitive to IVD injury and associated with pain in humans (25, 38). Rats received lateral X-rays at 55 kV for 10 seconds (UltraFocus Faxitron, Tucson, AZ) before surgery and at six weeks (Figure 2A). Vertebral borders were manually defined in Fiji (National Institutes of Health, Bethesda, MD), and IVD height calculated in MATLAB (MathWorks, Natick, MA) (25). Average IVD height for punctured IVDs were averaged for each time point (Figure 2A - box) and percentage change from baseline calculated.
Figure 2. Both 1x and 3x IVD injury reduced IVD height 6 weeks after injury, with no significant differences between male and female IVDs or between 1x and 3x injuries.
(A) IVD height was calculated from lateral X-rays of the L3/4, L4/5, and L5/6 IVD space (box). (B) Pre-operative comparisons between lumbar IVD levels by sex. Significant sex differences in IVD height were only observed at the L3/4 and L2/3 IVD. (C) The percentage change in the average IVD height across these three levels was significantly different from pre-operative baseline for 1x and 3x, but did not differ between males and females. (n=6–8, * p<0.05, *** p<0.001, **** p<0.0001 from baseline, p values for 2-way ANOVA effects shown below each graph).
Biomechanics
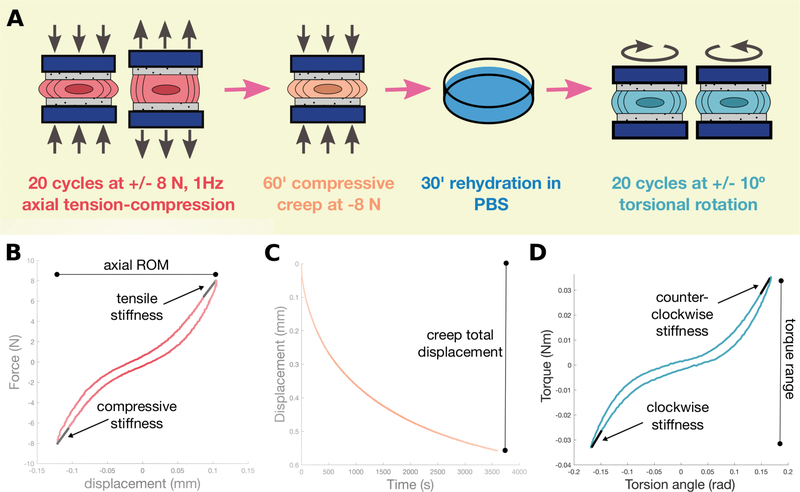
L5/6 motion segments (vertebra-IVD-vertebra) were used for biomechanical testing (Figure 3A). Posterior elements were removed, and vertebrae potted in stainless steel cylindrical pots with cyanoacrylate.
Figure 3. Biomechanical testing workflow and representative curves.
(A) Each IVD was subjected to an identical protocol of axial tension-compression, compressive creep, rehydration, and torsional rotation. “Minutes” is abbreviated as ‘ (single apostrophe). Representative curves from axial (B), creep (C), and torsional testing (D).
Axial biomechanics and creep
Axial biomechanics are reflective of physiological loading and were used to evaluate NP pressurization, AF tension, and IVD laxity. Creep is known to be sensitive to alterations in short and long-time viscoelastic behaviors. Axial and creep testing used an ElectroForce 3200 instrument (TA Instruments, New Castle, DE). Samples were hydrated in PBS with protease inhibitor tablets (ThermoScientific, Rockford, IL). Motion segments underwent 20 cycles ±8N of tension-compression at 1Hz followed by 60 minutes of compressive creep at −8N and 30 minutes of unloaded rehydration. Compressive stiffness, tensile stiffness, axial range of motion, and axial hysteresis were calculated from the 20th cycle in MATLAB (Figure 3B). Three independent reviewers manually calculated neutral zone (NZ) length and stiffness. Samples with undetectable NZ were excluded from NZ stiffness analysis. Creep was analyzed with MATLAB code measuring total displacement and applying a 5-parameter viscoelastic solid model to calculate elastic response (Se), fast response (τ1 and S1), and slow response (τ2 and S2) time constant and stiffness parameters, respectively (39) (Figure 3C).
Torsional biomechanics
Torsional biomechanics were used to measure AF structural integrity, without the influence of NP changes. Torsional biomechanical testing used an AR2000ex rheometer (TA Instruments, New Castle, DE). Motion segments equilibrated for five minutes at −8N compression before 20 cycles ±10° at 1Hz. Torsional stiffness, torque range, torsional hysteresis, NZ length and NZ stiffness were calculated from the 20th cycle using MATLAB (Figure 3D). Four curves were excluded from analysis due to unloading curves suggestive of improperly secured pots.
Histology
L3/4 IVD midsagittal 5μm paraffin-embedded sections were used for histology.
Degeneration grading
Sections were stained with picrosirius red (collagen) and alcian blue (glycoaminoglycans) (PR/AB) and imaged at 20x on a Leica DMB6 microscope (Leica Microsystems, Wetzlar, Germany) under brightfield and stitched as a single image. Three blinded independent evaluators performed degeneration scoring, using a semi-quantitative scoring system grading annulus fibrosus (AF) integrity, nucleus pulposus (NP) cellularity, NP matrix quality, interruption of AF/NP border, and endplate irregularity, that is sensitive to degenerative changes after IVD puncture injury (40, 41).
Second-harmonic generation imaging
Second-harmonic generation (SHG) imaging was used to evaluate AF collagen organization. Sections were imaged using an Olympus FV1000MPE two photon microscope with 910nm excitation wavelength (Olympus Corporation, Tokyo, Japan). SHG signal was collected in the backward direction at 25x and photomultiplier tube at 440±20nm. Maximum intensity z-projection was performed on mosaic images. Outer AF regions of interest were manually outlined in Fiji and background subtracted using 50 pixel rolling-ball radius. SHG pixel intensity was defined by mean gray value. OrientationJ Fiji plug-in was used for collagen organization analysis. Contrast enhancement was applied to minimize confounds from SHG intensity differences, fibril orientation calculated with a 1 pixel gaussian window for gaussian gradient, and orientation plotted in a histogram.
Immunofluorescence
Immunofluorescence was used to assess fibrotic markers in the IVD after injury. Sections were fluorescently co-labeled for alpha smooth muscle actin (α-SMA), fibronectin, and 4′,6-diamidino-2-phenylindole (DAPI) (Table 1 and see Data, Supplemental Digital Content 1, positive and negative controls). Fluorescent microscopy was performed at 10x using a Zeiss AxioImager.Z1 microscope with Apotome for optical sectioning (Zeiss, Thornwood, NY).
Table 1.
List of antibodies used for immunohistochemistry in Figure 9 and Supplemental Digital Content 1.
| antibody | vendor | product number | host species | fluorophore | dilution | positive control |
|---|---|---|---|---|---|---|
| anti-α-SMA (1°) | Abcam | ab7817 | mouse | N/A | 1:1000 | ovary |
| anti-mouse (2°) | Abcam | ab150120 | goat | Alexa Fluor 594 | 1:250 | ovary (paired with anti-α-SMA) |
| anti-fibronectin (1°) | Abcam | ab2413 | rabbit | N/A | 1:50 | kidney |
| anti-rabbit (2°) | Abcam | ab150081 | goat | Alexa Fluor 488 | 1:250 | kidney (paired with anti-fibronectin) |
Statistical analysis
Statistical analysis used Prism (GraphPad, La Jolla, CA). Differences between groups were evaluated with 2-way ANOVA (injury severity & sex) with Tukey’s post-hoc test, significance as p<0.05.
Results
Annular puncture induced lumbar IVD structural change
A sex effect was observed in baseline IVD height, but sex differences were only significant at L3/4 and L2/3 IVDs (Figure 2B). IVD height was averaged over three levels and measured as a percent difference from pre-operative baseline to control for level and sex effects. 1x and 3x injuries were sufficient to significantly reduce average IVD height from baseline, while no significant change was observed in sham controls at six weeks and no sex effect observed (Figure 2C).
Sex, but not annular puncture, influenced lumbar IVD biomechanical properties
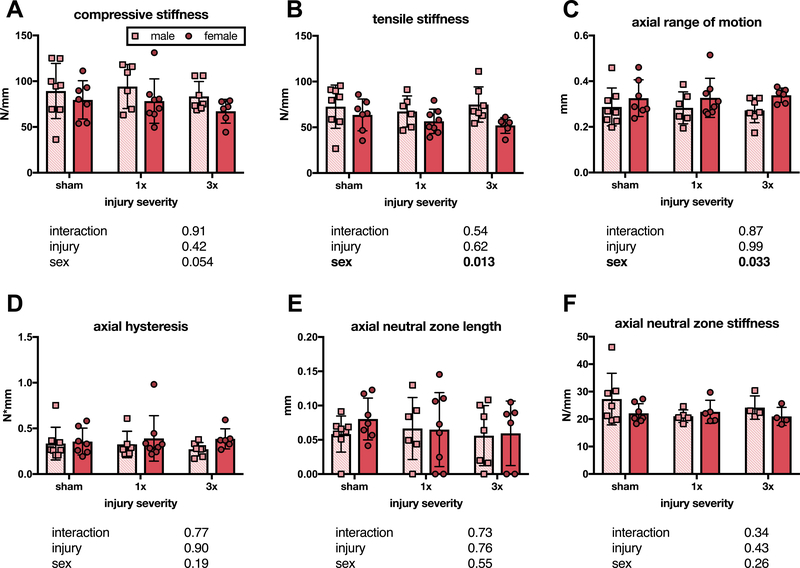
At six weeks, there were no significant changes in compressive stiffness across groups, suggesting no differences in NP pressurization (Figure 4A). Significant sex effects were seen on 2-way ANOVA for tensile stiffness and axial ROM, with male IVDs exhibiting greater stiffness and less axial ROM than female IVDs, but significant differences were not seen after post-hoc tests (Figure 4B–C). Axial hysteresis, axial NZ length, and axial NZ stiffness showed no differences between groups six weeks following injury (Figure 4D–F).
Figure 4. Annular puncture did not effect axial biomechanical properties at six weeks after injury.
Neither compressive stiffness (A), tensile stiffness (B), axial range of motion (C), axial hysteresis (D), axial neutral zone length (E), nor axial neutral zone stiffness (F) differed between sham and either 1x or 3x, or between males and females (n= 6–8 panels A-E, n = 4–6 panel F, p values for 2-way ANOVA effects shown below each graph).
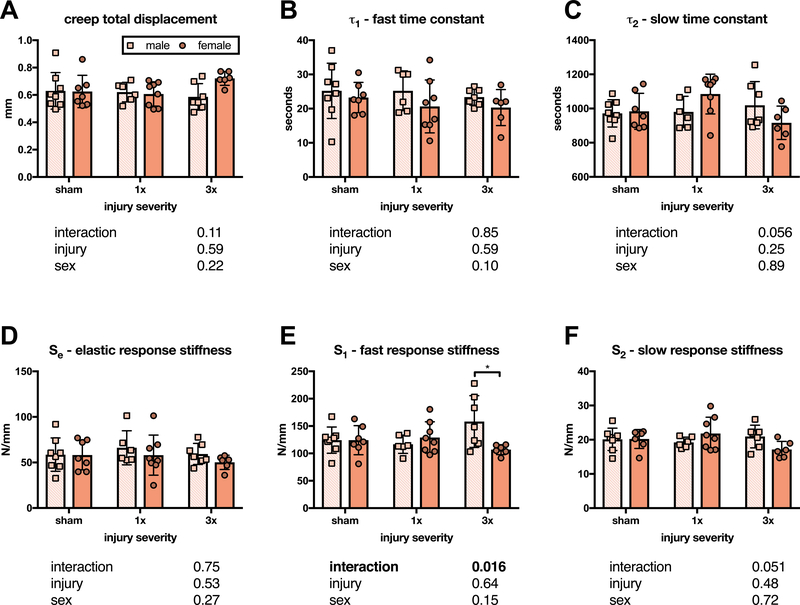
No significant differences were seen between groups for total displacement, τ1, τ2, or Se (Figure 5A–D). However, a significant interaction effect was observed for S1, which was significantly greater in male than female IVDs after 3x injury, but not after 1x or sham surgery (Figure 5E). A similar interaction effect was not observed for S2 (p = 0.0511) (Figure 5F).
Figure 5. Annular puncture injury did not affect creep biomechanical properties at six weeks after injury, as measured with a 5 parameter viscoelastic solid model.
Neither total displacement (A), fast time constant (B), slow time constant (C), nor elastic response stiffness (D) differed between sham and either 1x or 3x, or between males and females. (E) A significant interaction effect was found for fast response stiffness (associated with IVD bulge), with male IVDs exhibiting a significantly greater fast response stiffness than female IVDs after 3x injury. (F) Unlike fast response stiffness, slow response stiffness (associated with fluid exudation) did not have a significant interaction effect (p = 0.0511) (n = 6–8, p values for 2-way ANOVA effects shown below each graph).
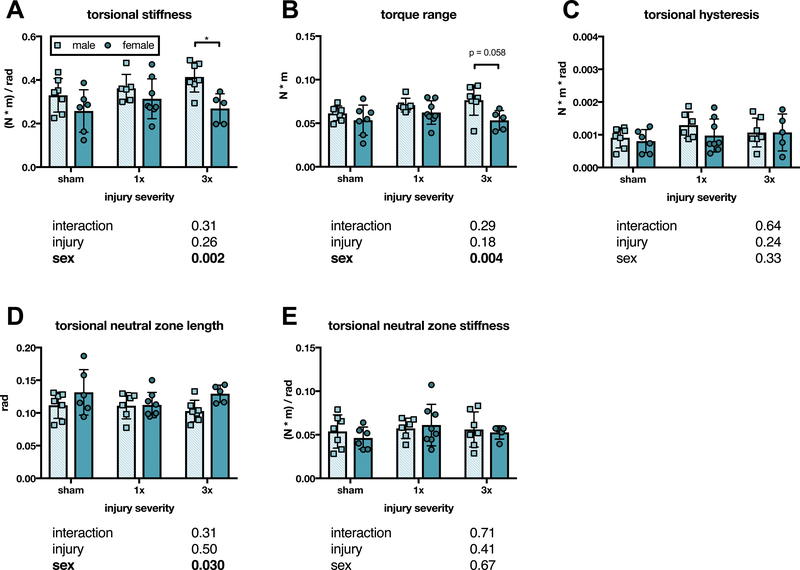
At six weeks, torsional stiffness had a significant sex effect on 2-way ANOVA, and after 3x injury, male IVDs were significantly stiffer than female IVDs (Figure 6A). Torque range exhibited a similar pattern, with a significant sex effect (Figure 6B). However, 3x sex differences were not significant after post-hoc tests (p = 0.058) (Figure 6B). No differences were observed for torsional hysteresis (Figure 6C). Torsional NZ had a significant sex effect on 2-way ANOVA, but a corresponding sex effect was not seen in torsional NZ stiffness (Figure 6D–E).
Figure 6. IVDs exhibited sex differences in torsional biomechanical properties six weeks after 3x annular puncture injury.
(A) Torsional stiffness did not differ between sham, 1x, and 3x six weeks after injury, but torsional stiffness was significantly greater in male IVDs than female IVDs after 3x injury (p < 0.05). (B) Torque range exhibited a similar pattern to torsional stiffness, but the sex difference after 3x injury was not significant (p = 0.058). Neither torsional hysteresis (C), torsional neutral zone length (D), nor torsional neutral zone stiffness (E) differed between groups six weeks after annular puncture. (n = 5–8, p values for 2-way ANOVA effects shown below each graph).
Annular puncture changed lumbar IVDs histologically
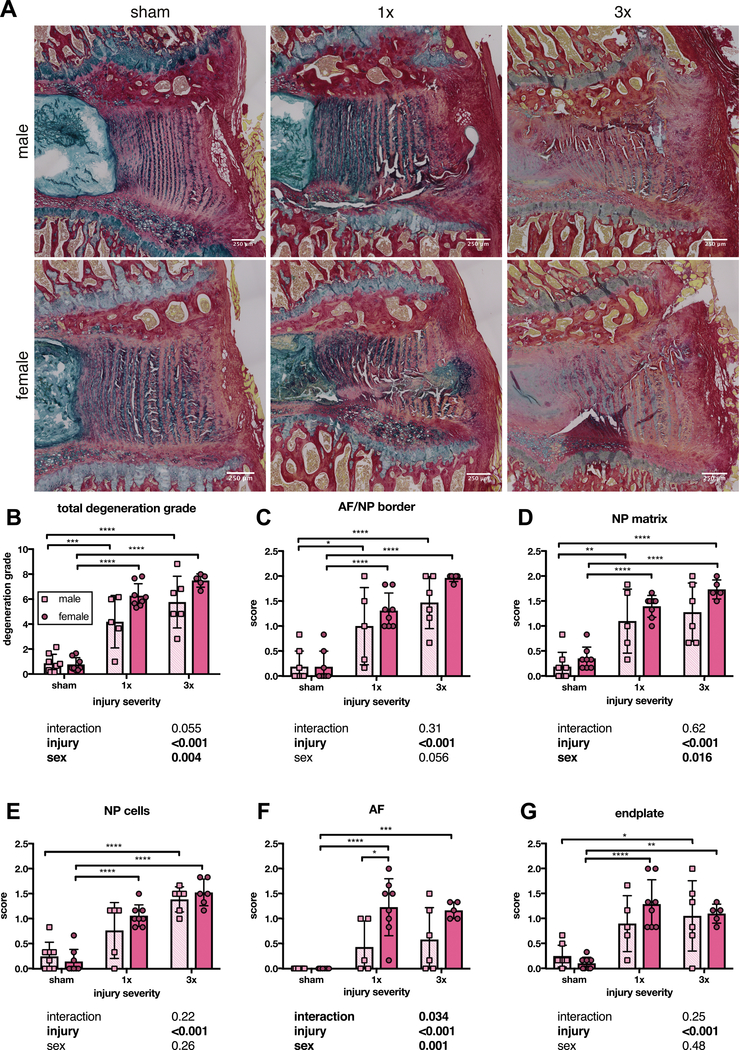
Structural histology showed disruption of annular fibers at six weeks for 1x and 3x, and a qualitative glycoaminoglycan reduction after 3x injury (Figure 7A). Total degeneration grade had sex and injury effects on 2-way ANOVA (Figure 7B). After post-hoc tests, 1x and 3x had significantly greater degeneration grade compared to sham and no sex differences were seen (Figure 7B).
Figure 7. Both 1x and 3x annular puncture significantly increased degeneration grade in male and female IVDs compared to sham at six weeks after injury.
(A) Representative 5 um PR/AB stained paraffin sections. (B) Total degeneration score was significantly increased across both 1x and 3x, and did not differ between male and female IVDs or between 1x and 3x. Scoring for the integrity of the AF/NP border (C) and NP matrix (D) exhibited the same pattern as the total degeneration score. (E) NP cellularity scoring differed between sham and 3x injury in both male and female IVDs, but only female IVDs had a significantly increased NP cellularity score compared to sham after 1x injury. (F) Scoring for AF integrity was significantly greater in female IVDs after both 1x and 3x injury compared to sham, but injured male IVDs did not significantly differ from sham. Female IVDs scored higher for AF integrity grading than males after 1x injury. (G) Scoring for endplate quality was significantly greater in female IVDs after both 1x and 3x injury compared to sham, but endplate quality scores in male IVDs only differed from sham after 3x injury. (n = 5–8, p values for 2-way ANOVA effects shown below each graph).
AF/NP border and NP matrix scoring showed an injury effect and 1x and 3x scored significantly higher than sham (Figure 7C–D). NP matrix was affected by injury and sex (Figure 7E). Scoring for AF quality had sex, injury, and interaction effects (Figure 7F). Female IVDs scored significantly higher after 1x and 3x injury than sham, and scored significantly higher than male IVDs for 1x (Figure 7F). Endplate scoring showed an injury effect, and female 1x and 3x scored significantly greater than sham, but only male 3x scored higher than sham (Figure 7G). Overall, scoring for individual degeneration criteria are consistent with total degeneration score, although sex effects are limited to AF and NP matrix scoring.
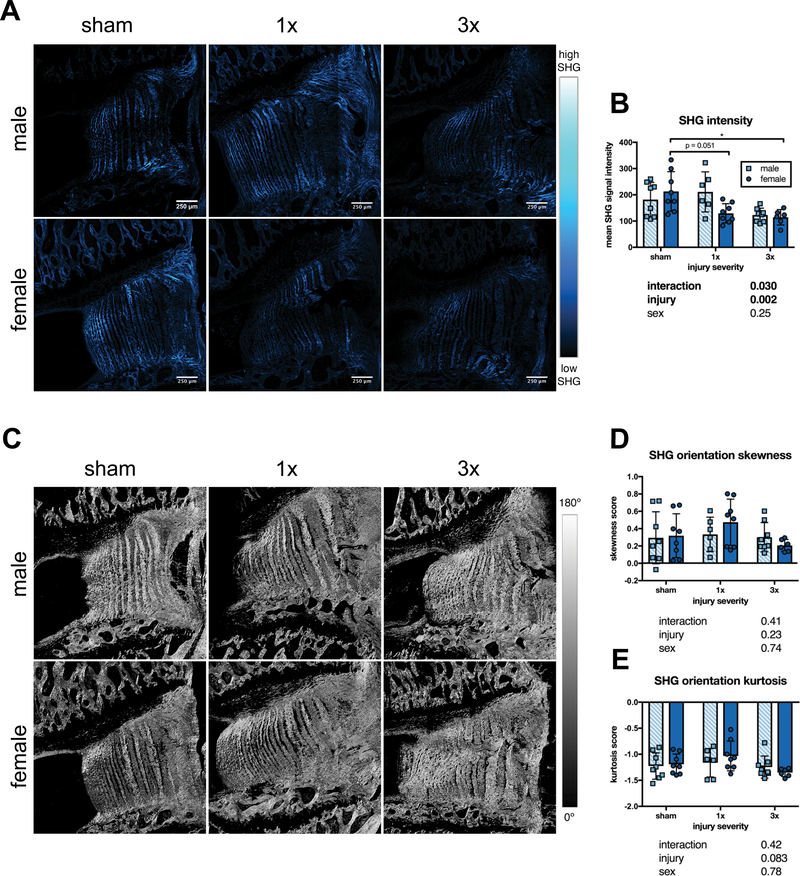
AF collagen microstructure was assessed using SHG signal intensity (Figure 8A). There was a significant injury effect on SHG intensity on 2-way ANOVA, however, after post-hoc tests, only female IVDs had significantly reduced SHG intensity from sham after both injuries (Figure 8B). Collagen organization contribution to SHG intensity was assessed by measuring collagen fibril orientations (Figure 8C). There were no injury or sex effects in either skewness or kurtosis of outer AF fibril orientation histograms, indicating that collagen disorganization was similar between groups (Figure 8D–E).
Figure 8. SHG signal intensity was reduced after injury only in female IVDs.
(A) Representative SHG images. (B) SHG signal mean intensity was reduced in both 1x and 3x in female, but not male IVDs. (C) Representative images of fibril orientation distribution generated using Orientation J. (D) Skewness of fibril orientation histograms did not differ between groups. (E) Kurtosis of orientation histograms did not differ between groups (n=6–8, * p < 0.05, p values for 2-way ANOVA effects shown below each graph).
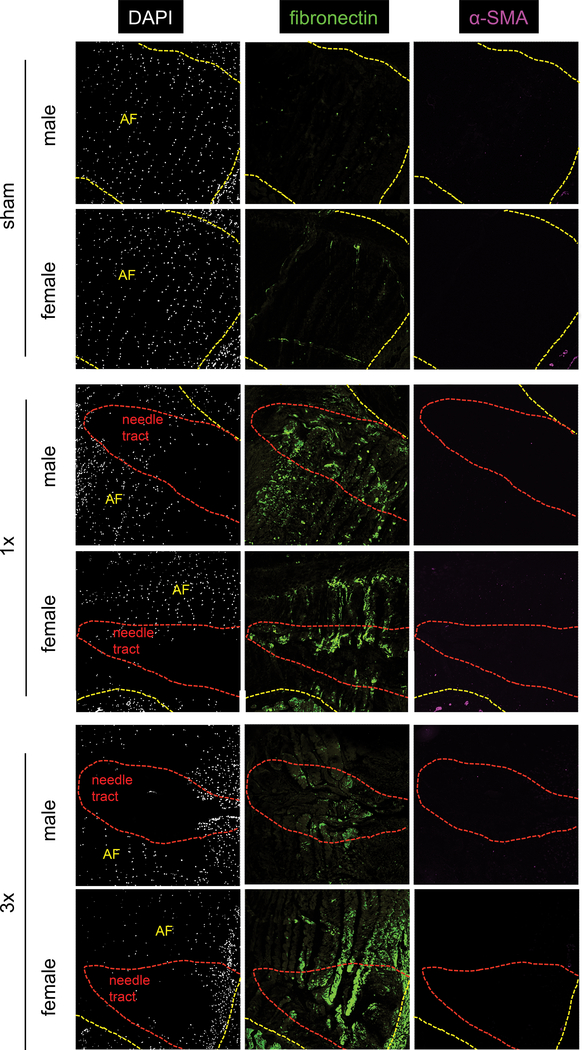
Immunofluorescence for fibrosis markers fibronectin and α-SMA and nuclear stain DAPI were qualitatively assessed. Sham IVDs had organized AF cells, but DAPI staining showed loss of cellularity in the needle tract after annular puncture (Figure 9). Fibronectin was minimally expressed in sham IVDs, but fibronectin increased in 1x and 3x, particularly within the needle tract (Figure 9). α-SMA expression was not seen in sham, 1x, or 3x AF (Figure 9). No sex differences in fibronectin or α-SMA expression were observed.
Figure 9. An increase in fibronectin and loss of cellularity was seen in the needle tract in both males and females after both 1x and 3x injury.
Cellularity was determined by DAPI nuclear staining (white). Fibronectin expression (green) was most pronounced in the needle tract (red outline). No change in alpha SMA expression (magenta) in the AF was seen with injury. AF is outlined in yellow to distinguish from vertebral bone and surrounding tissue.
Discussion
This study investigated sex differences in IVD injury responses by applying 1x and 3x annular puncture with TNFα injection to three lumbar IVDs in a rat IVD degeneration model. We hypothesized there would be sex-specific responses to IVD injury since sex differences are seen in LBP (10, 11) and degeneration-related change in spinal biomechanics (33). This rodent model determined if sex differences existed within injured IVDs to distinguish sex effects from gender/culture effects. The most important finding of this study is that sex differences exist in IVD degeneration and healing response following injury. Most biomechanical changes were restored at six weeks, consistent with increased fibronectin in the needle tract indicating fibrotic healing. Reduced SHG intensity after injury in outer AF in females IVDs, combined with larger torsional stiffness, torque range, and fast-response creep stiffness in male IVDs supports the concept that males heal with improved function and collagen quality.
Sex differences in outer AF ultimate tensile stress are seen in human IVD (42). Outer AF sex differences may also reflect distinct immune responses to injury, as this is likely a site for immune cell infiltration. Since AF integrity is strongly associated with LBP (43, 44), we infer that sex differences in IVD degeneration and healing responses are a potential source for increased pain prevalence in females. However, these biomechanical and structural changes were relatively mild compared to IVD height loss and degeneration.
IVD height loss and increased degeneration grade were similar for males and females after annular puncture, similar to prior studies that did not stratify by sex (25, 41, 45, 46). IVD height loss is a parameter shown to be strongly associated with IVD degeneration and LBP in human patients (47). LBP is multifactorial, involving spinal and neural components. Prior work has shown sex differences in LBP (10, 11) and in the neural “pain pathway” (12, 13, 15, 16, 48). Since males and females had similar IVD height loss, we expect sex-specific interactions between spinal and nervous systems are also likely to play a role in increased LBP prevalence in females.
Biomechanical properties were restored six weeks after injury, as there were no significant differences in any axial, creep, or torsional biomechanical property between sham, 1x, and 3x, suggesting possible collagen remodeling. Biomechanical restoration was surprising and contrasts many acute IVD injury studies (49–51). Large biomechanical changes are known to occur following ex vivo IVD injury and are dependent on puncture injury size (49, 51). Martin et al. showed that mouse caudal IVDs did not restore biomechanical properties several weeks after in vivo injury (50). We believe improved mechanical performance following injury in this study is due to differences between the loading environments of lumbar and caudal IVDs. Additionally, differences in species, anatomy (36), proximity to lymph nodes and other immune tissues (54) may also account for improved biomechanical properties in this study. However, ex vivo biomechanical testing of the IVD is not a perfect reflection of the in vivo condition, as adjacent structures have been removed, and testing sequence may influence biomechanical parameters (52, 53). Since IVD height loss and degeneration remained, we conclude that degeneration dominated any healing response.
Sex effects were seen in axial, creep, and torsional biomechanical parameters, and outer AF degeneration and structure following injury. These sex differences demonstrate that male and female IVDs cannot be used interchangeably in biomechanical studies. This is an important consideration in study design, yet we note that the majority of properties were similar across sex and these sex differences were smaller effect size than IVD injury effects.
Injury caused a significant reduction in SHG intensity in the outer AF only in female IVDs. SHG intensity is reflective of collagen organization (55), collagen density (56), fibril diameter (57), and molecular damage (58). Collagen organization, measured by fibril orientation, did not differ across sex or injury groups, suggesting the reduced SHG intensity in females is likely not due to disorganized collagen fibrils. The lack of difference in SHG intensity after injury in the males may have been due to an increase in collagen density or fibril diameter, further suggestive of increased fibrotic healing. Greater fibrosis in males has been found across other tissues, including liver (59, 60), kidney (61), heart (62–64), and lungs (65–67), so a similar effect may occur within IVD. However, no sex differences in immunofluorescence for fibronectin and α-SMA were found. Fibronectin increased after 1x and 3x injury across sexes, indicating a fibrotic response to IVD degeneration (68–70) and suggesting active tissue remodeling (71). α-SMA expression did not differ across groups. Although α-SMA is classically considered a fibrosis marker (72, 73), it has been found to be an inconsistent marker of fibrogenic activity (74, 75). Taken together, SHG and immunofluorescence results support the concept that sex differences may be more from collagen density and quality than fibrillar organization.
Some limitations of this study warrant discussion. This study prioritized chronic changes in IVDs at a six week time point. Six weeks was sufficient to induce chronic, stable IVD degeneration in prior studies (25, 45), and long-term degeneration was a key investigation parameter. Future time course investigations, and mechanistic studies to determine if sex differences in biomechanical properties and collagen quality are hormonally mediated are warranted.
In conclusion, this work identified sex differences in IVD degeneration and healing responses after IVD injury that may be an important contributing factor to LBP. This work demonstrates the need to consider sex effects and including both males and females in preclinical studies of spinal pathology.
Supplementary Material
Acknowledgements
We gratefully acknowledge Olivia M. Torre for assistance with biomechanical protocols and Kristen Howell for assistance with immunofluorescence staining.
The manuscript submitted does not contain information about medical device(s)/drug(s).
NIH (R01AR064157, F30AT010088P, and T32GM007280) funds were received in support of this work.
Footnotes
No relevant financial activities outside the submitted work.
References
- 1.Murray CJL et al. , The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 310, 591–608 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos T et al. , Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 380, 2163–2196 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palazzo C, Ravaud J-F, Papelard A, Ravaud P, Poiraudeau S, The burden of musculoskeletal conditions. PLoS ONE. 9, e90633 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubin DI, Epidemiology and risk factors for spine pain. Neurol Clin. 25, 353–371 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Freburger JK et al. , The rising prevalence of chronic low back pain. Arch Intern Med. 169, 251–258 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartvigsen J et al. , What low back pain is and why we need to pay attention. The Lancet. 391, 2356–2367 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Madigan L, Vaccaro AR, Spector LR, Milam RA, Management of symptomatic lumbar degenerative disk disease. J Am Acad Orthop Surg. 17, 102–111 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Foster NE et al. , Prevention and treatment of low back pain: evidence, challenges, and promising directions. The Lancet. 391, 2368–2383 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Tsang A et al. , Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain. 9, 883–891 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL, Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 10, 447–485 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gautschi OP et al. , Sex differences in subjective and objective measures of pain, functional impairment, and health-related quality of life in patients with lumbar degenerative disc disease. Pain. 157, 1065–1071 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Mogil JS, Bailey AL, Sex and gender differences in pain and analgesia. Prog Brain Res. 186, 141–157 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Sorge RE, Totsch SK, Sex differences in pain. J Neurosci Res. 95, 1271–1281 (2017). [DOI] [PubMed] [Google Scholar]
- 14.LaCroix-Fralish ML, Rutkowski MD, Weinstein JN, Mogil JS, Deleo JA, The magnitude of mechanical allodynia in a rodent model of lumbar radiculopathy is dependent on strain and sex. Spine. 30, 1821–1827 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Sorge RE et al. , Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 18, 1081–1083 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McEwen BS, Milner TA, Understanding the broad influence of sex hormones and sex differences in the brain. J Neurosci Res. 95, 24–39 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luoma K et al. , Low back pain in relation to lumbar disc degeneration. Spine. 25, 487–492 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Luoma K, Vehmas T, Kerttula L, Grönblad M, Rinne E, Chronic low back pain in relation to Modic changes, bony endplate lesions, and disc degeneration in a prospective MRI study. Eur Spine J. 25, 2873–2881 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Livshits G et al. , Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: the UK Twin Spine Study. Ann Rheum Dis. 70, 1740–1745 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samartzis D et al. , A population-based study of juvenile disc degeneration and its association with overweight and obesity, low back pain, and diminished functional status. J Bone Joint Surg Am. 93, 662–670 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Adams MA, Biomechanics of back pain. Acupunct Med. 22, 178–188 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Mosley GE, Evashwick-Rogler TW, Lai A, Iatridis JC, Looking beyond the intervertebral disc: the need for behavioral assays in models of discogenic pain. Ann N Y Acad Sci. 1409, 51–66 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin L, Balian G, Li XJ, Animal models for disc degeneration-an update. Histol Histopathol. 33, 543–554 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh K, Masuda K, An HS, Animal models for human disc degeneration. Spine J. 5, 267S–279S (2005). [DOI] [PubMed] [Google Scholar]
- 25.Lai A et al. , Annular puncture with tumor necrosis factor-alpha injection enhances painful behavior with disc degeneration in vivo. Spine J. 16, 420–431 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ulrich JA, Liebenberg EC, Thuillier DU, Lotz JC, ISSLS prize winner: repeated disc injury causes persistent inflammation. Spine. 32, 2812–2819 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Bailey JF, Sparrey CJ, Been E, Kramer PA, Morphological and postural sexual dimorphism of the lumbar spine facilitates greater lordosis in females. J Anat. 229, 82–91 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hay O et al. , The lumbar lordosis in males and females, revisited. PLoS ONE. 10, e0133685 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalichman L, Li L, Hunter DJ, Been E, Association between computed tomography-evaluated lumbar lordosis and features of spinal degeneration, evaluated in supine position. Spine J. 11, 308–315 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yukawa Y et al. , Normative data for parameters of sagittal spinal alignment in healthy subjects: an analysis of gender specific differences and changes with aging in 626 asymptomatic individuals. Eur Spine J. 27, 1–7 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Merrill RK et al. , Differences in fundamental sagittal pelvic parameters based on age, sex, and race. Clinical spine surgery : a Spine publication (2017), doi: 10.1097/BSD.0000000000000555. [DOI] [PubMed] [Google Scholar]
- 32.Asai Y et al. , Sagittal spino-pelvic alignment in adults: The Wakayama Spine Study. PLoS ONE. 12, e0178697 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujiwara A et al. , The effect of disc degeneration and facet joint osteoarthritis on the segmental flexibility of the lumbar spine. Spine. 25, 3036–3044 (2000). [DOI] [PubMed] [Google Scholar]
- 34.Muriuki MG et al. , Effects of motion segment level, Pfirrmann intervertebral disc degeneration grade and gender on lumbar spine kinematics. J Orthop Res. 34, 1389–1398 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Gruber HE, Gordon B, Williams C, Norton HJ, Hanley EN, Vertebral endplate and disc changes in the aging sand rat lumbar spine: cross-sectional analyses of a large male and female population. Spine. 32, 2529–2536 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Elliott DM, Sarver JJ, Young Investigator Award Winner: Validation of the Mouse and Rat Disc as Mechanical Models of the Human Lumbar Disc. Spine. 29, 713–722 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Jaumard NV et al. , Relevant anatomic and morphological measurements of the rat spine: considerations for rodent models of human spine trauma. Spine. 40, E1084–92 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Battié MC, Videman T, Levalahti E, Gill K, Kaprio J, Heritability of low back pain and the role of disc degeneration. Pain. 131, 272–280 (2007). [DOI] [PubMed] [Google Scholar]
- 39.O’Connell GD, Jacobs NT, Sen S, Vresilovic EJ, Elliott DM, Axial creep loading and unloaded recovery of the human intervertebral disc and the effect of degeneration. J Mech Behav Biomed Mater. 4, 933–942 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evashwick-Rogler TW et al. , Inhibiting tumor necrosis factor-alpha at time of induced intervertebral disc injury limits long-term pain and degeneration in a rat model. JOR Spine. 1 (2018), doi: 10.1002/jsp2.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masuda K et al. , A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine. 30, 5–14 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Skrzypiec D, Tarala M, Pollintine P, Dolan P, Adams MA, When are intervertebral discs stronger than their adjacent vertebrae? Spine. 32, 2455–2461 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Guterl CC et al. , Challenges and strategies in the repair of ruptured annulus fibrosus. Eur Cell Mater. 25, 1–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adams MA, Dolan P, Intervertebral disc degeneration: evidence for two distinct phenotypes. J Anat. 221, 497–506 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lai A et al. , Assessment of functional and behavioral changes sensitive to painful disc degeneration. J Orthop Res. 33, 755–764 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Millecamps M, Stone LS, Delayed onset of persistent discogenic axial and radiating pain after a single-level lumbar intervertebral disc injury in mice. Pain. 159, 1843–1855 (2018). [DOI] [PubMed] [Google Scholar]
- 47.de Schepper EIT et al. , The association between lumbar disc degeneration and low back pain: the influence of age, gender, and individual radiographic features. Spine. 35, 531–536 (2010). [DOI] [PubMed] [Google Scholar]
- 48.Rosen S, Ham B, Mogil JS, Sex differences in neuroimmunity and pain. J Neurosci Res. 95, 500–508 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Elliott DM et al. , The effect of relative needle diameter in puncture and sham injection animal models of degeneration. Spine. 33, 588–596 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Martin JT et al. , Needle puncture injury causes acute and long-term mechanical deficiency in a mouse model of intervertebral disc degeneration. J Orthop Res. 31, 1276–1282 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michalek AJ, Funabashi KL, Iatridis JC, Needle puncture injury of the rat intervertebral disc affects torsional and compressive biomechanics differently. Eur Spine J. 19, 2110–2116 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amin DB et al. , The effect of six degree of freedom loading sequence on the in-vitro compressive properties of human lumbar spine segments. J Biomech. 49, 3407–3414 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Costi JJ, Stokes IA, Gardner-Morse MG, Iatridis JC, Frequency-dependent behavior of the intervertebral disc in response to each of six degree of freedom dynamic loading: solid phase and fluid phase contributions. Spine. 33, 1731–1738 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tilney NL, Patterns of lymphatic drainage in the adult laboratory rat. J Anat. 109, 369–383 (1971). [PMC free article] [PubMed] [Google Scholar]
- 55.Reiser KM et al. , Quantitative analysis of structural disorder in intervertebral disks using second harmonic generation imaging: comparison with morphometric analysis. J Biomed Opt. 12, 064019 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Raub CB, Putnam AJ, Tromberg BJ, George SC, Predicting bulk mechanical properties of cellularized collagen gels using multiphoton microscopy. Acta Biomater. 6, 4657–4665 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bancelin S et al. , Determination of collagen fibril size via absolute measurements of second-harmonic generation signals. Nat Commun. 5, 4920 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Hwang J et al. , Molecular assessment of collagen denaturation in decellularized tissues using a collagen hybridizing peptide. Acta Biomater. 53, 268–278 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poynard T, Bedossa P, Opolon P, Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. The Lancet. 349, 825–832 (1997). [DOI] [PubMed] [Google Scholar]
- 60.Yasuda M, Shimizu I, Shiba M, Ito S, Suppressive effects of estradiol on dimethylnitrosamine-induced fibrosis of the liver in rats. Hepatology. 29, 719–727 (1999). [DOI] [PubMed] [Google Scholar]
- 61.Silbiger SR, Neugarten J, The impact of gender on the progression of chronic renal disease. Am J Kidney Dis. 25, 515–533 (1995). [DOI] [PubMed] [Google Scholar]
- 62.Kararigas G et al. , Sex-dependent regulation of fibrosis and inflammation in human left ventricular remodelling under pressure overload. Eur J Heart Fail. 16, 1160–1167 (2014). [DOI] [PubMed] [Google Scholar]
- 63.Montalvo C et al. , Androgens contribute to sex differences in myocardial remodeling under pressure overload by a mechanism involving TGF-β. PLoS ONE. 7, e35635 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coronado MJ et al. , Testosterone and interleukin-1β increase cardiac remodeling during coxsackievirus B3 myocarditis via serpin A 3n. Am J Physiol Heart Circ Physiol. 302, H1726–36 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carrington CB, Gaensler EA, Coutu RE, FitzGerald MX, Gupta RG, Natural history and treated course of usual and desquamative interstitial pneumonia. N Engl J Med. 298, 801–809 (1978). [DOI] [PubMed] [Google Scholar]
- 66.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G, Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 174, 810–816 (2006). [DOI] [PubMed] [Google Scholar]
- 67.Turner-Warwick M, Burrows B, Johnson A, Cryptogenic fibrosing alveolitis: clinical features and their influence on survival. Thorax. 35, 171–180 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oegema TR, Johnson SL, Aguiar DJ, Ogilvie JW, Fibronectin and its fragments increase with degeneration in the human intervertebral disc. Spine. 25, 2742–2747 (2000). [DOI] [PubMed] [Google Scholar]
- 69.Nerlich AG, Bachmeier BE, Boos N, Expression of fibronectin and TGF-beta1 mRNA and protein suggest altered regulation of extracellular matrix in degenerated disc tissue. Eur Spine J. 14, 17–26 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yee A et al. , Fibrotic-like changes in degenerate human intervertebral discs revealed by quantitative proteomic analysis. Osteoarthr Cartil. 24, 503–513 (2016). [DOI] [PubMed] [Google Scholar]
- 71.Betz P et al. , Immunohistochemical localization of fibronectin as a tool for the age determination of human skin wounds. Int J Legal Med. 105, 21–26 (1992). [DOI] [PubMed] [Google Scholar]
- 72.Yamaoka K, Nouchi T, Marumo F, Sato C, ?-Smooth-muscle actin expression in normal and fibrotic human livers. Dig Dis Sci. 38, 1473–1479 (1993). [DOI] [PubMed] [Google Scholar]
- 73.Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G, Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 122, 103–111 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao W, Wang X, Sun K-H, Zhou L, α-smooth muscle actin is not a marker of fibrogenic cell activity in skeletal muscle fibrosis. PLoS ONE. 13, e0191031 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun K-H, Chang Y, Reed NI, Sheppard D, α-Smooth muscle actin is an inconsistent marker of fibroblasts responsible for force-dependent TGFβ activation or collagen production across multiple models of organ fibrosis. Am J Physiol Lung Cell Mol Physiol. 310, L824–36 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.