Abstract
Despite recent advances in the treatment of cancer, pancreatic ductal adenocarcinoma (PDAC) still retains the worst survival rate of common malignancies. Late diagnosis and lack of curative therapeutic options are the most pressing clinical problems for this disease. Therefore, there is a need for patient models and biomarkers that can be applied in the clinic to identify the most effective therapy for a patient. Pancreatic ductal organoids are ex-vivo models of PDAC that can be established from very small biopsies, enabling the study of localized, advanced and metastatic patients. Organoids models have been applied to pancreatic cancer research and offer a promising platform for precision medicine approaches.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) remains one of the deadliest and most aggressive cancer types, with a five-year survival rate of less than 8% [1]. The poor outcomes of PDAC patients owe, in part, to the fact that the majority of patients (>70%) are diagnosed with advanced and metastatic disease, and are ineligible for surgical resection, the only potentially curative treatment [2, 3]. Even for patients diagnosed with localized tumors who are eligible for surgery, many patients’ disease will recur after surgical intervention [4]. Most surgical patients are treated with systemic neoadjuvant or adjuvant cytotoxic chemotherapy [5–7*]. For those patients with locally advanced or metastatic disease, the standard-of-care treatments are the combination chemotherapy regimens Gemcitabine/nab-Paclitaxel [8] or FOLFIRINOX (5-Fluorouracil, Leucovorin, Irinotecan, Oxaliplatin) [9]. These cytotoxic regiments unfortunately do not cure the vast majority of advanced patients, and the median overall survival for these patients is less than one year [8, 9]. In addition, prolonged systemic treatment of chemotherapy is associated with toxicity in patients, leading to challenges in maintaining patients on these regimens. Furthermore, many patients have chemo-refractory disease, and there is currently no personalized approach for treatment selection for such patients.
As the majority of PDAC patients do not present clinical symptoms until the cancer has advanced to the locally advanced or metastatic stage, there is a pressing need for clinically actionable, specific and sensitive early detection biomarkers for PDAC. Despite recent advances [10], CA19-9 remains one of the best biomarkers for assessing PDAC progression, but is not specific enough to be used for early detection [11]. One challenge for developing novel early detection strategies is the lack of normal, and pre-malignant models to identify cancer- specificity biomarkers.
Current precision medicine approaches to treating PDAC apply to a very limited subset of patients. For instance, while PDAC patients with mismatch repair deficiencies have been shown to respond to immunotherapy, only 1% of PDAC patients harbor alterations in these pathways [12, 13]. Similarly, patients with BRCA2 and KRASG12C mutations have been found to benefit from targeted therapies, but these patients only constitute a few percent of all PDAC patients, and overall the genetic makeup of PDAC lacks actionable driver mutation [14, 15]. This highlights the need for both novel effective treatments for the majority of pancreatic cancer patients as well as precision medicine approaches to stratify each patient into the most effective treatment regime.
Patient-derived organoid models
Pancreatic cancer research has benefited from the availability of genetically engineered mouse models, monolayer cell lines, conditionally reprogrammed cells, patient derived xenografts, and more recently, three dimensional ex vivo culture systems (see reviews [16–18]). These 3D primary cultures are termed “organoids” in reference to the ability of such cultures to maintain cell types and architecture resembling the organ from which they were derived as well as their ability to regenerate tissue from that organoid when transplanted orthotopically [19]. While organoid culture systems were initially described to study healthy, non-transformed tissue, the technology has subsequently been applied to isolate and study tumors, including models of PDAC. For instance, the Clevers and Tuveson laboratories have utilized Wnt-ligand-rich conditions to stimulate indefinite propagation of pancreas normal and cancer organoids [19, 20**]. In contrast, the Muthuswamy and Skala laboratories propagate PDAC-derived organoids in medium that lacks Wnt ligands [21, 22]. More recently, the Sato laboratory identified Wnt-dependent and independent subpopulations of PDAC organoids highlighting the need for continuing refinements to organoid culture methods [23**]. Organoid culture methodology and protocols are readily available, enabling the research community to adopt the technology [24, 25].
Organoid models can be generated from surgically resected tumors as well as from the limited material present in small biopsies, such as fine needle aspirates [19, 20, 26]. In a large cohort study, the success rate of isolating and propagating PDAC organoids models was fairly similar (>70%) between resected tumor specimens and fine- needle biopsies, allowing organoid models to be generated from all stages of PDAC [20, 26]. These advanced culturing methods have enabled researchers to study cancer patients with localized disease as well as metastatic disease for many gastrointestinal tumors [20, 26–28]. The study of organoid cohorts representing a spectrum of tumor stages has led to important disease progression findings such as the identification of enhancer programs that promote metastasis [29].
Genomics and transcriptomics of PDAC organoids
Studies of gastrointestinal organoids has not revealed the acquisition of new genomic alterations following extensive passaging in culture [30, 31]. For cancer patients, organoids faithfully capture the genomic alterations present in the tumors from which they were derived. Recently, multiple groups have performed targeted exome and whole genome sequencing to demonstrate good genomic concordance between primary PDAC tumors and their derived models [20, 32, 33]. While these studies generally demonstrate recapitulation of small nucleotide variants and structural variations in the organoids, thorough clonal analysis using single-cell or clonal organoid sequencing approaches remains unexplored for PDAC patients. Such clonal analysis performed on colorectal patient models yielded insight into organoid culture heterogeneity and its association with significant phenotypic consequences [34*]. Importantly, established organoid models of pancreatic cancer are predominantly composed of neoplastic cells enabling scientists to study low frequency nucleotide variants and copy number alterations that would be difficult to discern in primary tumor tissue with low neoplastic cellularity.
Molecular subtyping using transcriptome analyses of patient tumors has been described by multiple collaborative groups, and two major subtypes have been consistently identified [35–37]. One subtype has been described as a Basal-like, Squamous or Quasi-mesenchymal subtype which identifies PDAC patients with poor prognosis and is characterized by expression of TP63 and other basal markers. In contrast, a second Classical or Pancreatic Progenitor subtype has been described, that is characterized by expression of ductal differentiation markers such as GATA6 and identifies patients with a better prognosis [35–40]. Reassuringly, these subtypes were identified in independent cohorts of patient-derived organoids indicating that these transcriptional programs are maintained in ex-vivo cultures even in a Wnt-ligand- and growth factor-rich milieu [20, 23]. Seino and colleagues took advantage of the high neoplastic cellularity of the organoids to define functional subtypes of PDAC and demonstrated an inverse correlation between GATA6 expression (associated with classical subtype) and strict requirement for WNT-signaling, thus postulating that GATA6 acts as a master regulator of niche-dependency [23]. With inhibitors of the Wnt O-acyltransferase Porcupine in clinical trials, this important finding highlights the need for precision approaches for patient selection when considering therapeutics approaches to the Wnt pathway.
Therapeutic testing of PDAC organoids
Ex-vivo cellular models of cancer, such as monolayer cell lines, are commonly used to test therapeutic approaches. However, translation of these studies into clinical benefit has been challenging [41*]. Patient-derived organoid cultures are an attractive model for therapeutic studies. As discussed above, organoids can be isolated from patients at all stages of pancreas cancer progression with as little starting tissue as the limited tissue available in a fine needle tumor biopsy. Secondly, organoids offer a pure neoplastic population of cells enabling study of cancer-intrinsic sensitivities and resistances. Finally, beyond the scope of basic research, organoids could potentially serve as a personalized medicine platform (Figure 1). Using a large cohort of patient-derived organoids, Tiriac and colleagues have established a robust platform for testing single agent chemotherapy and targeted agents [20]. They demonstrate, in retrospective case studies, that organoid response to therapeutic testing, termed “pharmacotyping,” parallels patient sensitivity to chemotherapy. Through correlation of the drug sensitivity profile and the transcriptome of each organoid in the cohort, the group identified transcriptomic signatures of chemo-sensitivity. These RNA signatures were predictive of clinical outcome in adjuvant- and neoadjuvant- treated cohorts of PDAC patients. Other researchers have established similar platforms to test targeted and combination therapies, and these groups also found concordance between matched patient-derived PDAC organoids and patient sensitivity to treatment [21, 33]. Similar comparisons of organoid response to patient treatment response have been conducted in other gastrointestinal malignancies such as colorectal, intestinal and liver cancers demonstrating the robustness of this approach [28, 42, 43]. High-throughput drug screening of organoids requires either the adaptation of 3D culture methods to existing automation systems or the development of new automation systems designed to work with 3D cultures, but has the potential to discover transformative treatment strategies [44]. Clonal heterogeneity in pancreas cancer organoids remains to be explored and could be an underling factor leading to chemo-refractory disease in patients. In colorectal cancer organoids, Roerink and colleagues have shown that clonal organoid cultures derived from single cells from a patient tumor can display heterogeneous responses to drug treatment [34]. In repeat biopsy-derived organoids taken over multiple years in a metastatic PDAC patient, the Tuveson laboratory has shown increased organoid resistance to chemotherapy coinciding with treatment refractory disease [20]. which could be due in part to clonal selection. These important finding should be a focus of future studies of pancreas cancer organoids, as they may affect how clinical testing of organoids is conducted in the future.
Figure 1:
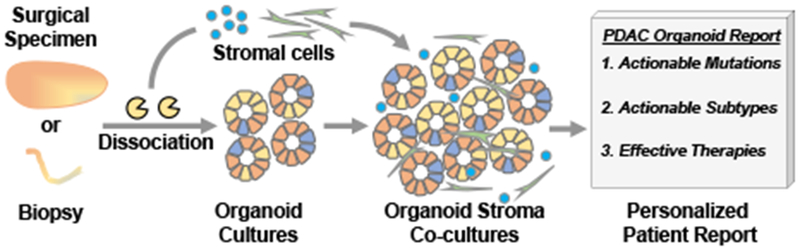
Patient-derived organoids and stromal cells, such as immune cells and cancer associated fibroblasts, can be isolated from small and large tissue samples. Organoids cultures can recapitulate clonal heterogeneity of the neoplastic tissue. the Molecular (DNA/RNA) and phenotypic (drug testing) characterization of mono- and co-culture systems can provide actionable clinical insights for cancer patients at all stages of the disease.
Patient-derived organoid therapeutic testing platforms warrant further evaluation in both retrospective and prospective clinical trials to demonstrate efficacy and, importantly, usability in a clinical setting where patients are diagnosed late and must rapidly begin therapy. Organoid isolation, expansion and testing currently requires weeks of effort in a specialized laboratory environment. To effectively couple organoid testing to the clinical care of advanced and metastatic patients, researchers must first refine their methodologies to accelerate testing of valuable compounds. Establishing the reproducibility and sensitivity of pharmacotyping platforms will be key to success, while establishing a clear threshold of drug-sensitivity will be needed to make assay results easily translatable for patient care. Finally, the identification of biomarkers of sensitivity or resistance using organoids provides additional tools for personalized medicine that may alleviate the requirement to establish an organoid model prior to the selection of initial therapies.
Co-culture models
Pancreatic ductal adenocarcinoma is characterized by a desmoplastic reaction resulting in dense, fibrotic stroma [45]. Monocultures of cancer organoids, while useful for determining cancer-intrinsic sensitivities, miss these important stromal cues. To address this issue, Ohlund and colleagues set out to establish co-cultures of organoids and the resident fibroblasts of the pancreas, pancreatic stellate cells. They identified a minimal set of culture conditions in which the PDAC organoids and fibroblasts were mutually supportive to one another such that the co-cultures, but not mono-cultures, could survive and thrive [46**]. Using this system they identified two novel sub-populations of fibroblasts present in the PDAC microenvironment that may act in distinctive tumor-supportive and tumor-restrictive roles. In a separate study, Biffi and colleagues uncovered IL-1 and TGF-beta as PDAC-derived signals underlying fibroblast heterogeneity [47]. These results suggest novel approaches for treating PDAC by blocking tumor-supportive and promoting tumor-restraining fibroblasts. In parallel, Tsai and colleagues established a patient-matched triple co-culture system which includes PDAC organoids, cancer-associated fibroblasts and T-cells [48]. Inclusion of immune cells in the ex-vivo culture systems is a critical step to establish a platform for the study of immunotherapy in pancreas cancer. With a robust patient-matched co-culture system, researchers and clinicians will be able to assess various immunotherapy strategies [49] prior to patient administration.
Future Research and clinical outlook
Dissemination of organoid methodology has greatly stimulated pancreatic cancer research and already culminated in important findings, some described in this review, which may directly impact clinical care. Pharmacotyping is clearly an important area for clinical evaluation, and current methodology limitations will have to be overcome for pharmacotyping to benefit most patients. Biomarker discovery for treatment selection, should be prioritized, and organoids offer an ideal setting for such studies. The availability of published protocols, advanced organoid training courses, and commercially available reagents are making the organoid system approachable and accessible to the research community. Recent refinements in culturing conditions such as the elimination of animal serum from the organoid medium [50, 51], and development of defined synthetic matrices [52] are important advances that are essential for establishing reliable clinical assays. Current efforts to create an organoid repository such as the NIH/NCI supported Human Cancer Models Initiative will greatly benefit the research community by making validated models and protocols [53] easily available. Novel protocols which enable neuroendocrine differentiation of pancreatic ductal organoids could lead to advances in regenerative medicine [54, 55]. These findings are important for the many patients, including pancreatic cancer patients, who suffer from pancreatic endocrine insufficiency. As researchers have made dramatic breakthroughs using organoid models, care must be taken to translate these important findings to the clinic through rigorously conducted and controlled clinical trials.
Acknowledgements
The authors declare no conflicts of interest. We are grateful to Hans Clevers for an ongoing and productive collaboration to develop pancreatic cancer organoids, and to Mona S. Spector and Sylvia F. Boj for initial development of the murine PDA organoid methods. This work was supported by the Lustgarten Foundation. Additional support was from the Cold Spring Harbor Laboratory Association, the David Rubinstein Center for Pancreatic Cancer Research at MSKCC. D.P. is supported by the Deutsche Forschungsgemeinschaft (PL 894/1-1). H.T., D.P., and D.A.T. are supported by SWOG ITSC (5U10CA180944-04). In addition, D.A.T. was supported by NIH awards P30CA045508, R01CA190092, R01CA188134, P20CA192996, P50CA101955, U01CA168409, U10CA180944, U01CA224013, U01CA210240, and R33CA206949, D.O.D. award W81XWH-14-1-0145; a gift from the Simons Foundation (552716); the STARR Cancer Consortium (I7-A718); the V Foundation (T2016-010); the Thompson Family Foundation; Stand Up to Cancer/KWF (SU2C-AACR-PS09); the Precision Medicine Research Associates; the Sackler Foundation, the Cold Spring Harbor Laboratory and Northwell Health Affiliation; and by the Lustgarten Foundation, where D.A.T. is a distinguished scholar and Director of the Lustgarten Foundation-designated Laboratory of Pancreatic Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
special interest (*)
outstanding interest (**)
- 1.Siegel RL, Miller KD, and Jemal A, Cancer statistics, 2018. CA Cancer J Clin, 2018. 68(1): p. 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Khorana AA, et al. , Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol, 2016. 34(21): p. 2541–56. [DOI] [PubMed] [Google Scholar]
- 3.Winter JM, et al. , 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg, 2006. 10(9): p. 1199–210; discussion 1210-1. [DOI] [PubMed] [Google Scholar]
- 4.Groot VP, et al. , Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann Surg, 2017. [DOI] [PubMed] [Google Scholar]
- 5.Neoptolemos JP, et al. , Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet, 2017. 389(10073): p. 1011–1024. [DOI] [PubMed] [Google Scholar]
- 6.Sinn M, et al. , CONKO-005: Adjuvant Chemotherapy With Gemcitabine Plus Erlotinib Versus Gemcitabine Alone in Patients After R0 Resection of Pancreatic Cancer: A Multicenter Randomized Phase III Trial. J Clin Oncol, 2017. 35(29): p. 3330–3337. [DOI] [PubMed] [Google Scholar]
- 7*.Conroy T, et al. , FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med, 2018. 379(25): p. 2395–2406. [DOI] [PubMed] [Google Scholar]; Conroy et al 2018 NEJM: The authors performed an adjuvant clinical trial comparing FOLFIRNIOX to Gemcitabine mono-therapy and found a marked increase in overall survival and progression-free survival for the FOLFIRNINOX arm of the study. This important study should shift the adjuvant standard-of-care for pancreas cancer.
- 8.Von Hoff DD, et al. , Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med, 2013. 369(18): p. 1691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conroy T, et al. , FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med, 2011. 364(19): p. 1817–25. [DOI] [PubMed] [Google Scholar]
- 10.Cohen JD, et al. , Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. Proc Natl Acad Sci U S A, 2017. 114(38): p. 10202–10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Safi F, et al. , High sensitivity and specificity of CA 19-9 for pancreatic carcinoma in comparison to chronic pancreatitis. Serological and immunohistochemical findings. Pancreas, 1987. 2(4): p. 398–403. [DOI] [PubMed] [Google Scholar]
- 12.Hu ZI, et al. , Evaluating Mismatch Repair Deficiency in Pancreatic Adenocarcinoma: Challenges and Recommendations. Clin Cancer Res, 2018. 24(6): p. 1326–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le DT, et al. , Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science, 2017. 357(6349): p. 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waddell N, et al. , Whole genomes redefine the mutational landscape of pancreatic cancer. Nature, 2015. 518(7540): p. 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostrem JM, et al. , K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature, 2013. 503(7477): p. 548–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker LA, et al. , Modeling pancreatic cancer with organoids. Trends Cancer, 2016. 2(4): p. 176–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krempley BD and Yu KH, Preclinical models of pancreatic ductal adenocarcinoma. Chin Clin Oncol, 2017. 6(3): p. 25. [DOI] [PubMed] [Google Scholar]
- 18.Moreira L, et al. , Pancreas 3D Organoids: Current and Future Aspects as a Research Platform for Personalized Medicine in Pancreatic Cancer. Cell Mol Gastroenterol Hepatol, 2018. 5(3): p. 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boj SF, et al. , Organoid models of human and mouse ductal pancreatic cancer. Cell, 2015. 160(1-2): p. 324–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20**.Tiriac H, et al. , Organoid Profiling Identifies Common Responders to Chemotherapy in Pancreatic Cancer. Cancer Discov, 2018. 8(9): p. 1112–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tiriac et al 2018 Cancer Discovery: The authors generated a large cohort of patient-derived PDAC organoids and performed genomic, transcriptomic and phenotypic characterization. They demonstrated that organoids recapitulate genomic and transcriptomic features of PDAC. Importantly they provided retrospective and correlative evidence from two independent patient cohorts that organoids can predict tumor sensitivity to chemotherapy.
- 21.Huang L, et al. , Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat Med, 2015. 21(11): p. 1364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh AJ, et al. , Optical Imaging of Drug-Induced Metabolism Changes in Murine and Human Pancreatic Cancer Organoids Reveals Heterogeneous Drug Response. Pancreas, 2016. 45(6): p. 863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23**.Seino T, et al. , Human Pancreatic Tumor Organoids Reveal Loss of Stem Cell Niche Factor Dependence during Disease Progression. Cell Stem Cell, 2018. 22(3): p. 454–467 e6. [DOI] [PubMed] [Google Scholar]; Seino et al 2018 Cell Stem Cell: The authors generated a large cohort of patient-derived PDAC organoids and performed deep genomic, transcriptomic and phenotypic characterization. They demonstrated an inverse correlation between GATA6 expression and niche-dependency. They generated isogenic PDAC organoids using CRISPR gene editing to show that Wnt-independence is acquired over time by genetic and epigenetic mechanisms.
- 24.Baker LA, Tiriac H, and Tuveson DA, Generation and Culture of Human Pancreatic Ductal Adenocarcinoma Organoids from Resected Tumor Specimens. Methods Mol Biol, 2019. 1882: p. 97–115. [DOI] [PubMed] [Google Scholar]
- 25.Broutier L, et al. , Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat Protoc, 2016. 11(9): p. 1724–43. [DOI] [PubMed] [Google Scholar]
- 26.Tiriac H, et al. , Successful creation of pancreatic cancer organoids by means of EUS-guided fine-needle biopsy sampling for personalized cancer treatment. Gastrointest Endosc, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nuciforo S, et al. , Organoid Models of Human Liver Cancers Derived from Tumor Needle Biopsies. Cell Rep, 2018. 24(5): p. 1363–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weeber F, et al. , Preserved genetic diversity in organoids cultured from biopsies of human colorectal cancer-metastases. Proc Natl Acad Sci U S A, 2015. 112(43): p. 13308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roe JS, et al. , Enhancer Reprogramming Promotes Pancreatic Cancer Metastasis. Cell, 2017. 170(5): p. 875–888 e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blokzijl F, et al. , Tissue-specific mutation accumulation in human adult stem cells during life. Nature, 2016. 538(7624): p. 260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huch M, et al. , Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell, 2015. 160(1-2): p. 299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gendoo DMA, et al. , Whole genomes define concordance of matched primary, xenograft, and organoid models of pancreas cancer. PLoS Comput Biol, 2019. 15(1): p. e1006596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romero-Calvo I, et al. , Human Organoids Share Structural and Genetic Features with Primary Pancreatic Adenocarcinoma Tumors. Mol Cancer Res, 2019. 17(1): p. 70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34*.Roerink SF, et al. , Intra-tumour diversification in colorectal cancer at the single-cell level. Nature, 2018. 556(7702): p. 457–462. [DOI] [PubMed] [Google Scholar]; Roerink et al 2018 Nature: This important study demonstrates the heterogeneous nature of organoid cultures. Single colorectal cancer cells were cloned and expanded to clonal organoid cultures that show surprising molecular and phenotypic diversity. Importantly the authors showed that within a heterogeneous culture there can be very sensitive and resistant clones to a particular drug.
- 35.Bailey P, et al. , Genomic analyses identify molecular subtypes of pancreatic cancer. Nature, 2016. 531(7592): p. 47–52. [DOI] [PubMed] [Google Scholar]
- 36.Collisson EA, et al. , Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med, 2011. 17(4): p. 500–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moffitt RA, et al. , Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet, 2015. 47(10): p. 1168–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cancer Genome Atlas Research Network. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell, 2017. 32(2): p. 185–203 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aung KL, et al. , Genomics-Driven Precision Medicine for Advanced Pancreatic Cancer: Early Results from the COMPASS Trial. Clin Cancer Res, 2018. 24(6): p. 1344–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Somerville TDD, et al. , TP63-Mediated Enhancer Reprogramming Drives the Squamous Subtype of Pancreatic Ductal Adenocarcinoma. Cell Rep, 2018. 25(7): p. 1741–1755 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Ben-David U, et al. , Genetic and transcriptional evolution alters cancer cell line drug response. Nature, 2018. 560(7718): p. 325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors analyzed the genetic evolution of monolayer cancer cells grown in multiple laboratories over many passages. They found rapid genetic changes occurred in the cell lines which were accompanied by transcriptomic and phenotypic changes. This study highlights the need for ex-vivo models that provide robust, repeatable and clinically accurate observations.
- 42.Broutier L, et al. , Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med, 2017. 23(12): p. 1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan HHN, et al. , A Comprehensive Human Gastric Cancer Organoid Biobank Captures Tumor Subtype Heterogeneity and Enables Therapeutic Screening. Cell Stem Cell, 2018. 23(6): p. 882–897 e11. [DOI] [PubMed] [Google Scholar]
- 44.Hou S, et al. , Advanced Development of Primary Pancreatic Organoid Tumor Models for High-Throughput Phenotypic Drug Screening. SLAS Discov, 2018. 23(6): p. 574–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moir JA, Mann J, and White SA, The role of pancreatic stellate cells in pancreatic cancer. Surg Oncol, 2015. 24(3): p. 232–8. [DOI] [PubMed] [Google Scholar]
- 46**.Ohlund D., et al. , Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med, 2017. 214(3): p. 579–596. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a novel co-culture system comprising both cancer organoids and cancer associated fibroblasts, the authors identified two reversible CAF subtypes. These data demonstrate that the microenvironment of PDAC is more complex than initially understood, and also highlights the power of ex-vivo organoid cultures for biological research.
- 47.Biffi G, et al. , IL-1-induced JAK/STAT signaling is antagonized by TGF-beta to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai S, et al. , Development of primary human pancreatic cancer organoids, matched stromal and immune cells and 3D tumor microenvironment models. BMC Cancer, 2018. 18(1): p. 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sahin IH, et al. , Immunotherapy in pancreatic ductal adenocarcinoma: an emerging entity? Ann Oncol,2017. 28(12): p. 2950–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mihara E, et al. , Active and water-soluble form of lipidated Wnt protein is maintained by a serum glycoprotein afamin/alpha-albumin. Elife, 2016. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janda CY, et al. , Surrogate Wnt agonists that phenocopy canonical Wnt and beta-catenin signalling. Nature, 2017. 545(7653): p. 234–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gjorevski N, et al. , Designer matrices for intestinal stem cell and organoid culture. Nature, 2016. 539(7630): p. 560–564. [DOI] [PubMed] [Google Scholar]
- 53.Clinton J and McWilliams-Koeppen P, Initiation, Expansion, and Cryopreservation of Human Primary Tissue-Derived Normal and Diseased Organoids in Embedded Three-Dimensional Culture. Curr Protoc Cell Biol, 2018: p. e66. [DOI] [PubMed] [Google Scholar]
- 54.Azzarelli R, et al. , Neurogenin3 phosphorylation controls reprogramming efficiency of pancreatic ductal organoids into endocrine cells. Sci Rep, 2018. 8(1): p. 15374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loomans CJM, et al. , Expansion of Adult Human Pancreatic Tissue Yields Organoids Harboring Progenitor Cells with Endocrine Differentiation Potential. Stem Cell Reports, 2018. 10(3): p. 712–724. [DOI] [PMC free article] [PubMed] [Google Scholar]


