Abstract
Sickle cell disease (SCD), an inherited blood disorder that primarily affects individuals of African descent, is associated with serious medical complications as well as numerous social-environmental risk factors. These social-environmental factors are linked to long-standing social inequities, such as financial hardship and racial discrimination, both of which impact cognitive and behavioral functioning in youth. Previous research on the relationship between social-environmental risk and psychological functioning has primarily relied on non-modifiable, unidimensional measures of socioeconomic status (SES), such as income and parental education, as a proxy for social-environmental risk. The current study aimed to address the limitations associated with typical SES-type measures by comparing the unique and shared association of SES and more targeted and modifiable social-environmental factors (e.g., parent and family functioning) with specific areas of cognitive and behavioral adjustment in pediatric SCD. Seventy children ages 4 to 8 years old and their parents completed measures of social-environmental risk and psychological adjustment. Exploratory factor analysis indicated parent and family functioning measures were largely independent from SES. Parent and family functioning predicted phonological processing and ADHD symptoms above and beyond SES alone. In addition, the predictive ability of social-environmental risk factors appears to vary by genotype severity for measures of social functioning and math problem solving ability. Future studies are needed to explore more specific and well-supported models of modifiable social-environmental risk and the relative impact of social-environmental risk on cognitive and behavioral functioning.
Keywords: Sickle-cell disease, socioeconomic status, neuropsychologic, family functioning
Sickle cell disease (SCD) is a complex genetic disorder associated with the potential for multiple medical complications that occurs in a population disproportionately burdened by social challenges (Barbarin & Christian, 1999; Edwards et al., 2005). SCD refers to a group of inherited blood disorders affecting approximately 1 of every 365 children of African descent born in the United States (Hassell, 2010). A number of serious medical complications can result, including cerebrovascular disease, vaso-occlusive pain, and anemia (National Heart, Lung and Blood Institute, 2014). Given the myriad medical complications associated with pediatric SCD, genotypic differences are often used as a proxy for overall biomedical risk, with high-risk genotypes (e.g., HbSS) experiencing more severe disease-related complications than lower risk genotypes (e.g., HbSC) (Saraf et al., 2014).
One of the areas of functioning particularly affected by these medical complications is cognition. Cognitive deficits have been identified across a range of domains and include impairments in overall intellectual ability as well as in specific domains such as executive functioning, attention, processing speed, and language compared to similar peers without SCD (Hijmans et al., 2010; Schatz, Finke, Kellett, & Kramer, 2002; Schatz, Puffer, Sanchez, Stancil, & Roberts, 2009). These cognitive deficits are present even in the absence of cerebral infarction, suggesting other disease-related and environmental processes play an important role (Schatz et al., 2002). Children with SCD also evidence poorer performance on measures of academic attainment (e.g., grade retention) and academic achievement, in areas such as reading, writing, and math (Berkelhammer et al., 2007; Schatz, Brown, Pascual, Hsu, & DeBaun, 2001). Lower cognitive ability and academic achievement scores among children with SCD show a small, but potentially meaningful, relationship with behavioral and social-emotional functioning, particularly for externalizing behaviors (Thompson et al., 1999).
Behavioral and social-emotional functioning is also impacted, with studies showing increased risk for internalizing problems and impaired social functioning in children with SCD compared with similar peers without SCD (Bakri, Ismail, Elsedfy, Amr, & Ibrahim, 2014; Hijmans et al., 2009). Research on externalizing behaviors is more mixed, with some studies showing evidence of increased externalizing problems for youth with SCD, while others have found no differences or even decreased externalizing problems compared to similar peers without SCD (Bakri et al., 2014; Noll, Kiska, Reiter-Purtill, Gerhardt, & Vannatta, 2010). Such findings highlight the complex relationship between pediatric SCD and behavioral outcomes as well as the need for further clarification of the nature of sickle cell related behavioral problems.
It is becoming increasingly apparent that the etiology of these cognitive and behavioral deficits in pediatric SCD is complex and multifactorial. Research has shown cognitive and behavioral outcomes in pediatric SCD are influenced by both disease-related phenomena and social-environmental factors (Burlew, Telfair, Colangelo, & Wright, 2000; King et al., 2013). Families of children with SCD must also contend with risk factors stemming from the cumulative disadvantage of social inequities (e.g. financial hardship, racial discrimination and stigmatization) and chronic disease (Hankins & Wang, 2009; Jenerette & Valrie, 2010; Robinson, Daniel, O’Hara, Szabo, & Barakat, 2014). The detrimental impact of this cumulative burden is evidenced in marked financial insecurity, with approximately 60% of children with SCD receiving public insurance (King et al., 2013; McCavit, Xuan, Zhang, Flores, & Quinn, 2013), as well as decreased employment opportunities for caregivers due to the demands of caring for a child with a chronic illness (Brandow, Brousseau, & Panepinto, 2009; Smith et al., 2002).
Many studies exploring the relative contribution of social-environmental factors on cognitive functioning in pediatric SCD have relied on traditional indicators of SES. Research has revealed links between parental education, income, and cognitive outcomes across a range of domains of functioning, including intelligence, memory, attention, and academic achievement (Oluwole Olubusola B., Noll Robert B., Winger Daniel G., Akinyanju Olu, & Novelli Enrico M., 2016; Smith et al., 2002; Tarazi, Grant, Ely, & Barakat, 2007). Research has also suggested that the influence of disease-related factors on cognitive functioning varies based on SES (Schatz, Finke, & Roberts, 2004). Although research using metrics other than SES are limited, modifiable social-environmental variables have been identified as important indicators of cognitive functioning in children with SCD. For instance, studies have identified a significant relationship between psychosocial factors, such as home environment and parental stress levels, and cognitive functioning (Drazen, Abel, Gabir, Farmer, & King, 2016; Fields et al., 2016; Tarazi et al., 2007).
Although SES has been commonly used as the primary indicator of social-environmental risk for cognitive deficits, there are a number of important limitations to consider. The use of single, broad variables as proxies for SES diminishes the multidimensional and complex nature of social-environmental risk. In addition, SES measures such as income and education level are generally static, thereby making it difficult to translate research findings into intervention. In light of these limitations, research is needed that directly measures downstream and modifiable social-environmental characteristics, such as family dynamics, parenting, and parent-child interactions. A recent study by Yarboi and colleagues (2017) addressed many of these concerns by examining the relationship between social-environmental risk, parenting, and specific cognitive and behavioral outcomes in pediatric SCD. The researchers found financial stress was a significant predictor of cognitive functioning; however, there were mixed findings regarding the relationships between parenting variables such as maternal depressive symptoms and positive parenting and cognitive outcomes. In addition, the parenting measures primarily focused on parents’ use of discipline, leaving open questions about the effects of a cognitively simulating environment (Yarboi et al., 2017). Given these varied findings and generally scant research on the topic, additional investigations are needed to better understand the complex relationships between social-environmental risk, parenting, and cognitive and behavioral outcomes.
The current study aims to compare the unique and shared association of traditional, broad measures of SES and more targeted and modifiable social-environmental factors with specific areas of cognitive and behavioral outcomes in pediatric SCD. Exploratory factor analysis was used to assess the degree of covariation between SES and other social variables and to provide a parsimonious set of constructs to relate to cognitive and behavioral outcomes. Data were collected as part of a developmental screening program that included a range of measures of social-environmental risk such as parenting, family functioning, stress, and home environment as well as standard SES measures such as parent income and education level. The developmental screening program also included cognitive and behavioral outcome measures that targeted specific domains of functioning that have been previously identified as sensitive to SCD-related disease processes (Schatz et al., 2009). We hypothesized that modifiable social-environmental factors would provide unique information above and beyond parent income and education in explaining cognitive and behavioral outcomes. Interactions of social and biomedical risk factors were also explored.
Methods
Participants
Participants were children with SCD attending routine hematological preventative care visits at a pediatric hematology/oncology outpatient clinic in the southeastern United States. Children and their parents were recruited through a developmental screening program offered to all children with SCD five to seven years of age. The inclusion criteria were a diagnosis of SCD and parental consent to use data for research purposes. Children with a previous history of overt stroke were excluded from the current study. However, as children did not receive routine MRI scans as part of their clinical care, children with a history of silent stroke were not excluded from the current sample.
Procedure
Families were approached by the principal investigator or co-principal investigator of the study, both of whom were doctoral level psychologists. A parent of all study participants completed informed consent as approved by an Institutional Review Board. Study participation occurred either before or after the child was seen by the pediatric hematologist for a health maintenance visit at the clinic site. Children who were experiencing pain or fatigue on the day of testing were rescheduled for their next clinic visit. Cognitive testing was completed one-on-one by a trained psychometrician according to standardized methods provided in the test manuals and lasted approximately 45 minutes. A primary caregiver for the children completed parent-report social-environmental and behavioral measures concurrently with the child’s cognitive testing. There were 10 missing values on cognitive scores or behavioral rating scales (out of 980 possible data points) due to children or parents failing to complete measures for a 0.7% rate of missing values. No child had more than two missing data points. Medical record review was conducted after the child testing to keep examiners blind to SCD genotype and medical history.
Social-environmental Measures
Socioeconomic Status.
Yearly household income and years of parental education were recorded in a categorical manner from parent reports for all children. Household income data were collected in brackets of $10,000 from less than $10,000 to $50,000 or more. Parental education was recorded according to years of education for parents living in the household. The higher of the two values was used for households with more than one parent. Mean parental education was then categorized according to less than high school diploma, high school diploma, some college, bachelor’s degree, or graduate degree to provide more meaningful distinctions between levels of education.
Family and Parent Functioning.
Family and parent functioning were measured through reports from a primary caregiver. A parenting questionnaire comprised of 7 items from the Warmth/Involvement subscale of the Parent Practices Scale (Strayhorn & Weidman, 1988) was used to assess frequency of positive parent-child interactions. The 5-item version of the Parenting Self-Agency Measure was administered to assess parenting self-efficacy (Dumka, Stoerzinger, Jackson, & Roosa, 1996). Chronbach’s alpha for the Warmth/Involvement subscale and the Self-Agency measure in the current sample was .64 and .60, respectively.
Parental support of academic socialization and engagement in activities supporting cognitive skill development were measured using the Home Involvement scale of the Family Involvement Questionnaire (FIQ; Fantuzzo, Tighe, & Childs, 2000). The internal consistency for this FIQ scale in the present sample was α= .84. Two parent-report measures were used to assess parent and family distress. Cohen’s Perceived Stress Scale (PSS) was used to assess subjective stress and distress of the primary caregiver (Cohen, Kamarck, & Mermelstein, 1983). The measure correlates with the extent of psychological distress, physical symptoms of stress, and elevated stress in the absence of psychopathology symptoms (Cohen & Williamson, 1987; Cohen, 1986; Cohen et al., 1983). The internal consistency for the PSS in the present sample was α = .82.
Family functioning was measured using the general functioning subscale of the McMaster Family Assessment Device (FAD; Byles, Byrne, Boyle, & Offord, 1988). The internal consistency for the FAD in the present sample was α = .85. Descriptive data for the parent report measures is shown in Table 2. Parent depression was measured using the 10-item version of the Center for Epidemiologic Studies of Depression Scale (CES-D). The internal consistency for the CES-D in the present sample was α =.62.
Table 2.
Bivariate Correlations Between Social-Environmental Variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1. Single parent household | - | |||||||
| 2. Family income | −.22 | - | ||||||
| 3. Parent education | −.15 | .53** | - | |||||
| 4. Parenting Warmth/Involvement | .08 | .21 | −.08 | - | ||||
| 5. Parenting Self Agency | .17 | .08 | .10 | .14 | - | |||
| 6. PSS | .14 | −.25* | −.16 | −.15 | −.15 | - | ||
| 7. CES | −.08 | −.18 | −.14 | −.23 | −.24* | .54** | - | |
| 8. FAD | −.01 | −.28 | −.10 | −.28* | −.21 | .31** | .21 | - |
| 9. FIQ | .08 | .31** | .18 | .29* | .10 | −.40** | −.28* | −.28* |
Note.
CES = Center for Epidemiologic Studies of Depression Scale; FAD = Family Assessment Device; FIQ = Family Involvement Questionnaire; PSS = Cohen’s Perceived Stress Scale.
p < .05,
p < .01
Parent-Report Data.
A structured interview was used to assess who the primary caregivers of the child were, history of preterm birth, other pre- and peri-natal birth complications, and history of painful episodes not requiring a hospital or emergency department visit. Questionnaires were completed on a computer using audio sound files to present both text and audio presentations of the instructions, questions, and response alternatives. An examiner was present during the questionnaire phase to address any questions by the parent.
Cognitive Measures
Language Skills.
Six tests from the Test of Language Development-Primary: Third Edition (TOLD-P:3; Newcomer & Hammill, 1998) were administered with two tests from each of three language domains. The TOLD-P:3 was selected due to its design for children in this age range, reliability at the subtest and domain level, supporting validity data, and lack of cultural bias for African-American children (Newcomer & Hammill, 1998). The semantic domain was measured via Picture Vocabulary and Oral Vocabulary which yields a Semantic Quotient. Picture Vocabulary assesses one-word receptive vocabulary by requiring the child to choose a picture that matches an orally presented word. Oral Vocabulary requires the child to provide definitions for orally presented words. A Syntactic Quotient which reflects syntactic processing was measured via Grammatical Understanding and Sentence Imitation. Grammatical Understanding requires that the child select the picture that best demonstrates the meaning of sentences that have increasingly complex syntax. Sentence Imitation requires the child to repeat back sentences with increasingly complex syntax. Phonological processing was measured via Word Discrimination and Phonemic Analysis which yields a Phonemic Quotient. Word Discrimination requires the child to assess whether two similarly sounding words are the same word or different words (e.g., pig – pig versus big – pig). Phonemic Analysis requires children to segment words into phonemes and repeat back only part of the word to the examiner. Age adjusted standardized scores were used for all tests.
Early Academic skills.
Academic/pre-academic skills were measured using the Letter-Word Identification and Applied Problems subtests of the Woodcock-Johnson Tests of Achievement, 3rd edition. Letter-Word Identification is a measure of early reading skills and requires the child to identify letters and words without having to know the meaning of a word. Applied Problems serves as a measure of quantitative knowledge and reasoning and requires children to analyze and solve relatively simple math problems. Age adjusted standardized scores were used for both subtests.
Visual-Motor Skills.
Visual–motor skills were assessed using the Beery Developmental Test of Visual-Motor Integration, 5th edition (Beery, 2004), and the Hand Movements subtest of the Kaufman Assessment Battery for Children (Kaufman & Kaufman, 1983). The Beery Developmental Test of Visual-Motor Integration requires children to imitate and copy geometric forms of increasing complexity using pencil and paper and the Hand Movement subtest requires children to copy a series of taps performed by the examiner. The age-adjusted standardized scores from these two measures were averaged to create a more concise score for visual-motor ability.
Other Attentional Skills.
The Memory for Words subtest from the Woodcock-Johnson Tests of Cognitive Abilities, 3rd edition was included as a measure of short-term word span that does not require syntactic processing (McGrew & Woodcock, 2001). This subtest requires the child to repeat lists of unrelated words in the correct sequence. Processing speed was assessed using the Decision Speed subtest of the Woodcock-Johnson Tests of Cognitive Abilities, 3rd edition, which requires children to identify the two pictures from a row of stimuli that are most conceptually similar. Age adjusted standardized scores were used for all subtests.
Behavioral Measures
Parents completed the Strengths and Difficulties Questionnaire (SDQ) (Goodman, 2001) as part of the screening procedure. The SDQ is a brief behavioral screening questionnaire designed for children aged 2-16 years. It is comprised of 25 items intended to measure five scales: Emotional Symptoms, Conduct Problems, Hyperactivity/Inattention, Peer Relationship Problems, and Pro-social Behavior. Each scale yields a raw score for the specific dimension being assessed; additionally, the scores from the first four scales can be summed to yield a Total Difficulties Score. The SDQ has demonstrated satisfactory internal consistency reliability (mean Cronbach’s α = .73), as well as cross-informant correlation (mean r = 0.34), and retest stability after 4 to 6 months (mean r = 0.62) in community samples (Goodman, 2001).
Statistical Analyses
The Statistical Package for the Social Sciences, 25th Edition (SPSS) was used to conduct all statistical analyses. Preliminary analyses were conducted to provide descriptive information on the demographic characteristics of the sample. Exploratory factor analysis (EFA) using principal component analysis was conducted to extract main factors for the social-environmental measures. A cutoff value of .5 for Measures of Sampling Adequacy (MSA) was set as the basic inclusion criteria for EFA (Yong & Pearce, 2013). Bivariate correlations were run to examine the relationship between identified factors and child cognitive and behavioral outcomes. The outcomes from these exploratory correlational analyses were used to select subsequent hierarchal linear regression models, with extracted social-environmental factors and genotype severity as univariate predictors. A total of nine hierarchal linear regression models were run to examine the unique contribution of each social-environmental factor, genotype risk (high-risk vs. low-risk) and their interaction. Genotype has been shown to be a reliable indicator of degree of risk for neurologic complications from sickle cell disease and is more strongly related to cognitive outcomes in young children than alternate measures such as anemia severity (Gill et al., 1995; Schatz et al., 2009). In the first step, extracted factors and genotype were entered as independent variables to determine their combined univariate predictive abilities. In the second step, the interaction terms between each factor and genotype were added. The alpha level was set as p < .05 for these analyses. To test the appropriateness of the regression analyses, we examined collinearity diagnostics, ran casewise diagnostics, plotted standardized residuals and normal probability plots to assess for multicollinearity, outliers, homoscedasticity, and the normality of the distribution of residuals.
Results
Particpant Characteristics
A total of 70 children between the ages of 4.90 and 8.20 years (M = 6.47, SD = 1.11, % female = 55.80) were recruited for the study. One child was tested before their 5th birthday due to a clerical error and two children were tested after their 8th birthday due to the rescheduling of appointments (see Table 1). This sample represents 70 of 80 consecutive children seen for routine preventative care visits. For the ten children not completing screenings, the parents of two children were not interested in receiving a developmental screening; four children’s parents were interested, but testing was not completed due to scheduling conflicts at multiple visits; three children had a history of overt stroke; and one child completed the developmental screening, but the parent did not consent for the data to be used for research purposes. Sixty-seven of the primary caregivers (96%) reported being the child’s mother, two primary caregivers (3%) reported being the child’s guardian/grandmothers, and one primary caregiver (1%) reporting being the child’s father. The cognitive outcomes for a subset of these children were described by Schatz and colleagues (2009), the results of which indicated that children with SCD with high neurologic risk showed deficits across multiple cognitive domains and that children with SCD-related cognitive deficits could be accurately identified with a screening algorithm.
Table 1.
Participants’ Demographic and Clinical Characteristics
| Variable | Total group (n =70) |
|---|---|
| Mean (SD) | |
| Age in years | 6.47 (1.11) |
| n (%) | |
| Ethnicity (% African American) | 70 (100) |
| Gender (% Female) | 39 (55.8) |
| Household income | |
| <$10,000 | 16 (22.9) |
| $10,000-20,000 | 23 (32.9) |
| $20,000-30,000 | 12 (17.1) |
| $30,000-40,000 | 6 (8.6) |
| >$40,000 | 13 (18.6) |
| Single parent household | 22 (31.4) |
| SCD type | |
| HbSS | 40 (55.6) |
| HbSC | 17 (24.3) |
| HbSBeta0 | 3 (.04) |
| HbSBeta+ | 7 (.10) |
| Other (HbSO Arab/HbSD-Punjab) | 3 (.04) |
| Preterm birth (%) | 16 (22.9) |
| # of past hospitalizations (%) | |
| 0 | 44 (62.9) |
| 1 | 15 (21.4) |
| >1 | 11 (15.7) |
Note.
SCD= Sickle Cell Disease
Exploratory Factor Analysis
Pearson correlations were run for all social-environmental variables (see Table 2). Principal component analysis was used to reduce the number of variables to develop a parsimonious model for analysis and interpretation and to address the inter-correlation among measures. Initial evaluation of variables indicated the single-parent household variable did not meet a basic inclusion criteria for EFA of Measures of Sampling Adequacy (MSA) values of greater than .5 (MSA = .428). As such, the principal component analysis was run excluding the single parent household variable, which revealed two factors with eigenvalues greater than 1.00. The remaining factors had eigenvalues ranging from .982-.375. Visual inspection of the scree plot also suggested a two-factor solution; as such, a two factor solution explaining 48.79% of the variance was chosen. Factor 1 accounted for 32.67% of the variance and was comprised of measures assessing parent and family functioning and included the Warmth/Involvement subscale of the Parent Practices Scale, Home Involvement scale of the FIQ, PSS, FAD, and CES-D depression scale. This factor was labeled “Parent and Family Functioning (PFF)”. Factor 2 accounted for 16.12% of variance and included parent income and education level. This factor was labeled “Socioeconomic Status (SES)”. Factor weights for each variable were multiplied by the value of each variable for each participant and summed to create summary variables for the two factors for each participant.
Hierarchal Regression Analyses
Bivariate Pearson correlations were run to examine the relationship between the previously defined two factors and child cognitive functioning and behavioral outcomes (see Table 3). Results from collinearity diagnostics, casewise diagnostics, plotted standardized residuals, and normal probability plots indicated there were no violations of the underlying assumptions for the analyses. As the social-environmental factors had a small degree of inter-correlation, we ran the main effects of the regression analyses including both social-environmental factors (as well as genotype severity) to add additional statistical control for the unique effects of each social environmental factor (see Table 4). PFF uniquely accounted for variance in the TOLD Phonemic Quotient and the SDQ Hyperactivity/Inattention scores even when statistically covarying SES. For SES, the TOLD Semantic Quotient and Syntactic Quotient scores, WJ-III Letter-Word Identification score, and SDQ Peer Relationship Problems scales were uniquely associated with SES after covarying PFF in the regression (all p’s < .05). None of the three variables (i.e., PFF, SES, genotype) were significant predictors of scores on the Applied Problems subtest.
Table 3.
Bivariate Correlations of Cognitive Measures, Parenting and Family Functioning, and Socioeconomic Status
| Cognitive measures | PFF (Factor 1) | SES (Factor 2) |
|---|---|---|
| TOLD Semantic Quotient | −.01 | .35** |
| TOLD Syntactic Quotient | −.13 | .31** |
| TOLD Phonemic Quotient | −.29* | .20 |
| DVTMI & Hand Movements | −.19 | .19 |
| WJ-III Decision Speed | −.13 | −.02 |
| WJ-III Memory for Words | −.03 | .15 |
| WJ-III Letter Word Identification | −.07 | .37** |
| WJ-III Applied Problems | −.27* | .27* |
| SDQ Emotional Symptoms | −.07 | −.03 |
| SDQ Conduct Problems | .17 | −.13 |
| SDQ Inattention/Hyperactivity | .34** | −.24* |
| SDQ Peer Relationship Problems | .26* | −.43** |
| SDQ Prosocial Behavior | −.29* | .00 |
| SDQ Total Score | .30* | −.19 |
Note.
DVTMI = Beery Developmental Test of Visual-Motor Integration; PFF = Parent and Family Functioning; SDQ = Strengths and Difficulties Questionnaire; SES= Socioeconomic Status; TOLD = Test of Language Development-Primary; WJ = Woodcock Johnson.
p < .05,
p < .01
Table 4.
Summary of Hierarchal Regression Analyses for Parent and Family Functioning, Socioeconomic Status, Genotype Risk, and Interactions
| Variable | B | t | Overall Model F | ΔR2 |
|---|---|---|---|---|
| TOLD Semantic Quotient | ||||
| Step 1 | 7.94** | .27** | ||
| HvL genotype | −8.00 | −3.46** | ||
| PFF | 1.22 | 1.06 | ||
| SES | 3.12 | 2.66* | ||
| Step 2 | 4.93** | .01 | ||
| HvL genotype | 3.91 | .29 | ||
| PFF | −.35 | −.18 | ||
| SES | 3.14 | 1.67 | ||
| HvL × PFF | .43 | 1.03 | ||
| HvL × SES | −.02 | −.02 | ||
| TOLD Syntactic Quotient | ||||
| Step 1 | 3.58* | .14* | ||
| HvL genotype | −6.57 | −1.81 | ||
| PFF | −.47 | −.26 | ||
| SES | 3.84 | 2.08* | ||
| Step 2 | 2.84** | .04* | ||
| HvL genotype | −40.01 | −1.89 | ||
| PFF | .49 | .16 | ||
| SES | .27 | .09 | ||
| HvL × PFF | −.26 | −.40 | ||
| HvL × SES | 1.81 | 1.59 | ||
| TOLD Phonemic Quotient | ||||
| Step 1 | 4.39** | .17** | ||
| HvL genotype | −7.59 | −2.30* | ||
| PFF | − 3.38 | −2.10* | ||
| SES | .96 | .57 | ||
| Step 2 | 3.11* | .03 | ||
| HvL genotype | −29.62 | −1.54 | ||
| PFF | −.16 | −.06 | ||
| SES | 1.26 | .47 | ||
| HvL × PFF | −.89 | −1.50 | ||
| HvL × SES | −.13 | −.13 | ||
| WJ-III Letter-Word Identification | ||||
| Step 1 | 3.69* | .14* | ||
| HvL genotype | −2.37 | −.74 | ||
| PFF | .58 | .37 | ||
| SES | 4.81 | 2.97** | ||
| Step 2 | 3.08* | .05 | ||
| HvL genotype | −6.31 | −.34 | ||
| PFF | −2.29 | −.88 | ||
| SES | 1.34 | .53 | ||
| HvL × PFF | .79 | 1.39 | ||
| HvL × SES | 1.73 | 1.75 | ||
| WJ-III Applied Problemsa | ||||
| Step 1 | 3.60* | .14* | ||
| HvL genotype | −4.36 | −1.44 | ||
| PFF | −2.58 | −1.72 | ||
| SES | 2.16 | 1.41 | ||
| Step 2 | 7.44** | .17** | ||
| HvL genotype | −53.25 | −4.31** | ||
| PFF | −2.56 | −1.90 | ||
| SES | −4.48 | −2.09* | ||
| HvL × SES | 3.34 | 4.05** | ||
| SDQ Inattention/Hyperactivity | ||||
| Step 1 | 3.64* | .14* | ||
| HvL genotype | −.25 | −.49 | ||
| PFF | .65 | 2.55* | ||
| SES | −.35 | 1.37 | ||
| Step 2 | 2.26 | .01 | ||
| HvL genotype | −2.47 | −.81 | ||
| PFF | .88 | 2.06* | ||
| SES | −.42 | −1.01 | ||
| HvL × PFF | −.06 | −.67 | ||
| HvL × SES | .04 | .22 | ||
| SDQ Peer Relationship Problemsa | ||||
| Step 1 | 5.63** | .20** | ||
| HvL genotype | .09 | .31 | ||
| PFF | .19 | 1.28 | ||
| SES | −.49 | − 3.24** | ||
| Step 2 | 5.90** | .06* | ||
| HvL genotype | −3.11 | −2.24* | ||
| PFF | .62 | 2.67* | ||
| SES | −.48 | −3.32** | ||
| HvL × PFF | −.12 | −2.36* | ||
| SDQ Prosocial Behavior | ||||
| Step 1 | 2.45 | .10 | ||
| HvL genotype | −.30 | −.80 | ||
| PFF | −.47 | −2.60* | ||
| SES | −.22 | −1.06 | ||
| Step 2 | 1.54 | .01 | ||
| HvL genotype | −1.80 | −.84 | ||
| PFF | −.35 | −1.17 | ||
| SES | −.29 | −1.01 | ||
| HvL × PFF | −.03 | −.47 | ||
| HvL × SES | .05 | .36 | ||
| SDQ Total Score | ||||
| Step 1 | 2.32 | .10 | ||
| HvL genotype | .27 | .24 | ||
| PFF | 1.18 | 2.10* | ||
| SES | −.44 | −.67 | ||
| Step 2 | 1.46 | .01 | ||
| HvL genotype | −4.32 | −.64 | ||
| PFF | 1.52 | 1.66 | ||
| SES | −.67 | −.75 | ||
| HvL × PFF | −.09 | −.43 | ||
| HvL × SES | .15 | .38 |
Note.
For the Applied Problems and SDQ Peer Relationship Problems subtests, there were collinearity concerns if both interactions were included in the model. As the inclusion of the second interaction term did not substantially impact outcomes, only the significant interaction term is reported.
HvL = High vs Low; PFF = Parent and Family Functioning; SDQ = Strengths and Difficulties Questionnaire; SES = Socioeconomic Status; TOLD = Test of Language Development-Primary; WJ = Woodcock Johnson.
p < .05,
p < .01
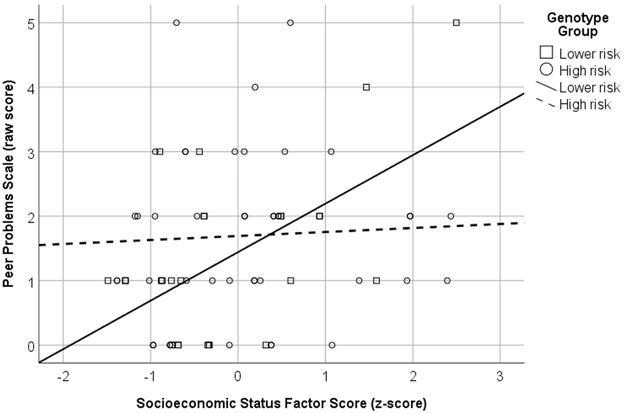
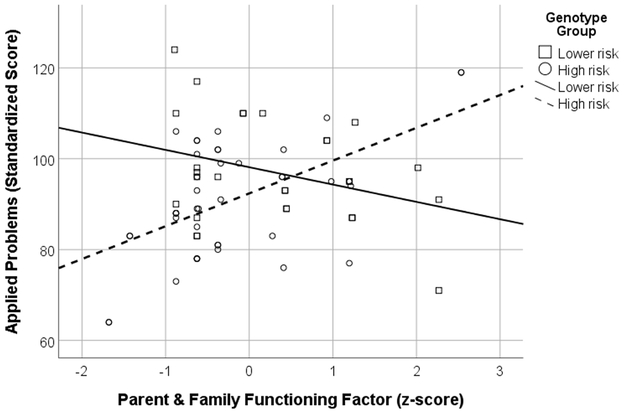
There were two outcomes that showed interaction effects. For scores on the SDQ Peer Relationship Problems scale, the interaction between PFF and genotype accounted for significantly more variance than genotype and PFF alone. Follow-up analyses were run on the interaction revealing PFF was a significant predictor of scores in the low risk genotype group, B= .13, t(26) = 3.43, p = .002, but not in the high risk genotype group, B = .01, t(42) = .32, p = .755 (see Figure 1). There was also a significant interaction between SES and genotype for scores on the WJ-III Applied Problems test. Follow up analyses revealed SES was a significant predictor of scores for the high-risk genotype group only, B = 2.19, t(42) = 4.25, p < .001 (see Figure 2).
Figure 1.
Interaction between Parent and Family Functioning (PFF) and genotype severity for the SDQ peer relationship scale.
Figure 2.
Interaction between Socioeconomic Status (SES) and genotype severity for the WJ-III applied problems subscale.
Discussion
Although previous research has established the association between commonly used SES variables and cognitive and behavioral outcomes in pediatric SCD, there is limited research assessing the association of more downstream and modifiable social-environmental factors. The current study predicted that modifiable social-environmental factors would provide unique explanatory value for psychological outcomes above and beyond SES. We also examined interactions between social and biomedical risk factors as they relate to cognitive and behavioral functioning in pediatric SCD. To create a more parsimonious model, we first conducted exploratory factor analysis to identify core constructs from social-environmental variables collected as part of a larger developmental screening battery. Exploratory factor analysis identified two largely independent factors; variables measuring parent and family functioning (PFF) emerged as a distinct factor from variables typically associated with SES (parent income and education), thereby supporting our hypothesis that SES does not fully capture social-environmental risks for psychological outcomes in pediatric SCD.
This finding was further supported by distinct associations of the two factors with cognitive and behavioral outcomes. As hypothesized, PFF predicted cognitive and behavioral functioning that was not accounted for by SES alone. Specifically, PFF predicted performance on measures of phonological processing and ADHD symptoms, whereas SES predicted scores on semantic and syntactic processing and early reading skills. Given the exploratory nature of these findings, some caution is needed in interpreting these results. However, the pattern of results suggests that SES may primarily predict language-related outcomes (e.g., vocabulary, reading skills) while parenting factors predict more externalizing symptomatology. This is in line with previous research showing an association between increased family conflict and more mother-reported problem behaviors in pediatric SCD (Thompson et al., 2003). These findings suggest that family functioning may represent an important target for interventions to improve behavioral adjustment in children with SCD.
Although the association between SES and language-related ability is not a novel finding (Hoff, 2013), results from the current study indicate that this relationship may be distinct from other parent and family factors measured in the present study. These findings differ from a previous study by Drazen and colleagues (2016) who found that a less enriching home environment was directly related to expressive language and general cognitive delays whereas SES was correlated with home environment but not cognitive outcomes. However, given the large age difference between participants in Drazen et al (2016) and the current study (eight months vs six years respectively), age-related differences may play a substantial role. As such, parent and family-related factors such as home environment may be of greater importance for language skills in the first few years of life, with SES-type factors playing a larger role later in development.
Both PFF and SES were associated with math problem solving skills, ADHD symptoms, and social functioning; however, follow up analyses that included both factors revealed SES as the only unique predictor of social functioning, PFF as the sole unique predictor of ADHD symptoms, and neither factor as uniquely predictive of math problem solving skills. These differential associations suggest that SES and PFF may denote largely separate risks for cognitive and behavioral outcomes. As such, these findings illustrate that the common practice of only measuring SES in many prior studies underestimates the impact of social-environmental risk factors on child outcomes.
The interaction between biological risk, as measured by genotype-derived disease severity, and the two social-environmental factors was also explored. Findings revealed that lower PFF only predicted peer relationship problems for children at lower biological risk, suggesting that for children with higher-risk genotypes, disease-related factors may outweigh the impact of parent and family-related social-environmental factors. A different pattern was observed for SES and math problem solving skills, with lower SES predicting poorer math problem solving skills for children at higher biological risk for SCD morbidity. Similar interactions between biological risk factors and social-environmental factors, as measured by SES, were reported in a study by Schatz et al. (2004) for cognitive outcomes; however, the specific type of interactions were different across these two studies. Overall, this suggests that biological risk may interact with social-environmental factors to impact cognitive and behavioral outcomes, but the reliability of specific interactions has not been established.
There are a number of limitations associated with the current study. First, the broad nature of our single factor measuring modifiable social-environmental risk (i.e., PFF) does not allow for the investigation of the unique effects of the variables that comprise parent and family functioning. However, the general lack of empirical data on modifiable social-environmental risk for pediatric SCD necessitates the confirmation of basic concepts before more complicated models are explored. Second, although many measures assessing social-environmental factors were included, there are a number of dimensions of social-environmental risk that were not captured in our measures. For instance, risk factors such as race-related stress (e.g., discrimination and stigmatization) are particularly salient given the population affected by pediatric SCD in the United States is predominantly African-American (Yarboi et al., 2017). We also used a unidimensional measure of family functioning and more nuanced measures of dimensions of family functioning could be important.
A third limitation of the current study is reliance on proxy reports. Measures of social-environmental risk and behavioral functioning relied primarily on parent-reports of child functioning. Given previous research demonstrating the bidirectional relationship between parent stress and behavioral concerns, it is possible some of the reported associations could reflect transactional parent-child patterns (Neece, Green, & Baker, 2012). These concerns are difficult to address as the age of youth in the sample precludes the collection of self-report data; however, it is important to be aware of such limitations when interpreting results. Additionally, internal consistency for the Parenting Self-Agency Measure and CES-D were relatively weak in the current sample (.60-.64), which brings into question the reliability of the two measures.
Lastly, there are limitations related to size and age range of the current sample. The relatively small sample size coupled with the number of analyses run limits statistical power and, therefore, the interpretability of the findings. In addition, the inclusion of only younger children limits the generalizability of findings to other age demographics. Therefore, future studies will be needed with older children and adolescents to determine if the impact of parent and family-related factors on psychological functioning varies based on age.
In conclusion, the results from the current study expand on previous research on social-environmental risk in pediatric SCD by demonstrating that parent and family-related functioning predicts psychological functioning above and beyond that of SES alone. This adds to the limited research exploring modifiable social-environmental risk factors in pediatric SCD by providing a parsimonious and more comprehensive model of the impact of social-environmental risk on child functioning. This more nuanced conceptualization of risk factors has a number of strengths. For instance, these findings can help identify aspects of parent and family functioning that would be the best targets for future interventions. Ultimately, this could help clinicians link specific dysfunctions in family functioning with distinct cognitive and behavioral outcomes, thereby allowing for the better of alignment of services. In addition, these findings provide a foundation for more complex investigations to tease apart the relative contribution of specific family or social factors (e.g., stimulating home environments, racial discrimination) to child functioning. Thus, future research should focus on the development of more complete models of risk that take into account the dynamic interplay between modifiable social-environmental and medical factors with the end goal of improving functioning in pediatric SCD.
Acknowledgments
Funding
This publication was made possible in part by Grant Number T32-GM081740 from NIH-NIGMS. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIGMS or NIH.
Footnotes
Disclosure statement
No potential conflict of interest
References
- Bakri MH, Ismail EA, Elsedfy GO, Amr MA, & Ibrahim A (2014). Behavioral impact of sickle cell disease in young children with repeated hospitalization. Saudi Journal of Anaesthesia, 8(4), 504 https://doi.org10.4103/1658-354X.140867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarin OA, & Christian M (1999). The social and cultural context of coping with sickle cell disease: I. A review of biomedical and psychosocial issues. Journal of Black Psychology, 25(3), 277–293. 10.1177/0095798499025003002 [DOI] [Google Scholar]
- Beery KE (2004). The Beery-Buktenica developmental test of visual-motor integration: Beery VMI, with supplemental developmental tests of visual perception and motor coordination, and stepping stones age norms from birth to age six. NCS Pearson Minneapolis, MN. [Google Scholar]
- Brandow AM, Brousseau DC, & Panepinto JA (2009). Postdischarge pain, functional limitations and impact on caregivers of children with sickle cell disease treated for painful events. British Journal of Haematology, 144(5), 782–788. 10.1111/j.1365-2141.2008.07512.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlew K, Telfair J, Colangelo L, & Wright EC (2000). Factors that influence adolescent adaptation to sickle cell disease. Journal of Pediatric Psychology, 25(5), 287–299. 10.1093/jpepsy/25.5.287 [DOI] [PubMed] [Google Scholar]
- Byles J, Byrne C, Boyle MH, & Offord DR (1988). Ontario Child Health Study: reliability and validity of the general functioning subscale of the McMaster Family Assessment Device. Family Process, 27(1), 97–104. 10.1111/j.1545-5300.1988.00097.x [DOI] [PubMed] [Google Scholar]
- Cohen S, & Williamson G (1987). Perceived stress in a probability sample of the United States In Spacapan S, & Oskamp S, (Eds.), The social psychology of health: The Claremont symposium on applied social psychology (pp. 31–67). ThousandOaks CA: Sage Publications, Inc. [Google Scholar]
- Cohen S (1986). Contrasting the Hassles Scale and the Perceived Stress Scale: Who’s really measuring appraised stress? American Psychologist, 41(6), 716–718. 10.1037/0003-066X.41.6.716 [DOI] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 385–396. [PubMed] [Google Scholar]
- Drazen CH, Abel R, Gabir M, Farmer G, & King AA (2016). Prevalence of developmental delay and contributing factors among children with sickle cell disease. Pediatric Blood & Cancer, 63(3), 504–510. 10.1002/pbc.25838 [DOI] [PubMed] [Google Scholar]
- Dumka LE, Stoerzinger HD, Jackson KM, & Roosa MW (1996). Examination of the cross-cultural and cross-language equivalence of the parenting self-agency measure. Family Relations, 216–222. 10.2307/585293 [DOI] [Google Scholar]
- Edwards CL, Scales MT, Loughlin C, Bennett GG, Harris-Peterson S, De Castro LM, … Johnson S (2005). A brief review of the pathophysiology, associated pain, and psychosocial issues in sickle cell disease. International Journal of Behavioral Medicine, 12(3), 171–179. https://doi.org10.1207/s15327558ijbm1203_6 [DOI] [PubMed] [Google Scholar]
- Fantuzzo J, Tighe E, & Childs S (2000). Family Involvement Questionnaire: A multivariate assessment of family participation in early childhood education. Journal of Educational Psychology, 92(2), 367–376. 10.1037/0022-0663.92.2.367 [DOI] [Google Scholar]
- Fields ME, Hoyt-Drazen C, Abel R, Rodeghier MJ, Yarboi JM, Compas BE, & King AA (2016). A Pilot Study of Parent Education Intervention Improves Early Childhood Development among Toddlers with Sickle Cell Disease. Pediatric Blood & Cancer, 63(12), 2131–2138. 10.1002/pbc.26164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill F, Sleeper L, Weiner S, Brown A, Bellevue R, Grover R, … Vichinsky E (1995). Clinical events in the first decade in a cohort of infants with sickle cell disease. Cooperative Study of Sickle Cell Disease. Blood, 86(2), 776–783. [PubMed] [Google Scholar]
- Goodman R (2001). Psychometric properties of the strengths and difficulties questionnaire. Journal of the American Academy of Child & Adolescent Psychiatry, 40(11), 1337–1345. [DOI] [PubMed] [Google Scholar]
- Hankins J, & Wang W (2009). The painful face of poverty. Pediatric Blood & Cancer, 52(2), 157–158. 10.1002/pbc.21856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell KL (2010). Population estimates of sickle cell disease in the US. American Journal of Preventive Medicine, 38(4), S512–S521. 10.1016/j.amepre.2009.12.022 [DOI] [PubMed] [Google Scholar]
- Hijmans CT, Grootenhuis MA, Oosterlaan J, Last BF, Heijboer H, Peters M, & Fijnvandraat K (2009). Behavioral and emotional problems in children with sickle cell disease and healthy siblings: Multiple informants, multiple measures. Pediatric Blood & Cancer, 53(7), 1277–1283. 10.1002/pbc.22257 [DOI] [PubMed] [Google Scholar]
- Hijmans CT, Fijnvandraat K, Grootenhuis MA, van Geloven N, Heijboer H, Peters M, & Oosterlaan J (2010). Neurocognitive deficits in children with sickle cell disease: a comprehensive profile. Pediatric Blood & Cancer, 56(5), 783–788. 10.1002/pbc.22879 [DOI] [PubMed] [Google Scholar]
- Hoff E (2013). Interpreting the early language trajectories of children from low SES and language minority homes: Implications for closing achievement gaps. Developmental Psychology, 49(1), 4–14. 10.1037/a0027238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollocks MJ, Kok TB, Kirkham FJ, Gavlak J, Inusa BP, DeBaun MR, & de Haan M (2012). Nocturnal oxygen desaturation and disordered sleep as a potential factor in executive dysfunction in sickle cell anemia. Journal of the International Neuropsychological Society, 18(1), 168–173. 10.1017/S1355617711001469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenerette CM, & Valrie CR (2010). The influence of maternal behaviors during childhood on self-efficacy in individuals with sickle cell disease. Journal of Family Nursing, 16(4), 422–434. 10.1177/1074840710385000 [DOI] [PubMed] [Google Scholar]
- Kaufman AS, & Kaufman NL (1983). K-ABC: Kaufman assessment battery for children: Interpretive manual. American Guidance Service. [Google Scholar]
- King AA, Strouse JJ, Rodeghier MJ, Compas BE, Casella JF, McKinstry RC, … DeBaun MR (2013). Parent education and biologic factors influence on cognition in sickle cell anemia. American Journal of Hematology, 89(2), 162–167. 10.1002/ajh.23604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCavit TL, Xuan L, Zhang S, Flores G, & Quinn CT (2013). National trends in incidence rates of hospitalization for stroke in children with sickle cell disease. Pediatric Blood & Cancer, 60(5), 823–827. 10.1002/pbc.24392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrew KS, & Woodcock R (2001). Technical Manual. Woodcock-Johnson III. Itasca, IL: Riverside Publishing [Google Scholar]
- National Heart, Lung, and Blood Institute. (2014). Evidence-based management of sickle cell disease: expert panel report, 2014. Washington, DC: National Institutes of Health. [Google Scholar]
- Neece CL, Green SA, & Baker BL (2012). Parenting stress and child behavior problems: A transactional relationship across time. American Journal on Intellectual and Developmental Disabilities, 117(1), 48–66. 10.1352/1944-7558-117.1.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer PL, & Hammill DD (1988). Test of language development-primary. Austin, TX: Pro-ed. [Google Scholar]
- Noll RB, Kiska R, Reiter-Purtill J, Gerhardt CA, & Vannatta K (2010). A controlled, longitudinal study of the social functioning of youth with sickle cell disease. Pediatrics, peds-2009. 10.1542/peds.2009-2996 [DOI] [PubMed] [Google Scholar]
- Oluwole OB, Noll RB, Winger DG, Akinyanju O, & Novelli EM (2016). Cognitive functioning in children from Nigeria with sickle cell anemia. Pediatric Blood & Cancer, 63(11), 1990–1997. 10.1002/pbc.26126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panepinto JA, O’mahar KM, DeBaun MR, Loberiza FR, & Scott J (2005). Health-related quality of life in children with sickle cell disease: Child and parent perception. British Journal of Haematology, 130(3), 437–444. 10.1111/j.1365-2141.2005.05622.x [DOI] [PubMed] [Google Scholar]
- Robinson MR, Daniel LC, O’hara EA, Szabo MM, & Barakat LP (2014). Insurance status as a sociodemographic risk factor for functional outcomes and health-related quality of life among youth with sickle cell disease. Journal of Pediatric Hematology/Oncology, 36(1), 51 10.1097/MPH.0000000000000013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraf SL, Molokie RE, Nouraie M, Sable CA, Luchtman-Jones L, Ensing GJ, … Gordeuk VR (2014). Differences in the clinical and genotypic presentation of sickle cell disease around the world. Paediatric Respiratory Reviews, 15(1), 4–12. 10.1016/j.prrv.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz J, Finke RL, Kellett JM, & Kramer JH (2002). Cognitive functioning in children with sickle cell disease: a meta-analysis. Journal of Pediatric Psychology, 27(8), 739–748. 10.1093/jpepsy/27.8.739 [DOI] [PubMed] [Google Scholar]
- Schatz J, Finke R, & Roberts CW (2004). Interactions of biomedical and environmental risk factors for cognitive development: a preliminary study of sickle cell disease. Journal of Developmental & Behavioral Pediatrics, 25(5), 303–310. [DOI] [PubMed] [Google Scholar]
- Schatz J, Puffer ES, Sanchez C, Stancil M, & Roberts CW (2009). Language processing deficits in sickle cell disease in young school-age children. Developmental Neuropsychology, 34(1), 122–136. 10.1080/87565640802499191 [DOI] [PubMed] [Google Scholar]
- Smith LA, Romero D, Wood PR, Wampler NS, Chavkin W, & Wise PH (2002). Employment barriers among welfare recipients and applicants with chronically ill children. American Journal of Public Health, 92(9), 1453–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strayhorn JM, & Weidman CS (1988). A parent practices scale and its relation to parent and child mental health. Journal of the American Academy of Child & Adolescent Psychiatry, 27(5), 613–618. 10.1097/00004583-198809000-00016 [DOI] [PubMed] [Google Scholar]
- Tarazi RA, Grant ML, Ely E, & Barakat LP (2007). Neuropsychological functioning in preschool-age children with sickle cell disease: the role of illness-related and psychosocial factors. Child Neuropsychology, 13(2), 155–172. 10.1080/09297040600611312 [DOI] [PubMed] [Google Scholar]
- Thompson RJ Jr, Armstrong FD, Kronenberger WG, Scott D, McCabe MA, Smith B, … Islam S (1999). Family functioning, neurocognitive functioning, and behavior problems in children with sickle cell disease. Journal of Pediatric Psychology, 24(6), 491–498. 10.1093/jpepsy/24.6.491 [DOI] [PubMed] [Google Scholar]
- Yarboi J, Compas BE, Brody GH, White D, Rees Patterson J, Ziara K, & King A (2017). Association of social-environmental factors with cognitive function in children with sickle cell disease. Child Neuropsychology, 23(3), 343–360. 10.1080/09297049.2015.1111318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong AG, & Pearce S (2013). A beginner’s guide to factor analysis: Focusing on exploratory factor analysis, 9(2). 10.20982/tqmp.09.2.p079 [DOI] [Google Scholar]
- Berkelhammer LD, Williamson AL, Sanford SD, Dirksen CL, Sharp WG, Margulies AS, & Prengler RA (2007). Neurocognitive sequelae of pediatric sickle cell disease: A review of the literature. Child Neuropsychology, 13(2), 120–131. [DOI] [PubMed] [Google Scholar]
- Schatz J, Brown R, Pascual J, Hsu L, & DeBaun M (2001). Poor school and cognitive functioning with silent cerebral infarcts and sickle cell disease. Neurology, 56(8), 1109–1111. [DOI] [PubMed] [Google Scholar]
- Thompson R Jr., Armstrong J, Link FD, Pegelow CL, Moser CH, & Wang WC (2003). A prospective study of the relationship over time of behavior problems, intellectual functioning, and family functioning in children with sickle cell disease: A report from the cooperative study of sickle cell disease. Journal of Pediatric Psychology, 28(1), 59–65 [DOI] [PubMed] [Google Scholar]