Abstract
Background
Surgery is a common treatment modality for stress urinary incontinence (SUI), usually offered to women for whom conservative treatments have failed. Midurethral tapes have superseded colposuspension because cure rates are comparable and recovery time is reduced. However, some women will not be cured after midurethral tape surgery. Currently, there is no consensus on how to manage the condition in these women.
This is an update of a Cochrane Review first published in 2013.
Objectives
To assess the effects of interventions for treating recurrent stress urinary incontinence after failed minimally invasive synthetic midurethral tape surgery in women; and to summarise the principal findings of economic evaluations of these interventions.
Search methods
We searched the Cochrane Incontinence Specialised Register, which contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE In‐Process, MEDLINE Epub Ahead of Print, ClinicalTrials.gov, WHO ICTRP and handsearching of journals and conference proceedings (searched 9 November 2018). We also searched the reference lists of relevant articles.
Selection criteria
We included randomised and quasi‐randomised controlled trials in women who had recurrent stress urinary incontinence after previous minimally invasive midurethral tape surgery. We included conservative, pharmacological and surgical treatments.
Data collection and analysis
Two review authors checked the abstracts of identified studies to confirm their eligibility. We obtained full‐text reports of relevant studies and contacted study authors directly for additional information where necessary. We extracted outcome data onto a standard proforma and processed them according to the guidance in the Cochrane Handbook for Systematic Reviews of Interventions.
Main results
We included one study in this review. This study was later reported in an originally unplanned secondary analysis of 46 women who underwent transobturator tape for recurrent SUI after one or more previous failed operations. We were unable to use the data, as they were not presented according to the nature of the first operation.
We excluded 12 studies, five because they were not randomised controlled trials (RCTs) and four because previous incontinence surgery was not performed using midurethral tape. We considered a further three to be ineligible because neither the trial report nor personal communication with the trialists could confirm whether any of the participants had previously undergone surgery with tape.
We had also planned to develop a brief economic commentary summarising the principal findings of relevant economic evaluations but supplementary systematic searches did not identify any such studies.
Authors' conclusions
There were insufficient data to assess the effects of any of the different management strategies for recurrent or persistent stress incontinence after failed midurethral tape surgery. No published papers have reported exclusively on women whose first operation was a midurethral tape. Evidence from further RCTs and economic evaluations is required to address uncertainties about the effects and costs of these treatments.
Plain language summary
Treatment of recurrent stress urinary incontinence in women after a failed midurethral tape operation
Review question
What is the best way to treat women whose stress urinary incontinence is not cured or recurs after surgery to insert a tape underneath the bladder outlet (midurethral tape)?
Background
Stress urinary incontinence (SUI) is the loss of urine when a person coughs or exercises. It can be caused by damage to the pelvic floor muscles or their nerve supply, particularly during childbirth. Simple treatments, such as exercising pelvic floor muscles or medication, may be tried at first. If these methods have not worked, surgery is often performed, which can be done using a midurethral tape. This usually involves placing a tape made from polypropylene (a synthetic material like nylon that is used in some surgical stitches and other medical devices) underneath the bladder outlet. This operation is usually very successful, but not all women will be cured. There is currently no agreement amongst experts on how to treat women with recurrent stress urinary incontinence problems following unsuccessful midurethral tape surgery.
How up‐to‐date is this review?
The evidence is current up to 9 November 2018.
Study characteristics
Our search identified one study for this review, including a total of 341 women. Of these, just 46 women met our inclusion criteria by having undergone previous continence surgery with a midurethral tape or colposuspension (a type of surgery used to support the tissues around the neck of the bladder with stitches). This review only focused on the results from these 46 women, extracted from the overall trial results.
Study funding sources
The one included study was funded by the Henry Smith Charity. A published correction indicated commercial support from the manufacturer of a device used in the study, which could be a source of bias.
Key results
We wanted to assess the effects of conservative treatment (such as pelvic floor muscle training or bladder training), surgery and medication on the number of women who reported that their incontinence was improved or cured after treatment, along with other outcomes such as quality of life and adverse events. We were also interested in the effects on our outcomes of different types of midurethral tapes.
Of the 46 eligible women in the included study, two‐thirds were reported to have received a midurethral tape in their first surgery. However, the data in the report did not differentiate between women who had previously undergone surgery with a midurethral tape and those who had had colposuspension. This means that we cannot be certain that the results were due to the midurethral tape, so we could not use the data in this review.
We planned to summarise evidence about which treatments might be considered worthwhile uses of healthcare resources but we did not identify any studies that asked this question.
Certainty of the evidence
The lack of useable data means that we were unable to assess the certainty of the body of evidence.
Authors' conclusions
We did not find enough data to accurately assess the effects of any of the different management strategies for recurrent or persistent stress incontinence after failed midurethral tape surgery. Evidence from high‐quality studies is required to address this area of uncertainty.
Summary of findings
Background
For a glossary of medical terms, see Appendix 1.
Description of the condition
Urinary incontinence (UI) has been estimated to have a prevalence rate in the range of 25% to 45%, with many studies reporting a prevalence rate of 10% to 39% (Milsom 2017). Stress urinary incontinence (SUI) is defined as the involuntary loss of urine on effort or physical exertion (e.g. sporting activities) or on sneezing or coughing (Haylen 2010). It can be a debilitating condition for women, severely affecting their quality of life (Margalith 2004).
Conservative treatment by pelvic floor muscle exercise should be the first line approach to managing the condition (NICE 2015), although surgery is usually offered to women for whom this treatment has not been effective. The synthetic midurethral sling was introduced in 1996, initially as the tension‐free vaginal tape (TVT) (Ulmsten 1996). This rapidly superseded the previous standard operation of colposuspension, and has been the most commonly used procedure for SUI in the UK since 2001 (Hilton 2008). It has been shown to have a cure rate for incontinence comparable to colposuspension (Ward 2008). Alternative midurethral slings, including those inserted via the obturator foramen and single incision slings, were subsequently developed. Prospective, observational cohort studies have suggested that surgery using tape to support the midurethra can result in cure rates of 80% to 90% (Rovner 2017).
A small proportion of women will not be cured after midurethral sling surgery, and recurrent SUI is reported to occur in 2% to 16% of women after a TVT procedure (Merlin 2001). Some of the risk factors for failure may include the presence of an immobile urethra, using two or more pads per day prior to treatment, high body mass index (BMI), a weight of greater than 80 kg and intraoperative blood loss of over one litre (Alcalay 1995; Cammu 2009).
SUI is also associated with a number of direct and indirect economic costs. For example, one US‐based study found that women about to undergo Burch or fascial sling surgery for SUI had mean out‐of‐pocket costs (for supplies, laundry and dry cleaning) equivalent to 19 US dollars per week in today's terms (2019 USD; converted from 2012 USD (Shemilt 2010) at baseline (SD = 30) (Subak 2014). The women who participated in this study had an average (mean) age of 53 years (SD = 10) and an average (mean) baseline frequency of urinary UI episodes of 23 per week (SD = 21); 48% had undergone prior nonsurgical treatment for UI; and 16% had had undergone prior surgery for UI. Another study estimated that, in a single year (2012) in Spain alone, a national total of over 350,000 quality‐adjusted life years were lost among women aged 60 years and over, due to UI (Villoro 2016).
Description of the intervention
Historically, traditional sling procedures have been the surgical treatment of choice for managing recurrent SUI (McGuire 1992), although there are many considerations and choices facing both women and practitioners when thinking about treatment for recurrent SUI following failed surgery (MacLachlan 2014).
It is recommended that conservative management options should be used as a first line of therapy for those with recurrent UI following surgery (Lovatsis 2017). Conservative interventions are considered to be non‐invasive therapies, which can be used alone or with the addition of biofeedback (Dumoulin 2017). These therapies can include pelvic floor muscle training (Dumoulin 2018), mechanical devices such as pessaries (Bugge 2004), and electrical stimulation (Stewart 2017). Pharmacological treatments for SUI include serotonin nonadrenaline reuptake inhibitors (SNRIs) such as duloxetine (Mariappan 2005), imipramine or alpha‐adrenoceptor agonists (Andersson 2017). Possible surgical interventions include open or laparoscopic colposuspension (Dean 2017; Lapitan 2017), traditional tape procedures (Rehman 2017), periurethral bulking agents (Kirchin 2017), and repeat midurethral tape surgery.
How the intervention might work
Conservative interventions for SUI work in a variety of ways. For example, with pelvic floor muscle training, lifting and contracting the pelvic floor muscles both prior to and during exertion increases urethral pressure, thus reducing the risk of leakage (Dumoulin 2018).
Although pharmacological interventions are unlikely to treat structural abnormalities, it is thought that SUI in women is characterised by a decrease in urethral transmission closure pressure (Andersson 2017). Pharmacological treatments such as duloxetine may contribute to an alternative mechanism for improving intraurethral closure by increasing the resting tone in the urethral smooth and striated muscle. A randomised controlled trial followed by an open‐label extension run was carried out in 342 centres across 16 European countries (Cardozo 2010). The trial examined the effects of duloxetine for women with predominant SUI, and found that the intervention was associated with decreased pad use and increases in quality of life.
It is also theorised that UI (including SUI) can be caused by laxity, either in the vagina or its supporting ligaments, as a result of altered connective tissues (Papa Petros 2010). Surgical procedures generally aim to lift or support the urethro‐vesical junction (or both); for example, colposuspension involves inserting sutures into the vaginal tissues on either side of the neck of the bladder which are then attached to the ileo‐pectineal ligaments on the pelvic brim (Dean 2017). Sling procedures (both traditional and synthetic midurethral types) aim to restore or enhance urethral support when coughing or exercising, by providing a "backboard" against which the urethra is compressed by the increase in intra‐abdominal pressure (Nambiar 2017; Rehman 2017).
It has been recommended that managing these women should include a complete assessment to determine any predisposing conditions, such as compromises to the urethral sphincter mechanism, detrusor overactivity and voiding dysfunction (Lovatsis 2017), as well as discussion of treatment options with a multidisciplinary team (NICE 2015). However, even with this evaluation, there is currently little consensus on how best to manage women with recurrent SUI, particularly those who have previously had failed tape surgery.
Why it is important to do this review
The lack of consensus on how to manage women with recurrent SUI following failed tape surgery constitutes a major problem, not only for the women with SUI, but also for the clinician, who is faced with offering all reasonable alternative treatment options. Furthermore, there is no consensus about whether the previously inserted tape should be excised or if a second tape should simply be placed over the existing tape. The decision about whether to use a tape inserted via a different route (retropubic versus transobturator versus single incision) has also not been addressed. Following concerns about the safety of surgical procedures using mesh, NHS England recently placed a pause on the use of tape procedures to treat SUI and mesh for pelvic organ prolapse (NHS England 2018), and this has also been taken up in Wales, Northern Ireland and the Republic of Ireland.
The main purpose of this review is to identify evidence addressing this uncertainty, and that supporting best practice for the management of recurrent SUI following failed midurethral tape surgery. This review addresses a separate question to other existing reviews. Other Cochrane Reviews relating to primary surgery for SUI focus on laparoscopic colposuspension (Dean 2017), open retropubic colposuspension (Lapitan 2017), midurethral sling operations (Ford 2017), single incision slings (Nambiar 2017), traditional slings (Rehman 2017), bladder neck needle suspension (Glazener 2017), and urethral injection therapy (Kirchin 2017). Secondarily, the review will also summarise published evidence for both the impacts of the interventions on resource use (costs) and for their comparative efficiency (cost‐effectiveness).
Objectives
To assess the effects of interventions for treating recurrent stress urinary incontinence after failed minimally invasive synthetic midurethral tape surgery in women; and to summarise the principal findings of economic evaluations of these interventions.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised or quasi‐randomised controlled studies comparing conservative treatment and medical or further surgical treatments, or comparing two different surgical treatments, after failed midurethral tape surgery.
We included studies treating women with recurrent SUI regardless of whether they had solely recruited recurrent cases or reported subsets of women with recurrent SUI.
Types of participants
We included studies of adult women with persistent or recurrent SUI after any failed midurethral tape surgery.
We did not exclude women with de novo detrusor overactivity or overactive bladder (OAB) from this review, but we would have carried out a subgroup analysis if we had identified large enough numbers of women with this condition from included studies.
Types of interventions
We included studies looking at the following interventions: further surgery (including injectables), administration of medication or conservative treatment (e.g. pelvic floor muscle training) in women who had previously had unsuccessful midurethral tape surgery. Any form of previous midurethral tape was included (retropubic, transobturator of either direction and single incision).
The following comparisons were deemed to be of importance to women, clinicians and stakeholders, with the last three of particular interest for the 'Summary of findings' tables.
Conservative treatment (e.g. pelvic floor muscle training or bladder retraining) versus surgical treatment (any route)
Conservative treatment versus pharmacological treatment (e.g. duloxetine or anticholinergic medication, or both)
Pharmacological treatment versus surgical treatment (any route)
Surgical treatment (e.g. traditional sling, colposuspension, injectables) versus placebo or sham treatment
Repeat midurethral tape (any type) versus any other non‐tape surgical treatment (e.g. traditional sling, colposuspension, injectables)
One type of repeat midurethral tape versus another type of repeat midurethral tape
Surgery with excision of failed tape versus surgery without excision of tape
Types of outcome measures
Primary outcomes
Number of women whose incontinence was improved or cured (assessed by subjective report, urinary diary or validated incontinence questionnaires in the short‐term (less than 12 months) and longer‐term (more than 12 months))
Secondary outcomes
Quantification of symptoms (assessed using incontinence episodes (from self‐completed bladder chart), pad changes (from self‐reported number of pads used), pad tests of quantified leakage (mean volume or weight of urine loss))
Number of incontinence episodes (assessed by self‐completed bladder chart)
Number of pad changes (from self‐reported number of pads used)
Pad tests of quantified leakage (mean volume or weight of urine loss)
Objective cure rates in the short‐term (less than 12 months) and long‐term (more than 12 months), assessed using observed leakage during repeat urodynamics
General health status measures (e.g. Short‐Form 36) (Ware 1993)
Condition‐specific instruments designed to assess incontinence (e.g. Bristol Female Lower Urinary Tract Symptoms questionnaire (BFLUTS) (Jackson 1996))
Repeat continence surgery
Adverse events e.g. operative and postoperative complications including bladder or bowel injury, blood loss, nerve injury, de novo detrusor overactivity
Main outcomes for 'Summary of findings' tables
Where data allowed, we planned to include the following outcomes in 'Summary of findings' tables:
Number of women whose incontinence was improved or cured in the longer‐term (more than 12 months, assessed with validated incontinence questionnaires)
Objective cure rates in the longer‐term (more than 12 months, assessed with urodynamics)
General health status measures (e.g. Short‐Form 36) (Ware 1993)
Condition‐specific instruments designed to assess incontinence (e.g. Bristol Female Lower Urinary Tract Symptoms questionnaire (BFLUTS) (Jackson 1996))
Repeat continence surgery
Adverse events
Search methods for identification of studies
We did not impose any restrictions, for example language or publication status, on the searches detailed below.
Electronic searches
Search for clinical effectiveness studies
We drew on the search strategy developed for Cochrane Incontinence. We identified relevant trials from the Cochrane Incontinence Specialised Register. For more details of the search methods used to build the Specialised Register, please see the Group's webpages for details of the Register's development (from inception) and the most recent searches performed to populate the Register. To summarise, the Register contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE In‐Process, MEDLINE Epub Ahead of Print, ClinicalTrials.gov, WHO ICTRP, UK Clinical Research Network Portfolio and handsearching of journals and conference proceedings. Many of the trials in the Cochrane Incontinence Specialised Register are also contained in CENTRAL.
The terms used to search the Cochrane Incontinence Specialised Register are given in Appendix 2.
The date of the last search was 9 November 2018.
Authors of the first version of this review also sought non‐randomised studies (Bakali 2013). Appendix 3 contains details of those searches.
Search for economic evaluations
We also performed supplementary electronic searches designed to identify published reports of relevant economic evaluations to inform the brief economic commentary (BEC) (see 'Incorporating economic evidence' in the Methods). We searched the following databases on 28 January 2019:
MEDLINE on OvidSP (covering 1 January 1946 to January Week 3 2019);
Embase on OvidSP (covering 1 January 1974 to 2019 Week 04); and
NHS Economic Evaluation Database (NHS EED) on the Centre for Reviews and Dissemination website (covering from the earliest record in NHS EED, dating from 1968, up to and including 31 December 2014 when their coverage ended).
Appendix 4 contains details of these supplementary electronic searches, including the search terms we used.
Searching other resources
Search for clinical effectiveness studies
We searched the reference lists of relevant articles including those of the included studies and those of other completed reviews, especially those of previous Cochrane Reviews, for randomised or quasi‐randomised studies treating people with recurrent incontinence, either as the sole population or a subset.
Search for economic evaluations
We also conducted forwards and backwards citation searching from reports of included studies identified by Electronic searches using Microsoft Academic Graph (MAG), with the aim of identifying any further published reports of relevant economic evaluations to inform the BEC (see 'Incorporating economic evidence' in the Methods). Had any published reports of relevant economic evaluations been identified by any of the search methods described in the Search methods for identification of studies, we would also have applied the same citation searching method to those reports, for the same purpose.
Data collection and analysis
Selection of studies
Two review authors scanned the search results to identify trials which appeared to meet the inclusion criteria. Two review authors accessed and read the full text reports of potentially eligible studies and independently applied the inclusion criteria. Another review author acted as the arbiter and resolved any differences of opinion. Native speakers assessed papers in languages other than English for eligibility and subsequent data extraction.
Data extraction and management
Two review authors independently extracted data from the included trials, using a standard form containing prespecified outcomes. Where data from the study were not provided, we contacted the trial author(s) requesting further information. We planned to process included trial data as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any differences were resolved by discussion between the two review authors and, if necessary, referred to a third review author for arbitration.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias in the included trials using the Cochrane 'Risk of bias' tool. Factors considered included: quality of random sequence generation and concealment of allocation; description of dropouts, withdrawals and missing data; blinding during intervention and at outcome assessment (where appropriate); and description of and protection against possible other sources of bias (where appropriate). A third review author acted as the arbiter and resolved any differences of opinion.
Measures of treatment effect
Had sufficient data been available, we would have reported risk ratios (RRs) for dichotomous data and mean differences (MDs) with 95% confidence intervals (CIs) for continuous data where relevant.
Unit of analysis issues
We planned to take into consideration any possible unit of analysis issues e.g. due to cluster randomisation or in cross‐over trials. These issues would have been handled as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
Where possible, we contacted study authors to request missing data if insufficient data were included in trial reports.
Assessment of heterogeneity
Had data allowed, we would only have combined trial data if there was no clinical heterogeneity. We would have investigated differences between trials if significant heterogeneity was found from the Chi² test or the I² statistic (Higgins 2003), or had appeared obvious from visual inspection of the results. We would have regarded statistical heterogeneity as substantial if either I² was greater than 30% or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
Had sufficient studies been identified, we planned to generate funnel plots to assess reporting bias where appropriate.
Data synthesis
We planned to process included data as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We would have used a fixed‐effect model in data analysis unless there was evidence of marked heterogeneity, in which case we would have used a random‐effects model. Where quantitative data synthesis and meta‐analysis were not appropriate, due to of the nature of reported data or because of evident heterogeneity, we would have presented a narrative review of the evidence.
Subgroup analysis and investigation of heterogeneity
If the studies had reported adequate data, we would have used subgroup analyses to consider differences in outcomes between subgroups defined by criteria such as type of urinary incontinence (stress or urgency), race, comorbidity, concurrent treatment for comorbidities, different types of surgical operations, different types of anaesthetic procedures, and different types of conservative and medical treatment.
Sensitivity analysis
Had data allowed, we would have performed a sensitivity analysis to assess the effect of possible bias associated with individual trials on the outcome of the meta‐analysis.
'Summary of findings' tables
We prepared ‘Summary of findings’ tables for our main comparisons as specified in the Types of interventions and included the outcomes prespecified in the Types of outcome measures. If we had identified any relevant data, we would have used GRADEpro software to generate these (GRADEpro GDT 2015).
Had data allowed, we would have adopted the GRADE approach to assess the certainty of evidence related to these outcomes (Guyatt 2013a; Guyatt 2013b).The four levels of evidence certainty would have been 'high,' 'moderate,' 'low' or 'very low.' The following factors would have been considered for assessing the certainty of evidence: limitations in the study design, inconsistency of results, indirectness of evidence, imprecision and publication bias.
Incorporating economics evidence
We planned to develop a brief economic commentary (BEC) to summarise the availability and principal findings of economic evaluations comparing conservative treatment and medical or further surgical treatments, or comparing two different surgical treatments, of recurrent SUI after failed midurethral tape surgery, in women. This BEC would have encompassed full economic evaluations (that is, cost‐effectiveness analyses, cost‐utility‐analyses and/or cost‐benefit analyses), conducted either alongside (or based upon) one or more randomised or quasi‐randomised controlled studies included in the main review of intervention effects (that is, [primarily] trial‐based economic evaluations), or using a modelling framework (that is, [primarily] model‐based economic evaluations). We planned to develop this BEC in accordance with current Cochrane methods guidance (Shemilt In Press) (see also Search methods for identification of studies).
Results
Description of studies
Results of the search
The literature search produced 58 records. We screened these and obtained 48 full‐text reports that appeared to be eligible for inclusion. On further assessment, 17 reports of 12 studies did not meet the eligibility criteria for the review and we excluded these, for reasons given in the Characteristics of excluded studies (Barber 2008; Cardozo 2002; Enzelsberger 1993; Enzelsberger 1996; Hilton 2002; Kociszewski 2016; Maher 2004; Smithling 2017; Tincello 2017; Wallwiener 1995; Watson 2002; Zimmern 2016). It was unclear whether the eight reports of one further study were eligible, so this study was assessed as awaiting classification while clarification was sought from the trialists (Carr 2017). Twenty‐three reports of one study met the eligibility criteria, and we included these in the review (Abdel‐Fattah 2010). Figure 1 shows the flow of literature through the assessment process.
1.
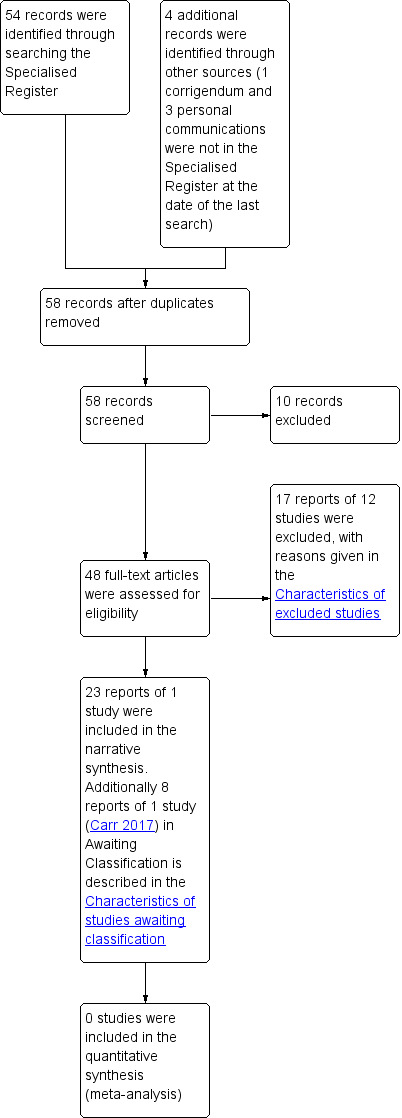
PRISMA study flow diagram ‐ search for clinical effectiveness studies
Searches for economic evaluations to inform the development of the brief economic commentary (BEC) produced 313 unique records, all of which were excluded based on their titles and/or abstracts, with no corresponding full‐text articles retrieved for closer examination. Figure 2 shows the flow of records through the BEC searching and study selection process.
2.
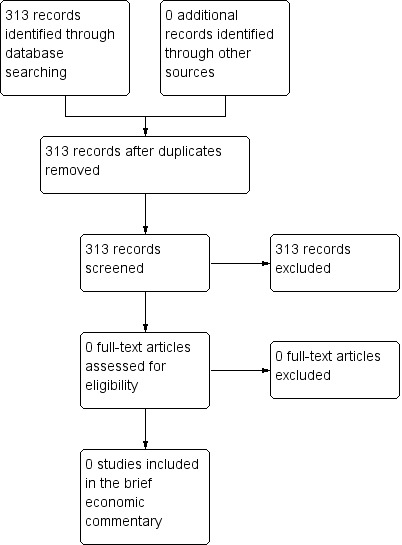
PRISMA study flow diagram ‐ search for economic evaluations for the brief economic commentary
Included studies
We included one study (Abdel‐Fattah 2010), although the previous version of this review had excluded it (Bakali 2013). We assessed it as eligible for this version because we identified a report on a subset of 46 women who had undergone previous unsuccessful continence surgery, which is the specific population of interest to this review (Abdel‐Fattah 2011a). The data for this subset of women did not differentiate between women who had previously undergone midurethral tape and those who had undergone colposuspension, so we were unable to use the data from either report in this review.
From this point, we have presented characteristics for Abdel‐Fattah 2011a as this report contains the population of interest. Further details can be found in the Characteristics of included studies.
Design
The one included study was a secondary analysis of a prospective RCT (Abdel‐Fattah 2011a).
Sample sizes
This secondary analysis included 46 women who had undergone primary continence surgery, with 28 women in one intervention arm and 18 women in the other (Abdel‐Fattah 2011a).
Setting
The setting for the included secondary analysis was a tertiary urogynaecology centre in Scotland, UK (Abdel‐Fattah 2011a).
Participants
The participants in the included study were women who had previously received incontinence surgery, and were aged between 29 to 78 years with a mean age of 55.22 years in the TVT™‐Obturator System (Ethicon, Somerville, NJ) (henceforth known as TVT‐O) arm and 56.73 years in the Aris® (Coloplast, Minneapolis, MN) (henceforth TOT‐ARIS) arm (Abdel‐Fattah 2011a). Of the 46 women in the subset, 15 had previously undergone colposuspension, 15 had previous retropubic TVT, 11 women had previous transobturator tapes and five women had undergone both colposuspension and midurethral tape. A total of 23 women had also previously undergone a hysterectomy.
Abdel‐Fattah 2011a undertook a range of baseline assessment methods, including a detailed history, a pelvic examination, urodynamic assessment (e.g. free uroflowmetry, subtracted multichannel cystometry, urethral pressure profile), completion of the King's Health Questionnaire (KHQ), Birmingham Bowel Urinary Symptom Questionnaire and the Pelvic Organ Prolapse/Incontinence Sexual Function Questionnaire (PISQ‐12).
Inclusion criteria
The secondary analysis by Abdel‐Fattah 2011a included women with SUI or mixed incontinence with predominantly bothersome SUI symptoms, who had previously declined pelvic floor muscle training.
Exclusion criteria
The study excluded women who had concomitant surgery, uterovaginal prolapse (pelvic organ prolapse quantification stage 2 or greater), predominantly bothersome OAB symptoms or specific comorbidities (e.g. multiple sclerosis, diabetes) (Abdel‐Fattah 2011a).
Interventions
Abdel‐Fattah 2011a compared two different types of midurethral sling. One group received "inside‐out" TVT‐O (n = 28) and the other group received "outside‐in" TOT‐ARIS (n = 18). "Inside‐out" and "outside‐in" refers to the direction of travel of the trocar insertion: from vagina to groin incision via the obturator muscles for the former and from groin incision to vagina for the latter.
Outcomes
Primary outcomes
The primary outcome for the secondary analysis of the included study was patient‐reported success as assessed by the Patient Global Impression of Improvement (PGI‐I) as either "very much improved" or "much improved" (Abdel‐Fattah 2011a).
Secondary outcomes
The secondary outcomes for the study were objective cure (defined as negative pad test findings of ≤ 1 g gain), the effect on quality of life (assessed by changes in King's Health Questionnaire (KHQ) score) and effect on sexual life (assessed as changes in Pelvic Organ Prolapse/Incontinence Sexual Function Questionnaire (PISQ‐12) total score) (Abdel‐Fattah 2011a).
Excluded studies
We excluded 12 studies from this review. Of these, five were not RCTs (Barber 2008; Kociszewski 2016; Smithling 2017; Tincello 2017; Zimmern 2016); and four were not eligible because the previous incontinence surgery was not performed using a midurethral tape (Cardozo 2002; Enzelsberger 1993; Enzelsberger 1996; Wallwiener 1995). We excluded three studies because neither the trial report nor personal communication with the trialists could confirm whether any of the participants had previously undergone surgery with midurethral tape (Hilton 2002; Maher 2004; Watson 2002).
Reasons for the exclusion of studies are listed in the Characteristics of excluded studies.
Risk of bias in included studies
We identified one eligible study for this review (Abdel‐Fattah 2010). Although the population of interest is reported only by Abdel‐Fattah 2011a, we made judgements about the risk of bias based on the full trial report as this gives a more complete indication of how the study was conducted.
The Characteristics of included studies gives details of the risk of bias for the included study, with summaries in Figure 3 and Figure 4.
3.
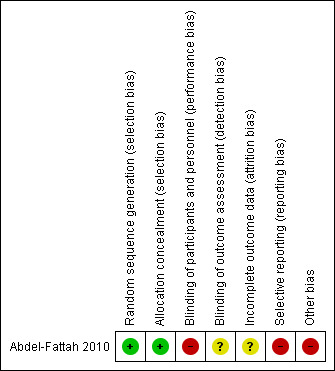
Risk of bias summary: review authors' judgements about each risk of bias item for the included study.
4.
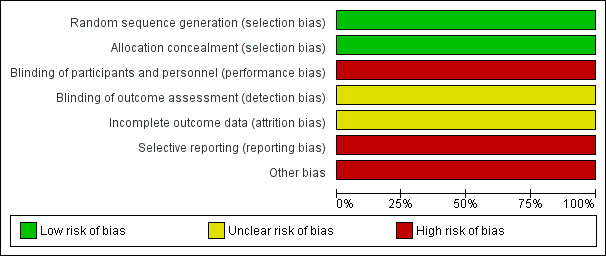
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
Abdel‐Fattah 2010 was at low risk of bias for this domain as the sequence was generated by a computer.
Allocation concealment
Abdel‐Fattah 2010 used opaque sealed envelopes only opened by nursing staff on the morning of the patient's procedure. As such, we judged the study to be at low risk of bias for allocation concealment.
Blinding
We judged Abdel‐Fattah 2010 to be at high risk of performance bias, as the women participating were given the option of knowing to which of the two procedures they were assigned. Although an independent clinician undertook the postoperative assessment, it is possible that the women who knew which procedure they had undergone could reveal this information despite being instructed not to. As such, we judged the study to be at unclear risk of detection bias.
Incomplete outcome data
We judged Abdel‐Fattah 2010 to be at an unclear risk of attrition bias. There was an 11.4% loss to follow‐up, as 317/341 women completed the six‐month follow‐up. In addition, 15 of these 317 women declined to undertake postoperative urodynamic studies.
Selective reporting
Although the study reported the outcome measures they had detailed, these differed from those the trial registration had predefined (Abdel‐Fattah 2005; Abdel‐Fattah 2010). We therefore judged the study as having a high risk of reporting bias.
Other potential sources of bias
Although none of the previously published publications or abstracts declared commercial funding for Abdel‐Fattah 2010, a revised funding statement was published in 2019, indicating historical grant support from the manufacturer of one of the devices used in the study (Coloplast) (Abdel‐Fattah 2019). Although it was stated that "Coloplast had no input into any aspect of the study at any time from design to publication", this poses a conflict of interest or form of bias potentially affecting the quality of the evidence; and we judged the study to be at high risk of bias for this domain as a result.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Repeat midurethral tape (any type) versus any other non‐tape surgical treatment (e.g. traditional sling, colposuspension, injectables).
| Repeat midurethral tape (any type) versus any other non‐tape surgical treatment (e.g. traditional sling, colposuspension, injectables) for recurrent stress urinary incontinence in women with failed minimally invasive midurethral tape surgery | ||||||
|
Patient or population: women with recurrent stress urinary incontinence after failed minimally invasive midurethral tape surgery Settings: secondary or tertiary urogynaecology centre Intervention: repeat midurethral tape (any type) Comparison: any other non‐tape surgical treatment (e.g. traditional sling, colposuspension, injectables) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Risk with other non‐tape surgical treatment | Risk with repeat midurethral sling | |||||
| Number of women whose incontinence was improved or cured ‐ assessed with validated incontinence questionnaires | ‐ | ‐ | ‐ | ‐ | ‐ | No studies identified |
| Objective cure rates in the longer‐term ‐ more than 12 months, assessed with urodynamics | ‐ | ‐ | ‐ | ‐ | ‐ | No studies identified |
| General health status measures ‐ e.g. Short‐Form 36 | ‐ | ‐ | ‐ | ‐ | ‐ | No studies identified |
| Condition‐specific instruments designed to assess incontinence ‐ e.g. BFLUTS | ‐ | ‐ | ‐ | ‐ | ‐ | No studies identified |
| Repeat continence surgery | ‐ | ‐ | ‐ | ‐ | ‐ | No studies identified |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | No studies identified |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; BFLUTS: Bristol Female Lower Urinary Tract Symptoms questionnaire | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
Summary of findings 2. One type of repeat midurethral tape versus another type of repeat midurethral tape.
| One type of repeat midurethral tape compared with another type of repeat midurethral tape for recurrent stress urinary incontinence in women with failed minimally invasive midurethral tape surgery | ||||||
|
Patient or population: women with recurrent stress urinary incontinence after failed minimally invasive midurethral tape surgery Settings: secondary or tertiary urogynaecology centre Intervention: one type of repeat midurethral tape Comparison: another type of repeat midurethral tape | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Risk with another type of repeat midurethral tape | Risk with one type of repeat midurethral tape | |||||
| Number of women whose incontinence was improved or cured ‐ assessed with validated incontinence questionnaires | ‐ | ‐ | ‐ | 46 (1 secondary analysis of RCT) | ‐ | One secondary analysis of an RCT for relevant population identified; no usable data |
| Objective cure rates in the longer‐term ‐ more than 12 months, assessed with urodynamics | ‐ | ‐ | ‐ | ‐ | ‐ | No studies identified |
| General health status measures ‐ e.g. Short‐Form 36 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Condition‐specific instruments designed to assess incontinence ‐ e.g. BFLUTS | ‐ | ‐ | ‐ | 46 (1 secondary analysis of RCT) | ‐ | One secondary analysis of an RCT for relevant population identified; no usable data |
| Repeat continence surgery | ‐ | ‐ | ‐ | ‐ | ‐ | No studies identified |
| Adverse events | ‐ | ‐ | ‐ | 46 (1 secondary analysis of RCT) | ‐ | One secondary analysis of an RCT for relevant population identified, which reported on adverse events narratively within the text. However, there was no usable data |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RCT: randomised controlled trial; BFLUTS: Bristol Female Lower Urinary Tract Symptoms questionnaire | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
Summary of findings 3. Surgery with excision of failed tape versus surgery without excision of tape.
| Surgery with excision of failed tape compared with surgery without excision of tape for women with recurrent stress urinary incontinence after failed minimally invasive midurethral tape surgery | ||||||
|
Patient or population: women with recurrent stress urinary incontinence after failed minimally invasive midurethral tape surgery Settings: secondary or tertiary urogynaecology centre Intervention: surgery with excision of failed tape Comparison: surgery without excision of tape | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Risk of surgery without excision of tape | Risk of surgery with excision of failed tape | |||||
| Number of women whose incontinence was improved or cured ‐ assessed with validated incontinence questionnaires | ‐ | ‐ | ‐ | ‐ | ‐ | No studies identified |
| Objective cure rates in the longer‐term ‐ more than 12 months, assessed with urodynamics | ‐ | ‐ | ‐ | ‐ | ‐ | No studies identified |
| General health status measures ‐ e.g. Short‐Form 36 | ‐ | ‐ | ‐ | ‐ | ‐ | No studies identified |
| Condition‐specific instruments designed to assess incontinence ‐ e.g. BFLUTS | ‐ | ‐ | ‐ | ‐ | ‐ | No studies identified |
| Repeat continence surgery | ‐ | ‐ | ‐ | ‐ | ‐ | No studies identified |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | No studies identified |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; BFLUTS: Bristol Female Lower Urinary Tract Symptoms questionnaire | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1. Conservative treatment (e.g. pelvic floor muscle training or bladder retraining) versus surgical treatment (any route)
No trials were identified for this comparison.
2. Conservative treatment versus pharmacological treatment (e.g. duloxetine or anticholinergic medication, or both)
No trials were identified for this comparison.
3. Pharmacological treatment versus surgical treatment (any route)
No trials were identified for this comparison.
4. Surgical treatment (e.g. traditional sling, colposuspension, injectables) versus placebo or sham treatment
No trials were identified for this comparison.
5. Repeat midurethral tape (any type) versus any other non‐tape surgical treatment (e.g. traditional sling, colposuspension, injectables, other)
No trials were identified for this comparison.
6. One type of repeat midurethral tape versus another type of repeat midurethral tape
One trial was identified for this comparison (Abdel‐Fattah 2010), which reported on a subset of 46 women who had previously undergone continence surgery in a secondary analysis (Abdel‐Fattah 2011a).
As the secondary analysis did not report data for the participants by type of primary continence surgery, we were not able to use the data.
7. Surgery with excision of failed tape versus surgery without excision of tape
No trials were identified for this comparison.
Discussion
Summary of main results
One study met the inclusion criteria for this review. This study was published multiple times, with one of these publications being a secondary analysis of the 46 women who presented with recurrent SUI (Abdel‐Fattah 2011a). Due to the lack of separation by previous surgery type, we were not able to use the data (Table 2).
We did not find any studies relating to any other prespecified comparison for this review (Table 1, Table 3).
Overall completeness and applicability of evidence
The RCTs that we identified mostly included women whose previous surgery was either unknown or were procedures other than midurethral tapes. Authors rarely described the exact nature of previous surgeries. Although several studies had considered the efficacy of secondary midurethral tape surgery, the overwhelming majority of women in many of the studies had undergone non‐tape surgery as the primary procedure.
We only identified one RCT with potentially relevant data. However, the numbers of participants in each group were small (28 and 18, respectively) and only two‐thirds of each group had undergone primary midurethral tape as surgery. The study did not report data separately by type of primary procedure, so we could not use it. Clearly, there is a gap in the evidence surrounding the effectiveness of interventions for women with recurrent SUI after previous midurethral tape surgery.
This review includes no evidence from RCTs to provide guidance on other surgical options such as urethral bulking agents, colposuspension, autologous fascial sling or artificial urinary sphincters and there is no evidence from RCTs on the efficacy of non‐surgical interventions in this patient group.
Quality of the evidence
We did not find any RCTs which included women on the basis of failed midurethral tape surgery and randomised them to alternative treatment strategies. We found one RCT which randomised women with primary or recurrent SUI to different surgical procedures; the authors published a secondary analysis of those with previously failed continence surgery. However, this analysis did not analyse outcomes by specific primary procedure and only compared midurethral tapes of one type, albeit using different routes of insertion. As the data from the study were unusable, we did not perform a GRADE assessment.
The sample size of the secondary analysis was small (46 women), was of unclear risk of bias for blinding of outcome assessment and selective reporting, and high risk of bias for blinding of participants and personnel, attrition bias and other bias.
Potential biases in the review process
We conducted the review according to Cochrane’s standard methodology and made attempts to minimise bias in the process. Two review authors independently reviewed all studies for inclusion and two review authors also independently assessed ‘Risk of bias’, with a third review author arbitrating any disagreements .
We made a decision to include a further comparison in the review, ‘surgical treatment (e.g. traditional sling, colposuspension, injectables) versus placebo or sham treatment’, which had not been included in the previous version (Bakali 2013). Although we deemed this comparator to be clinically important, it could be seen to have introduced bias into the process as it led to us including one study which would not otherwise have been eligible (Carr 2017). Although we are now aware of a full report, it was published after the search date for this review, and we therefore classed it as ‘awaiting assessment'. As a result, this has not impacted on the findings of this review.
Agreements and disagreements with other studies or reviews
The conclusions of this review are consistent with those of the previous version (Bakali 2013).
Information from non‐randomised studies
Non‐randomised studies are open to bias and their findings must be interpreted with caution. Yet, in the absence of randomised trials, they provide the only evidence available to inform clinical decisions. Thus, in order to consider what is known about the management of failed midurethral tape surgery, it is worth considering the literature relating to non‐randomised studies.
A number of studies have considered specific methods for the management of failed midurethral tape insertion. Periurethral bulking agents achieved subjective cure in 8 of 23 women (35%) with persistent or recurrent incontinence after midurethral tape insertion, which is in keeping with the primary success rates for bulking agents (Lee 2010). Han 2012 reported a retrospective comparison of 36 women having secondary midurethral tape surgery, with 30 women having the original tape shortened. The cure rates (unspecified time frame) were 72% for repeat surgery and 47% for tape shortening. Colposuspension was reported to be a successful option for secondary surgery in two small non‐RCTs, with subjective cure rates of 93% and 85% after laparoscopic or open surgery (de Cuyper 2008; Giarenis 2012).
Few studies have reported comparative data on different tape insertion routes following women with failed midurethral tape surgery exclusively. Stav 2010 reported data from a large cohort of 1225 women, comparing outcomes between primary and secondary midurethral tape. At a mean follow‐up of 50 months, the subjective cure rate was 62% for the 77 women having repeat surgery compared to 86% for the 1035 women having primary surgery. In this non‐randomised comparison, repeat retropubic surgery was more successful than repeat transobturator surgery (71% versus 48%, P = 0.04). Urgency and urge incontinence were both more common after secondary surgery (30% versus 14%, and 22% versus 5%, respectively, P < 0.001 for both).
A number of single cohort studies have reported surgical outcomes exclusively after primary midurethral tape surgery. Liapis 2009 reported outcomes from 31 women after retropubic midurethral tape insertion as secondary surgery; 15 women had received transobturator tapes, six women a retropubic tape and 10 women a single incision tape. The study authors did not mention whether the previous tape was removed or not. Objective cure, based on a 1‐hour pad test at 12 months, was 74%, and a negative cough stress test during cystometry was seen in 77% of women. The number of women with each individual primary procedure was 10 or fewer, and no difference in secondary cure rate was seen by primary procedure. Parker 2016 reported a cohort study of 59 women having autologous fascial sling after failed midurethral tape, compared to 229 women having this as a primary procedure. The authors found that, at a median follow up of 14.7 months, there was no difference in objective (54.2% versus 66.8%, P = 0.09) or subjective (52.5% versus 51.1%, P = 0.89) cure rates between those who did or did not have previous midurethral tapes. More women with previous surgery developed urinary retention (8.5% versus 3.1%, P < 0.001) and more women required further additional procedures (urethral bulking) (13.6% versus 3.5%, P = 0.01).
Systematic reviews
One systematic review of the literature on midurethral tapes for recurrent incontinence identified the same studies that we did (Pradhan 2012). However, the review’s conclusions, based on one randomised trial and 11 other papers, were that midurethral tape surgery appears less effective as a secondary procedure than a primary procedure, and retropubic tapes appear to be more effective than transobturator tapes. The authors highlighted the lack of prospective randomised studies.
Agur 2013 conducted a systematic review of all treatments for recurrent stress incontinence, regardless of primary operation. They identified 183 articles for full text review. Only one had a population of entirely recurrent cases, but the primary intervention was anterior vaginal repair so the study was excluded (Enzelsberger 1996). Agur 2013 identified 10 RCTs with a subpopulation of recurrent cases, but reported that only two studies included primary cases which had midurethral tapes (Abdel‐Fattah 2010; Maher 2004). We included Abdel‐Fattah 2010 in our review, but excluded Maher 2004. For all recurrent cases in Agur 2013, there was no difference in subjective (odds ratio (OR) 0.84, 95% confidence intervals (CI) 0.41 to 1.69) or objective cure (OR 1.75, 95% CI 0.86 to 3.54) after transobturator or retropubic midurethral tape (123 women). From two trials (25 participants), there was no difference between autologous fascial slings and retropubic midurethral tape (subjective cure: OR 0.83, 95% CI 0.11 to 6.26; objective cure: OR 1.43, 95% CI 0.22 to 9.26). One study (51 participants) reported similar subjective (OR 0.33, 95% CI 0.01 to 8.57) and objective (OR 0.52, 95% CI 0.13 to 2.05) cure rates between colposuspension and retropubic midurethral tape. One other study of 93 women showed no difference between colposuspension and fascial sling in objective cure (OR 0.62, 95% CI 0.27 to 1.41).
Nikolopoulos 2015 carried out a systematic review of literature on currently practiced procedures for recurrent SUI, without restriction on methodology, focusing on study quality and treatment outcome. They reviewed 52 studies from an initial 732 identified from searches. Using both the Oxford Centre for Evidence‐Based Medicine (OCEBM) and GRADE criteria, they found significant heterogeneity and many studies were low‐quality. The common characteristic of all procedures for recurrent SUI was a lower success rate compared to the primary procedure.
Brief economic commentary
To supplement the main systematic review of intervention effects, we searched for economic evaluations comparing conservative treatment with medical or further surgical treatments, or comparing two different surgical treatments, of recurrent SUI after failed midurethral tape surgery in women.
These searches identified no eligible economic evaluations. Although we encountered published economic evaluations that have assessed interventions for treating SUI in women (for example, Boyers 2013; Kunkle 2015), none have investigated interventions among women who have previously had a failed midurethral tape surgery. This finding highlights a current lack of evidence for the impacts on costs (resource use) of interventions for managing recurrent stress urinary incontinence among this specific group of women, as well as evidence for their cost‐effectiveness.
Authors' conclusions
Implications for practice.
To date there is no high‐quality, trial‐based evidence that can usefully inform treatment decisions on the management of recurrent stress urinary incontinence (SUI) after a failed midurethral tape (MUT). No comparative randomised studies exist with sufficient participants and long‐term follow‐up to generate enough robust data to identify clinically important differences in cure rates, complications or adverse events after the different available treatment options. Given the high prevalence of MUT surgery for primary incontinence over the last 20 years, it is likely that even this weak evidence is no longer relevant. In view of the absence of any evidence comparing the alternative management options for failed primary midurethral tape surgery, clinicians must rely largely on expert opinion or personal experience when advising women about treatment options.
Implications for research.
None of the prestated objectives of this review have been satisfactorily addressed by the published trials. The absence of evidence in the management of recurrent or persistent stress incontinence after a failed midurethral tape indicates the need for well‐designed randomised controlled trials comparing interventions to answer this question. Such studies should attempt to randomise women who have previously undergone midurethral tape surgery, or clearly separate data for this subgroup of participants. When presenting such subgroup data, studies will need to be adequately powered.
Recent reports of national health service datasets from England and Scotland report the rate of reoperation for SUI surgery following a primary tape procedure to be 4% to 5%, although it is not entirely clear what management options specialists currently offer (Gurol‐Urganci 2018; Morling 2017). However, given the number of midurethral tapes inserted globally each year, it is likely that the numbers involved are significant and that research is warranted. Such surgical trials will necessitate multicentre collaboration. They should include careful pre‐ and post‐surgical assessment to allow evaluation of factors which may influence cure, such as positioning of the original tape, the presence of detrusor overactivity or significant symptoms of urgency, voiding function and the presence or recurrence of coexisting urogenital prolapse.
Suggested recommendations have been published (Smith 2011). Possible treatment options which need to be evaluated include conservative treatment options (lifestyle advice, pelvic floor muscle training, bladder training) and drugs (medication) in addition to further surgery. Surgical treatment options may include retropubic colposuspension, urethral bulking agents, a fascial sling procedure, artificial urethral sphincter or repeat midurethral tape.
Much more robust evidence is therefore urgently required. This encompasses evidence for the effectiveness of alternative management strategies (which is currently lacking), and their comparative costs and cost‐effectiveness.
What's new
| Date | Event | Description |
|---|---|---|
| 19 August 2019 | New search has been performed | For this update, published in 2019, the following changes were made: 1. The search was updated to 09 November 2018 and one study included. 2. The review has been substantially updated in accordance with current Cochrane guidance, including the assessment of risk of bias, development of 'Summary of findings' tables and plans to adopt the GRADE approach to assess the certainty of evidence. 3. A brief economic commentary was added to the review. 4. To reflect updated terminology and compliance with Cochrane recommendations, the title has been changed to 'Interventions for treating recurrent stress urinary incontinence after failed minimally invasive synthetic midurethral tape surgery in women'. |
| 19 August 2019 | New citation required but conclusions have not changed | 1. There are insufficient data from high‐quality trials to recommend or refute any of the different management strategies for recurrent or persistent stress incontinence after failed midurethral tape surgery. |
History
Protocol first published: Issue 10, 2011 Review first published: Issue 2, 2013
| Date | Event | Description |
|---|---|---|
| 19 December 2012 | New citation required and conclusions have changed | New review about the treatment of recurrent stress urinary incontinence after failed minimally invasive synthetic suburethral tape surgery in women |
Acknowledgements
The review authors would like to acknowledge the support and help of Luke Vale, Sheila Wallace and Lindsey Elstub (Cochrane Incontinence); and Priya Madhuvrata and Marilyn Walsh for valuable comments on drafts of this review.
Appendices
Appendix 1. Glossary of terms
Adverse event: Any untoward medical occurrence in a patient or clinical trial participant who has been administered an intervention (i.e. a drug, an investigational product, or an operation); it can be an unfavourable and unintended symptom, physical sign or laboratory finding, or a disease linked in time to the use of the intervention; it does not necessarily have to be caused by the intervention. An adverse event is considered serious if it results in death or is life‐threatening; requires or extends hospitalisation; results in persistent or significant disability; is a congenital anomaly or birth defect.
Detrusor overactivity (DO): The occurrence of involuntary contractions in the detrusor (bladder muscle) during filling cystometry (see ‘urodynamics’). These contractions are seen as a wave form on the investigation output (computer screen or paper trace), and are of variable duration and strength. The person may or may not be aware of bladder sensations, e.g. urgency or urgency incontinence at the time of the contractions. When detrusor overactivity is found during bladder function testing after a treatment, but was not recognised prior to that treatment (usually an operation), this is sometimes referred to as ‘de novo’ detrusor overactivity.
Laparoscopic: This term is used to describe surgical operations otherwise referred to as ‘keyhole’ or ‘minimally invasive’ surgery, in which a viewing instrument (laparoscope – one type of endoscope) with a fine fibre optic light cable is inserted through the abdominal wall to view internal organs or permit a surgical procedure. This requires one or more small incisions to be made, as opposed to the larger incision often required for conventional ‘open’ surgery; as a result there may be less pain, bleeding, and shorter recovery with laparoscopic operations, although they often take longer to carry out than the equivalent open procedures.
Obturator foramen: An opening in the front of the pelvis which lies between two of the main bones of the pelvis – the pubis and the ischium. The opening (foramen) is largely covered by a fibrous membrane (the obturator membrane); there is a gap in this covering (the obturator canal) through which nerves (the obturator nerve) and blood vessels (the obturator artery and vein) pass out of the pelvis into the thigh.
Overactive bladder syndrome (OABS): The report by a person of symptoms of urinary urgency, usually accompanied by going to the toilet more often during the day (frequency) or night (nocturia), or both, with or without urgency urinary incontinence; the term should only be used in the absence of urinary tract infection or other obvious condition associated with these symptoms.
Periurethral: This refers to the tissues surrounding or the area around the urethra (the bladder outlet tube – from which urine leaves the body).
Periurethral bulking agents: The injection of one of several paste‐like materials into the are surrounding the urethra to add bulk to the tissues and thereby reduce the lumen (opening), with the aim of relieving stress urinary incontinence.
Pessaries: Devices inserted into the vagina for medical purposes; they may be used for the administration of medications (analgesics, antibiotics, hormones etc.), in which case they are absorbable; more commonly they are synthetic non‐absorbable devices to provide support to the vaginal walls or uterus, or both, in women where these organs are prolapsed (dropping down as a result of childbirth or aging, and causing discomfort).
Pharmacological: Relating to the action of drugs or medications.
Randomised controlled trials: Clinical trials where participants are randomly assigned (by chance, e.g. by a computer‐generated random number sequence, or drawing lots) to one of two (usually) or more treatments or management strategies. For most areas of uncertainty, this is usually considered to provide the best quality of scientific evidence.
Retropubic space: A ‘potential’ space outside the peritoneal (abdominal) cavity, lying between the back of the pubic bones and the front surface of the bladder. The insertion of some types of midurethral tape (e.g. tension‐free vaginal tape) for the treatment of stress urinary incontinence involves passing them through the retropubic space ‐ the retropubic route.
Retropubic tape: A generic term for all midurethral tapes inserted by the retropubic route.
Stress urinary incontinence (SUI): The complaint of involuntary leakage of urine on sneezing or coughing, or on effort or physical exertion (e.g. sporting activities). Whilst not yet in widespread use, the term ‘activity‐related incontinence’ might be preferred to avoid confusion with psychological stress.
Transobturator tape: Strictly, this is a trade name for one specific version of transobturator foramen midurethral tape (the TOT®); the term tends to be used generically to refer to all devices inserted by the transobturator foramen route.
Trocar or trochar: Medical instrument originally used to relieve pressure in body cavities by draining excess fluid. Now most commonly used to insert the endoscope or other instruments during laparoscopic surgery, or to insert retropubic or transobturator foramen midurethral tapes.
Urethro‐vesical junction: The point at which the urethra (bladder outlet tube) and the bladder itself meet. This is otherwise known as the bladder neck, and has historically (prior to the introduction of midurethral tape procedures) been thought to be crucial to the prevention and treatment of stress urinary incontinence.
Urgency: The complaint of a sudden, compelling desire to pass urine, which is difficult to defer.
Urgency urinary incontinence (UUI): The complaint of involuntary loss of urine associated with the symptom of urgency.
Urodynamics: The science of bladder function testing; it includes a number of investigations carried out with the aim of reproducing a patient’s symptoms in the laboratory, to provide an explanation for their complaints, and to inform management decisions in terms of treatment options, prognosis, and side effects. The term ’urodynamics’ is often used synonymously with ‘cystometry’, a test during which the pressure/volume relationships of the bladder are monitored during the micturition cycle (the sequence of bladder filling, storage, and emptying).
Urodynamic stress incontinence (USI): This is the involuntary leakage of urine during filling cystometry (see ‘urodynamics’), associated with an increase in abdominal pressure (e.g. coughing, straining, movement), in the absence of a detrusor contraction (see ‘detrusor overactivity’).
Appendix 2. Search terms for location of randomised or quasi‐randomised studies
Cochrane Incontinence Specialised Register – search terms
The search terms used to search the Cochrane Incontinence Specialised Register are given below:
(design.cct* OR design.rct*)
AND
(topic.urine.incon.recurrent. OR topic.urine.incon.stress.recurrent.)
(All searches were of the keyword field of EndNote 2018).
The date of the last search was 9 November 2018.
Appendix 3. Search methods for location of non‐randomised studies for the first version of this review
For the first version of this review (Bakali 2013) the following searches were undertaken by one of the review authors to identify non‐randomised studies:
MEDLINE using the terms: (previous surgery OR repeat surgery) AND (mid‐urethral tape OR sub‐urethral tape);
Web of Knowledge using Science Citation Index was 'forward' searched for papers which had cited some of the papers.
Appendix 4. Electronic search methods for the brief economic commentary
We performed supplementary electronic searches designed to identify published reports of relevant economic evaluations to inform development of the brief economic commentary (BEC). We searched the following databases on 28 January 2019:
MEDLINE on OvidSP (covering 1 January 1946 to January Week 3 2019);
Embase on OvidSP (covering 1 January 1974 to 2019 Week 04); and
NHS Economic Evaluation Database (NHS EED) on the Centre for Reviews and Dissemination website (covering from the earliest record in NHS EED, dating from 1968, up to and including 31 December 2014 when their coverage ended).
The economic evaluation search filters applied to our MEDLINE and Embase search strategies for the BEC (reproduced below) are those formerly used by the UK Centre for Reviews and Dissemination (CRD) to identify published reports of full economic evaluations for indexing on NHS EED. These economic evaluation search filters remain freely available on the CRD Databases web‐pages (CRD 2015). The other lines of search syntax in these MEDLINE and Embase search strategies for the BEC are adapted from the electronic search strategies run for our Cochrane Incontinence Specialised Register along with additional terms for this population developed specifically for this review (see Appendix 2 and Appendix 3). Similarly, our NHS EED search strategy for the BEC (also reproduced below) was adapted from search strategies run for our Specialised Register and based on textword and MESH terms (capturing relevant P‐I‐C concepts) used to identify eligible studies of intervention effects. In accordance with current methods guidance (Shemilt In Press), the dates of electronic searches conducted to inform the development of the BEC are as close as practically possible to the dates of electronic searches conducted to identify eligible studies of intervention effects.
MEDLINE
MEDLINE on OvidSP (covering 1 January 1946 to January Week 3 2019) was searched on 28 January 2019 using the following search strategy:
| Set | Search Statement |
| 1. | Economics/ |
| 2. | exp "costs and cost analysis"/ |
| 3. | Economics, Dental/ |
| 4. | exp economics, hospital/ |
| 5. | Economics, Medical/ |
| 6. | Economics, Nursing/ |
| 7. | Economics, Pharmaceutical/ |
| 8. | (economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. |
| 9. | (expenditure$ not energy).ti,ab. |
| 10. | value for money.ti,ab. |
| 11. | budget$.ti,ab. |
| 12. | or/1‐11 |
| 13. | ((energy or oxygen) adj cost).ti,ab. |
| 14. | (metabolic adj cost).ti,ab. |
| 15. | ((energy or oxygen) adj expenditure).ti,ab. |
| 16. | or/13‐15 |
| 17. | 12 not 16 |
| 18. | letter.pt. |
| 19. | editorial.pt. |
| 20. | historical article.pt. |
| 21. | or/18‐20 |
| 22. | 17 not 21 |
| 23. | exp animals/ not humans/ |
| 24. | 22 not 23 |
| 25. | (incontinen$ or continen$).tw. |
| 26. | exp urinary incontinence/ |
| 27. | nycturia.tw. |
| 28. | ((bladder or detrusor or vesic$) adj5 (instability or stab$ or unstable or irritab$ or hyperreflexia or dys?ynerg$ or dyskinesi$ or irritat$)).tw. |
| 29. | (urin$ adj2 (leak$ or urge$ or frequen$)).tw. |
| 30. | dribbl$.tw. |
| 31. | bladder, neurogenic/ |
| 32. | ((bladder or detrusor or vesic$) adj2 (hyper$ or overactiv$)).tw. |
| 33. | (spinal adj2 bladder$).tw. |
| 34. | (bladder$ adj2 (neuropath$ or neurogen$ or neurolog$)).tw. |
| 35. | (nervous adj1 (pollakisur$ or pollakiur$)).tw. |
| 36. | urinary bladder, overactive/ |
| 37. | exp enuresis/ |
| 38. | enure$.tw. |
| 39. | bedwet$.tw. |
| 40. | bed‐wet$.tw. |
| 41. | (bed adj5 wet$).tw. |
| 42. | (diurnal adj5 wet$).tw. |
| 43. | diurnal‐wet$.tw. |
| 44. | ((daytime or day‐time or nighttime or night‐time or nightime) adj5 wet$).tw. |
| 45. | (void$ adj2 dysfunct$).tw. |
| 46. | ((urin$ or bladder) adj5 sphincter$).tw. |
| 47. | (urethra$ adj2 sphincter$).tw. |
| 48. | (bladder adj2 neck).tw. |
| 49. | (vesic$ adj1 (neck$ or cervi$)).tw. |
| 50. | (detrusor adj1 sphincter$).tw. |
| 51. | or/25‐50 |
| 52. | 24 and 51 |
| 53. | 2014$.ed. |
| 54. | 2015$.ed. |
| 55. | 2016$.ed. |
| 56. | 2017$.ed. |
| 57. | 2018$.ed. |
| 58. | 2019$.ed. |
| 59. | 53 or 54 or 55 or 56 or 57 or 58 |
| 60. | 52 and 59 |
| 61. | exp recurrence/ |
| 62. | secondary prevention/ |
| 63. | recur*.tw. |
| 64. | ((Fail* or repeat* or unsuccessful or previous) adj6 (tape* or sling* or surger* or operation* or surgical or treatment*)).tw. |
| 65. | persist*.tw. |
| 66. | relaps*.tw. |
| 67. | return*.tw. |
| 68. | recrudescence*.tw. |
| 69. | (symptom* adj2 flar*).tw. |
| 70. | or/61‐69 |
| 71. | 60 and 70 |
Embase
Embase on OvidSP (covering 1 January 1974 to 2019 Week 04) searched on 28 January 2019 using the following search strategy:
| Set | Search Statement |
| 1. | Health Economics/ |
| 2. | exp Economic Evaluation/ |
| 3. | exp Health Care Cost/ |
| 4. | pharmacoeconomics/ |
| 5. | (econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. |
| 6. | (expenditure$ not energy).ti,ab. |
| 7. | (value adj2 money).ti,ab. |
| 8. | budget$.ti,ab. |
| 9. | or/1‐8 |
| 10. | letter.pt. |
| 11. | editorial.pt. |
| 12. | note.pt. |
| 13. | or/10‐12 |
| 14. | 9 not 13 |
| 15. | (metabolic adj cost).ti,ab. |
| 16. | ((energy or oxygen) adj cost).ti,ab. |
| 17. | ((energy or oxygen) adj expenditure).ti,ab. |
| 18. | 15 or 16 or 17 |
| 19. | 14 not 18 |
| 20. | animal/ |
| 21. | exp animal experiment/ |
| 22. | nonhuman/ |
| 23. | (rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. |
| 24. | 20 or 21 or 22 or 23 |
| 25. | exp human/ |
| 26. | human experiment/ |
| 27. | 25 or 26 |
| 28. | 24 not (24 and 27) |
| 29. | 19 not 28 |
| 30. | conference abstract.pt. |
| 31. | 29 not 30 |
| 32. | incontinence/ or mixed incontinence/ or stress incontinence/ or urge incontinence/ or urine incontinence/ |
| 33. | continence/ |
| 34. | overactive bladder/ |
| 35. | micturition disorder/ or lower urinary tract symptom/ or pollakisuria/ |
| 36. | urinary dysfunction/ or bladder instability/ or detrusor dyssynergia/ or neurogenic bladder/ or urinary urgency/ or urine extravasation/ |
| 37. | (incontinen$ or continen$).tw. |
| 38. | ((bladder or detrusor or vesic$) adj5 (instab$ or stab$ or unstab* or irritab$ or hyperreflexi$ or dys?ynerg$ or dyskinesi$ or irritat$)).tw. |
| 39. | (urin$ adj2 leak$).tw. |
| 40. | ((bladder or detrusor or vesic$) adj2 (hyper$ or overactiv$)).tw. |
| 41. | (bladder$ adj2 (neuropath$ or neurogen* or neurolog$)).tw. |
| 42. | (nervous adj pollakisur$).tw. |
| 43. | or/32‐42 |
| 44. | 31 and 43 |
| 45. | "2015".yr. |
| 46. | "2016".yr. |
| 47. | "2017".yr. |
| 48. | "2018".yr. |
| 49. | 45 or 46 or 47 or 48 |
| 50. | 44 and 49 |
| 51. | 44 and 49 |
| 52. | limit 51 to (conference abstracts or embase) |
| 53. | limit 52 to embase |
| 54. | recurrent disease/ |
| 55. | secondary prevention/ |
| 56. | recur*.tw. |
| 57. | ((Fail* or repeat* or unsuccessful or previous) adj6 (tape* or sling* or surger* or operation* or surgical or treatment*)).tw. |
| 58. | persist*.tw. |
| 59. | relaps*.tw. |
| 60. | return*.tw. |
| 61. | recrudescence*.tw. |
| 62. | (symptom* adj2 flar*).tw. |
| 63. | 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 |
| 64. | 52 and 63 |
| 65. | limit 64 to (conference abstracts or embase) |
NHS Economic Evaluation Database (NHS EED)
The NHS Economic Evaluation Database (NHS EED) was searched on the Centre for Reviews and Dissemination website (covering from the earliest record in NHS EED, dating from 1968, up to and including 31 December 2014 when their coverage ended) on 28 January 2019 using the following search strategy (searching all fields):
| Line | Search | Hits |
| 1 | MeSH DESCRIPTOR pelvic floor EXPLODE ALL TREES IN NHSEED | 11 |
| 2 | MeSH DESCRIPTOR pelvic floor disorders EXPLODE ALL TREES IN NHSEED | 1 |
| 3 | MeSH DESCRIPTOR Urinary Bladder, Neurogenic EXPLODE ALL TREES IN NHSEED | 7 |
| 4 | MeSH DESCRIPTOR Urinary Bladder, overactive EXPLODE ALL TREES IN NHSEED | 27 |
| 5 | ((incontinen* ) OR (continen*)) IN NHSEED | 208 |
| 6 | ((floor adj2 pelvi* ) OR (pelvi* adj2 floor)) IN NHSEED | 21 |
| 7 | ((nycturia)) IN NHSEED | 0 |
| 8 | (((urin* or bladder) adj5 sphincter*) OR (sphincter* adj5 (urin* or bladder))) IN NHSEED | 6 |
| 9 | (((bladder OR detrusor OR vesic*) ADJ5 (instability OR stab* OR unstable OR irritab* OR hyperreflexia OR dysynerg* OR dyskinesi* OR irritat*)) OR ((instability OR stab* OR unstable OR irritab* OR hyperreflexia OR dysynerg* OR dyskinesi* OR irritat*) ADJ5 (bladder OR detrusor OR vesic*) )) IN NHSEED | 5 |
| 10 | ((urethra* ADJ2 sphincter*) OR (sphincter* ADJ2 urethra* )) IN NHSEED | 0 |
| 11 | ((bladder ADJ2 neck) OR (neck ADJ2 bladder )) IN NHSEED | 16 |
| 12 | ((urin* ADJ2 (leak* OR urge* OR frequen*)) OR ((leak* OR urge* OR frequen*) ADJ2 urin* )) IN NHSEED | 21 |
| 13 | (dribbl*) IN NHSEED | 0 |
| 14 | ((vesic* ADJ1 (neck* OR cervi*)) OR ((neck* OR cervi*) ADJ1 vesic*)) IN NHSEED | 0 |
| 15 | (((bladder OR detrusor OR vesic*) ADJ2 (hyper* OR overactiv*)) OR ((hyper* OR overactiv*) ADJ2 (bladder OR detrusor OR vesic*))) IN NHSEED | 36 |
| 16 | ((detrusor ADJ1 sphincter*) OR (sphincter* ADJ1 detrusor)) IN NHSEED | 0 |
| 17 | ((spinal ADJ2 bladder*) OR (bladder* ADJ2 spinal)) IN NHSEED | 4 |
| 18 | ((bladder* ADJ2 (neuropath* OR neurogen* OR neurolog*)) OR ((neuropath* OR neurogen* OR neurolog*) ADJ2 bladder*)) IN NHSEED | 11 |
| 19 | ((nervous ADJ1 (pollakisur* OR pollakiur*)) OR ((pollakisur* OR pollakiur*) ADJ1 nervous)) IN NHSEED | 0 |
| 20 | (MeSH DESCRIPTOR urinary incontinence EXPLODE ALL TREES) IN NHSEED | 70 |
| 21 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 | 253 |
| 22 | (MeSH DESCRIPTOR Recurrence EXPLODE ALL TREES ) IN NHSEED | 354 |
| 23 | (MeSH DESCRIPTOR Secondary prevention EXPLODE ALL TREES ) IN NHSEED | 130 |
| 24 | ((((Fail* or repeat* or unsuccessful or previous) adj6 (tape* or sling* or surger* or operation* or surgical or treatment*))) OR (((tape* or sling* or surger* or operation* or surgical or treatment*) adj6 (Fail* or repeat* or unsuccessful or previous))) ) IN NHSEED | 962 |
| 25 | (Persist*) IN NHSEED | 457 |
| 26 | (Return* OR Relaps* OR recrudescence*) IN NHSEED | 1120 |
| 27 | ((symptom* adj2 flar*) OR (flar* adj2 symptom*)) IN NHSEED | 1 |
| 28 | (recur*) IN NHSEED | 1175 |
| 29 | #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 | 3150 |
| 30 | #21 AND #29 | 67 |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdel‐Fattah 2010.
| Methods |
Study design: prospective RCT with secondary analysis reporting on the population of interest (Abdel‐Fattah 2011a) Dates study conducted: April 2005 to April 2007 |
|
| Participants |
Setting: tertiary urogynaecology centre Country: Scotland, UK Total number of participants: 341 Number of women eligible for review: 46 (participants had previously undergone continence surgery (Group I (inside‐out TVT‐O): n = 28; Group II (outside‐in TOT ARIS): n = 18)), as reported in 2011 secondary analysis (Abdel‐Fattah 2011a) Age: Group I mean = 51.5 years; Group II mean = 52.1 years (for secondary analysis, Group I mean = 55.22 years (SD 9.70); Group II mean = 56.73 years (SD 11.66)) Sex: female Any relevant details of health status of participants: overall, 46 participants had previously undergone continence surgery (Group I n = 28; Group II n = 18). 88 participants had previously undergone hysterectomy, 25 participants had undergone anterior repair and 61 participants had previously undergone antimuscarinic treatment. For the participants in the secondary analysis, the median duration of years between previous and repeat continence surgery was 4.8 years, ranging from 6 months to 12 years. Four women described persistent SUI from initial surgery. Fifteen participants had previously undergone colposuspension, 15 women had previous retropubic TVT, 11 women had previously had transobturator tapes and five women had previously received both colposuspension and midurethral tape. 23/46 had also had a previous hysterectomy. Inclusion criteria: women with SUI or MUI but with predominantly bothersome SUI. Women with previous surgery for incontinence were included. All of the participants had either failed or declined pelvic floor muscle training. Exclusion criteria: unwilling to be randomised, had predominant OAB symptoms, specific comorbidities (e.g. MS, diabetes), pelvic organ prolapse (≥ stage 2 POP‐Q) or concomitant surgery Baseline assessment methods: detailed history, pelvic examination, urodynamic assessment (e.g. free uroflowmetry, subtracted multichannel cystometry and urethral pressure profile), completion of KHQ, Birmingham Bowel Urinary Symptom Questionnaire and PISQ‐12 |
|
| Interventions |
Group I (n = 170; secondary analysis n = 28): inside‐out TVT‐O Group II (n = 171; secondary analysis n = 18): outside‐in TOT ARIS Procedures mostly took place in a day surgery unit under general anaesthesia. All participants received intraoperative prophylactic antibiotics. Intraoperative cough stress tests and cystoscopies were not routinely performed. |
|
| Outcomes | Outcomes of the secondary analysis are detailed below (Abdel‐Fattah 2011a). Primary outcomes: patient‐reported success (assessed by Patient Global Impression of Improvement as "very much improved" or "much improved") Secondary outcomes: objective cure (defined as negative pad test findings of ≤ 1 g gain); quality of life (changes in KHQ score); effect on sexual life (changes in PISQ‐12 total score) |
|
| Notes |
Study funding sources: Henry Smith Charity (Abdel‐Fattah 2011a); a late published correction indicated commercial support from the manufacturer of a device used in the study (Abdel‐Fattah 2019) Intention‐to‐treat: not reported Follow‐up: the secondary analysis reports 12 month follow‐up. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Women were assigned to either procedure by random allocation (computer generated)" Comment: adequate method of randomisation, probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation was concealed using opaque sealed envelopes which were opened by the nursing staff on the morning of the operation" Comment: adequate allocation concealment method, probably done. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Women were informed about the type of operation if they wished, for ethical considerations" Comment: participants had the option to be informed of which of the two arms they had been assigned to. It would have been impossible to blind the personnel performing the surgery of the specific intervention in each procedure. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Post‐operative assessment at 6 months was performed by an independent clinician who was blinded to the type of surgery" Comment: the outcome assessor was initially adequately blinded. However, although they had been instructed not to do so, there is a possibility that any participants who had been informed of which intervention they had received could have revealed this information during their assessment, potentially introducing bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Six‐month follow‐up was completed by 317 women but 15 participants declined post‐operative UDS" Comment: there was a high level of attrition. There was a 11.4% loss to follow‐up. Additionally, not all of these 317 women undertook UDS. |
| Selective reporting (reporting bias) | High risk | The outcome measures as detailed by the study have been reported. However, there were changes in the outcome measures predefined in the trial registration (Abdel‐Fattah 2005). |
| Other bias | High risk | Although commercial funding for the study was not declared in any of the publications or abstracts from this study (Abdel‐Fattah 2008a; Abdel‐Fattah 2008b; Abdel‐Fattah 2010a; Abdel‐Fattah 2010b; Abdel‐Fattah 2010c; Abdel‐Fattah 2011a; Abdel‐Fattah 2011b; Abdel‐Fattah 2011c; Abdel‐Fattah 2012; Abdel‐Fattah 2014; Abdel‐Fattah 2017a; Abdel‐Fattah 2017b; Abdel‐Fattah 2017c; Abdel‐Fattah 2017d; Hopper 2013a; Hopper 2013b; Karmakar 2015a; Karmakar 2015b; Karmakar 2017a; Karmakar 2017b; Mostafa 2011), a revised funding statement was published in 2019 indicating grant support from one of the device manufacturers concerned (Abdel‐Fattah 2019). This poses a conflict of interest or form of bias potentially affecting the quality of the evidence. |
KHQ = King's Health Questionnaire MS = multiple sclerosis MUI = mixed urinary incontinence OAB = overactive bladder PISQ‐12 = Pelvic Organ Prolapse/Incontinence Sexual Function Questionnaire POP‐Q = Pelvic Organ Prolapse Quantification System RCT = randomised controlled trial SD = standard deviation SUI = stress urinary incontinence TOT = transobturator tape TVT = tension‐free vaginal tape UDS = urodynamic study
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barber 2008 | Not an RCT. This is a secondary analysis using logistic regression to determine risk factors for recurrent UI. The treatment of recurrent UI is not addressed. |
| Cardozo 2002 | Authors were contacted but the participants had a previous colposuspension and not a tape procedure. |
| Enzelsberger 1993 | Women had hysterectomies with an anterior repair. There was no mention of previous tape surgery. |
| Enzelsberger 1996 | Women had hysterectomies with an anterior repair. There was no mention of previous tape surgery. |
| Hilton 2002 | Ineligible participants: neither the trial report or personal communication with the trialists could confirm whether any of the participants had previously undergone surgery with tape. |
| Kociszewski 2016 | Not an RCT. |
| Maher 2004 | Ineligible participants: neither the trial report or personal communication with the trialists could confirm whether any of the participants had previously undergone surgery with tape. |
| Smithling 2017 | Not an RCT. |
| Tincello 2017 | Not an RCT. |
| Wallwiener 1995 | Previous incontinence surgery was not tape. |
| Watson 2002 | Ineligible participants: neither the trial report or personal communication with the trialists could confirm whether any of the participants had previously undergone surgery with tape. |
| Zimmern 2016 | Not an RCT. This is a secondary analysis of two separate RCTs. |
RCT = randomised controlled trial UI = urinary incontinence
Characteristics of studies awaiting assessment [ordered by study ID]
Carr 2017.
| Methods |
Study design: RCT Dates study conducted: between February 2012 and January 2015 |
| Participants | Setting: tertiary care centre Total number of participants: 143 Sex: female Number of women eligible for review: 2 participants had previously undergone TVT/TOT, as reported in the conference abstract (Ismail 2017). Inclusion criteria: women with predominant SUI who experienced ≥ 3 stress incontinence episodes in 3 days |
| Interventions |
Group I (n = 50): vehicle placebo
Group II (n = 93): Autologous Muscle Derived Cells for Urinary Sphincter Repair (AMDC‐USR) Randomisation was 2:1 to receive intrasphincteric injection of 150 x 106 AMDC‐USR or vehicle placebo and 1:1 to receive 1 or 2 treatments. Second treatments were administered 6 months after first treatments. Intrasphincteric injections were administered at 9 circumferential locations; re‐injections were undertaken at 6 months. |
| Outcomes | The primary composite endpoint was the percentage of participants with ≥ 50% reduction in incontinence episode frequency (IEF) from a 3‐day diary or ≥ 50% reduction in either 24 hour or in‐office pad test 12 months post‐treatment. No secondary outcomes were defined, although the authors used alternative outcomes in a post‐hoc analysis in an effort to show a reduced placebo effect. |
| Notes |
Follow‐up: for 2 years after initial treatment (141 participants completed their 12‐month visit and 127 completed the 2‐year visit). Recruitment halted prematurely in view of high placebo response rate. We are aware that there is a full report of this study published after the search date for this review. Authors were contacted for additional information regarding population of interest on 27 November 2018; no response was obtained by 25 February 2019 (Hilton 2018 [pers comm]). |
AMDC‐USR = Autologous Muscle Derived Cells for Urinary Sphincter Repair IEF = incontinence episode frequency RCT = randomised controlled trial SUI = stress urinary incontinence TOT = transobturator tape TVT = tension‐free vaginal tape
Differences between protocol and review
For the 2019 update
The title of the review has been changed to comply with Cochrane standards and "suburethral" has been changed to "midurethral" for standardisation with other Cochrane Reviews (Ford 2017).
Methods
The review has been substantially updated in accordance with current Cochrane guidance, including the assessment of 'Risk of bias', development of 'Summary of findings' tables and the adoption of the GRADE approach to assess the certainty of evidence.
For the first version of the review, non‐randomised controlled trials were sought (Bakali 2013). The protocol had not prespecified this search and, for this version of the review, the search for non‐randomised studies has not been updated.
One comparison (repeat suburethral sling versus single incision sling) has been removed from this version as this area is already covered by the other proposed comparisons in the review.
A new comparison has been added (surgical treatment (e.g. traditional sling, colposuspension, injectables) versus placebo or sham treatment) as it was agreed that this was a clinically important comparison for this topic area.
We have revised some of the outcome measures from the previous version, in particular by splitting general quality of life measures and condition‐specific quality of life measures into two separate outcomes and further defining adverse events, making repeat continence surgery a separate secondary outcome measure. These decisions were taken based on their clinical importance.
Study selection
On screening titles and abstracts, three studies excluded in the first version of this review (Bakali 2013) could be readily assessed as not meeting the eligibility criteria (Ashok 2010; Lovatsis 2010; Schraffordt 2006). The three studies have now been removed from the Characteristics of excluded studies.
One study that had been excluded from the first version of this review (Bakali 2013) has now been found to meet the eligibility criteria and has been included (Abdel‐Fattah 2010)
Contributions of authors
All review authors contributed to the conducting and writing of this version of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Incontinence. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health. The NIHR Cochrane Infrastructure grant is the single largest funder of Cochrane Incontinence.
Declarations of interest
EB: Wellbeing of Women training fellowship. EJ: is Editorial Assistant for Cochrane Incontinence, whose single largest funder is the UK National Institute for Health Research (NIHR). She did not participate in the editorial process for this review. BB: none known PH: has no current financial interests to declare. Within the last 20 years he has received commercial research funding for trials of surgery for SUI from Gynecare (1998 to 2003) and Gynae Ideas (2001 to 2003), and received reimbursement of travel expenses to attend meetings in connection with these studies. He was chair of the NICE guideline development group on urinary incontinence in women (2004 to 2007), and received an honorarium and travel expenses in association with this role. He has previously been a member of the NICE Interventional Procedures Advisory Committee, National Coordinating Centre for Health Technology Assessment Therapeutic Procedures Panel (2007 to 2008) and Clinical Evaluations and Trials Prioritisation Group (2008 to 2010), and the International Consultation on Incontinence section on surgery in women (2007 to 2009). Reimbursement of, or contribution to, travel expenses was received in respect of these activities. He was a member of The Scottish Independent Review of the Use, Safety and Efficacy of Transvaginal Mesh Implants in the Treatment of Stress Urinary Incontinence and Pelvic Organ Prolapse in Women (2014‐2017), and was commissioned by the NHS National Services Scotland Central Legal Office, to provide expert advice in relation to legal claims relating to the use of mesh in gynaecological surgery (2014‐2018). BW: none known DT: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Abdel‐Fattah 2010 {published data only}
- Abdel‐Fattah M. Authors' reply re: Long‐term outcomes of transobturator tapes in women with stress urinary incontinence: E‐TOT randomized controlled trial [letter]. BJOG: an International Journal of Obstetrics & Gynaecology 2017;124(10):1626. [sr‐incont76721] [DOI] [PubMed] [Google Scholar]
- Abdel‐Fattah M. No data available for the subset of participants with failed tape [personal communication]. Email to: D Tincello Exact date unknown: late 2017 to 2018. [NCT00136071; TRIALID.ETOT; sr‐incont41485]
- Abdel‐Fattah M, Cao G, Mostafa A. Long‐term outcomes for transobturator tension‐free vaginal tapes in women with urodynamic mixed urinary incontinence. Neurourology and Urodynamics 2017;36(4):902‐8. [sr‐incont74214] [DOI] [PubMed] [Google Scholar]
- Abdel‐Fattah M, Cao G, Mostafa A. Long‐term outcomes of transobturator tension‐free vaginal tapes as secondary continence procedures. World Journal of Urology 2017;35(7):1141‐8. [sr‐incont74555] [DOI] [PubMed] [Google Scholar]
- Abdel‐Fattah M, Familusi A, Ramsay I, Ayansina D, Mostafa A. Preoperative determinants for failure of transobturator tapes in the management of female urodynamic stress incontinence. International Journal of Gynaecology and Obstetrics 2010;110(1):18‐22. [NCT00136071; sr‐incont40971] [DOI] [PubMed] [Google Scholar]
- Abdel‐Fattah M, Familusi A, Ramsay I, N'Dow J. A randomised prospective single‐blinded study comparing "inside‐out" versus "outside‐in" transobturator tapes in the management of female stress urinary incontinence (E‐TOT study); 3 years follow‐up. Neurourology and Urodynamics 2011;30(6):825‐6. [Abstract number 18; NCT00136071; sr‐incont42168] [Google Scholar]
- Abdel‐Fattah M, Hasafa Z, Mostafa A. Correlation of three validated questionnaires for assessment of outcomes following surgical treatment of stress urinary incontinence in women. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2011;157(2):226‐9. [NCT00136071; sr‐incont41753] [DOI] [PubMed] [Google Scholar]
- Abdel‐Fattah M, Hopper LR, Mostafa A. Evaluation of transobturator tension‐free vaginal tapes in the surgical management of mixed urinary incontinence: 3‐year outcomes of a randomized controlled trial. Journal of Urology 2014;191(1):114‐9. [NCT00136071; sr‐incont50402] [DOI] [PubMed] [Google Scholar]
- Abdel‐Fattah M, Mostafa A, Familusi A, Ramsay I, N'Dow J. Corrigendum re "Prospective Randomised Controlled Trial of Transobturator Tapes in Management of Urodynamic Stress Incontinence in Women: 3‐Year Outcomes from the Evaluation of Transobturator Tapes Study" [Eur Urol 2012;62:843‐51. PMID: 22534058]. European Urology 2019;75(4):e119. [DOI: 10.1016/j.eururo.2019.01.007; sr‐incont78146] [DOI] [PubMed] [Google Scholar]
- Abdel‐Fattah M, Mostafa A, Familusi A, Ramsay I, N'Dow J. Prospective randomised controlled trial of transobturator tapes in management of urodynamic stress incontinence in women: 3‐year outcomes from the evaluation of transobturator tapes study [Corrigendum in: European Urology 2019;75(4):e119, doi: 10.1016/j.eururo.2019.01.007. PMID: 30665811]. European Urology 2012;62(5):843‐51. [NCT00136071; sr‐incont45947] [DOI] [PubMed] [Google Scholar]
- Abdel‐Fattah M, Ramsay I, NCT00136071. Transvaginal tension free vaginal tape‐obturator (TVT‐O) versus transobturator tape‐mentor (TOT) in the management of urodynamic stress urinary incontinence [Randomised prospective blinded trial comparing transvaginal tension free vaginal tape‐obturator (outside‐in) with transobturator tape‐mentor (inside‐out) in surgical management of urodynamic stress urinary incontinence]. clinicaltrials.gov/show/NCT00136071 (first received 26 August 2005). [NCT00136071; sr‐incont40211]
- Abdel‐Fattah M, Ramsay I, Pringle S, Hardwick C, Ali H. Evaluation of transobturator tapes (E‐TOT) study: randomised prospective single‐blinded study comparing inside‐out vs. outside‐in transobturator tapes in management of urodynamic stress incontinence: short term outcomes. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2010;149(1):106‐11. [NCT00136071; sr‐incont39661] [DOI] [PubMed] [Google Scholar]
- Abdel‐Fattah M, Ramsay I, Pringle S, Hardwick C, Ali H, Young D, et al. Evaluation of transobturator tension‐free vaginal tapes in management of women with recurrent stress urinary incontinence. Urology 2011;77(5):1070‐5. [NCT00136071; TRIALID.ETOT; sr‐incont41485] [DOI] [PubMed] [Google Scholar]
- Abdel‐Fattah M, Ramsay I, Pringle S, Hardwick C, Ali H, Young D, et al. Randomised prospective single‐blinded study comparing 'inside‐out' versus 'outside‐in' transobturator tapes in the management of urodynamic stress incontinence: 1‐year outcomes from the E‐TOT study. BJOG: an International Journal of Obstetrics & Gynaecology 2010;117(7):870‐8. [NCT00136071; sr‐incont39920] [DOI] [PubMed] [Google Scholar]
- Abdel‐Fattah M, Ramsay I, Pringle S, Hardwick C, Tierney J, Ali H. (E‐TOT) Study: a randomised prospective single‐blinded study of two transobturator tapes in management of urodynamic stress incontinence: objective & patient reported outcomes. International Urogynecology Journal 2008;19(Suppl 2):S2‐3. [Abstract number 2; NCT00136071; sr‐incont31053] [Google Scholar]
- Abdel‐Fattah M, Ramsay I, Pringle S, Hardwick C, Tierney J, Young D. (E‐TOT) Study: a randomised prospective single blinded study of two transobturator tapes in management of urodynamic stress incontinence: quality of life; sexual function at 1‐year. International Urogynecology Journal 2008;19(Suppl 2):S168‐9. [Abstract number 200; NCT00136071; sr‐incont31050] [Google Scholar]
- Hopper LR, Mostafa A, Abdel‐Fattah M. Evaluation of transobturator tapes in the surgical management of women with mixed urinary incontinence at 3 years follow‐up (Abstract). Journal of Obstetrics and Gynaecology 2013;33(8):920. [NCT00136071; sr‐incont62157] [Google Scholar]
- Hopper LR, Mostafa A, Abdel‐Fattah M. The effectiveness of transobturator tapes in the surgical management of women with mixed urinary incontinence: 3 year outcomes. 43rd Annual Meeting of the International Continence Society (ICS), 2013 Aug 26‐30, Barcelona, Spain. 2013. [Abstract number 612; NCT00136071; sr‐incont60036]
- Karmakar D, Mostafa A, Abdel‐Fattah M. A new validated score for detecting patient‐reported success on postoperative ICIQ‐SF: a novel two‐stage analysis from two large RCT cohorts. International Urogynecology Journal 2017;28(1):95‐100. [sr‐incont73074] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmakar D, Mostafa A, Abdel‐Fattah M. A new validated score for detecting patient‐reported success on postoperative ICIQ‐SF: a novel two‐stage analysis from two large RCT cohorts. International Urogynecology Journal and Pelvic Floor Dysfunction 2015;26(1 Suppl 1):S81‐2. [Abstract number PP 54; NCT00136071; sr‐incont68575] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmakar D, Mostafa A, Abdel‐Fattah M. Long‐term outcomes (8‐years) from the prospective randomized control trial of trans‐obturator tapes for stress incontinence in women (the ETOT study). International Urogynecology Journal and Pelvic Floor Dysfunction 2015;26(1 Suppl 1):S74‐5. [Abstract number PP 48; NCT00136071; sr‐incont68577] [Google Scholar]
- Karmakar D, Mostafa A, Abdel‐Fattah M. Long‐term outcomes of transobturator tapes in women with stress urinary incontinence: E‐TOT randomised controlled trial. BJOG: an International Journal of Obstetrics & Gynaecology 2017;124(6):973‐81. [sr‐incont74221] [DOI] [PubMed] [Google Scholar]
- Mostafa A, Madhuvrata P, Abdel‐Fattah M. Preoperative urodynamic predictors of short‐term voiding dysfunction following a transobturator tension‐free vaginal tape procedure. International Journal of Gynaecology and Obstetrics 2011;115(1):49‐52. [NCT00136071; sr‐incont42671] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Barber 2008 {published data only}
- Barber MD, Kleeman S, Karram MM, Paraiso MF, Ellerkmann M, Vasavada S, et al. Risk factors associated with failure 1 year after retropubic or transobturator midurethral slings. American Journal of Obstetrics and Gynecology 2008;199:666.e1‐e7. [sr‐incont29239] [DOI] [PubMed] [Google Scholar]
Cardozo 2002 {published data only (unpublished sought but not used)}
- Cardozo L, Rufford J. Comparative study of the efficacy, acceptability, morbidity and cost‐effectiveness of the 'Tension Free Vaginal Tape' and the periurethral injection of collagen in the management of recurrent stress incontinence. The National Research Register 2001, Issue 4. [N0116091776; sr‐incont16380]
- Cardozo L, Rufford J. Comparative study of the efficacy, acceptability, morbidity and cost‐effectiveness of the 'Tension Free Vaginal Tape' and the periurethral injection of collagen in the management of recurrent stress incontinence ‐ additional information [personal communication]. Email to: Cochrane Incontinence Information Specialist June 2002. [N0116091776; sr‐incont16380]
Enzelsberger 1993 {published data only}
- Enzelsberger H, Kurz C, Seifert M, Raimann H, Schatten C. Surgical treatment of recurrent stress incontinence: Burch versus lyodura sling operation‐‐a prospective study [Zur operativen Behandlung der Rezidivstreßinkontinenz: Burch‐versus Lyoduraschlingenoperation ‐ eine prospektive Studie]. Geburtshilfe Frauenheilkunde 1993;53:467‐71. [sr‐incont100] [DOI] [PubMed] [Google Scholar]
Enzelsberger 1996 {published data only}
- Enzelsberger H, Helmer H, Schatten C. Comparison of Burch and Lyodura sling procedures for repair of unsuccessful incontinence surgery. Obstetrics and Gynecology 1996;88(2):251‐6. [sr‐incont2759] [DOI] [PubMed] [Google Scholar]
Hilton 2002 {unpublished data only}
- Hilton P. A prospective randomised comparative trial of a tension‐free vaginal tape (TVT) and fascial sling procedure for 'secondary' genuine stress incontinence. The National Research Register 2001, Issue 1. [N0503016202; sr‐incont16383]
- Hilton P. Additional information about an ongoing trial. Email to: Cochranae Incontinence Information Specialist [personal communication] 11 June 2002. [N0503016202; sr‐incont16383]
- Hilton P. Re: Cochrane: Treatment of recurrent stress urinary incontinence after failed minimally invasive synthetic suburethral tape surgery in women (098) [personal communication]. Email to: Lindsey Elstub and Evangelia Bakali 31 August 2018. [N0503016202; sr‐incont16383] [DOI] [PubMed]
Kociszewski 2016 {published data only}
- Kociszewski J, Majkusiak W, Pomian A, Tomasik P, Horosz E, Kuszka A, et al. The outcome of repeated mid urethral sling in SUI treatment after vaginal excisions of primary failed sling: preliminary study. BioMed Research International 2016;2016:1242061. [DOI: 10.1155/2016/1242061; sr‐incont75467] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maher 2004 {published data only}
- Maher C. No further data available [personal communication]. Email to: D Tincello Exact date unknown late 2017 to 2018. [sr‐incont19005]
- Maher C, Qatawneh A, Baessler K, Cropper M, Schluter P. Laparoscopic colposuspension or tension‐free vaginal tape for recurrent stress urinary incontinence and/or intrinsic sphincter deficiency – a randomised controlled trial. Neurourology and Urodynamics 2004;23(5/6):433‐4. [Abstract number 25; sr‐incont19005] [Google Scholar]
Smithling 2017 {published data only}
- Smithling KR, Tran AM, Dave B, Chu CM, Chan R, Antosh DD, et al. Efficacy of repeat mid‐urethral sling for persistent or recurrent stress urinary incontinence. American Journal of Obstetrics and Gynecology 2017;216(3 Suppl 1):S612‐3. [Abstract number 66; sr‐incont77288] [Google Scholar]
Tincello 2017 {published data only}
- Tincello D, Bach F, Toozs‐Hobson P. Surgery for recurrent stress incontinence in the UK 2007‐2015. Neurourology and Urodynamics 2017;36(Suppl S3):S200‐1. [Abstract number 239; sr‐incont77698] [Google Scholar]
Wallwiener 1995 {published data only}
- Wallwiener D, Grischke EM, Rimbach S, Maleika A, Bastert G. Endoscopic retropubic colposuspension: "Retziusscopy" versus laparoscopy‐‐a reasonable enlargement of the operative spectrum in the management of recurrent stress incontinence?. Endoscopic Surgery and Allied Technologies 1995;3(2‐3):115‐8. [sr‐incont2928] [PubMed] [Google Scholar]
Watson 2002 {unpublished data only}
- Doyle PM. Comparison of two surgical methods for curing stress incontinence (recurrent) ‐ additional information [personal communication]. Email to: Cochrane Incontinence Information Specialist 13 June 2002. [N0280055971; sr‐incont16382]
- Watson C. Comparison of two surgical methods for curing stress incontinence (recurrent). The National Research Register 2001, Issue 4. [N0280055971; sr‐incont16382]
Zimmern 2016 {published data only}
- Zimmern PE, Gormley EA, Stoddard AM, Lukacz ES, Sirls L, Brubaker L, et al. Management of recurrent stress urinary incontinence after burch and sling procedures. Neurourology and Urodynamics 2016;35(3):344‐8. [sr‐incont74150] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Carr 2017 {published data only}
- Carr L, Mai TL, Robert M, Quinlan D, Carlson K, Herschorn S, et al. Randomized, double‐blind, placebo‐controlled study of autologous muscle derived cells for urinary sphincter repair. Journal of Urology 2017;197(4 Suppl 1):e609‐10. [Abstract number PNFBA‐03; sr‐incont77566] [Google Scholar]
- Carr L, Tu L, Robert M, Quinlan D, Carlson K, Herschorn S, et al. Autologous muscle derived cells for urinary sphincter repair in women with stress urinary incontinence: preliminary assessment of single and multiple treatment modalities. International Gynecology Journal and Pelvic Floor Dysfunction 2017; Vol. 28, issue 1 Suppl 1:S41‐2. [Abstract number 046; sr‐incont77978]
- Carr L, Tu LM, Magali R, Quinlan D, Carlson K, Herschorn S, et al. Autologous muscle derived cells for urinary sphincter repair for recurrent or persistent stress urinary incontinence after continence surgery. Journal of Urology 2017;197(4 Suppl 1):e982. [Abstract number PD50‐08; sr‐incont77215] [Google Scholar]
- Carr L, Tu LM, Robert M, Quinlan D, Carlson KV, Herschorn S, et al. A randomized, double‐blind, multicenter, placebo‐controlled study of autologous muscle derived cells for urinary sphincter repair (AMDC‐USR). Neurourology and Urodynamics 2017;36(Suppl S1):S35‐6. [Abstract number: Poster #M9; sr‐incont75956] [Google Scholar]
- Carr LK, Tu L, Robert M, Quinlan D, Carlson KV, Herschorn S, et al. Autologous muscle derived cells for urinary sphincter repair to treat women with recurrent or persistent stress urinary incontinence after continence surgery. International Urogynecology Journal and Pelvic Floor Dysfunction 2017; Vol. 28, issue 1 Suppl 1:S131‐2. [Abstract number 210; sr‐incont77979]
- Carr LK, Tu LM, Robert M, Quinlan D, Carlson KV, Jankowski R, et al. Autologous muscle‐derived cells for urinary sphincter repair in women with stress urinary incontinence: results of a randomized, double‐blind, placebo‐controlled study. Canadian Urological Association Journal 2017;11(6 Suppl 4):S182‐3. [Abstract number POD‐03.05; sr‐incont77218] [Google Scholar]
- Ismail S, Tu L, Morin M. Predictive factors for the efficacy of autologous muscle derived cell injections in women with stress urinary incontinence. Neurourology and Urodynamics 2017;36(Suppl S3):S273‐4. [Abstract number 293; sr‐incont77697] [Google Scholar]
- Pruchnic R, Jankowski R, Kaufman M. Lessons learned from a multicenter, randomized, double‐blind, placebo controlled study of autologous muscle derived cells for urinary sphincter repair. Neurourology and Urodynamics 2017;36(Suppl S3):S65‐7. [Abstract number 27; sr‐incont77516] [Google Scholar]
Additional references
Abdel‐Fattah 2005
- Abdel‐Fattah M, Ramsay I, NCT00136071. Transvaginal tension free vaginal tape‐obturator (TVT‐O) versus transobturator tape‐mentor (TOT) in the management of urodynamic stress urinary incontinence [Randomised prospective blinded trial comparing transvaginal tension free vaginal tape‐obturator (outside‐in) with transobturator tape‐mentor (inside‐out) in surgical management of urodynamic stress urinary incontinence]. clinicaltrials.gov/show/NCT00136071 (first received 26 August 2005). [NCT00136071; sr‐incont40211]
Abdel‐Fattah 2008a
- Abdel‐Fattah M, Ramsay I, Pringle S, Hardwick C, Tierney J, Ali H. (E‐TOT) Study: a randomised prospective single‐blinded study of two transobturator tapes in management of urodynamic stress incontinence: objective & patient reported outcomes. International Urogynecology Journal 2008;19(Suppl 2):S2‐3. [Abstract number 2] [Google Scholar]
Abdel‐Fattah 2008b
- Abdel‐Fattah M, Ramsay I, Pringle S, Hardwick C, Tierney J, Young D. (E‐TOT) Study: a randomised prospective single blinded study of two transobturator tapes in management of urodynamic stress incontinence: quality of life; sexual function at 1‐year. International Urogynecology Journal 2008;19(Suppl 2):S168‐9. [Abstract number 200] [Google Scholar]
Abdel‐Fattah 2010a
- Abdel‐Fattah M, Ramsay I, Pringle S, Hardwick C, Ali H, Young D, et al. Randomised prospective single‐blinded study comparing ‘inside‐out’ versus ‘outside‐in’ transobturator tapes in the management of urodynamic stress incontinence: 1‐year outcomes from the E‐TOT study. BJOG: an International Journal of Obstetrics & Gynaecology 2010;117(7):870‐8. [NCT00136071; TRIALID.ETOT; sr‐incont39920] [DOI] [PubMed] [Google Scholar]
Abdel‐Fattah 2010b
- Abdel‐Fattah M, Familusi A, Ramsay I, Ayansina D, Mostafa A. Preoperative determinants for failure of transobturator tapes in the management of female urodynamic stress incontinence. International Journal of Gynaecology and Obstetrics 2010;110(1):18‐22. [DOI] [PubMed] [Google Scholar]
Abdel‐Fattah 2010c
- Abdel‐Fattah M, Ramsay I, Pringle S, Hardwick C, Ali H. Evaluation of transobturator tapes (E‐TOT) study: randomised prospective single‐blinded study comparing inside‐out vs. outside‐in transobturator tapes in management of urodynamic stress incontinence: short term outcomes. BJOG: an International Journal of Obstetrics & Gynaecology 2010;117(7):870‐8. [DOI] [PubMed] [Google Scholar]
Abdel‐Fattah 2011a
- Abdel‐Fattah M, Ramsay I, Pringle S, Hardwick C, Ali H, Young D, et al. Evaluation of transobturator tension‐free vaginal tapes in management of women with recurrent stress urinary incontinence. Urology 2011;77(5):1070‐5. [NCT00136071; TRIALID.ETOT; sr‐incont41485] [DOI] [PubMed] [Google Scholar]
Abdel‐Fattah 2011b
- Abdel‐Fattah M, Familusi A, Ramsay I, N'Dow J. A randomised prospective single‐blinded study comparing "inside‐out" versus "outside‐in" transobturator tapes in the management of female stress urinary incontinence (E‐TOT study); 3 years follow‐up. Neurourology and Urodynamics 2011;30(6):825‐6. [Abstract number 18] [Google Scholar]
Abdel‐Fattah 2011c
- Abdel‐Fattah M, Hasafa Z, Mostafa A. Correlation of three validated questionnaires for assessment of outcomes following surgical treatment of stress urinary incontinence in women. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2011;157(2):226‐9. [DOI] [PubMed] [Google Scholar]
Abdel‐Fattah 2012
- Abdel‐Fattah M, Mostafa A, Familusi A, Ramsay I, N'Dow J. Prospective randomised controlled trial of transobturator tapes in management of urodynamic stress incontinence in women: 3‐year outcomes from the evaluation of transobturator tapes study. European Urology 2012;62(5):843‐51. [DOI] [PubMed] [Google Scholar]
Abdel‐Fattah 2014
- Abdel‐Fattah M, Hopper LR, Mostafa A. Evaluation of transobturator tension‐free vaginal tapes in the surgical management of mixed urinary incontinence: 3‐year outcomes of a randomized controlled trial. Journal of Urology 2014;191(1):114‐9. [DOI] [PubMed] [Google Scholar]
Abdel‐Fattah 2017a
- Abdel‐Fattah M, Cao G, Mostafa A. Long‐term outcomes for transobturator tension‐free vaginal tapes in women with urodynamic mixed urinary incontinence. Neurourology and Urodynamics 2017;36(4):902‐8. [DOI] [PubMed] [Google Scholar]
Abdel‐Fattah 2017b
- Abdel‐Fattah M, Cao G, Mostafa A. Long‐term outcomes of transobturator tension‐free vaginal tapes as secondary continence procedures. World Journal of Urology 2017;35(7):1141‐8. [DOI] [PubMed] [Google Scholar]
Abdel‐Fattah 2017c
- Abdel‐Fattah M. Authors' reply re: Long‐term outcomes of transobturator tapes in women with stress urinary incontinence: E‐TOT randomized controlled trial [letter]. BJOG: an International Journal of Obstetrics & Gynaecology 2017;124(10):1626. [DOI] [PubMed] [Google Scholar]
Abdel‐Fattah 2017d
- Abdel‐Fattah M. Interim title: no data available for the subset of participants with failed tape [personal communication]. Email to: D Tincello Exact date unknown: late 2017 to 2018.
Abdel‐Fattah 2019
- Abdel‐Fattah M, Mostafa A, Familusi A, Ramsay I, N'Dow J. Corrigendum re "Prospective Randomised Controlled Trial of Transobturator Tapes in Management of Urodynamic Stress Incontinence in Women: 3‐Year Outcomes from the Evaluation of Transobturator Tapes Study" [European Urology 2012;62:843‐51; PMID: 22534058]. European Urology 2019;75(4):e119. [DOI] [PubMed] [Google Scholar]
Agur 2013
- Agur W, Riad M, Secco S, Litman H, Madhuvrata P, Novara G, et al. Surgical treatments of recurrent stress urinary incontinence in women: a systematic review and meta‐analysis of randomised controlled trials. European Urology 2013;64:323‐36. [DOI] [PubMed] [Google Scholar]
Alcalay 1995
- Alcalay M, Monga A, Stanton SL. Burch colposuspension: a 10–20 year follow up. British Journal of Obstetrics and Gynaecology 1995;102:740‐5. [DOI] [PubMed] [Google Scholar]
Andersson 2017
- Andersson K‐E, Cardozo L, Cruz F, Lee K‐S, Sahai A, Wein AJ. Pharmacological treatment of urinary incontinence. In: Abrams P, Cardozo L, Wagg A, Wein A editor(s). Incontinence: 6th International Consultation on Incontinence. 6th Edition. Bristol, UK: ICI‐ICS. International Continence Society, 2017:805‐957. [ISBN: 978‐0956960733] [Google Scholar]
Ashok 2010
- Ashok K, Wang A. Recurrent urinary stress incontinence: an overview. Journal of Obstetrics and Gynaecology Research 2010;36:467‐73. [DOI] [PubMed] [Google Scholar]
Boyers 2013
- Boyers D, Kilonzo M, Mostafa A, Abdel‐Fattah M. Comparison of an adjustable anchored single‐incision mini‐sling, Ajust(®), with a standard mid‐urethral sling, TVT‐O(TM): a health economic evaluation. British Journal of Urinary Incontinence 2013;112(8):1169‐77. [DOI] [PubMed] [Google Scholar]
Bugge 2004
- Bugge C, Adams EJ, Gopinath D, Reid F. Pessaries (mechanical devices) for pelvic organ prolapse in women. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD004010.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cammu 2009
- Cammu H, Abbeele E, Nagel H, Haentjens P. Factors predictive of outcome in tension‐free vaginal tape procedure for urinary stress incontinence in a teaching hospital. International Urogynaecology Journal and Pelvic Floor Dysfunction 2009;20(7):775‐80. [DOI] [PubMed] [Google Scholar]
Cardozo 2010
- Cardozo L, Lange R, Voss S, Beardsworth A, Manning M, Viktrup L, et al. Short‐ and long‐term efficacy and safety of duloxetine in women with predominant stress urinary incontinence. Current Medical Research and Opinion 2010;26(2):253‐61. [DOI] [PubMed] [Google Scholar]
CRD 2015
- Centre for Reviews and Dissemination (CRD). CRD databases: search strategies. 2015. crd.york.ac.uk/crdweb/searchstrategies.asp (accessed 28 June 2019).
de Cuyper 2008
- Cuyper EM, Ismail R, Maher CF. Laparoscopic Burch colposuspension after failed sub‐urethral tape procedures: a retrospective audit. International Urogynecology Journal 2008;19:681‐5. [DOI] [PubMed] [Google Scholar]
Dean 2017
- Dean N, Ellis G, Herbison GP, Wilson D, Mashayekhi A. Laparoscopic colposuspension for urinary incontinence in women. Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD002239.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dumoulin 2017
- Dumoulin C, Adewuyi T, Booth J, Bradley C, Burgio K, Hagen S, et al. Adult conservative management. In: Abrams P, Cardozo L, Wagg A, Wein A editor(s). Incontinence: 6th International Consultation on Incontinence. 6th Edition. Bristol, UK: ICI‐ICS. International Continence Society, 2017:1443‐628. [ISBN: 978‐0956960733] [Google Scholar]
Dumoulin 2018
- Dumoulin C, Cacciari LP, Hay‐Smith JC. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database of Systematic Reviews 2018, Issue 10. [DOI: 10.1002/14651858.CD005654.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
EndNote 2018 [Computer program]
- Clarivate Analytics. EndNote X8.2. Version X8.2. Philadelphia (PA): Clarivate Analytics, 2018.
Ford 2017
- Ford AA, Rogerson L, Cody JD, Aluko P, Ogah JA. Mid‐urethral sling operations for stress urinary incontinence in women. Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD006375.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Giarenis 2012
- Giarenis I, Mastoroudes H, Cardozo L, Robinson D. Rediscovery of open colposuspension as a salvage continence operation. International Urogynecology Journal 2012;23(8):1117‐22. [DOI] [PubMed] [Google Scholar]
Glazener 2017
- Glazener CM, Cooper K, Mashayekhi A. Bladder neck needle suspension for urinary incontinence in women. Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD003636.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime Inc). GRADEpro GDT. Hamilton (ON), Canada: GRADE Working Group, McMaster University, 2015. Available at: http://gradepro.org (accessed 9 March 2017).
Gurol‐Urganci 2018
- Gurol‐Urganci I, Geary RS, Mamza JB, Duckett J, El‐Hamamsy D, Dolan L, et al. Long‐term rate of mesh sling removal following midurethral mesh sling insertion among women with stress urinary incontinence. JAMA 2018;320(16):1659‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2013a
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables ‐ binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158‐72. [DOI] [PubMed] [Google Scholar]
Guyatt 2013b
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles ‐ continuous outcomes. Journal of Clinical Epidemiology 2013;66(2):173‐83. [DOI] [PubMed] [Google Scholar]
Han 2012
- Han J‐Y, Moon KH, Park CM, Choo M‐S. Management of recurrent stress urinary incontinence after failed midurethral sling: tape tightening or repeat sling?. International Urogynecology Journal and Pelvic Floor Dysfunction 2012;23:1279‐84. [DOI] [PubMed] [Google Scholar]
Haylen 2010
- Haylen BT, Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. International Urogynaecology Journal 2010;21:5‐26. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Review of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hilton 2008
- Hilton P. Long‐term follow‐up studies in pelvic floor dysfunction: the Holy Grail or a realistic aim. BJOG: an International Journal of Obstetrics & Gynaecology 2008;115(2):135‐43. [DOI] [PubMed] [Google Scholar]
Hilton 2018 [pers comm]
- Hilton P. Information request [personal communication]. Email to: K Alberico 27 November 2018.
Hopper 2013a
- Hopper LR, Mostafa A, Abdel‐Fattah M. Evaluation of transobturator tapes in the surgical management of women with mixed urinary incontinence at 3 years follow‐up (Abstract). Journal of Obstetrics and Gynaecology 2013;33(8):920. [Google Scholar]
Hopper 2013b
- Hopper LR, Mostafa A, Abdel‐Fattah M. The effectiveness of transobturator tapes in the surgical management of women with mixed urinary incontinence: 3 year outcomes (Abstract number 612). 43rd Annual Meeting of the International Continence Society (ICS), 2013 Aug 26‐30, Barcelona, Spain. 2013.
Ismail 2017
- Ismail S, Tu L, Morin M. Predictive factors for the efficacy of autologous muscle derived cell injections in women with stress urinary incontinence (Abstract number 293). Neurourology and Urodynamics 2017; Vol. 36, issue Suppl S3:S273‐4.
Jackson 1996
- Jackson S, Donovan J, Brookes S, Eckford S, Swithinbank L, Abrams P. The Bristol Female Lower Urinary Tract Symptoms questionnaire: development and psychometric testing. British Journal of Urology 1996;77(6):805‐12. [DOI] [PubMed] [Google Scholar]
Karmakar 2015a
- Karmakar D, Mostafa A, Abdel‐Fattah M. A new validated score for detecting patient‐reported success on postoperative ICIQ‐SF: a novel two‐stage analysis from two large RCT cohorts (Abstract number PP 54). International Urogynecology Journal and Pelvic Floor Dysfunction 2015;26(1 Suppl 1):S81‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karmakar 2015b
- Karmakar D, Mostafa A, Abdel‐Fattah M. Long‐term outcomes (8‐years) from the prospective randomized control trial of trans‐obturator tapes for stress incontinence in women (the ETOT study) (Abstract number PP 48). International Urogynecology Journal and Pelvic Floor Dysfunction 2015;26(1 Suppl 1):S74‐5. [Google Scholar]
Karmakar 2017a
- Karmakar D, Mostafa A, Abdel‐Fattah M. A new validated score for detecting patient‐reported success on postoperative ICIQ‐SF: a novel two‐stage analysis from two large RCT cohorts. International Urogynecology Journal 2017;28(1):95‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karmakar 2017b
- Karmakar D, Mostafa A, Abdel‐Fattah M. Long‐term outcomes of transobturator tapes in women with stress urinary incontinence: E‐TOT randomised controlled trial. BJOG: an International Journal of Obstetrics and Gynaecology 2017;124(6):973‐81. [DOI] [PubMed] [Google Scholar]
Kirchin 2017
- Kirchin V, Page T, Keegan PE, Atiemo K, Cody JD, McClinton S, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD003881.pub4] [DOI] [PubMed] [Google Scholar]
Kunkle 2015
- Kunkle CM, Hallock JL, Hu X, Blomquist JL. Cost utility analysis of urethral bulking agents versus midurethral sling in stress urinary incontinence. Female Pelvic Medicine & Reconstructive Surgery 2015;21(3):154‐9. [DOI] [PubMed] [Google Scholar]
Lapitan 2017
- Lapitan MCM, Cody JD, Mashayekhi A. Open retropubic colposuspension for urinary incontinence in women. Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD002912.pub7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2010
- Lee HN, Lee YS, Han JY, Jeong JY, Choo MS, Lee KS. Transurethral injection of bulking agent for treatment of failed mid‐urethral sling procedures. International Urogynecology Journal 2010;21(12):1479‐83. [DOI] [PubMed] [Google Scholar]
Liapis 2009
- Liapis A, Bakas P, Creatsas G. Tension‐free vaginal tape in the management of recurrent urodynamic stress incontinence after previous failed midurethral tape. European Urology 2009;55:1450‐8. [DOI] [PubMed] [Google Scholar]
Lovatsis 2010
- Lovatsis D, Easton W, Wilkie D, et al. Guidelines for the evaluation and treatment of recurrent urinary incontinence following pelvic floor surgery. Journal of Obstetrics and Gynaecology Canada 2010;32:893‐8. [DOI] [PubMed] [Google Scholar]
Lovatsis 2017
- Lovatsis D, Easton W, Wilkie D. No. 248‐Guidelines for the evaluation and treatment of recurrent urinary incontinence following pelvic floor surgery. Journal of Obstetrics and Gynaecology Canada 2017;39(9):e309‐14. [DOI] [PubMed] [Google Scholar]
MacLachlan 2014
- MacLachlan LS, Rovner ES. Management of failed stress urinary incontinence surgery. Current Urology Reports 2014;15(8):Article number 429. [DOI: 10.1007/s11934-014-0429-y] [DOI] [PubMed] [Google Scholar]
Margalith 2004
- Margalith I, Gillon G, Gordon D. Urinary incontinence in women under 65: quality of life, stress related to incontinence and patterns of seeking health care. Quality of Life Research 2004;13:1381‐90. [DOI] [PubMed] [Google Scholar]
Mariappan 2005
- Mariappan P, Alhasso AA, Grant A, N'Dow JM. Serotonin and noradrenaline reuptake inhibitors (SNRI) for stress urinary incontinence in adults. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD004742.pub2] [DOI] [PubMed] [Google Scholar]
McGuire 1992
- McGuire EJ, Wan J. Pubovaginal slings. In: Hurt WG editor(s). Urogynecological Surgery (Principles and techniques in gynecologic surgery series). Gaithersburg (MD): Aspen Publishers Inc, 1992:97‐105. [ISBN: 978‐0834203396] [Google Scholar]
Merlin 2001
- Merlin, T, Arlnold E, Petros P, et al. A systematic review of tension‐free urethropexy for stress urinary incontinence: intravaginal slingplasty and the tension‐free vaginal tape procedures. British Journal of Urology 2001;88:871‐80. [DOI] [PubMed] [Google Scholar]
Milsom 2017
- Milsom I, Altman D, Cartwright R, Lapitan MC, Nelson R, Sjöström S, et al. Epidemiology of urinary incontinence (UI) and other lower urinary tract symptoms (LUTS), pelvic organ prolapse (POP) and anal (AI) incontinence. In: Abrams P, Cardozo L, Wagg A, Wein A editor(s). Incontinence: 6th International Consultation on Incontinence. 6th Edition. Bristol, UK: ICI‐ICS. International Continence Society, 2017:1‐141. [ISBN: 978‐0956960733] [Google Scholar]
Morling 2017
- Morling JR, McAllister DA, Agur W, Fischbacher CM, Glazener CM, Guerrero K, et al. Adverse events after first, single, mesh and non‐mesh surgical procedures for stress urinary incontinence and pelvic organ prolapse in Scotland, 1997‐2016: a population‐based cohort study. Lancet 2017;389(10069):629‐40. [DOI] [PubMed] [Google Scholar]
Mostafa 2011
- Mostafa A, Madhuvrata P, Abdel‐Fattah M. Preoperative urodynamic predictors of short‐term voiding dysfunction following a transobturator tension‐free vaginal tape procedure. International Journal of Gynaecology and Obstetrics 2011;115(1):49‐52. [DOI] [PubMed] [Google Scholar]
Nambiar 2017
- Nambiar A, Cody JD, Jeffery ST, Aluko P. Single‐incision sling operations for urinary incontinence in women. Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD008709.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
NHS England 2018
- NHS England. Vaginal mesh: high vigilance restriction period: immediate action required, all cases should be postponed if it is clinically safe to do so [letter]. i.emlfiles4.com/cmpdoc/9/7/2/8/1/1/files/47633_mesh‐letter‐to‐acute‐ceos‐and‐mds.pdf (Accessed 26 November 2018).
NICE 2015
- National Institute for Health and Care Excellence (NICE). Urinary incontinence in women: management. Clinical guideline [CG171]. www.nice.org.uk/guidance/cg171 Last updated 2015 (accessed 26 November 2018).
Nikolopoulos 2015
- Nikolopoulos KI, Betschart C, Doumouchtsis SK. The surgical management of recurrent stress urinary incontinence: a systematic review. Acta Obstetricia et Gynecologica Scandinavica 2015;94(6):568‐76. [DOI] [PubMed] [Google Scholar]
Papa Petros 2010
- Papa Petros PE. The integral theory system. A simplified clinical approach with illustrative case histories. Pelviperineology 2010;29(2):37‐51. [Google Scholar]
Parker 2016
- Parker WP, Gomelsky A, Padmanabhan P. Autologous fascia pubovaginal slings after prior synthetic anti‐incontinence procedures for recurrent incontinence: A multi‐institutional prospective comparative analysis to de novo autologous slings assessing objective and subjective cure. Neurourology and Urodynamics 2016;35(5):604‐8. [DOI] [PubMed] [Google Scholar]
Pradhan 2012
- Pradhan A, Jain P, Latthe PM. Effectiveness of midurethral slings in recurrent stress urinary incontinence: a systematic review and meta‐analysis. International Urogynecology Journal 2012;23:831‐41. [DOI] [PubMed] [Google Scholar]
Rehman 2017
- Rehman H, Bezerra CA, Bruschini H, Cody JD, Aluko P. Traditional suburethral sling operations for urinary incontinence in women. Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD001754.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rovner 2017
- Rovner E, Athanasiou S, Choo M‐S, Cosson M, Dmochowski R, Gomelsky A, et al. Surgery for urinary incontinence in women. In: Abrams P, Cardozo L, Wagg A, Wein A editor(s). Incontinence: 6th International Consultation on Incontinence. 6th Edition. Bristol, UK: ICI‐ICS. International Continence Society, 2017:1741‐854. [ISBN: 978‐0956960733] [Google Scholar]
Schraffordt 2006
- Schraffordt Koops SE, Bisseling TM, Heintz AP, Vervest HA. The effectiveness of tension‐free vaginal tape (TVT) and quality of life measured in women with previous urogynecologic surgery: analysis from The Netherlands TVT database. American Journal of Obstetrics and Gynecology 2006;195:439‐44. [DOI] [PubMed] [Google Scholar]
Shemilt 2010
- Shemilt I, Thomas J, Morciano M. A web‐based tool for adjusting costs to a specific target currency and price year. Evidence and Policy 2010;6(1):51‐9. [Google Scholar]
Shemilt In Press
- Shemilt I, Aluko P, Graybill E, Craig D, Henderson C, Drummond M, Wilson ECF, Robalino S, Vale L, Campbell and Cochrane Economics Methods Group. Chapter 20: Incorporating economic evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions (Second Edition, Version 6.0). Chichester (UK): John Wiley & Sons, In Press [Draft version available online to Cochrane members from: https://training.cochrane.org/handbook ‐ Archie login required]:507‐23. [Google Scholar]
Smith 2011
- Smith AR, Artibani W, Drake MJ. Managing unsatisfactory outcome after mid‐urethral tape insertion. Neurourology and Urodynamics 2011;30:771‐4. [DOI] [PubMed] [Google Scholar]
Stav 2010
- Stav K, Dwyer PL, Rosamilia A, Schierlitz L, Lim YN, Chao F, et al. Repeat synthetic mid urethral sling procedure for women with recurrent stress urinary incontinence. Journal of Urology 2010;183(1):241‐6. [DOI] [PubMed] [Google Scholar]
Stewart 2017
- Stewart F, Berghmans B, Bø K, Glazener CM. Electrical stimulation with non‐implanted devices for stress urinary incontinence in women. Cochrane Database of Systematic Reviews 2017, Issue 12. [DOI: 10.1002/14651858.CD012390.pub2; CD012390] [DOI] [PMC free article] [PubMed] [Google Scholar]
Subak 2014
- Subak LL, Goode PS, Brubaker L, Kusek JW, Schembri M, Lukacz ES. Urinary incontinence management costs are reduced following Burch or sling surgery for stress incontinence. American Journal of Obstetrics & Gynecology 2014;211(2):171.e171‐177. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ulmsten 1996
- Ulmsten U, Henriksson L, Johnson P, Varhos G. An ambulatory surgical procedure under local anesthesia for treatment of female urinary incontinence. International Urogynecology Journal and Pelvic Floor Dysfunction 1996;7(2):81‐6. [DOI] [PubMed] [Google Scholar]
Villoro 2016
- Villoro R, Merino M, Hidalgo‐Vega A, Jimenez M, Martinez L, Aracil J. Women with urinary incontinence in Spain: health‐related quality of life and the use of healthcare resources. Maturitas 2016;94:52‐7. [DOI: ] [DOI] [PubMed] [Google Scholar]
Ward 2008
- Ward KL, Hilton P. Tension‐free vaginal tape versus colposuspension for primary urodynamic stress incontinence: 5‐year follow up. International Journal of Obstetrics and Gynaecology 2008;115(2):226‐33. [DOI] [PubMed] [Google Scholar]
Ware 1993
- Ware JE, Snow KK, Kosinski M, Gandek B. SF‐36 Health survey manual and interpretation guide. Boston (MA): The Health Institute, New England Medical Centre, 1993. SF‐36 Health survey manual and interpretation guide. Boston (MA): The Health Institute, New England Medical Centre, 1993. [Google Scholar]
References to other published versions of this review
Bakali 2011
- Bakali E, Buckley BS, Hilton P, Tincello DG. Treatment of recurrent stress urinary incontinence after failed minimally invasive synthetic suburethral tape surgery in women. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD009407] [DOI] [PubMed] [Google Scholar]
Bakali 2013
- Bakali E, Buckley BS, Hilton P, Tincello DG. Treatment of recurrent stress urinary incontinence after failed minimally invasive synthetic suburethral tape surgery in women. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD009407.pub2] [DOI] [PubMed] [Google Scholar]


