Abstract
We previously presented the protein-protein interaction network of schizophrenia associated genes, and from it, the drug-protein interactome which showed the drugs that target any of the proteins in the interactome. Here, we studied these drugs further to identify whether any of them may potentially be repurposable for schizophrenia. In schizophrenia, gene expression has been described as a measurable aspect of the disease reflecting the action of risk genes. We studied each of the drugs from the interactome using the BaseSpace Correlation Engine, and shortlisted those that had a negative correlation with differential gene expression of schizophrenia. This analysis resulted in 12 drugs whose differential gene expression (drug versus normal) had an anti-correlation with differential expression for schizophrenia (disorder versus normal). Some of these drugs were already being tested for their clinical activity in schizophrenia and other neuropsychiatric disorders. Several proteins in the protein interactome of the targets of several of these drugs were associated with various neuropsychiatric disorders. The network of genes with opposite drug-induced versus schizophrenia-associated expression profiles were significantly enriched in pathways relevant to schizophrenia etiology and GWAS genes associated with traits or diseases that had a pathophysiological overlap with schizophrenia. Drugs that targeted the same genes as the shortlisted drugs, have also demonstrated clinical activity in schizophrenia and other related disorders. This integrated computational analysis will help translate insights from the schizophrenia drug-protein interactome to clinical research - an important step, especially in the field of psychiatric drug development which faces a high failure rate.
Subject terms: Drug discovery, Computational biology and bioinformatics
Introduction
Schizophrenia is a complex disorder with a cumulative impact of variable genetic effects coupled with environmental factors1. The Schizophrenia Working Group of the Psychiatric Genomics Consortium (PGC) had identified 108 genetic loci that likely confer risk for schizophrenia. Prior to this, around 25 genes were being studied for their association with the disorder2. While the role of genetics has been clearly validated by the genome-wide association studies (GWAS), the functional impact of the risk variants is not well understood. Several of the schizophrenia genes, especially those implicated by the GWAS have unknown functions and/or pathways. To discover the functional role of these genes, and promote discovery of novel therapeutics, we had carried out a computational analysis of the protein-protein interactions (PPI) network, or the interactome, of schizophrenia associated genes3. The schizophrenia interactome, comprising 101 schizophrenia genes and about 1,900 PPIs, provided valuable results highlighting the functions and pathways tied to schizophrenia genes through their protein interactome3. A valuable result from this study was the drug-target interactome that showed a total of 524 drugs targeting 53 proteins in the schizophrenia interactome. Many of these drugs were labeled for therapeutic value for nervous system as expected, but there were several drugs that were labeled for other anatomical systems in the human body.
As drug approvals for psychiatric indications have been facing a high failure rate in the last few years4, it would be beneficial to study whether these drugs that target proteins from the schizophrenia interactome could be repurposed for treatment of schizophrenia. Finding alternate uses for approved drugs would be optimal, and such uses are being found in recent years5–7.
Diseases are often considered to be driven by an abnormal or perturbed expression of a multitude of genes which together constitute unique differential (gene) expression signatures (DES)8–12. Drugs administered to treat these diseases often revert the expression of these genes to their normal levels13,14. DES for disease versus normal are quantified using gene expression analysis based on microarrays and RNA sequencing methods, and are deposited in online repositories, which make the data freely available for integrated computational analyses15. Similarly, DES for drug-treated versus untreated is made available through Connectivity Map (CMAP)16. In order to analyze the suitability of these drugs for repurposing, we build over the results from our previous work on schizophrenia interactome discovery and analysis3, utilizing large transcriptomic databases such as CMAP and Gene Expression Omnibus (GEO), and employing a bioinformatics data analysis software suite named BaseSpace Correlation Engine17. The approach of repurposing drugs based on the negative correlation of drug-induced versus disease-associated gene expression profiles has resulted in some valuable results in the past. Topiramate, an anti-convulsant drug used in the treatment of epilepsy, was identified to be potentially repurposable for inflammatory bowel disease (IBD), based on the negative correlation of drug-induced profiles extracted from CMAP and disease-associated profile from GEO18. They further validated the efficacy of this drug in a rodent model of IBD18.
Many genes harboring variants associated with schizophrenia, such as DTNBP1, DAOA, NRG1 and RGS4, show differential gene expression in post-mortem brain samples obtained from schizophrenia patients compared with normal controls19. In schizophrenia, it has been pointed out that the effect of genetic variants may, in fact, be reflected on gene expression rather than on the structure of the proteins coded by these genes20. Gene expression has been described as a ‘psychiatric endophenotype’ in schizophrenia19. A psychiatric endophenotype may broadly be defined as a measurable phenotype, namely, any neuroanatomical, physiological, psychological, biochemical or molecular aspect of brain function, having some definitive disease-associated genetic component, and contributing to a larger behavioral trait such as ‘cognitive dysfunction’ or ‘psychosis’ underlying a complex disorder such as schizophrenia19. The ‘definitive genetic component’, in this case, could be a set of disease susceptibility genes harboring sequence variants affecting the expression of the susceptibility genes themselves, or a set of genes differentially expressed in patients compared with healthy subjects. These genes may uncover novel pathways underlying some behavioral trait contributing to disease etiology. For example, it was recently shown that expression of genes associated with immunological processes vary with cognitive performance in familial schizophrenia21. So, our method to identify repurposable drugs may be tested on schizophrenia, in which differential gene expression plays a critical role.
Results
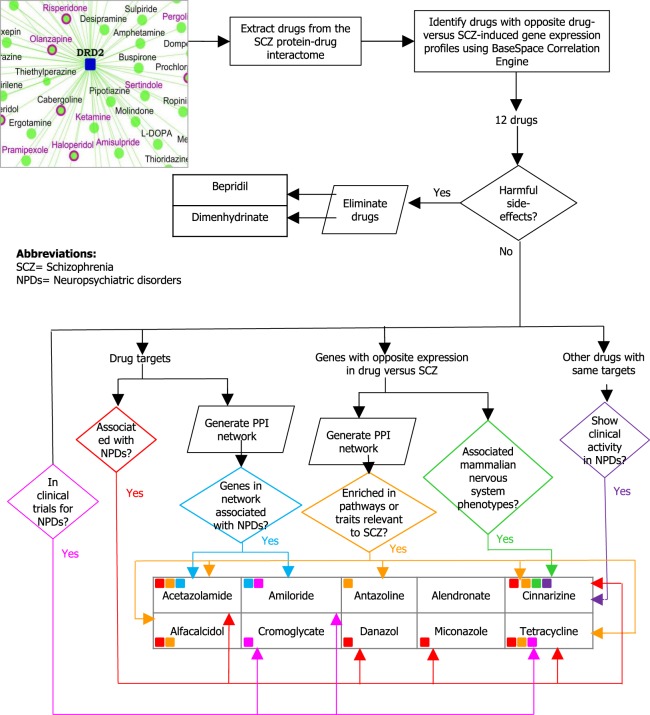
In our prior work3, we presented 524 drugs that target any of the proteins in the Schizophrenia Interactome3. We pruned this large list of drugs by comparing differential expression profiles induced by drug to profiles associated with schizophrenia, using our in silico protocol, and shortlisted drugs that had a negative correlation between these expression profiles22. We carried out bioinformatics analysis on the shortlist of drugs identified thus, to answer the following questions on their biological validity to schizophrenia (see Fig. 1): Have any of these drugs been considered for clinical trials? Are the genes targeted by these drugs associated with neuropsychiatric disorders? Are the genes with opposite expression in drug versus schizophrenia associated with morphological or physiological phenotypes of the mammalian nervous system? Do other drugs targeting the same genes as the shortlisted drugs show clinical activity in neuropsychiatric disorders? Are any genes in the PPI network of the genes targeted by the shortlisted drugs associated with neuropsychiatric disorders? Are any genes in the PPI network of genes with opposite expression in drug versus schizophrenia involved in pathways relevant to schizophrenia? Are they also GWAS genes associated with traits or diseases having a pathophysiological overlap with schizophrenia? These questions were based on the fact that genes associated with traits related to the nervous system and genes linked to neuropsychiatric disorders have been shown to converge in specific co-expression modules, indicating shared genetic basis and disease mechanisms23. Drugs used for treatment of a neuropsychiatric disorder may be repurposable for schizophrenia by virtue of shared genes and mechanisms. Each of these sources of information is assessed separately in parallel, highlighting which of the drugs have multiple sources of supporting information.
Figure 1.
Graphical abstract depicting the steps taken in this study to assess the biological validity of the shortlisted drugs. Drugs were extracted from the schizophrenia drug-protein interactome and screened for negative correlation of drug-induced versus disease-associated gene expression profiles. Drugs shortlisted in this manner were further checked for their toxicity, and eliminated if they were found to have harmful side effects. The targets of the remaining drugs and their network of protein-protein interactions were checked for their association with schizophrenia (SCZ)/other neuropsychiatric disorders (NPDs) using DisGeNET. Genes with opposite expression in drug-induced versus disease-associated profile were analyzed for their association with nervous system phenotypes (Mammalian Phenotype Ontology). Their networks were analyzed for enrichment of SCZ-associated pathways/GWAS traits. Apart from this, it was checked whether the shortlisted drugs are already being tested against NPDs (NIH Clinical Trials), and whether other drugs with the same targets show clinical activity in NPDs. Different sources of supporting information are shown by lines of different colors. Each of the drugs is also tagged with little squares of colors of corresponding supporting information. For example, amiloride is supported by “genes in network associated with neuropsychiatric disorders” (blue) and “in clinical trials for neuropsychiatric disorders” (bright pink). Acetazolamide, cinnarizine and tetracycline each are supported by 3 sources of supporting information.
We followed an established approach to identify drugs that have opposite differential expression to the differential expression of schizophrenia (i.e., genes over-expressed in schizophrenia are under-expressed by drug treatment and vice versa)8. We identified such drugs using the BaseSpace Correlation Engine software suite, a data analysis platform used to study the effect of diseases and/or drugs on publicly available gene expression data17. This analysis resulted in 12 drugs. Although in each case, there are some genes that are differentially expressed in the same direction for both the drug and disorder, the overall effect on the entire transcriptome has an anti-correlation, leading to 12 drugs as potential candidates for further studies (Table 1 and Fig. 2). The top 5 drugs by the score of anti-correlation are cromoglicic acid, bepridil, acetazolamide, dimenhydrinate, cinnarizine, of which bepridil and dimenhydrinate may be excluded due to their side-effects related to nervousness and hallucinations (see Table 1), thus leaving cromoglicic acid, acetazolamide and cinnarizine as top candidates. There were 30 drugs indicated for schizophrenia in DrugBank24. 23 out of these occur in the schizophrenia drug-protein interactome (77%). We checked the overlap of drugs indicated for other diseases to infer the specificity of this result, namely, coronary heart disease (25%), lung cancer (50%), diabetes (33%), chronic kidney disease (0%), post-traumatic stress disorder (75%) and bipolar disorder (66%). As expected, there was a larger overlap with neurological disorders compared to other unrelated disorders. 50% overlap with lung cancer drugs may be explained by the large number of drug targets implicated in cancers, and their vital role in numerous basic cellular functions. Eleven of these did not have relevant datasets in BaseSpace, or even though a negative correlation was found, the p-value was insignificant for schizophrenia gene expressions studies. Of 23 known schizophrenia drugs – six of them, namely, clozapine, haloperidol, molindone, perphenazine, amitriptyline and nortriptyline, had negative correlation with schizophrenia and 6 others had a positive correlation with schizophrenia. Sources of datasets in which differential expression is observed is listed in Data File 1.
Table 1.
Details of known schizophrenia drugs and drugs identified as potentially repurposable for schizophrenia: Pharmacokinetic information is collected from DrugBank (www.drugbank.ca). Known schizophrenia drugs are shown in italics.
| Drug | Drug class | Original therapeutic purpose(s) | Pharmacokinetic details: dosage form, delivery route, half-life | Toxicity | Correlation with all data types, Overall correlation score | Correlation with SCZ gene expression study, Correlation score | Bs1 | Bs2 | Bs1 & Bs2 up | Bs1 & Bs2 down | Bs1 up & Bs2 down | Bs1 down & Bs1 up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amitriptyline | Dibenzo-cycloheptenes | Major depressive disorder and anxiety disorders, treatment of secondary depression in schizophrenia | Tablet, oral, 25 hours | Abnormally low blood pressure, confusion, convulsions, dilated pupils and other eye problems on overdosing, and withdrawal symptoms including gastrointestinal disturbances, anxiety, and insomnia | Negative, 76 | Negative, 100 | HL60 cells + amitriptyline, 12.8 uM _vs_ DMSO vehicle | Hippocampus tissues from schizophrenia patients _vs_ normals | 9 | 3 | 19 | 31 |
| Haloperidol | Alkyl-phenyl-ketone | Schizophrenia and other psychoses, delusional disorders, ballism, and Tourette syndrome, adjunctive therapy in mental retardation, chorea associated with Huntington’s disease | Solution/tablet, oral, 24 hours | Cardiovascular effects, extrapyramidal symptoms, tardive dyskinesia, neuroleptic malignant syndrome, hematologic effects | Negative, 70 | Negative, 68 | HL60 cells + haloperidol, 10 μM vs. DMSO vehicle | Prefrontal cortex Brodmann area 46 of schizophreniacs with short DOI vs. helathy controls | 3 | 6 | 30 | 1 |
| Molindone | Indoles and derivatives | Schizophrenia, other psychoses and aggressive type of undersocialized conduct disorder | Tablet, oral, not available | Not available | Negative, 76 | Negative, 57 | MCF7 + molindone, 12.8 μM vs. DMSO vehicle | Hippocampus tissues from schizophrenia patients _vs_ normals | 22 | 23 | 174 | 21 |
| Clozapine | Dibenzo-diazepines | Atypical antipsychotic drug used in schizophrenia | Tablet, oral, 4 to 12 hours | Black-box warning for agranulocytosis | Negative, 59 | Negative, 100 | HL60 cells + clozapine, 10 μM vs. DMSO vehicle | Prefrontal cortex Brodmann area 46 of schizophreniacs with short DOI vs. helathy controls | 10 | 23 | 101 | 2 |
| Nortriptyline | Dibenzo-cycloheptenes | Clinical depression, treatment of depressive symptoms in schizophrenia (dose adjustments are necessary to safely use the drug in schizophrenia, as it has been shown to exacerbate psychosis) | Capsule, oral, 26 hours | Cardiac dysrhythmias, severe hypotension, shock, congestive heart failure, pulmonary edema, convulsions, and CNS depression, including coma on overdosing, and withdrawal symptoms include gastrointestinal disturbances, anxiety, and insomnia | Negative, 50 | Negative, 89 | HL60 cells + nortriptyline, 13.4 uM _vs_ DMSO vehicle | Hippocampus tissues from schizophrenia patients _vs_ normals | 6 | 6 | 26 | 3 |
| Perphenazine | Phenothiazines | Schizophrenia and the manic phases of bipolar disorder | Tablet, oral, 8 to 12 hours | Stupor or coma, convulsive seizures in children | Negative, 80 | Negative, 100 | HL60 cells + perphenazine, 10 μM vs. DMSO vehicle | Prefrontal cortex Brodmann area 46 of schizophreniacs with short DOI vs. helathy controls | 4 | 7 | 78 | 7 |
| Acetazo-lamide | Thiadiazole sulfonamides | Glaucoma, mountain sickness | Tablet, oral, 3 to 9 hours | Not available | Negative, 76 | Negative, 100 | MCF7 cells + acetazolamide, 18 uM _vs_ DMSO vehicle | Whole blood from schizophrenic patients _vs_ healthy controls_GPL6947 | 67 | 38 | 95 | 119 |
| Alendronate | Bisphos-phonates | Osteoporosis | Tablet, oral, 10 years | Damage of oesophagus | Negative, 68 | Negative, 57 | Heart of rats + ALENDRONIC ACID at 138 mg-kg-d in CMC by oral gavage 5d _vs_ vehicle | Associative striatum tissues from schizophrenia patients _vs_ normals | 1 | 14 | 12 | 8 |
| Alfacalcidol | Vitamin D and derivatives | Vitamin D supplement | Capsule, oral, not available | Hypercalcemia | Negative, 46 | Negative, 100 | Liver of rats + ALFACALCIDOL at 043 mg-kg-d in CMC by oral gavage 1d _vs_ vehicle | Prefrontal cortex Brodmann area 46 - schizophrenics with short DOI _vs_ healthy controls | 22 | 120 | 125 | 25 |
| Amiloride | Aminop-yrazines | Hypertension, heart failure, edema | Tablet, oral, 6 to 9 hours | Dehydration and electrolyte imbalance | Negative, 58 | Positive, 66 | HL60 cells + amiloride, 13.2 uM _vs_ DMSO vehicle | Whole blood from schizophrenic patients _vs_ healthy controls_GPL6947 | 36 | 26 | 14 | 18 |
| Antazoline | Phenylben-zamines | Nasal congestion, allergic conjunctivitis | Liquid, opthalmic, not available | Not available | Negative, 60 | Negative, 91 | HL60 cells + antazoline, 13.2 uM _vs_ DMSO vehicle | Whole blood from schizophrenic patients _vs_ healthy controls_GPL6947 | 5 | 10 | 10 | 24 |
| Bepridil | Phenylben-zamines | Angina | Tablet, oral, 24 to 50 hours | Gastrointestinal problems, dizziness,asthenia, nervousness | Negative, 77 | Negative, 40 | HL60 cells + bepridil, 10 uM _vs_ DMSO vehicle | Neural progenitors derived from donor stably expressing GFP - schizophrenia _vs_ normal | 36 | 51 | 73 | 68 |
| Cinnarizine | Diphenyl-methanes | Motion sickness, vertigo | Tablet, oral, 3 to 4 hours | Drowsiness, skin problems, lethargy, movement problems | Negative, 64 | Negative, 100 | HL60 cells + cinnarizine, 10.8 uM _vs_ DMSO vehicle | Whole blood from schizophrenic patients _vs_ healthy controls_GPL6947 | 20 | 15 | 21 | 57 |
| Cromoglicic acid | Chromones | Asthma prophylaxis, aerosol | Solution, oral, 1.3 hours | Cough, nasal congestion, nausea, sneezing and wheezing | Negative, 84 | Negative, 64 | MCF7 cells + cromoglicic acid, 7.8 uM _vs_ DMSO vehicle | Neural progenitors derived from donor stably expressing GFP - schizophrenia _vs_ normal | 15 | 18 | 36 | 13 |
| Danazol | Estrane steroids | Endometriosis, fibrocystic breast disease, hereditary angioedema | Capsule, oral, 24 hours | Not available | Negative, 61 | Negative, 100 | HL60 cells + danazol, 11.8 uM _vs_ DMSO vehicle | Whole blood from schizophrenic patients _vs_ healthy controls_GPL6947 | 173 | 335 | 460 | 1264 |
| Dimenhy-drinate | Diphenyl-methanes | Motion sickness, nausea | Solution, intramuscular or intravenous, 1 to 4 hours | Delerium, hallucinations, excitement | Negative, 64 | Negative, 50 | HL60 cells + dimenhydrinate, 8.6 uM _vs_ DMSO vehicle | Whole blood from schizophrenic patients _vs_ healthy controls_GPL6947 | 22 | 10 | 35 | 6 |
| Miconazole | Benzylethers | Antifungal medication used in vaginal infections | Tablet, buccal, not available | Oral toxicity in mice at LD50 = 3800 mg/kg | Negative, 60 | Negative, 50 | HL60 cells + miconazole, 9.6 uM _vs_ DMSO vehicle | Hippocampus tissues from schizophrenia patients _vs_ normals | 11 | 19 | 66 | 20 |
| Tetracycline | Tetracyclines | Antibiotic used in acne, cholera, brucellosis, plague, malaria, and syphilis | Capsule, oral, 6 to 12 hours | Oral toxicity in mice at LD50 = 808 mg/kg | Negative, 49 | Negative, 100 | HL60 cells + tetracycline, 8.4 uM _vs_ DMSO vehicle | Whole blood from schizophrenic patients _vs_ healthy controls_GPL6947 | 26 | 20 | 44 | 149 |
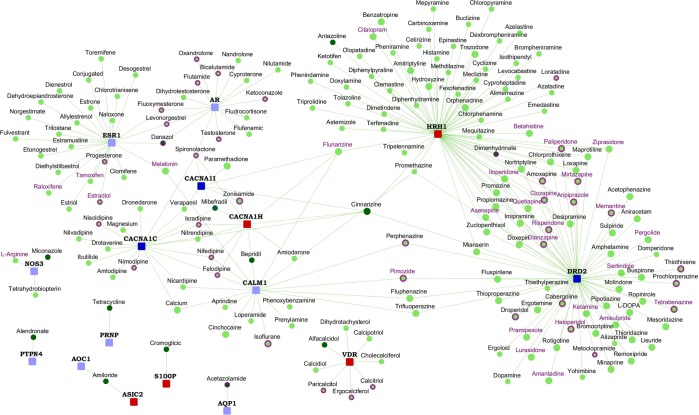
Figure 2.
Drugs potentially repurposable for schizophrenia: The network highlights the shortlisted drugs that may be potentially repurposed for schizophrenia. The shortlisted drugs are shown as round nodes colored in dark green, and other drugs are shown as light green nodes. FDA approved drugs are shown with purple borders. Drugs with purple labels are in clinical trials for schizophrenia. Schizophrenia genes are square nodes colored in dark blue, known interactors are colored in light blue and novel interactors in red.
Acetazolamide
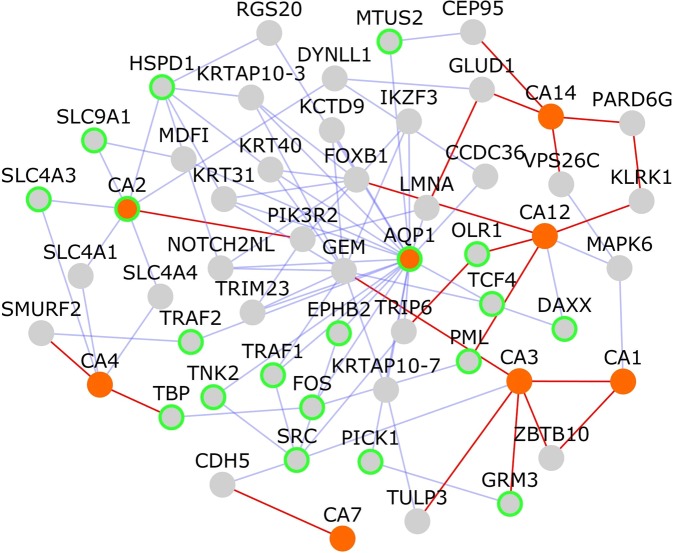
The protein targets of acetazolamide are carbonic anhydrases (CA*) and aquaporin (AQP1). We collected known and computationally predicted PPIs of these targets of acetazolamide and queried the DisGeNet25 database whether any of the proteins in this interaction network are associated with neuropsychiatric disorders. While Fig. 2 shows the protein targets only from schizophrenia interactome, Fig. 3 shows the network of all protein targets (orange colored nodes) of acetazolamide and their PPIs. Nineteen genes within this network are associated with various neuropsychiatric disorders (nodes with green border in Fig. 3; Data File 2): AQP1 and CA2, which are acetazolamide targets, DAXX, EPHB2, HSPD1, SLC4A3, SLC9A1, SRC, TCF4, TNK2, TRAF1, TRAF2, MTUS2, PICK1, GRM3, OLR1, TBP, PML and FOS, giving credence to the consideration that it has a potential application to schizophrenia. Acetazolamide has been shown to have high inhibitory activity against human CA2 (hCA II), the ubiquitous cytosolic enzyme (inhibition constant, Ki = 12 nM) and human CA7 (Ki = 2.5 nM), the brain-specific form of the enzyme26. Human CA2 was found to be catalytically highly active (defined in terms of Kcat/Km for CO2 hydration described by two ionizations at pKa 6.2 and 7.5, with a maximum approaching 8 × 107 M−1 s−1)27. Kcat/Km for human CA2 is 1.5 × 108 27. The increase in extracellular pH which accompanies neural activity is generated by the exchange of external H+ for cytosolic Ca2+. This process, and its impact on the glutamate receptors, NMDARs, has been shown to be regulated by CA14 in the synaptic microenvironment28. On these lines, it is interesting to note that CA3 has been predicted to be a novel interactor of the glutamate receptor, GRM3, mutations in which have been associated with schizophrenia29.
Figure 3.
Network of PPIs among targets of acetazolamide: The network shows protein-protein interactions that connect the targets of acetazolamide, which are shown as orange colored nodes. Nodes that connect these target genes are shown as grey colored nodes. Nodes with light green borders are genes associated with neuropsychiatric disorders. Novel interactions are shown as red edges and known interactions as blue edges.
We assembled the network of PPIs of genes that are differentially expressed by each of the shortlisted drugs and carried out network and enrichment analysis using a tool called LENS30. The networks of genes found to be differentially expressed in acetazolamide, antazoline and cinnarizine, having an anti-correlation in schizophrenia, were shown to be enriched in ubiquitination and proteasome degradation pathways (Data File 3). The ubiquitin proteasome system has been identified as an important pathway in several genetic studies of neuropsychiatric disorders including Alzheimer’s disease, Parkinson’s disease, psychosis and bipolar disorder31. Many gene expression studies performed on blood collected from schizophrenia patients, and on post-mortem samples of hippocampus, prefrontal cortex and temporal cortex of patients have pointed at abnormalities in the ubiquitin proteasome pathway, which targets protein for degradation in the cell31. Moreover, reduced protein ubiquitination, reduced levels of ubiquitin and ubiquitin-like activases and ligases, were identified in a region of the brain called the left superior temporal gyrus in schizophrenia patients31. Left superior temporal gyrus, the volume of which has been shown to decrease in schizophrenia patients, is involved in the development of auditory hallucinations and thought process abnormalities seen in schizophrenia31. Interestingly, acetazolamide which has been shown to mediate diuretic effects through its action on AQP1, induces AQP1 ubiquitination, and a proteasome inhibitor reversed its downregulatory action on AQP132. RAD51AP1 and AQR are novel interactors of the calcium channel CACNA1C and the nicotinic receptor CHRNA7 respectively in the schizophrenia interactome, found to have an anti-correlated expression in schizophrenia and acetazolamide treatment. It has been shown that UAF1, an interaction partner of USP1 deubiquitinating enzyme, associates with RAD51AP1, which interacts with RAD51 to mediate homologous recombination repair33. NEDD4-1, an ubiquitin ligase, has been shown to promote the sorting of newly synthesized calcium voltage gated channels for proteasomal degradation34. Suppression of AQR in HepG2, a liver cancer line, has been shown to inhibit protein ubiquitination35. It has been shown that the expression of nicotinic receptors on the cell surface is regulated by the ubiquitin proteasomal system36. The networks of genes found to be differentially expressed in alfacalcidol and tetracycline, having an anti-correlation in schizophrenia, were shown to be enriched in the neutrophil degranulation pathway (Data File 3). Degranulating activity of neutrophils has been attributed to dysfunctional permeability of the blood-brain barrier in schizophrenia37.
Network of genes which were differentially expressed in acetazolamide and had an anti-correlation with schizophrenia were found to be significantly enriched for association to rheumatoid arthritis (Data File 3). Recently, the reduced prevalence of rheumatoid arthritis observed in schizophrenia patients was attributed to SNPs (single nucleotide polymorphisms) in the HLA region that conferred differential risk for schizophrenia and rheumatoid arthritis38. The interactomes of schizophrenia and rheumatoid arthritis genes also showed a significant overlap even outside of HLA genes, and shared common pathways38.
Alfacalcidol
Alfacalcidol targets the protein VDR which was found to be overexpressed in whole blood obtained from schizophrenic patients compared to healthy controls (fold change (FC) = 2.21, p-value = 0.0037)39. The network of genes differentially expressed in alfacalcidol was enriched in GWAS genes associated with inflammatory bowel disease (Data File 3). The incidence of schizophrenia has been shown to be high in patients with immune-mediated inflammatory diseases such as inflammatory bowel disease, rheumatoid arthritis and multiple sclerosis40.
Amiloride
With our focus on candidate drugs for repurposing (i.e. those that exhibited a negative correlation to schizophrenia but are not currently labeled for this use), we queried the ClinicalTrials.gov database (https://clinicaltrials.gov/) and found that amiloride is being tested in clinical trials for its efficacy in attention deficit hyperactivity disorder.
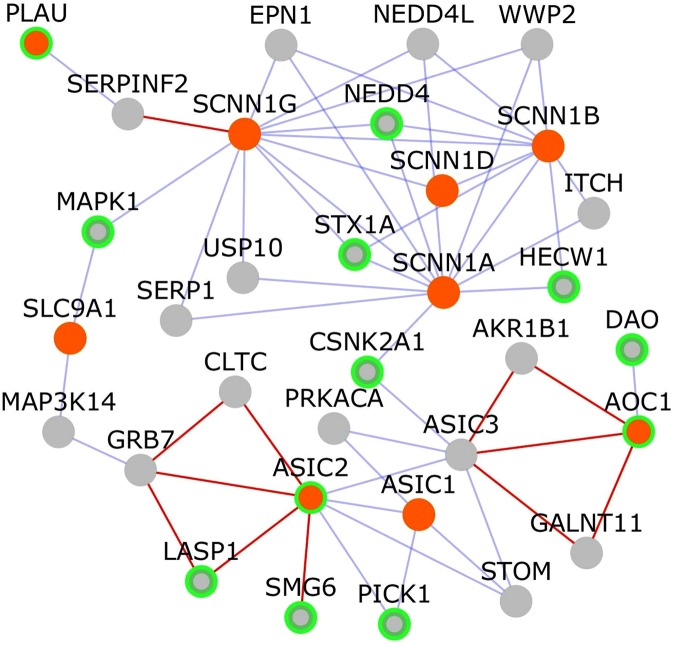
We analyzed the PPI network of proteins targeted by the drug amiloride (Fig. 4), despite its positive correlation with schizophrenia gene expression because its overall correlation with a range of schizophrenia datasets was negative, and because of the biological characteristics of its targets. The protein targets of amiloride are ASIC1, ASIC2, AOC1, SLC9A1, PLAU, SCNN1A, SCNN1B, SCNN1G and SCNN1D (orange nodes in Fig. 4). The network of PPIs among these targets of amiloride shows that 12 genes, including ASIC2, AOC1 and PLAU, which are amiloride targets, NEDD4, STX1A, MAPK1, HECW1, DAO, CSNK2A1, LASP1, SMG6 and PICK1 are associated with various neuropsychiatric disorders (nodes with green border in Fig. 4; Data File 2). ASIC2 was a computationally predicted interactor of the gene SMG6, structural variants in which have been associated with schizophrenia or bipolar disorder in a Spanish population41,42. SMG6 is located in the chromosomal region 17p13.3, linked to lissencephaly, a neuronal migration disorder arising from incomplete neuronal migration to the cerebral cortex during gestation, and characterized by an absence of normal convolutions in the cerebral cortex and an abnormally small head (or microcephaly)42,43. ASICs (acid-sensing ion channels) are members of the epithelial Na+ channel (ENaC) family of ion channels, expressed in the nervous system44. It was shown in a study that ASIC2 is not expressed at the cell surface of high grade glioma (brain tumor) cells and this may be responsible for the constitutively activated inward Na+ current, which promotes increased cell growth and migration in these cells44. In such glioma cells, compounds such as glycerol and the transcriptional regulator, sodium 4-phenylbutyrate, were shown to inhibit the constitutively activated inward Na+ current and reduce cell growth and migration44. These compounds were shown to induce the movement of ASIC2 to the plasma membrane, and prevent the active inward current through negative regulatory mechanisms, reducing the ability of glioma cells to proliferate and migrate44.
Figure 4.
Network of PPIs among targets of amiloride: The network shows protein-protein interactions that connect the targets of amiloride, which are shown as orange colored nodes. Nodes that connect these target genes are shown as grey colored nodes. Nodes with light green borders are genes associated with neuropsychiatric disorders. Novel interactions are shown as red edges and known interactions as blue edges.
Antazoline
The networks of genes found to be differentially expressed in antazoline having an anti-correlation in schizophrenia, were shown to be enriched in ubiquitination and proteasome degradation pathways (Data File 3). The network of genes differentially expressed in antazoline, and with an opposite expression in schizophrenia, was significantly enriched in GWAS genes associated with brain connectivity (Data File 3). Abnormal interactions between brain networks have been pointed out to be an important contributing factor in schizophrenia etiology45.
Cinnarizine
The networks of genes found to be differentially expressed in cinnarizine having an anti-correlation in schizophrenia, were shown to be enriched in ubiquitination and proteasome degradation pathways (Data File 3). We checked whether any of the genes having anti-correlated expression on cinnarizine treatment and in schizophrenia were associated with mammalian phenotype ontology (MPO) terms related to various morphological or physiological aspects of the nervous system (http://www.informatics.jax.org/)46. It was found that mutations in 13 genes were associated with relevant MPO terms, namely, AHI1, ENTPD1, IFNGR1, NAP1L1, NPTN, PIK3CA, PKN2, PRKDC, PTGS2, RBM12, SEC. 23 A, SS18L1 and UBE3A. Two of these genes, IFNGR1 and AHI1, both linked to ‘abnormal depression-related behavior’, are predicted to have a novel interaction between them. Depressive symptoms have been observed in schizophrenia patients47. IFNGR1 has been found to be necessary for the induction of IDO, the tryptophan synthesizing enzyme, which plays a role in depressive behavior, induced by inflammation47. AHI1 is associated with susceptibility to schizophrenia and autism47. Mice lacking neuronal expression of AHI1 had reduced levels of tyrosine kinase receptor B and a depressive phenotype, which was alleviated by antidepressants and overexpression of TRKB47. BDNF/TRKB signaling has been shown to play a key role in depression. Altered BDNF/TRKB signaling in the prefrontal cortex, hippocampus and nucleus accumbens has been shown to give rise to depressive phenotype induced by inflammation48. Another gene, UBE3A, was associated with increased dopamine and serotonin levels, abnormal brain wave pattern, cerebral cortex morphology, dendrite morphology, GABA-mediated receptor currents, long term potentiation and nervous system electrophysiology. Yet another gene, NPTN, was linked to abnormal synaptic transmission in the central nervous system and abnormal dendritic spine morphology.
The network of genes differentially expressed in cinnarizine was enriched in GWAS genes associated with inflammatory bowel disease (Data File 3).
We queried Drug Bank24 to find drugs that targeted the same genes as the shortlisted drugs, and checked whether they demonstrated any clinical activity in schizophrenia or other neuropsychiatric disorders. Risperidone, nimodipine, nilvadipine, flunarizine, nifedipine, cannabidiol and clozapine target the same genes as cinnarizine. Flunarizine (targeting CALM1, CACNA1H) showed good efficacy and tolerability for the treatment of schizophrenia49. Nifedipine (which targets CALM1, CACNA1H) enhanced learning and memory in schizophrenic patients with tardive dyskinesia50. Cannabidiol (which targets CACNA1H) shows beneficial effects as an adjunctive drug along with existing anti-psychotic medication in schizophrenia51. Risperidone (targeting DRD2) is used to treat schizophrenia, bipolar disorder, and irritability in autistic patients52–54. Nimodipine (CACNA1C) has been found effective for treating resistant bipolar mood disorder55. Nilvadipine (CACNA1C) was found to be effective in treatment of schizophrenia56. Clozapine (targeting HRH1) is effective in treatment-resistant schizophrenia57.
Cinnarizine targets CACNA1H which is found to be overexpressed in neural progenitor cells differentiated for 2 days from induced pluripotent stem cells of schizophrenia patients versus healthy subjects (FC = 3.1227, p-value = 4.10E-20)58. Cinnarizine targets HRH1, which has been linked to schizophrenia etiology. It also targets CACNA1C, associated with bipolar disorder, schizophrenia and depressive disorder, and CACNA1H, associated with epilepsy and autism. It targets DRD2, linked to bipolar disorder, schizophrenia, depressive disorder, Parkinson’s disease and attention deficit hyperactivity disorder.
Cromoglicic acid
Cromoglicic acid is being tested in clinical trials for its efficacy in Alzheimer’s disease. It has been reported that cromoglicic acid in combination with ibuprofen reduces the levels of amyloid-beta protein levels, a pathological biomarker in Alzheimer’s disease, and promotes a neuroprotective state by activating microglia and inducing phagocytosis of amyloid-beta proteins59. Based on this work, cromoglicic acid has been considered for further study by our clinical collaborators and is currently in clinical trials (ClinicalTrials.gov Identifier: NCT03794076).
Danazol and miconazole
Danazol and miconazole target ESR1 and NOS3, both associated with Alzheimer’s disease. NOS3 was also identified as a potential target for schizophrenia based on its druggability, membership in schizophrenia-related biological pathways and differential expression in schizophrenia60.
Tetracycline
Minocycline, a broad spectrum tetracycline antibiotic (where tetracycline is one of the shortlisted drugs), has been shown to be effective as an adjunctive drug, improving the effect of antipsychotic drugs in schizophrenia61. Tetracycline targets PRNP, linked to depressive disorder, Huntington disease-like 1 and Alzheimer’s disease. The network of genes differentially expressed in tetracycline was enriched in GWAS genes associated with inflammatory bowel disease (Data File 3).
In summary, clinical trial data, network-based analyses and literature review support the biological validity of 9 out of the 12 drugs proposed to be repurposable for schizophrenia, namely, acetazolamide, alfacalcidol, amiloride, antazoline, cinnarizine, cromoglicic acid, danazol, miconazole and tetracycline.
Discussion
In this section, we discuss cinnarizine and alfacalcidol further due to abundant evidences in literature pointing at their potential utility as repurposable drugs for schizophrenia.
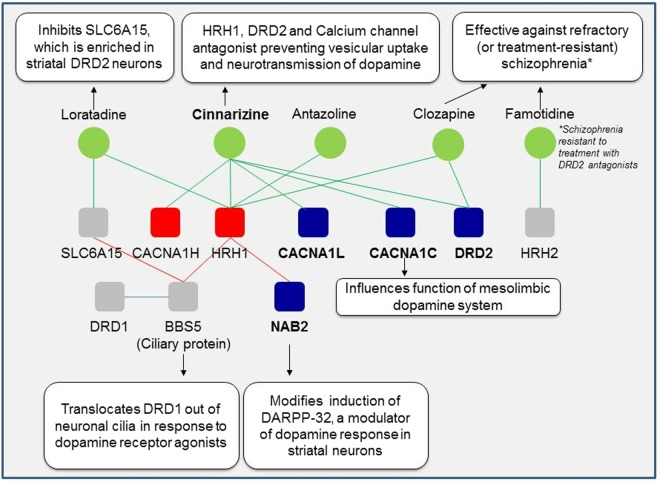
Cinnarizine, an HRH1 (histamine receptor H1), DRD2 (dopamine receptor D2) and calcium channel antagonist commonly used to treat motion sickness, may be re-purposed to treat symptoms of schizophrenia (see Fig. 5)62. Histamine receptors are highly expressed in brain regions associated with the higher cognitive functions disturbed in schizophrenia63. Leu49Ser mutation in HRH1 was associated with susceptibility to schizophrenia64. Schizophrenia patients have elevated levels of n-tele-methylhistamine, a histamine metabolite, in their cerebrospinal fluid and reduced HRH1 binding in their frontal cortex and cingulate gyrus65. According to the revised dopamine hypothesis of schizophrenia, hyperactive dopamine transmission in the mesolimbic areas such as the ventral tegmental area and ventral striatum including nucleus accumbens contribute to disease etiology66. Many studies have demonstrated a crosstalk between the dopaminergic and the histamine neuron systems. Compounds acting at histamine receptors have been shown to modulate extracellular striatal dopamine levels67. Enhanced release of neuronal histamine was observed on DRD2 activation and in methamphetamine or phencyclidine-induced animal models of schizophrenia68,69. Histamine antagonists inhibit behavioral sensitization arising from increased levels of extracellular dopamine69–72. The fact that refractory schizophrenia may be treated with clozapine, an HRH1 antagonist, indicates that extra-dopaminergic systems, namely, the histamine neuron system, contribute to schizophrenia etiology57,69. Clozapine also exhibits strong affinity to dopaminergic receptors and decreases hyperactivity of the mesolimbic dopaminergic pathway by blocking 5-HT2A (5-hydroxytryptamine receptor 2A)66. Famotidine, an HRH2 antagonist, significantly reduced psychotic symptoms in schizophrenia patients73. The examples of clopazine and famotidine indicate that a drug such as cinnarizine acting as a DRD2 and HRH1 antagonist may serve to alleviate psychotic symptoms arising from the interplay of dopaminergic and histamine neuron systems. Cinnarizine prevents vesicular uptake of dopamine74. It shows antagonistic activity at the calcium channel, CACNA1C, whose reduced levels attenuate the function of the mesolimbic dopaminergic pathway and impair behavioral responses to dopamine stimulants75. Calcium channel antagonists reduce neurotransmission of dopamine76. Even though our computational analysis supports the repurposing of cinnarizine to treat schizophrenia symptoms, its clinical utility can only be validated after experiments in pre-clinical models such as cell lines or animal models, and in clinical trials. Being an anti-histamine, cinnarizine causes drowsiness and its anti-dopaminergic activity may induce Parkinsonism and depression77. HRH1, targeted by cinnarizine, was predicted to interact with the schizophrenia gene NAB2. NAB2 modifies the induction of DARPP-32, which modulates the response to dopamine in striatal neurons78. HRH1 has also been predicted to interact with BBS5, a ciliary protein. BBS5 interacts with DRD1 and is involved in translocating DRD1 out of the cilia in response to dopamine receptor agonists, thereby implicating neuronal cilia in dopamine signaling79. BBS5 was predicted to interact with SLC6A15, which is enriched in striatal DRD2 neurons and inhibited by loratadine, an HRH1 antagonist80,81.
Figure 5.
Cinnarizine and its targets in the schizophrenia interactome: The drug cinnarizine is shown here with the proteins it targets from the schizophrenia interactome. 4 additional proteins (BBS5, DRD1, HRH2 and SLC6A15) and 3 additional drugs (loratadine, clozapine and famotidine) that are relevant to the hypothesis are also shown. Cinnarizine, targets 3 schizophrenia genes and 2 novel interactors which constitute calcium channels, and histamine & dopamine receptors. Since histamine antagonists are known to reduce dopamine levels through their action on dopamine receptors, and calcium channel antagonists are known to reduce dopamine neurotransmission, the HRH1, DRD2 and calcium channel antagonist, cinnarizine, may be repurposable for schizophrenia. Another shortlisted drug, antazoline, is not part of the reasoning presented here even though it is an HRH1 antagonist. Schizophrenia genes are shown as dark blue colored nodes, novel interactors are red colored nodes and genes relevant to the hypothesis, which are not in the schizophrenia interactome, are shown as grey colored nodes.
The drug alfacalcidol, an analog of vitamin D, commonly used as a vitamin D supplement, or to treat conditions involving imbalance in calcium metabolism such as hypercalcemia and imbalance in bone metabolism such as osteoporosis, may be potentially re-purposed to treat dopaminergic symptoms in schizophrenia, possibly in combination with dopamine receptor antagonists such as clozapine82,83. Deficiency of vitamin D exerting its effects through VDR (vitamin D receptor) has been observed in schizophrenia patients84. Dopaminergic aspects of schizophrenia etiology as proposed by the dopamine hypothesis of schizophrenia may, at least in part, be treated by vitamin D supplementation66. In a study based on 9,114 subjects from the Northern Finland 1966 birth cohort, vitamin D supplementation in the first year of life was associated with reduced risk of schizophrenia in males85. Several studies have noted an interplay between vitamin D and dopaminergic systems86. VDR is highly expressed in brain regions associated with schizophrenia, namely, the hippocampus, prefrontal cortex and dopaminergic neurons in substantia nigra of rats and humans87. During early stages of development, VDR is expressed in the mesencephalon precisely at the time when monoamine cells differentiate to dopaminergic cells and dopaminergic systems are innervated86. Mice pups with vitamin D deficiency have reduced levels of the enzyme COMT (catechol-O-methyltransferase), which converts the dopamine metabolite DOPAC (3,4-Dihydroxyphenylacetic acid) into HVA (homovanillic acid) and affects the dopamine turnover86. In rats with vitamin D deficiency, the effect of MK-801, an NMDA (N-methyl-D-aspartate) receptor antagonist which indirectly activates dopaminergic activity and also induces hyperlocomotion in animals, was found to be attenuated with the use of haloperidol, a DRD2 (Dopamine Receptor D2) anatgonist88. In SH-SY5Y cells routinely used to model neural functions, VDR overexpression resulted in increased dopamine levels, overexpression of TH (tyrosine hydroxylase) which is an enzyme involved in the production of the precursor of dopamine called L-DOPA and overexpression of DRD2 whose increased activity has been noted in schizophrenia models, among other regulatory effects on genes associated with the dopaminergic system89,90. On treatment of these SH-SY5Y cells with calcitriol, a biologically active form of vitamin D, increased levels of dopamine metabolites such as HVA, increased COMT levels and reduced DRD2 expression were observed90,91. Even though there are several studies supporting the efficacy of vitamin D supplementation in treating schizophrenia symptoms85,92, several groups have argued that these studies were irreplicable and that randomized controlled trials in larger cohorts would be necessary to ascertain its clinical utility, if any93.
In this study, we shortlisted several drugs potentially repurposable for schizophrenia based on the negative correlation of drug-induced versus disease-associated gene expression profiles. Even though this approach has resulted in some valuable results in the past, it has several limitations. The gene expression profiles analyzed in this study were induced by drugs in cancer cell lines16, and not in cell lines relevant to schizophrenia. The biological validity of our study will be strengthened if we perform our analysis with gene expression profiles induced by drugs in neuronal cell lines such as SH-SY5Y, in patient-derived induced pluripotent stem cells or in animal models of schizophrenia. However it is to be noted that such data has been shown to be valuable for repurposing drugs even for non-cancer diseases. Specific examples include repurposing of topiramate, an anti-epileptic drug, for inflammatory bowel disease18, repurposing of drugs for schizophrenia94 and repurposing of drugs for bipolar disorder95. These studies show that the drug-induced profiles generated in non-neural cells and deposited in CMAP are amenable to analysis involving neuropsychiatric disorders. Our future analysis will also focus on interrogating gene expression datasets of larger sample sizes. In summary, we showed that the drugs repurposable for schizophrenia may be identified from the schizophrenia drug-protein interactome based on gene expression profiles induced by the drug versus associated with the disease, and augmented our findings with clinical trial data, network-based analyses, and literature review. Through this study, we disseminate this list of drugs potentially repurposable drugs for schizophrenia to the scientific community so as to enable clinical translation of these results.
Methods
Identification of potentially repurposable drugs using BaseSpace correlation engine
In an earlier work, we constructed the protein-protein interaction network of schizophrenia genes, and then identified the drugs that target any of the proteins in this interactome3. Several of these drugs were known to have therapeutic value for nervous system, but there were several drugs that targeted other anatomical systems in the human body3. In this work, as a mechanism of shortlisting drugs for further analysis, we selected those that targeted more than two proteins in the schizophrenia interactome or those that target proteins that are also targeted by many drugs. While the first criterion helps in selecting drugs with the capacity to exert several pharmacological actions, a feature that is critical to targeting a disease as multifactorial as schizophrenia, the second criterion may point in the direction of highly druggable targets. For identifying repurposable drugs, it is essential that we tap into undiscovered regions of the PPI network. So, we also included drugs targeting novel proteins predicted to interact with known schizophrenia-associated genes96. Next step involved identifying the drugs that have opposite differential expression to the differential expression of schizophrenia (i.e., genes over-expressed in schizophrenia are under-expressed by drug treatment and vice versa). We studied each of these drugs in comparison to gene expression profiles of schizophrenia by using the software suite called BaseSpace (http://www.nextbio.com/b/nextbio.nb). BaseSpace Correlation Engine is used to study the effect of diseases and/or drugs on publicly available gene expression data17. Bioset 1 (‘BS1’) or a particular cell line, tissue or blood sample in which differential expression by drug has been studied was compared with a bioset 2 (‘BS2’), another cell line, tissue or blood sample in which differential expression in schizophrenia patients was studied. A correlation score is generated by the tool based on the strength of the overlap or enrichment, between the two biosets. Additional statistical criteria such as correction for multiple hypothesis testing are applied and the correlated biosets are then ranked by statistical significance. A numerical score of 100 is assigned to the most significant result, and the scores of the other results are normalized with respect to the top-ranked result. We excluded drugs with unacceptable levels of toxicity or undesirable pharmacokinetics.
Network analysis using LENS
LENS (Lens for Enrichment and Network Studies of human proteins) is a web-based tool which may be used to identify pathways and diseases that are significantly enriched among the genes submitted by users30. The LENS algorithm finds the nearest neighbor of each gene in the interactome and includes the intermediate interactions that connect them. LENS then computes the statistical significance of the overlap of genes in the network and genes with annotations pertaining to pathways, diseases, drugs and GWASs, and reports a p-value computed from Fisher’s exact test.
Shortlisted drugs which are being tested in clinical trials against various neuropsychiatric disorders were identified from NIH Clinical Trials (https://clinicaltrials.gov/).
Differential expression of the novel interactor VDR in whole blood obtained from schizophrenia patients was identified from GSE3848539, and that of CACNA1H in induced pluripotent stem cells of schizophrenia patients was identified from GSE9287458.
Association of the various genes in the network of PPIs among targets of the shortlisted drugs was identified from DisGeNET, a database that integrates human gene-disease associations from expert curated databases and text-mining derived associations25.
Drugs that targeted the same genes as the shortlisted drugs were identified from DrugBank (https://www.drugbank.ca/)24.
Preprint publication
An earlier version of this article was deposited into preprint server bioRxiv, where it appeared online on October 13, 201897.
Supplementary information
Acknowledgements
This work has been funded by the Biobehavioral Research Awards for Innovative New Scientists (BRAINS) grant R01MH094564 awarded to MKG by the National Institute of Mental Health of National Institutes of Health (NIMH/NIH) of USA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, the National Institutes of Health. Article processing charges have been paid by University Library System, University of Pittsburgh. MKG thanks Ansuman Chattopadhyay of University of Pittsburgh Health Sciences Library System for licensing and consultation for the commercial software suite used in this study. MKG and KBK thank Prof. N. Balakrishnan of Indian Institute Science for his support.
Author Contributions
The study has been designed by M.K.G. and K.B.K. S.C. carried out correlation analysis of drugs against diseases. K.B.K. carried out literature study and further bioinformatics analysis of the shortlisted drugs. Manuscript has been prepared by K.B.K. and edited by M.K.G.
Data Availability
Data sharing is not applicable to this article as no datasets were generated during the current study. Source of data that was analyzed in this study has been described in Methods and Data File 1.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-48307-w.
References
- 1.Corvin AP. Two patients walk into a clinic…a genomics perspective on the future of schizophrenia. BMC biology. 2011;9:77. doi: 10.1186/1741-7007-9-771741-7007-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farrell MS, et al. Evaluating historical candidate genes for schizophrenia. Molecular psychiatry. 2015 doi: 10.1038/mp.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganapathiraju MK, et al. Schizophrenia interactome with 504 novel protein-protein interactions. NPJ Schizophr. 2016;2:16012. doi: 10.1038/npjschz.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas, D. W. et al. Clinical development success rates 2006–2015. San Diego: Biomedtracker/Washington, DC: BIO/Bend: Amplion (2016).
- 5.Athauda D, et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. The Lancet. 2017;390:1664–1675. doi: 10.1016/S0140-6736(17)31585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinnings SL, et al. Drug discovery using chemical systems biology: repositioning the safe medicine Comtan to treat multi-drug and extensively drug resistant tuberculosis. PLoS computational biology. 2009;5:e1000423. doi: 10.1371/journal.pcbi.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. Bmj. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sirota M, et al. Discovery and preclinical validation of drug indications using compendia of public gene expression data. Science translational medicine. 2011;3:96ra77–96ra77. doi: 10.1126/scitranslmed.3001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maertzdorf J, et al. Common patterns and disease-related signatures in tuberculosis and sarcoidosis. Proceedings of the National Academy of Sciences. 2012;109:7853–7858. doi: 10.1073/pnas.1121072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaussabel D, et al. Analysis of significance patterns identifies ubiquitous and disease-specific gene-expression signatures in patient peripheral blood leukocytes. Annals of the New York Academy of Sciences. 2005;1062:146–154. doi: 10.1196/annals.1358.017. [DOI] [PubMed] [Google Scholar]
- 11.Kumar, A. et al. (Am Soc Hematology, 2014).
- 12.Chiu IM, et al. A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell reports. 2013;4:385–401. doi: 10.1016/j.celrep.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duran-Frigola M, Mateo L, Aloy P. Drug repositioning beyond the low-hanging fruits. Current Opinion in. Systems Biology. 2017;3:95–102. [Google Scholar]
- 14.Pushpakom S, et al. Drug repurposing: progress, challenges and recommendations. Nature Reviews Drug Discovery. 2019;18:41. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 15.Barrett T, et al. NCBI GEO: archive for functional genomics data sets—update. Nucleic acids research. 2012;41:D991–D995. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamb J, et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. science. 2006;313:1929–1935. doi: 10.1126/science.1132939. [DOI] [PubMed] [Google Scholar]
- 17.Kupershmidt I, et al. Ontology-based meta-analysis of global collections of high-throughput public data. PloS one. 2010;5:e13066. doi: 10.1371/journal.pone.0013066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dudley JT, et al. Computational repositioning of the anticonvulsant topiramate for inflammatory bowel disease. Science translational medicine. 2011;3:96ra76–96ra76. doi: 10.1126/scitranslmed.3002648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bray NJ. Gene expression in the etiology of schizophrenia. Schizophrenia bulletin. 2008;34:412–418. doi: 10.1093/schbul/sbn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huo Y, Li S, Liu J, Li X, Luo X-J. Functional genomics reveal gene regulatory mechanisms underlying schizophrenia risk. Nature communications. 2019;10:670. doi: 10.1038/s41467-019-08666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ukkola-Vuoti L, et al. Gene expression changes related to immune processes associate with cognitive endophenotypes of schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2019;88:159–167. doi: 10.1016/j.pnpbp.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Chattopadhyay A, Ganapathiraju MK. Demonstration Study: A Protocol to Combine Online Tools and Databases for Identifying Potentially Repurposable Drugs. Data. 2017;2:15. doi: 10.3390/data2020015. [DOI] [Google Scholar]
- 23.Li M, et al. Integrative functional genomic analysis of human brain development and neuropsychiatric risks. Science. 2018;362:eaat7615. doi: 10.1126/science.aat7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wishart DS, et al. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic acids research. 2006;34:D668–D672. doi: 10.1093/nar/gkj067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piñero, J. et al. DisGeNET: a discovery platform for the dynamical exploration of human diseases and their genes. Database2015 (2015). [DOI] [PMC free article] [PubMed]
- 26.Carta F, et al. Carbonic anhydrase inhibitors: inhibition of cytosolic carbonic anhydrase isozymes II and VII with simple aromatic sulfonamides and some azo dyes. Chemical biology & drug design. 2009;74:196–202. doi: 10.1111/j.1747-0285.2009.00842.x. [DOI] [PubMed] [Google Scholar]
- 27.Vullo D, et al. Carbonic anhydrase inhibitors. Inhibition of the human cytosolic isozyme VII with aromatic and heterocyclic sulfonamides. Bioorganic & medicinal chemistry letters. 2005;15:971–976. doi: 10.1016/j.bmcl.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 28.Makani S, et al. NMDA receptor-dependent afterdepolarizations are curtailed by carbonic anhydrase 14: regulation of a short-term postsynaptic potentiation. Journal of Neuroscience. 2012;32:16754–16762. doi: 10.1523/JNEUROSCI.1467-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egan MF, et al. Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia. Proceedings of the National Academy of Sciences. 2004;101:12604–12609. doi: 10.1073/pnas.0405077101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Handen A, Ganapathiraju MK. LENS: web-based lens for enrichment and network studies of human proteins. BMC medical genomics. 2015;8:S2. doi: 10.1186/1755-8794-8-S4-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubio MD, Wood K, Haroutunian V, Meador-Woodruff JH. Dysfunction of the ubiquitin proteasome and ubiquitin-like systems in schizophrenia. Neuropsychopharmacology. 2013;38:1910. doi: 10.1038/npp.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, et al. Aquaporin-1 translocation and degradation mediates the water transportation mechanism of acetazolamide. Plos one. 2012;7:e45976. doi: 10.1371/journal.pone.0045976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cukras S, et al. The USP1-UAF1 complex interacts with RAD51AP1 to promote homologous recombination repair. Cell Cycle. 2016;15:2636–2646. doi: 10.1080/15384101.2016.1209613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rougier J-S, Albesa M, Abriel H, Viard P. Neuronal precursor cell-expressed developmentally down-regulated 4-1 (NEDD4-1) controls the sorting of newly synthesized CaV1. 2 calcium channels. Journal of biological chemistry. 2011;286:8829–8838. doi: 10.1074/jbc.M110.166520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song C, et al. AQR is a novel type 2 diabetes-associated gene that regulates signaling pathways critical for glucose metabolism. Journal of Genetics and Genomics. 2018;45:111–120. doi: 10.1016/j.jgg.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Christianson JC, Green WN. Regulation of nicotinic receptor expression by the ubiquitin–proteasome system. The EMBO journal. 2004;23:4156–4165. doi: 10.1038/sj.emboj.7600436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shcherbakova I, et al. Activation of kallikrein-kinin system, degranulating activity of neutrophils and blood-brain barrier in schizophrenia. Zhurnal nevrologii i psikhiatrii imeni SS Korsakova. 1998;98:38–41. [PubMed] [Google Scholar]
- 38.Malavia TA, et al. Generating testable hypotheses for schizophrenia and rheumatoid arthritis pathogenesis by integrating epidemiological, genomic, and protein interaction data. NPJ schizophrenia. 2017;3:11. doi: 10.1038/s41537-017-0010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Jong S, et al. A gene co-expression network in whole blood of schizophrenia patients is independent of antipsychotic-use and enriched for brain-expressed genes. PloS one. 2012;7:e39498. doi: 10.1371/journal.pone.0039498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marrie RA, et al. Increased incidence of psychiatric disorders in immune-mediated inflammatory disease. Journal of psychosomatic research. 2017;101:17–23. doi: 10.1016/j.jpsychores.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 41.Yu H, et al. Association study of suppressor with morphogenetic effect on genitalia protein 6 (SMG6) polymorphisms and schizophrenia symptoms in the Han Chinese population. Neuropsychiatry (London) 2016;6:223–228. doi: 10.4172/Neuropsychiatry.1000143. [DOI] [Google Scholar]
- 42.Tabares-Seisdedos R, et al. Evidence for association between structural variants in lissencephaly-related genes and executive deficits in schizophrenia or bipolar patients from a Spanish isolate population. Psychiatric genetics. 2008;18:313–317. doi: 10.1097/YPG.0b013e3283118725. [DOI] [PubMed] [Google Scholar]
- 43.Dobyns W, Truwit C. Lissencephaly and other malformations of cortical development: 1995 update. Neuropediatrics. 1995;26:132–147. doi: 10.1055/s-2007-979744. [DOI] [PubMed] [Google Scholar]
- 44.Vila-Carriles WH, et al. Surface expression of ASIC2 inhibits the amiloride-sensitive current and migration of glioma cells. Journal of Biological Chemistry. 2006;281:19220–19232. doi: 10.1074/jbc.M603100200. [DOI] [PubMed] [Google Scholar]
- 45.Yu Q, et al. Brain connectivity networks in schizophrenia underlying resting state functional magnetic resonance imaging. Current topics in medicinal chemistry. 2012;12:2415–2425. doi: 10.2174/156802612805289890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith CL, Eppig JT. The Mammalian Phenotype Ontology as a unifying standard for experimental and high-throughput phenotyping data. Mammalian genome. 2012;23:653–668. doi: 10.1007/s00335-012-9421-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu X, et al. Neuronal Abelson helper integration site-1 (Ahi1) deficiency in mice alters TrkB signaling with a depressive phenotype. Proceedings of the National Academy of Sciences. 2010;107:19126–19131. doi: 10.1073/pnas.1013032107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J-c, Yao W, Hashimoto K. Brain-derived neurotrophic factor (BDNF)-TrkB signaling in inflammation-related depression and potential therapeutic targets. Current neuropharmacology. 2016;14:721–731. doi: 10.2174/1570159X14666160119094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bisol LW, et al. Is flunarizine a long-acting oral atypical antipsychotic? A randomized clinical trial versus haloperidol for the treatment of schizophrenia. The Journal of clinical psychiatry. 2008;69:1572–1579. doi: 10.4088/JCP.v69n1007. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz BL, Fay-McCarthy M, Kendrick K, Rosse RB, Deutsch SI. Effects of nifedipine, a calcium channel antagonist, on cognitive function in schizophrenic patients with tardive dyskinesia. Clinical neuropharmacology. 1997;20:364–370. doi: 10.1097/00002826-199708000-00009. [DOI] [PubMed] [Google Scholar]
- 51.McGuire P, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. American Journal of Psychiatry. 2017;175:225–231. doi: 10.1176/appi.ajp.2017.17030325. [DOI] [PubMed] [Google Scholar]
- 52.Scott LJ, Dhillon S. Spotlight on risperidone in irritability associated with autistic disorder in children and adolescents. CNS drugs. 2008;22:259–262. doi: 10.2165/00023210-200822030-00006. [DOI] [PubMed] [Google Scholar]
- 53.Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. The American Journal of Psychiatry. 1994;151:825. doi: 10.1176/ajp.151.6.825. [DOI] [PubMed] [Google Scholar]
- 54.Sajatovic M, Subramoniam M, Fuller MA. Risperidone in the treatment of bipolar mania. Neuropsychiatric disease and treatment. 2006;2:127. doi: 10.2147/nedt.2006.2.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goodnick PJ. The use of nimodipine in the treatment of mood disorders. Bipolar disorders. 2000;2:165–173. doi: 10.1034/j.1399-5618.2000.020303.x. [DOI] [PubMed] [Google Scholar]
- 56.YAMADA K, et al. Effectiveness of nilvadipine in two cases of chronic schizophrenia. Psychiatry and clinical neurosciences. 1995;49:237–238. doi: 10.1111/j.1440-1819.1995.tb01891.x. [DOI] [PubMed] [Google Scholar]
- 57.Humbert-Claude M, Davenas E, Gbahou F, Vincent L, Arrang J-M. Involvement of histamine receptors in the atypical antipsychotic profile of clozapine: a reassessment in vitro and in vivo. Psychopharmacology. 2012;220:225–241. doi: 10.1007/s00213-011-2471-5. [DOI] [PubMed] [Google Scholar]
- 58.Narla S, et al. Common developmental genome deprogramming in schizophrenia—Role of Integrative Nuclear FGFR1 Signaling (INFS) Schizophrenia research. 2017;185:17–32. doi: 10.1016/j.schres.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang C, et al. Cromolyn Reduces Levels of the Alzheimer’s Disease-Associated Amyloid β-Protein by Promoting Microglial Phagocytosis. Scientific reports. 2018;8:1144. doi: 10.1038/s41598-018-19641-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chellappa SA, et al. Meta-analysis of genomic variants and gene expression data in schizophrenia suggests the potential need for adjunctive therapeutic interventions for neuropsychiatric disorders. Journal of Genetics. 2019;98:60. doi: 10.1007/s12041-019-1101-6. [DOI] [PubMed] [Google Scholar]
- 61.Liu F, et al. Minocycline supplementation for treatment of negative symptoms in early-phase schizophrenia: a double blind, randomized, controlled trial. Schizophrenia research. 2014;153:169–176. doi: 10.1016/j.schres.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 62.Wilder-Smith C, Schimke J, Osterwalder B, Senn H. Cinnarizine for prevention of nausea and vomiting during platin chemotherapy. Acta Oncologica. 1991;30:731–734. doi: 10.3109/02841869109092448. [DOI] [PubMed] [Google Scholar]
- 63.Jin C, Panula P. The laminar histamine receptor system in human prefrontal cortex suggests multiple levels of histaminergic regulation. Neuroscience. 2005;132:137–149. doi: 10.1016/j.neuroscience.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 64.García-Martín E, Ayuso P, Luengo A, Martínez C, Agúndez JA. Genetic variability of histamine receptors in patients with Parkinson’s disease. BMC medical genetics. 2008;9:15. doi: 10.1186/1471-2350-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mahmood D. Histamine H3 receptors and its antagonism as a novel mechanism for antipsychotic effect: a current preclinical & clinical perspective. International journal of health sciences. 2016;10:564. doi: 10.12816/0048906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brisch, R. et al. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: old fashioned, but still in vogue. Frontiers in psychiatry5 (2014). [DOI] [PMC free article] [PubMed]
- 67.Hussain N, Flumerfelt B, Rajakumar N. Muscarinic, adenosine A 2 and histamine H 3 receptor modulation of haloperidol-induced c-fos expression in the striatum and nucleus accumbens. Neuroscience. 2002;112:427–438. doi: 10.1016/S0306-4522(02)00069-6. [DOI] [PubMed] [Google Scholar]
- 68.Benveniste EN. Cytokine actions in the central nervous system. Cytokine & growth factor reviews. 1998;9:259–275. doi: 10.1016/S1359-6101(98)00015-X. [DOI] [PubMed] [Google Scholar]
- 69.Ito C. The role of the central histaminergic system on schizophrenia. Drug News Perspect. 2004;17:383–387. doi: 10.1358/dnp.2004.17.6.829029. [DOI] [PubMed] [Google Scholar]
- 70.Baucum AJ, Rau KS, Riddle EL, Hanson GR, Fleckenstein AE. Methamphetamine increases dopamine transporter higher molecular weight complex formation via a dopamine-and hyperthermia-associated mechanism. Journal of Neuroscience. 2004;24:3436–3443. doi: 10.1523/JNEUROSCI.0387-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hernandez L, Auerbach S, Hoebel BG. Phencyclidine (PCP) injected in the nucleus accumbens increases extracellular dopamine and serotonin as measured by microdialysis. Life sciences. 1988;42:1713–1723. doi: 10.1016/0024-3205(88)90037-9. [DOI] [PubMed] [Google Scholar]
- 72.Galosi R, et al. Dopaminergic effects of histamine administration in the nucleus accumbens and the impact of H1-receptor blockade. Neuropharmacology. 2001;40:624–633. doi: 10.1016/S0028-3908(00)00181-7. [DOI] [PubMed] [Google Scholar]
- 73.Meskanen K, et al. A randomized clinical trial of histamine 2 receptor antagonism in treatment-resistant schizophrenia. Journal of clinical psychopharmacology. 2013;33:472–478. doi: 10.1097/JCP.0b013e3182970490. [DOI] [PubMed] [Google Scholar]
- 74.Terland O, Flatmark T. Drug-induced parkinsonism: cinnarizine and flunarizine are potent uncouplers of the vacuolar H+-ATPase in catecholamine storage vesicles. Neuropharmacology. 1999;38:879–882. doi: 10.1016/S0028-3908(98)00233-0. [DOI] [PubMed] [Google Scholar]
- 75.Terrillion, C. E. et al. Reduced levels of Cacna1c attenuate mesolimbic dopamine system function. Genes, Brain and Behavior (2017). [DOI] [PMC free article] [PubMed]
- 76.Mena MA, et al. Effects of calcium antagonists on the dopamine system. Clinical neuropharmacology. 1995;18:410–426. doi: 10.1097/00002826-199510000-00004. [DOI] [PubMed] [Google Scholar]
- 77.Teive HA, Troiano AR, Germiniani FM, Werneck LC. Flunarizine and cinnarizine-induced parkinsonism: a historical and clinical analysis. Parkinsonism & related disorders. 2004;10:243–245. doi: 10.1016/j.parkreldis.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 78.Chandwani S, et al. Induction of DARPP-32 by brain-derived neurotrophic factor in striatal neurons in vitro is modified by histone deacetylase inhibitors and Nab2. PloS one. 2013;8:e76842. doi: 10.1371/journal.pone.0076842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Domire JS, et al. Dopamine receptor 1 localizes to neuronal cilia in a dynamic process that requires the Bardet-Biedl syndrome proteins. Cellular and Molecular Life Sciences. 2011;68:2951–2960. doi: 10.1007/s00018-010-0603-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cuboni S, et al. Loratadine and Analogues: Discovery and Preliminary Structure–Activity Relationship of Inhibitors of the Amino Acid Transporter B0AT2. Journal of medicinal chemistry. 2014;57:9473–9479. doi: 10.1021/jm501086v. [DOI] [PubMed] [Google Scholar]
- 81.Chandra R, et al. Reduced Slc6a15 in nucleus accumbens D2-neurons underlies stress susceptibility. Journal of Neuroscience. 2017;37:6527–6538. doi: 10.1523/JNEUROSCI.3250-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shiraishi A, et al. The advantage of alfacalcidol over vitamin D in the treatment of osteoporosis. Calcified tissue international. 1999;65:311–316. doi: 10.1007/s002239900704. [DOI] [PubMed] [Google Scholar]
- 83.Meltzer HY. Clinical studies on the mechanism of action of clozapine: the dopamine-serotonin hypothesis of schizophrenia. Psychopharmacology. 1989;99:S18–S27. doi: 10.1007/BF00442554. [DOI] [PubMed] [Google Scholar]
- 84.Kočovská, E., Gaughran, F., Krivoy, A. & Meier, U.-C. Vitamin-D Deficiency As a Potential Environmental Risk Factor in Multiple Sclerosis, Schizophrenia, and Autism. Frontiers in psychiatry8 (2017). [DOI] [PMC free article] [PubMed]
- 85.McGrath John, Saari Kaisa, Hakko Helinä, Jokelainen Jari, Jones Peter, Järvelin Marjo-Riitta, Chant David, Isohanni Matti. Vitamin D supplementation during the first year of life and risk of schizophrenia: a Finnish birth cohort study. Schizophrenia Research. 2004;67(2-3):237–245. doi: 10.1016/j.schres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 86.Kesby, J. P., Cui, X., Burne, T. H. & Eyles, D. W. Altered dopamine ontogeny in the developmentally vitamin D deficient rat and its relevance to schizophrenia. Frontiers in cellular neuroscience 7 (2013). [DOI] [PMC free article] [PubMed]
- 87.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1α-hydroxylase in human brain. Journal of chemical neuroanatomy. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 88.Kesby JP, Burne TH, McGrath JJ, Eyles DW. Developmental vitamin D deficiency alters MK 801-induced hyperlocomotion in the adult rat: An animal model of schizophrenia. Biological psychiatry. 2006;60:591–596. doi: 10.1016/j.biopsych.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 89.Krabbe S, et al. Increased dopamine D2 receptor activity in the striatum alters the firing pattern of dopamine neurons in the ventral tegmental area. Proceedings of the National Academy of Sciences. 2015;112:E1498–E1506. doi: 10.1073/pnas.1500450112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pertile RA, Cui X, Eyles DW. Vitamin D signaling and the differentiation of developing dopamine systems. Neuroscience. 2016;333:193–203. doi: 10.1016/j.neuroscience.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 91.Steddon SJ, Schroeder NJ, Cunningham J. Vitamin D analogues: how do they differ and what is their clinical role? Nephrology Dialysis Transplantation. 2001;16:1965–1967. doi: 10.1093/ndt/16.10.1965. [DOI] [PubMed] [Google Scholar]
- 92.Eyles DW, et al. The association between neonatal vitamin D status and risk of schizophrenia. Scientific reports. 2018;8:17692. doi: 10.1038/s41598-018-35418-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brown HE, Roffman JL. Vitamin supplementation in the treatment of schizophrenia. CNS drugs. 2014;28:611–622. doi: 10.1007/s40263-014-0172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao, K. & So, H.-C. Drug repositioning for schizophrenia and depression/anxiety disorders: A machine learning approach leveraging expression data. IEEE journal of biomedical and health informatics (2018). [DOI] [PubMed]
- 95.Kidnapillai, S. et al. The use of a gene expression signature and connectivity map to repurpose drugs for bipolar disorder. The World Journal of Biological Psychiatry, 1–9 (2018). [DOI] [PubMed]
- 96.Kondej M, Stępnicki P, Kaczor A. Multi-target approach for drug discovery against schizophrenia. International journal of molecular sciences. 2018;19:3105. doi: 10.3390/ijms19103105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karunakaran, K. B., Chaparala, S. & Ganapathiraju, M. K. Potentially repurposable drugs for schizophrenia identified from its interactome. bioRxiv, 442640 (2018). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated during the current study. Source of data that was analyzed in this study has been described in Methods and Data File 1.