Abstract
A novel polyvinyl alcohol (PVA)-degrading strain Bacillus cereus RA23 was isolated from an oil sludge sample and environmental factors affecting its PVA degradation efficiency were optimized in detail. Inorganic nitrogen source, ammonium chloride (NH4Cl), was found to be the best nitrogen source and enhanced the PVA degradation rate greatly. The optimal medium for PVA biodegradation consisted of (g/L) PVA 1, NH4Cl 1, K2HPO4 1.6, MgSO4·7H2O 0.05, FeSO4·6H2O 0.02, CaCl2 0.05, NaCl 0.02. The optimal temperature and pH for PVA biodegradation by strain RA23 was 28 °C and 7.0, respectively, and 85% of 0.1% PVA was degraded after 5 days under these conditions. FTIR studies showed that the carboxylic acids (possibly including aldehyde or ketone) could be the intermediate product of PVA biodegradation. The investigation of strain RA23 for PVA degradation will provide important information to facilitate the removal of wastewater pollution in industrial zones.
Keywords: Biodegradation, Polyvinyl alcohol, Bacillus cereus RA23, Culture condition optimization, Structural changes
Introduction
Polyvinyl alcohol (PVA) is an important synthetic polymer that can be used in various industrial applications, e.g., biomedical field, paper coatings, adhesives and textile industries (Ben Halima 2016; Bian et al. 2019). The heavy use of PVA causes pollution in both terrestrial and aquatic ecosystems, because PVA is directly discharged into industrial wastewater (Kim et al. 2003). For example, various sizing agents including PVA are employed to protect cotton fibers during the weaving process in the textile industries (Larking et al. 1999). The cotton fabric is subsequently dyed, and the sizing agent used during the finishing process must be removed to increase the dyeing efficiency. The industrial water drainage is remarkably polluted due to the spontaneous release of PVA, and this is problematic not only because is the biodegradation of PVA extremely slow, but also its accumulation blocks the supply of oxygen to aquatic organisms (Kim et al. 2003; Chiellini et al. 2003).
It is very difficult to remove all PVA via simple wastewater treatment techniques. Therefore, various physical and chemical treatments such as ultrasonic techniques (Gronroos et al. 2001), adsorption (Behera et al. 2008), photocatalytic oxidation method (Chen et al. 2001) or radiation-induced degradation (Zhang and Yu 2004) have been used to treat PVA in wastewater. However, the operational costs of using such methods are expensive and additionally, unnecessary sludge is generated as a consequence of this treatment. Therefore, the use of a biological method to solve this problem has attracted much attention (Ben Halima 2016). For efficient removal of PVA polymer, microbial treatment techniques are considered to be environmentally friendly and economic (Mori et al. 1996), and to date, many studies on PVA biodegradation have been published. Previous studies have shown that PVA-degrading strains can be bacteria such as Pseudomonas sp. O-3 (Suzuki et al. 1973), Alcaligenes faecalis (Matsumura et al. 1994), Sphingomonas sp. (Tokiwa et al. 2001), Sphingopyxis sp. PVA3 (Yamatsu et al. 2006), Povalibacter uvarum (Nogi et al. 2014a), Thalassospira povalilytica (Nogi et al. 2014b), and Stenotrophomonas rhizophila (Wei et al. 2018), as well as fungi belonging to the genera of Penicillium (Qian et al. 2004), Aspergilus (Stoica-Guzun et al. 2010), Fomitopsis (Tsujiyama and Okada 2013). Other researchers have isolated several symbiotic PVA-degrading isolates, including Pseudomonas sp. VM15C and its symbiotic Pseudomonas sp. VM15A (Sakazawa et al. 1981), Bacillus megaterium and the bacterial strain PN19 (Mori et al. 1996), and Sphingomonas sp. SA3 and SA2 (Kim et al. 2003). However, the degradation rate of PVA still needs to be improved by screening for more efficient PVA degraders. In some studies, different environmental conditions were also found to influence PVA degradation such as composting (Chiellini et al. 2003), aqueous environments (Corti et al. 2002) and anaerobic environments (Matsumura and Shimokobe 1993). Therefore, the eventual biological fate of PVA removal is largely dependent on environmental conditions. However, only a few reports have focused on the culture conditions of the strain, such as nutrients, temperature, and pH, to enhance PVA biodegradation rate.
In the present study, a bacterial strain, Bacillus cereus RA23, showed an ability to degrade PVA, and its culture conditions were optimized to enhance the PVA degradation rate.
Materials and methods
Isolation of PVA-degrading microbial strains
Bacterial strain RA23 was obtained from an oil sludge sample from Shengli Oil Field (Shandong, China). For the isolation of PVA-degrading microbes, the screening medium contained the following components: 1.0 g of PVA 1799, 1 g of yeast extract, 1.0 g of NH4NO3, 1.6 g of K2HPO4, 0.05 g of MgSO4·7H2O, 0.05 g of CaCl2, 0.02 g of FeSO4·7H2O and 0.02 g of NaCl per 1000 mL prepared at an initial pH of 7.0 (Chen et al. 2007) and the agar screening plates were supplemented with 1.5% agar. One gram of sludge sample was added into 9 mL 0.8% (w/w) NaCl solution. The mixture was shaken on a shaker for 10 min and 1 mL sample solution was transferred into 50 mL PVA medium and incubated on a shaker at 30 °C, 200 rpm for 24 h. After enrichment culture, the PVA-degrading strains were initially spread on PVA agar plates and incubated at 30 °C for 48 h, and their PVA-degrading ability was evaluated by a solution of iodine and boric acid (Du et al. 2007). The selected strains were purified on LB (0.5% yeast extract, 1% tryptone, 1% NaCl) agar plates. The strain RA23 showed the maximum PVA degradation activity and was chosen as the candidate PVA degrader in further experiments.
16S ribosomal RNA (rRNA) gene sequence analysis of the strain RA23
The 16S rRNA of strain RA23 was analyzed by PCR amplification. Strain RA23 was cultured in LB broth, cells were harvested by centrifugation at 6000×g for 5 min, and chromosomal DNA was extracted using the TIANamp Bacteria DNA Kit (TianGen, Beijing, China) according to manufacturer’s instructions. The 16S rRNA gene sequence was amplified using genomic DNA as the PCR template with 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5′-TACGGTTACCTTGTTACGACTT-3′) as primers (Rahman et al. 2009; Weisburg et al. 1991). Both primers were synthesized by Sangon Biotech (Shanghai, China). The reaction mixture had a total volume of 50 µL and contained 1 µL of genomic DNA, 1 µL of each primer (10 mmol/L), 4 µL of dNTP mixture (each 2.5 mmol/L), 5 µL of buffer, 37 µL of distilled water and 1 µL of Taq DNA polymerase. PCR reaction was carried out in the thermal cycler, and the main cycle procedure containing denaturation (94 °C for 30 s), primer annealing (56 °C for 45 s), and product extension (72 °C for 90 s) steps, 30 cycles. The amplified products were analyzed on a gel imaging system, and the visualized band was purified by a gel purification kit (Favorprep, Favorgen). The commercial DNA sequencing of 16S rRNA gene was performed by Sangon Biotech (Shanghai, China) and the obtained sequence was subjected to an online BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) (Altschul et al. 1990) tool on NCBI website. A phylogenetic tree for strain RA23 was constructed using the program ClustalX version 2.0 (Larkin et al. 2007) and MEGA 6 software (Tamura et al. 2013) with the neighbor-joining method to calculate the evolutionary distances and bootstrap resampling (1000 replicates each) to assess robustness of phylogenetic trees.
Cell morphological and biochemical characteristics
The colony morphology was observed on LB media. Gram staining was performed using a Gram Staining Kit (Qingdao Hope Bio-Technology Co., Ltd., Qingdao, China) according to the manufacturer’s instructions. The cell morphology of the bacterium was observed by a field emission scanning electron microscope (Hitachi S-4800, Ibraraki, Japan) using the same method as described in our previous work (Ullah et al. 2018). Furthermore, the bacterial isolate was biochemically characterized using the B. cereus biochemical identification kit (Qingdao Hope Bio-Technology Co., Ltd., Qingdao, China) according to the manual of the manufacture; the tests included catalase activity, citrate utilization (Simmons), fermentation of carbohydrates, nitrate reduction, indole formation, Voges–Proskauer reaction, liquefaction of gelatin and motility. Other tests such as oxidase activity, β-galactosidase activity, H2S production, etc., were also performed using related kits (Qingdao Hope Bio-Technology Co., Ltd., Qingdao, China).
Analysis of PVA degradation for strain RA23
The bacteria involved in PVA degradation were cultured for 1 week. For each strain, 1 mL of the seed culture was transfered to 30-mL screening medium in a 250-mL flask and cultured at 30 °C, 200 rpm for 7 days. During the culture process, cell growth and PVA degradation were determined every day to confirm the role of bacterial cells in PVA degradation. After centrifugation of the culture broth at 10,000×g for 10 min, the pellet was discarded, and the resulting supernatant was filtered through a membrane filter (0.45 µm; Millipore) and was further used as the PVA samples to be tested. After the proper dilution of samples, the PVA concentration was estimated according to the reported method (Finley 1961) using the spectrophotometric assay based on the blue products generated by the reaction between PVA and iodine in the presence of boric acid. At first, the calibration curve was determined. In brief, 1 g/L of standard PVA solution was prepared and further diluted to different concentrations, followed by the addition of 0.75 mL of 4% boric acid and 0.15 mL of KI–I2 (12.7 g/L I2 added into 25 g/L KI). The above solution was diluted to a total volume of 2.5 mL with distilled water and then equilibrated for 30 min at room temperature. The optical density at 690 nm was detected using a double-beam spectrophotometer (UV-2450, Shimadzu, Japan) to obtain the calibration curve. The absorbance of PVA samples treated with the same method was measured, and the PVA concentration was calculated from the calibration curve.
Culture condition optimization of strain RA23
To investigate the effects of different carbon sources on cell growth and PVA degradation rate, eight carbon sources including glucose, fructose, galactose, maltose, sucrose, lactose, cellulose, and dextrin at a concentration of 1% were added independently to the PVA medium. The strain RA23 was cultured in different media at 30 °C, 200 rpm for 5 days and the final PVA concentration after degradation was determined. Similarly, different nitrogen sources at a concentration of 0.1% as the sole nitrogen source were also tested, including inorganic nitrogen sources, such as ammonium chloride, ammonium sulphate, ammonium nitrate, ammonium acetate, and urea, as well as organic nitrogen sources, such as yeast extract, beef extract and peptone. Different incubation temperatures ranging from 5 to 50 °C and different pH values ranging from 5 to 9 were used to identify the optimal temperature and pH.
Other analytical methods
The culture broth was centrifuged at 10,000×g for 10 min, washed twice with deionized water and dried to a constant weight in an oven at 105 °C. The total biomass was calculated as g of DCW per L (g/L). Fourier-transform infrared (FTIR) spectra were determined in a KBr disc using a Nicolet 6700 spectrometer (Thermo Scientific, Waltham, MA, USA), and transmission spectra were obtained in the region from 500 to 4000 cm−1.
Results and discussion
Isolation of PVA-degrading strain RA23 and analysis of the biodegradation rate
Previously, many PVA-degrading microbes, particularly bacteria, have been isolated from soils contaminated with PVA, wastewater, and activated sludge (Chen et al. 2007; Zhang 2009; Kim et al. 2003). However, the PVA degradation efficiency of many single cultures is not satisfactory. Therefore, the current research was conducted to exploit potential bacterial strains for the biodegradation of PVA. Microbes were obtained from an oil sludge sample from Shengli Oil Field P.R China after the enrichment culture in the screening medium and isolation on the PVA agar plates. Strain RA23 presented the highest PVA degradation capability in all isolated strains. Therefore, the time course for PVA degradation by strain RA23 was determined to confirm its PVA degradation ability. Both cell growth and PVA degradation of RA23 were examined after a fresh seed culture of strain RA23 was inoculated in the screening medium containing 0.1% PVA as the sole source of carbon. As shown in Fig. 1, the cell concentration increased exponentially after a very short lag time and peaked at 5 days. Similar to the cell growth rate, the PVA degradation rate increased over time, and half of the total PVA concentration was removed at 5 days; however, the removal of PVA was at a standstill as the cell growth entered the death phase. These results may indicate that PVA degradation was related to cell growth, which was inconsistent with the results of previous studies (Du et al. 2007; Kim et al. 2003). Thus, strain RA23 was finally selected as a candidate for PVA biodegradation in this study.
Fig. 1.
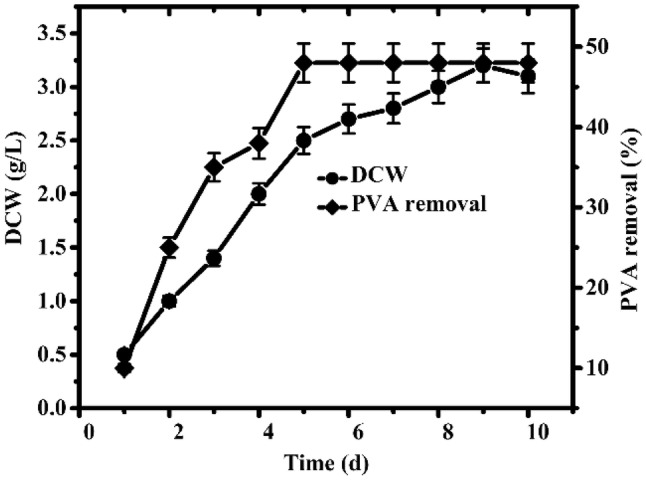
Time course of cell growth and PVA degradation of the strain RA23 using the screening medium
Identification of strain RA23
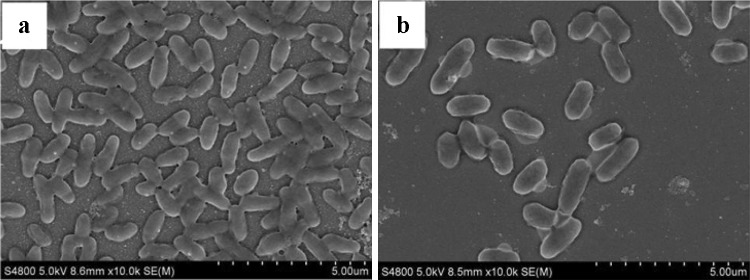
Phenotypic and genotypic characterization of strain RA23 was performed. First, strain RA23 was identified by 16S rRNA sequence (1376 bp, GenBank accession number MN122695) analysis, and a phylogenetic tree was constructed (Fig. 2). B. cereus was the closest species to strain RA23. Strain RA23 exhibited 99% 16S rRNA sequence similarity to different strains of B. cereus. Further morphological and biochemical characteristics of strain RA23 in logarithmic growth phase were determined. Cells in early and middle stages of logarithmic growth phase cultured for 1 day and 3 days were chosen and their morphology were observed under light microscopy and scanning electron microscopy with an S-4800 scanning electron microscope (Fig. 3). Strain RA23 had Gram-positive rods. The strain appeared to be aerobic and motile. It was also positive for urease, oxidase, catalase, lysine decarboxylase, ornithine decarboxylase, beta-galactosidase, and arginine hydrolase. Strain RA23 also utilized citrate and gelatine and could produce acid from rhamnose and amygdalin; however, it could not produce acid from glucose, sucrose, lactose, cellulose, mannose, sorbitol, inositol, arabinose, or melibiose (Table 1). These morphological and biochemical characteristics are similar to those of B. cereus. Therefore, this strain was named B. cereus RA23 and the strain in this species has not previously been reported as a PVA degrader.
Fig. 2.
Phylogenetic analysis of B. cereus RA23 based on the 16S rRNA gene sequence. The phylogenetic tree was constructed with the MEGA6 program using the neighbor-joining method. Bootstrap values, the percentages of 1000 replications, were given at branching points. Scale bar 0.005 amino acid substitutions/site
Fig. 3.
SEM of B. cereus RA23 cultured in PVA medium for 1 day (a) and 3 days (b) at 30 °C. Bar 5 μm
Table 1.
Biochemical characteristics of B. cereus RA23
| Test | Reaction* | Test | Reaction* |
|---|---|---|---|
| Gram | + | Lysine decarboxylase (LDH) | + |
| Motility | + | Ornithine decarboxylase (ODC) | + |
| Urease reaction | + | Mannose | – |
| Catalase reaction | + | Sorbitol | – |
| Oxidase reaction | + | Inositol | – |
| Voges–Proskauer (VP) | – | Rhamnose | + |
| Gelatine hydrolysis | + | Arabinose | – |
| Hydrogen sulphide (H2S) | + | Sucrose | – |
| Indole production | – | Amygdalin | + |
| Citrate utilization | + | Glucose | – |
| Beta-galactosidase (ONPG) | + | Melibiose | – |
| Arginine dihydrolase (ADH) | + | Lactose | – |
* + Positive reaction, – negative reaction
Effects of environmental factors on PVA biodegradation by B. cereus RA23
Previous studies have shown that different nutrient sources, including nitrogen, carbon sources and other environmental conditions, also affect the PVA degradation rate. One of the most possible reasons may be that these nutrients or environmental conditions are required for bacterial cell growth and PVA-degrading enzyme synthesis. For these reasons, the composition of the culture medium for strain B. cereus RA23 was optimized to enhance the PVA removal rate by adding different carbon and nitrogen sources to the medium.
For the proper selection of carbon source, eight carbon sources were initially selected at a concentration of 1% (Table 2). The supplementation of carbon sources in the culture medium enhanced cell growth; however, the PVA removal rate was slightly decreased. It seems reasonable to suppose that the addition of carbon sources inhibited the production of PVA-degrading enzymes, although it promoted the growth of bacteria. Differently, nitrogen sources greatly influenced B. cereus RA23 cell growth and PVA removal. Inorganic nitrogen sources resulted in higher percentages of PVA degradation than did organic nitrogen sources. The similar effects of inorganic nitrogen sources on PVA degradation were also found in our previous studies (Ullah et al. 2018; Kim et al. 2003). The PVA degradation rate of B. cereus RA23 was highest when ammonium chloride was used (Table 3). It was concluded that the addition of inorganic nitrogen sources was useful for PVA degradation of strain RA23, and ammonium chloride was chosen as the optimal nitrogen source.
Table 2.
Effects of carbon source on cell growth and PVA removal of B. cereus RA23
| Carbon source | PVA removal (%) | DCW (g/L) |
|---|---|---|
| Glucose | 25 | 1.4 |
| Sucrose | 28 | 1.8 |
| Maltose | 30 | 2.0 |
| Galactose | 32 | 2.2 |
| Fructose | 40 | 2.5 |
| Cellulose | 41 | 2.7 |
| Dextrin | 44 | 2.9 |
| Lactose | 45 | 3 |
Table 3.
Effects of nitrogen source on cell growth and PVA removal of B. cereus RA23
| Nitrogen source | PVA removal (%) | DCW (g/L) |
|---|---|---|
| Yeast extract | 40 | 2.4 |
| Beef extract | 34 | 2.2 |
| Peptone | 30 | 2.0 |
| Urea | 50 | 1.8 |
| NH4NO3 | 65 | 2 |
| (NH4)2AC | 55 | 1.8 |
| (NH4)2SO4 | 63 | 1.9 |
| NH4Cl | 75 | 1.8 |
Besides, some possible environmental factors that affect PVA degradation were also studied. In previous studies, pH and temperature were considered to be important factors that demonstrated their influence on biological features, particularly microbial growth and degradation ratio (Zhang 2009). To ensure the optimal pH and temperature, the effects of different pH values ranging from 5 to 10 and temperatures ranging from 15 to 50 °C on PVA biodegradation were studied in detail (Fig. 4). Cell growth and degradation activities were assessed after 5 days of incubation. The optimal pH and temperature values for B. cereus RA23 were 7.0 and 28 °C, respectively. B. cereus RA23 can also grow at 45 °C and 50 °C, but it did not grow well. Using these optimized temperature and pH values, PVA removal was enhanced and reached 85% after 5 days of incubation (Fig. 5).
Fig. 4.
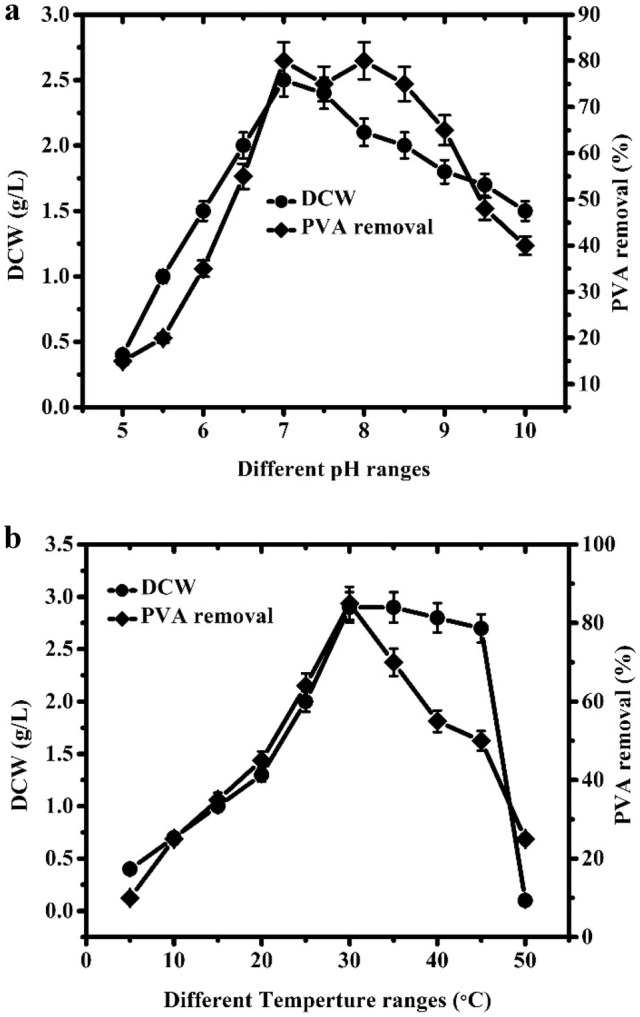
Effects of pH (a) and temperature (b) on cell growth and PVA removal of B. cereus RA23
Fig. 5.
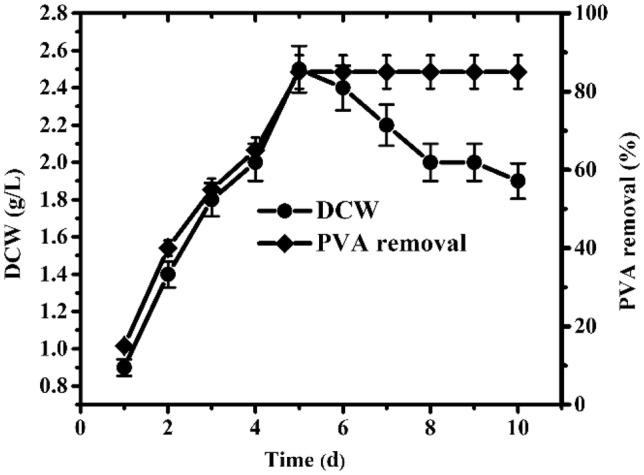
Time course of cell growth and PVA degradation by the strain B. cereus RA23 under the optimized conditions: the optimal medium consisted of (g/L) PVA 1, NH4Cl 1, K2HPO4 1.6, MgSO4·7H2O 0.05, FeSO4·6H2O 0.02, CaCl2 0.05, NaCl 0.02, and the pH and temperature was 7 and 28 °C, respectively
Thus, B. cereus RA23 exhibited the maximum degradation rate at 28 °C when 0.1% PVA and 0.1% ammonium chloride were used as the carbon source and nitrogen source with a pH of 7.0. Strain RA23 will be very useful in industrial wastewater treatment for the removal of PVA pollution, because it can degrade PVA efficiently and independently. Therefore, the present strain for PVA removal has great advantages for the reduction of environmental contamination.
FTIR spectroscopy of PVA 1799 before and after degradation
PVA1799 used in this study was known as the sizing resin in textile industries with an average polymerization degree of 1700 and a saponification degree of 99%. To determine the effect of bacteria on the PVA degradation, structural changes during the PVA degradation course were investigated using FTIR spectroscopy after 5 days of incubation with B. cereus RA23 under the optimized conditions. FTIR spectra were measured at wavelengths ranging from 500 to 4000 cm−1 (Fig. 6). Compared with the FTIR spectrum of PVA at 0 h, after 5 days of degradation, an absorption band at 1740 cm−1 attributed to carbonyl groups and an additional absorption at 1600 cm−1 probably resulting from the presence of carboxylic iron groups were observed. Therefore, the carboxylic acids (possibly including aldehyde or ketone) might appear during PVA degradation process and they formed the possible intermediate products of PVA degradation. Similar finding was also reported in other study (Chen et al. 2007). The supposed formation pathway of carboxylic acids or other products through the degradation of PVA in the PVA-utilizing microorganisms comprised two steps: the first step is the oxidation of hydroxyl groups of PVA to form diketone or monoketone structures catalyzed by secondary alcohol oxidases (SAO) or PVA dehydrogenase (PVADH), and the second step is the hydrolysis of a C–C bond at diketone structures with the production of methyl ketone, carboxylic acid, and acetic acid, etc., catalyzed by especially oxidized PVA hydrolase (OPH) or β-diketone hydrolase (BDH) (Kawai and Hu 2009). The PVA-degrading enzymes of the strain will be detected and characterized in our next work to elucidate the possible PVA degradation mechanism.
Fig. 6.

FTIR spectra of PVA samples during degrading process by strain B. cereus RA23. (a) Control, (b) after 5 days of degradation
Conclusions
A new PVA-degrading strain RA23 was isolated from oil sludge sample and it was identified to be B. cereus. The culture medium and environmental factors that affect the PVA degradation efficiency of the strain were optimized systematically. The inorganic nitrogen source NH4Cl was found to be the optimal nitrogen source and enhanced the PVA degradation rate greatly. The optimal medium for PVA biodegradation consisted of (g/L) PVA 1, NH4Cl 1, K2HPO4 1.6, MgSO4·7H2O 0.05, FeSO4· 6H2O 0.02, CaCl2 0.05, NaCl 0.02, and the optimal pH and temperature was 7 and 28 °C, respectively. After the optimization of culture conditions, 85% of 0.1% PVA was degraded by B. cereus RA23 after 5 days. FTIR spectroscopy showed that the carboxylic acids (possibly including aldehyde or ketone) may be the intermediate product of PVA degradation. Our investigation of B. cereus RA23 for PVA degradation provides important information to facilitate the removal of wastewater pollution in industrial zones.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (U1805234 and 31800075), 863 Program (No. 2015AA020925), Natural Science Foundation of Fujian Province of China (2019J01264), Minjiang Scholar (No. 2013A13), Startup fund for high-level talent at Fujian Normal University (No. 004828), and Fundamental Research Funds for the Central Universities (No. 18CX02124A), the Study of Bioresources Protection and Restoration of the Ecological Environment of the Typical Coastal Zone in Shandong Province, and partially supported by the Open Funding Project of the State Key Laboratory of Bioreactor Engineering.
Compliance with ethical standards
Conflict of interest
All authors hereby declare that there is no conflict of interest.
Ethical approval
This article does not include any studies with human participants or animals performed by any of the authors.
Informed consent
This article does not involve any informed consent.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Behera SK, Kim JH, Guo X, Park HS. Adsorption equilibrium and kinetics of polyvinyl alcohol from aqueous solution on powdered activated carbon. J Hazard Mater. 2008;153:1207–1214. doi: 10.1016/j.jhazmat.2007.09.117. [DOI] [PubMed] [Google Scholar]
- Ben Halima N. Poly(vinyl alcohol): review of its promising applications and insights into biodegradation. RSC Adv. 2016;6:39823–39832. doi: 10.1039/C6RA05742J. [DOI] [Google Scholar]
- Bian H, Cao M, Wen H, Tan Z, Jia S, Cui J. Biodegradation of polyvinyl alcohol using cross-linked enzyme aggregates of degrading enzymes from bacillus niacini. Int J Biol Macromol. 2019;124:10–16. doi: 10.1016/j.ijbiomac.2018.11.204. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sun Z, Yang Y, Ke Q. Heterogeneous photocatalytic oxidation of polyvinyl alcohol in water. J Photochem Photobiol A. 2001;142:85–89. doi: 10.1016/S1010-6030(01)00477-4. [DOI] [Google Scholar]
- Chen J, Zhang Y, Du GC, Hua ZZ, Zhu Y. Biodegradation of polyvinyl alcohol by a mixed microbial culture. Enzyme Microb Technol. 2007;40:1686–1691. doi: 10.1016/j.enzmictec.2006.09.010. [DOI] [Google Scholar]
- Chiellini E, Corti A, D’Antone S, Solaro R. Biodegradation of poly (vinyl alcohol) based materials. Prog Polym Sci. 2003;28:963–1014. doi: 10.1016/S0079-6700(02)00149-1. [DOI] [Google Scholar]
- Corti A, Cinelli P, D’Antone S, Kenawy ER, Solaro R. Biodegradation of poly(vinyl alcohol) in soil environment: influence of natural organic fillers and structural parameters. Macromol Chem Phys. 2002;203:1526–1531. doi: 10.1002/1521-3935(200207)203:10/11<1526::AID-MACP1526>3.0.CO;2-R. [DOI] [Google Scholar]
- Du G, Liu L, Song Z, Hua Z, Zhu Y, Chen J. Production of polyvinyl alcohol-degrading enzyme with Janthinobacterium sp. and its application in cotton fabric desizing. Biotech J. 2007;2:752–758. doi: 10.1002/biot.200600121. [DOI] [PubMed] [Google Scholar]
- Finley JH. Spectrophotometric determination of polyvinyl alcohol in paper coatings. Anal Chem. 1961;33:1925–1927. doi: 10.1021/ac50154a044. [DOI] [Google Scholar]
- Gronroos A, Pirkonen P, Heikkinen J, Ihalainen J, Mursunen H, Sekki H. Ultrasonic depolymerization of aqueous polyvinyl alcohol. Ultrason Sonochem. 2001;8:259–264. doi: 10.1016/S1350-4177(01)00086-4. [DOI] [PubMed] [Google Scholar]
- Kawai F, Hu X. Biochemistry of microbial polyvinyl alcohol degradation. Appl Microbiol Biotechnol. 2009;84:227–237. doi: 10.1007/s00253-009-2113-6. [DOI] [PubMed] [Google Scholar]
- Kim BC, Sohn CK, Lim SK, Lee JW, Park W. Degradation of polyvinyl alcohol by Sphingomonas sp. SA3 and its symbiote. J Ind Microbiol Biotechnol. 2003;30:70–74. doi: 10.1007/s10295-002-0010-4. [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Larking DM, Crawford RJ, Christie GB, Lonergan GT. Enhanced degradation of polyvinyl alcohol by Pycnoporus cinnabarinus after pretreatment with fenton’s reagent. Appl Environ Microbiol. 1999;65:1798–1800. doi: 10.1128/aem.65.4.1798-1800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura S, Shimokobe HK. Anaerobic biodegradability of polyvinyl alcohol. Biotechnol Lett. 1993;15:749–754. doi: 10.1007/BF01080150. [DOI] [Google Scholar]
- Matsumura S, Shimura Y, Terayama K. Effects of molecular weight and stereoregularity on biodegradation of poly (vinyl alcohol) by Alcaligenes faecalis. Biotechnol Lett. 1994;16:1205–1210. doi: 10.1007/BF01020852. [DOI] [Google Scholar]
- Mori T, Sakimoto M, Kagi T, Sakai T. Isolation and characterization of a strain of Bacillus megaterium that degrades poly(vinyl alcohol) Biosci Biotechnol Biochem. 1996;60:330–332. doi: 10.1271/bbb.60.330. [DOI] [PubMed] [Google Scholar]
- Nogi Y, Yoshizumi M, Hamana K, Miyazaki M, Horikoshi K. Povalibacter uvarum gen. Nov., sp. Nov., a polyvinyl-alcohol-degrading bacterium isolated from grapes. Int J Syst Evol Microbiol. 2014;64:2712–2717. doi: 10.1099/ijs.0.062620-0. [DOI] [PubMed] [Google Scholar]
- Nogi Y, Yoshizumi M, Miyazaki M. Thalassospira povalilytica sp. Nov., a polyvinyl-alcohol-degrading marine bacterium. Int J Syst Evol Microbiol. 2014;64:1149–1153. doi: 10.1099/ijs.0.058321-0. [DOI] [PubMed] [Google Scholar]
- Qian D, Du G, Chen J. Isolation and culture characterization of a new polyvinyl alcohol-degrading strain: Penicillium sp. WSH02-21. World J Microb Biot. 2004;20:587. doi: 10.1023/B:WIBI.0000043172.83610.08. [DOI] [Google Scholar]
- Rahman N, Xiaohong C, Meiqin F, Mingsheng D. Characterization of the dominant microflora in naturally fermented camel milk shubat. World J Microb Biotechnol. 2009;25:1941–1946. doi: 10.1007/s11274-009-0092-5. [DOI] [Google Scholar]
- Sakazawa C, Shimao M, Taniguchi Y, Kato N. Symbiotic utilization of polyvinyl alcohol by mixed cultures. Appl Environ Microbiol. 1981;41:261–267. doi: 10.1128/aem.41.1.261-267.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica-Guzun A, Jecu L, Gheorghe A, Raut I, Stroescu M, Ghiurea M, Danila M, Jipa I, Fruth V. Biodegradation of poly(vinyl alcohol) and bacterial cellulose composites by Aspergillus niger. J Polym Environ. 2010;19:69–79. doi: 10.1007/s10924-010-0257-1. [DOI] [Google Scholar]
- Suzuki T, Ichihara Y, Yamada M. Some characteristics of Pseudomonas O-3 which utilizes polyvinyl alcohol. Agric Biol Chem. 1973;37:747–756. [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokiwa Y, Kawabata G, Jarerat A. A modified method for isolating poly(vinyl alcohol)-degrading bacteria and study of their degradation patterns. Biotechnol Lett. 2001;23:1937–1941. doi: 10.1023/A:1013785817554. [DOI] [Google Scholar]
- Tsujiyama S, Okada A. Biodegradation of polyvinyl alcohol by a brown-rot fungus, Fomitopsis pinicola. Biotechnol Lett. 2013;35:1907–1911. doi: 10.1007/s10529-013-1281-8. [DOI] [PubMed] [Google Scholar]
- Ullah M, Weng CH, Li H, Sun SW, Zhang H, Song AH, Zhu H. Degradation of polyvinyl alcohol by a novel bacterial strain Stenotrophomonas sp. SA21. Environ Technol. 2018;39:2056–2061. doi: 10.1080/09593330.2017.1349189. [DOI] [PubMed] [Google Scholar]
- Wei Y, Fu J, Wu J, Jia X, Zhou Y, Li C, Dong M, Wang S, Zhang J, Chen F. Bioinformatics analysis and characterization of highly efficient polyvinyl alcohol (PVA)-degrading enzymes from the novel PVA degrader Stenotrophomonas rhizophila QL-P4. Appl Environ Microbiol. 2018;84:e01898. doi: 10.1128/AEM.01109-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16s ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamatsu A, Matsumi R, Atomi H, Imanaka T. Isolation and characterization of a novel poly(vinyl alcohol)-degrading bacterium, Sphingopyxis sp. PVA3. Appl Microbiol Biotechnol. 2006;72:804–811. doi: 10.1007/s00253-006-0351-4. [DOI] [PubMed] [Google Scholar]
- Zhang HZ. Influence of pH and C/N ratio on poly (vinyl alcohol) biodegradation in mixed bacterial culture. J Polym Environ. 2009;17:286–290. doi: 10.1007/s10924-009-0151-x. [DOI] [Google Scholar]
- Zhang SJ, Yu HQ. Radiation-induced degradation of polyvinyl alcohol in aqueous solutions. Water Res. 2004;38:309–316. doi: 10.1016/j.watres.2003.09.020. [DOI] [PubMed] [Google Scholar]