Abstract
This article displays raw data linked to the research article “Compositional Analysis and Biological Characterization of Cornus officinalis on Human 1.1B4 Pancreatic β Cells” [1]. This data was generated by utilizing HPLC/(+and -)ESI-MSn on Cornus officinalis (CO) from four independent sources [1]. The aim was to identify the chemical profile of CO from multiple sources to compare the similarities and differences resulting from various processing methods, and compile a list of known and novel constituents to elucidate the bioactive ingredients. This report contains the full chromatogram and a raw list of the constituents found in CO including chemical name, retention time, and molecular weight from all four sources. All data from HPLC/MS analysis is raw and unprocessed.
Keywords: Cornus officinalis, HPLC, Mass spectrometry, Chemical analysis, Traditional Chinese medicine
Specifications Table
| Subject area | Chemistry |
|---|---|
| More specific subject area | Spectroscopy |
| Type of data | Tables, Figures |
| How data was acquired | HPLC/(+ and −)ESI-MSn |
| Data format | Raw |
| Experimental factors | The chemical profile of Cornus officinalis from four independent sources (sources 1–4) were individually analyzed by HPLC/MS. |
| Experimental features | Approximately 0.5 g of dried powder from each source were extracted with 1.5 mL H2O + 1.5 mL methanol. After sonication and centrifugation, some of the supernatant was transferred to autosampler vials and analyzed via C8 HPLC/(+ and −)ESI-MSn. |
| Data source location | Cell Biology, Microbiology and Molecular Biology. University of South Florida, Tampa, Florida, United States. |
| Data accessibility | Data is within this article |
| Related research article | Sharp-Tawfik, A.E., Coiner, A.M., MarElia, C.B., Kazantzis, M., Zhang, C., and Burkhardt, B.R. (2019). Compositional analysis and biological characterization of Cornus officinalis on human 1.1B4 pancreatic beta cells. Mol Cell Endocrinol 494, 110491 [1]. |
Value of the data
|
1. Data
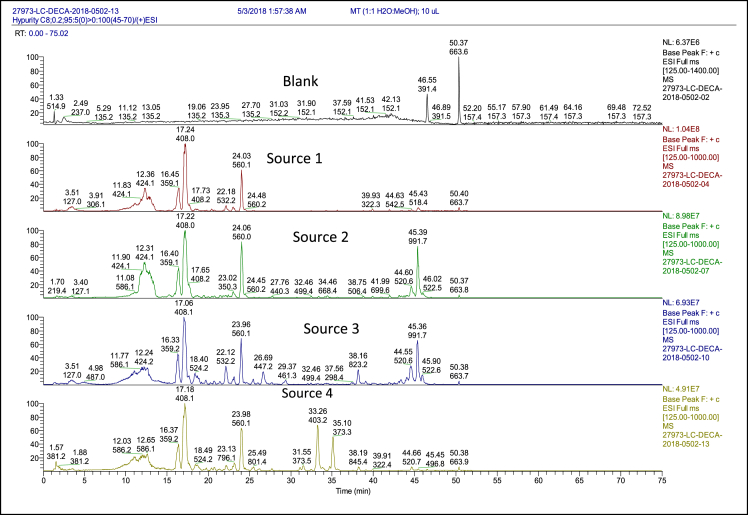
HPLC/(+ and −)ESI-MSn was performed on four sources of CO obtained from independent companies of which all follow good manufacturing processes (GMP) and quality control. Sources 1–4 include Guangdong Yifang Pharmaceutical Co., Ltd, Treasure of the East Tianjiang Pharmaceutical Co., Ltd, Min Tong Co, and Evergreen Herbs International, LLC. The individual preparations of CO differed by extraction/purification method. Source 1 has been processed using rice wine followed by water extraction to increase active ingredients. Sources 2–4 were all processed using traditional water-extraction followed by high pressure, low temperature vacuum extraction and granulation. After vacuum extraction, sources 1 and 2 performed a spray drying process to protect the active ingredients followed by a re-introduction of volatile oils by source 1. During the granulation process, source 3 utilizes non-GMO cornstarch as an addition to prevent caking and ensure stability of the extract. All four sources perform quality control assessments to examine bacteria levels, heavy metals, and pesticide residue. The chromatogram showing peaks of all compounds over 75 minutes from all four companies is displayed in Fig. 1. The Supplementary Tables 1 and 2 include the raw ion-peak detected in all samples with identified chemical name (if known), molecular weight, retention time and peak area.
Fig. 1.
HPLC/MS Chromatogram from all four sources of Cornus officinalis. The blank (1:1 H2O:MeOH) is shown at the top of this figure.
2. Experimental design, materials, and methods
2.1. HPLC/MS
Approximately 0.5 g of dried powder were extracted with 1.5 mL H2O + 1.5 mL methanol. After sonication and centrifugation, some of the supernatant was transferred to autosampler vials and analyzed via C8 HPLC/(+ and −)ESI-MSn. The analysis was carried out on Agilent (Palo Alto, CA) 1100 series system consisting of G1313A autosampler, G1322A degasser and G1312A binary pump followed by ThermoFinnigan (now ThermoFisher Scientific; San Jose, CA) LCQ DECA quadrupole ion trap mass spectrometer with electrospray ionization (ESI) operating with XCALIBUR 2.0.7. SP1. The chromatographic parameters were Thermoscientific Hypurity C8 (5 μm; 2.1 × 100 mm + guard column). Mobile phase (A): water + 0.2% acetic acid and mobile phase (B): methanol + 0.2% acetic acid with the following gradient: 0.2 mL/min: A:B(min) = 95:5(0) => 0:100(45–70) => 95:5(80–90). The + ESI parameters were as follows: spray voltage = 4.0 kV; heated capillary voltage = −18 V; tube lens offset = +8 V. The –ESI parameters were: spray voltage = 4.5 kV; heated capillary voltage = +6 V; tube lens offset = −2 V. The duration was 75 min with 4 scan events. The spectra of ion-peaks were examined and from those, related ions were plotted to create new ion-peaks in order to determine which ions correlated with each other. An ion-peak which was characteristic of a compound peaks was then integrated for the different samples.
Acknowledgments
This study was supported by grant NIH S10 OD021758-01A1 from the Mass Spectrometry Research and Education Center at the University of Florida.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dib.2019.104401.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Sharp-Tawfik A.E., Coiner A.M., MarElia C.B., Kazantzis M., Zhang C., Burkhardt B.R. Compositional analysis and biological characterization of Cornus officinalis on human 1.1B4 pancreatic beta cells. Mol. Cell. Endocrinol. 2019;494:110491. doi: 10.1016/j.mce.2019.110491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.