Abstract
Background
Phenylketonuria (PKU) leads to an accumulation of phenylalanine (Phe) in the blood and subsequent neurologic, cognitive, psychiatric, and behavioral dysfunction. Many patients report social isolation and decreased quality of life. Pegvaliase is an enzyme substitution therapy that reduces blood Phe levels in patients with PKU and is associated with a risk of hypersensitivity reactions.
Objective
To define the minimum acceptable benefit (MAB) of pegvaliase, i.e., the minimum probability of achieving a blood Phe level <360 μmol/L, which patients require to tolerate the risks of hypersensitivity associated with pegvaliase.
Methods
Adult, pegvaliase-naïve patients with blood Phe levels >600 μmol/L participated in a patient-preference web survey using two surveys: adapted swing-weighting and thresholding. Participants were asked to make ordinal choices between varying clinical benefit and severity levels for hypersensitivity. Disease effects and treatment satisfaction were also assessed.
Results
Among 45 participants, the mean (standard deviation) self-reported blood Phe level was 976.9 (429.9) μmol/L; only 28.8% reported satisfaction with their current treatment. Most (84.4%) indicated difficulty in following a PKU diet; 60% reported that the PKU diet was burdensome, and 58% reported feeling socially isolated. Most (≥69%) reported their MAB to be less than the expected clinical benefit provided by pegvaliase; the mean MAB was 22.7% and 34.4% in the swing-weighting and thresholding surveys, respectively.
Conclusion
Most participants felt the burden of PKU on their daily lives, were dissatisfied with current treatments, and were willing to accept the risks of hypersensitivity reactions to achieve recommended blood Phe levels with pegvaliase treatment.
Keywords: Phenylketonuria, PKU, Pegvaliase, Minimum acceptable benefit, Benefit-risk analysis, Patient preference
Abbreviations: ANOVA, analysis of variance; FDA, Food and Drug Administration; IgE, immunoglobin E; MAB, minimum acceptable benefit; Phe, phenylalanine; PKU, phenylketonuria; SD, standard deviation; SE, standard error
1. Introduction
Phenylketonuria (PKU) is a rare autosomal recessive disorder that results in elevated phenylalanine (Phe) levels in the blood [1]. In the U.S., the prevalence of PKU is approximately 1 case per 25,000 individuals, and the incidence is approximately 1 case per 2900 live births [2,3]. Classic PKU, the most severe form, is characterized by complete enzyme deficiency and an untreated blood Phe level of >1200 μmol/L (vs. a normal range of 50 to 110 μmol/L) [1,4]. Guidelines from the American College of Medical Genetics recommend the maintenance of blood Phe levels within the range of 120 to 360 μmol/L. If left untreated, high Phe levels are associated with significant neurocognitive disability.
According to U.S. guidelines, adult PKU patients with blood Phe concentrations >360 μmol/L should receive treatment [1]. Non-pharmacologic treatment options include a diet that is severely restricted in Phe and medically prescribed Phe-free or Phe-restricted food [5]. However, 88% of patients report trouble adhering to dietary restrictions [6,7], and blood Phe levels typically may remain elevated despite adherence to the PKU diet [8,9]. Until recently, the only available pharmacologic treatment option was sapropterin dihydrochloride (KUVAN®, BioMarin Pharmaceutical Inc.); however, it requires residual Phe hydroxylase enzyme activity. Only 25% to 50% of PKU patients are responsive to this treatment, indicating that there is an unmet need for treatment of PKU [1,10].
Pegvaliase-pqpz (PALYNZIQ™, BioMarin Pharmaceutical Inc.) was recently approved by the US Food and Drug Administration (FDA) as an enzyme substitution therapy for PKU [11,12]. The efficacy and safety of pegvaliase have been established in two phase 3 clinical studies, PRISM-1 (NCT01819727) and PRISM-2 (NCT01889862) [13,14]. All patients enrolled in the study had blood Phe concentrations of >600 μmol/L for the 6 months prior to enrollment. Blood Phe levels were estimated to reduce to ≤360 μmol/L in 44.0% of patients by 12 months, and in 60.7% of patients by 24 months [14].
Set against these benefits, 93.5% of patients reported hypersensitivity-related adverse events, with the majority (98.7%) being mild to moderate, including arthralgia (70.5%), rash (34.5%), and pruritus (27.6%). Only a small proportion (4.6%) of these were acute systemic hypersensitivity reactions identified by National Institute of Allergy and Infectious Diseases and Food Allergy and Anaphylaxis Network clinical anaphylaxis criteria [15], which were further assessed by an independent allergist/immunologist. These events resolved without clinical sequelae, were not associated with elevated immunoglobulin E (IgE) levels in any of the patients, and were thought to have had a type III hypersensitivity response. Therefore, patients with these events were frequently successfully rechallenged without further reactions [14].
The objective of this study was to identify the minimum acceptable benefit (MAB) of pegvaliase, defined as the minimum probability of achieving a reduction in blood Phe level to <360 μmol/L, which patients require to accept the potential risks of hypersensitivity/anaphylaxis associated with pegvaliase.
2. Methods
2.1. Data collection
An online survey was used to elicit the preferences of PKU patients regarding clinical benefit and potential risks associated with pegvaliase. Participants were recruited via patient organizations and clinical treatment sites. If they consented to the study, participants were asked to complete the self-reported eligibility criteria and, if eligible, they proceeded to an online survey that took approximately 30 min to complete.
Patients could participate in the study if they met the following self-reported inclusion criteria: ≥18 years of age; residence in the U.S.; PKU diagnosis; previous measurement of blood Phe level; most recent blood Phe level of ≥600 μmol/L within the last 6 months; naïve to pegvaliase treatment; and able to comply with study procedures (e.g., proficiency in English). Participants were excluded from the study if they were pregnant or planning to become pregnant.
The survey included questions on MAB and demographic and clinical characteristics, including age, gender, ethnicity, education, and employment; ethnic background; blood Phe level; current treatment; perceived satisfaction with treatment; and perceived control of Phe level. The effect of PKU on daily life was also assessed, including challenges of and compliance with diet.
MAB questions focused on two attributes (Supplementary Table 1): the proportion of patients achieving the target blood Phe level of <360 μmol/L, and the proportion of patients experiencing reactions of three types of severity (emergency allergic reactions requiring immediate medical care [e.g., anaphylaxis], severe allergic reactions not requiring immediate medical care [e.g., intense itchy skin and generalized rash, or swelling of the face], and mild allergic reactions [e.g., joint pain or injection-site reactions]).
Two types of choice questions were used to evaluate participants' MAB for pegvaliase: adapted swing-weighting and thresholding. Participants' understanding of the attribute descriptions and elicitations tasks were tested with 15 participants before the main surveys were administered. In the adapted swing-weighting survey, participants were asked to rank changes in performance on attributes [16]. The task was iterated five times, with the blood Phe level improvement updated according to the response to the previous question (Supplementary Fig. 1 and Supplementary Fig. 2).
In the thresholding survey, participants were asked to select between two hypothetical treatment options (current treatment and a new treatment) with varying probabilities of achieving target blood Phe levels and having hypersensitivity reactions. The new treatment was described as an injectable that would not cost more than the current treatment. Again, the question was iterated five times with the benefit-to-risk profile of the “new treatment” updated for each subsequent question according to the participant's response to the previous question/scenario (Supplementary Fig. 1 and Supplementary Fig. 3). The goal was to identify the threshold for the minimum amount of clinical benefit required given the treatment risks.
2.2. Analysis
Participant data were excluded from the analyses if the total time spent on the survey was ≤10 min. Continuous variables were summarized using descriptive analyses (mean, standard deviation, and range). Categorical variables were summarized using frequency statistics. All analyses were performed using SAS version 9.4. P-values were generated using analysis of variance (ANOVA) or Fisher's exact test.
The elicitation exercises allowed the MAB to be identified within a range of a few percentage points (Supplementary Fig. 1 and Supplementary Table 2). The mid-point of this range was then taken as a point estimate of the participant's MAB. Participants needed to complete all of the choice questions to be included in the analyses.
Upon review of responses, high MAB was defined as ≥91% (participants would effectively not take pegvaliase regardless of its clinical benefit) and low MAB was defined as ≤4% (participants would take pegvaliase regardless of the associated probability of hypersensitivity reactions).
At the end of the thresholding exercise, participants were asked to answer a free-text question about their thought process for selecting a treatment option. After responses were reviewed, motivations were coded as follows: 1) avoidance of hypersensitivity reactions; 2) improving blood Phe level; 3) concern about administration method — fear of injectables; and 4) desire to try a new treatment. Participants were removed from the analysis if their MAB was not consistent with their answers. For example, if their MAB was high, participants were excluded if they did not identify a negative element of treatment that would stop them from taking it (e.g., priority to avoid hypersensitivity reactions). In cases where a large difference (>33%) was observed between the two MABs, participants were excluded unless their motivation justified their different responses.
3. Results
A total of 57 participants were eligible for the study and completed the survey. Upon data review and validation, data from 45 participants were included in the analyses. Reasons for exclusion included time for survey completion being ≤10 min (n = 3), previous use of pegvaliase (n = 3), and inconsistency between MAB responses and reasoning provided in the free-text response (n = 6).
Demographic characteristics are presented in Table 1. The mean age of participants was 34.4 years (range, 18–59). The majority were female (n = 28; 62.2%) and white (n = 43; 95.6%). Additional demographic information is provided in Table 1.
Table 1.
Patient self-reported demographics, background, and disease characteristics (N = 45).
| Variable | n (%)a |
|---|---|
| Gender | |
| Female | 28 (62.2%) |
| Age, Years | |
| Mean (SD) | 34.4 (9.5) |
| 18–30 | 17 (37.8%) |
| 31–40 | 12 (26.7%) |
| >40 | 16 (35.6%) |
| Race | |
| White | 43 (95.6%) |
| Asian | 1 (2.2%) |
| Otherb | 1 (2.2%) |
| Ethnicity | |
| Hispanic or Latino | 5 (11.1%) |
| Not Hispanic or Latino | 40 (88.9%) |
| Education | |
| Secondary/high school | 5 (11.1%) |
| Some college | 11 (24.4%) |
| College degree | 21 (46.7%) |
| Postgraduate degree | 4 (8.9%) |
| Otherc | 4 (8.9) |
| Employment status | |
| Full time | 21 (46.7%) |
| Unemployed | 5 (11.1%) |
| Homemaker | 3 (6.7%) |
| Student | 3 (6.7%) |
| Student and unemployed | 3 (6.7%) |
| Part time | 2 (4.4%) |
| Part time and studentd | 2 (4.4%) |
| Disablee | 2 (4.4%) |
| Part time and homemaker and unemployed | 1 (2.22%) |
| Full-time and disabled | 1 (2.22%) |
| Full-time and student | 1 (2.22%) |
| Full-time and part-timef | 1 (2.22%) |
| Living situation | |
| Living alone | 7 (15.6%) |
| Living with a partner or spouse, family, or friends | 35 (77.8%) |
| Otherg | 3 (6.7%) |
| Phe level | |
| μmol/L, mean (SD) [range] | 976.9 (429.9) [600–2301] |
| Phe range | |
| 00–900 μmol/L | 27 (60.0%) |
| >900–1200 μmol/L | 9 (20.0%) |
| >1200 μmol/L | 9 (20.0%) |
| How often do you feel your PKU is under control? | |
| Always in control | 1 (2.2%) |
| In control most of the time | 19 (42.2%) |
| Sometimes in control | 14 (31.1%) |
| Often not under control | 8 (17.8%) |
| Rarely under control | 3 (6.7%) |
| Current PKU treatment | |
| Medical food and low-protein diet | 11 (24.4%) |
| Sapropterin dihydrochloride, medical food, and low-protein diet | 9 (20.0%) |
| Sapropterin dihydrochloride alone | 7 (15.6%) |
| Low-protein diet alone | 5 (11.1%) |
| Medical food alone | 2 (4.4%) |
| Sapropterin dihydrochloride and low-protein diet | 2 (4.4%) |
| Medical food, low-protein diet, and other treatments | 2 (4.4%) |
| Other treatments; sapropterin dihydrochloride and medical food; sapropterin dihydrochloride and other treatments; low-protein diet and other treatments; sapropterin dihydrochloride, medical food, and other treatments; sapropterin dihydrochloride, low-protein diet, and other treatments; no treatment | 1 (2.2%) each |
| Satisfied with current PKU treatment | |
| Strongly agree | 6 (13.3%) |
| Agree | 11 (24.4%) |
| Neither agree nor disagree | 15 (33.3%) |
| Disagree | 11 (24.4%) |
| Strongly disagree | 2 (4.4%) |
| Is it difficult to follow a PKU diet? | |
| Yes (reasons below) | 38 (84.4%) |
| Cost | 23 (51.1%) |
| Availability (whether your local shops have any food options) | 23 (51.1%) |
| Taste/Texture | 18 (40.0%) |
| Meeting family/friends, eating out | 22 (48.9%) |
| Limited options (e.g., few flavors) | 22 (48.9%) |
| Work constraints | 11 (24.4%) |
| Other | 10 (22.2%) |
| No | 7 (15.6%) |
| General health in the past week | |
| Very good | 10 (22.2%) |
| Good | 26 (57.8%) |
| Fair | 9 (20.0%) |
| Poor | 0 (0.0%) |
| Very poor | 0 (0.0%) |
| Experienced a significant hypersensitivity reaction in the last 6 months | |
| No | 42 (93.3%) |
| Yes, but did not require emergency medical treatment | 2 (4.4%) |
| Yes, required emergency medical treatment | 1 (2.2%) |
Phe, phenylalanine; PKU, phenylketonuria; SD, standard deviation.
Except where indicated.
Other race: “Prefer not to discuss.”
Other education status included as free-text answers: “BSIT” (n = 1), “High school and cosmetology license” (n = 1), “NBCC college certificate” (n = 1), and “some graduate classes” (n = 1).
Answer is indicative of how the participant described his/her employment status.
Answer is indicative of participants' response. Specific information regarding disabilities was not asked.
Answers are indicative of participants' selections.
Other living situation includes “In transition to a new home by myself” (n = 1) and “Living in a college dorm” (n = 2).
Participant-reported disease characteristics are also presented in Table 1. The mean (standard deviation) self-reported blood Phe level (within the previous 6 months of the study) was 976.9 (429.9) μmol/L (range, 600–2301 μmol/L). The proportion of participants with blood Phe level between 600 and 900 μmol/L was >50%. Reported PKU treatment included various combinations of sapropterin dihydrochloride, medical food, low-protein diet, and other treatments; 48.8% of participants were receiving sapropterin dihydrochloride as part of their treatment plan, and 77.5% were taking medical food and/or on a low-protein diet, with only 1 participant (2.2%) not seeking treatment. Only 28.8% reported satisfaction with their current treatment. Most participants (84.4%) indicated difficulty in following a PKU diet.
Greater than 50% of participants reported concerns for each of the following cognitive capacities: making careless mistakes; difficulty keeping attention; avoiding or delaying getting started when a task requires a lot of thought; getting distracted by noise or activity; and misplacing or having difficulty finding things at home or work. Participants reported the following effects of their PKU diet: feeling burdened most of the time to weigh/measure/estimate the protein content in their food (60%); feeling left out at least some of the time due to their disease (58%); and feeling less able to lead a normal social life with family and friends most of the time (48.9%). Also, 73% of participants felt that their PKU diet prevented them from getting sufficient nutrients (Supplementary Fig. 4). Non-compliance within the previous 7 days (lack of adherence to their PKU diet for ≥1 meal/week) was reported by 76% of participants, and lack of compliance ≥6 times in the last 7 days was reported by 18% of participants. The mean blood Phe level increased with the number of meals missed by participants, but the association was not statistically significant (Supplementary Table 3).
The mean MAB was 22.7% for the swing-weighting exercise and 34.4% for the thresholding exercise (Table 2), meaning that patients were willing to tolerate the risks of pegvaliase for a 22.7% and 34.4% chance of achieving target blood Phe levels <360 μmol/L. There were no obvious patterns of effect due to age, blood Phe level, perception of PKU control, or satisfaction with treatment on the MAB results.
Table 2.
Minimum acceptable benefit of the new treatment for PKU (N = 45).
| MAB, Mean (SE) |
||
|---|---|---|
| Adapted swing-weighting | Thresholding | |
| Overall sample | 22.7 (4.7) | 34.4 (5.9) |
| Age | ||
| ≤30 years (n = 17) | 23.2 (7.3) | 44.6 (9.3) |
| >30–40 years (n = 12) | 19.5 (8.8) | 23.5 (10.7) |
| >40 years (n = 16) | 24.4 (9.0) | 31.8 (10.4) |
| P-valuea | 0.9202 | 0.3524 |
| Phe level | ||
| 600–900 μmol/L (n = 27) | 25.8 (6.3) | 43.1 (7.7) |
| >900–1200 μmol/L (n = 9) | 21.7 (9.9) | 19.7 (9.2) |
| >1200 μmol/L (n = 9) | 14.3 (11.0) | 23.0 (14.6) |
| P-valuea | 0.6529 | 0.1907 |
| How often do you feel your PKU is under control? | ||
| Always or most of the time (n = 20) | 28.0 (8.2) | 35.4 (8.9) |
| Sometimes, often not, or rarely (n = 25) | 18.4 (5.5) | 33.6 (8.0) |
| P-valuea | 0.3201 | 0.8868 |
| Satisfaction with treatment | ||
| Dissatisfied (n = 17) | 16.2 (6.4) | 26.2 (9.1) |
| Neither dissatisfied nor satisfied (n = 15) | 29.8 (9.1) | 37.4 (10.5) |
| Satisfied (n = 13) | 22.9 (9.6) | 41.6 (11.4) |
| P-valuea | 0.4918 | 0.5444 |
MAB, minimum acceptable benefit; Phe, phenylalanine; PKU, phenylketonuria; SE, standard error.
ANOVA was used to generate P-values for these comparisons.
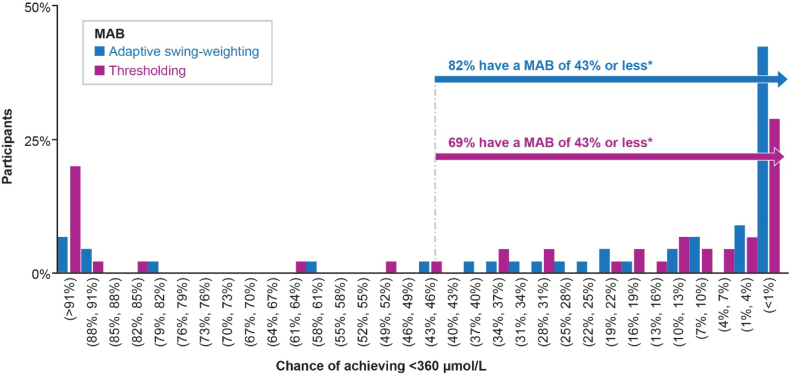
Fig. 1 shows the distribution of MAB across participants for the chance of achieving target blood Phe levels. Most participants (82% in the adapted swing-weighting exercise and 69% in the thresholding exercise) reported a MAB that was less than the estimated probability of achieving a blood Phe level of ≤360 μmol/L when using pegvaliase [14].
Fig. 1.
Minimum acceptable benefit for Phe reduction to <360 μmol/L.
The distributions of MAB across participants (N = 45) for the chance of achieving a blood Phe level < 360 μmol/L ranged from <1% to >91%. Eighty-two percent (adapted swing-weighting survey) and 69% (thresholding survey) of participants reported a MAB that was less than the chance of achieving a blood Phe level < 360 μmol/L (44%). MAB, minimum acceptable benefit; Phe, phenylalanine.
*The chance of achieving a blood Phe level of ≤360 μmol/L with pegvaliase treatment.
Of the 8 participants who reported a MAB of >91% in either exercise, reasons for their unwillingness to receive the new treatment included that the new treatment was an injectable (n = 1); concern about the cost of the treatment (n = 1); the risk of hypersensitivity reaction was not worth tolerating (n = 3); and concern about both injectables and hypersensitivity reactions (n = 3).
The MAB increased by >33 points from the adapted swing-weighting exercise to the thresholding exercise for 8 participants. Refusal to take an injectable (n = 4), which was part of the thresholding exercise but not part of the swing-weighting exercise, was a main explanation for this difference. Another reason was the risk of life-threatening reactions (n = 2), which suggested that the safety risks might have been more apparent to participants in the thresholding exercise (where a treatment choice was being made) than in the adapted swing-weighting exercise. For the remaining participants (n = 2), the reason for the increase in MAB from the adapted swing-weighting exercise to the thresholding exercise was not specified.
For 1 participant, the MAB was decreased by approximately 30% from the adapted swing-weighting exercise to the thresholding exercise. The swing-weighting exercise elicited preferences for changes in outcomes independent of the cause of the change. The thresholding exercise introduced a cause – a new treatment. The participant whose MAB changed by 30% referenced this cause as the reason for their responses to the thresholding exercise. That is, the patients would be willing to access a smaller benefit to access a new treatment. As stated earlier, treatment characteristics were not part of the adapted swing-weighting exercise.
4. Discussion
Patients with PKU are willing to tolerate a risk of hypersensitivity reactions associated with pegvaliase in exchange for an increased likelihood of achieving an improvement in blood Phe level to ≤360 μmol/L. Most participants in both the adapted swing-weighting and thresholding exercises indicated that they were willing to tolerate a risk of hypersensitivity reactions, demonstrating that PKU patients have a strong desire to control blood Phe levels and are willing to accept some risk to do so.
Consistent with previous studies, a large proportion of participants felt the burden of the disease on their daily lives, including problems with neuropsychological function, lifestyle, and social challenges associated with their PKU diet [6,7,17]. In a survey of 474 PKU patients, >90% of respondents indicated that the development of new treatments for PKU was important to them [5,18]. The results of the current study indicate that PKU patients are not generally satisfied with their treatment options and desire new treatment options due to the perceived difficulties with current standard-of-care of a severely restrictive diet. Notably, the participants appear to indicate that they are willing to accept a treatment option that has significant risks over a predominantly dietary restriction approach with relatively limited risks. A similar MAB trend was observed in patients with other metabolic disorders like type 2 diabetes mellites. In an online discrete-choice experiment survey conducted in Germany and Spain, responders were willing to have lower rates (or improvements) of side effects over efficacy, route of administration, and dosing frequency [19].
There is no “gold standard” method for collecting patient-preference data as part of a benefit-risk assessment, and recent FDA guidance acknowledged that several methods may be used [20]. In this study, the elicitation instruments were selected for three reasons: the minimum acceptable level of a single benefit endpoint could be determined; a large sample size was not required; and simple questions could be used, which reduced the cognitive burden on participants [21].
A limitation of the study is the relatively small sample size (n = 45), which did not allow for meaningful subgroup analyses. Furthermore, the sample differed from the overall PKU population and that seen in clinical trials, including a lower mean blood Phe level (976.9 μmol/L vs. 1200 μmol/L in the general PKU adult population in the U.S. [8]; the survey findings may be different in a population with more severe disease. Despite only enrolling PKU patients with a blood Phe level of >600 μmol/L, the proportion of participants with Phe levels between 600 and 900 μmol/L was relatively high (>50%), which suggests that participants had better control than the average adult patient with a blood Phe level of >600 μmol/L. This may be due to the sample being recruited from patient organizations and clinical sites, a population that might be expected to have better metabolic control vs. patients who are lost to follow-up. It is estimated that >70% of PKU patients in the 19 to 45-year age group are not being followed in the clinic [22].
Patients with PKU may lack accurate self-perception regarding their health and cognitive impairment and/or may struggle with focus/attention for an extended period [[23], [24], [25]]. Given this and the subjectivity of self-reports, patient responses to certain health-related questions may underreport their actual health situation. The effect of this limitation may be less for lifestyle-related questions, and the tests of consistency within the survey suggest that these factors do not invalidate the results.
5. Conclusion
The treatment burdens of PKU disease are high, and there is an unmet need for effective clinical treatment. This survey shows that PKU patients value the clinical benefits provided by pegvaliase. In exchange for the chance to reduce blood Phe levels, participants were willing to accept the risk of hypersensitivity reactions and the delivery of pegvaliase via a subcutaneous injection.
Funding
This study was funded by BioMarin Pharmaceutical Inc. The authors confirm that the content of the article reflects the results of the patient survey and has not been influenced by the sponsor.
Author contributions
All authors contributed to the study design and interpretation of the data. SS and KM collected and analyzed the data. All authors advised on the manuscript content and critically revised the manuscript during its development.
Ethics
All procedures performed in studies involving human patients were conducted in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Institutional review board approval or waiver was obtained from Chesapeake Institutional Review Board prior to distribution of invitation emails, survey announcements, and activation of the electronic survey URL. Informed consent was obtained from all individual patients included in this study at the beginning of the survey; proof of informed consent is available upon request.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declaration of Competing Interest
AQ, HHW, and AG are employees and stockholders of BioMarin Pharmaceutical Inc. NL and JT have received clinical trial support with personnel and salary support from BioMarin Pharmaceutical Inc. for the phase III pegvaliase clinical trial and were on the trial steering committee, the pegvaliase consensus program, and the BioMarin Market Access Advisory Board. RZ is on the steering committee for the phase III pegvaliase clinical trial and has received grants and personal fees from BioMarin Pharmaceutical Inc. SS and KM are employees of Evidera; Evidera received funding from BioMarin Pharmaceutical Inc. to conduct the study.
Acknowledgments
Medical writing and editing support were provided by Nora Tu, PharmD and Melanie Jardim, PhD (Evidera, Morrisville, NC) and were funded by BioMarin Pharmaceutical Inc.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ymgmr.2019.100507.
Appendix A. Supplementary data
Supplementary material
References
- 1.Vockley J., Andersson H.C., Antshel K.M. Phenylalanine hydroxylase deficiency: diagnosis and management guideline. Genet. Med. 2014;16:188–200. doi: 10.1038/gim.2013.157. [DOI] [PubMed] [Google Scholar]
- 2.Regier D.S., Greene C.L. Phenylalanine hydroxylase deficiency. In: Adam M.P., Ardinger H.H., Pagon R.A., editors. GeneReviews® [Internet] University of Washington; Seattle: 2000. (updated 2017 Jan 5) [Google Scholar]
- 3.Rush E.T. Phenylketonuria (PKU) 2017. https://emedicine.medscape.com/article/947781-overview#a5 (accessed 29 May 2018)
- 4.Blau N., van Spronsen F.J., Levy H.L. Phenylketonuria. Lancet. 2010;376:1417–1427. doi: 10.1016/S0140-6736(10)60961-0. [DOI] [PubMed] [Google Scholar]
- 5.Singh R.H., Cunningham A.C., Mofidi S. Updated, web-based nutrition management guideline for PKU: an evidence and consensus based approach. Mol. Genet. Metab. 2016;118:72–83. doi: 10.1016/j.ymgme.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Bik-Multanowski M., Didycz B., Mozrzymas R. Quality of life in noncompliant adults with phenylketonuria after resumption of the diet. J. Inherit. Metab. Dis. 2008;31:S415–S418. doi: 10.1007/s10545-008-0978-7. [DOI] [PubMed] [Google Scholar]
- 7.Jurecki E.R., Cederbaum S., Kopesky J. Adherence to clinic recommendations among patients with phenylketonuria in the United States. Mol. Genet. Metab. 2017;120:190–197. doi: 10.1016/j.ymgme.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Bilder D.A., Noel J.K., Baker E.R. Systematic review and meta-analysis of neuropsychiatric symptoms and executive functioning in adults with phenylketonuria. Dev. Neuropsychol. 2016;41:245–260. doi: 10.1080/87565641.2016.1243109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koch R., Burton B., Hoganson G. Phenylketonuria in adulthood: a collaborative study. J. Inherit. Metab. Dis. 2002;25:333–346. doi: 10.1023/a:1020158631102. 12408183 [DOI] [PubMed] [Google Scholar]
- 10.Harding C.O. New era in treatment for phenylketonuria: pharmacologic therapy with sapropterin dihydrochloride. Biologics. 2010;4:231–236. doi: 10.2147/btt.s3015. (20714359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell S.M., Wendt D.J., Zhang Y. Formulation and PEGylation optimization of the therapeutic PEGylated phenylalanine ammonia lyase for the treatment of phenylketonuria. PLoS One. 2017;12 doi: 10.1371/journal.pone.0173269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahan K.C., Gandhi M.A., Anand S. Pegvaliase: a novel treatment option for adults with phenylketonuria. Curr. Med. Res. Opin. 2019;35:647–651. doi: 10.1080/03007995.2018.1528215. [DOI] [PubMed] [Google Scholar]
- 13.Harding C.O., Amato R.S., Stuy M. Pegvaliase for the treatment of phenylketonuria: a pivotal, double-blind randomized discontinuation phase 3 clinical trial. Mol. Genet. Metab. 2018;124:20–26. doi: 10.1016/j.ymgme.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Thomas J., Levy H., Amato S. Pegvaliase for the treatment of phenylketonuria: results of a long-term phase 3 clinical trial program (PRISM) Mol. Genet. Metab. 2018;124:27–38. doi: 10.1016/j.ymgme.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Sampson H.A., Muñoz-Furlong A., Campbell R.L. Second symposium on the definition and management of anaphylaxis: summary report - second national institute of allergy and infectious disease/food allergy and anaphylaxis network symposium. J. Allergy Clin. Immunol. 2006;117:391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 16.Postmus D., Mavris M., Hillege H.L. Incorporating patient preferences into drug development and regulatory decision making: results from a quantitative pilot study with cancer patients, carers, and regulators. Clin. Pharmacol. Ther. 2016;99:548–554. doi: 10.1002/cpt.332. [DOI] [PubMed] [Google Scholar]
- 17.Brown C.S., Lichter-Konecki U. Phenylketonuria (PKU): a problem solved? Mol. Genet. Metab. Rep. 2015;6:8–12. doi: 10.1016/j.ymgmr.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National PKU Alliance 2015 PKU Patient Survey Results. 2015. https://www.npkua.org/Portals/0/PDFs/NPKUA-WP_FINAL.pdf?ver=2016-02-10-123840-590 (accessed 6 August 2018)
- 19.Mansfield C., Sikirica M.V., Pugh A. Patient preferences for attributes of type 2 diabetes mellitus medications in Germany and Spain: an online discrete-choice experiment survey. Diabetes. Ther. 2017;8:1365–1378. doi: 10.1007/s13300-017-0326-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Department of Health and Human Services, Food and Drug Administration Patient Preference Information – Voluntary Submission, Review in Premarket Approval Applications, Humanitarian Device Exemption Applications, and De Novo Requests, and Inclusion in Decision Summaries and Device Labeling. Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders. 2016. https://www.fda.gov/media/92593/download (accessed 6 August 2019)
- 21.Ho M.P., Gonzalez J.M., Lerner H.P. Incorporating patient-preference evidence into regulatory decision making. Surg. Endosc. 2015;29:2984–2993. doi: 10.1007/s00464-014-4044-2. [DOI] [PubMed] [Google Scholar]
- 22.Berry S.A., Brown C., Grant M. Newborn screening 50 years later: access issues faced by adults with PKU. Genet. Med. 2013;15:591–599. doi: 10.1038/gim.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartus A., Palasti F., Juhasz F. The influence of blood phenylalanine levels on neurocognitive function in adult PKU patients. Metab. Brain Dis. 2018;33:1609–1615. doi: 10.1007/s11011-018-0267-6. [DOI] [PubMed] [Google Scholar]
- 24.Bone A., Kuehl A.K., Angelino A.F. A neuropsychiatric perspective of phenylketonuria I: overview of phenylketonuria and its neuropsychiatric sequelae. Psychosomatics. 2012;53:517–523. doi: 10.1016/j.psym.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Jahja R., Huijbregts S.C.J., de Sonneville L.M.J. Cognitive profile and mental health in adult phenylketonuria: a PKU-COBESO study. Neuropsychology. 2017;31:437–447. doi: 10.1037/neu0000358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.