Abstract
Bisphosphonates are commonly used in patients with metastatic bone disease to prevent skeletal related events. Atypical femur fracture is a known complication of long-term bisphosphonate use but the incidence in cancer patients and pathogenesis are not well known. Several mechanisms of pathogenesis have been proposed including altered angiogenesis, altered bone mechanical properties, micro damage and bone remodeling suppression. Atypical femur fractures are atraumatic or minimally traumatic fractures in the sub trochanteric region or the femoral shaft. Awareness of atypical femur fractures is critical to diagnose and treat them in a timely manner. There is a paucity of data regarding the management of atypical femur fracture in patients with malignancy. Management options of atypical femur fractures include stopping bisphosphonates, initiating calcium/vitamin D supplementation and either surgery with internal fixation or conservative management. In the future, it will be important to explore the effect of continuous vs. intermittent exposure, cumulative dose and length of exposure on the incidence of this complication. Herein, we review the epidemiology, risk factors, management options and proposed mechanisms of pathogenesis of atypical femur fractures.
Keywords: Atypical femur fracture, Bisphosphonates, Bone remodeling, Bone metastasis, Denosumab
Abbreviations: AFF, atypical femur fracture; AGE, advanced glycation end products; ASBMR, American Society of Bone and Mineral Research; BP, bisphosphonate; CI, confidence interval; CT, computed tomography; GGPPS, geranyl geranyl pyrophosphate synthase Her2, human epidermal growth factor receptor; IM, intramedullary; IV, intravenous; MGUS, monoclonal gammopathy of unknown significance; MRI, magnetic resonance imaging; ORIF, open reduction internal fixation; ONJ, osteonecrosis of the jaw; OR, odds ratio; RCT, randomized clinical trial; VEGF, vascular endothelial growth factor
1. Case presentation
A 52-year old woman was treated with standard-of-care treatment for metastatic breast cancer, which was hormone receptor and HER-2 receptor positive. The patient had bone metastatic disease and had been treated with monthly I.V. Zoledronic acid for 8 years. She received a total of 50 doses of 4 mg IV infusions, initially on a monthly basis then every three months for the last four years. During the course of her disease, the patient received repeated doses of steroids as a premedication prior to chemotherapy administration and an extended course of aromatase inhibitors. The patient presented to an outside facility with severe pain in the left thigh. The pain was acute in nature with sudden onset. X-ray examination revealed fracture of the left femoral diaphysis. (Fig. 1) The patient underwent retrograde intramedullary (IM) nail fixation at the outside facility. At that time chemotherapy and zoledronic acid were halted. The patient then underwent physical rehabilitation and later resumed standard chemotherapy, at which time zoledronic acid was permanently discontinued. Six months later the patient sustained a fracture of right femur the result of a minor, relatively non-traumatic twisting injury. X-ray of the right femur demonstrated a displaced transverse mid-shaft fracture (Fig. 1). The patient underwent open reduction internal fixation (ORIF) of the fracture and received an IM nail. Pathological examination of the bone obtained from the fracture sites showed fragments of mature bone and bone marrow but was negative for malignancy in both cases. Work up showed very low vitamin D level (Vit D, 25-Hydroxy 7.9 ng/ml; Nl: 20–50) that was corrected to normal (32.3 ng/ml) with aggressive vitamin D3 supplementation. Alkaline phosphatase was normal throughout the course of these events. No other turnover markers were available.
Fig. 1.
X-rays of the right (A) and left femur fractures (B).
Four months later the patient presented with pain in her left knee. X-ray of bilateral femurs at that time revealed persistent lucent fracture lines bilaterally despite callus formation. On the left side there was mild angulation of the left femoral interlocking screw close to the knee concerning for screw fracture. CT of left femur showed mid femoral diaphyseal fracture nonunion along with incomplete bridging of the fragments. The patient then underwent revision of the retrograde IM nail and antegrade IM nail fixation using a compression technique for nonunion. Surgery was followed by rehabilitation and the patient resumed normal activities. The patient was last seen five months after the revision surgery and was free of any pain in lower extremities with good function and range of motion in both legs.
2. Introduction
Patients with malignancies involving bone are often treated with long term bisphosphonates (BPs) to prevent skeletal related events, such as pathologic fractures [1]. The efficacy of BP treatment in the bone metastatic setting is well established. Indeed, as treatment of breast cancer has improved, patients are living longer with metastatic breast cancer [2]. Because of the longer overall survival, patients are often subjected to prolonged courses of BPs, even over several years. Along with patients being exposed to bisphosphonates for longer amounts of time, the dosages of bisphosphonates used in the oncology setting are higher than those used in osteoporosis [3]. It is now well acknowledged that long-term BP use is not without some risk. In this context, osteonecrosis of the jaw (ONJ) and atypical femur fracture (AFF) are potential complications of long-term BP use. Although rare, AFF is a potentially serious complication that is important to recognize and treat early. The incidence and pathogenesis of AFF in patients with cancer are not well known [4]. We recently had a case of a 52 y/o female with hormone receptor positive and Her2 receptor positive breast cancer with bone metastasis who developed bilateral atypical femur fractures after receiving i.v. zoledronic acid for 8 years. This case prompted a review of AFF to help better understand this entity in the oncology setting.
3. Epidemiology and risk factors of atypical femur fractures
After the development of case definition criteria for AFF by The American Society of Bone and Mineral Research (ASBMR) [4], several studies have been published on risk factors for AFF and their relation to the use of bisphosphonates (BP). Many of these studies are in non-cancer BP user (e.g., osteoporosis) [5], [6], [7]. Similar studies in the setting of BP use in breast cancer patients are increasing.
Among these, an early retrospective study demonstrated that four patients out of 327 patients who had malignant involvement of skeleton received a minimum of twenty-four doses of intravenous BPs between 2004 and 2007 developed AFF. The calculated incidence was 1.2% [8]. A single center case control study by Edwards et al. identified 23 cases of AFF out of 10,587 patients treated with BPs as part of cancer treatment with an estimated incidence of AFF in BP users of 0.05 per 100,000 person-years. Most cases had breast cancer followed by multiple myeloma, leukemia, lymphoma, monoclonal gammapathy of unknown significance (MGUS) and skin cancer. Among patients who developed AFF and received alendronate, the odds ratio (OR) for development of AFF was higher for those who were on drug for more than 3 years than those who were on for less than 3 years (OR 6.3 [95% CI, 1.49 to 26.72, p = 0.013]). OR for AFF with vitamin D deficiency versus AFF without vitamin D deficiency was 1.82 (95% CI, 0.71–4.66, p = 0.21) [9]. Another retrospective study by Chang and colleagues identified six AFFs (five with bilateral lesions) among 62 patients who developed femoral fractures after receiving intravenous BPs for breast cancer or myeloma. These investigators reported that the duration of intravenous BP therapy in patients with AFFs was longer than that in those with non-atypical fractures (5.9 years [5.7–7.3] vs 1.6 [0.5–3.6] years, p<0.01). In addition, AFF patients received significantly more intravenous BP doses (55 [28–59] vs 15 [6–35], p = 0.01) compared to those with non-atypical fractures [10].
Recently, a study by Ota et al. compared the frequency of AFF in 32 breast cancer patients with bone metastasis who received antiresorptive agents to 32 breast cancer patients without bone metastasis who did not receive antiresorptive agents. Denosumab (anti-RANKL antibody) and the BP zoledronic acid were the most common antiresorptive agents used. The average administration periods for zoledronic acid and denosumab were 43.5 months and 27.6 months, respectively. Of the 32 patients who received antiresorptive agents for breast cancer with bone metastasis, 3 patients developed AFF in 5 limbs (7.8%) compared to 0 patients in the control arm. In those 32 patients who received antiresorptive therapy, 8 limbs in 6 patients (12.5%) showed “beaking” in the subtrochanteric region. The average time from start of antiresorptive therapy to “beaking” on imaging was 48.4 months. The average time from “beaking” to complete fracture was 23 months [11].
A recent retrospective study by Yang et al. [12] showed that the incidence of AFF was 0.4% (1/253; 95% CI, 0.1%−2.2%). Skeletal images of 66 patients who had received at least 21 doses of denosumab were reviewed to look for asymptomatic atypical stress reactions. The incidence of atypical femoral stress reactions was 4.5% (3/66; 95% CI 1.6%−12.5%). In this study, the median duration of treatment was 23 months and median number of doses was 17. The patient that developed AFF received 23 doses of denosumab over 33 months. Prior to denosumab, she had received 5 years of alendronate and 1 year of risendronate. The 2 patients that developed atypical stress reaction had been exposed to denosumab for 28 doses over 27 months and 21 doses over 21 months [12]. There are also case reports describing AFFs with use of Denosumab though the true incidence and frequency with their use compared to that from BP use is unknown due to lack of comparative trials [13].
The true incidence of AFF among cancer patients on BP therapy remains unknown due to lack of large studies. The specific risk factors involved in the development of AFF in BP-treated cancer patients are also unclear.
4. Pathogenesis
Although several mechanisms for AFFs have been proposed, the exact pathogenesis remains largely unclear [4]. Some clinical, radiological and pathological features of AFFs provide insight into pathogenesis [14], [15]. AFFs occur after trivial or minimal/no trauma and are often located in the sub-trochanteric or diaphyseal region of the femur with a transverse pattern, without comminution (JBMR Taskforce). The fractures appear to originate in the lateral cortex of the femur, which is subjected to high levels of tensile stress [16]. There is retrospective evidence that AFFs initially develop as periosteal callus which over long time, evolves into transverse cortical fracture, the pattern which is reminiscent of the development of a stress fracture [4], [17].
4.1. Micro damage and bone remodeling suppression
Evidence from studies that attempted to measure bone turnover from biopsies at the site of AFF suggest that there is reduced or absent populations of osteoclasts and osteoblasts suggesting that bone turnover is suppressed. Stress fractures heal by initial stabilization by endosteal or periosteal bridging of the crack, followed by repair by normal intra cortical bone remodeling. BPs localize at sites of high bone turnover such as stress fracture sites and by suppressing remodeling, can adversely affect the intracortical repair allowing the crack to grow to a critical size [16]. Several pre-clinical studies have demonstrated that BPs lead to micro damage accumulation by inhibiting bone remodeling, clinical studies have failed to confirm this [18]. Indeed, some concerns have prevailed regarding the continued retention of BPs in bone leading to bone saturation and subsequent diminished bone resorption. In a recent post hoc analysis Hortobagy and colleagues [19] pooled pharmacokinetic data from three independent studies, OPTIMIZE-2 (patients receiving ≥9 doses of BPs) and two phase I studies, CZOL4460503 and CZOL4460506 (patients who were BP naïve/BP free for ≥1 year after previous dosing). The analysis showed that prolonged BP (zoledronate) administration did not cause bone saturation. In addition, reducing BP dosing frequency did not affect BP retention in bone, providing some relief to persistent physician concerns regarding prolonged BP administration to oncology patients [19].
4.2. Geometry of lower extremity
There is some evidence to suggest that there may be a relationship between the axis of lower extremity and risk and location of AFF. A study presented at the 2012 American Society for Bone and Mineral Research (ASBMR) annual meeting and another Japanese study suggest that there may be correlation between the axis or curvature of femoral diaphysis and risk of diaphyseal AFF. Bilateral incidence of AFFs and similar fracture location in such cases further strengthen such a relation [20], [21].
4.3. Altered bone mechanical properties
Bone toughness is a measure of the intrinsic energy-absorption capacity of bone that is largely determined by the composition of the bone organic matrix. Type I collagen is the major protein constituent of bone organic matrix and consists of two different components, enzymatic and nonenzymatic collagen cross-links. BPs appear to have both positive and negative effects on bone's organic matrix by altering collagen maturity and the extent of cross-linking. Pre-clinically, BPs increase the pyridinoline/deoxypyridinoline ratio to improve enzymatic collagen cross-links and thus bone strength and stiffness [22]. Nonenzymatic cross-links (formed through the interaction of collagen and sugars via oxidation reactions) are associated with the accumulation of advanced glycation end products (AGEs) in bone. Increased AGEs have been associated with bones that are more brittle with reduced toughness, and thus reduced energy required to fracture [23], [24]. In vivo studies have shown that BPs increase the accumulation of AGEs by reducing bone turnover [25]. However, there are limited human data on effect of BPs on the type and extent of collagen cross-links.
4.4. Altered angiogenesis
Vascular endothelial growth factor (VEGF) levels have also been shown to be decreased in breast cancer patients treated with BPs [26]. Anti-angiogenic effects of BPs has been demonstrated in animal studies [27] and angiogenesis has been shown to play an essential role during bone formation and fracture healing. It is conceivable that BPs may impair the repair of stress fractures by negatively regulating VEGF. However, there is no convincing evidence demonstrating that BPs can lead to AFFs through anti-angiogenesis. It has also been suggested that other concomitant therapies or comorbidities (such as osteoporosis) may also result in fracture non-healing [28]. Clearly, much more investigation is required to clarify this possibility.
5. Case definition
In 2013, the ASBMR revised case definition criteria for AFFs (Table 1). AFFs are atraumatic or minimally traumatic fractures located in the sub trochanteric region or the femoral shaft. They should be differentiated from the more common high trauma fractures, fractures of the femoral neck, intertrochanteric region and pathological fractures associated with primary or metastatic bone tumors. AFFs are typically not comminuted. Characteristic radiographic features of AFFs include a transverse fracture line that usually originates in the lateral cortex. The fracture line may cross the diaphysis to reach the medial cortex when the fracture line may appear oblique. Focal periosteal reaction at the fracture origin in the lateral cortex may appear as cortical “beaking” or “flaring”. Interestingly, in a recent study [29] the ASBMR AFF criteria were shown to be useful for classifying AFF, although several features were somewhat difficult to interpret. In sum, the lateral cortical transverse fracture line and the associated new-bone formation with no or minimal comminution were identified as critical features for the definition of AFF [29].
Table 1.
Definition criteria for the diagnosis of Atypical Femur Fractures.
| The American Society for Bone and Mineral Research (ASBMR) case definition criteria for Atypical Femoral Fractures (AFFs) | |
|---|---|
| Major features | 1. The fracture is associated with minimal or no trauma, as in a fall from a standing height or less |
| 2. The fracture line originates at the lateral cortex and is substantially transverse in its orientation, although it may become oblique as it progresses medially across the femur | |
| 3. Complete fractures extend through both cortices and may be associated with a medial spike; incomplete fractures involve only the lateral cortex | |
| 4. The fracture is noncomminuted or minimally comminuted | |
| 5. Localized periosteal or endosteal thickening of the lateral cortex is present at the fracture site (“beaking” or “flaring”) | |
| Minor features | 1. Generalized increase in cortical thickness of the femoral diaphysis |
| 2. Unilateral or bilateral prodromal symptoms such as dull or aching pain in the groin or thigh | |
| 3. Bilateral incomplete or complete femoral diaphysis fractures | |
| 4. Delayed fracture healing | |
To satisfy the case definition of AFF, the fracture must be located along the femoral diaphysis from just distal to the lesser trochanter to just proximal to the supracondylar flare. In addition, at least four of five major features must be present. None of the minor features are required but have sometimes been associated with these fractures.
6. Management
To diagnose and treat AFF in a timely manner, it is critical to be aware of the potential complications of BP use. Indeed, the FDA in 2010 issued guidance calling for monitoring of patients on BP therapy that report leg or groin pain that are amongst the most common symptoms [30]. Patients on BPs or Denosumab (especially those on long term treatment e.g., 3–5 years) should be educated to report such symptoms and treating physicians should routinely ask about thigh or groin pain at follow-up. If symptoms are reported, the first step is to obtain a plain radiograph of the femur, although it should be acknowledged that radiographic findings are variable. If complete fracture is present, it shows characteristic medial unicortical beak and further DXA assessment [31] or CT and MRI studies may be indicated for surgical planning but may not add additional information [32]. Incomplete fractures may be seen as periosteal callus on the lateral aspect of the femur or more commonly cortical thickening. However, many radiographs are normal. If the plain radiograph features are normal or suggest cortical thickening, further imaging with magnetic resonance imaging (MRI) should be obtained and surgical intervention considered. Computerized tomography (CT) can also be helpful if MRI is not available. MRI features can classify the damage as stress reaction to complete fracture (see Table 2). Bone scan can show metabolic activity but cannot demonstrate fracture [4].
Table 2.
Four of five major features should be present to designate a fracture as atypical, regardless of the presence of minor features in individual cases.
| Stress reaction | Incomplete fracture | Complete fracture | |
|---|---|---|---|
| MRI findings | Hyperemia only | Cortical lucency +/- hyperemia | Fracture line with marrow edema and/or hyperemia |
| No lucency or fracture line | No fracture line |
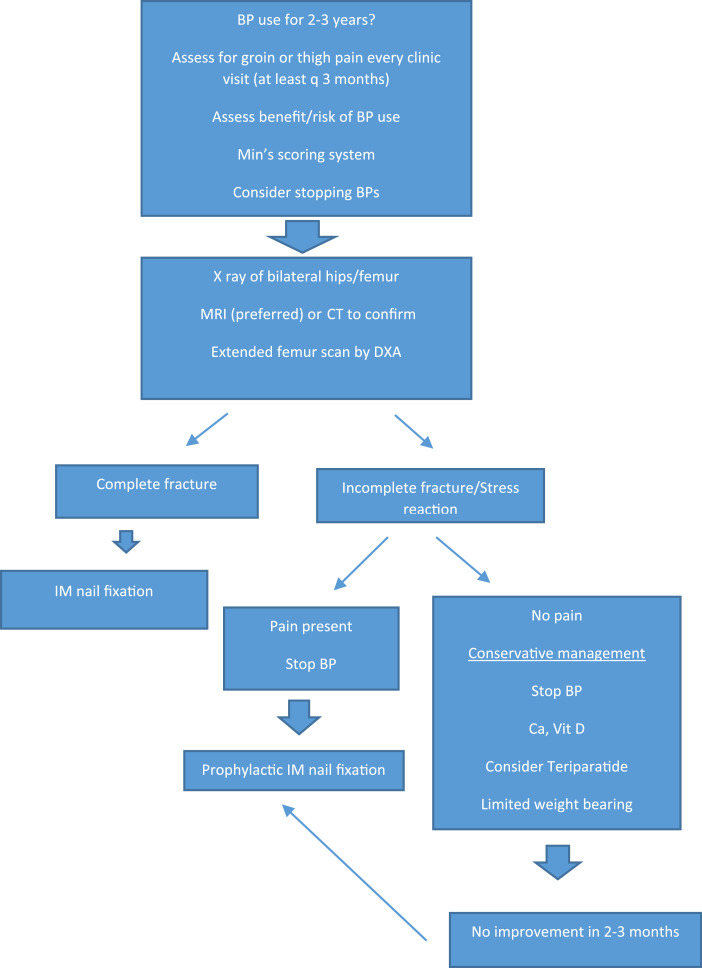
It is also essential to obtain imaging of the contralateral femur, even if asymptomatic, since AFFs are often bilateral (Fig. 2).
Fig. 2.
Flow chart for the management and follow up of patients with AFF.
7. Medical management
There is a sparsity of data regarding the management of AFF in the setting of malignancy. The available literature on the management of AFF comes predominantly from patients treated with BPs for osteoporosis.
7.1. Stopping bisphosphonate therapy
There is no convincing evidence that stopping BP therapy facilitates healing of AFFs. In a systematic review of case reports/case series studies of AFFs by Giusti et al., there was no significant difference in the delay of healing of AFFs between patients who continued using the BP (6/13) compared to those who discontinued it (5/8; p = 0.659) [33]. However, stopping BPs may decrease the contralateral or recurrent AFFs. A prospective Swedish study showed a 70% reduction in AFF risk for every year from discontinuation (multivariable-adjusted odds ratio, 0.28; 95% CI, 0.21 to 0.38) [34]. In another study by Dell et al., the incidence of contralateral femur fracture was only 19.3% in those who stopped BP after first AFF but was 53.9% in those who continued BP 3 years after the first AFF [35].
Based on these data, it is prudent to consider stopping BP especially if risk of AFF outweighs the benefits of continuing and perhaps reduced BP dosing schedules should be considered [36], [37]. Moreover since no prospective clinical RCT data exist to support the continuation of bone-modifying agent therapy beyond 1 year in breast cancer patients with skeletal metastases, it is not unreasonable to have risk benefit discussion in the setting of AFF.
7.2. Calcium and vitamin d supplementation
Patients should continue vitamin D and calcium supplementation if already in use. The ASBMR guidelines recommend that dietary calcium and vitamin D status should be assessed and adequate supplementation be prescribed [4].
7.3. Teriparatide
Teriparatide (human parathyroid hormone 1–34) is an anabolic agent that has been reported in AFF, in the setting of osteoporosis. A systematic review of the use of teriparatide in AFF in osteoporotic patients, which mostly included case reports, case series and only one prospective study by Gun-II Im et al., concluded that teriparatide has positive effects on AFF healing [38]. However, it is well recognized that teriparatide is not approved for use in the oncology setting or after therapeutic radiation. A prospective study by Chiang et al. showed that 20 μg of teriparatide subcutaneously daily for 6 months, administered to 5 of the 14 osteoporosis patients with AFF was associated with 2–3 fold increase in bone remodeling markers and promoted fracture healing. In that study 7 of the 9 patients who were managed conservatively had poor fracture healing with ongoing pain and 1 patient sustained a contralateral AFF [39]. However, some case series report that only some patients appear to respond to teriparatide. For example, in a study of 13 Canadian women with AFFs who were treated with teriparatide, Cheung et al. reported that only 5 patients improved while 3 patients required surgery and the rest did not improve or even worsened [40].
In view of the lack of convincing evidence from randomized studies, ASBMR guidelines propose that no definite conclusion can be reached regarding the efficacy of teriparatide in the treatment of osteoporosis patients with AFF and recommends considering it only in those who fail conservative osteoporosis therapy. Furthermore, this drug was not approved to treat patients with cancer.
8. Surgical management
Patients with complete fractures require internal fixation, commonly with an IM nail. Incomplete fractures accompanied by pain should be treated with prophylactic IM nail fixation. Patients with no or minimal pain and incomplete fractures and those with stress reaction can be managed conservatively with no or limited weight bearing with a crutch, cane or walker along with consideration of stopping BPs, and optimization of calcium and Vitamin D supplementation. After 2–3 months of such conservative management, if there is no symptomatic and radiographic improvement, prophylactic IM nail fixation should be considered.4 [4] In another recent study [41] a new scoring system to identify impending incomplete AFFs was reported. If validated, and the progression to complete fracture can be predicted it may indeed change patient management and surgical planning.
Some studies have shown a higher rate of both intraoperative and postoperative complications following fixation of AFFs. In a retrospective study by Prasarn et al., rates of iatrogenic fracture, implant failure, nonunion and malunion were higher among patients with BP related AFFs when compared to those not associated with BP use [42]. Also, time to fracture union was delayed in the BP treated group compared to non BP treated group (26 weeks vs. 19 weeks). In another single center study by Weil et al., 46% of patients treated for BP related AFFs required revision surgery [43].
9. Management algorithm
9.1. Conclusions
AFFs are a rare but known complication of long term BP use. Attending oncologists should be aware and actively monitoring patients for this potential complication. The pathogenesis of AFF is unclear, but several potential mechanisms have been proposed. A better understanding of the pathogenesis of AFF is needed. The recent introduction of denosumab to the oncology armamentarium to treat patients with cancer-related bone disease may involve some risk that remains to be identified and is likely lower in osteoporosis than in cancer patients due to the much lower doses used in the former patients. If the pathogenesis of AFF is related to inhibiting osteoclasts leading to decreased bone remodeling, it would be expected that outcomes with long term use of this agent would be similar. However, in a recent study of 3425 postmenopausal patients with early, hormone receptor-positive, non-metastatic breast cancer, who completed initial adjuvant treatment pathway and were receiving adjuvant aromatase inhibitors, no confirmed AFFs were recorded [44]. In addition, the pathogenic consideration for the development of AFF may also include anabolic agents, since treatment of osteoporosis patients with the sclerostin antibody (romosozumab) resulted in one adjudicated AFFs in 3581 treated patients [45]. Such data raise the intriguing possibility that the mechanism of AFF is not solely related to the inhibition of bone resorption.
In the future, it will be important to understand whether this increased risk of AFF with prolonged exposure to bone modifying agents is related to the cumulative dose or the length of exposure to these therapies. It is also important to explore the effect of continuous vs. intermittent exposure on the incidence of these serious but rare complications.
Patients on long term BPs should be assessed periodically for thigh pain or groin pain. The risks and benefits of continuing BPs should be discussed after 2–3 years of their use. Patients with thigh or groin pain should promptly be investigated with DXA ± X-ray ± MRI of femur. Anti-resorptive therapy should be stopped and prompt surgical fixation is indicated in patients who present with complete fracture or symptomatic incomplete fracture and those who failed conservative management.
Despite this specific guidance, the biological mechanism responsible for the association of antiresorptive therapies with AFF remains elusive. Indeed, both bisphosphonates and Denosumab are successful in patients with early breast cancer and clearly maintain bone health while counteracting the considerable bone loss induced by cancer therapy. As a result, there is much interest but little understanding of the pathways underlying AFF. Perhaps the most compelling information comes from Roca et al. who showed that patients suffering from AFF might have an underlying genetic background that contributed to the development of these fractures [46]. In a small family of three sisters with AFF, whole-exome sequencing was performed compared with BP-treated patients with no AFF, to identify possible genetic variants involved in the apparent increased risk. Interestingly, a number of rare mutations in 34 genes were identified. Among those identified was a novel p.Asp188Tyr substitution in geranylgeranyl pyrophosphate synthase (GGPPS), the same enzyme that is targeted for inhibition by bisphosphonates in the mevalonate pathway. Should similar mutations exist in Denosumab-treated AFF patients, then it may be that specific targets of the treatments will guide eventual understanding of the cause.
Other biological advantages may also come from translational research projects addressing the prognostic value of bone turnover markers, hormone serum concentrations, tumor biomarkers and patient-derived covariates (e.g., body-mass index) that may clarify the anticancer mechanism(s) of antiresorptive therapies. Such information will be invaluable and has the potential to assist clinicians with optimum patient selection. One such target is the ongoing investigation of the MAF gene [47]. MAF is a transcription factor of the AP-1 family, and a putative biomarker of bone relapse in early breast cancer [48]. Coleman et al. suggested associations between MAF expression and bone relapse in post-hoc analysis of the AZURE trial. [47]. Importantly, MAF positivity was associated with increased extraskeletal recurrence in the zoledronic acid group and may be an indicator of a skeletal response associated with the development of AF. That said, it is clear that much work is required before any definitive biological mechanism driving AFF and the association (or not) with antiresorptive therapy can be claimed.
Our patient received a large dose of zoledronic acid in addition to steroids and aromatase inhibitors. Furthermore, her vitamin D replacement was not adequate and it was only after an aggressive vitamin D supplementation that her vitamin D level was normalized. It is possible that the patient transferred her weight to the opposite leg and an inactive fracture became symptomatic causing the right femur to fracture. It is clear that our screening for the early signs of imminent fracture was not adequate. So, close observation of the opposite side is needed.
Declaration of Competing Interest
None.
Funding
This review was partially supported by funds from the Laura F. Hutchins, M.D. Distinguished Chair for Hematology and Oncology.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jbo.2019.100259.
Contributor Information
Matthew Lockwood, Email: mblockwood@uams.edu.
Larry J. Suva, Email: lsuva@cvm.tamu.edu.
Issam Makhoul, Email: makhoulissam@uams.edu.
Appendix. Supplementary materials
References
- 1.Makhoul I., Montgomery C.O., Gaddy D., Suva L.J. The best of both worlds [mdash] managing the cancer, saving the bone. Nat. Rev. Endocrinol. 2016;12:29–42. doi: 10.1038/nrendo.2015.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mariotto A.B., Etzioni R., Hurlbert M., Penberthy L., Mayer M. Estimation of the number of women living with metastatic breast cancer in the united states. Cancer Epidemiol. Biomark. Prev. 2017;26:809–815. doi: 10.1158/1055-9965.EPI-16-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman R.E., McCloskey E.V. Bisphosphonates in oncology. Bone. 2011;49:71–76. doi: 10.1016/j.bone.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Shane E., Burr D., Abrahamsen B., Adler R.A., Brown T.D., Cheung A.M., Cosman F., Curtis J.R., Dell R., Dempster D.W. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American society for bone and mineral research. J. Bone Miner. Res. 2014;29:1–23. doi: 10.1002/jbmr.1998. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen H.H., van de Laarschot, Denise M., Verkerk A.J., Milat F., Zillikens M.C., Ebeling P.R. Genetic risk factors for atypical femoral fractures (AFFs): a systematic review. JBMR Plus. 2018;2:1–11. doi: 10.1002/jbm4.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black D.M., Abrahamsen B., Bouxsein M.L., Einhorn T., Napoli N. Atypical femur fractures: review of epidemiology, relationship to bisphosphonates, prevention, and clinical management. Endocr. Rev. 2018;40:333–368. doi: 10.1210/er.2018-00001. [DOI] [PubMed] [Google Scholar]
- 7.Koh J., Myong J., Yoo J., Lim Y., Lee J., Kwok S., Park S., Ju J. Predisposing factors associated with atypical femur fracture among postmenopausal korean women receiving bisphosphonate therapy: 8 years’ experience in a single center. Osteoporosis Int. 2017;28:3251–3259. doi: 10.1007/s00198-017-4169-y. [DOI] [PubMed] [Google Scholar]
- 8.Puhaindran M.E., Farooki A., Steensma M.R., Hameed M., Healey J.H., Boland P.J. Atypical subtrochanteric femoral fractures in patients with skeletal malignant involvement treated with intravenous bisphosphonates. J. Bone Joint Surg. Am. 2011;93:1235–1242. doi: 10.2106/JBJS.J.01199. [DOI] [PubMed] [Google Scholar]
- 9.Edwards B.J., Sun M., West D.P., Guindani M., Lin Y.H., Lu H., Hu M., Barcenas C., Bird J., Feng C. Incidence of atypical femur fractures in cancer patients: The md anderson cancer center experience. J. Bone Miner. Res. 2016;31:1569–1576. doi: 10.1002/jbmr.2818. [DOI] [PubMed] [Google Scholar]
- 10.Chang S.T., Tenforde A.S., Grimsrud C.D., O'Ryan F.S., Gonzalez J.R., Baer D.M., Chandra M., Lo J.C. Atypical femur fractures among breast cancer and multiple myeloma patients receiving intravenous bisphosphonate therapy. Bone. 2012;51:524–527. doi: 10.1016/j.bone.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Ota S., Inoue R., Shiozaki T., Yamamoto Y., Hashimoto N., Takeda O., Yoshikawa K., Ito J., Ishibashi Y. Atypical femoral fracture after receiving antiresorptive drugs in breast cancer patients with bone metastasis. Breast Cancer. 2017;24:601–607. doi: 10.1007/s12282-016-0746-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang S.P., Kim T.W., Boland P.J., Farooki A. Retrospective review of atypical femoral fracture in metastatic bone disease patients receiving denosumab therapy. Oncologist. 2017;22:438–444. doi: 10.1634/theoncologist.2016-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paparodis R., Buehring B., Pelley E., Binkley N. A case of an unusual subtrochanteric fracture in a patient receiving denosumab. Endocr. Pract. 2013;19:e64–e68. doi: 10.4158/EP12367.CR. [DOI] [PubMed] [Google Scholar]
- 14.Starr J., Tay Y.K.D., Shane E. Current understanding of epidemiology, pathophysiology, and management of atypical femur fractures. Curr. Osteoporosis Rep. 2018;16:519–529. doi: 10.1007/s11914-018-0464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adler R.A. Management of endocrine disease: atypical femoral fractures: risks and benefits of long-term treatment of osteoporosis with anti-resorptive therapy. Eur. J. Endocrinol. 2018;178:R81–R87. doi: 10.1530/EJE-17-1002. [DOI] [PubMed] [Google Scholar]
- 16.Gustafsson A., Schilcher J., Grassi L., Aspenberg P., Isaksson H. Strains caused by daily loading might be responsible for delayed healing of an incomplete atypical femoral fracture. Bone. 2016;88:125–130. doi: 10.1016/j.bone.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Compston J. Pathophysiology of atypical femoral fractures and osteonecrosis of the jaw. Osteoporosis Int. 2011;22:2951–2961. doi: 10.1007/s00198-011-1804-x. [DOI] [PubMed] [Google Scholar]
- 18.Zheng N., Tang N., Qin L. Atypical femoral fractures and current management. J. Orthop. Transl. 2016;7:7–22. doi: 10.1016/j.jot.2016.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hortobagyi G.N., Zheng M., Mohanlal R. Indirect evaluation of bone saturation with zoledronic acid after long-term dosing. Oncologist. 2019;24:178–184. doi: 10.1634/theoncologist.2018-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saita Y., Ishijima M., Mogami A., Kubota M., Baba T., Kaketa T., Nagao M., Sakamoto Y., Sakai K., Kato R., Nagura N. The fracture sites of atypical femoral fractures are associated with the weight-bearing lower limb alignment. Bone. 2014;66:105–110. doi: 10.1016/j.bone.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki S., Miyakoshi N., Hongo M., Kasukawa Y., Shimada Y. Low-energy diaphyseal femoral fractures associated with bisphosphonate use and severe curved femur: a case series. J. Bone Miner. Metab. 2012;30:561–567. doi: 10.1007/s00774-012-0358-0. [DOI] [PubMed] [Google Scholar]
- 22.Allen M.R., Gineyts E., Leeming D.J., Burr D.B., Delmas P.D. Bisphosphonates alter trabecular bone collagen cross-linking and isomerization in beagle dog vertebra. Osteoporosis Int. 2008;19:329–337. doi: 10.1007/s00198-007-0533-7. [DOI] [PubMed] [Google Scholar]
- 23.Vashishth D. Advanced glycation end-products and bone fractures. IBMS BoneKEy. 2009;6:268–278. doi: 10.1138/20090390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viguet-Carrin S., Roux J., Arlot M., Merabet Z., Leeming D., Byrjalsen I., Delmas P., Bouxsein M. Contribution of the advanced glycation end product pentosidine and of maturation of type i collagen to compressive biomechanical properties of human lumbar vertebrae. Bone. 2006;39:1073–1079. doi: 10.1016/j.bone.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Saito M., Mori S., Mashiba T., Komatsubara S., Marumo K. Collagen maturity, glycation induced-pentosidine, and mineralization are increased following 3-year treatment with incadronate in dogs. Osteoporosis Int. 2008;19:1343–1354. doi: 10.1007/s00198-008-0585-3. [DOI] [PubMed] [Google Scholar]
- 26.Santini D., Vincenzi B., Dicuonzo G., Avvisati G., Massacesi C., Battistoni F., Gavasci M., Rocci L., Tirindelli M.C., Altomare V., Tocchini M., Bonsignori M., Tonini G. Zoledronic acid induces significant and long-lasting modifications of circulating angiogenic factors in cancer patients. Clin. Cancer Res. 2003;9:2893–2897. [PubMed] [Google Scholar]
- 27.Fournier P., Boissier S., Filleur S., Guglielmi J., Cabon F., Colombel M., Clezardin P. Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res. 2002;62:6538–6544. [PubMed] [Google Scholar]
- 28.Cheng C., Shoback D. Mechanisms underlying normal fracture healing and risk factors for delayed healing. Curr. Osteoporos. Rep. 2019;17:36–47. doi: 10.1007/s11914-019-00501-5. [DOI] [PubMed] [Google Scholar]
- 29.LeBlanc E.S., Rosales A.G., Black D.M., Genant H.K., Dell R.M., Friess D.M., Boardman D.L., Bauer D.C., de Papp A., Santora A.C. Evaluating atypical features of femur fractures: how change in radiological criteria influenced incidence and demography of atypical femur fractures in a community setting. J. Bone Miner. Res. 2017;32:2304–2314. doi: 10.1002/jbmr.3221. [DOI] [PubMed] [Google Scholar]
- 30.FDA . Vol. 2019. 2010. (Safety Update for Osteoporosis Drugs, Bisphosphonates, and Atypical Fractures). [Google Scholar]
- 31.van de Laarschot, Denise M., Smits A.A., Buitendijk S.K., Stegenga M.T., Zillikens M.C. Screening for atypical femur fractures using extended femur scans by DXA. J. Bone Miner. Res. 2017;32:1632–1639. doi: 10.1002/jbmr.3164. [DOI] [PubMed] [Google Scholar]
- 32.Haworth A., Webb J. Skeletal complications of bisphosphonate use: what the radiologist should know. Br J Radiol. 2012;85(1018):1333–1342. doi: 10.1259/bjr/99102700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giusti A., Hamdy N.A., Papapoulos S.E. Atypical fractures of the femur and bisphosphonate therapy: a systematic review of case/case series studies. Bone. 2010;47:169–180. doi: 10.1016/j.bone.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 34.Schilcher J., Michaëlsson K., Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N. Engl. J. Med. 2011;364:1728–1737. doi: 10.1056/NEJMoa1010650. [DOI] [PubMed] [Google Scholar]
- 35.Dell R.M., Adams A.L., Greene D.F., Funahashi T.T., Silverman S.L., Eisemon E.O., Zhou H., Burchette R.J., Ott S.M. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J. Bone Miner. Res. 2012;27:2544–2550. doi: 10.1002/jbmr.1719. [DOI] [PubMed] [Google Scholar]
- 36.Amadori D., Aglietta M., Alessi B., Gianni L., Ibrahim T., Farina G., Gaion F., Bertoldo F., Santini D., Rondena R. Efficacy and safety of 12-weekly versus 4-weekly zoledronic acid for prolonged treatment of patients with bone metastases from breast cancer (ZOOM): a phase 3, open-label, randomised, non-inferiority trial. Lancet Oncol. 2013;14:663–670. doi: 10.1016/S1470-2045(13)70174-8. [DOI] [PubMed] [Google Scholar]
- 37.Himelstein A.L., Loprinzi C.L., Shapiro C.L. Zoledronic acid dosing interval for metastatic cancer—reply. JAMA. 2017;317 doi: 10.1001/jama.2017.2566. 1478–1478. [DOI] [PubMed] [Google Scholar]
- 38.Im G., Lee S. Effect of teriparatide on healing of atypical femoral fractures: a systemic review. J. Bone Metabolism. 2015;22:183–189. doi: 10.11005/jbm.2015.22.4.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiang C.Y., Zebaze R.M., Ghasem-Zadeh A., Iuliano-Burns S., Hardidge A., Seeman E. Teriparatide improves bone quality and healing of atypical femoral fractures associated with bisphosphonate therapy. Bone. 2013;52:360–365. doi: 10.1016/j.bone.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 40.Cheung A.M., Tile L., Bleakney R., Khan A., Cardew S., Ridout R., McDonald-Blumer H., Syed K., Chang J., Hu H., Morin S., Papaioannou A., Josse R., Bogoch E., Adachi J. Effect of teriparatide on fracture healing in patients with non‐displaced incomplete atypical femur fractures. J Bone Miner Res. 2012;27 (Suppl 1) [Google Scholar]
- 41.Min B., Koo K., Park Y., Oh C., Lim S., Kim J., Lee K., Lee Y. Scoring system for identifying impending complete fractures in incomplete atypical femoral fractures. J. Clin. Endocrinol. Metab. 2016;102:545–550. doi: 10.1210/jc.2016-2787. [DOI] [PubMed] [Google Scholar]
- 42.Prasarn M.L., Ahn J., Helfet D.L., Lane J.M., Lorich D.G. Bisphosphonate-associated femur fractures have high complication rates with operative fixation. Clin. Orthop. Relat. Res. 2012;470:2295–2301. doi: 10.1007/s11999-012-2412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weil Y.A., Rivkin G., Safran O., Liebergall M., Foldes A.J. The outcome of surgically treated femur fractures associated with long-term bisphosphonate use. J. Trauma. 2011;71:186–190. doi: 10.1097/TA.0b013e31821957e3. [DOI] [PubMed] [Google Scholar]
- 44.Gnant M., Pfeiler G., Steger G.G., Egle D., Greil R., Fitzal F., Wette V., Balic M., Haslbauer F., Melbinger-Zeinitzer E. Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:339–351. doi: 10.1016/S1470-2045(18)30862-3. [DOI] [PubMed] [Google Scholar]
- 45.Cosman F., Crittenden D.B., Adachi J.D., Binkley N., Czerwinski E., Ferrari S., Hofbauer L.C., Lau E., Lewiecki E.M., Miyauchi A. Romosozumab treatment in postmenopausal women with osteoporosis. N. Engl. J. Med. 2016;375:1532–1543. doi: 10.1056/NEJMoa1607948. [DOI] [PubMed] [Google Scholar]
- 46.Roca-Ayats N., Balcells S., Garcia-Giralt N., Falcó-Mascaró M., Martínez-Gil N., Abril J.F., Urreizti R., Dopazo J., Quesada-Gómez J.M., Nogués X. GGPS1 mutation and atypical femoral fractures with bisphosphonates. N. Engl. J. Med. 2017;376:1794–1795. doi: 10.1056/NEJMc1612804. [DOI] [PubMed] [Google Scholar]
- 47.Coleman R., Hall A., Albanell J., Hanby A., Bell R., Cameron D., Dodwell D., Marshall H., Jean-Mairet J., Tercero J. Effect of MAF amplification on treatment outcomes with adjuvant zoledronic acid in early breast cancer: a secondary analysis of the international, open-label, randomised, controlled, phase 3 azure (BIG 01/04) trial. Lancet Oncol. 2017;18:1543–1552. doi: 10.1016/S1470-2045(17)30603-4. [DOI] [PubMed] [Google Scholar]
- 48.Pavlovic M., Arnal-Estapé A., Rojo F., Bellmunt A., Tarragona M., Guiu M., Planet E., Garcia-Albéniz X., Morales M., Urosevic J. Enhanced maf oncogene expression and breast cancer bone metastasis. J. Natl. Cancer Inst. 2015;107(12) doi: 10.1093/jnci/djv256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.