Abstract
The main obstacle in antimycobacterial discovery is the extremely slow growth rates of pathogenic mycobacteria that lead to the long incubation times needed in antimycobacterial screening. Some in vitro testings has been developed and are currently available for antimycobacterial screening. The aim of the study was to compare Resazurin Microplate Assay (REMA) and Crystal Violet Decolorization Assay (CVDA) for testing mycobacteria susceptibility to isoniazid and rifampicin as well as for antimycobacterial screening of natural products (NP). Mycobacterium tuberculosis strain H37Rv and Mycobacterium smegmatis strain mc2 155 were used as tested mycobacteria. Serial two-fold dilutions from 0.0625 to 1.0 μg/mL for the isoniazid and rifampicin and from 6.25 to 100.0 μg/mL for the NP A and B were prepared. Tested mycobacteria were then incubated with tested drugs or NPs in each growth medium at 37 °C for 7 days for M. tuberculosis and 3 days for M. smegmatis. MIC values against M. tuberculosis were interpreted 24–48 h after adding resazurin or at least 72 h after adding crystal violet, whereas MIC values against M. smegmatis were interpreted 1 h after adding resazurin or 24 h after adding crystal violet. The MIC values against M. tuberculosis interpreted by REMA were 0.0625, 0.0625, 6.25, and >100 μg/mL for rifampicin, isoniazid, NP A, and NP B, respectively, and those interpreted by CVDA were 0.0625, 0.0625, 6.25, and >100 μg/mL for rifampicin, isoniazid, NP A, and NP B, respectively. Moreover, the MIC values against M. smegmatis interpreted by REMA were 0.0625, >1, 6.25, and 100 μg/mL for rifampicin, isoniazid, NP A, and NP B, respectively, and those interpreted by CVDA were 0.125, >1, 6.25, and >100 μg/mL for rifampicin, isoniazid, NP A, NP B respectively. In conclusion, REMA is faster and easier than CVDA to interpret MIC values, however CVDA produces higher MIC values than REMA for rifampicin and NP B in M. smegmatis susceptibility testing. Therefore, REMA and CVDA can be used for antimycobacterial screening.
Keywords: Resazurin microplate assay, Mycobacterium, Natural product screening, Crystal violet decolorization assay, Antimicrobial susceptibility testing
1. Introduction
Although the global tuberculosis (TB) incidence rate slowly declined in the last decade, TB remains a major health problem worldwide (WHO, 2017). Several major problems are associated with the currently available TB treatment including the duration and complexity of treatment protocols which lead to lack of patient adherence, the emergence of adverse drug reactions and the increasing incidence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) TB (Laurenzi et al., 2007; Boogaard et al., 2009). To overcome the problems in TB treatment, new antimycobacterial agents that are more effective, faster-acting, safe, easier to use, tolerable and accessible are urgently needed (Mdluli et al., 2015; Kumar et al., 2017). Unfortunately, the interest in new antimycobacterial discovery and development is very limited.
The major obstacle in antimycobacterial discovery is the extremely slow growth rates of pathogenic mycobacteria that lead to the long incubation times needed in antimycobacterial screening (Sharma et al., 2014). Additionally, the nature of the lipid-rich cell walls makes single cell culture and homogenous growth of this organism difficult to obtain (Kumar et al., 2017). Drug susceptibility testing (DST) using egg or agar-based media continues to be the standard method for evaluating the activity of antimycobacterial compounds. However, this method is very labor-intensive and time-consuming, requiring up to two months obtaining the results. Moreover, the results are only qualitative, not quantitative (Sirgel et al., 2008).
In the early stage of antimycobacterial discovery, accurate, rapid, and affordable methods in DST play an important role in the determination of minimal inhibitory concentration (MIC) or minimal bactericidal concentration (MBC) during the process of screening, prioritizing, and optimizing of a compound series (Klingeren et al., 2007; Wells et al., 2013). Some in vitro methods have been developed and are currently available for monitoring viable mycobacteria and antimycobacterial screening. Resazurin-based microplate assay (REMA) is a new method using the colorimetric indicator resazurin for rapid drug resistance detection in M. tuberculosis (Palomino et al., 2007). Resazurin is a redox indicator dye that is reduced to resofurin by metabolically viable cells. A positive direct correlation between the resazurin reduction in the growth medium and mycobacteria proliferation is reported. The resazurin is blue in color when it is in an oxidized state and turns pink when reduced by viable mycobacteria. The REMA has been used successfully for determination of MIC (Schon et al., 2017; Webster et al., 2010). However, the resazurin availability as the main active ingredient of the method is limited in developing countries due to not being routinely used. Another new colorimetric method, crystal violet decolorization assay (CVDA), has been developed to solve the limited availability of resazurin. The crystal violet dye is inexpensive and is commonly used for Gram staining in clinical microbiology laboratories. Therefore, it can be easily obtained in many laboratories (Coban, 2014).
Studies on the comparison of REMA and CVDA methods have been carried out previously for detection of M. tuberculosis resistance to primary drugs isoniazid and rifampicin. The results showed that both methods have the same sensitivity and specificity (Coban et al., 2015). However, comparison of both methods for DST anti M. smegmatis has never been conducted, yet. In this study we compared REMA and CVDA for testing mycobacteria susceptibility to isoniazid and rifampicin as well as for antimycobacterial screening of natural products. M. tuberculosis and M. smegmatis were used as tested mycobacteria in this study. M. tuberculosis is pathogenic and a slow growth mycobacteria (SGM). In contrast, M. smegmatis is non-pathogenic and a rapid growth mycobacteria (RGM), however it can cause infection in an immunocompromised person. Moreover, these mycobacteria are naturally highly resistant to antimicrobials (Brown-Elliott et al., 2012; Ingen et al., 2012).
2. Materials and methods
2.1. Tested mycobacteria
Standard reference strains used for DST were M. tuberculosis H37Rv (ATCC 27294) obtained from the Department of Microbiology, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta and M. smegmatis mc2 155 (ATCC 700084) obtained from Research Center for Biotechnology, Indonesia Institute of Sciences, Bogor.
2.2. Drug stocks and indicator color reagents
Pure isoniazid and rifampicin powder (Sigma Aldrich ref I3377 and R3501) were dissolved in methanol (Merck). Drug stocks solutions were aseptically prepared at concentrations of 4.0 μg/mL. The natural product used was actinomycetes extract that was dissolved in 5% DMSO, and aseptically prepared at concentration 400 μg/mL. The final test concentrations used were obtained by serial two-fold dilutions from 0.0625 to 1.0 μg/mL for the isoniazid and rifampicin, and from 6.25 to 100.0 μg/mL for the natural product.
Resazurin sodium salt (Sigma Aldrich ref R7017) solution was prepared at 0.01–0.02 g/100 mL in distilled water (Martin et al., 2011) and stock solution of crystal violet (Merck ref CI 42555) was prepared at 0.0025 g/100 mL with sterile distilled water (Coban et al., 2016a). The solutions were sterilized by filtration through a sterilized 0.2 μm nylon (Puradisc ref 6750–2502) and keep at 4 °C for 1–2 weeks and protected from exposure to light until use.
2.3. Medium preparation
2.3.1. Growth medium
Mycobacterium tuberculosis H37Rv strains were cultured using Lowenstein Jensen Agar, with the age of isolates used for DST was 21–28 d (Martin et al., 2011). Whereas M. smegmatis was cultured using Mueller Hinton Agar with the age of isolates used for DST was 2–3 d (Silva et al., 2009).
2.3.2. Drug susceptibility test medium
The medium used to perform DST for M. tuberculosis was Middlebrook 7H9–S that contains Middlebrook 7H9 broth (DifcoTM ref 271310) supplemented with 0.1% bacto casitone (Becton Dickinson ref 225930), 0.5% glycerol (Merck ref 1.04094) and 10% oleic acid, albumin, dextrose and catalase-OADC (Becton Dickinson ref 245116) (Martin et al., 2011). The medium used to perform DST for M. smegmatis was Mueller Hinton Broth (MHB), which was prepared by diluting 21.0 g/L (Merck ref 1.10293) in purified water. All mediums used were sterilized with an autoclave 121 °C for 15 min (CLSI, 2011; Brown-Elliott et al., 2012; Hatakeyama et al., 2017).
2.4. Preparation of standardized inoculum
2.4.1. Mycobacterium tuberculosis inoculum preparation
The suspensions of Mycobacterium tuberculosis for the REMA and CVDA were prepared from 21 to 28-day-old Lowenstein–Jensen (LJ) subcultures. Using a sterile loop, M. tuberculosis colonies were transferred to a tube containing 3–5 glass beads in 3 drops 3% tween. Clumps were disrupted by vortexing for 60 s in the presence of the beads and the suspension was allowed to sediment for 15 min. Part of the supernatant was transferred to a new tube. The suspension was adjusted to a turbidity equivalent to that of a 1.0 McFarland standard and the suspension was diluted 1:10 in M7H9S broth supplemented with 10% OADC (Martin et al., 2011; Schena et al., 2016).
2.4.2. Mycobacterium smegmatis inoculum preparation
For M. smegmatis, bacterial inoculums were adjusted with sterile distilled water to a 1 McFarland standard with an organism density of approximately 3 × 108 colony forming units (CFU)/mL, then the suspension was diluted with MH broth to make a 1 : 300 bacterial dilution (1 × 106 CFU/mL) (Pang et al., 2015).
2.5. Resazurin and crystal violet assay
One-hundred μL of suspension of mycobacteria were used as inoculum per well and 100 μL of serial 2-fold dilutions of drug and extract working solution were added to each well of a sterile, polystyrene 96-well flat-bottom plate (Iwaki). A drug free growth control and a mycobacteria-free sterility control of the medium were included in each plate. Two-hundred μL of sterile water were added to all outer perimeter wells to avoid evaporation during incubation. Plates were then covered with self-adhesive membranes and incubated at 37 °C. For M. tuberculosis DST, the assay was stained by adding 30 μL of a freshly prepared solution of resazurin 0.01 g/100 mL (Sigma–Aldrich) aquadest after 7 d incubation. The plates were re-incubated for an additional 24–72 h at 37 °C. For the M. smegmatis DST, the assay was stained by adding resazurin after 2–3 d incubation, and were re-incubated for an additional 0.5–24 h at 37 °C. A change in color from blue (oxidized state) to pink (reduced state) indicated growth of the bacteria. The MIC was defined as the lowest concentration of drug that prevented this change in color. Result was interpreted when there was a color change to pink in drug free control mycobacteria growth (Martin et al., 2011).
For CVDA 100 μL of bacterial suspension were inoculated into each well. One-hundred μL of serial 2-fold dilutions of drugs and extracts working solution were added to each well of a sterile, polystyrene 96-well flat-bottom plate (Iwaki). For M. tuberculosis DST, the assay was stained by adding 25 μL of a freshly prepared solution of crystal violet 25 mg/L (Merck) aquadest after 7 d incubation. The plates were re-incubated for an additional 24–120 h at 37 °C. For the M. smegmatis DST, the assay was stained by adding crystal violet after 2–3 d incubation, and were re-incubated for an additional 0.5–24 h at 37 °C. The incubation was then continued until decolorized of crystal violet in the drug free control mycobacteria growth well. MIC was defined as the lowest drug concentration without decolorization. If the MIC value exceeds the susceptibility breakpoint value, the isolate was considered to be resistant to that tested antibiotics. Susceptibility breakpoints values were 0.125 and 0.5 mg/L for isoniazid and rifampicin, respectively (Coban et al., 2016b).
2.6. Ensuring absence of contamination
Although having the benefit of a shorter duration of test, REMA and CVDA are prone to contamination when opening the microplate lid for the addition of resazurin or crystal violet (Bwanga et al., 2010). Contaminants such as other bacteria, mold, and yeast can be present due to non-aseptically performed work (Ryan, 2002; Bykowski and Stevenson, 2008). The presence of a negative control was used to detect any contaminants. Contamination with non-tuberculosis bacteria or RGM in performing anti M. tuberculosis in this research was confirmed by performing an LJ-PNB test followed by a niacin test.
Inoculum for the LJ-PNB test was prepared from suspension series of anti M. tuberculosis DST, either from the REMA and CVDA methods. The inoculum was first incubated for 7 days at 37 °C, before being used for the LJ-PNB test. Ten microliter suspension of each well in the dilution serial anti M. tuberculosis DST was inoculated into LJ medium with or without PNB (para-nitrobenzoic acid). The PNB concentration in LJ-PNB medium was 500 μg/mL. The LJ medium with or without PNB tubes then incubated into an incubator at 37 °C. Reading of the LJ tubes was started on day 12 after incubation and then followed with reading after incubated at 37 °C for 28 d and finally for 42 d. The results for the LJ-PNB test confirmed there was no contamination by NTM strains because there was no growth on the PNB containing tube (Affolabi et al., 2013; Giampaglia et al., 2007; Grange et al., 1996).
For the niacin test, the strain growth in PNB free LJ medium was punctured with a loop several times thus allowing niacin to accumulate in the medium. One mL of sterile distilled water was added to the PNB free LJ medium and the tube cap was tightened. The tube was laid horizontally, so that the fluid was in contact with the entire surface of the medium. The tilted tube was then kept for up to 30 min at 37 °C. About 0.5 mL of the fluid was transferred to the sterile screw cap test tube. A niacin reagent strip was inserted in the tube with marked end at the top. The tube was immediately closed tightly and incubated for 15–20 min at 37 °C with occasional side-to-side shaking to mix the fluid with the reagent strip on the bottom of the strip. The test result was considered positive M. tuberculosis if the yellow color of the liquid appeared (Palomino et al., 2007).
3. Results
The REMA was faster than the CVDA for testing mycobacteria susceptibility to antimycobacterial drugs and for antimycobacterial screening of natural products against M. tuberculosis H37Rv and M. smegmatis mc2 155 strains (Table 1). Moreover, the REMA was more sensitive than the CVDA in DST of rifampicin and NP B against M. smegmatis, as demonstrated by the lower MIC values obtained by the REMA.
Table 1.
Susceptibility test of Mycobacterium against isoniazid, rifampicin, and natural products using REMA and CVDA.
| Microorganism Test | Drug Test | Time added color indicator (d)* | REMA |
CVDA |
||
|---|---|---|---|---|---|---|
| Positive in growth control (h)** | MIC (μg/mL) | Positive in growth control (h)** | MIC (μg/mL) | |||
| M. tuberculosis strain H37Rv | Isoniazid | 7 | 24 | 0.0625 | 72 | 0.0625 |
| Rifampicin | 0.0625 | 0.0625 | ||||
| NP A | 6.25 | 6.25 | ||||
| NP B | >100 | >100 | ||||
| M. smegmatis | Isoniazid | 3 | 1 | >1 | 24 | >1 |
| Rifampicin | 0.0625 | 0.125 | ||||
| NP A | 6.25 | 6.25 | ||||
| NP B | 100 | >100 | ||||
*Time (in days) to add color indicator resazurin/crystal violet, counted from first day 37 °C incubation; **Time (in hours) to get positive result in mycobacteria control growth (blue turn into pink in REMA and blue turn into colorless in CVDA), counted from color indicator added.
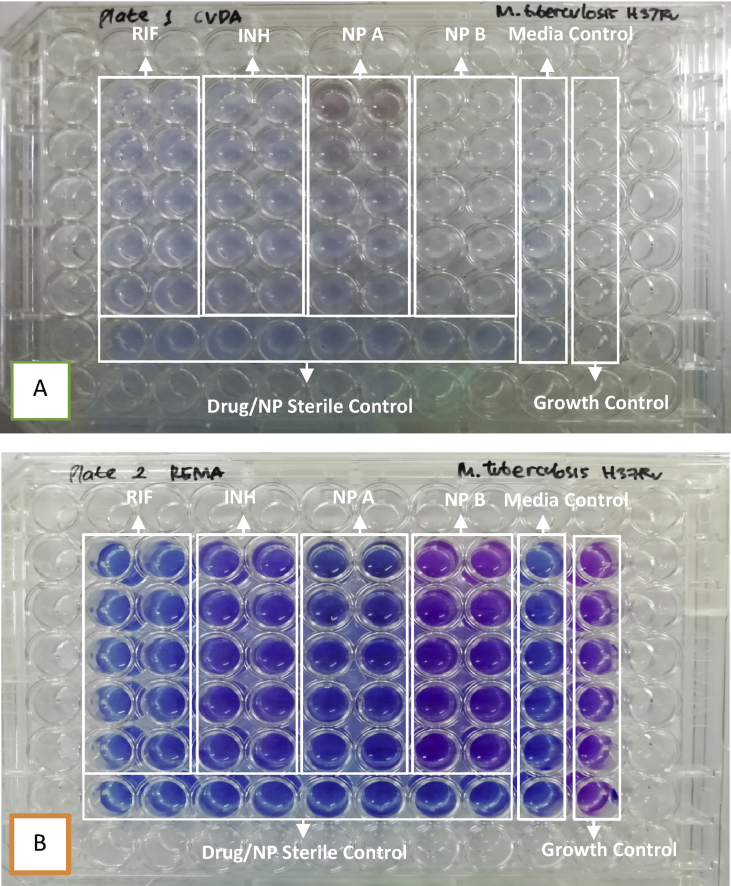
The determination of the MIC value using the REMA or the CVDA method is based on the change of color indicator resulting from interaction between indicators with the growing test bacteria. The REMA method produces color indicator change from blue to pink purple if there is growth of mycobacteria, while the CVDA produces decolorization changes in blue color to colorless. The changes in the color from blue to pink purple can be more easily observed, so the process of interpretation of the MIC value is easier to do. An example of the color indicator change in the CVDA method can be seen from Figs. 1A and 2A, while the REMA method can be seen from Figs. 1B and 2B.
Fig. 1.
(A) Result in rifampicin and natural product A susceptibility testing against M. tuberculosis strains H37Rv, observation in 72 h after added crystal violet (B) Result in rifampicin and NP A susceptibility testing against M. tuberculosis strains H37Rv, observation in 24 h after added resazurin. Note rifampicin serial dilution concentration from above (1, 0.5, 0.25, 0.125, and 0.0625 μg/mL), NP serial dilution concentration from above (100, 50, 25, 12.5, and 6.25 μg/mL).
Fig. 2.
(A) Result in isoniazid, rifampicin, natural product A and B susceptibility testing against M. smegmatis strains mc2 155, observation in 24 h after added crystal violet (B) Result in isoniazid, rifampicin. natural product A and B susceptibility testing against M. smegmatis strains mc2 155, observation in 1 h after added resazurin. Note rifampicin and isoniazid serial dilution concentration from above (1, 0.5, 0.25, 0.125, and 0.0625 μg/mL), NP serial dilution concentration from above (100, 50, 25, 12.5, and 6.25 μg/mL).
4. Discussion
4.1. DST of M. tuberculosis
REMA is one method that can be used for drug susceptibility testing (DST) against M. tuberculosis. It is an inexpensive, easy, and fast method compared to the standard proportion method using Lowenstein Jensen medium. Moreover, REMA is also cheaper and easier compared to the direct method such as BACTEC MGIT 960 due to not needing specific equipment (Campanerut et al., 2011). However, the availability of resazurin as the main active ingredient of the method is a problem in developing countries. Resazurin is not routinely used although a high-number of TB cases are occurring in these countries (Parsons et al., 2011). A new method, crystal violet decolorization assay (CVDA), has been developed to answer the problem of resazurin availability required as a reliable color indicator (Coban, 2014).
REMA is a fast and reliable method for DST, and in the case of M. tuberculosis, it is possible to obtain the results with an incubation of, at least, eight days. This method is recommended by the WHO for M. tuberculosis DST, although the results obtained take 8–16 d which can slow down the handling of patients infected by resistant TB. A modification of this classic REMA has been conducted to simplify procedures and enable more rapid results. However, this modification reduced its specificity (Katawera et al., 2014).
The time needed for interpretation of DST results was 8 d or 24 h after administration of resazurin for the sensitivity detection of isoniazid, rifampicin, or pyrazinamide using standard M. tuberculosis H37Rv strain (Campanerut et al., 2011). Other researchers reported that interpretation of results using REMA can be observed in 10 d or 72 h after resazurin was added (Hillemann et al., 2007), or on average 8–14 d (Dixit et al., 2012). In this study, the optimal time for reading interpretations of MIC values was 24 h after resazurin administration or 24 h after re-incubation. Resazurin was given after 7 d of incubation treatment at 37 °C. However, some replication resulted in differences in the interpretation time of reading MIC values. One replication resulted in a reading of MIC values 24 h after re-incubation, but the administration of resazurin was done after the incubation treatment for 6 d at 37 °C. Other replications resulted in readings 48 h after re-incubation, when administration of resazurin was done after incubation treatment for 7 d at 37 °C. The test protocol referred to in this study suggested adding resazurin after 5–7 d of incubation at 37 °C and interpretation of MIC values after re-incubation for 24–48 h at 37 °C (Martin et al., 2011).
The time needed for interpretation of MIC values with the CVDA method (14 d) was longer than the REMA method (8 d) using M. tuberculosis H37Rv standard strain. However, if using clinical isolates, the time needed was 8–10 d by CVDA method (Coban et al., 2016a). In this study, shorten in the time needed for interpretation (10 d) was observed using standard M. tuberculosis H37Rv strain compared to previous studies. We tried to optimize the speed of the time needed for interpretation by adding crystal violet after 6 d of incubation at 37 °C, but the results remain observed on day 10.
In this study, MIC values of rifampicin, isoniazid, or natural products can be interpreted using both REMA and CVDA methods. The MIC values of rifampicin, isoniazid, and natural products interpreted using the REMA method were the same as those value obtained using the CVDA method (Table 1). These results are in accordance with the study conducted by Coban et al. (2015), who reported that REMA has the same values as CVDA, in terms of specificity, sensitivity, positive predictive value, and negative predictive value on susceptibility testing of isoniazid and rifampicin.
The DST using REMA or CVDA method is considered impractical and prone to contamination because it involves opening the lid of the microplate for the resazurin or crystal violet addition (Bwanga et al., 2010). Therefore, in this study confirmation of contamination of non-tuberculosis mycobacteria or specifically RGM was conducted using LJ-PNB and niacin testing. Contamination can produce bias results in DST using REMA. The REMA method is considered to be less specific because resazurin can be reduced by all active bacterial cells to resorufin, so when contamination occurred negative results are produced (Elshikh et al., 2016).
REMA is reported can be used as growth indicator of several microorganism like B. subtilis (Balouiri et al., 2016), N. Gonorrhoeae (Foerster et al., 2017), Chlamydia trachomatis (Osaka and Hefty, 2013), S. aureus and E. faecalis (March-Rosello et al., 2015), Klebsiella pneumonia (Kim and Jang, 2017), Nocardia spp (Zhao et al., 2017), and Paracoccidioides (Oliveira et al., 2013). In yeasts, growth indicator results can be achieved with an incubation up to 48 h (Pfalleri and Barry, 1996; To and Fothergill, 1995). Resazurin, in a period of time ranged from 24 to 53 h, is also can be used to determine the cytotoxicity of different drugs against parasites, such as Leishmania spp. and Trypanosoma cruzi (Mikus and U, 2000; Rolón et al., 2006). Meanwhile, the CVDA method is considered more specific for antimycobacterial DST. Crystal violet can inhibit bacterial growth both negative and positive bacteria, as well as candida (Fung and Miller, 1973; Maley and Arbiser, 2015). Only mycobacteria have been reported resistant to crystal violet. Mycobacteria are classified acid fast Gram-positive, but typically do not retain the crystal violet stain (Reynolds et al., 2009). It is because mycobacteria can absorb crystal violet into lipid cell fractions which causes decolorization of crystal violet (Jones and Falkinham, 2003; Coban, 2014).
4.2. DST of M. smegmatis
The M. smegmatis has advantages for new drug screening compared to M. tuberculosis such as faster regeneration time and less risk of contamination for laboratory workers (Altaf et al., 2010; Reyrat and Kahn, 2001). In the DST using M. smegmatis, it is well known that the variation of the time of resazurin addition has an effect on the interpretation of MIC values. In some studies, variation at the time resazurin addition was applied, starting from early resazurin addition along with the inoculum and the drug tested until resazurin addition after incubation of the inoculum for 24–72 h. The early resazurin addition did not affect the interpretation of MIC values. Moreover, it eliminated contaminants due to not opening the microplate. The early resazurin addition was also faster for the interpretation of MIC values than the standard turbidity method recommended by CLSI in which resazurin addition is conducted after 72 h of incubation (Castilho et al., 2015).
In this study, the resazurin or crystal violet addition after 2 d of incubation have the same result in terms of the time needed to interpret MIC values, when compared to the indicator addition after 3 d of incubation assays. The REMA method reaches results for the interpretation after 1 h addition of resazurin, and the CVDA method after 24 h addition of crystal violet, in either 2 and 3 d incubation assays. However, there are differences in the MIC values between 2 and 3 d incubation screening. For the 3 d incubation assays (Table 1), it can be concluded that the CVDA method resulted in greater MIC values than the REMA method for rifampicin and NP B. However, if the test is done for 2 d, both CVDA and REMA methods produce the same MIC value for all drugs and NP test. Lower MIC value was obtained for the NP B, which was 50 μg/mL compared to 100 or >100 μg/mL in REMA and CVDA 3 d assays, respectively. For rifampicin, although MIC value on 3 d incubation of the CVDA method was higher than the REMA method, the value was still below the resistant breakpoint value of rifampicin.
From our study, M. smegmatis was considered to be resistant to isoniazid as reported by Li et al. (2013) who tested nontuberculous mycobacteria (NTM) strains using Microplate Alamar Blue Assay (MABA). All NTM strains (12 RGM and 12 SGM) were reported resistant to isoniazid. In addition, the time needed to obtain the interpretation of MIC values was 6 days. It was also reported DST for RGM using MHB could be used with the interpretation of MIC values obtained on day 5 as seen from turbidity. Ramis et al. (2015) reported that the use of resazurin indicators either in MHB or M7H9 media can accelerate the interpretation of MIC values which was only in 1–3 days. Based on the results in this study, we recommend conducting an anti M. smegmatis DST using CVDA and REMA methods for both 2 and 3 d assays.
5. Conclusion
In conclusion, REMA is faster than CVDA for DST of rifampicin, isoniazid and natural products against M. tuberculosis and M. smegmatis. REMA is also easier than CVDA in the interpretation of MIC values due to easier interpretation of changes in color indicator. However, CVDA has a greater MIC value than REMA in DST against M. smegmatis. Therefore, REMA and CVDA can be used for DST of antimycobacterial against M. tuberculosis and M. smegmatis. Both methods are safe from contaminants, when used in a standard and aseptic laboratory.
Declarations
Author contribution statement
Maya Dian Rakhmawatie, Tri Wibawa, Mustofa, Woro Rukmi Pratiwi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Puspita Lisdiyanti: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Ministry of Research, Technology and Higher Education of the Republic of Indonesia through the Excellence Higher Education Institution Research grant.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank the Head of Department of Microbiology, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta for his permission to conduct this study. We would also like to thank the Head of Research Center for Biotechnology, Indonesia Institute of Sciences for providing the test bacteria M. smegmatis mc2 155.
References
- Affolabi D., Sanoussi N.D., Odoun M., Martin A., Koukpemedji L., Palomino J.C., Kestens L., Anagonou S., Portaels F. Rapid low-cost identification of Mycobacterium tuberculosis complex using p-nitro-benzoic acid (PNB) as inhibitor and the resazurin microplate assay (REMA): a preliminary study. Afr. J. Microbiol. Res. 2013;7(24):3135–3138. [Google Scholar]
- Altaf M., Miller C.H., Bellows D.S., O'Toole R. Evaluation of the Mycobacterium smegmatis and BCG models for the discovery of Mycobacterium tuberculosis inhibitors. Tuberculosis. 2010;90(6):333–337. doi: 10.1016/j.tube.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Balouiri M., Sadiki M., Ibnsouda S.K. Methods for in vitro evaluating antimicrobial activity : a review. J. Pharm. Anal. 2016;6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boogaard J. Van Den, Kibiki G.S., Kisanga E.R., Boeree M.J., Aarnoutse R.E. New drugs against tuberculosis: problems, progress, and evaluation of agents in clinical development. Antimicrob. Agents Chemother. 2009;53(3):849–862. doi: 10.1128/AAC.00749-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Elliott B.A., Nash K.A., Wallace R.J. Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous Mycobacteria. Clin. Microbiol. Rev. 2012;25(3):545–582. doi: 10.1128/CMR.05030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bwanga F., Joloba M.L., Haile M., Hoffner S. Evaluation of seven tests for the rapid detection of multidrug-resistant tuberculosis in Uganda. Int. J. Tuberc. Lung Dis. 2010;14(7):890–895. [PubMed] [Google Scholar]
- Bykowski T., Stevenson B. Aseptic technique. Curr. Protoc. Microbiol. Supp. 2008;11:1–11. doi: 10.1002/9780471729259.mca04ds11. [DOI] [PubMed] [Google Scholar]
- Campanerut P.A.Z., Ghiraldi L.D., Spositto F.L.E., Sato D.N., Leite C.Q.F., Hirata M.H., Hirata R.D.C., Cardoso R.F. Rapid detection of resistance to pyrazinamide in Mycobacterium tuberculosis using the resazurin microtitre assay. J. Antimicrob. Chemother. 2011;66:1044–1046. doi: 10.1093/jac/dkr057. [DOI] [PubMed] [Google Scholar]
- Castilho A.L., Ferracioli K.R., Canezin P.H., Siqueira V.L.D., Scodro R.B.L., Cardoso R.F. Detection of drug susceptibility in rapidly growing mycobacteria by resazurin broth microdilution assay. J. Microbiol. Methods. 2015;111:119–121. doi: 10.1016/j.mimet.2015.02.007. [DOI] [PubMed] [Google Scholar]
- CLSI . Approved Standard—Second Edition; 2011. M24-A2: Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes. [PubMed] [Google Scholar]
- Coban A.Y. A new rapid colourimetric method for testing Mycobacterium tuberculosis susceptibility to isoniazid and rifampicin: a crystal violet decolourisation assay. Mem. Inst. Oswaldo Cruz. 2014;109(2):246–249. doi: 10.1590/0074-0276140297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coban A.Y., Akbal A.U., Bicmen C., Albay A., Sig A.K., Uzun M., Selale D.S., Ozkutuk N., Surucuoglu S., Albayrak N., Ucarman N., Ozkutuk A., Esen N., Ceyhan I., Ozyurt M., Bektore B., Aslan G., Delialioglu N., Alp A. Multicenter evaluation of crystal violet decolorization assay (CVDA) for rapid detection of isoniazid and rifampicin resistance in Mycobacterium tuberculosis. Sci. Rep. 2016;6:1–5. doi: 10.1038/srep39050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coban A.Y., Akbal A.U., Uzun M., Cayci Y.T., Birinci A., Durupinar B. Evaluation of crystal violet decolorization assay for minimal inhibitory concentration detection of primary antituberculosis drugs against Mycobacterium tuberculosis isolates. Mem. Inst. Oswaldo Cruz. 2016;111(7):454–459. doi: 10.1590/0074-02760160082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coban A.Y., Akbal A.U., Uzun M., Durupinar B. Evaluation of four colourimetric susceptibility tests for the rapid detection of multidrug-resistant Mycobacterium tuberculosis isolates. Mem. Inst. Oswaldo Cruz. 2015;110(5):649–654. doi: 10.1590/0074-02760150136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit P., Singh U., Sharma P., Jain A. Evaluation of nitrate reduction assay , resazurin microtiter assay and microscopic observation drug susceptibility assay for fi rst line antitubercular drug susceptibility testing of clinical isolates of M . tuberculosis. J. Microbiol. Methods. 2012;88(1):122–126. doi: 10.1016/j.mimet.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Elshikh M., Ahmed S., Funston S., Dunlop P., McGaw M., Marchant R., Banat I.M. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016;38(6):1015–1019. doi: 10.1007/s10529-016-2079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerster S., Desilvestro V., Hathaway L.J., Althaus C.L., Unemo M. A new rapid resazurin-based microdilution assay for antimicrobial susceptibility testing of Neisseria gonorrhoeae. J. Antimicrob. Chemother. 2017;72:1961–1968. doi: 10.1093/jac/dkx113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung D.Y.C., Miller R.D. Effect of dyes bacterial growth. J. Appl. Microbiol. 1973;25(5):793–799. doi: 10.1128/am.25.5.793-799.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giampaglia C.M.S., Martins M.C., Chimara E., Oliveira R.S., Vieira G.B.D.O., Marsico A.G., Mello F.C.Q., Fonseca L.D.S., Kritski A., Telles M.A.S. Differentiation of Mycobacterium tuberculosis from other mycobacteria with p -nitrobenzoic acid using MGIT960. Int. J. Tuberc. Lung Dis. 2007;11:803–807. [PubMed] [Google Scholar]
- Grange J.M., Yates M.D., de Kantor I.N. second ed. WHO; Geneva: 1996. World Health Organization: Guidelines for Speciation within the Mycobacterium tuberculosis Complex. [Google Scholar]
- Hatakeyama S., Ohama Y., Okazaki M., Nukui Y., Moriya K. Antimicrobial susceptibility testing of rapidly growing mycobacteria isolated in Japan. BMC Infect. Dis. 2017;17(197):1–7. doi: 10.1186/s12879-017-2298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillemann D., Gerdes S.R., Richter E. Evaluation of the GenoType MTBDRplus assay for rifampin and isoniazid susceptibility testing of Mycobacterium tuberculosis strains and clinical Specimens. J. Clin. Microbiol. 2007;45(8):2635–2640. doi: 10.1128/JCM.00521-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingen J.Van, Boeree M.J., Soolingen D.Van, Mouton J.W. Resistance mechanisms and drug susceptibility testing of nontuberculous mycobacteria. Drug Resist. Updates. 2012;15(3):149–161. doi: 10.1016/j.drup.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Jones J.J., Falkinham J.O. Decolorization of malachite green and crystal violet by waterborne pathogenic mycobacteria. Antimicrob. Agents Chemother. 2003;47(7):2323–2326. doi: 10.1128/AAC.47.7.2323-2326.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katawera V., Siedner M., Ii Y.B. Evaluation of the modified colorimetric resazurin microtiter plate-based antibacterial assay for rapid and reliable tuberculosis drug susceptibility testing. BMC Microbiol. 2014;14(259):1–4. doi: 10.1186/s12866-014-0259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.J., Jang S. Optimization of a resazurin - based microplate assay for large - scale compound screenings against Klebsiella pneumoniae. 3 Biotech. 2017:1034–1039. doi: 10.1007/s13205-017-1034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingeren B.Van, Dessens-kroon M., Laan T.Van Der, Kremer K., Soolingen D.Van. Drug susceptibility testing of Mycobacterium tuberculosis complex by use of a high-throughput, reproducible, absolute concentration method. J. Clin. Microbiol. 2007;45(8):2662–2668. doi: 10.1128/JCM.00244-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Chettiar S., Parish T. Current challenges in drug dscovery for tuberculosis. Expert Opin. Drug Discov. 2017;12(1):1–4. doi: 10.1080/17460441.2017.1255604. [DOI] [PubMed] [Google Scholar]
- Laurenzi M., Ginsberg A., Spigelman M. Challenges associated with current and futur TB treatment. Infect. Disord. - Drug Targets. 2007;7:105–119. doi: 10.2174/187152607781001817. [DOI] [PubMed] [Google Scholar]
- Li G., Lian L., Wan L., Zhang J., Zhao X., Jiang Y., Zhao L., Liu H., Wan K. Antimicrobial susceptibility of standard strains of nontuberculous Mycobacteria by microplate alamar blue assay. PLoS One. 2013;8(12):4–9. doi: 10.1371/journal.pone.0084065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maley A.M., Arbiser J.L. Gentian violet: a 19th century drug re-emerges in the 21th century. Exp. Dermatol. 2015;22:775–780. doi: 10.1111/exd.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March-Rosello G.A., Rodriguez-Guiterrez M.P., Simarro-Grande M., Orduña-Domingo A., Bratos-Perez M.Á. A two-hour procedure for determining the susceptibility of enterococci and staphylococci to antibiotics by a colourimetric method. Rev. Española Quimioter. 2015;28:247–255. [PubMed] [Google Scholar]
- Martin A., Paasch F., Docx S., Fissette K., Imperiale B., Ribon W., Gonzalez L.A., Werngren J., Engstrom A., Skenders G., Jureen P., Hoffner S., Portillo P., Morcillo N., Palomino J.C. Multicentre laboratory validation of the colorimetric redox indicator (CRI) assay for the rapid detection of extensively drug-resistant (XDR) Mycobacterium tuberculosis. J. Antimicrob. Chemother. 2011;66:827–833. doi: 10.1093/jac/dkq527. [DOI] [PubMed] [Google Scholar]
- Mdluli K., Kaneko T., Upton A. The tuberculosis drug discovery and development pipeline and emerging drug targets. Cold Spring Harb. Perspect. Med. 2015;5(a021154):1–24. doi: 10.1101/cshperspect.a021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikus J., U D.S. A simple colorimetric method to screen drug cytotoxicity against Leishmania using the dye Alamar Blue. Parasitol. Int. 2000;48:265–269. doi: 10.1016/s1383-5769(99)00020-3. [DOI] [PubMed] [Google Scholar]
- Oliveira H.C., Silva J.F., Scorzoni L. Microplate alamarBlue assay for Paracoccidioides susceptibility testing. J. Clin. Microbiol. 2013;51(4):1250–1252. doi: 10.1128/JCM.02914-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaka I., Hefty P.S. Simple resazurin-based microplate assay for measuring chlamydia infection. J. Antimicrob. Chemother. 2013;57(6):2838–2840. doi: 10.1128/AAC.00056-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomino J.C., Martin A., Portaels F. Rapid drug resistance detection in Mycobacterium tuberculosis : a review of colourimetric methods. Clin. Microbiol. Infect. 2007;13:754–762. doi: 10.1111/j.1469-0691.2007.01698.x. [DOI] [PubMed] [Google Scholar]
- Pang H., Li G., Zhao X., Liu H., Wan K., Yu P. Drug susceptibility testing of 31 antimicrobial agents on rapidly growing Mycobacteria isolates from China. BioMed Res. Int. 2015;2015:1–8. doi: 10.1155/2015/419392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons L.M., Somoskovi A., Gutierrez C., Lee E., Paramasivan C.N., Abimiku A., Spector S., Roscigno G., Nkengasong J. Laboratory diagnosis of tuberculosis in resource-poor countries : challenges and opportunities. Clin. Microbiol. Rev. 2011;24(2):314–350. doi: 10.1128/CMR.00059-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfalleri M.A., Barry A.L. Evaluation of a novel colorimetric broth microdilution method for antifungal susceptibility testing of yeast isolates. J. Clin. Microbiol. 1996;32(8):1992–1996. doi: 10.1128/jcm.32.8.1992-1996.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramis I.B., Cnockaert M., Groll A.Von., Nogueira C.L., Leao S.C., Andre E., Simon A., Palomino J.C., da Silva P.E.A., Vandamme P., Martin A. Antimicrobial susceptibility of rapidly growing mycobacteria using the rapid colorimetric method. Eur. J. Clin. Microbiol. Infect. Dis. 2015;34(7):1403–1413. doi: 10.1007/s10096-015-2365-2. [DOI] [PubMed] [Google Scholar]
- Reynolds J., Moyes R.B., Breakwell D.P. Differential staining of Bacteria : acid fast stain. Curr. Protoc. Microbiol. Supp. 2009;15:1–5. doi: 10.1002/9780471729259.mca03hs15. [DOI] [PubMed] [Google Scholar]
- Reyrat J., Kahn D. Mycobacterium smegmatis: an absurd model for tuberculosis ? Trends Microbiol. 2001;9(10):472–473. doi: 10.1016/s0966-842x(01)02168-0. [DOI] [PubMed] [Google Scholar]
- Rolón M., Vega C., Escario J.A., Gómez-barrio A. Development of resazurin microtiter assay for drug sensibility testing of Trypanosoma cruzi epimastigotes. Parasitol. Res. 2006;99:103–107. doi: 10.1007/s00436-006-0126-y. [DOI] [PubMed] [Google Scholar]
- Ryan J. Corning Incorporated; 2002. Understanding and Managing Cell Culture Contamination.https://safety.fsu.edu/safety_manual/supporting_docs/Understanding and Managing Cell Culture Contamination.pdf [Google Scholar]
- Schena E., Nedialkova L., Borroni E., Battaglia S., Cabibbe A.M., Niemann S., Utpatel C., Merker M., Trovato A., Hofmann-thiel S., Hoffmann H., Cirillo D.M. Delamanid susceptibility testing of Mycobacterium tuberculosis using the resazurin microtitre assay and the BACTEC. J. Antimicrob. Chemother. 2016;71:1532–1539. doi: 10.1093/jac/dkw044. [DOI] [PubMed] [Google Scholar]
- Schon T., Miotto P., Koser C., Viveiros M., Bottger E., Cambau E. Mycobacterium tuberculosis drug-resistance testing : challenges, recent developments, and perspectives. Clin. Microbiol. Infect. 2017;23:154–160. doi: 10.1016/j.cmi.2016.10.022. [DOI] [PubMed] [Google Scholar]
- Sharma S., Gelman E., Narayan C., Bhattacharjee D., Achar V., Humnabadkar V., Humnabadkar V., Balasubramanian V., Ramachandran V., Dhar N., Dinesh N. Simple and rapid method to determine antimycobacterial poency of compounds by using autoluminescent Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2014;58(10):5801–5808. doi: 10.1128/AAC.03205-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J.L., Mesquita A.R.C., Ximenes E.A. In vitro synergic effect of β -lapachone and isoniazid on the growth of Mycobacterium fortuitum and Mycobacterium smegmatis. Mem. Inst. Oswaldo Cruz. 2009;104(4):580–582. doi: 10.1590/s0074-02762009000400008. [DOI] [PubMed] [Google Scholar]
- Sirgel F.A., Wiid I.J.F., Helden D. P. Van. Measuring minimum inhibitory concentrations in mycobacteria. Mycobact. Protoc. 2008;465:173–186. doi: 10.1007/978-1-59745-207-6_11. [DOI] [PubMed] [Google Scholar]
- To W., Fothergill A.W. Comparative evaluation of macrodilution and alamar colorimetric microdilution broth methods for antifungal susceptibility testing of yeast isolates. J. Clin. Microbiol. 1995;33(10):2660–2665. doi: 10.1128/jcm.33.10.2660-2664.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster D., Lee T.D.G., Moore J., Manning T., Kunimoto D., Leblanc D., Johnson J.A., Gray C.A. Antimycobacterial screening of traditional medicinal plants using the microplate resazurin assay. Can. J. Microbiol. 2010;56:487–494. doi: 10.1139/w10-035. [DOI] [PubMed] [Google Scholar]
- Wells W.A., Boehme C.C., Cobelens F.G.J., Daniels C., Dowdy D., Gardiner E., Gheuens J., Kim P., Kimerling M.E., Kreiswirth B., Lienhardt C., Mdluli K., Pai M., Perkins M.D., Peter T., Zignol M., Zumla A., Schito M. Alignment of new tuberculosis drug regimens and drug susceptibility testing : a framework for action. Lancet Infect. Dis. 2013;13(5):449–458. doi: 10.1016/S1473-3099(13)70025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 2017. Global Tuberculosis Report. [Google Scholar]
- Zhao P., Zhang X., Du P., Li G., Li L., Li Z. Susceptibility profiles of Nocardia spp . to antimicrobial and antituberculotic agents detected by a microplate Alamar Blue assay. Nat. Publ. 2017:1–8. doi: 10.1038/srep43660. [DOI] [PMC free article] [PubMed] [Google Scholar]