Abstract
Since primary Sjögren's syndrome (pSS) is an autoummune disease of B cell hyperactivity and pathologic autoantibody response, follicular helper T (Tfh) cells and follicular regulatory T (Tfr) cells are suggested to be key players in pSS. We examined subsets of Tfh and Tfr cells from the blood in pSS patients, and whether these subsets represent disease activity, glandular inflammation, or autoantibody responses in pSS. Circulating Tfh and Tfr cells, along with their specific subsets, were identified from the peripheral blood of 18 pSS patients and 14 age- and sex-matched healthy controls (HCs) using flow cytometry analysis. Blood Tfr and Tfh cell ratios were increased in pSS patients compared with HCs. The CCR7loPD-1hi subset of circulating Tfh cells was increased in pSS patients with high degree of focal lymphocytic sialadenitis; whereas circulating Tfh cells did not differ between pSS patients and HCs. The frequency of CCR7loPD-1hi Tfh cells was significantly correlated with disease activity scores and differentiated B cells. PD-1 expression on blood Tfh and Tfr cells showed positive correlations with IL-21 in pSS. Increasing trend of blood Tfr cells was observed in pSS patients, and blood Tfr cells (particularly Th1 and Th17 subsets) represented hypergammaglobulinemia in pSS. In summary, circulating CCR7loPD-1hi Tfh cells indicated disease activity and glandular inflammation in pSS. Circulating Tfr cells, shifted toward Th1 and Th17 subsets, indicated ongoing IgG production in pSS. Subsets of circulating Tfh or Tfr cells could be biomarkers for disease monitoring and patient stratification in pSS.
Keywords: Sjögren's syndrome; T-lymphocytes; T-lymphocyte subsets; T-lymphocytes, regulatory; Autoantibodies
INTRODUCTION
Sjögren's syndrome (SS) is an autoimmune disease of B-cell hyperactivity, which is reflected by pathogenic autoantibody production. B-cell Ab production requires help from T cells. Follicular helper T (Tfh) cells are a subset of CD4+ T cells that have migrated into B-cell follicles of secondary lymphoid organs in order to participate in germinal center (GC) reactions (1). Tfh cells stimulate B cells to produce high-affinity Abs through affinity maturation and class switch recombination within the GC. IL-21, a cytokine produced by Tfh cells, works as a signal for B-cell maturation and differentiation (2). The interaction between Tfh cells and B cells, in concert with IL-21, may play a crucial role in SS pathogenesis (3,4,5,6).
Follicular regulatory T (Tfr) cells, a recently discovered subset of Tregs, regulate GC reactions (7,8,9). Tfr cells are derived from thymic Treg cells and gain access to the GC (7,8,9). Tfr cells share a phenotype with Tfh cells: Tfr cells express surface markers of CXCR5, inducible costimulator (ICOS), and PD-1, and transcriptional factor Bcl-6 (10). CXCR5+ CD4+ T cells may contain both Tfh and Tfr cells, despite their different functions on GC B cells. Since the SS represents a dysregulated GC response, the characteristics of Tfh and Tfr cells need explored in SS. Several studies have investigated the Tfh and Tfr cell populations in peripheral blood of SS; however, little is currently known about Tfr cells in SS (11,12,13).
Circulating Tfr and Tfh cells are a distinct subset differing from Tfr and Tfh cells found in secondary lymphoid tissues. Notably, circulating Tfr and Tfh cells develop independently to have memory cell properties (14). Blood Tfr and Tfh cells circulate until they become fully activated and enter the GC (14). Due to challenges in obtaining cells from human secondary lymphoid tissues, circulating Tfr cells and Tfh cells are used in human studies as counterparts to Tfr and Tfh cells from lymphoid tissues (15,16,17,18). Studies have investigated that specific subsets of circulating CXCR5+ CD4+ T cells are Tfh cell precursors, which can enter the GC and support B-cell response (19,20,21). He and colleagues had found that the CCR7loPD-1hi subset of circulating CXCR5+ CD4+ T cells differentiate into mature Tfh cells and promote GC responses in mice and humans. Thus, this subset indicates active Tfh cell differentiation in secondary lymphoid organs (21). In addition, the CCR7loPD-1hi subset was expanded in patients with systemic lupus erythematosus (SLE) or rheumatoid arthritis and was correlated with disease activity scores and autoantibody titers (21).
We examined circulating Tfr and Tfh cell populations and their ratios in patients with primary Sjögren's syndrome (pSS). In addition, we examined the specific subsets of circulating Tfr and Tfh cell populations, with a focus on the CCR7loPD-1hi subset and the Th1-like, Th2-like, or Th17-like subsets. Association of circulating Tfr and Tfh cells and their specific subsets with SS disease activity, target organ inflammation, autoantibody responses, and IL-21 secretion were also investigated. The CCR7loPD-1hi subset of circulating Tfh cells may be used as a biomarker for disease activity and glandular inflammation, and blood Tfr cell subsets may be used as an indicator of IgG production in patients with pSS.
MATERIALS AND METHODS
Study population and human blood samples
Patients with pSS (n=18) who fulfilled the 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for pSS (22) and healthy control individuals (n=14) were recruited for this study. The mean age of the pSS patients and healthy controls (HCs) were 51.7 and 48.3 years (p>0.05), respectively. All study participants were women (Supplementary Table 1). Peripheral blood was collected from patients with pSS and HCs. Heparinized blood (10 ml) and serum samples were obtained from each study participant. Informed consent was obtained from the study participants. This study was approved by the Ethics Committee of Seoul St. Mary's Hospital, The Catholic University of Korea (approval ID: KC12TISI0501). All study procedures were performed according to the Declaration of Helsinki.
Clinical and immunological variables of study population
Data on disease duration, European League Against Rheumatism Sjögren's Syndrome Disease Activity Index (ESSDAI), and focus scores of labial salivary gland (LSG) biopsies, along with levels of serum rheumatoid factor (RF), anti-Sjögren's-syndrome-related antigen A (anti-SSA)/Ro Abs, IgG, were collected at the time of blood sampling. Patients with pSS had a median disease duration of 3.6 months and a median ESSDAI score of 2 (Supplementary Table 1). Ninety-two percent of the 12 SS patients who underwent LSG biopsy showed focus scores (FSs) of >1. Patients with SS did not take any disease-modifying antirheumatic drugs other than hydroxychloroquine or predinisolone equivalent > 5 mg per day.
PBMC isolation and flow cytometry
PBMCs were freshly isolated by centrifugation with Ficoll-Hypaque (GE Healthcare, Chicago, IL, USA). PBMCs were re-suspended in PBS solution containing 0.5% BSA (Amresco, Solon, OH, USA) and 2 mM EDTA (Sigma-Aldrich, St Louis, MO, USA). Cells were then stained with fluorescence-conjugated Abs: FITC conjugated anti-CD4 Ab (A161A1), PerCP-Cy5.5 conjugated anti-CD185 (CXCR5) Ab (J252D4), PE-Cy7 conjugated anti-CD25 Ab (BC96), allophycocyanin (APC)-Cy7 conjugated anti-CD127 Ab (A019D5), APC conjugated anti-ICOS Ab (C398.4A), Alexa488 conjugated anti-CD38 Ab (HIT2), PE conjugated anti-CCR7 Ab (G043H7), and PE conjugated anti-CD183(CXCR3) Ab (G025H7; BioLegend, San Diego, CA, USA); APC conjugated anti-CD19 Ab (HIB19) and APC conjugated anti-CD279 (PD-1) Ab (eBioJ105; Invitrogen, Carlsbad, CA, USA); and Brilliant Violet 605 conjugated anti-CD138 Ab (MI15) and APC conjugated anti-CCR6 Ab (11A9; BD Biosciences, Franklin Lakes, NJ, USA).
ELISA
Serum IL-21 was measured using the Human IL-21 ELISA MAX Deluxe kit (BioLegend) according to the manufacturer's instruction.
Statistical analysis
Data were compared between 2 (or 3) independent groups using an independent t-test (or 1-way ANOVA with post-hoc Bonferroni test) for continuous variables. An independent t-test with Welch's correction was used when the variance was not equal between groups. Results were expressed as the mean±SEM. Spearman's correlation analysis was performed to determine the correlation of Tfr cells, Tfh cells, and their respective subsets with SS clinical and immunological variables. GraphPad Prism version 5 (GraphPad Software, San Diego, CA, USA) was used to make graphs and perform statistical analysis. A heatmap image for correlation was drawn using the “corrplot” package from R software 3.5.1 (R Project, Vienna, Austria). The p values <0.05 were considered statistically significant.
RESULTS
Tfh and Tfr cells and their ratios in peripheral blood of SS patients
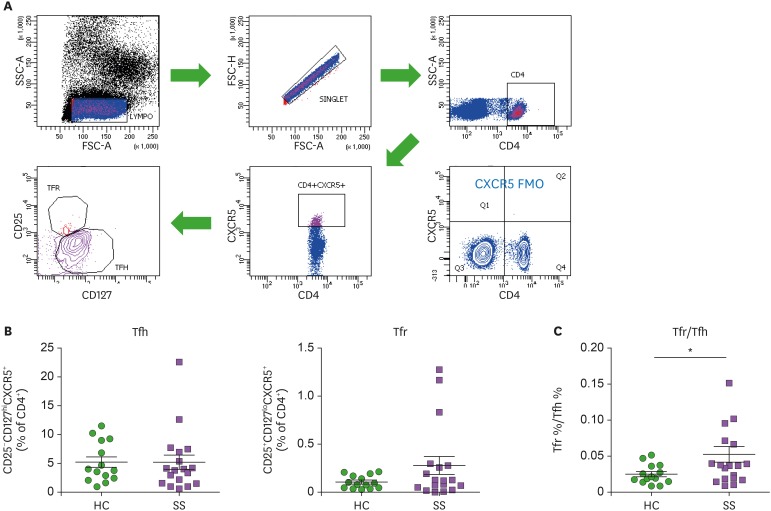
Blood CD4+CXCR5+ T cells consist of Tfh and Tfr cells, and they can be identified based on expression of surface markers, CD25 and CD127 (23). CD25+CD127loCXCR5+CD4+ Tfr and CD25-CD127hiCXCR5+CD4+ Tfh cells in peripheral blood were identified (Fig. 1A) and compared between pSS patients and HCs (Fig. 1B). Blood Tfr cells tended to increase in patients with pSS, whereas blood Tfh cells did not differ between pSS patients and HCs. Blood Tfr and Tfh cell ratios were significantly elevated in patients with pSS compared to HCs (p<0.05, Fig. 1C).
Figure 1. Circulating Tfh cells, Tfr cells, and Tfr/Tfh ratios in patients with pSS. (A) Gating strategies for circulating Tfh and Tfr cell populations representing CD25−CD127hiCXCR5+CD4+ Tfh cells and CD25+CD127loCXCR5+CD4+ Tfr cells in the peripheral blood of pSS patients (n=18) and HCs (n=14). (B) Frequencies of Tfh and Tfr cells are compared between pSS patients and HCs. (C) The Tfr/Tfh ratio is increased in pSS patients compared to HCs. Bars indicate the means±SEMs.
*p<0.05.
To examine the differentiation of B cell subsets in peripheral blood, circulating plasmablasts and plasma cells were identified from flow cytometry analysis (Supplementary Fig. 1A). Frequencies of CD38+CD138−CD27+CD19+ plasmablasts (Supplementary Fig. 1B) and CD38+CD138+CD27+CD19+ plasma cells (Supplementary Fig. 1C) were significantly increased in pSS patients compared to HCs.
PD-1 expression on Tfh and Tfr cells in peripheral blood and correlations with serum IL-21 and autoantibody levels
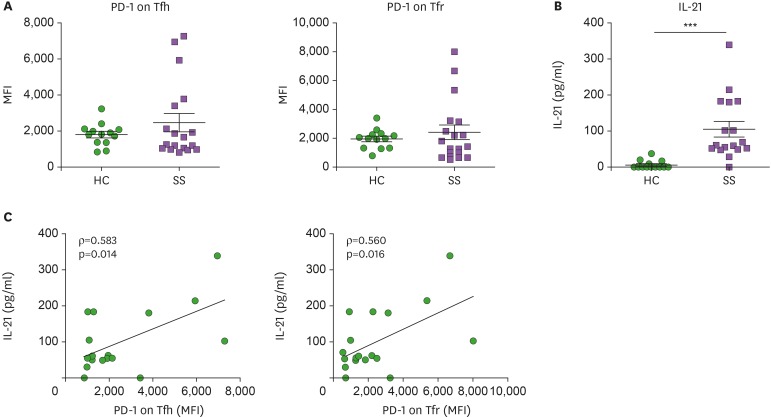
Since PD-1 and ICOS are activation markers of T cells, we examined their expression on Tfh and Tfr cells in peripheral blood of pSS patients and HCs. Mean fluorescence intensity (MFI) of PD-1 expression on blood Tfh and Tfr cells was increased in pSS patients, but was not statistically significant (Fig. 2A). Serum IL-21 levels were markedly increased in pSS patients compared to HCs (Fig. 2B). We examined whether PD-1 and ICOS expression on blood Tfh and Tfr cells may represent disease indices of SS. PD-1 expression on Tfh and Tfr cells in peripheral blood was significantly correlated with serum IL-21 levels (Fig. 2C). Although it was not significant, PD-1 expression on Tfh and Tfr cells showed positive correlations with anti-SSA/Ro and RF titers (Supplementary Fig. 2). In contrast, ICOS expression on Tfh and Tfr cells showed negative or negligible correlations with serum IL-21 levels and autoantibody titers in SS (Supplementary Fig. 3). PD-1 expression, and not ICOS expression, on Tfh and Tfr cells was positively correlated with serum IL-21 and autoantibodies in patients with pSS. Our findings suggest that PD-1 expression on circulating CXCR5+CD4+ T cells can characterize SS disease status.
Figure 2. PD-1 expression on Tfh and Tfr cells in peripheral blood is positively correlated with serum IL-21 levels in pSS. (A) PD-1 expression on circulating Tfh and Tfr cells from pSS patients (n=18) and HCs (n=14). (B) Serum IL-21 levels were increased in patients with pSS (n=17) as compared to HCs (n=14). (C) Correlation of intensity of PD-1 expression on Tfh and Tfr cells with serum IL-21 levels (n=17). Bars indicate the means±SEMs.
***p<0.001.
The CCR7loPD-1hi subset of blood Tfh cells reflects SS glandular inflammation and correlates with SS disease activity
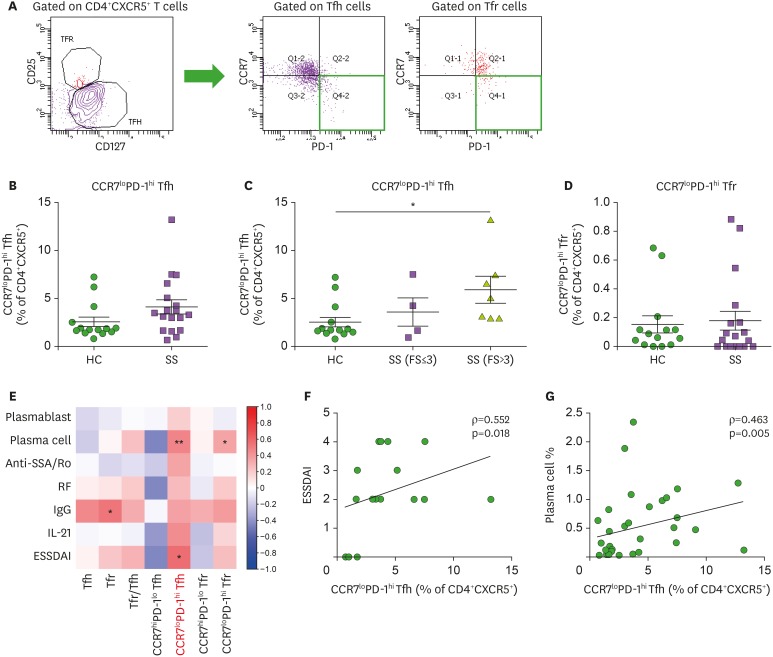
Here, we examined specific subsets of blood Tfh and Tfr cells. Previous studies have examined ICOS+PD-1+ Tfh cells in the peripheral blood of pSS patients and have found a correlation with ESSDAI (12,13). We focused on the CCR7loPD-1hi subset of blood Tfh and Tfr cells in pSS patients. Blood CXCR5+CD4+ T cells comprise 2 distinct subsets: CCR7hiPD-1lo and CCR7loPD-1hi (21). We found that the majority of blood CXCR5+CD4+ T cells express CCR7 at high levels in HCs; however, blood CXCR5+CD4+ T cells shift to populations expressing low amounts of CCR7 and high amounts of PD-1 in pSS patients (data not shown). The CCR7loPD-1hi subset of circulating Tfh cells tended to increase in pSS patients, but was not statistically significant (Fig. 3B). To determine whether these blood Tfh and Tfr cell subsets are affected by lymphocytic infiltration in salivary gland tissue, patients with pSS were divided into subgroups according to FS from the LSG biopsy. The CCR7loPD-1hi subset of blood Tfh cells significantly increased as the degree of focal lymphocytic infiltration increased in the salivary gland, suggesting an association of CCR7loPD-1hi Tfh cells with salivary gland inflammation (Fig. 3C). The CCR7loPD-1hi subset of blood Tfr cells did not show any difference between pSS patients and HCs (Fig. 3D).
Figure 3. The CCR7loPD-1hi subset of circulating Tfh cells correlates with disease activity of pSS and represents glandular inflammation of pSS. (A) Gating strategies for CCR7loPD-1hi and CCR7hiPD-1lo subsets from circulating Tfh and Tfr cells. (B) Circulating CCR7loPD-1hi Tfh cells tended to increase in pSS patients (n=18) as compared to HCs (n=14), although circulating Tfh cells showed similar frequencies between pSS patients and HCs. (C) The CCR7loPD-1hi subset of circulating Tfh cells was markedly increased in pSS patients with FS >3 versus HCs (1-way ANOVA with post-hoc Bonferroni test). (D) Frequencies of circulating CCR7loPD-1hi Tfr cells are similar between pSS patients (n=18) and HCs (n=14). (E) Correlation plot of circulating Tfh and Tfr cells and their subsets with circulating plasmablast and plasma cell populations, serum anti-SSA/Ro Ab titers, serum RF titers, serum IgG levels, serum IL-21 levels, and the ESSDAI. (F) Correlation of circulating CCR7loPD-1hi Tfh cells with ESSDAI scores (n=18). (G) Correlation of circulating CCR7loPD-1hi Tfh cells with circulating plasma cells (n=32). Bars indicate the means±SEMs.
*p<0.05; **p<0.01.
Next, we examined the correlation of blood Tfh and Tfr cells and their subsets with disease activity, blood B-cell subsets, serum autoantibody titers, serum IgG levels, and serum IL-21 levels in pSS (Fig. 3E). Notably, the CCR7loPD-1hi subset of blood Tfh cells was correlated with ESSDAI (ρ=0.552, p=0.018) (Fig. 3F). The CCR7loPD-1hi subset of blood Tfh cells showed a significant correlation with blood plasma cells (Fig. 3G). In short, the CCR7loPD-1hi subset, and not the CCR7hiPD-1lo subset, of blood Tfh cells was positively correlated with a differentiated B-cell subset in the peripheral blood as well as with SS disease activity.
Th1-like and Th17-like subsets of blood Tfr cells and hypergammaglobulinemia in SS
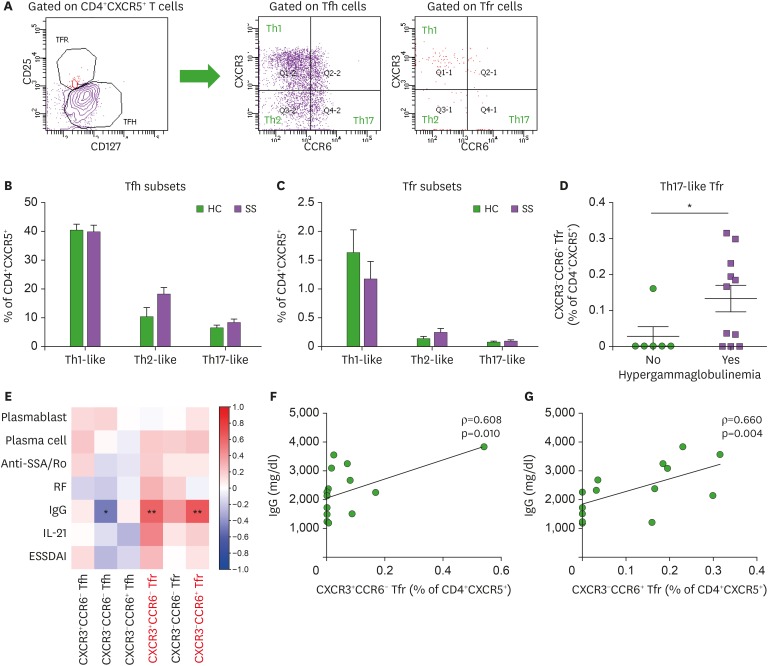
Blood CXCR5+CD4+ T cells can be divided into subsets based on CXCR3 and CCR6 expression (19). CXCR3+CCR6− (Th1-like), CXCR3−CCR6− (Th2-like), and CXCR3−CCR6+ (Th17-like) subsets were identified in blood Tfh and Tfr cells, respectively (Fig. 4A). CXCR3−CCR6− (Th2-like) and CXCR3−CCR6+ (Th17-like) subsets tended to increase in blood Tfh and Tfr cells in pSS patients compared to HCs, while the CXCR3+CCR6− (Th1-like) subset tended to decrease in blood Tfr cells from pSS patients (statistically not significant, Fig. 4B and C). Interestingly, the CXCR3−CCR6+ (Th17-like) subset of blood Tfr cells was higher in pSS patients with hypergammaglobulinemia than those without hypergammaglobulinemia (Fig. 4D).
Figure 4. Th1 and Th17 subsets of circulating Tfr cells indicate hypergammaglobulinemia in pSS. (A) Gating strategies for Th1-like, Th2-like, and Th17-like subsets of circulating Tfh and Tfr cells. (B and C) Th1-like, Th2-like, and Th17-like subsets of circulating Tfh and Tfr cells are compared between pSS patients (n=18) and HCs (n=13). Th1-like Tfh = CXCR3+CCR6− Tfh cells; Th2-like Tfh = CXCR3−CCR6− Tfh cells; Th17-like Tfh = CXCR3-CCR6+ Tfh cells; Th1-like Tfr = CXCR3+CCR6- Tfr cells; Th2-like Tfr = CXCR3-CCR6- Tfr cells; Th17-like Tfr = CXCR3-CCR6+ Tfr cells. (D) Circulating CXCR3−CCR6+ (Th17-like) Tfr cells in pSS patients with and without hypergammaglobulinemia. (E) Correlation plot of circulating Tfh and Tfr cell subsets with circulating plasmablast and plasma cell populations, serum anti-SSA/Ro Ab titers, serum RF titers, serum IgG levels, serum IL-21 levels, and the ESSDAI. (F) Correlation of circulating CXCR3+CCR6− (Th1-like) Tfr cells with serum IgG levels (n=17). (G) Correlation of circulating CXCR3-CCR6+ (Th17-like) Tfr cells with serum IgG levels (n=17). Bars indicate the means±SEMs.
*p<0.05; **p<0.01.
As shown previously in Fig. 1B, blood Tfr cells tended to increase in pSS patients. We examined the association of blood Tfr cells and their Th1, Th2, and Th17-like subsets with SS clinical and immunological variables (Fig. 4E). Blood Tfr cells, particularly CXCR3+CCR6− (Th1-like) and CXCR3−CCR6+ (Th17-like) subsets, were significantly correlated with serum IgG levels in pSS patients (Fig. 4F and G). Thus, Th1-like and Th17-like subsets of blood Tfr cells indicate hypergammaglobulinemia in pSS.
DISCUSSION
The present study explored Tfh and Tfr cells and their subsets in the peripheral blood of pSS patients, along with associations with differentiated B-cell subsets, autoantibodies, IL-21 secretion, disease activity score, and target organ inflammation. Since dysregulated B-cell response is a hallmark of pSS, finding blood markers which represent disease activity and autoantibody production could be promising. We found that the CCR7loPD-1hi subset of blood Tfh cells was associated with disease activity score (ESSDAI) and salivary gland inflammation. Furthermore, blood Tfr cells and their Th1-like and Th17-like subsets correlated with hypergammaglobulinemia in SS.
Previous studies have found that circulating Tfh-like cells (defined as CXCR5+CD4+ T cells and CD4+CXCR5+ICOS+PD-1+ T cells) were increased in patients with pSS (3,24,25). However, circulating CXCR5+CD4+ T cells are a heterogeneous population that contains both Tfh and Tfr cells. Our study defined circulating Tfh and Tfr cells as distinct populations and found increased proportions of circulating Tfr cells in pSS patients and similar proportions of circulating Tfh cells between patients and HCs. Thus, the ratio of Tfr and Tfh cells was higher in pSS patients than in HCs. This result is consistent with results from other recent studies, which have shown a blood component of Tfr cells in pSS patients (12,13,26). However, the clinical utility of Tfr/Tfh ratio as a blood biomarker in pSS is not clear. One study showed that Tfr/Tfh ratio indicated lymphocytic infiltration in salivary glands of SS, whereas another study using blood samples from larger cohort did not show a correlation between Tfr/Tfh ratio and lymphocytic sialadenitis (12,13).
Although frequencies of circulating Tfh cells did not differ between SS patients and HCs, expansion of circulating CCR7loPD-1hi Tfh cells was observed in patients with pSS. The expansion of circulating CCR7loPD-1hi Tfh cells was prominent in SS patients with increased lymphocytic infiltration in salivary glands, thus supporting their role as an indicator of glandular inflammation in SS. Interestingly, circulating CCR7loPD-1hi Tfh cells also represented SS disease activity. CCR7loPD-1hi Tfh cells are a subset of circulating CXCR5+CD4+ T cells, which are able to differentiate into mature Tfh cells and induce GC response upon antigen re-exposure (21). When cocultured with memory B cells, CCR7loPD-1hi subset, not CCR7hiPD-1lo subset or naïve CD4+ T cells, potently induced plasmablast or plasma cell differentiation and IgG production (21). The CCR7loPD-1hi subset supports rapid and efficient Ab responses in humoral immunity; however, they may play a pathogenic role in autoimmune diseases such as SLE and SS. Our study found that circulating CCR7loPD-1hi Tfh cells could be a biomarker for disease activity and target organ inflammation in SS. Our findings are supported by another study, which demonstrated that blood cell components, such as activated CD4+ and CD8+ T cells, can be used to divide SS patients into clusters with distinct disease activity and glandular inflammation (27).
The conflicting results on the correlation of PD-1 and ICOS expression with IL-21 and autoantibodies in pSS showed that PD-1 expression in blood Tfh or Tfr cells is more likely to indicate IL-21 secretion and autoantibody responses in SS. In a study using an SS animal model, CD4+ T cells that had infiltrated mouse lacrimal glands included subsets which highly express PD-1 (28). Likewise, PD-1+CD4+ T cells were abundant in salivary glands from patients with pSS, but were not expressed in the lupus kidney or normal salivary glands (28). Modulating the lymphotoxin β receptor (LTβR) signaling pathway with LTβR-Ig treatment in non-obese diabetic (NOD) mice had selectively inhibited recruitment of PD-1- naïve, but PD-1+ effector, T cells to the target organ (28). This result could explain the negative results of the baminercept (LTβR-Ig) trial in patients with pSS (29). Thus, we support targeting PD-1-positive cells as an effective treatment strategy for pSS.
Expression of PD-1 on Tfh or Tfr cells in the peripheral blood was significantly correlated with serum IL-21 concentration. IL-21 is a signature cytokine of Tfh cells, and co-expression of CXCR5 and IL-21 in salivary glands of SS patients suggest a critical role of Tfh cells in IL-21 secretion in SS (4). However, not all circulating Tfh cell subsets were correlated with increased IL-21 concentration in SS patients. In addition, there are cells other than Tfh cells that can produce IL-21. IL-21+CCR9+CD4+ T cells have been abundantly observed in the salivary glands of NOD mice and in the circulation of SS patients (30). Another study had identified a novel subset of IL-21+ Th1 cells in the lacrimal glands of NOD mice (28). Taken together, SS is characterized by an expansion of IL-21 producing PD-1hi T cells; reduction of these cells from target tissues and/or from circulation should be considered for the treatment of SS.
Blood CXCR5+CD4+ T cells have subsets divided into Th1-like, Th2-like, or Th17-like cells. Morita and colleagues found that Th2 and Th17 subsets of blood CXCR5+CD4+ T cells help B-cell differentiation and Ig production via IL-21 (19). CXCR5+CD4+ T cell subsets in the peripheral blood of juvenile dermatomyositis patients were shifted towards Th2-like and Th17-like cells (19). In the present study, Th2-like and Th17-like subsets of blood Tfh and Tfr cells tended to increase in patients with SS. Another study showed that the Th17-like subset of blood CXCR5+CD4+ T cells increased in SS patients and these cells may participate in Ab responses in SS (31). However, the proportions of Th1, Th2, and Th17 subsets were different between circulating Tfh and Tfr cells (32). Moreover, correlation of Th1, Th2, and Th17 subsets with B-cell function differed between circulating Tfh and Tfr cells in our study, suggesting a different role in indicating autoantibody responses.
We demonstrated that blood Tfr cells, particularly Th1 and Th17 subsets, were correlated with serum IgG levels and hypergammaglobulinemia in SS. Blood Tfr cells, unlike tissue Tfr cells, have limited suppressive capacity on the humoral response, with no impact on IgG and IgA production (11). Blood Tfr cells leave secondary lymphoid tissues in the immature state and circulate before fully activated into mature GC Tfr cells (11,14). Because circulating Tfr cells are increased seven days after vaccination, blood Tfr cells indicate ongoing humoral responses (11,33). Therefore, peripheral blood expansion of specific Tfr cell subsets suggests ongoing humoral responses and class-switch recombination in pSS.
In conclusion, blood components of Tfh and Tfr cells reflect disease status of pSS. The CCR7loPD-1hi subset of circulating Tfh cells displays disease activity and glandular inflammation of SS. Subsets of circulating Tfr cells represent ongoing humoral responses in SS. Our findings suggest these blood subsets could be potential pSS biomarkers.
ACKNOWLEDGEMENTS
This study was supported by a grant from the Korean Health Technology R&D Project of the Ministry of Health & Welfare, Republic of Korea (HI13C0016).
Abbreviations
- APC
allophycocyanin
- anti-SSA
anti-Sjögren's-syndrome-related antigen A
- ESSDAI
European League Against Rheumatism Sjögren's Syndrome Disease Activity Index
- FMO
fluorescence minus one
- FS
focus score
- FSC-A
forward scatter area
- FSC-H
forward scatter height
- GC
germinal center
- HC
healthy control
- ICOS
inducible costimulatory
- LSG
labial salivary gland
- LTβR
lymphotoxin β receptor
- MFI
median fluorescence intensity
- NOD
non-obese diabetic
- pSS
primary Sjögren's syndrome
- RF
rheumatoid factor
- SLE
systemic lupus erythematosus
- SS
Sjögren's syndrome
- SSC-A
side scatter area
- Tfh
follicular helper T
- Tfr
follicular regulatory T
Footnotes
Conflicts of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Kim JW, Lee J1, Lee J2, Cho ML, Park SH.
- Data curation: Kim JW.
- Formal analysis: Kim JW, Lee J1, Hong SM.
- Investigation: Kim JW, Lee J1, Hong SM.
- Methodology: Kim JW, Lee J1, Lee J2.
- Supervision: Park SH, Lee J2, Cho ML.
- Writing - original draft: Kim JW, Lee J1.
- Writing - review & editing: Kim JW, Hong SM, Lee J2, Cho ML, Park SH.
1Lee Jaeseon; 2Lee Jennifer
SUPPLEMENTARY MATERIALS
Characteristics of HC and patients with pSS
(A) Gating strategies for circulating CD38+CD138-CD27+CD19+/low plasmablasts and CD38+CD138+CD27+CD19+/low plasma cells in the peripheral blood of pSS patients (n=18) and HCs (n=14). (B and C) Plasmablasts and plasma cells are significantly increased in pSS patients compared to HCs. Bars indicate the means±SEMs.
Correlation of PD-1 expression on Tfh and Tfr cells in peripheral blood with autoantibody titers in pSS. (A and C) Correlation of intensity of PD-1 expression on Tfh and Tfr cells with serum anti-SSA/Ro titers (n=18). (B and D), Correlation of intensity of PD-1 expression on Tfh and Tfr cells with serum RF titers (n=15).
ICOS expression on Tfh and Tfr cells in the peripheral blood has negative or weak correlations with serum IL-21 levels and autoantibody titers in pSS. (A and D) Correlation of intensity of ICOS expression on Tfh and Tfr cells with serum IL-21 levels (n=17). (B and E) Correlation of intensity of ICOS expression on Tfh and Tfr cells with serum anti-SSA/Ro titers (n=18). (C and F) Correlation of intensity of ICOS expression on Tfh and Tfr cells with serum RF titers (n=14).
References
- 1.Craft JE. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol. 2012;8:337–347. doi: 10.1038/nrrheum.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwok SK, Lee J, Yu D, Kang KY, Cho ML, Kim HR, Ju JH, Lee SH, Park SH, Kim HY. A pathogenetic role for IL-21 in primary Sjögren syndrome. Nat Rev Rheumatol. 2015;11:368–374. doi: 10.1038/nrrheum.2014.225. [DOI] [PubMed] [Google Scholar]
- 3.Jin L, Yu D, Li X, Yu N, Li X, Wang Y, Wang Y. CD4+CXCR5+ follicular helper T cells in salivary gland promote B cells maturation in patients with primary Sjögren's syndrome. Int J Clin Exp Pathol. 2014;7:1988–1996. [PMC free article] [PubMed] [Google Scholar]
- 4.Kang KY, Kim HO, Kwok SK, Ju JH, Park KS, Sun DI, Jhun JY, Oh HJ, Park SH, Kim HY. Impact of interleukin-21 in the pathogenesis of primary Sjögren's syndrome: increased serum levels of interleukin-21 and its expression in the labial salivary glands. Arthritis Res Ther. 2011;13:R179. doi: 10.1186/ar3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gong YZ, Nititham J, Taylor K, Miceli-Richard C, Sordet C, Wachsmann D, Bahram S, Georgel P, Criswell LA, Sibilia J, et al. Differentiation of follicular helper T cells by salivary gland epithelial cells in primary Sjögren's syndrome. J Autoimmun. 2014;51:57–66. doi: 10.1016/j.jaut.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Szabo K, Papp G, Dezso B, Zeher M. The histopathology of labial salivary glands in primary Sjögren's syndrome: focusing on follicular helper T cells in the inflammatory infiltrates. Mediators Inflamm. 2014;2014:631787. doi: 10.1155/2014/631787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, Wang YH, Lim H, Reynolds JM, Zhou XH, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wollenberg I, Agua-Doce A, Hernández A, Almeida C, Oliveira VG, Faro J, Graca L. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J Immunol. 2011;187:4553–4560. doi: 10.4049/jimmunol.1101328. [DOI] [PubMed] [Google Scholar]
- 10.Sage PT, Sharpe AH. T follicular regulatory cells. Immunol Rev. 2016;271:246–259. doi: 10.1111/imr.12411. [DOI] [PubMed] [Google Scholar]
- 11.Fonseca VR, Agua-Doce A, Maceiras AR, Pierson W, Ribeiro F, Romão VC, Pires AR, da Silva SL, Fonseca JE, Sousa AE, et al. Human blood Tfr cells are indicators of ongoing humoral activity not fully licensed with suppressive function. Sci Immunol. 2017;2:2. doi: 10.1126/sciimmunol.aan1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonseca VR, Romão VC, Agua-Doce A, Santos M, López-Presa D, Ferreira AC, Fonseca JE, Graca L. The ratio of blood T follicular regulatory cells to T follicular helper cells marks ectopic lymphoid structure formation while activated follicular helper T cells indicate disease activity in primary Sjögren's syndrome. Arthritis Rheumatol. 2018;70:774–784. doi: 10.1002/art.40424. [DOI] [PubMed] [Google Scholar]
- 13.Verstappen GM, Nakshbandi U, Mossel E, Haacke EA, van der Vegt B, Vissink A, Bootsma H, Kroese FG. Is the t follicular regulatory: follicular helper t cell ratio in blood a biomarker for ectopic lymphoid structure formation in Sjögren's syndrome? Comment on the article by Fonseca et al. Arthritis Rheumatol. 2018;70:1354–1355. doi: 10.1002/art.40488. [DOI] [PubMed] [Google Scholar]
- 14.Sage PT, Alvarez D, Godec J, von Andrian UH, Sharpe AH. Circulating T follicular regulatory and helper cells have memory-like properties. J Clin Invest. 2014;124:5191–5204. doi: 10.1172/JCI76861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shan Y, Qi C, Zhao J, Liu Y, Gao H, Zhao D, Ding F, Wang J, Jiang Y. Higher frequency of peripheral blood follicular regulatory T cells in patients with new onset ankylosing spondylitis. Clin Exp Pharmacol Physiol. 2015;42:154–161. doi: 10.1111/1440-1681.12330. [DOI] [PubMed] [Google Scholar]
- 16.Wen Y, Yang B, Lu J, Zhang J, Yang H, Li J. Imbalance of circulating CD4+CXCR5+FOXP3+ Tfr-like cells and CD4+CXCR5+FOXP3− Tfh-like cells in myasthenia gravis. Neurosci Lett. 2016;630:176–182. doi: 10.1016/j.neulet.2016.07.049. [DOI] [PubMed] [Google Scholar]
- 17.Xu B, Wang S, Zhou M, Huang Y, Fu R, Guo C, Chen J, Zhao J, Gaskin F, Fu SM, et al. The ratio of circulating follicular T helper cell to follicular T regulatory cell is correlated with disease activity in systemic lupus erythematosus. Clin Immunol. 2017;183:46–53. doi: 10.1016/j.clim.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu C, Wang D, Song Y, Lu S, Zhao J, Wang H. Increased circulating CD4+CXCR5+FoxP3+ follicular regulatory T cells correlated with severity of systemic lupus erythematosus patients. Int Immunopharmacol. 2018;56:261–268. doi: 10.1016/j.intimp.2018.01.038. [DOI] [PubMed] [Google Scholar]
- 19.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, et al. Human blood CXCR5+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, Su LF, Cubas R, Davis MM, Sette A, et al. Human circulating PD-1+CXCR3−CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39:758–769. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He J, Tsai LM, Leong YA, Hu X, Ma CS, Chevalier N, Sun X, Vandenberg K, Rockman S, Ding Y, et al. Circulating precursor CCR7loPD-1hi CXCR5+ CD4+ T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. 2013;39:770–781. doi: 10.1016/j.immuni.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, Rasmussen A, Scofield H, Vitali C, Bowman SJ, et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification criteria for primary Sjögren's syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. 2017;69:35–45. doi: 10.1002/art.39859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei Y, Feng J, Hou Z, Wang XM, Yu D. Flow cytometric analysis of circulating follicular helper T (Tfh) and follicular regulatory T (Tfr) populations in human blood. Methods Mol Biol. 2015;1291:199–207. doi: 10.1007/978-1-4939-2498-1_17. [DOI] [PubMed] [Google Scholar]
- 24.Szabó K, Papp G, Szántó A, Tarr T, Zeher M. A comprehensive investigation on the distribution of circulating follicular T helper cells and B cell subsets in primary Sjögren's syndrome and systemic lupus erythematosus. Clin Exp Immunol. 2016;183:76–89. doi: 10.1111/cei.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brokstad KA, Fredriksen M, Zhou F, Bergum B, Brun JG, Cox RJ, Skarstein K. T follicular-like helper cells in the peripheral blood of patients with primary Sjögren's syndrome. Scand J Immunol. 2018;88:e12679. doi: 10.1111/sji.12679. [DOI] [PubMed] [Google Scholar]
- 26.Ivanchenko M, Aqrawi LA, Björk A, Wahren-Herlenius M, Chemin K. FoxP3+ CXCR5+ CD4+ T cell frequencies are increased in peripheral blood of patients with primary Sjögren's syndrome. Clin Exp Immunol. 2019;195:305–309. doi: 10.1111/cei.13244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mingueneau M, Boudaoud S, Haskett S, Reynolds TL, Nocturne G, Norton E, Zhang X, Constant M, Park D, Wang W, et al. Cytometry by time-of-flight immunophenotyping identifies a blood Sjögren's signature correlating with disease activity and glandular inflammation. J Allergy Clin Immunol. 2016;137:1809–1821.e12. doi: 10.1016/j.jaci.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 28.Haskett S, Ding J, Zhang W, Thai A, Cullen P, Xu S, Petersen B, Kuznetsov G, Jandreski L, Hamann S, et al. Identification of novel CD4+ T cell subsets in the target tissue of Sjögren's syndrome and their differential regulation by the lymphotoxin/LIGHT signaling axis. J Immunol. 2016;197:3806–3819. doi: 10.4049/jimmunol.1600407. [DOI] [PubMed] [Google Scholar]
- 29.St Clair EW, Baer AN, Wei C, Noaiseh G, Parke A, Coca A, Utset TO, Genovese MC, Wallace DJ, McNamara J, et al. Clinical efficacy and safety of baminercept, a lymphotoxin β receptor fusion protein, in primary Sjögren's syndrome: results from a phase II randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 2018;70:1470–1480. doi: 10.1002/art.40513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGuire HM, Vogelzang A, Ma CS, Hughes WE, Silveira PA, Tangye SG, Christ D, Fulcher D, Falcone M, King C. A subset of interleukin-21+ chemokine receptor CCR9+ T helper cells target accessory organs of the digestive system in autoimmunity. Immunity. 2011;34:602–615. doi: 10.1016/j.immuni.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 31.Li XY, Wu ZB, Ding J, Zheng ZH, Li XY, Chen LN, Zhu P. Role of the frequency of blood CD4+ CXCR5+ CCR6+ T cells in autoimmunity in patients with Sjögren's syndrome. Biochem Biophys Res Commun. 2012;422:238–244. doi: 10.1016/j.bbrc.2012.04.133. [DOI] [PubMed] [Google Scholar]
- 32.Fonseca VR, Graca L. Contribution of FoxP3+ Tfr cells to overall human blood CXCR5+ T cells. Clin Exp Immunol. 2019;195:302–304. doi: 10.1111/cei.13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhaeze T, Peelen E, Hombrouck A, Peeters L, Van Wijmeersch B, Lemkens N, Lemkens P, Somers V, Lucas S, Broux B, et al. Circulating follicular regulatory T cells are defective in multiple sclerosis. J Immunol. 2015;195:832–840. doi: 10.4049/jimmunol.1500759. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of HC and patients with pSS
(A) Gating strategies for circulating CD38+CD138-CD27+CD19+/low plasmablasts and CD38+CD138+CD27+CD19+/low plasma cells in the peripheral blood of pSS patients (n=18) and HCs (n=14). (B and C) Plasmablasts and plasma cells are significantly increased in pSS patients compared to HCs. Bars indicate the means±SEMs.
Correlation of PD-1 expression on Tfh and Tfr cells in peripheral blood with autoantibody titers in pSS. (A and C) Correlation of intensity of PD-1 expression on Tfh and Tfr cells with serum anti-SSA/Ro titers (n=18). (B and D), Correlation of intensity of PD-1 expression on Tfh and Tfr cells with serum RF titers (n=15).
ICOS expression on Tfh and Tfr cells in the peripheral blood has negative or weak correlations with serum IL-21 levels and autoantibody titers in pSS. (A and D) Correlation of intensity of ICOS expression on Tfh and Tfr cells with serum IL-21 levels (n=17). (B and E) Correlation of intensity of ICOS expression on Tfh and Tfr cells with serum anti-SSA/Ro titers (n=18). (C and F) Correlation of intensity of ICOS expression on Tfh and Tfr cells with serum RF titers (n=14).