Abstract
The purpose of this study was to determine the regulatory role of intravenous Ig (IVIg) in Th17 cytokine–induced RANK ligand (RANKL) expression and osteoclast (OC) differentiation from OC precursors (pre-OC). Human CD14+ monocytes were isolated and stimulated by Th17 cytokines (IL-17, IL-21, and IL-22) and RANKL expression was investigated using a real-time PCR. CD14+ monocytes were incubated with RANKL, Th17 cytokines, and M-CSF, with/without IVIg, and OC differentiation was determined by counting tartrate-resistant acid phosphatase-positive multinucleated cells. OC differentiation was investigated after monocytes were cocultured with Th17 cells in the presence of IVIg. Th17 cell differentiation was determined using enzyme-linked immunosorbent assay and flow cytometry after CD4+ T cells were cultured with IVIg under Th17 condition. Th17 cytokines stimulated monocytes to express RANKL and IVIg suppressed the Th17 cytokine-induced RANKL expression. OCs were differentiated when pre-OC were cocultured with RANKL or Th17 cytokines and IVIg reduced the osteoclastogenesis. IVIg also decreased osteoclastogenesis when pre-OC were cocultured with Th17 cells. IVIg decreased both Th17 and Th1 cell differentiation while it did not affect Treg cell differentiation. In summary, IVIg inhibited Th17 cytokine-induced RANKL expression and OC differentiation. IVIg reduced osteoclastogenesis when monocytes were cocultured with Th17 cells. IVIg also reduced Th17 polarization. IVIg could be a new therapeutic option for Th17 cell–mediated osteoclastogenesis.
Keywords: IVIg, Osteoclastogenesis, RANK ligand, IL-17, Th17 cells
INTRODUCTION
Many pathological bone diseases are characterized by progressive and excessive bone resorption (1). Therefore, identification of agents to block osteoclast (OC) differentiation and resorption is a common and successful strategy for the development of therapeutic drugs for treatment of OC-related diseases (2).
OCs are specialized bone-resorbing cells regulated by M-CSF and RANK ligand (RANKL) (3).
Th17 cells induce rheumatoid arthritis (RA) on OC activation in published articles by Pöllinger et al. (4) They reported that Th17 cells drive arthritic bone destruction in mice and humans. In our previous studies, Th17 cytokines promoted RANKL production and directly stimulated OC differentiation, causing bone destructive process in RA (5,6).
Intravenous Ig (IVIg) is a biologic agent with immune modulatory, anti-inflammatory, and immune supplementary effects. It is used as a first-line treatment in patients with Kawasaki disease, dermatomyositis, and immune-mediated thrombocytopenia. IVIg also has extensive off-label indications for various autoimmune diseases (7). In Kawasaki disease, IVIg controls the imbalance of Th17/Treg cells through the downregulation of the number of Th17 cells, and the production of IL-17/Treg cells (8). Th17 induces RA on OC activation. Therefore, there can be potential linkage between IVIg and osteoclastogenesis.
CD4+ T cells, mononuclear phagocytes, fibroblasts, OCs, and neutrophils play major cellular roles in the immunopathology of RA, as does the abnormal production of numerous cytokines, chemokines, and other inflammatory mediators. The autoantibodies contribute directly to the pathogenesis of synovial inflammation and joint destruction via the activation of the complement and Fcγ receptor pathways (9).
Previous research has demonstrated that IVIg suppresses the differentiation and amplification of Th17 cells, as well as the secretion of their effector molecules IL-17, IL-21, IL-22, and CCL20 (10,11). These effects of IVIg are associated with inhibition of RAR Related Orphan Receptor C, phosphorylation of STAT3, and reciprocal regulation of Tregs. Since Th17 cells are implicated in the pathogenesis of various diseases where IVIg is also beneficial, our results indicate that inhibition of Th17 cytokine (IL-17, IL-21, and IL-22)–induced osteoclastogenesis represents one of the major mechanisms of action of IVIg. In a previous study, IVIg attenuates tumor necrosis factor-induced bone resorption and it suppressed osteoclastogenesis by induction of A20 expression (12).
In this study, we determined the regulatory effect of IVIg in the Th17 cytokine-induced RANKL expression and OC differentiation, and the inhibitory effect of IVIg in the Th17 differentiation. This study provided important insights into the potential applications of IVIg as a new therapeutic option for preventing bony destructive diseases, such as RA.
MATERIALS AND METHODS
Peripheral blood of healthy donors
Informed consent was obtained from healthy donors, and the experimental protocol was approved by Institutional Review Board (IRB) for Human Research, Konkuk University Medical Center, Seoul, Korea (IRB No. KUH1010186).
Reagents
IL-17, IL-21, IL-22, RANKL, M-CSF, anti-human IL-17, and anti-IFN-γ were obtained from R&D Systems (Minneapolis, MN, USA). IVIg was obtained from IVIg-SN (Green Cross Corp., Yongin, Korea).
Expression of RANKL mRNA by real-time PCR
Peripheral blood monocytes were stimulated with Th17 cytokines. Monocytes were stimulated with or without IVIg for 3 h before treatment of Th17 cytokines. After 72 h, mRNA was measured using real-time PCR as before (13).
Cell isolation from blood
PBMCs were collected from healthy blood by density gradient separation. The monocytes (OC precursors) were prepared from PBMC.
Th17-differentiation conditions
To create Th17-differentiation conditions, PBMCs were cultured for 48 h as before (14,15). To examine the immunosuppressive effects of IVIg, PBMCs were pre-incubated for 1 h with IVIg and then stimulated as described above.
Flow cytometric protocol
Cells were stained to detect with mAbs to CD4, CD25, IL-17, IFN-γ, and Foxp3 (eBioscience, San Diego, CA, USA). Cells were analyzed on a FlowJo software (Tree Star, Ashland, OR, USA).
ELISA
IL-17 and IFN-γ levels in the culture supernatants from PBMCs were measured using sandwich ELISA according to the manufacturer's instructions.
OC formation
Monocytes were pretreated with IVIg for 1 h, after which monocytes were added to each well along with Th17 cytokines. RANKL was used as the positive control. For coculture experiments with CD4+ T cells, human monocytes were seeded in 48-well plates at 5×104 cells/well with 1 ml of medium, and pre-incubated for 1 h with IVIg (0.1, 1, 10, and 20 mg/ml). On day 21, tartrate-resistant acid phosphatase (TRAP)-positive cells were identified as before (13).
Western blot analysis in CD4+ T cells
CD4+ T cells were isolated from the PBMCs as described earlier (16). For the effect of IVIg, CD4+ T cells were pretreated with or without IVIg (10 mg/ml) and cocultured with Th17-differentiation conditions for 1 h. The first Abs to phosphorylate mTOR, AMP-activated protein kinase (AMPK), protein kinase B (Akt), ribosomal protein S6 kinase (S6K), STAT3 (727), and STAT3 (705) (Cell Signaling Technology Inc., Danvers, MA, USA) were diluted in buffers (13).
Statistical analysis
The data are expressed as means±SEM. Statistical analysis was performed using the 1 way ANOVA and Bonferroni's multiple comparison test. In all analyses, p<0.05 indicated statistical significance.
RESULTS
IVIg reduced Th17 cytokine–induced RANKL expression in CD14+ monocytes
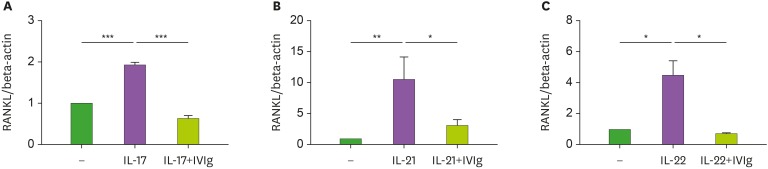
To investigate the suppressive effect of IVIg in Th17 cytokine-induced RANKL expression, CD14+ monocytes were incubated with IVIg (10 mg/ml) for 1 h. The CD14+ monocytes were then cultured with Th17 cytokines, such as IL-17, IL-21, and IL-22 for 72 h. The Th17 cytokines increased the expression of RANKL mRNA. IVIg reduced the Th17 cytokines–induced expression of RANKL mRNA (Fig. 1).
Figure 1. The suppressive effect of IVIg on RANKL mRNA level in peripheral blood monocytes. CD14+ monocytes were pretreated with IVIg and then cultured with 20 ng/ml IL-17 (A), IL-21 (B), and IL-22 (C) for 72 h. RANKL mRNA level was quantified by real-time PCR.
*p<0.05; **p<0.01; ***p<0.001.
The inhibitory effect of IVIg in Th17 cytokine–induced OC differentiation
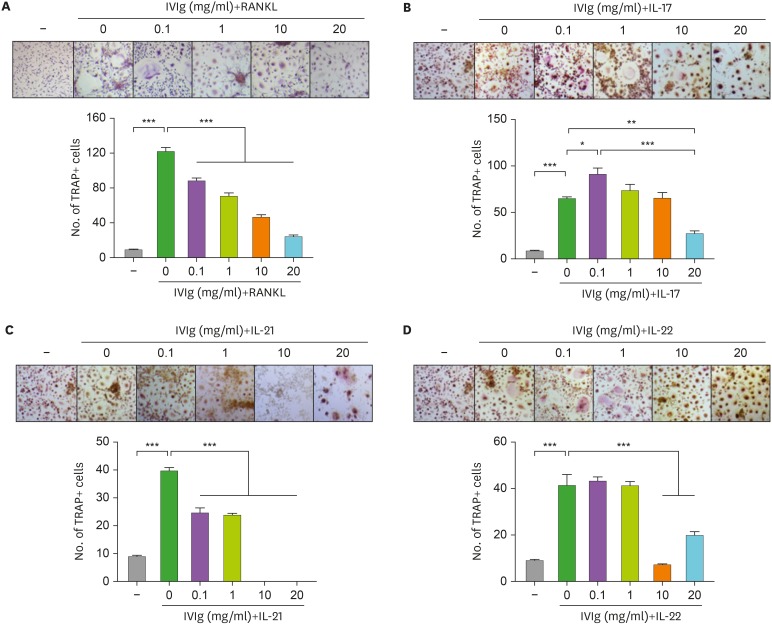
The isolated CD14+ monocytes were cultured with RANKL and M-CSF. After 21 days of culture, OCs were differentiated from their precursors. However, pre-incubation with IVIg significantly reduced the RANKL-induced osteoclastogenesis (Fig. 2A). To evaluate the effects of IVIg on Th17 cytokine–induced OC differentiation, isolated CD14+ monocytes were cultured with IL-17, IL-21, IL-22, and M-CSF in the absence of RANKL. After 21 days of culture, OCs were differentiated in the Th17 cytokines and M-CSF culture system. However, IVIg significantly decreased the Th17 cytokine-induced osteoclastogenesis (Fig. 2B-D).
Figure 2. The effect of IVIg in OC differentiation from peripheral blood monocytes. CD14+ monocytes were pretreated with IVIg for 1 h, and then cultured with 25 ng/ml of M-CSF, and (A) 10 ng/ml of RANKL, and 20 ng/ml of (B) IL-17, (C) IL-21, or (D) IL-22.
*p<0.05; **p<0.01; ***p<0.001.
The regulatory effect of IVIg in Th17 cell–induced OC differentiation
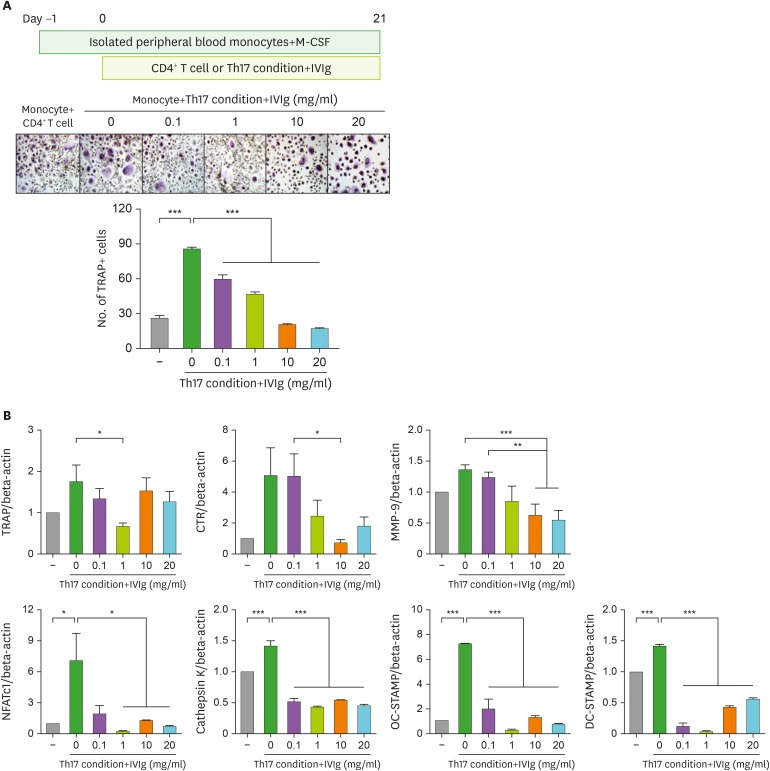
When CD14+ monocytes were cultured with Th17 cells in the presence of M-CSF, OCs were differentiated and IVIg decreased the OC differentiation induced by Th17 cells in a dose-dependent manner (Fig. 3A). The gene expression of OC markers, such as TRAP, calcitonin receptor, matrix metallopeptidase 9, cathepsin K, NFATc1, OC-stimulatory transmembrane protein (STAMP) and dendritic cell (DC)-STAMP was increased with Th17 cell addition but their gene expression was reduced by IVIg treatment (Fig. 3B).
Figure 3. The effect of IVIg in OC differentiation by Th17 condition prestimulated CD4+ T cells. CD14+ monocytes were pretreated. (A) CD4+ T cells were pretreated with IVIg for 1 h, then cultured with Th17 condition for 72 h and then cocultured with CD14+ monocytes in the presence of M-CSF. (B) The gene expression of TRAP, calcitonin receptor, MMP-9, NFATc1, cathepsin K, OC-STAMP, and DC-STAMP from differentiated OCs was measured by real-time PCR. Data were normalized to beta-actin and reported in relative expression units.
DC, dendritic cell.
*p<0.05; **p<0.01; ***p<0.001.
Suppressive effect of IVIg on Th17 cell polarizing
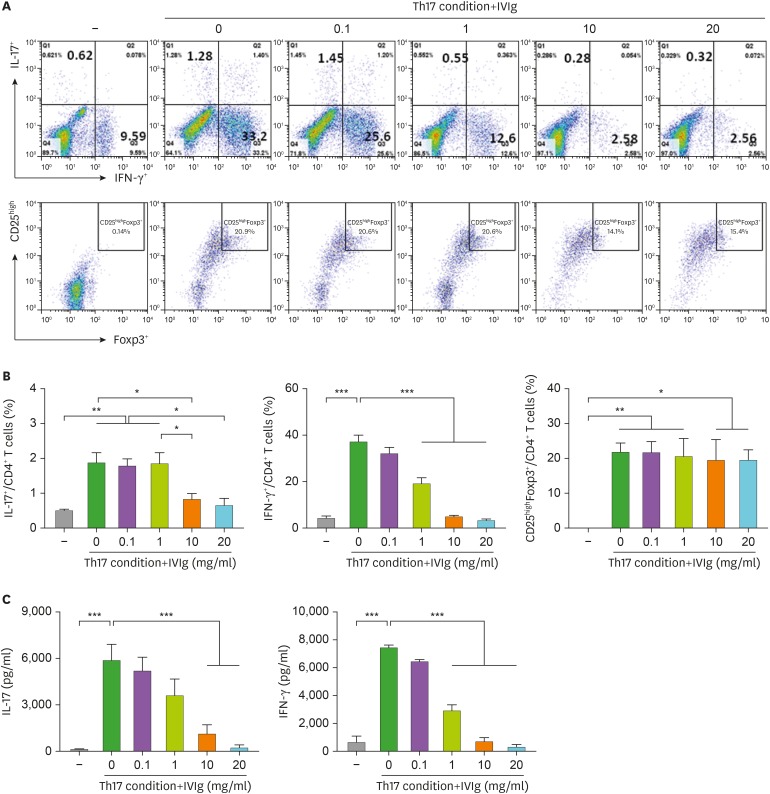
The proportions of Th17 (CD4+ IL-17+), Th1 (CD4+ IFN-γ+), and Treg (CD25high Foxp3+) cells out of the total CD4+ T cell population are presented in Fig. 4A. Both Th17 cells and Th1 cells were differentiated in Th17 condition. IVIg inhibited the differentiation of IL-17 and IFN-γ expressing cells; however, IVIg did not affect the differentiation of Foxp3 expressing Treg cells (Fig. 4B). IVIg also reduced the production of IL-17 and IFN-γ in the culture medium (Fig. 4C).
Figure 4. Effect of IVIg on CD4+ T cells isolated from the PBMCs of healthy donors and cultured under Th17-polarizing conditions. Human PBMCs (n=6) were isolated from healthy subjects and pre-incubated with IVIg (0.1, 1, 10, and 20 mg/ml) for 1 h, and then cultured under Th17-polarizing conditions for 72 h. (A) CD4+ T cells were gated for further analysis. Next, the percentage of (B) IL-17+/CD4+ T cells, IFN-γ+/CD4+ T cells, and CD25highfoxp3+/CD4+ T cells was measured by flow cytometry. The production of (C) IL-17 and IFN-γ by Th17-polarizing CD4+ T cells and secretion into the culture supernatant by ELISA.
*p<0.05; **p<0.01; ***p<0.001.
The signaling molecules involved in the regulation of IVIg in Th17 polarization
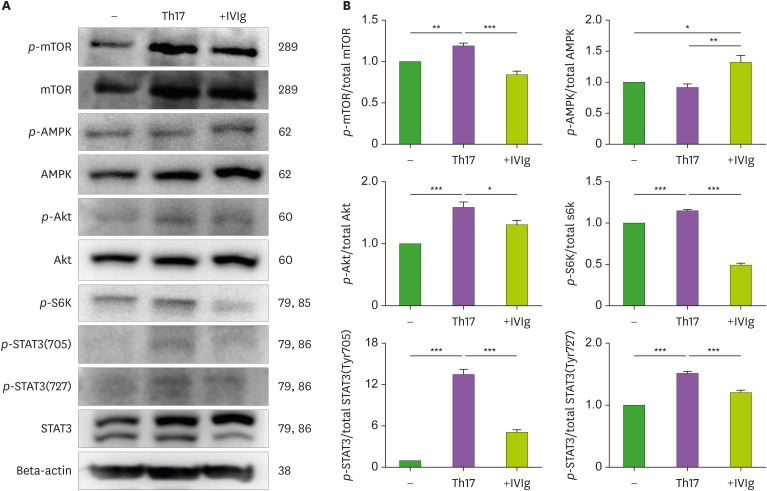
To investigate the signaling involved in the regulation of IVIg in Th17 cell differentiation, CD4+ T cells were incubated with Th17 condition and IVIg. As shown in Fig. 5, the phosphorylation of mTOR, Akt, and S6K increased significantly under Th17 conditions. IVIg significantly inhibited the level of phosphorylated mTOR, its downstream protein pS6K, and Akt, compared with Th17 condition (p-value <0.05 for each).
Figure 5. Effects of IVIg on the expression of mTOR and AMPK proteins on CD4+ T cells isolated from the PBMCs of healthy donors and cultured under Th17-polarizing conditions. (A) Immunoblotting of p-mTOR, mTOR, p-AMPK, AMPK, p-Akt, Akt, p-S6K, p-STAT3(727), p-STAT3(705), and STAT3 in the CD4+ T cells pretreated with IVIg (10 mg/ml) and then cultured under Th17-differentiation conditions for 1 h. (B) Stimulation of CD4+ T cells under Th17-differentiation conditions, activated phosphorylation of mTOR, Akt, S6K, STAT3 (705), and STAT3 (727) as detected by Western blotting and shown by the ratio of phosphorylated proteins to total proteins.
*p<0.05; **p<0.01; ***p<0.001.
DISCUSSION
In this study, we found that IVIg reduced Th17 cytokine–induced RANKL expression and protein production in CD14+ monocytes. In a previous study that we recently conducted, we found that other Th17 cytokines, such as IL-21 and IL-22, induce RANKL expression in RA synovial fibroblasts (6). RANKL and M-CSF are important cytokines in OC formation from the precursor cells (17). This result suggests that IVIg indirectly interrupts the initial process of the Th17 cytokine-associated bony destructive pathways through the inhibition of RANKL production.
In addition to the indirect effect of IVIg on osteoclastogenesis, we determined the direct effect of IVIg in OC formation from their precursors. IVIg directly inhibited the RANKL and Th17-cytokine induced OC differentiation in a dose-dependent manner. This result suggests that IVIg can directly ameliorate the cytokine-induced osteoclastogenesis and that there is a possibility for using it therapeutically.
Next, we determined whether IVIg modulates Th17 cells in direct stimulation of OC differentiation. When monocytes were cocultured with Th17 condition, OC differentiation was increased compared to when they were cocultured with CD4+ T cells. Without RANKL, Th17 cells directly induced OC differentiation and IVIg inhibited the Th17 cell induced osteoclastogenesis. The final goal of treatment in patients with RA is the prevention of joint destruction. Bone erosion caused by activated OCs induces joint destruction and patients’ disability. These results suggest that IVIg can prevent bony destructive processes in RA.
To determine the relationship between IVIg and Th17, we investigated the regulatory role of IVIg in Th17 cell differentiation. IVIg downregulated the number of Th17 cells and IL-17 production, without affecting the number of Treg cells. IVIg reduced osteoclastogenesis via 3 mechanisms. First, IVIg reduced Th17 cytokine–induced RANKL expression in monocytes. Second, IVIg directly inhibited Th17 cytokine–induced OC differentiation from peripheral blood-derived monocytes. Third, IVIg downregulated Th17 cell differentiation. In RA, the clinical efficacy and safety of anti-IL-17 monoclonal Abs, such as secukinumab and ixekizumab, have been under clinical investigation (18). Although dosage does not show the effectiveness of IVIg in clinical trials of low-dose IVIg among patients with RA, the combination therapy of IVIg and IL-17 inhibitors could be a new therapeutic option in patients with severe RA (19).
The previous study and our study share similar results which suggest “IVIg controls the imbalance of Th17/Treg polarization.” However, we did not find IVIg promotes Treg polarization. There are several differences between the 2 studies: 1) We used human peripheral CD4+ T cells, however, they used mouse splenocytes; 2) We used T cells of healthy donors, however they used splenocytes of diseased mouse “collagen-induced arthritis (CIA)”; and 3) Splenocytes from CIA is already exposed by immunologic stimulation, so the focus of immune regulation is both decreasing Th17 response and increasing regulatory system. However, in healthy condition, the focus of immune regulation could be only prevention of abnormal immune response such as Th17 polarization. It does not need to perform regulatory process (20).
Next, we investigated the molecular signaling pathway involved in the suppressive effects of IVIg with CD4+ T cell. We focused on the mTOR/Akt/S6K pathway, which has been shown to have an essential role in the development and differentiation of Th17 cells in many previous studies, as well as in a previous report that we published (20,21). We also investigated the activity of Akt and p-S6K, which are located either upstream or downstream of the mTOR/STAT3 pathway, and are significantly associated with mTOR activity (22,23). This result showed that the enhanced suppressive effect of IVIg was mediated by an effective regulation of mTOR/Akt/S6K signaling. PI3K/mTOR signaling has been shown to promote effector T-cell activation and differentiation, and our group and others have demonstrated the reorganization of this pathway during the differentiation of naive T-cells into functional suppressor Tregs (24,25). Indeed, T-cell specific loss of mTOR correlates with a diminished generation of effector Th1, Th2, and Th17 cells, and enhanced generation of Tregs. Tregs play a major role in the prevention of autoimmunity by suppressing T-cell responses to self-Ags and by limiting the response to foreign Ags.
Additionally, IVIg activates AMPK, which functions to counteract the mTOR signal. In RA, inhibition of mTOR complex 1 (mTORC1) alters the ability of IL-17 to induce mTORC1-dependent proliferation of RA synovial fibroblasts (26).
In conclusion, we found that IVIg significantly suppressed Th17 cytokine–induced RANKL mRNA level and osteoclastogenesis and attenuated Th17 differentiation. Our findings provide important insights into the potential applications of IVIg as a new therapeutic option for preventing OC-associated bony destructive diseases, such as RA.
ACKNOWLEDGEMENTS
This work was supported by a grant of the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, Republic of Korea (NRF-2017R1A2B4006015, NRF-2015R1D1A1A01056763, and NRF-2015R1D1A1A09058510).
Abbreviations
- Akt
protein kinase B
- AMPK
AMP-activated protein kinase
- CIA
collagen-induced arthritis
- DC
dendritic cell
- IVIg
intravenous Ig
- MMP-9
matrix metallopeptidase 9
- mTORC1
mTOR complex 1
- OC
osteoclast
- Pre-OC
osteoclast precursors
- RA
rheumatoid arthritis
- RANKL
RANK ligand
- S6K
ribosomal protein S6 kinase
- STAMP
stimulatory transmembrane protein
- TRAP
tartrate-resistant acid phosphatase
Footnotes
Conflicts of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Lee SH.
- Data curation: Kim KW, Kim HR.
- Formal analysis: Kim KW, Kim HR.
- Funding acquisition: Kim KW, Kim HR, Lee SH.
- Investigation: Kim KW, Kim HR, Kim BM, Won JY, Lee KA.
- Methodology: Kim KW, Kim HR, Lee SH.
- Resources: Kim BM, Won JY, Lee KA.
- Validation: Kim BM, Won JY, Lee KA.
- Visualization: Kim KW, Kim HR.
- Writing - original draft: Kim KW, Kim HR, Lee SH.
- Writing - review & editing: Kim KW, Kim HR, Kim BM, Won JY, Lee KA, Lee SH.
References
- 1.Lee JH, Jin H, Shim HE, Kim HN, Ha H, Lee ZH. Epigallocatechin-3-gallate inhibits osteoclastogenesis by down-regulating c-Fos expression and suppressing the nuclear factor-kappaB signal. Mol Pharmacol. 2010;77:17–25. doi: 10.1124/mol.109.057877. [DOI] [PubMed] [Google Scholar]
- 2.Qi B, Cong Q, Li P, Ma G, Guo X, Yeh J, Xie M, Schneider MD, Liu H, Li B. Ablation of Tak1 in osteoclast progenitor leads to defects in skeletal growth and bone remodeling in mice. Sci Rep. 2014;4:7158. doi: 10.1038/srep07158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gravallese EM. Bone destruction in arthritis. Ann Rheum Dis. 2002;61(Suppl 2):ii84–ii86. doi: 10.1136/ard.61.suppl_2.ii84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pöllinger B, Junt T, Metzler B, Walker UA, Tyndall A, Allard C, Bay S, Keller R, Raulf F, Di Padova F, et al. Th17 cells, not IL-17+ γδ T cells, drive arthritic bone destruction in mice and humans. J Immunol. 2011;186:2602–2612. doi: 10.4049/jimmunol.1003370. [DOI] [PubMed] [Google Scholar]
- 5.Kim KW, Kim HR, Park JY, Park JS, Oh HJ, Woo YJ, Park MK, Cho ML, Lee SH. Interleukin-22 promotes osteoclastogenesis in rheumatoid arthritis through induction of RANKL in human synovial fibroblasts. Arthritis Rheum. 2012;64:1015–1023. doi: 10.1002/art.33446. [DOI] [PubMed] [Google Scholar]
- 6.Kim KW, Kim HR, Kim BM, Cho ML, Lee SH. Th17 cytokines regulate osteoclastogenesis in rheumatoid arthritis. Am J Pathol. 2015;185:3011–3024. doi: 10.1016/j.ajpath.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Bayry J, Negi VS, Kaveri SV. Intravenous immunoglobulin therapy in rheumatic diseases. Nat Rev Rheumatol. 2011;7:349–359. doi: 10.1038/nrrheum.2011.61. [DOI] [PubMed] [Google Scholar]
- 8.Jia S, Li C, Wang G, Yang J, Zu Y. The T helper type 17/regulatory T cell imbalance in patients with acute Kawasaki disease. Clin Exp Immunol. 2010;162:131–137. doi: 10.1111/j.1365-2249.2010.04236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benoist C, Mathis D. A revival of the B cell paradigm for rheumatoid arthritis pathogenesis? Arthritis Res. 2000;2:90–94. doi: 10.1186/ar73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maddur MS, Vani J, Hegde P, Lacroix-Desmazes S, Kaveri SV, Bayry J. Inhibition of differentiation, amplification, and function of human TH17 cells by intravenous immunoglobulin. J Allergy Clin Immunol. 2011;127:823–830.e1-e7. doi: 10.1016/j.jaci.2010.12.1102. [DOI] [PubMed] [Google Scholar]
- 11.Maddur MS, Kaveri SV, Bayry J. Comparison of different IVIg preparations on IL-17 production by human Th17 cells. Autoimmun Rev. 2011;10:809–810. doi: 10.1016/j.autrev.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Lee MJ, Lim E, Mun S, Bae S, Murata K, Ivashkiv LB, Park-Min KH. Intravenous immunoglobulin (IVIG) attenuates TNF-induced pathologic bone resorption and suppresses osteoclastogenesis by inducing A20 expression. J Cell Physiol. 2016;231:449–458. doi: 10.1002/jcp.25091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HR, Kim KW, Kim BM, Lee KA, Lee SH. N-acetyl-l-cysteine controls osteoclastogenesis through regulating Th17 differentiation and RANKL production in rheumatoid arthritis. Korean J Intern Med. 2019;34:458. doi: 10.3904/kjim.2016.329.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganjalikhani Hakemi M, Ghaedi K, Andalib A, Hosseini M, Rezaei A. Optimization of human Th17 cell differentiation in vitro: evaluating different polarizing factors. In Vitro Cell Dev Biol Anim. 2011;47:581–592. doi: 10.1007/s11626-011-9444-1. [DOI] [PubMed] [Google Scholar]
- 15.Chung BH, Kim BM, Doh KC, Min JW, Cho ML, Kim KW, Yang CW. Suppressive effect of 1α,25-dihydroxyvitamin D3 on Th17-immune responses in kidney transplant recipients with tacrolimus-based immunosuppression. Transplantation. 2017;101:1711–1719. doi: 10.1097/TP.0000000000001516. [DOI] [PubMed] [Google Scholar]
- 16.Han SH, Hur MS, Kim MJ, Kim BM, Kim KW, Kim HR, Choe YB, Ahn KJ, Lee YW. Preliminary study of histamine H4 receptor expressed on human CD4+ T cells and its immunomodulatory potency in the IL-17 pathway of psoriasis. J Dermatol Sci. 2017;88:29–35. doi: 10.1016/j.jdermsci.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 18.Koenders MI, van den Berg WB. Secukinumab for rheumatology: development and its potential place in therapy. Drug Des Devel Ther. 2016;10:2069–2080. doi: 10.2147/DDDT.S105263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz-Agranov N, Khattri S, Zandman-Goddard G. The role of intravenous immunoglobulins in the treatment of rheumatoid arthritis. Autoimmun Rev. 2015;14:651–658. doi: 10.1016/j.autrev.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Lee SY, Jung YO, Ryu JG, Kang CM, Kim EK, Son HJ, Yang EJ, Ju JH, Kang YS, Park SH, et al. Intravenous immunoglobulin attenuates experimental autoimmune arthritis by inducing reciprocal regulation of Th17 and Treg cells in an interleukin-10-dependent manner. Arthritis Rheumatol. 2014;66:1768–1778. doi: 10.1002/art.38627. [DOI] [PubMed] [Google Scholar]
- 21.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoerning A, Wilde B, Wang J, Tebbe B, Jing L, Wang X, Jian F, Zhu J, Dolff S, Kribben A, et al. Pharmacodynamic monitoring of mammalian target of rapamycin inhibition by phosphoflow cytometric determination of p70S6 kinase activity. Transplantation. 2015;99:210–219. doi: 10.1097/TP.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 23.Yang CS, Song CH, Lee JS, Jung SB, Oh JH, Park J, Kim HJ, Park JK, Paik TH, Jo EK. Intracellular network of phosphatidylinositol 3-kinase, mammalian target of the rapamycin/70 kDa ribosomal S6 kinase 1, and mitogen-activated protein kinases pathways for regulating mycobacteria-induced IL-23 expression in human macrophages. Cell Microbiol. 2006;8:1158–1171. doi: 10.1111/j.1462-5822.2006.00699.x. [DOI] [PubMed] [Google Scholar]
- 24.Ellis GI, Reneer MC, Vélez-Ortega AC, McCool A, Martí F. Generation of induced regulatory T cells from primary human naïve and memory T cells. J Vis Exp. 2012:3738. doi: 10.3791/3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reneer MC, Estes DJ, Vélez-Ortega AC, Norris A, Mayer M, Marti F. Peripherally induced human regulatory T cells uncouple Kv1.3 activation from TCR-associated signaling. Eur J Immunol. 2011;41:3170–3175. doi: 10.1002/eji.201141492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saxena A, Raychaudhuri SK, Raychaudhuri SP. Interleukin-17-induced proliferation of fibroblast-like synovial cells is mTOR dependent. Arthritis Rheum. 2011;63:1465–1466. doi: 10.1002/art.30278. [DOI] [PubMed] [Google Scholar]