Abstract
Immunotherapy has been introduced into cancer treatment methods, but different problems have restricted the efficacy of these protocols in clinical trials such as the presence of various immunomodulatory factors in the tumor microenvironment. Adenosine is an immunosuppressive metabolite produced by the tumor to promote growth, invasion, metastasis, and immune evasion. Many studies about adenosine and its metabolism in cancer have heightened interest in pursuing this treatment approach. It seems that targeting the adenosine pathway in combination with immunotherapy may lead to efficient antitumor response. In this review, we provide information on the roles of both adenosine and CD73 in the immune system and tumor development. We also describe recent studies about combination therapy with both purinergic inhibitors and other immunotherapeutic methods.
Keywords: Adenosine CD73 tumor immunotherapy
INTRODUCTION
Both chemotherapy and radiotherapy continue to be used as routine cancer treatment protocols, even though cancer is considered the second leading cause of death worldwide (1). Immunotherapy is a new approach and potential treatment for various cancers. Immunotherapeutic strategies aim to inhibit tumors by suppressing tumor tolerance mechanisms and augmenting the immune response that enable both identify and destruction of cancerous cells. Although the first series of cancer vaccines were shown to be safe and significantly induced specific immune responses, these vaccines were unable to provide effective results in the clinic (2,3,4). Tumors employ several mechanisms to escape immune responses by producing a variety of tumor-derived factors that could exert immunosuppressive effects to promote both invasion and metastasis (5).
Clinical trials recently changed to combination protocols of a tumor vaccine with other protocols such as anti-cancer drugs or radiation therapy. The rationale for this approach is that radiotherapy or chemotherapy may help tumor vaccines exert their desired effects. This approach aligns with the concept of conventional anti-cancer therapies and may be in accordance with therapeutically appropriate antitumor immune responses (6,7).
In 1863, Virchow was the first to discuss the relationship between inflammation that is promoted by cancerous tissue and tumor genesis (8). Inflammatory compounds contain reactive oxygen species and nitrogen intermediates, prostaglandins, growth factors, inflammatory cytokines, chemokines, and adenosine. All of these inflammatory entities are involved in tumor development by stimulating different mutations, resisting apoptosis, inducing angiogenesis, and facilitating a survival benefit to a sensitive cell (8,9).
In a cancer response, immune cells have binary actions with the potential to either remove or induce malignancy (10,11). Cancer cells use the help of immune cells, like tumor-associated macrophages (TAMs), NKT cells, Tregs, and myeloid-derived suppressor cells (MDSCs) to generate locally in tumor-bearing hosts. Treg-mediated immunosuppression is both one of the principal tumor immune-evasion mechanisms and a crucial barrier to successful tumor immunotherapy (11,12,13). MDSCs are a population of myeloid cell progenitors that differentiated to macrophages, dendritic cells (DCs), or other granulocytes. These MDSCs execute suppressive functions on T cell responses through nitric oxide, reactive oxygen species, prostaglandin E2 (PGE2), adenosine, and TGF-β production while also inducing Treg and anti-inflammatory responses (14,15). It has become apparent that efficient cancer treatments need a different application, which mainly targets multiple pro-tumorigenic pathways and disrupts immunosuppressive networks (2).
Adenosine is a purine nucleoside with different well-known functions in various pathophysiological processes that is released from cells or generated extracellular (7,16). Adenosine can prevent immune cells involved in anti-tumor responses and stimulate the development of immunosuppressive cells such as Treg and MDSCs by adenosine receptors binding (17,18). Adenosine and its receptors attracted researchers' attention in cancer pathology studies and create new research area in therapy purposes (19,20). Here, we discuss both the pharmacologic and immunologic inhibition of adenosine metabolism, which along with other immunotherapies, can induce effective anti-tumor responses.
MOLECULAR BIOLOGY OF ADENOSINE
There are low levels of adenosine in unstressed tissues under physiological conditions, but they can rapidly rise in response to hypoxia-ischemia, inflammation, or trauma (21). When adenosine is released from intracellular sources into the extracellular space, it acts as an ‘alarm’ or danger signal. In addition, through its effects on cell surface receptors, it triggers numerous cellular responses that target tissue homeostatic mechanisms (22).
Adenosine is produced by the ectoenzyme CD73 from AMP, which is generated by the ectoenzyme CD39 from ATP. Adenosine fulfills its biological functions via four subtypes of adenosine receptors (A1, A2A, A2B, and A3), which belong to the G-protein-coupled family of receptors (23).
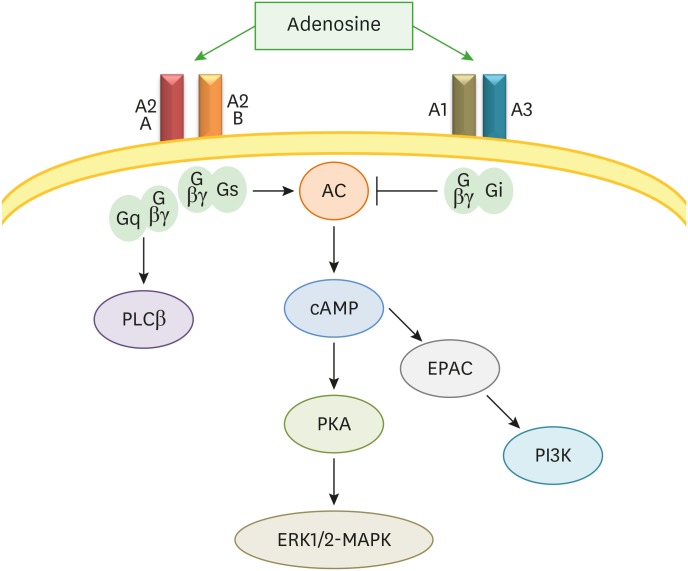
Adenosine receptors' mechanisms of action are inhibition or stimulation of adenylyl-cyclase to decrease or increase amounts of intracellular cyclic AMP (cAMP).Both A1 and A3 receptors decrease cAMP levels, whereas A2A and A2B increase cAMP contents. Adenosine receptors activate MAPK pathways and, in some cells, A1, A3, and A2B receptors direct phosphatidylinositol 3-kinase (PI3K) or Gq/phospholipase C (PLC) pathways (Fig. 1). The biological activity of adenosine is regulated by an ecto-adenosine deaminase, which converts adenosine into inosine molecules. Between these adenosine receptors, the A2A receptor plays a definitive role in the regulation of inflammatory responses. Different studies presented evidence about the activity of A2A receptor agonists as both anti-inflammatory and immunosuppressive mediators (24).
Figure 1. Adenosine signaling pathways.
AC, adenylate cyclase; EPAC, exchange protein activated by cyclic AMP; PI3K, phosphatidylinositol 3-kinase; PKA, protein kinase A; PLC, phospholipase C.
EFFECTS OF ADENOSINE ON THE IMMUNE SYSTEM
T cells
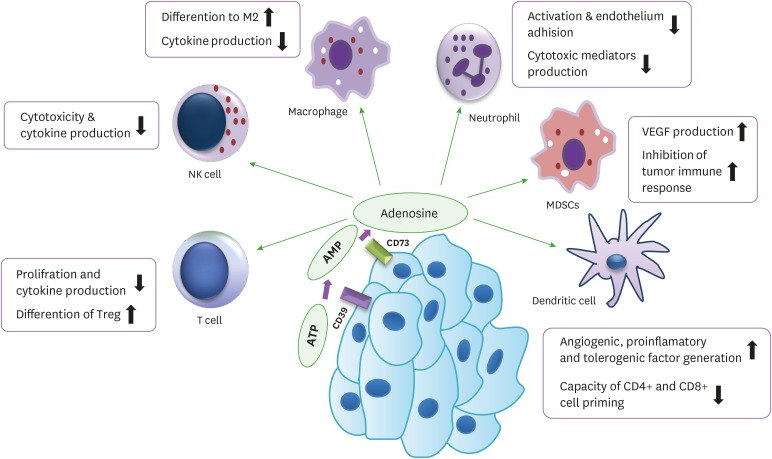
Extracellular adenosine via the A2A receptor can inhibit both activation and proliferation of CD4+ T cells and has been proposed not only to prevent Th type 1 (Th1) responses in vivo, but also, to prepare induction of Treg (25). T cells cultured in the presence of an A2A receptor agonist could neither proliferate nor generate IL-2, TNF-α, and IFN-γ. Other immunosuppressive roles of adenosine A2A receptor antagonists include the up regulation of T-cell negative regulatory molecules such as CTLA4, PD-1, and down regulation of the co-stimulatory molecule CD-40L. Stimulation of A2A with agonist induced T-cell tolerance and promoted Tregs and lymphocyte-activation gene 3 expression (26).
Tregs
A2A receptor stimulation enhanced fork head box P3 (FoxP3) mRNA and the immunosuppressor function of CD4+ FoxP3+cells. Since Tregs selectively co-express both CD39 and CD73, they may represent new specific markers of Tregs. Recent studies examined the relationship between the transcription factor FOXP3 and CD39, and found that FOXP3 up regulates the expression of CD39 (27).
A2A receptor stimulation in mixed-lymphocyte cultures raised the number of CD4+ FoxP3+ cell populations with superior immunoregulatory potency, while activation of effector T cells was mainly reduced so that this mechanism may be in accordance with the addition of Tregs within the tumor's microenvironment (28,29).
Recently, Di Gennaro et al. (30) established that Treg isolated from different sites (peripheral blood, lymph nodes, and tumor infiltrating lymphocytes) of melanoma patients in stage III–IV, mediated immunosuppression by adenosine molecule. This study divided Tregs subsets with different CD39/CD73 expression into CD39+CD73− natural Tregs, CD39+CD73+ induced Tregs, CD39−CD73+ Tregs, and CD39−CD73– Tregs .The CD39−CD73− subset is unable to generate adenosine and its immunoregulatory activation might be exerted by secretion of TGF-β and/or IL-10, or cell-contact mechanisms (30).
DCs
DCs are professional Ag-presenting cells that up take Ags then process and present them to T cells. Therefore, DCs act as a connection between the innate and the adaptive immune systems (31). Activation of the A2A receptor in mature DCs leads to diminished IL-12, IL-6, and IFN-α but increases IL-10 secretion, so that it switches their cytokine pattern from a pro-inflammatory to an anti-inflammatory type (32).Adenosine actually controls TNF-β and IL-12 production; whereas it intensifies the production of IL-10 from LPS matured DCs. Adenosine matured DCs had a weakened ability to induce the differentiation of Th1 from naïve CD4+ T lymphocytes (33). DCs that generated in the presence of adenosine have altered function and produce high amounts of pro-inflammatory, angiogenic, immune suppressor, and tolerogenic components including IL-6, IL-8, and IL-10, cyclooxygenase-2, VEGF, TGF-β, and indoleamine-2, 3-dioxygenase; and promote tumor growth, if given to animal models (34). N-ethylcarboxamidoadenosine (adenosine analog) and forskolin (cAMP elevating-agent) skewed DCs differentiation to tolerogenic or regulatory subsets, which produced different immunosuppressor materials, and expressed myeloid/monocytic lineage markers. These types of DCs could not prime Ag-specific responses by CD8+ T-cells (35).
MDSCs
Cells with immunosuppressive properties have been stabilized and are called MDSCs. In mice; these cells display a population of myeloid cell lineages with both monocytic and granulocytic morphology and are usually distinguished by CD11b and Gr-1 markers (36). CD11b+Gr-1+ cells accumulate in the tumor microenvironment and lymphoid organs and are considered major contributors to tumor immunotolerance (37).
This observation indicates that MDSCs express high levels of the CD73 molecule, which is linked to high amounts of ecto-5′-nucleotidase enzymatic activity. This result suggested that targeting CD73 might reduce the population of MDSCs within the tumor medium and overcome their inhibitory condition (38). Ryzhov et al. (39) indicated that TGF-β signaling could induce the maturation of tumor-infiltrated MDSCs into terminally differentiated myeloid mononuclear cells (TDMMCs). These cells presented high levels of cell surface CD39 and CD73 and had a capacity of adenosine production. Also, deletion of the TGF-β receptor reduced TDMMCs' numbers, tumor growth rate, and metastasis (39). A study has shown that adenosine promotes MDSC function with the activation of A2B receptors, administration of A2B antagonist inhibited the accumulation of tumor-infiltrating MDSCs and enhanced Th1-like responses in the tumor environment and significantly delayed tumor growth (17). It was also demonstrated that A2B blockade raised the number of CD11b+Gr1+ cells in tumors and exclusion of MDSCs in mice significantly decreased VEGF production especially through A2B receptors (40).
NK cells
Adenosine prevents the capability of activated NK cells to destroy tumor cells and Perforin- and FasL-mediated cytotoxicity (41). Also, it was demonstrated that adenosine could be a main factor in blocking the ability of lymphokine-activated killer cells to kill tumor cells (42). Beavis et al. (43) suggested that the A2A antagonist has an anti-metastatic effect because it enhanced NK cell function. Hatfield and colleagues (44) established that hyperoxic therapy has an anti-metastatic impact, which was related to the hypoxia-adenosinergic immunosuppression of NK cells. A subset of NK cell peripheral blood CD16−CD56bright NK cells, produce high amounts of CD38-mediated adenosine and inhibit CD4+ T cell proliferation, and act as regulatory cells (45). NKT cells are a population of T lymphocytes, which are presented NK-associated molecules, such as NK1.1 and TCR on the surface. Like Treg, NKT cells express both ecto-nucleotidases CD39 and CD73, and expression of both enzymes plays a role in the regulatory functions of these cells. In addition, a subset of NKT cells termed invariant NKT cells requires adenosine A2A activation to generate both IL-4 and IL-10 (46).
Macrophages
The stimulation of adenosine receptors affects cytokine production by macrophages; it shows that the adenosine pathway mediates inhibition of TNF-α release mainly via A2A and A2B receptors (47). Activation of A2A receptors primarily has multiple inhibitory effects on the M1 macrophage subset, while adenosine receptors induce the M2 macrophage subset by up regulating the expression of several markers such as arginase 1, tissue inhibitor of matrix metalloproteinase 1, and macrophage galactose-type C lectin 1 (48). Several lines of evidence also support this idea that adenosine can increase VEGF secretion by macrophages through the activation of A2A receptors (49). Cekic et al. (50) investigated A2A expression on myeloid cells, specifically TAMs, and found indirectly mediated suppression of T cells and NK cell in the tumor microenvironment.
Neutrophils
It has been shown that stimulation of neutrophils by adenosine prevented attachment to vascular endothelial cells and damage to the endothelium by these cells (51). In addition, other studies found that the mechanisms of this inhibition by adenosine were through both selectin and integrin molecules (52,53). Adenosine decreased secretion of oxygen radicals and other inflammatory mediators by neutrophils. Also, adenosine via A2A receptors diminished both the phagocytosis and apoptosis of neutrophils. Results of investigation indicate that cAMP-protein kinase A (PKA)-independent pathways are responsible for neutrophil inhibition by adenosine A2A receptors (Fig. 2) (54,55,56).
Figure 2. Inhibition of anti-tumor immune response. The purinergic pathway through adenosine molecules creates an immunosuppressive and tolerogenic condition in the tumor medium. The biologic function of different immune cells such as T cells, DCs, MDSCs, NK cells, macrophages, and neutrophils are influenced by adenosine, and aid in tumor progression.
ADENOSINE IN TUMORS ENVIROMENT
Extracellular amounts of adenosine in tumor tissues are higher than in normal tissues because of accumulation of ATP in the case of ischemia, damage, and stress. As mentioned previously, alteration of ATP to adenosine by the enzymatic activity of CD39 and CD73 in tumor tissues eventuates in immune response inhibition (57,58). On the other hand, hypoxic media, which exist in the tumor medium, enhanced the breakdown of adenine nucleotides to adenosine (59). Binding of the transcription factor, hypoxia-inducible factor 1-alpha (HIF-1α), to the hypoxia response element containing the promoter region of the CD73 gene, induced transcription and protein expression (60,61). Adenosine acting as an anti-inflammatory mediator down regulates the functions of infiltrating immune cells, thus preventing tissue destruction but this mechanism leads to tumor progression (62).
The expression of CD73 has been unregulated in many types of cancers, such as ovarian carcinoma (63), melanoma (64), prostate cancer (65), breast cancer (66), colon cancer (67), head and neck cancer (68,69), leukemia (70), hepatocellular carcinoma (71), and glioblastoma (72). Many studies reported that CD73 expression in cancer is connected with several outcomes, such as progression, poor prognosis, metastasis, and weak response to chemotherapy agents (72,73,74).
One of the new areas of interest in cancer research is MicroRNAs. Short non-coding RNAs bind to the 3′-untranslated regions of different genes and regulate their expression (75). Xie et al. demonstrated that miR-30a is a negative regulator of CD73 expression in colorectal cancer cells and leads to apoptosis and growth inhibition of tumor cells (76).
Different studies have shown that both expression and enzymatic activity of ecto-5′-nucleotidase (CD73) are elevated in various metastatic carcinomas (77,78,79). Beavis et al. (43) explained that metastatic capability of CD73+ tumors is mediated by an NK cell-dependent mechanism. Recently, one study established that a BRA mutation is associated with increased CD73 expression in melanoma patients. In addition, adenosine enhanced lymph node pigmentation and promoted lymph node metastasis. Also, combining BRAF and A2A receptor inhibition decreased tumor growth rate and metastasis in experimental BRAF-mutant melanoma (80).
Previous studies found that if the activity of CD73 in cancer cells were inhibited, angiogenesis of the tumor would decrease, suggesting that this enzyme is involved in tumor angiogenesis (81,82). Moreover, knockdown of CD73 expression in a breast tumor model diminished their metastatic potential for lung tissue (66).
Both CD39 and CD73 represent by the endothelial cells and can, therefore, be an origin of adenosine production. In addition, the presence of hypoxia in tumors increases the expression of both CD39 and CD73 on endothelial cells and leads to an increase in adenosine levels. The genetic or pharmacological deletion of CD39 and CD73 results in defects in the generation of tumor neovascularization (83). Further mechanistic investigations showed that adenosine can regulate the production of pro-and anti-angiogenic factors in tumor cells (84,85). Indeed, adenosine induces the secretion of VEGF, IL-8, and angiopoietin 2 by several human cancer cell lines through A2B or A3 receptors (86). A2B receptor stimulation promotes the release of VEGF by host immune cells that infiltrated the tumor. Ryzhov et al (39). showed tumor-infiltrating CD45+ immune cells, by engaging A2B receptors, involved in VEGF production in a mouse lung carcinoma model. Deletion of Cd39/Entpd-1 in mice not only disrupted the angiogenesis process but also delayed the development of melanoma tumors. These data indicated that CD39/ENTPD-1 expression was associated with the formation of new vessels and tumor growth (87). The described pro-angiogenic roles of CD73 might be related to sustaining an immunosuppressive network in the tumor microenvironment.
Recent evidence proposed that mesenchymal stem cells (MSCs) in the tumor site play essential roles in the progression, invasion, metastasis, and induction of epithelial-to-mesenchymal transition of malignant cells. These cells are distinguished by 3 markers including CD73, CD105, and CD90; so, they might be a good source of adenosine generation (88). Although, the expression of CD39 on MSCs was not reported, other CD39+ cells like Treg can produce AMP in tumors (89). Also, some studies indicated that cancer derived MSCs can inhibit the function of antitumor-effective immune cells through adenosine production (90,91).
ADENOSINE INHIBITION AND CANCER TREATMENT
Monoclonal Abs
Cancer treatment using monoclonal Abs has been demonstrated as one of the most successful therapeutic approaches in several tumors in the last 20 years (92).Several preclinical studies showed that pharmacological blockade of CD73, as well as treatment with anti-CD73 monoclonal Abs, is effective in preventing both tumor growth and metastatic spread in animal models (65,72). One study argued that anti-human CD73 Abs, which targeted adhesion and catalytic activity of CD73, could inhibit the development of metastasis in a breast cancer model (93). The study by Allard et al. showed that blockade of CD73 with a monoclonal Ab significantly diminished tumor VEGF production, and prevented tumor angiogenesis in vivo (83). MEDI9447 is a human, high-affinity Ab that inhibits the hydrolysis of CD73 to AMP. An in vivo study showed that MEDI9447 could impair the suppression of immune effector cells by adenosine and lead to tumor growth inhibition. Moreover, blockade of CD73 with MEDI9447 resulted in elevated Ag presentation and enhanced lymphocyte activation; and therefore, led to higher production of inflammatory cytokines such as IFN-γ, IL-1β, and TNF by Th1 cells (94). Young et al. identified that targeting A2A receptor antagonism in association with an anti-CD73 Ab that employs Fcγ receptors, limited tumor development and metastasis. This study demonstrated that combined inhibition of CD73 and A2A receptor is more effective than inhibition of either alone (16).
Pharmacological inhibitors
Different adenosine receptor antagonists have been developed for numerous therapeutic applications, such as cardiovascular, inflammatory, and neurodegenerative diseases without any unwanted side effect (95,96). Many studies showed that pharmacologic inhibition of adenosine especially through A2A and A2B, or CD73 and CD39 are clinically useful treatments in cancer (Table 1). Also, there are some studies about effect of A1 and A3 agonist on tumor development. It is established that specific agonist of A1 and A3 receptor could delay melanoma growth in CD73 knockout mice but increased angiogenesis (85).
Table 1. The effects of adenosine A2A and A2B receptors antagonist on animal cancer models.
| Target | Drug | Tumor | Effect | Ref |
|---|---|---|---|---|
| A2AR | ZM241385, Caffeine | Lung | Enhanced antitumor effect of CD8+ T cells | (97) |
| Caffeine | Melanoma | Limitation of tumor neovascularization and increased apoptosis | (97) | |
| SCH58261 | Ovary | Prolong the survival of tumor-bearing mice | (63) | |
| Breast | Increase of doxorubicin activity against tumor cells | (73) | ||
| Melanoma | Inhibition of metastasis | (43) | ||
| Breast | Inhibition of metastasis | (43) | ||
| Melanoma | Inhibition of metastasis | (16) | ||
| Melanoma | Inhibition of tumor growth, induction tumor infiltration of NK and CD8+ cells | (80) | ||
| A2BR | ATL801 | Breast | Inhibition of tumor growth and metastasis | (98) |
| ATL801 | Bladder | Inhibition of tumor growth and Inducing T cell immune response | (98) | |
| PSB1115 | Melanoma | Inhibition of tumor growth and Inducing T cell immune response | (17) | |
| CVT-6883 | Lung | Decrease of VEGF and cAMP production | (99) | |
| CD73 | APCP | Breast | Inhibition of tumor migration | (43) |
| Melanoma | Enhanced tumor regression by production of Th1 cell-associated and Th17 cell-associated cytokines and CD8+ T cell infiltration in the tumor microenvironment. | (100) | ||
| Melanoma | Inhibition of tumor growth | (101) | ||
| Ovary | Increased survival of tumor-bearing mice | (63) | ||
| Melanoma | Inhibition of lung metastasis | (74) | ||
| Breast | Decrease of micro vessel formation in tumors | (83) | ||
| Breast | Inhibition of tumor growth | (102) | ||
| Melanoma | Inhibition of tumor growth | (103) | ||
| Melanoma | Inhibition of tumor growth and angiogenesis | (104) | ||
| Melanoma | Inhibition of tumor metastasis | (16) | ||
| Glioblastoma | Inhibition of tumor growth, migration and invasion | (105) |
Short interfering RNA (siRNA)
siRNA is used to regulate gene expression in various therapeutic approaches (106). Zhi and colleagues (82) demonstrated that blocking CD73 by siRNA suppressed CD73 gene and protein expression in the breast cancer cell line MB-MDA-231, leading to inhibition of both growth and metastasis inhibition in vivo. In another study by this group, CD73 siRNA delayed breast cancer growth both in vivo and in vitro by arresting the cell cycle in the synthesis phase and inhibited the apoptosis pathway (107). Jadidi-Niaragh et al. (108) designed CD73-siRNA encapsulated into chitosan-lactate nanoparticles, which were applied to inhibit CD73 molecules in an animal model of human metastatic breast cancer.
SIMULTANEOUS REMOVAL OF ADENOSINE AND CANCER IMMUNOTHERAPY
Because of the robust nature of the immune system such as its ability for memory and specificity, it is anticipated that cancer immunotherapy can achieve total, long-lasting remissions and cancer rejection with few or no side effects (109).
However, the presence of different factors with immunosuppressive capacity in the tumor microenvironment is a formidable obstacle in effective cancer immunotherapy. The presence of these factors indicated that immune regulatory cells such as Tregs, MDSCs, NKT cells, and TAMs are the important immunoregulatory cells that disrupt effective responses against tumors (9,110).
Additionally, multiple soluble components such as HIF-1α, VEGF, and PGE2, inhibitory cytokines like IL-10 and TGF-β, and adenosine can also debilitate the efficacy of anti-tumor responses (9,111). Therefore, the reduced amount of adenosine in the tumor medium may improve the effectiveness of cancer vaccine immunotherapy.
The progress in tumor biology regarding both the conception and potency of immune system-based cancer vaccines may derive from evidence demonstrating that genetic deletions of the A2A receptor or the blockade of A2A receptor signaling by A2A receptor antagonists both restored suppression of anti-tumor T cells and induced tumor rejection (97).
Components which target the A2A receptor pathway can induce antitumor immunity by limiting results of extracellular adenosine generated from tissues and Tregs. This observation provides considerable evidence for the high expression of both CD39 and CD73 ectoenzymes on Tregs, MDSCs, and MSCs that secrete adenosine and have various therapeutic applications (112).
T cell-based therapy and adenosine
T lymphocytes are the effector arms in the response to cancer and immunosurveillance. Accordingly, numerous therapeutic approaches have been generated to augment effector T cells against tumors (113). Ohta et al. (97) found that adoptively transferred CD8+ T cells in mice that received ZM241, 385 (A2A receptor antagonists) decreased metastasis in a CL8-1 melanoma model. In a study by Jin et al. (63) inhibition of the A2A adenosine receptor with the antagonist (SCH 58261and caffeine) rescued tumor-specific immune response and enhanced the efficacy of adoptive T-cell therapy. The combination of SCH58261 and adoptive T-cell therapy significantly could improve survival in mice compared with T-cell therapy or SCH58261 alone (63). Wang et al. (103) showed that a combination of T-cell immunotherapy with a CD73 inhibitor (APCP) inhibited tumor growth in a melanoma model compared with immunotherapy or APCP treatment alone. In this study, treatment with anti-CD73 and T-cell therapy in a peritoneal ovarian model increased survival of mice and was more effective than mono-therapy.
T lymphocytes, which were modified genetically to express a chimeric Ag receptor (CAR), have been successful in the treatment of some malignancies especially; hematologic cancers (114,115). Beavis et al (116). showed that activation of the CAR unregulated expression of the A2A receptor. Also, genetic or pharmacological blockade of the A2A receptor increased CAR T-cell efficacy remarkably when associated with PD-1. This protocol leads to IFN-γ production of CD8+ CAR T-cells and induction of CD8+ and CD4+ CAR T-cells. This study showed that the A2A receptor pathway restricts the activity of patient-derived CAR T cells because CAR T cells that were also A2A receptor-deficient had a significantly better therapeutic influence than did the wild-type of CAR T (Fig. 3A) (116).
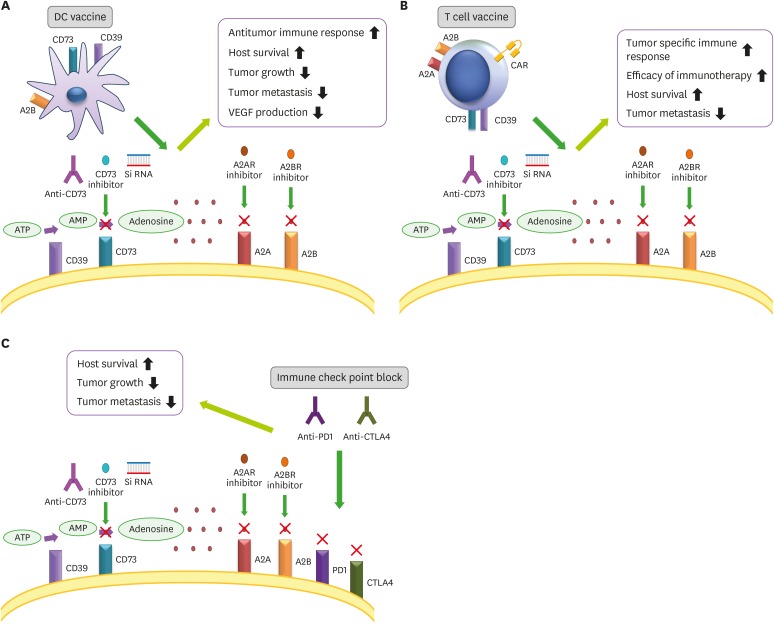
Figure 3. Efficacy of Immunotherapy. Inhibition of adenosine production and function, along with immunotherapeutic methods, may provide many advantages. DC vaccination and adenosine blockage could induce a tumor-specific T-cell response and increase efficacy of the vaccine. T cell or CAR T-cell immunotherapy in combination with CD73 inhibitor, or an A2 receptor antagonist could lead to tumor regression. An immune check point inhibitor, such as anti-PD-1, anti-CTLA-4, combined with adenosine inhibitors could tumor progression and metastasis.
DC immunotherapy and adenosine
DCs are the primary target of different protocols of cancer immunotherapy because they can induce an effective and specific tumor immune response (117). In our study, we used an A2A adenosine receptor antagonist (SCH58261) and a APCP in combination with a DCs vaccine in a 4T1 breast cancer model. Combination therapy with SCH, APCP, and DC reduced tumor growth and VEGF production, improved survival of tumor-bearing mice, and induced specific antitumor immune responses (118).
Jadidi-Niaragh et al. (108) examined CD73-specific siRNA-loaded chitosan-lactate nano-particles with DC vaccine in 4T1 breast cancer model that led to reduced expression of CD73 on tumor cells, tumor growth, metastasis, and improved mice survival (119). Already, this nano-particle suppressed the expression of the CD73 molecule on tumor cells, in vitro (Fig. 3B).
As mentioned previously, activities of HIF-1α have a direct relationship to the adenosine pathway. The combination of HIF-1α inhibition and the DC vaccine also provided a cytotoxic T cell response, IFN-γ production, and increased efficacy of immunotherapy (120).
Immune check point blockade and adenosine
Immune check point blockades have good results in cancer treatment such as anti-CTLA-4 (ipilimumab) and anti-PD-1/PDL-1 (nivolumab, MK-3475/MPDL3280A, MDX-1105). Allard et al. (57) study can reveal that blockade of CD73 reinforced therapeutic activity of immune checkpoint inhibitors anti-CTLA-4 and anti-PD-1 monoclonal Abs. It proved that combination therapy in mice with the A2A receptor antagonist (SCH) and anti-PD-1 monoclonal Abs substantially reduced both experimental and spontaneous metastases, and prolonged the survival of mice when compared with monotherapy. This study suggested that inhibition of metastasis was dependent on NK, T CD8+, IFN-γ, and perforin (76).
Beavis et al. utilized co-blockade of PD-1 and adenosine receptor 2A in cancer treatment led to the significant expression of IFN-γ and Granzyme B by tumor-infiltrating CD8+ T cells, growth inhibition of CD73+ tumors, and extension of survival in mice. This study suggested that adenosine receptor 2A antagonists could improve the efficacy of anti-PD-1 monoclonal Abs in cancer therapy (77).
A recently published study has shown that combination therapy with anti-CD73 andanti-PD-1 improved survival, induced both an Ab-mediated response and an infiltration by T CD8+ cells in a murine ovarian cancer model (Fig. 3C) (4).
A number of clinical trials are investigating adenosine receptor antagonists or anti-CD73 and check point inhibitors. Novartis/Palobiofarma is testing a class of A2Areceptor antagonists PFB509 and PDR001 (anti-PD-1) in a Phase I trial in patients with advanced non-small cell lung cancer (NSCLC) (121). CPI-444, a small oral molecule, is an antagonist of the A2A receptor. Corvus Pharmaceuticals is studying single-agent therapy with CPI-444 for renal cell carcinoma (RCC) and in combination with atezolizumab (anti PD-L1 monoclonal Ab) for both RCC and NSCLC (122). Another study is in phase 1b with the combination of AZD4635 (A2A receptor antagonist) and durvalumab (anti-PD-L1 Ab) for NSCLC, metastatic castrate-resistant prostate carcinoma, and colorectal carcinoma (CRC) (123). In addition, combination therapy withMEDI9447 (anti-CD73) and MEDI4736 (human anti-PD-L1 IgG1 Ab) in advanced solid tumors, which are selected between adults, is being examined (124). Also, Corvus Pharmaceuticals designed a clinical trial to determine the efficacy of CD73 inhibitor (CPI-006) alone or in combination with an A2A receptor antagonist (CPI-444) and an anti-PD-1 Ab in several advanced solid tumors (Table 2) (125).
Table 2. Clinical trials with adenosine pathway inhibitors combined with an immune check inhibitor in cancer.
| Target | Drug | Company | Study phase | Tumor | Combination agent | Code |
|---|---|---|---|---|---|---|
| A2AR | PFB509 | Novartis/Palobiofarma | I, Ib | NSCLC | PDR001 (anti-PD-1) | NCT02403193 |
| CPI-444 | Corvus Pharmaceutical | I, Ib | NSCLC, Melanoma, renal cell carcinoma, TNBC, colorectal cancer, bladder cancer | MPDL3280A atezolizumab (anti-PD-1) | NCT02655822 | |
| AZD4635 | AstraZeneca | I | NSCLC, metastatic castrate-resistant prostate carcinoma, colorectal cancer | MEDI4736, durvalumab (anti-PD-L1) | NCT02740985 | |
| PFB509 (NIR178) | Novartis | Ib | Solid tumors and non-Hodgkin lymphoma | PDR001 (anti-PD-1) | NCT03207867 | |
| CD73 | MEDI9447 | MedImmune | I | Selected solid tumor | MEDI4736, durvalumab (anti-PD-L1) | NCT02503774 |
| CPI-006 | Corvus Pharmaceutical | I | NSCLC, RCC, colorectal cancer, TNBC, cervical cancer, ovarian cancer, pancreatic cancer, endometrial cancer, sarcoma, SCC of head and neck, bladder cancer, metastatic castrate-resistant prostate carcinoma | CPI-004 (A2AR antagonist), pembrolizumab (anti-PD-1) | NCT03454451 |
SCC, squamous cell carcinoma; TNBC, triple-negative breast cancer.
CONCLUSION
Different treatments were defined by targeting important pathways or particular molecules that are essential for both tumor cell development and invasion. Many recent studies offer combination therapies that present suitable options in cancer immunotherapy for patients. Also, blocking any suppressor molecule or cell in the tumor microenvironment will increase the performance of different immunotherapeutic methods. The CD39/CD73 purinergic pathway can now be distinguished as one of the most substantial immunosuppressive regulatory mechanisms in the tumor medium. It is essential to try to develop approaches to reduce adenosine receptors and CD73 expression on both tumor and immune cells for subsequent adoptive immunotherapy. It seems that further studies are necessary to assess anti-tumor immune responses and design an effective targeted therapy to both disrupt and modulate the immunosuppressive network. These studies may suggest new strategic direction as well as provide insight for innovative approaches to overcome specific difficulties encountered in cancer therapy.
ACKNOWLEDGEMENTS
This study was supported by a grant from Tehran University of Medical Sciences (grant number: 92-03-87-23034).
Abbreviations
- cAMP
cyclic AMP
- CAR
chimeric Ag receptor
- DC
dendritic cell
- FoxP3
fork head box P3
- HIF-1α
hypoxia-inducible factor 1-alpha
- MDSC
myeloid-derived suppressor cell
- MSC
mesenchymal stem cell
- NSCLC
non-small cell lung cancer
- PGE2
prostaglandin E2
- PI3K
phosphatidylinositol 3-kinase
- PKA
protein kinase A
- PLC
phospholipase C
- RCC
renal cell carcinoma
- siRNA
short interfering RNA
- TAM
tumor-associated macrophage
- TDMMC
terminally differentiated myeloid mononuclear cell
- Th1
Th type 1
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Hajati J.
- Writing - original draft: Arab S.
- Writing - review & editing: Arab S.
References
- 1.Ma Y, Kepp O, Ghiringhelli F, Apetoh L, Aymeric L, Locher C, Tesniere A, Martins I, Ly A, Haynes NM, et al. Chemotherapy and radiotherapy: cryptic anticancer vaccines. Semin Immunol. 2010;22:113–124. doi: 10.1016/j.smim.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Zhou J. Advances and prospects in cancer immunotherapy. New J Sci. 2014;2014:745808 [Google Scholar]
- 3.Kimura H, Matsui Y, Ishikawa A, Nakajima T, Yoshino M, Sakairi Y. Randomized controlled phase III trial of adjuvant chemo-immunotherapy with activated killer T cells and dendritic cells in patients with resected primary lung cancer. Cancer Immunol Immunother. 2015;64:51–59. doi: 10.1007/s00262-014-1613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Virani NA, Thavathiru E, McKernan P, Moore K, Benbrook DM, Harrison RG. Anti-CD73 and anti-OX40 immunotherapy coupled with a novel biocompatible enzyme prodrug system for the treatment of recurrent, metastatic ovarian cancer. Cancer Lett. 2018;425:174–182. doi: 10.1016/j.canlet.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 5.Devaud C, John LB, Westwood JA, Darcy PK, Kershaw MH. Immune modulation of the tumor microenvironment for enhancing cancer immunotherapy. OncoImmunology. 2013;2:e25961. doi: 10.4161/onci.25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drake CG. Combination immunotherapy approaches. Ann Oncol. 2012;23(Suppl 8):viii41–viii46. doi: 10.1093/annonc/mds262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schindler U, Seitz L, Ashok D, Piovesan D, Tan J, DiRenzo D, Yin F, Leleti M, Rosen B, Miles D, et al. AB928, a dual antagonist of the A2aR and A2bR adenosine receptors, leads to greater immune activation and reduced tumor growth when combined with chemotherapy. Eur J Cancer. 2018;92(Suppl 1):S14–S15. [Google Scholar]
- 8.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 9.Stewart TJ, Smyth MJ. Improving cancer immunotherapy by targeting tumor-induced immune suppression. Cancer Metastasis Rev. 2011;30:125–140. doi: 10.1007/s10555-011-9280-5. [DOI] [PubMed] [Google Scholar]
- 10.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mao FY, Kong H, Zhao YL, Peng LS, Chen W, Zhang JY, Cheng P, Wang TT, Lv YP, Teng YS, et al. Increased tumor-infiltrating CD45RA−CCR7− regulatory T-cell subset with immunosuppressive properties foster gastric cancer progress. Cell Death Dis. 2017;8:e3002. doi: 10.1038/cddis.2017.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 2011;7:651–658. doi: 10.7150/ijbs.7.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao L, Fan TF, Wu L, Yu GT, Deng WW, Chen L, Bu LL, Ma SR, Liu B, Bian Y, et al. Selective blockade of B7-H3 enhances antitumour immune activity by reducing immature myeloid cells in head and neck squamous cell carcinoma. J Cell Mol Med. 2017;21:2199–2210. doi: 10.1111/jcmm.13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trikha P, Carson WE., 3rd Signaling pathways involved in MDSC regulation. Biochim Biophys Acta. 2014;1846:55–65. doi: 10.1016/j.bbcan.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka T, Fujita M, Hasegawa H, Arimoto A, Nishi M, Fukuoka E, Sugita Y, Matsuda T, Sumi Y, Suzuki S, et al. Frequency of myeloid-derived suppressor cells in the peripheral blood reflects the status of tumor recurrence. Anticancer Res. 2017;37:3863–3869. doi: 10.21873/anticanres.11766. [DOI] [PubMed] [Google Scholar]
- 16.Young A, Ngiow SF, Barkauskas DS, Sult E, Hay C, Blake SJ, Huang Q, Liu J, Takeda K, Teng MW, et al. Co-inhibition of CD73 and A2AR adenosine signaling improves anti-tumor immune responses. Cancer Cell. 2016;30:391–403. doi: 10.1016/j.ccell.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 17.Iannone R, Miele L, Maiolino P, Pinto A, Morello S. Blockade of A2b adenosine receptor reduces tumor growth and immune suppression mediated by myeloid-derived suppressor cells in a mouse model of melanoma. Neoplasia. 2013;15:1400–1409. doi: 10.1593/neo.131748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun X, Wu Y, Gao W, Enjyoji K, Csizmadia E, Müller CE, Murakami T, Robson SC. CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology. 2010;139:1030–1040. doi: 10.1053/j.gastro.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Zhang T, Song Z, Li L, Zhang X, Liu J, Liu X, Qiu L, Qian Z, Zhou S, et al. Tumor CD73/A2aR adenosine immunosuppressive axis and tumor-infiltrating lymphocytes in diffuse large B-cell lymphoma: correlations with clinicopathological characteristics and clinical outcome. Int J Cancer. 2019;145:1414–1422. doi: 10.1002/ijc.32144. [DOI] [PubMed] [Google Scholar]
- 20.Perrot I, Michaud HA, Giraudon-Paoli M, Augier S, Docquier A, Gros L, Courtois R, Déjou C, Jecko D, Becquart O, et al. Blocking antibodies targeting the CD39/CD73 immunosuppressive pathway unleash immune responses in combination cancer therapies. Cell Reports. 2019;27:2411–2425.e9. doi: 10.1016/j.celrep.2019.04.091. [DOI] [PubMed] [Google Scholar]
- 21.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 23.Haskó G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Haskó G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sitkovsky M, Lukashev D, Deaglio S, Dwyer K, Robson SC, Ohta A. Adenosine A2A receptor antagonists: blockade of adenosinergic effects and T regulatory cells. Br J Pharmacol. 2008;153(Suppl 1):S457–S464. doi: 10.1038/bjp.2008.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, Drake CG, Powell JD. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Höpner S, Centonze D, Bernardi G, Dell'Acqua ML, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 28.Ohta A, Kini R, Ohta A, Subramanian M, Madasu M, Sitkovsky M. The development and immunosuppressive functions of CD4+ CD25+ FoxP3+ regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front Immunol. 2012;3:190. doi: 10.3389/fimmu.2012.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sundström P, Stenstad H, Langenes V, Ahlmanner F, Theander L, Ndah TG, Fredin K, Börjesson L, Gustavsson B, Bastid J, et al. Regulatory T cells from colon cancer patients inhibit effector T-cell migration through an adenosine-dependent mechanism. Cancer Immunol Res. 2016;4:183–193. doi: 10.1158/2326-6066.CIR-15-0050. [DOI] [PubMed] [Google Scholar]
- 30.Di Gennaro P, Gerlini G, Caporale R, Sestini S, Brandani P, Urso C, Pimpinelli N, Borgognoni L. T regulatory cells mediate immunosuppresion by adenosine in peripheral blood, sentinel lymph node and TILs from melanoma patients. Cancer Lett. 2018;417:124–130. doi: 10.1016/j.canlet.2017.12.032. [DOI] [PubMed] [Google Scholar]
- 31.Turtle CJ, Hart DN. Dendritic cells in tumor immunology and immunotherapy. Curr Drug Targets. 2004;5:17–39. doi: 10.2174/1389450043490640. [DOI] [PubMed] [Google Scholar]
- 32.Schnurr M, Toy T, Shin A, Hartmann G, Rothenfusser S, Soellner J, Davis ID, Cebon J, Maraskovsky E. Role of adenosine receptors in regulating chemotaxis and cytokine production of plasmacytoid dendritic cells. Blood. 2004;103:1391–1397. doi: 10.1182/blood-2003-06-1959. [DOI] [PubMed] [Google Scholar]
- 33.Panther E, Corinti S, Idzko M, Herouy Y, Napp M, la Sala A, Girolomoni G, Norgauer J. Adenosine affects expression of membrane molecules, cytokine and chemokine release, and the T-cell stimulatory capacity of human dendritic cells. Blood. 2003;101:3985–3990. doi: 10.1182/blood-2002-07-2113. [DOI] [PubMed] [Google Scholar]
- 34.Novitskiy SV, Ryzhov S, Zaynagetdinov R, Goldstein AE, Huang Y, Tikhomirov OY, Blackburn MR, Biaggioni I, Carbone DP, Feoktistov I, et al. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood. 2008;112:1822–1831. doi: 10.1182/blood-2008-02-136325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Challier J, Bruniquel D, Sewell AK, Laugel B. Adenosine and cAMP signalling skew human dendritic cell differentiation towards a tolerogenic phenotype with defective CD8+ T-cell priming capacity. Immunology. 2013;138:402–410. doi: 10.1111/imm.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanabe S, Deguchi K, Zheng R, Tamai H, Wang LX, Cohen PA, Shu S. Tumor-induced CD11b+Gr-1+ myeloid cells suppress T cell sensitization in tumor-draining lymph nodes. J Immunol. 2008;181:3291–3300. doi: 10.4049/jimmunol.181.5.3291. [DOI] [PubMed] [Google Scholar]
- 38.Ryzhov S, Novitskiy SV, Goldstein AE, Biktasova A, Blackburn MR, Biaggioni I, Dikov MM, Feoktistov I. Adenosinergic regulation of the expansion and immunosuppressive activity of CD11b+Gr1+ cells. J Immunol. 2011;187:6120–6129. doi: 10.4049/jimmunol.1101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryzhov SV, Pickup MW, Chytil A, Gorska AE, Zhang Q, Owens P, Feoktistov I, Moses HL, Novitskiy SV. Role of TGF-β signaling in generation of CD39+CD73+ myeloid cells in tumors. J Immunol. 2014;193:3155–3164. doi: 10.4049/jimmunol.1400578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sorrentino C, Miele L, Porta A, Pinto A, Morello S. Myeloid-derived suppressor cells contribute to A2B adenosine receptor-induced VEGF production and angiogenesis in a mouse melanoma model. Oncotarget. 2015;6:27478–27489. doi: 10.18632/oncotarget.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raskovalova T, Huang X, Sitkovsky M, Zacharia LC, Jackson EK, Gorelik E. Gs protein-coupled adenosine receptor signaling and lytic function of activated NK cells. J Immunol. 2005;175:4383–4391. doi: 10.4049/jimmunol.175.7.4383. [DOI] [PubMed] [Google Scholar]
- 42.Raskovalova T, Huang X, Sitkovsky M, Zacharia LC, Jackson EK, Gorelik E. Gs protein-coupled adenosine receptor signaling and lytic function of activated NK cells. J Immunol. 2005;175:4383–4391. doi: 10.4049/jimmunol.175.7.4383. [DOI] [PubMed] [Google Scholar]
- 43.Beavis PA, Divisekera U, Paget C, Chow MT, John LB, Devaud C, Dwyer K, Stagg J, Smyth MJ, Darcy PK. Blockade of A2A receptors potently suppresses the metastasis of CD73+ tumors. Proc Natl Acad Sci U S A. 2013;110:14711–14716. doi: 10.1073/pnas.1308209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hatfield SM, Kjaergaard J, Lukashev D, Schreiber TH, Belikoff B, Abbott R, Sethumadhavan S, Philbrook P, Ko K, Cannici R, et al. Immunological mechanisms of the antitumor effects of supplemental oxygenation. Sci Transl Med. 2015;7:277ra30. doi: 10.1126/scitranslmed.aaa1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morandi F, Horenstein AL, Chillemi A, Quarona V, Chiesa S, Imperatori A, Zanellato S, Mortara L, Gattorno M, Pistoia V, et al. CD56brightCD16− NK cells produce adenosine through a CD38-mediated pathway and act as regulatory cells inhibiting autologous CD4+ T cell proliferation. J Immunol. 2015;195:965–972. doi: 10.4049/jimmunol.1500591. [DOI] [PubMed] [Google Scholar]
- 46.Nowak M, Lynch L, Yue S, Ohta A, Sitkovsky M, Balk SP, Exley MA. The A2aR adenosine receptor controls cytokine production in iNKT cells. Eur J Immunol. 2010;40:682–687. doi: 10.1002/eji.200939897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kreckler LM, Wan TC, Ge ZD, Auchampach JA. Adenosine inhibits tumor necrosis factor-α release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. J Pharmacol Exp Ther. 2006;317:172–180. doi: 10.1124/jpet.105.096016. [DOI] [PubMed] [Google Scholar]
- 48.Haskó G, Pacher P. Regulation of macrophage function by adenosine. Arterioscler Thromb Vasc Biol. 2012;32:865–869. doi: 10.1161/ATVBAHA.111.226852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ernens I, Léonard F, Vausort M, Rolland-Turner M, Devaux Y, Wagner DR. Adenosine up-regulates vascular endothelial growth factor in human macrophages. Biochem Biophys Res Commun. 2010;392:351–356. doi: 10.1016/j.bbrc.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 50.Cekic C, Day YJ, Sag D, Linden J. Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Res. 2014;74:7250–7259. doi: 10.1158/0008-5472.CAN-13-3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cronstein BN, Levin RI, Belanoff J, Weissmann G, Hirschhorn R. Adenosine: an endogenous inhibitor of neutrophil-mediated injury to endothelial cells. J Clin Invest. 1986;78:760–770. doi: 10.1172/JCI112638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sullivan GW, Lee DD, Ross WG, DiVietro JA, Lappas CM, Lawrence MB, Linden J. Activation of A2A adenosine receptors inhibits expression of α4/β1 integrin (very late antigen-4) on stimulated human neutrophils. J Leukoc Biol. 2004;75:127–134. doi: 10.1189/jlb.0603300. [DOI] [PubMed] [Google Scholar]
- 53.Cronstein BN, Levin RI, Philips M, Hirschhorn R, Abramson SB, Weissmann G. Neutrophil adherence to endothelium is enhanced via adenosine A1 receptors and inhibited via adenosine A2 receptors. J Immunol. 1992;148:2201–2206. [PubMed] [Google Scholar]
- 54.Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–944. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 55.Haskó G, Cronstein B. Regulation of inflammation by adenosine. Front Immunol. 2013;4:85. doi: 10.3389/fimmu.2013.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barletta KE, Ley K, Mehrad B. Regulation of neutrophil function by adenosine. Arterioscler Thromb Vasc Biol. 2012;32:856–864. doi: 10.1161/ATVBAHA.111.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Allard D, Allard B, Gaudreau PO, Chrobak P, Stagg J. CD73-adenosine: a next-generation target in immuno-oncology. Immunotherapy. 2016;8:145–163. doi: 10.2217/imt.15.106. [DOI] [PubMed] [Google Scholar]
- 58.Allard B, Beavis PA, Darcy PK, Stagg J. Immunosuppressive activities of adenosine in cancer. Curr Opin Pharmacol. 2016;29:7–16. doi: 10.1016/j.coph.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 59.Hatfield SM, Kjaergaard J, Lukashev D, Belikoff B, Schreiber TH, Sethumadhavan S, Abbott R, Philbrook P, Thayer M, Shujia D, et al. Systemic oxygenation weakens the hypoxia and hypoxia inducible factor 1α-dependent and extracellular adenosine-mediated tumor protection. J Mol Med (Berl) 2014;92:1283–1292. doi: 10.1007/s00109-014-1189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang M, Ma C, Liu S, Shao Q, Gao W, Song B, Sun J, Xie Q, Zhang Y, Feng A, et al. HIF-dependent induction of adenosine receptor A2b skews human dendritic cells to a Th2-stimulating phenotype under hypoxia. Immunol Cell Biol. 2010;88:165–171. doi: 10.1038/icb.2009.77. [DOI] [PubMed] [Google Scholar]
- 61.Li J, Wang L, Chen X, Li L, Li Y, Ping Y, Huang L, Yue D, Zhang Z, Wang F, et al. CD39/CD73 upregulation on myeloid-derived suppressor cells via TGF-β-mTOR-HIF-1 signaling in patients with non-small cell lung cancer. OncoImmunology. 2017;6:e1320011. doi: 10.1080/2162402X.2017.1320011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 63.Jin D, Fan J, Wang L, Thompson LF, Liu A, Daniel BJ, Shin T, Curiel TJ, Zhang B. CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res. 2010;70:2245–2255. doi: 10.1158/0008-5472.CAN-09-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang H, Lee S, Nigro CL, Lattanzio L, Merlano M, Monteverde M, Matin R, Purdie K, Mladkova N, Bergamaschi D, et al. NT5E (CD73) is epigenetically regulated in malignant melanoma and associated with metastatic site specificity. Br J Cancer. 2012;106:1446–1452. doi: 10.1038/bjc.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang Q, Du J, Zu L. Overexpression of CD73 in prostate cancer is associated with lymph node metastasis. Pathol Oncol Res. 2013;19:811–814. doi: 10.1007/s12253-013-9648-7. [DOI] [PubMed] [Google Scholar]
- 66.Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, Dwyer KM, Smyth MJ. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci U S A. 2010;107:1547–1552. doi: 10.1073/pnas.0908801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu N, Fang XD, Vadis Q. CD73 as a novel prognostic biomarker for human colorectal cancer. J Surg Oncol. 2012;106:918–919. doi: 10.1002/jso.23159. [DOI] [PubMed] [Google Scholar]
- 68.Ren ZH, Lin CZ, Cao W, Yang R, Lu W, Liu ZQ, Chen YM, Yang X, Tian Z, Wang LZ, et al. CD73 is associated with poor prognosis in HNSCC. Oncotarget. 2016;7:61690–61702. doi: 10.18632/oncotarget.11435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma SR, Deng WW, Liu JF, Mao L, Yu GT, Bu LL, Kulkarni AB, Zhang WF, Sun ZJ. Blockade of adenosine A2A receptor enhances CD8+ T cells response and decreases regulatory T cells in head and neck squamous cell carcinoma. Mol Cancer. 2017;16:99. doi: 10.1186/s12943-017-0665-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao SX, Zhang HM, Dong SX, Liu JH, Zhou Z, Wang HJ, Zhu XF, Mi YC, Ru YX. Characteristics and clinical significance of CD73 expression in subtypes of leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2011;19:1141–1144. [PubMed] [Google Scholar]
- 71.Sciarra A, Monteiro I, Menetrier-Caux C, Caux C, Gilbert B, Halkic N, La Rosa S, Romero P, Sempoux C, de Leval L. CD73 expression in normal and pathological human hepatobiliopancreatic tissues. Cancer Immunol Immunother. 2019;68:467–478. doi: 10.1007/s00262-018-2290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quezada C, Garrido W, Oyarzún C, Fernández K, Segura R, Melo R, Casanello P, Sobrevia L, San Martín R. 5′-ectonucleotidase mediates multiple-drug resistance in glioblastoma multiforme cells. J Cell Physiol. 2013;228:602–608. doi: 10.1002/jcp.24168. [DOI] [PubMed] [Google Scholar]
- 73.Loi S, Pommey S, Haibe-Kains B, Beavis PA, Darcy PK, Smyth MJ, Stagg J. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc Natl Acad Sci U S A. 2013;110:11091–11096. doi: 10.1073/pnas.1222251110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stagg J, Divisekera U, Duret H, Sparwasser T, Teng MW, Darcy PK, Smyth MJ. CD73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res. 2011;71:2892–2900. doi: 10.1158/0008-5472.CAN-10-4246. [DOI] [PubMed] [Google Scholar]
- 75.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 76.Xie M, Qin H, Luo Q, Huang Q, He X, Yang Z, Lan P, Lian L. MicroRNA-30a regulates cell proliferation and tumor growth of colorectal cancer by targeting CD73. BMC Cancer. 2017;17:305. doi: 10.1186/s12885-017-3291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang L, Zhou X, Zhou T, Ma D, Chen S, Zhi X, Yin L, Shao Z, Ou Z, Zhou P. Ecto-5′-nucleotidase promotes invasion, migration and adhesion of human breast cancer cells. J Cancer Res Clin Oncol. 2008;134:365–372. doi: 10.1007/s00432-007-0292-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sadej R, Spychala J, Skladanowski AC. Expression of ecto-5′-nucleotidase (eN, CD73) in cell lines from various stages of human melanoma. Melanoma Res. 2006;16:213–222. doi: 10.1097/01.cmr.0000215030.69823.11. [DOI] [PubMed] [Google Scholar]
- 79.Kondo T, Nakazawa T, Murata SI, Katoh R. Expression of CD73 and its ecto-5′-nucleotidase activity are elevated in papillary thyroid carcinomas. Histopathology. 2006;48:612–614. doi: 10.1111/j.1365-2559.2005.02277.x. [DOI] [PubMed] [Google Scholar]
- 80.Young A, Ngiow SF, Madore J, Reinhardt J, Landsberg J, Chitsazan A, Rautela J, Bald T, Barkauskas DS, Ahern E, et al. Targeting adenosine in BRAF-mutant melanoma reduces tumor growth and metastasis. Cancer Res. 2017;77:4684–4696. doi: 10.1158/0008-5472.CAN-17-0393. [DOI] [PubMed] [Google Scholar]
- 81.Zhou P, Zhi X, Zhou T, Chen S, Li X, Wang L, Yin L, Shao Z, Ou Z. Overexpression of Ecto-5′-Nucleotidase (CD73) promotes T-47D human breast cancer cells invasion and adhesion to extracellular matrix. Cancer Biol Ther. 2007;6:426–431. doi: 10.4161/cbt.6.3.3762. [DOI] [PubMed] [Google Scholar]
- 82.Zhi X, Chen S, Zhou P, Shao Z, Wang L, Ou Z, Yin L. RNA interference of ecto-5′-nucleotidase (CD73) inhibits human breast cancer cell growth and invasion. Clin Exp Metastasis. 2007;24:439–448. doi: 10.1007/s10585-007-9081-y. [DOI] [PubMed] [Google Scholar]
- 83.Allard B, Turcotte M, Spring K, Pommey S, Royal I, Stagg J. Anti-CD73 therapy impairs tumor angiogenesis. Int J Cancer. 2014;134:1466–1473. doi: 10.1002/ijc.28456. [DOI] [PubMed] [Google Scholar]
- 84.Auchampach JA. Adenosine receptors and angiogenesis. Circ Res. 2007;101:1075–1077. doi: 10.1161/CIRCRESAHA.107.165761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koszałka P, Gołuńska M, Urban A, Stasiłojć G, Stanisławowski M, Majewski M, Składanowski AC, Bigda J. Specific activation of A3, A2A and A1 adenosine receptors in CD73-knockout mice affects B16F10 melanoma growth, neovascularization, angiogenesis and macrophage infiltration. PLoS One. 2016;11:e0151420. doi: 10.1371/journal.pone.0151420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Merighi S, Simioni C, Gessi S, Varani K, Mirandola P, Tabrizi MA, Baraldi PG, Borea PA. A2B and A3 adenosine receptors modulate vascular endothelial growth factor and interleukin-8 expression in human melanoma cells treated with etoposide and doxorubicin. Neoplasia. 2009;11:1064–1073. doi: 10.1593/neo.09768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goepfert C, Sundberg C, Sévigny J, Enjyoji K, Hoshi T, Csizmadia E, Robson S. Disordered cellular migration and angiogenesis in cd39-null mice. Circulation. 2001;104:3109–3115. doi: 10.1161/hc5001.100663. [DOI] [PubMed] [Google Scholar]
- 88.Poggi A, Varesano S, Zocchi MR. How to hit mesenchymal stromal cells and make the tumor microenvironment immunostimulant rather than immunosuppressive. Front Immunol. 2018;9:262. doi: 10.3389/fimmu.2018.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kerkelä E, Laitinen A, Räbinä J, Valkonen S, Takatalo M, Larjo A, Veijola J, Lampinen M, Siljander P, Lehenkari P, et al. Adenosinergic immunosuppression by human mesenchymal stromal cells requires co-operation with T cells. Stem Cells. 2016;34:781–790. doi: 10.1002/stem.2280. [DOI] [PubMed] [Google Scholar]
- 90.de Lourdes Mora-García M, García-Rocha R, Morales-Ramírez O, Montesinos JJ, Weiss-Steider B, Hernández-Montes J, Ávila-Ibarra LR, Don-López CA, Velasco-Velázquez MA, Gutiérrez-Serrano V, et al. Mesenchymal stromal cells derived from cervical cancer produce high amounts of adenosine to suppress cytotoxic T lymphocyte functions. J Transl Med. 2016;14:302. doi: 10.1186/s12967-016-1057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schuler PJ, Westerkamp AM, Kansy BA, Bruderek K, Dissmann PA, Dumitru CA, Lang S, Jackson EK, Brandau S. Adenosine metabolism of human mesenchymal stromal cells isolated from patients with head and neck squamous cell carcinoma. Immunobiology. 2017;222:66–74. doi: 10.1016/j.imbio.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 92.Scott AM, Allison JP, Wolchok JD. Monoclonal antibodies in cancer therapy. Cancer Immun. 2012;12:14. [PMC free article] [PubMed] [Google Scholar]
- 93.Terp MG, Olesen KA, Arnspang EC, Lund RR, Lagerholm BC, Ditzel HJ, Leth-Larsen R. Anti-human CD73 monoclonal antibody inhibits metastasis formation in human breast cancer by inducing clustering and internalization of CD73 expressed on the surface of cancer cells. J Immunol. 2013;191:4165–4173. doi: 10.4049/jimmunol.1301274. [DOI] [PubMed] [Google Scholar]
- 94.Hay CM, Sult E, Huang Q, Mulgrew K, Fuhrmann SR, McGlinchey KA, Hammond SA, Rothstein R, Rios-Doria J, Poon E, et al. Targeting CD73 in the tumor microenvironment with MEDI9447. OncoImmunology. 2016;5:e1208875. doi: 10.1080/2162402X.2016.1208875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Preti D, Baraldi PG, Moorman AR, Borea PA, Varani K. History and perspectives of A2A adenosine receptor antagonists as potential therapeutic agents. Med Res Rev. 2015;35:790–848. doi: 10.1002/med.21344. [DOI] [PubMed] [Google Scholar]
- 97.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cekic C, Sag D, Li Y, Theodorescu D, Strieter RM, Linden J. Adenosine A2B receptor blockade slows growth of bladder and breast tumors. J Immunol. 2012;188:198–205. doi: 10.4049/jimmunol.1101845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ryzhov S, Novitskiy SV, Zaynagetdinov R, Goldstein AE, Carbone DP, Biaggioni I, Dikov MM, Feoktistov I. Host A2B adenosine receptors promote carcinoma growth. Neoplasia. 2008;10:987–995. doi: 10.1593/neo.08478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Forte G, Sorrentino R, Montinaro A, Luciano A, Adcock IM, Maiolino P, Arra C, Cicala C, Pinto A, Morello S. Inhibition of CD73 improves B cell-mediated anti-tumor immunity in a mouse model of melanoma. J Immunol. 2012;189:2226–2233. doi: 10.4049/jimmunol.1200744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yegutkin GG, Marttila-Ichihara F, Karikoski M, Niemelä J, Laurila JP, Elima K, Jalkanen S, Salmi M. Altered purinergic signaling in CD73-deficient mice inhibits tumor progression. Eur J Immunol. 2011;41:1231–1241. doi: 10.1002/eji.201041292. [DOI] [PubMed] [Google Scholar]
- 102.Zhou X, Zhi X, Zhou P, Chen S, Zhao F, Shao Z, Ou Z, Yin L. Effects of ecto-5′-nucleotidase on human breast cancer cell growth in vitro and in vivo. Oncol Rep. 2007;17:1341–1346. [PubMed] [Google Scholar]
- 103.Wang L, Fan J, Thompson LF, Zhang Y, Shin T, Curiel TJ, Zhang B. CD73 has distinct roles in nonhematopoietic and hematopoietic cells to promote tumor growth in mice. J Clin Invest. 2011;121:2371–2382. doi: 10.1172/JCI45559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Koszałka P, Pryszlak A, Gołuńska M, Kolasa J, Stasiłojć G, Składanowski AC, Bigda JJ. Inhibition of CD73 stimulates the migration and invasion of B16F10 melanoma cells in vitro, but results in impaired angiogenesis and reduced melanoma growth in vivo. Oncol Rep. 2014;31:819–827. doi: 10.3892/or.2013.2883. [DOI] [PubMed] [Google Scholar]
- 105.Azambuja JH, Gelsleichter NE, Beckenkamp LR, Iser IC, Fernandes MC, Figueiró F, Battastini AM, Scholl JN, de Oliveira FH, Spanevello RM, et al. CD73 downregulation decreases in vitro and in vivo glioblastoma growth. Mol Neurobiol. 2019;56:3260–3279. doi: 10.1007/s12035-018-1240-4. [DOI] [PubMed] [Google Scholar]
- 106.Chakraborty C, Sharma AR, Sharma G, Doss CG, Lee SS. Therapeutic miRNA and siRNA: moving from bench to clinic as next generation medicine. Mol Ther Nucleic Acids. 2017;8:132–143. doi: 10.1016/j.omtn.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhi X, Wang Y, Zhou X, Yu J, Jian R, Tang S, Yin L, Zhou P. RNAi-mediated CD73 suppression induces apoptosis and cell-cycle arrest in human breast cancer cells. Cancer Sci. 2010;101:2561–2569. doi: 10.1111/j.1349-7006.2010.01733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jadidi-Niaragh F, Atyabi F, Rastegari A, Mollarazi E, Kiani M, Razavi A, Yousefi M, Kheshtchin N, Hassannia H, Hadjati J, et al. Downregulation of CD73 in 4T1 breast cancer cells through siRNA-loaded chitosan-lactate nanoparticles. Tumour Biol. 2016;37:8403–8412. doi: 10.1007/s13277-015-4732-0. [DOI] [PubMed] [Google Scholar]
- 109.Baxevanis CN, Perez SA, Papamichail M. Cancer immunotherapy. Crit Rev Clin Lab Sci. 2009;46:167–189. doi: 10.1080/10408360902937809. [DOI] [PubMed] [Google Scholar]
- 110.Facciabene A, Motz GT, Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72:2162–2171. doi: 10.1158/0008-5472.CAN-11-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lukashev D, Sitkovsky M, Ohta A. From “Hellstrom Paradox” to anti-adenosinergic cancer immunotherapy. Purinergic Signal. 2007;3:129–134. doi: 10.1007/s11302-006-9044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang B. Opportunities and challenges for anti-CD73 cancer therapy. Immunotherapy. 2012;4:861–865. doi: 10.2217/imt.12.83. [DOI] [PubMed] [Google Scholar]
- 113.Houot R, Schultz LM, Marabelle A, Kohrt H. T-cell-based immunotherapy: adoptive cell transfer and checkpoint inhibition. Cancer Immunol Res. 2015;3:1115–1122. doi: 10.1158/2326-6066.CIR-15-0190. [DOI] [PubMed] [Google Scholar]
- 114.Kalaitsidou M, Kueberuwa G, Schütt A, Gilham DE. CAR T-cell therapy: toxicity and the relevance of preclinical models. Immunotherapy. 2015;7:487–497. doi: 10.2217/imt.14.123. [DOI] [PubMed] [Google Scholar]
- 115.Magee MS, Snook AE. Challenges to chimeric antigen receptor (CAR)-T cell therapy for cancer. Discov Med. 2014;18:265–271. [PMC free article] [PubMed] [Google Scholar]
- 116.Beavis PA, Henderson MA, Giuffrida L, Mills JK, Sek K, Cross RS, Davenport AJ, John LB, Mardiana S, Slaney CY, et al. Targeting the adenosine 2A receptor enhances chimeric antigen receptor T cell efficacy. J Clin Invest. 2017;127:929–941. doi: 10.1172/JCI89455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Arab S, Kheshtchin N, Ajami M, Ashurpoor M, Safvati A, Namdar A, Mirzaei R, Mousavi Niri N, Jadidi-Niaragh F, Ghahremani MH, et al. Increased efficacy of a dendritic cell-based therapeutic cancer vaccine with adenosine receptor antagonist and CD73 inhibitor. Tumour Biol. 2017;39:1010428317695021. doi: 10.1177/1010428317695021. [DOI] [PubMed] [Google Scholar]
- 119.Jadidi-Niaragh F, Atyabi F, Rastegari A, Kheshtchin N, Arab S, Hassannia H, Ajami M, Mirsanei Z, Habibi S, Masoumi F, et al. CD73 specific siRNA loaded chitosan lactate nanoparticles potentiate the antitumor effect of a dendritic cell vaccine in 4T1 breast cancer bearing mice. J Control Release. 2017;246:46–59. doi: 10.1016/j.jconrel.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 120.Kheshtchin N, Arab S, Ajami M, Mirzaei R, Ashourpour M, Mousavi N, Khosravianfar N, Jadidi-Niaragh F, Namdar A, Noorbakhsh F, et al. Inhibition of HIF-1α enhances anti-tumor effects of dendritic cell-based vaccination in a mouse model of breast cancer. Cancer Immunol Immunother. 2016;65:1159–1167. doi: 10.1007/s00262-016-1879-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.US National Library of Medicine. Trial of PBF-509 and PDR001 in patients with advanced non-small cell lung cancer (NSCLC) (AdenONCO) [Internet] [accessed on 2016]. Available at https://www.clinicaltrials.gov/ct2/show/NCT02403193.
- 122.US National Library of Medicine. Phase 1/1b study to evaluate the safety and tolerability of CPI-444 alone and in combination with atezolizumab in advanced cancers [Internet] [accessed on 2018]. Available at https://www.clinicaltrials.gov/ct2/show/NCT02655822?term=CPI-444&rank=2.
- 123.US National Library of Medicine. A phase 1 clinical study of AZD4635 in patients with advanced solid malignancies [Internet] [accessed on 2018]. Available at https://www.clinicaltrials.gov/ct2/show/NCT02740985?term=AZD4635&rank=1.
- 124.US National Library of Medicine. MEDI9447 alone and in combination with MEDI4736 in adult subjects with select advanced solid tumors [Internet] [accessed on 2018]. Available at https://www.clinicaltrials.gov/ct2/show/NCT02503774?term=MEDI9447&rank=1.
- 125.US National Library of Medicine. CPI-006 alone and in combination with CPI-444 and with pembrolizumab for patients with advanced cancers [Internet] [accessed on 2019]. Available at https://clinicaltrials.gov/ct2/show/NCT03454451.