Abstract
Purpose
To report a case of a healthy, young male with recurrent herpes zoster ophthalmicus (HZO) and high-dose l-arginine supplementation.
Observations
A 39-year-old man presented to another institution with a HZO involving the right eye. He was treated with oral acyclovir with complete resolution. Four months later the patient had a recurrent HZO episode and was started again on oral acyclovir. After resolution of the episode, the patient was continued on 1 g of oral acyclovir daily as a prophylactic measure. He then presented to our institution for a second opinion. The patient was otherwise healthy, with no past medical history involving systemic immunosuppressant agents or HIV. However, the patient was an active weight lifter taking high doses of amino acids. A diet recall was performed prior to both HZO episodes, which calculated an average intake of 46.5 g of l-arginine a day. Examination revealed 20/40 best-corrected acuity, anterior stromal haze in the visual axis, and inferior superficial punctate keratitis in the right eye. The patient was treated for dry eye disease with punctual plugs and artificial tears. The patient was instructed to decrease all supplemental arginine consumption while continuing with oral acyclovir prophylaxis for one year.
Conclusions and importance
l-arginine is associated with the replication and virulence of a variety of viruses in vitro, including herpes simplex and varicella zoster. Although arginine consumption increased prior to the initial and recurrent HZO infection, further investigation needs to be performed to deem if a true association exists.
Keywords: Varicella zoster virus, Herpes zoster ophthalmicus, Herpes zoster keratitis, Recurrence, Immunocompetent, l-arginine
1. Background
l-arginine is an amino acid found naturally in poultry, red meat, fish, nuts, and dairy products. It is a conditional amino acid which becomes essential for developing infants, children, or adults experiencing catabolic stress. However, supplementation with l-arginine is widespread in the fitness community as its inherent properties may increase muscular performance and hypertrophy, especially when combined with other nutrients such as creatinine.1
In vitro experiments have shown arginine to be associated with the replication and virulence of a variety of viruses, including viruses from the herpes family.2, 3, 4, 5 These viruses are dependent on arginine-associated metabolic pathways in order to facilitate viral replication. Therefore, diets with increased l-arginine consumption may potentially affect herpes infection and replication.
Varicella Zoster Virus (VZV), also known as shingles, is part of the herpes virus family.
When zoster involves the ophthalmic division of the fifth cranial nerve (V1) it is known as herpes zoster ophthalmicus (HZO).
About 30% of individuals will develop shingles once in their lifetime, with a majority of individuals affected over the age of 65.6 A vast majority of these individuals will only have one episode of shingles, as the incidence of recurrence has ranged from 1.3% to 6.2%.7 Nevertheless, we present a case of recurrent zoster infection in a young, healthy, immunocompetent individual.
2. Case presentation
A 39-year-old male presented to another institution with a right-sided vesicular rash which involved the V1 dermatome. Herpes zoster ophthalmicus was confirmed by clinical exam and the patient was treated with oral acyclovir. Four months later the patient had a recurrent HZO episode involving the right eye and was started on a standard regimen of oral acyclovir within 72 hours of onset. After resolution of this subsequent HZO episode, the patient was started on 1 g oral acyclovir daily for HZO prophylaxis.
The patient presented to our institution for a second opinion. His past medical history was unremarkable. He denied taking any medication including immunosuppressant agents. However, he noted to take various supplements related to athletic performance, including large amounts of amino acids such as arginine. The patient was an active weight lifter with a diet high in meat, nuts, dairy, and supplemental amino acids. Based on a diet recall prior to both HZO episodes, his daily consumption of l-arginine averaged around 46.5 g (Table 1). He was 5 feet 9 inches and weighed 165 pounds with a body mass index of 24.4. The patient denied any sexually transmitted diseases including HIV as well as anabolic steroid abuse. Labratory testing confirmed normal androgen levels and negative HIV infection.
Table 1.
The typical daily diet of the patient prior to the development of HZO. The daily arginine intake ranged from 35.2 to 57.9 g, averaging 46.5 g. Arginine amount derived from the USDA National Nutrient Database. http://www.ars.usda.gov/nutrientdata
| Typical Diet | Average Amount | Arginine (grams) |
|---|---|---|
| Dry Roasted Nuts (peanuts, almonds, or walnuts) | 3 cups | 10.2–13.5 |
| Meat (chicken, fish, pork, or beef) | 4 cups | 9.6–24.0 |
| Whole cooked eggs | 3 large | 1.22 |
| Cottage cheese | 1 cup | 2.56 |
| Yogurt | 1 cup | 0.27 |
| Rice | 2 cups | 0.76 |
| Broccoli | 2 cups | 0.59 |
| Supplements (protein power, pre-workout drinks, and amino acids) | NA* | 10.0–15.0 |
| Patient Range | 35.2–57.9 | |
| Normal Range | 4.2–20.0 |
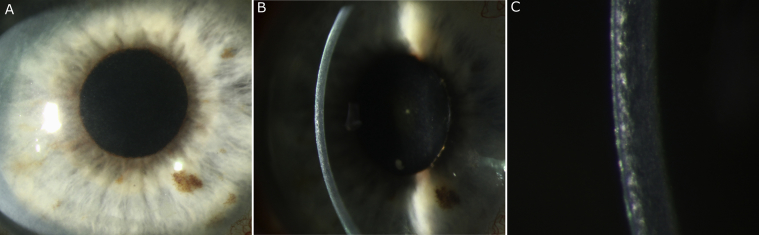
The patient's visual acuity was 20/40 for the right eye (OD) and 20/20 for the left eye (OS). Upon slit lamp examination of the right eye, there was anterior stromal haze in the visual axis from a resolved herpetic infection and inferior superficial punctate keratitis (Fig. 1). The anterior chamber was quiet with no signs of inflammation for both eyes (OU). Fundus examination revealed no posterior segment inflammation with the optic nerve, macula, and peripheral retina within normal limits OU. The slit lamp examination was remarkable for superficial punctate keratitis OS with a normal fundus exam. Optical coherence tomography (OCT) of the nerve and macula confirmed normal findings of the ophthalmoscopic exam.
Fig. 1.
A representative image was acquired from Jesse Vislisel, MD and Brice Critser, CRA from eyerounds. org at the University of Iowa (Creative Commons Attribution-NonCommercial-NoDerivs 3.0). Full permission was given for use in publication. A) An external photo depicting a resolved zoster keratitis with a corneal haze in the central visual axis. Low magnification B) and high magnification C) of the slit lamp through the corneal opacities, depicts anterior stromal haze from the resolve zoster virus keratitis.
Punctual plugs and artificial tears were used to treat the dry eye findings of the patient. The patient was also recommended to decrease l-arginine supplementation in his diet. The patient was also continued with oral acyclovir for one year. The patient stopped acyclovir after one year and remained compliant with his low arginine diet. Nine months later the patient remained asymptomatic with no recurrent herpetic infection.
3. Discussion
Herpes zoster virus (HZV) is a painful dermatomal rash resulting from the reactivation of latent varicella infection from childhood. Sequelae from herpes zoster infection may result in post-herpetic neuralgia or ocular complications, leading to significant morbidity.6 Clinicians often think herpes zoster primarily affects the elderly or immunosuppressed population without reoccurrence. However, a study of 1669 zoster patients followed over the course of 6–12 years found a recurrence rate of 6.2%.7 Interestingly, 85% of those who developed recurrent herpes zoster were not immunocompromised.7 Another study cohort involving 18 patients under 40 years old revealed a similar trend, finding a majority of patients affected by HZO were immunocompetent.8 Lastly, a study analyzing patients with recurrent HZO were also mostly healthy, non-immunocompromised individuals.9 It is important to note that the each of the above studies had a larger immunocompetent cohort and that immunocompromised individuals had a higher rate of reoccurrence. In spite of this, it is possible that other underlying properties may have led to primary or recurrent zoster infection in these immunocompetent individuals.
Although the majority of individuals affected by herpes zoster are over the age of 60,6 a decreasing mean age of onset has been documented in the recent decade.10 In a population-based study, Chan et al. compared patients diagnosed with zoster from 1996 to 2004 to patients in the 2005 to 2012 period. Analysis revealed that 32.3% of the patients with zoster were less than 60 years old in 1996–2004, compared with 44.8% in the 2005 to 2012 period, likely due to widespread childhood varicella vaccination. Chan et al. also found other risk factors associated with younger age of onset including immunosuppression from either pharmacological or pathological causes, autoimmunity, female gender, smoking, and diabetes.10 However, smoking was the strongest risk factor with patients presenting on average 11.5 years earlier than non-smokers.10
Although our patient was younger than the average patient affected by herpes zoster, he did not have any known risk factors listed above. Other factors may have contributed to his clinical presentation. In particular, our patient consumed high levels of the amino acid l-arginine, averaging 46.5 g a day, preceding both HZO infections. The average intake of l-arginine from food and supplements in the general population is approximately 4.2 g per day (g/day). Through a quantitative risk assessment based on published human clinical trial data, the observed safety level for arginine is up to 20 g per day.11 Although some studies have exceeded 20 g a day for a short time period, there is insufficient data to make a confident conclusion about the potential adverse effects.11
At doses from 4 to 20 g/day, l-arginine has been associated with beneficial effects on wound healing, immune function, and metabolism.11 However, l-arginine also serves an essential role in viral replication. Herpes simplex viruses (HSV-1 and HSV-2), varicella-zoster virus (VZV), cytomegalovirus (CMV), and adenovirus require arginine to replicate.2, 3, 4, 5 One in vitro study demonstrated suppression of HSV replication within cells lacking arginine, a phenomenon which was rapidly reversed with the introduction of l-arginine.2
Other in vitro studies have documented the effect of l-arginine by examining the function of arginase, an enzyme which degrades l-arginine to l-ornithine and urea. Increase in arginase and reduction in l-arginine led to the inhibition of herpes simplex 1 and 2 replication, cell-to-cell transmission, and virus-mediated cytopathic effects.12 In addition, arginase 1 metabolism appeared to increase 10 fold in a murine model of HSV-1 induced stromal keratitis infection,12 suggesting a correlation with symptom resolution and arginine levels. Kahan et al. also found that corneal herpes was accompanied by the accumulation of arginine in the corneal epithelium, a potential finding which they attributed to increased viral replication and virulence.13 Furthermore, the addition of topical ocular arginase led to a resolution of the herpetic episode, further substantiating arginine's role in infection and disease progression.13
On the other hand, some in vitro studies utilizing arginine or arginine derived compounds have also been found to be viricidal against the herpes simplex virus.14,15 Naito et al. showed that arginine was effective against inhibiting HSV-1 replication at moderate concentrations.14 In comparison, Yamasaki et al. reported that the arginine derivative, Nα-Cocoyl-l-arginine ethyl ester, was effective at inhibiting HSV-1 and HSV-2 extracellular virus particles and multiplication.15 Another important discrepancy involves a recent large meta-analysis which suggested arginine supplementation increased CD4+T cell proliferation responses and reduced infectious complications compared to controls (OR 0.40; 95% CI 0.17, 0.95; p < 0.05).16
The contrasting results may be due to the context in which arginine is utilized. Arginine becomes an essential amino acid during states of rapid depletion such as infection, tissue injury, or inflammation where cationic amino acid transporters are upregulated in myeloid cells.17 Arginine supplementation has been shown to be effective on critically ill patients. The use of immune-enhancing diets, such as arginine, omega-3 fatty acids, glutamine, nucleotides, and structured lipids, have been used to improve the outcome and recovery time of patients affected by trauma, sepsis, surgery, and HIV by replacing critical nutrients depleted during states of high catabolic stress.18 However, arginine supplementation in these studies generally does not exceed 30 g or 30 days. Secondly, little arginine is used by myeloid cells under normal catabolic states due to lack of expression of cell membrane transporters.17 Therefore, a decrease in cell utilization combined with increased arginine supplementation for the course of months may have impacted herpetic reactivation and infection in our patient. However, further observation and investigation needs to be performed to deem if a true association exists.
However as a single case report, we cannot make any definitive conclusions regarding arginine intake and HZO infection. The incidence of recurrent HZO preceded by high arginine consumption is an intriguing observation but may only be coincidental. Nevertheless, as nutrition is becoming an important topic in medicine, athletics, and overall quality of life, further investigation should take place to deem if a true associate exits, especially in at-risk individuals.
4. Conclusion
In summary, we present a case report of l-arginine supplementation, averaging 46.5 g/day, preceding a primary and recurrent herpes zoster ophthalmicus in a young, immunocompetent individual. In vitro studies have shown l-arginine to serve as an essential role in viral replication for the herpes virus, including HSV-1/2, VZV, CMV, and adenovirus. In vitro experiments have demonstrated conflicting results on the effect of arginine and herpes replication. Clinical trials on arginine supplementation are also limited but have shown to be beneficial up to 20 g for short intervals with patients experiencing high states of catabolic stress. The effect of high dose l-arginine, exceeding 30 days, has not been studied and requires further investigation. Although we present a case of recurrent HZO preceded by high dose arginine, our observations are limited to one case and may only be coincidental. Thus further investigation needs to be performed to deem if a true association exists between arginine consumption and HZO, especially in at-risk individuals.
Patient consent
The patient consented to publication of the case in writing. This report does not contain any personal information that could lead to the identification of the patient.
Funding
No funding or grant support.
Disclosure
The following authors have no financial disclosures: SAL, PT, SMC, FM, RAG, ES, TDL.
Conflicts of interest
None of the authors have any financial disclosures or potential conflicts of interest.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
None.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ajoc.2019.100547.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Little J.P., Forbes S.C., Candow D.G., Cornish S.M., Chilibeck P.D. Creatine, arginine alpha-ketoglutarate, amino acids, and medium-chain triglycerides and endurance and performance. Int J Sport Nutr Exerc Metab. 2008;18(5):493–508. doi: 10.1123/ijsnem.18.5.493. [DOI] [PubMed] [Google Scholar]
- 2.Tankersley R.W. Amino acid requirements of herpes simplex virus in human cells. J Bacteriol. 1964;87:609–613. doi: 10.1128/jb.87.3.609-613.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gönczöl E., Boldogh I., Váczi L. Effect of arginine deficiency on the reproduction of human cytomegalovirus. Acta Microbiol Acad Sci Hung. 1975;22(3):263–270. [PubMed] [Google Scholar]
- 4.Wigand R., Kümel G. Amino acid requirement of adenovirus multiplication. J Gen Virol. 1978;39(2):281–292. doi: 10.1099/0022-1317-39-2-281. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez M.D., Ochoa A.C., Foster T.P. Development and evaluation of a host-targeted antiviral that abrogates herpes simplex virus replication through modulation of arginine-associated metabolic pathways. Antivir Res. 2016;132:13–25. doi: 10.1016/j.antiviral.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harpaz R., Ortega-Sanchez I.R., Seward J.F. Advisory committee on immunization practices (ACIP) centers for disease control and prevention (CDC). Prevention of herpes zoster: recommendations of the advisory committee on immunization practices (ACIP) MMWR Recomm Rep (Morb Mortal Wkly Rep) 2008;57(RR-5):1–30. quiz CE2-4. [PubMed] [Google Scholar]
- 7.Yawn B.P., Wollan P.C., Kurland M.J., Sauver JL St, Saddier P. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc. 2011;86(2):88–93. doi: 10.4065/mcp.2010.0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta N., Sachdev R., Sinha R., Titiyal J.S., Tandon R. Herpes zoster ophthalmicus: disease spectrum in young adults. Middle East Afr J Ophthalmol. 2011;18(2):178–182. doi: 10.4103/0974-9233.80710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran K.D., Falcone M.M., Choi D.S. Epidemiology of herpes zoster ophthalmicus: recurrence and chronicity. Ophthalmology. 2016;123(7):1469–1475. doi: 10.1016/j.ophtha.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan A.Y., Conrady C.D., Ding K., Dvorak J.D., Stone D.U. Factors associated with age of onset of herpes zoster ophthalmicus. Cornea. 2015;34(5):535–540. doi: 10.1097/ICO.0000000000000362. [DOI] [PubMed] [Google Scholar]
- 11.Shao A., Hathcock J.N. Risk assessment for the amino acids taurine, L-glutamine and L-arginine. Regul Toxicol Pharmacol. 2008;50(3):376–399. doi: 10.1016/j.yrtph.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Mistry S.K., Zheng M., Rouse B.T., Morris S.M. Induction of arginases I and II in cornea during herpes simplex virus infection. Virus Res. 2001;73(2):177–182. doi: 10.1016/s0168-1702(00)00243-4. [DOI] [PubMed] [Google Scholar]
- 13.Kahán I.L., Hajas K., Halász A. The significance of the arginine and arginase of tears in experimentally-induced herpes simplex corneae. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1979;209(3):219–224. doi: 10.1007/BF00414614. [DOI] [PubMed] [Google Scholar]
- 14.Naito T., Irie H., Tsujimoto K., Ikeda K., Arakawa T., Koyama A.H. Antiviral effect of arginine against herpes simplex virus type 1. Int J Mol Med. 2009;23(4):495–499. doi: 10.3892/ijmm_00000156. [DOI] [PubMed] [Google Scholar]
- 15.Yamasaki H., Tsujimoto K., Ikeda K., Suzuki Y., Arakawa T., Koyama A.H. Antiviral and virucidal activities of nα-cocoyl-L-arginine ethyl ester. Adv Virol. 2011;2011 doi: 10.1155/2011/572868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang K., Shu X.-L., Zhong J.-X., Yu T.-T. Effect of L-arginine on immune function: a meta-analysis. Asia Pac J Clin Nutr. 2014;23(3):351–359. doi: 10.6133/apjcn.2014.23.3.09. [DOI] [PubMed] [Google Scholar]
- 17.Popovic P.J., Zeh H.J., Ochoa J.B. Arginine and immunity. J Nutr. 2007;137(6 Suppl 2):1681S–1686S. doi: 10.1093/jn/137.6.1681S. [DOI] [PubMed] [Google Scholar]
- 18.Bistrian B.R. Practical recommendations for immune-enhancing diets. J Nutr. 2004;134(10 Suppl):2868S–2872S. doi: 10.1093/jn/134.10.2868S. discussion 2895S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.