Abstract
Entamoeba histolytica and Giardia duodenalis are widespread intestinal protozoan parasites which both spread via cysts that have to be ingested to infect a new host. Their environment, the small intestine for G. duodenalis and the colon for E. histolytica, contains only very limited amounts of oxygen, so both parasites generate energy by fermentation and substrate level phosphorylation rather than by oxidative phosphorylation. They both contain reducing agents able to reduce and activate nitroimidazole drugs such as metronidazole which is the gold standard drug to treat Entamoeba or Giardia infections. Although metronidazole works well in the majority of cases, it has a number of drawbacks. In animal models, the drug has carcinogenic activity, and concerns about a possible teratogenic activity remain. In addition, the treatment of G. duodenalis infections is hampered by emerging metronidazole resistance. Plant-derived drugs play a dominant role in human medicine, therefore we tested the activity of 14 isolated plant compounds belonging to seven different classes in vitro against both parasites. The tests were performed in a new setting in microtiter plates under anaerobic conditions. The compound with the highest activity was methylgerambullin, a sulphur-containing amide found in Glycosmis species of the family Rutaceae with an EC50 of 14.5 μM (6.08 μg/ml) after 24 h treatment for E. histolytica and 14.6 μM (6.14 μg/ml) for G. duodenalis. The compound was successfully synthesised in the laboratory which opens the door for the generation of new derivatives with higher activity.
Keywords: Entamoeba histolytica, Giardia duodenalis, Glycosmis spp., Methylgerambullin, Sulphur-containing amide, Aglafoline
Graphical abstract
Highlights
-
•
Fourteen plant compounds of seven classes were isolated.
-
•
The activity against Entamoeba histolytica and Giardia duodenalis was tested.
-
•
Methylgerambullin had the highest activity against both parasites.
-
•
Methylgerambullin is a sulphur-containing amide from Glycosmis spp. (Citrus plants).
-
•
High cysteine concentrations interfered with methylgerambullin activity.
1. Introduction
Entamoeba histolytica and Giardia duodenalis are human protozoan parasites with a simple life cycle lacking intermediate hosts. Infectious cysts are passed in the stool of patients and have to reach uninfected individuals either via smear infection or via food or water. After passing through the stomach, they excyst as trophozoites. Whereas G. duodenalis trophozoites colonize the small intestine, E. histolytica trophozoites reside in the colon.
G. duodenalis infections can remain without symptoms, but can also lead to diarrhoea with greasy or foul-smelling stools, accompanied by abdominal pain, flatulence, bloating, nausea, and sometimes weight loss (Minetti et al., 2016). As the parasite interferes with the absorption of nutrients in the small intestine, it is not surprising that cases of giardiasis were associated with underweight and severe malnutrition in children in a study from Rwanda (Ignatius et al., 2012). An estimated 184 million of symptomatic cases per year occur worldwide (Havelaar et al., 2015), with a higher frequency in poor regions with a lack of clean drinking water. Deaths caused by G. duodenalis infections are extremely rare (Gargano et al., 2017).
In the colon, E. histolytica trophozoites are able to phagocytose bacteria and take up remaining nutrients from the host, thus the infection can remain asymptomatic, but they can also attach to the mucus and enterocytes and penetrate the protective layers into the intestinal wall. This can result in amoebic dysentery with abdominal pain, tenesms and diarrhoea, sometimes with blood-covered stools. Moreover, the amoebae can invade the mesenterial vessels to be carried to the liver where they can establish large abscesses (Stanley, 2003). The Global Burden of Disease 2010 Study of the University of Washington estimated 55,500 deaths and 2.2 million years of life lost from premature death or disability (DALYs) caused by amoebiasis (Turkeltaub et al., 2015). So amoebiasis remains a serious neglected infectious disease.
Both E. histolytica and G. duodenalis infections are treated with metronidazole, as the gold standard drug. To become active, the drug must be reduced at its nitro group (Müller, 1983). This reduction typically occurs in microaerophilic or anaerobic microorganisms. E. histolytica and G. duodenalis possess a key enzyme, pyruvate:ferredoxin oxidoreductase (PFOR) catalysing the oxidation of pyruvate to acetyl-CoA and CO2 with the concomitant generation of reduced ferredoxin, which is able to activate metronidazole (Moreno et al., 1984; Upcroft and Upcroft, 2001). An alternative mechanism to reduce and activate metronidazole is by thioredoxin reductase with the cofactor NADPH (Leitsch et al., 2007). In addition, a nitroreductase GINR1 with the ability to reduce metronidazole has been characterised in G. duodenalis (Nillius et al., 2011).
Considering that metronidazole has been used for more than 50 years in E. histolytica, the low level of resistance is surprising. Treatment failures have been reported (Hanna et al., 2000), and in the laboratory the amoebae could be adapted to metronidazole concentrations between 10 μM (Upcroft and Upcroft, 2001) and 40 μM (Wassmann et al., 1999), but to our knowledge, no resistant strain could be isolated from any patient. In G. duodenalis, metronidazole resistance is much more of a problem (Upcroft and Upcroft, 2001; Leitsch et al., 2012).
Metronidazole treatment is associated with some common adverse effects such as metallic taste, headache, nausea, and negative interaction with alcohol, and rarely, with central or peripheral neurotoxicity, pancreatitis or neutropenia (Gardner and Hill, 2001). The biggest concern, however, is that the International Agency for Research on Cancer (IARC) has listed metronidazole as animal carcinogenic and possible human carcinogenic (IARC, 1987). DNA damage has been observed in individuals treated with metronidazole, however, the exact mechanism how this occurs remains unknown, and no long-term studies have been carried out to resolve the question if metronidazole is carcinogenic for humans (Bendesky et al., 2002). Taken together, emerging resistance in the case of G. duodenalis and remaining concerns over its possible carcinogenic activity justify to continue the search for alternatives to metronidazole.
Throughout recorded human history, medicines derived from plants have been used to treat various diseases (Cragg and Newman, 2013), in particular infections with parasites (Wink, 2012). Malaria treatment by quinine, its derivatives chloroquine and mefloquine, and the current drug artemisine (Tu, 2016) and its derivatives has literally saved many millions of lives. These drugs, like all anti-infective agents, suffer from problems of resistance, even the best of them, artemisinin (Noedl et al., 2008), so the search has to continue. New compounds from plants often have complex structures, are extracted in small amounts and the ownership of intellectual property may raise disputes. In the last two decades of the previous century, high-throughput synthesis of compounds addressing very specific targets was hoped to quickly generate better drug candidates. With some disappointments of the pure chemical approach, and with a realistic view on drug development from plants, this field recovered in the new century (Balunas and Kinghorn, 2005). In a large review, Newman and Cragg (2016) analysed the 1562 new drugs approved in the period between 1981 and 2014. These included only 27% of chemically synthesised drugs. The vast majority is a complex list of pure or mixed natural products, mostly derived from plants, chemically modified natural products, synthetic drugs with a natural pharmacophore, mimics of natural products, as well as vaccines.
Previously we tested the activity of a small series of plant-derived compounds comprising several classes against several important protozoan parasites. The maturation of Plasmodium falciparum schizonts was inhibited by sub-micromolar concentrations of the flavaglines rocaglamide and aglafoline (Astelbauer et al., 2012). Two further studies, included, in addition, sulphur-containing amides from Glycosmis spp. (Rutaceae). These compounds were highly active in low micromolar concentrations against Trypanosoma cruzi epimastigotes (Astelbauer et al., 2010) and Leishmania infantum promastigotes (Astelbauer et al., 2011). In the present work we tested the activity of a similar set of 14 compounds against E. histolytica and G. duodenalis and found the sulphur-containing amide methylgerambullin to display the highest activity. This compound is easily accessible to chemical synthesis opening the door to study its mechanism of action and to generate improved derivatives.
2. Materials and Methods
2.1. Parasites and culture
The Entamoeba histolytica trophozoites (strain HM-1:IMSS, ATCC 30459) used in this study were axenically cultivated in TYI-S-33 medium (Diamond et al., 1978), containing 10% (v/v) complement-inactivated bovine serum, 1% (v/v) penicillin/streptomycin solution (10,000 units penicillin and 10 mg streptomycin per ml, Sigma-Aldrich) and 3% (v/v) of complete vitamin mixture (Diamond Vitamin Tween 80 Solution, SAFC Biosciences, KA, USA). Axenical cultivation of Giardia intestinalis WB clone 6 (ATCC 50803) cells was performed in Keister's modified TYI-S-33 medium (Keister, 1983), supplemented with 1% (v/v) penicillin/streptomycin solution and 10% (v/v) complement-inactivated bovine serum. Both media are rich in cysteine, 1 mg/ml for E. histolytica and 2 mg/ml for G. duodenalis. Entamoeba trophozoites were subcultured twice and Giardia trophozoites three times per week.
2.2. Compounds
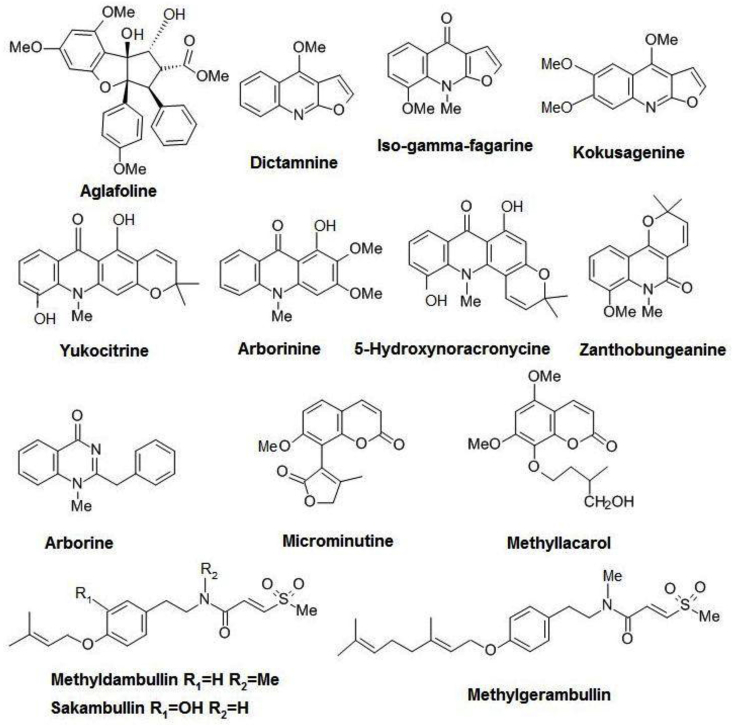
The fourteen tested compounds (Fig. 1, Table 1) belong to seven different chemical classes. Aglafoline is a flavagline from the family Meliaceae (mahogany family). The furoquinolines dictamnine, iso-gamma-fagarine and kokusagenine, the acridones yukocitrine, arborinine and 5-hydroxynoracronycine, the quinolinone zanthobungeanine and the quinazoline arborine are alkaloids found in members of the family Rutaceae (Citrus plants). Methyllacarol found in Asteraceae and microminutine from Rutaceae are coumarins, finally the sulphur-containing amides methyldambullin, sakambullin and methylgerambullin are again found in Rutaceae.
Fig. 1.
The 14 compounds tested against E. histolytica and G. duodenalis in this study.
Table 1.
List of compounds tested in this study, compound class, origin from plant family and species, plant organs, references.
| Compound | Compound class | Plant family | Plant species | Plant organ | References for isolation |
|---|---|---|---|---|---|
| Aglafoline | Flavagline | Meliaceae | Aglaia odorata | Stembark | Brem. (2002) |
| Greger et al. (2001) | |||||
| Dictamnine | Furoquinoline | Rutaceae | G. trichanthera | Rootbark | Vajrodaya et al. (1998) |
| Iso-gamma-fagarine | Furoquinoline | Rutaceae | G. sapindoides | Leaves | Vajrodaya (1998) |
| Kokusagenine | Furoquinoline | Rutaceae | G. sapindoides | Rootbark | Vajrodaya (1998) |
| Yukocitrine | Acridone | Rutaceae | G. trichanthera | Stembark | Vajrodaya et al. (1998) |
| Arborinine | Acridone | Rutaceae | G. sapindoides | Leaves | Vajrodaya (1998) |
| 5-Hydroxy-noracronycine | Acridone | Rutaceae | G. trichanthera | Stembark | Vajrodaya et al. (1998) |
| Zanthobungeanine | Quinolinone | Rutaceae | Zanthoxylum simulans | Rootbark | Brader et al. (1993) |
| Arborine | Quinazoline | Rutaceae | G. pentaphylla | Leaves | Vajrodaya (1998) |
| Microminutine | Coumarin | Rutaceae | Micromelum cf.minutum | Leaves | Grassi (1998) |
| Methyllacarol | Coumarin | Asteraceae | Artemisia laciniata | Leaves | Szabo et al. (1985) |
| Methyldambullin | S-amidea | Rutaceae | G. angustifolia | Leaves | Greger et al. (1994) |
| Sakambullin | S-amidea | Rutaceae | G. chlorosperma | Leaves | Hofer et al. (2000) |
| Methylgerambullin | S-amidea | Rutaceae | G. trichanthera | Leaves | Vajrodaya et al. (1998) |
S-amide = sulphur-containing amide.
The compounds were extracted from various plant organs (Table 1) as described before (Greger et al., 1994, 1996; Hofer et al., 2000). Briefly, the methanolic extract was concentrated and the aqueous residue extracted with CHCl3. The CHCl3 fractions were roughly separated by column chromatography (CC), and further separation was achieved by preparative medium pressure chromatography (MPLC). In some cases, preparative thin layer chromatography (TLC) was used for the final purification. The identity of the compounds was confirmed by comparison with authentic samples by high performance liquid chromatography (HPLC) and nuclear magnetic resonance (NMR) analysis. For stock solutions, the compounds were dissolved in dimethylsulphoxide DMSO (Sigma-Aldrich) at a concentration of 10 μg/ml.
2.3. Susceptibility assays
The assays were carried out in 96-well microplates. For E. histolytica assays, parasite viability was very poor, when the plates were only covered with parafilm and lid, or set in a candle jar as used in malaria research (Jensen and Trager, 1977) (results not shown). In contrast, cells remained viable and proliferated well in a rectangular air-tight plastic box with air-tight clamps (Komax, Korea) in which anaerobic conditions were ensured by Anaerocult A pouches (Merck Darmstadt, Germany). A box with the dimensions 19 cm length, 13 cm width and 4.5 cm height was suitable for up to two plates. Anaerobic conditions were tested with Anaerotest strips (Merck). The same system was also found suitable for G. duodenalis assays.
For the assays, the parasites were seeded at a concentration of 40,000 cells ml−1 in a volume of 300 μl. After incubation of 24 h or 48 h, to 20 μl of cells, released by vigorous pipetting, an equal volume of a 0.4% solution of Trypan blue (Sigma-Aldrich) was added and the number of the dead and living cells was counted in a Bürker-Türk haemocytometer.
Initially, the activity of the compounds was compared by quick tests with final drug concentrations of 2.5 μg/ml and 10 μg/ml. The tests were performed twice in triplicates and the percentage of growth inhibition GI was determined for each sample after incubation at 37 °C for 24 h or 48 h. GI [%] (percent growth inhibition) was calculated by the formula GI = [(Gc - Gp)/ Gc] x 100, where Gc equals the mean number of living cells per ml in control (no drug added), and Gp equals the mean number of living cells per ml at the different drug concentrations. Standard deviations σn were calculated.
When it turned out that methylgerambullin was by far the most active compound against E. histolytica and G. duodenalis, the compound was tested in the same manner, under the same conditions, only in a wider range of concentrations: 1 μg/ml, 2.5 μg/ml, 5 μg/ml, 7.5 μg/ml, 10 μg/ml and 20 μg/ml respectively. The EC50 (half maximal effective concentration) value of methylgerambullin was calculated via log-probit analysis (SPSS 16.0, IBM, Chicago, IL). All experiments were carried out three times with results counted in triplicates. The geometric means G [μM] of the EC50 values as well as the geometric standard deviations σg (Limpert et al., 2001) were calculated according to https://en.wikipedia.org/wiki/Geometric_standard_deviation. Metronidazole as positive reference compound was tested in the same way in two independent experiments and the EC50 values were calculated as well.
Finally, as aglafoline had also shown relevant activity against G. duodenalis, we tested this compound in two separate experiments, using the same concentrations in triplicate wells as above. Again, the effect was measured after 24 h and 48 h and the EC50 values were determined.
In order to test the influence of cysteine, present in the growth media of both parasites, on the activity of methylgerambullin, media were prepared containing different cysteine concentrations, 0 mg/ml, 0.125 mg/ml, 0.25 mg/ml, 0.5 mg/ml or 1 mg/ml for E. histolytica and 0 mg/ml, 0.25 mg/ml 0.5 mg/ml, 1 mg/ml or 2 mg/ml for G. duodenalis. The highest values (1 mg/ml for E. histolytica and 2 mg/ml for G. duodenalis as mentioned above) are the standard cysteine concentrations used in all the previous tests. Then the parasites were cultivated anaerobically in microtiter plates in each of the media with varied cysteine concentrations in the presence of 0 μg/ml (control), 1 μg/ml, 5 μg/ml or 20 μg/ml of methylgerambullin. After 24 h, the remaining cells were counted.
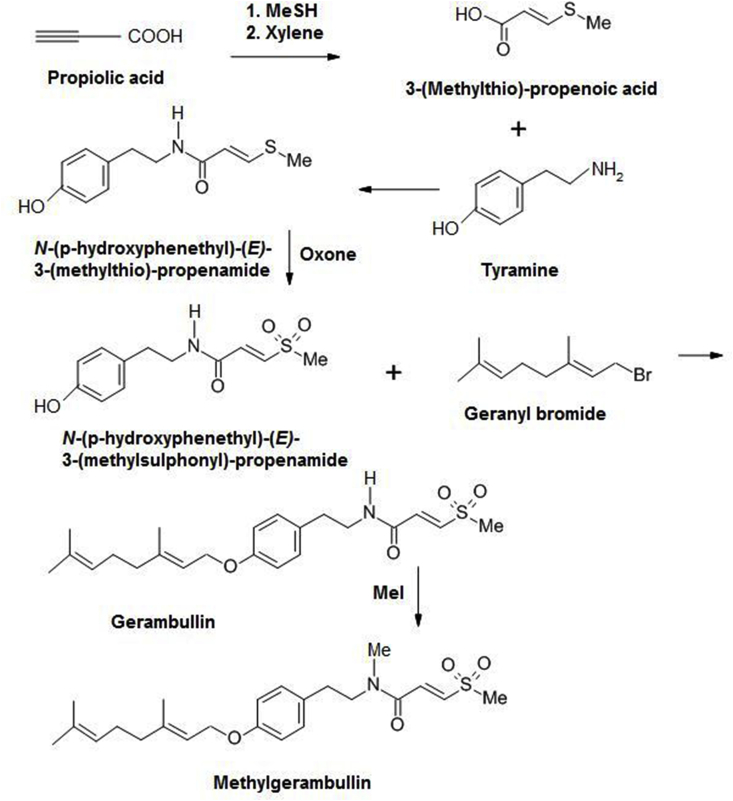
2.4. Chemical synthesis of methylgerambullin
Methylgerambullin was synthesised at the company Selvita (Krakow, Poland) in an analogous way to the synthesis of methylgerambullone (Moon et al., 2010). Briefly (Fig. 2), propiolic acid and methanethiol were condensed and isomerised in xylene to form 3-(methylthio)-(E)-propenoic acid (yield: 66%). An amide of this compound with tyramine was formed resulting in N-(p-hydroxyphenethyl)-(E)-3-(methylthio)-propenamide (yield: 52%). This compound was then oxidised to the sulfone (yield 67%). The geranyl group was introduced by geranyl bromide (yield: 69%), and finally the amide nitrogen was methylated using methyliodide (yield: 63%). The purity (>97%) and identity of the synthesised compound were assessed by HPLC and 1H and 13C NMR (Supplementary Figs. S1 and S2), and the data corresponded to the original results from the plant-derived compound (Greger et al., 1994).
Fig. 2.
Chemical synthesis of methylgerambullin from commercially available compounds.
3. Results
3.1. Selection of compounds
Fourteen purified compounds of various chemical classes and from different plant families were tested (Fig. 1, Table 1). Total plant extracts were not included, because it is easier to synthesise known compounds and in the future to generate improved derivatives.
3.2. Anaerobic assay and testing of the compounds in quick tests
For the examination of E. histolytica the most suitable system was to culture the amoebae in microtiter plates in an anaerobic environment created by Anaerocult A sachets in air-tight plastic boxes (results not shown), also G. duodenalis was examined in this anaerobic environment. All the compounds were tested in quick tests at final concentrations of 2.5 μg/ml and 10 μg/ml, and the cells were counted after 24 h and 48 h. The results are shown in Table 2. A (E. histolytica) and 2. B (G. duodenalis). Taken together, the compound methylgerambullin stood out as most active against both E. histolytica and G. duodenalis. At the higher concentration E. histolytica was inhibited by 96.5% after 24 and 96.8% after 48 h. G. duodenalis at the same concentration was inhibited by 97% after 24 h and by 99.5% after 48 h. In contrast, the other sulphur-containing amides methyldambullin and sakambullin were much less active. Whereas the compound aglafoline displayed significant and the second best activity against G. duodenalis, it was ineffective against E. histolytica.
Table 2.
Quick tests of the compounds against E. histolytica and G. duodenalis.
| A - Test of growth inhibition (GI) [%] of E. histolytica. | ||||
|---|---|---|---|---|
| Assay time |
24 h |
48 h |
||
| Concentration | 10 μg/ml | 2.5 μg/ml | 10 μg/ml | 2.5 μg/ml |
| Aglafoline | 16.3 ± 1.9 | 11.4 ± 4.2 | 7.8 ± 15.2 | 6.7 ± 6.3 |
| Dictamnine | 6.2 ± 13.2 | −5.4 ± 12.8 | 15.7 ± 3.3 | −6.4 ± 7.2 |
| Iso-gamma-fagarine | 5.9 ± 12.8 | 27.4 ± 21.2 | 16.1 ± 3.7 | −28.7 ± 11.3 |
| Kokusagenine | 8.8 ± 2.1 | 21.4 ± 5.1 | 0 ± 8.4 | −25.8 ± 27.8 |
| Yukocitrine | 11.1 ± 15.4 | 15.8 ± 9.3 | −41.9 ± 13.7 | −21.7 ± 14.0 |
| Arborinine | 15.3 ± 12.1 | 32.4 ± 5.6 | −33.2 ± 4.3 | −57.3 ± 19.4 |
| 5-Hydroxynoracronycine | 31.1 ± 5.9 | 26.6 ± 2.2 | 26.4 ± 6.1 | −41.8 ± 4.8 |
| Zanthobungeanine | 28.7 ± 4.2 | 9.7 ± 4.7 | 2.3 ± 17.2 | −7.1 ± 7.0 |
| Arborine | 22.9 ± 9.8 | 36.6 ± 7.3 | −51.5 ± 9.5 | −34.7 ± 14.7 |
| Microminutine | 26.5 ± 2.9 | 14.5 ± 1.6 | −6.6 ± 9.7 | 13.4 ± 9.2 |
| Methyllacarol | −13.6 ± 14.5 | 2.8 ± 11.1 | −16.1 ± 13.5 | −18.9 ± 9.8 |
| Methyldambullin | 27.4 ± 12.8 | 13.9 ± 9.8 | −1.0 ± 6.0 | −24.3 ± 21.6 |
| Sakambullin | 25.9 ± 10.4 | 8.1 ± 13.5 | −13.6 ± 25.5 | −56.8 ± 9.5 |
| Methylgerambullin |
96.5 ± 2.3 |
48.2 ± 14.9 |
96.8 ± 0.3 |
30.9 ± 4.9 |
|
| ||||
| B - Test of growth inhibition (GI) [%] of G.duodenalis | ||||
| Assay time |
24 h |
48 h |
||
| Concentration |
10 μg/ml |
2.5 μg/ml |
10 μg/ml |
2.5 μg/ml |
| Aglafoline | 62.6 ± 5.5 | 63.7 ± 2.6 | 73.9 ± 2.5 | 75.4 ± 2.8 |
| Dictamnine | 24.0 ± 9.5 | 11.8 ± 1.6 | 1.7 ± 3.5 | 0.5 ± 4.2 |
| Iso-gamma-fagarine | 36.2 ± 5.5 | 0.1 ± 3.14 | 2.7 ± 3.3 | −1.8 ± 2.2 |
| Kokusagenine | 18.4 ± 7.9 | 24.7 ± 1.4 | 36.1 ± 3.5 | 28.9 ± 2.3 |
| Yukocitrine | 7.5 ± 4.6 | 14.9 ± 9.3 | 7.7 ± 2.8 | 3.7 ± 10.1 |
| Arborinine | 17.0 ± 2.1 | 5.7 ± 3.8 | 12.2 ± 5.5 | 13.2 ± 3.6 |
| 5-Hydroxynoracronycine | −2.1 ± 14.1 | −0.1 ± 11.3 | 18.2 ± 5.4 | 16.1 ± 4.2 |
| Zanthobungeanine | 28.2 ± 7.4 | 0.1 ± 6.7 | 41.1 ± 7.8 | 40.1 ± 7.8 |
| Arborine | 27.8 ± 3.4 | 18.6 ± 4.8 | 9.1 ± 1.6 | 7.5 ± 3.7 |
| Microminutine | 21.5 ± 10.0 | 10.4 ± 7.7 | −2.9 ± 6.5 | −5.6 ± 9.6 |
| Methyllacarol | 27.9 ± 10.0 | 15.8 ± 5.3 | 19.2 ± 4.2 | 26.8 ± 13.9 |
| Methyldambullin | 54.8 ± 4.4 | 21.2 ± 11.7 | 26.4 ± 12.3 | 3.6 ± 5.0 |
| Sakambullin | 7.2 ± 2.2 | 7.1 ± 3.5 | 10.8 ± 1.9 | 0.7 ± 3.4 |
| Methylgerambullin | 96.9 ± 2.0 | 54.1 ± 12.9 | 99.5 ± 0.5 | 36.9 ± 9.3 |
The assays were carried out in triplicates in 96-well microplates in an air-tight plastic box under anaerobic conditions. The parasites were seeded at a concentration of 40,000 cells ml−1 in a volume of 300 μl. After incubation of 24 h or 48 h a sample was stained with Trypan blue and the number of the dead and living cells was counted. GI [%] (percent growth inhibition) ± standard deviation σn [%] was calculated as described in Materials and Methods.
3.3. Activity of methylgerambullin against E. histolytica and G. duodenalis and aglafoline against G. duodenalis
The sulphur-containing amide methylgerambullin, which had shown good activity against both protozoans, was tested at several concentrations and the cells were counted in three experiments each either after 24 h or after 48 h. The experiments were carried out with metronidazole as a control. The EC50 results are shown in Table 3. A (E. histolytica) and Table 3. B (G. duodenalis). The EC50s for E. histolytica after 24 h and 48 h were 14.5 μM (6.08 μg/ml) and 17.4 μM (7.33 μg/ml), respectively. The EC50s for G. duodenalis after 24 h and 48 h were 14.6 μM (6.14 μg/ml) and 36.5 μM (15.34 μg/ml), respectively. For unknown reasons, the first experiment on G. duodenalis showed rather poor activity (not shown) which increased the calculated mean. Taken together, the activity of methylgerambullin against both parasites was comparable.
Table 3.
Activity of methylgerambullin against E. histolytica (A) and methylgerambullin and aglafoline against G. duodenalis (B).
| A - Entamoeba histolytica | G [μM] | σg |
|---|---|---|
| Methylgerambullin | ||
| 24 h | 14.5 | 1.36 |
| 48 h | 17.5 | 1.59 |
| Control metronidazole | ||
| 24 h | 2.40 | |
| 48 h |
1.40 |
|
| B - Giardia duodenalis |
G [μM] |
σg |
| Methylgerambullin | ||
| 24 h | 14.6 | 2.85 |
| 48 h | 36.6 | 6.08 |
| Aglafoline | ||
| 24 h | 17.2 | |
| 48 h | 7.71 | |
| Control metronidazole | ||
| 24 h | 3.15 | |
| 48 h | 1.93 | |
Test of methylgerambullin and metronidazole (control) against E. histolytica (A) as well as methylgerambullin, aglafoline and metronidazole (control) against G. duodenalis (B). The geometric means G [μM] of EC50 values are shown for three experiments (methylgerambullin) or two experiments (metronidazole, aglafoline), in addition the geometric standard deviations σg are given where three experiments were performed.
The calculated EC50s for the metronidazole after 24 h and 48 h were 2.40 μM (0.41 μg/ml) and 1.40 μM (0.24 μg/ml) for E. histolytica (Table 3. A) and 3.16 μM (0.54 μg/ml) and 1.93 μM (0.33 μg/ml) for G. duodenalis (Table 3. B).
Although only methylgerambullin was active against both parasites, aglafoline did exhibit a significant activity against G. duodenalis in the quick tests (Table 2. B). With the remaining amount of aglafoline we were able to perform two EC50 measurements with triplicate samples, and the outcome is presented in Table 3. B, the EC50 for 24 h was 17.2 μM (8.47 μg/ml), and for 48 h as low as 7.71 μM (3.80 μg/ml).
3.4. Influence of the concentration of cysteine on the activity of methylgerambullin
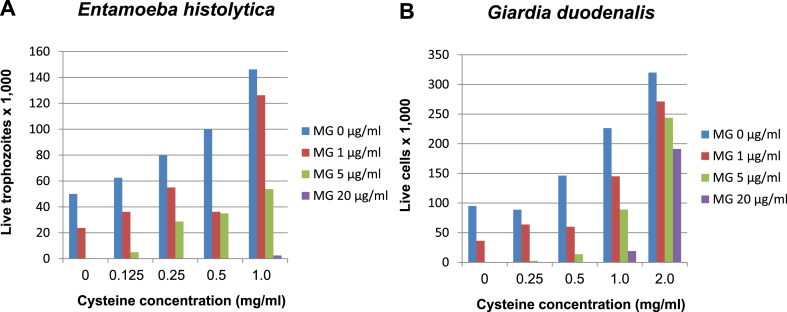
An ample supply of sulphur is provided as cysteine in media for E. histolytica (1 mg/ml) as well as G. duodenalis (2 mg/ml). We hypothesised that cysteine could influence the activity of methylgerambullin as it does for the activity of metronidazole (Leitsch et al., 2007). Therefore the activity of the sulphur-containing compound methylgerambullin in concentrations of 0 μg/ml, 1 μg/ml, 5 μg/ml or 20 μg/ml, respectively, was tested against both protozoans cultivated in media containing different cysteine concentrations, 0 mg/ml, 0.125 mg/ml, 0.25 mg/ml, 0.5 mg/ml or 1 mg/ml for E. histolytica and 0 mg/ml, 0.25 mg/ml 0.5 mg/ml, 1 mg/ml or 2 mg/ml for G. duodenalis. The surviving parasites were counted after 24 h.
The results are shown in Fig. 3A and 3B. Clearly, the activity of methylgerambullin (MG) is inhibited in rich media with high cysteine concentrations. Of course the growth of E. histolytica and G. duodenalis is also lower as less cysteine is present in the medium. When we look at the cysteine concentrations of 0.25 mg/ml for Entamoeba and 0.5 mg/ml for Giardia we can see, however, that the untreated cells still have enough cysteine to grow and divide successfully and at the same time even lower concentrations of methylgerambullin show good efficacy.
Fig. 3.
A.Entamoeba histolytica was cultivated for 24 h with different concentrations of methylgerambullin (MG) in medium with different cysteine concentrations and the number of surviving trophozoites was counted. B. The same experiment was performed for G. duodenalis.
3.5. Chemical synthesis of methylgerambullin
As among the sulphur-containing amides methylgerambullin stood out as the most active, it was desirable to be able to synthesise the compound and in the future to be able to generate derivatives as well. Fortunately, the compound could be synthesised rather easily from the commercially available compounds propriolic acid, methanethiol, tyramine and geranyl bromide (section 2.4 and Fig. 2).
4. Discussion
4.1. The sulphur-containing amide methylgerambullin and its origin
The fourteen compounds which were tested in this study against E. histolytica and G. duodenalis belong to seven different chemical classes and are shown in Fig. 1 and listed in Table 1. In preliminary tests with 2.5 μg/ml and 10 μg/ml (Tables 2A and 2B), the sulphur-containing amide methylgerambullin had the highest activity against both parasites. Therefore, the rest of this study was mainly focussed on this compound, although aglafoline had some lesser activity against G. duodenalis, but not against E. histolytica. The EC50s of methylgerambullin against E. histolytica (Table 3. A) after 24 h and 48 h were 14.5 μM (6.08 μg/ml) and 17.4 μM (7.33 μg/ml), respectively and the EC50s against G. duodenalis (Table 3. B) after 24 h and 48 h were 14.6 μM (6.14 μg/ml) and 36.5 μM (15.34 μg/ml), respectively. The EC50 of aglafoline against G. duodenalis after 24 h and 48 h was 17.2 μM (8.47 μg/ml) and 7.71 μM (3.80 μg/ml), respectively.
Methylgerambullin and the other sulphur-containing amides methyldambullin and sakambullin are found in the leaves of Glycosmis spp., a small genus of about 40 species in the family Rutaceae (Citrus plants). The plants grow in shrubs or small trees and develop small berries typically with a sweet taste giving the genus its name. In contrast, the leaves, from which the sulphur-containing amides were extracted, appear to have an unpleasant taste for grazing animals.
Chemically, the sulphur-containing acid moiety 3-(methylsulfonyl)-propenoic acid could be derived from cysteine and the p-hydroxyphenethyl amide part could be derived from tyrosine. This structure is linked to prenyloxy side chains in methyldambullin and sakambullin and to a geranyloxy side chain in methylgerambullin (Fig. 1) (Hofer and Greger, 2000). The sulphur in these prenylated amides is mostly oxidized to a sulfone or sulfoxide (Hofer et al., 2000).
4.2. Bioactivities of the sulphur-containing amides
The sulphur-containing amides methylgerambullin, sakambullin and methyldambullin were all highly active against T. cruzi epimastigotes (Astelbauer et al., 2010). Methylgerambullin had the lowest EC50 of 2.83 μM after 48 h of treatment, compared to 4.50 μM and 4.17 μM for sakambullin and methyldambullin. In a second study on L. infantum promastigotes, methyldambullin had an EC50 of 1.1 μM after 48 h of treatment (Astelbauer et al., 2011), and later, the activity of methylgerambullin after 48 h was tested at an EC50 of 0.56 μM (Astelbauer, unpublished data).
In further preliminary studies, methylgerambullin strongly inhibited the maturation of Plasmodium falciparum schizonts, but was inactive against Trichomonas vaginalis (Astelbauer, unpublished data).
Methylgerambullin showed cytotoxic activity against CEM-SS (T-lymphoblastic leukaemia), KU812F (chronic myelogenous leukaemia), HT29 (colon cancer) and UACC-62 (melanoma) cell lines, however, methylgerambullin was much less toxic against human peripheral blood mononuclear cells (Mohamed et al., 2000). Also, methylgerambullin showed no acivity against fish-pathogenic bacteria (Abdullah et al., 2006).
Taken together, methylgerambullin had a broad activity against protozoan parasites, and was superior to the other two sulphur-containing amides.
4.3. Properties of methylgerambullin
A potential orally active drug should be soluble and able to permeate to reach its target. Lipinski's “Rule of 5” (Lipinski et al., 1997) has become a widely-used tool to assess these desired properties. The rule states, that drug-like molecules should have a logP ≤5 (a measure of hydrophobicity), a molecular mass ≤ 500 Da, the number of hydrogen bond acceptors should be ≤ 10 and the number of hydrogen bond donors ≤ 5. We used the web tool www.molinspiration.com provided by the company Molinspiration Cheminformatics (Slovensky Grob, Slovak Republic) to test if the three sulphur-containing amides conform to the Rule of 5, and they all do. Although conforming to the Rule of 5 is a positive property of a molecule, many valuable drugs, in particular natural products, but sometimes even compounds designed by medicinal chemistry, do not conform to the Rule of 5 (Lipinski, 2016).
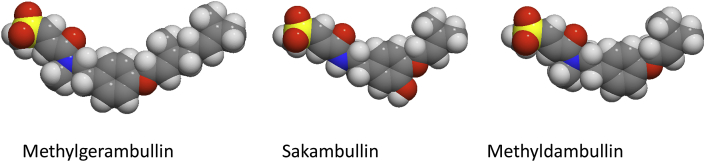
The Molinspiration website also provides the Galaxy 3D Structure Generator, which allows to visualize molecules without very unusual properties. The predicted structures of the three sulphur-containing amides are shown in Fig. 4. All three structures are bent, and there is a more hydrophilic part containing the sulfone and a more hydrophobic part with the prenyl or geranyl portion. In contrast to methylgerambullin, the compound methyldambullin with the same sulphur-containing amide part had much less activity against G. duodenalis and no activity against E. histolytica. Possibly the longer hydrophobic geranyl portion of methylgerambullin could interact with the plasma membrane of the parasites more strongly resulting in a higher activity.
Fig. 4.
Predicted structures of the sulphur-containing amides as generated by the Molinspiration Galaxy 3D Structure Generator (www.molinspiration.com).
A large advantage of the compounds is that they do not contain chiral centres and they can be synthesised from few commercially available starting materials. So it will be rather easy to generate derivatives from the compounds, such as increasing the hydrophobic side chain length to a farnesyl group. This might on the one hand increase the activity, but the logP calculation shows that the increased hydrophobicity does no longer conform to the Rule of 5.
Hydrophobicity of methylgerambullin was observed in this study. The compound dissolves extremely well in dimethylsulfoxide (DMSO) (>600 mg/ml), but no useful concentrations can be generated in H2O or ethanol. In contrast, it was possible to dissolve methylgerambullin at a concentration of 10 mg/ml in the non-toxic liquid polymer polyethylene glycol 300 (PEG 300). This required stirring overnight, however. Taken together, there will be few obstacles to synthesise a range of derivatives for methylgerambullin, but significant efforts will be needed to find the most suitable ones.
4.4. Influence of cysteine on the activity of methylgerambullin - a possible link to its mode of action
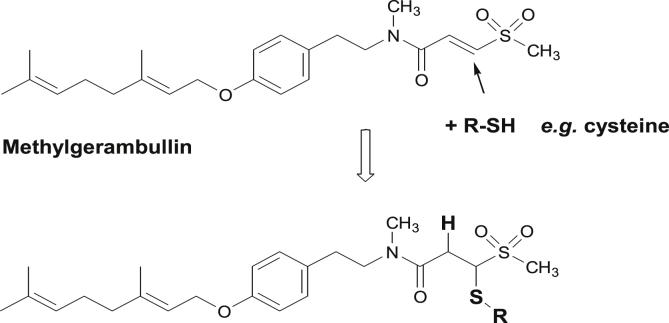
Cysteine, the important component of E. histolytica and G. duodenalis media, serves as an anti-oxidant but at least as importantly, as a source of sulphur for the biosynthesis of cysteine-rich proteins as well as iron-sulphur clusters. Under anaerobic conditions, however, E. histolytica proliferates better with cystine than with cysteine, and can do altogether without cysteine. In contrast, G. duodenalis needs cysteine, but the high concentrations are required due to the concomitant presence of bile in the medium, and without bile, G. duodenalis could do with much less cysteine (Leitsch, 2017). Importantly, cysteine inhibits the activity of various anti-parasitic compounds (Leitsch, 2017). So this was also the case for methylgerambullin (Fig. 3A and Fig. 3B). Whereas 5 μg/ml of methylgerambullin were sufficiently active at low cysteine concentrations, the standard medium concentrations of 1 mg/ml for E. histolytica and even more the 2 mg/ml for G. duodenalis drastically inhibited the drug activity. It may be imagined that cysteine is able to react with methylgerambullin (Fig. 5). In addition, this type of reaction could also occur with other free thiols in the parasite. The consequences could be inactivation of the drug or, alternatively, inactivation of proteins possessing reduced cysteine residues.
Fig. 5.
Proposed reaction scheme for the reaction of methylgerambullin with cysteine.
Inside the colon, E. histolytica has to compete for cysteine with various bacteria with an active metabolism of sulphur compounds, such as bacteria harbouring cysteine desulphydrase, which degrades cysteine to pyruvate, ammonia and hydrogen sulphide (Carbonero et al., 2012). When the amoebae invade the colonic mucosa, they encounter a cysteine concentration decreasing along the colon from about 27 mg/kg tissue in the ascending colon to about 13–15 mg/kg tissue in the transverse and descending colon and rectum (Ahlman et al., 1993). Amoebae invading into the bloodstream will encounter a plasma cysteine concentration of about 27 μg/ml (Ahlman et al., 1993). Taken together, these cysteine concentrations are much too low to significantly inhibit the activity of methylgerambullin.
4.5. On the activity of aglafoline against G. duodenalis
Although aglafoline was active only against G. duodenalis in this study, it should still be considered. It obeys Lipinski's “Rule of 5”, but it will be much more difficult to sythesize than methylgerambullin, as aglafoline possesses five centres of chirality. In our older studies it had shown activity against L. infantum (Astelbauer et al., 2011) and P. falciparum (Astelbauer et al., 2012), but not against T. cruzi.
4.6. Conclusions
From a panel of 14 plant compounds, methylgerambullin was identified as the compound with the highest activity against E. histolytica and G. intestinalis. The activity was lower than that of metronidazole, but part of the reason was that cysteine in the culture media of both parasites inhibited the action. The chemical synthesis of the compound is straightforward and this puts the discovery of derivatives with higher activity and favourable pharmacological properties within reach. The compound aglafoline was active only against G. duodenalis but should also be considered in the future.
Declarations of interest
None.
Acknowledgements
This study was supported by the Austrian Research Promotion Agency (Österreichische Forschungsförderungsgesellschaft FFG) [grant number 814280]. We thank Katarzyna Kaczorowska and her team at Selvita (Krakow) for the synthesis of methylgerambullin.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2019.08.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abdullah R., Rahmani M., Ali A.M., Ismail H.B.M., Sukari M.A., Lian G.E.C., Kulip J. Chemical constituents and biological actvities of Glycosmis chlorosperma var Elmeri (Merr.) stone (Rutaceae) Malays. J. Sci. 2006;25:45–51. [Google Scholar]
- Ahlman B., Leijonmarck C.E., Lind C., Vinnars E., Wernerman J. Free amino acids in biopsy specimens from the human colonic mucosa. J. Surg. Res. 1993;55:647–653. doi: 10.1006/jsre.1993.1198. [DOI] [PubMed] [Google Scholar]
- Astelbauer F., Obwaller A., Raninger A., Brem B., Greger H., Duchêne M., Wernsdorfer W., Walochnik J. High antitrypanosomal activity of plant derived sulphur-containing amides. Int. J. Antimicrob. Agents. 2010;36:570–572. doi: 10.1016/j.ijantimicag.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Astelbauer F., Obwaller A., Raninger A., Brem B., Greger H., Duchêne M., Wernsdorfer W., Walochnik J. Anti-leishmanial activity of plant-derived acridones, flavaglines, and sulfur-containing amides. Vector Borne Zoonotic Dis. 2011;11:793–798. doi: 10.1089/vbz.2010.0087. [DOI] [PubMed] [Google Scholar]
- Astelbauer F., Gruber M., Brem B., Greger H., Obwaller A., Wernsdorfer G., Congpuong K., Wernsdorfer W.H., Walochnik J. Activity of selected phytochemicals against Plasmodium falciparum. Acta Trop. 2012;123:96–100. doi: 10.1016/j.actatropica.2012.04.002. http://www.dx.doi:10.1016/j.actatropica.2012.04.002 [DOI] [PubMed] [Google Scholar]
- Balunas M.J., Kinghorn A.D. Drug discovery from medicinal plants. Life Sci. 2005;78:431–441. doi: 10.1016/j.lfs.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Bendesky A., Menéndez D., Ostrosky-Wengman P. Is metronidazole carcinogenic? Mutat. Res. 2002;511:133–144. doi: 10.1016/S1383-5742(02)00007-8. [DOI] [PubMed] [Google Scholar]
- Brader G., Wurz G., Greger H., Hofer O. Novel prenylated 2-quinolinones from East Asian Zanthoxylum species. Liebigs Ann. Chem. 1993;24:355–358. [Google Scholar]
- Brem B. University of Vienna; 2002. Distribution and Insecticidal Properties of Characteristic Plant Constituents from Tropical Aglaia and Stemona Species. Ph.D.Thesis. [Google Scholar]
- Carbonero F., Benefiel A.C., Alizadeh-Ghamsari A.H., Gaskins H.R. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front. Physiol. 2012;3:448. doi: 10.3389/fphys.2012.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg G.M., Newman D.J. Natural products: a continuing source of novel drug leads. Biochim. Biophys. Acta. 2013;1830:3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond L.S., Harlow D.R., Cunnick C.C. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans. R. Soc. Trop. Med. Hyg. 1978;72:431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- Gardner T.B., Hill D.R. Treatment of giardiasis. Clin. Microbiol. Rev. 2001;14:114–128. doi: 10.1128/CMR.14.1.114-128.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargano J.W., Adam E.A., Collier S.A., Fullerton K.E., Feinman S.J., Beach M.J. Mortality from selected diseases that can be transmitted by water - United States, 2003-2009. J. Water Health. 2017;15:438–450. doi: 10.2166/wh.2017.301. [DOI] [PubMed] [Google Scholar]
- Grassi P. University of Vienna; 1998. Vergleichende phytochemische Untersuchungen in der Gattung Micromelum (Rutaceae) Master Thesis. [Google Scholar]
- Greger H., Hofer O., Zechner G., Hadacek F., Gerald Wurz. Sulfones derived from methylthiopropenoic acid-amides from Glycosmis angustifolia. Phytochemistry. 1994;37:1305–1310. doi: 10.1016/S0031-9422(00)90403-5. [DOI] [Google Scholar]
- Greger H., Pacher T., Brem B., Bacher M., Hofer O. Insecticidal flavaglines and other compounds from Fijian Aglaia species. Phytochemistry. 2001;57:57–64. doi: 10.1016/S0031-9422(00)00471-4. [DOI] [PubMed] [Google Scholar]
- Greger H., Zechner G., Hofer O., Vajrodaya S. Bioactive amides from Glycosmis species. J. Nat. Prod. 1996;59:1163–1168. doi: 10.1021/np9604238. [DOI] [PubMed] [Google Scholar]
- Hanna R.M., Dahniya M.H., Badr S.S., El-Betagy A. Percutaneous catheter drainage in drug-resistant amoebic liver abscess. Trop. Med. Int. Health. 2000;5:578–581. doi: 10.1046/j.1365-3156.2000.00586.x. [DOI] [PubMed] [Google Scholar]
- Havelaar A.H., Kirk M.D., Torgerson P.R., Gibb H.J., Hald T., Lake R.J., Praet N., Bellinger D.C., de Silva N.R., Gargouri N., Speybroeck N., Cawthorne A., Mathers C., Stein C., Angulo F.J., Devleesschauwer B., World Health Organization Foodborne Disease Burden Epidemiology Reference Group World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001923. https://doi:10.1371/journal.pmed.1001923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer O., Greger H. Sulfur-containing amides from Glycosmis species (Rutaceae) In: Herz W., Falk H., Kirby G.W., Moore R.E., editors. vol. 80. Springer-Verlag; Vienna: 2000. pp. 187–223. (Progress in the Chemistry of Organic Natural Products). [DOI] [PubMed] [Google Scholar]
- Hofer O., Greger H., Lukaseder B., Vajrodaya S., Bacher M. Prenylated sulfonyl amides from Glycosmis species. Phytochemistry. 2000;54:207–213. doi: 10.1016/S0031-9422(00)00011-X. [DOI] [PubMed] [Google Scholar]
- IARC . International Agency for Research on Cancer; Lyon, France: 1987. Evaluation of Carcinogenic Risk to Humans; pp. 250–251. Suppl. 7. [Google Scholar]
- Ignatius R., Gahutu J.B., Klotz C., Steininger C., Shyirambere C., Lyng M., Musemakweri A., Aebischer T., Martus P., Harms G., Mockenhaupt F.P. High prevalence of Giardia duodenalis Assemblage B infection and association with underweight in Rwandan children. PLoS Neglected Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J.B., Trager W. Plasmodium falciparum in culture: use of outdated erythrocytes and description of the candle jar method. J. Parasitol. 1977;63:883–886. [PubMed] [Google Scholar]
- Keister D.B. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans. R. Soc. Trop. Med. Hyg. 1983;77:487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- Leitsch D., Kolarich D., Wilson I.B., Altmann F., Duchêne M. Nitroimidazole action in Entamoeba histolytica: a central role for thioredoxin reductase. PLoS Biol. 2007;5 doi: 10.1371/journal.pbio.0050211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitsch D., Schlosser S., Burgess A., Duchêne M. Nitroimidazole drugs vary in their mode of action in the human parasite Giardia lamblia. Int. J. Parasitol. Drugs Drug Resist. 2012;2:166–170. doi: 10.1016/j.ijpddr.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitsch D. Drug susceptibility testing in microaerophilic parasites: cysteine strongly affects the effectivities of metronidazole and auranofin, a novel and promising antimicrobial. Int. J. Parasitol. Drugs Drug Resist. 2017;7:321–327. doi: 10.1016/j.ijpddr.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limpert E., Stahel W.A., Abbt M. Log-normal distributions across the sciences: keys and clues. Bioscience. 2001;51:341–352. [Google Scholar]
- Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997;23:4–25. doi: 10.1016/S0169-409X(96)00423-1. [DOI] [PubMed] [Google Scholar]
- Lipinski C.A. Rule of five in 2015 and beyond: target and ligand structural limitations, ligand chemistry structure and drug discovery project decisions. Adv. Drug Deliv. Rev. 2016;101:34–41. doi: 10.1016/j.addr.2016.04.029. [DOI] [PubMed] [Google Scholar]
- Minetti C., Chalmers R.M., Beeching N.J., Probert C., Lamden K. Giardiasis. BMJ. 2016;355 doi: 10.1136/bmj.i5369. [DOI] [PubMed] [Google Scholar]
- Mohamed S.M., Ali A.M., Rahmani M., Wiart C., Dhaliwal J.S., Yusoff K. Apoptotic and necrotic cell death manifestations in leukemic cells treated with methylgerambullin a sulphone from Glycosmis calcicola. J. Biochem. Mol. Biol. Biophys. 2000;4:253–261. [Google Scholar]
- Moon J.T., Ha S.H., Lee S.H., Kwon T.H., Oh C.R., Kim Y.D., Kim J., Choo D.J., Lee J.Y. Total synthesis and biological evaluation of methylgerambullone. Bioorg. Med. Chem. Lett. 2010;20:52–55. doi: 10.1016/j.bmcl.2009.11.040. [DOI] [PubMed] [Google Scholar]
- Moreno S.N., Mason R.P., Docampo R. Distinct reduction of nitrofurans and metronidazole to free radical metabolites by Tritrichomonas foetus hydrogenosomal and cytosolic enzymes. J. Biol. Chem. 1984;259:8252–8259. [PubMed] [Google Scholar]
- Müller M. Mode of action of metronidazole on anaerobic bacteria and protozoa. Surgery. 1983;93:165–171. [PubMed] [Google Scholar]
- Newman D.J., Cragg G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Nillius D., Müller J., Müller N. Nitroreductase (GlNR1) increases susceptibility of Giardia lamblia and Escherichia coli to nitro drugs. J. Antimicrob. Chemother. 2011;66:1029–1035. doi: 10.1093/jac/dkr029. [DOI] [PubMed] [Google Scholar]
- Noedl H., Se Y., Schaecher K., Smith B.L., Socheat D., Fukuda M.M., Artemisinin Resistance in Cambodia 1 (ARC1) Study Consortium Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 2008;359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- Stanley S.L., Jr. Amoebiasis. Lancet. 2003;361:1025–1034. doi: 10.1016/S0140-6736(03)12830-9. [DOI] [PubMed] [Google Scholar]
- Szabo G., Greger H., Hofer O. Coumarin-hemiterpene ethers from Artemisia species. Phytochemistry. 1985;24:537–541. doi: 10.1016/S0031-9422(00)80763-3. [DOI] [Google Scholar]
- Tu Y. Artemisinin-a gift from traditional Chinese medicine to the world (Nobel Lecture) Angew Chem. Int. Ed. Engl. 2016;55:10210–10226. doi: 10.1002/anie.201601967. [DOI] [PubMed] [Google Scholar]
- Turkeltaub J.A., McCarty T.R., 3rd, Hotez P.J. The intestinal protozoa: emerging impact on global health and development. Curr. Opin. Gastroenterol. 2015;31:38–44. doi: 10.1097/MOG.0000000000000135. [DOI] [PubMed] [Google Scholar]
- Upcroft P., Upcroft J.A. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin. Microbiol. Rev. 2001;14:150–164. doi: 10.1128/CMR.14.1.150-164.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajrodaya S. University of Vienna; 1998. Comparative Analyses within the Genus Glycosmis (Rutaceae-Citroideae) Ph.D.Thesis. [Google Scholar]
- Vajrodaya S., Bacher M., Greger H., Hofer O. Organ-specific chemical differences in Glycosmis trichanthera. Phytochemistry. 1998;48:897–902. doi: 10.1016/S0031-9422(97)00986-2. [DOI] [Google Scholar]
- Wassmann C., Hellberg A., Tannich E., Bruchhaus I. Metronidazole resistance in the protozoan parasite Entamoeba histolytica is associated with increased expression of iron-containing superoxide dismutase and peroxiredoxin and decreased expression of ferredoxin 1 and flavin reductase. J. Biol. Chem. 1999;274:26051–26056. doi: 10.1074/jbc.274.37.26051. [DOI] [PubMed] [Google Scholar]
- Wink M. Medicinal plants: a source of anti-parasitic secondary metabolites. Molecules. 2012;17:12771–12791. doi: 10.3390/molecules171112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.