Abstract
Background
Cancer till date remains one of the world's most life threatening disease accompanied by risk of secondary infections. Therefore formulations carrying anticancer drugs which can also decrease the risk of secondary infection are inevitable. Chemotherapeutic drug doxorubicin along with flavonoids quercetin and epigallocatechin gallate (EGCG) is simultaneously loaded on liposomal formulation exploiting the amphiphilic property of the liposomes.
Results
Atomic force microscope imaging reveal the size of liposomal formulation loaded with doxorubicin, quercetin and EGCG to be greater than void liposome confirming the presence of drugs. Liposomal stability is improved by PEGylation; adding to the drug release time in vitro. The charge of phosphatidylcholine is rendered positive by coating the formulation with histone. The average size of the formulation is 342 nm. The encapsulation efficiency of doxorubicin, quercetin and EGCG is found to be 65.8%, 96.8% and 98% respectively. The above formulation demonstrated both anticancer and antimicrobial activity.
Conclusion
The formulation will provide dual anticancer and antimicrobial therapy thereby evading secondary infection in cancer patients along with chemotherapy.
Keywords: Biotechnology, Cancer research, Microbiology, Pharmaceutical science, Antimicrobial, Epigallocatechin gallate, Quercetin, Liposome, Atomic force microscopy, Anticancer, Doxorubicin
1. Introduction
Combating with secondary infections in cancer patients is a crucial challenge faced by scientist and clinicians worldwide. A combined anticancer and antimicrobial therapy is required in the present date for effective cancer treatment. Anthracyclin drugs combined with the versatile flavonoids entrapped in liposomes can provide effective cancer treatment eradicating deleterious side effects of chemotherapy. Liposomes discovered way back in 1961 by Bangham et al. is established as a suitable tool for encapsulating drugs with varying solubility [1]. Water soluble drugs occupy the aqueous core of the liposomes whereas drugs with reduced affinity for water are entrapped in the lipid bilayers. In addition liposomes when administered as drug carriers possess limited toxicity and do not elicit immunologic responses [2]. Liposome bound drugs, entering the lipophilic channels of the membrane have increased bioavailability. Surface modulations enable liposomes to deliver optimal amount of drugs in the tumor sites sparing normal cells from being exposed to chemotherapeutic agents [3]. Most normal tissues have perforated vascular system with pores smaller in size compared to tumor tissues [4]. Accumulation of drug loaded liposomes in normal tissues is minimal whereas in the fast growing tumors disorganized vascular endothelium has larger apertures, which enable increased leakage of liposomes [5]. Usually, significantly reduced volume of distribution and improved therapeutic index are achieved in liposomal delivery systems [6]. There are four major mechanisms by which liposomes interact with the cells [7]. Predominant among them are simple adsorption or subsequent endocytosis. Rarely liposomes fuse with the cell membrane and empties out drug payloads directly within the cell. Lipid exchange by which exchange of bilayer constituents such as lipids, cholesterol and membrane bound components take place may be the last possible mode of interaction.
Anthracyclines drugs are delivered to cells by the above mechanism and stop the growth of dividing cells by intercalating into the DNA and killing predominantly fast dividing cells. Encapsulation of these drugs into liposome limited their toxic effects on and enabled better survival of the experimental animals compared to free drugs. Several liposomal formulations of anthracyclin drugs such as doxorubicin (Doxil), daunorubicin (DaunoXome1) are in clinical practice [8, 9].
Our interest is to deliver more than one drug using liposomes as drug delivery vehicles. Tea polyphenols aids in radioprotection and chemoprevention by scavenging reactive oxygen species (ROS) [10]. EGCG (epigallocatechin gallate), inhibits growth of cancer cells and drives various types of tumor cells into programmed cell death due to their pro-oxidant activity and inhibition of enzyme such as catalase [11, 12]. Tea polyphenol EGCG demonstrated broad antimicrobial spectrum such as antifungal, antibacterial and antiviral effects [13]. Most of these micro-organisms are the source of secondary infections in cancer patients.
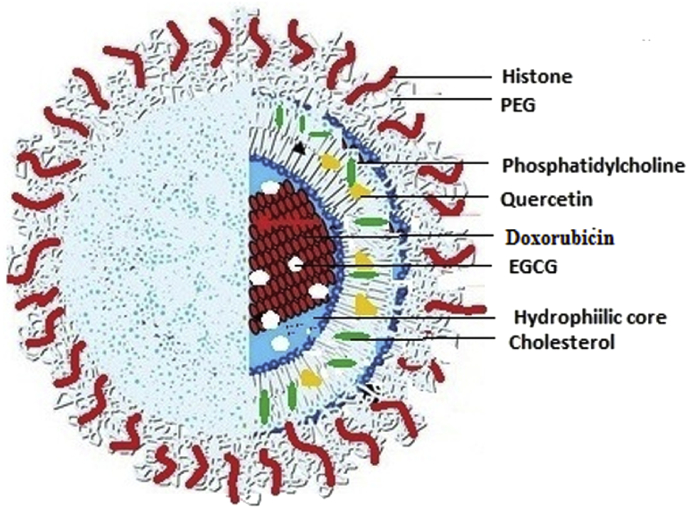
The formulation synthesised and characterized in the present study has doxorubicin and EGCG both being hydrophilic are encapsulated in the hydrophilic core of the liposome. Quercetin (Q), a hydrophobic flavonoid is trapped in the lamella of the liposome (Fig. 1). Quercetin has the ability to reduce the damaging effects of oxidation caused by ROS [14]. It can reverse the multidrug resistance (MDR) pathways [15, 16] and is also reported to inhibit CYP450, COX protein and tyrosine kinase family of enzymes leading to modulation of signal transduction and apoptosis [17].
Fig. 1.
Schematic diagram of histone coated PEGylated liposome with doxorubicin and EGCG entrapped in the hydrophilic core and quercetin trapped in the lamella.
The physiochemical characterization of the above formulation is done using different techniques including differential scanning calorimetry (DSC), atomic force microscopy (AFM), dynamic light scattering (DLS) and their applicable efficacy as anticancer and antimicrobial formulation is explored. The results obtained are expounded in the present article.
2. Materials and methods
2.1. Chemicals
L-α-phosphatidylcholine, cholesterol, DSPE-PEG (2000), EGCG and quercetin were obtained from Sigma-Aldrich, St. Louis, MO, USA. Chloroform and methanol were obtained from Merck. Milli Q water and phosphate buffer from sigma was used where ever necessary.
2.2. Preparations of drug loaded and void liposome
L-α-phosphatidylcholine, cholesterol and DSPE-PEG (2000) were taken in 3:1:0.15 M ratios and dissolved in a solvent mixture of chloroform and methanol (2:1 v/v) in a 250 ml round bottom flask. Organic solvents were evaporated at 43 °C. EGCG (5.4 mg) and 15 μl of 700 μM doxorubicin were then added.
Complete hydration was done at pH 6.7. Glass beads were added and the mixture was stirred for 30 min at 60 °C to bring the liposome into the aqueous phase and allowed to cool. Alternate freeze thaw cycles were repeated for 6–7 times and then freeze dried to be stored for further use.
Similarly void and quercetin loaded liposome was also prepared and stored for further experiments. Liposomes without PEGylation were also prepared.
PEGylated liposomes were further made positive by incubating liposomes with 1 ml histone (100 μM) overnight.
2.3. Atomic force microscope
Acquisition of AFM images was done on Bruker's diINNOVA AFM equipped with NanoDrive v8 real-time control & NanoScope Analysis system softwares. Void and formulated liposomes were diluted 10 times and were drop casted on glass slides. The samples were left to dry overnight before AFM imaging. Scanning mode used was tapping mode with scan rate of 3.41 μm/s and aspect ratio 1. Images were captured from random spots on the slides.
2.4. Particle size analysis and zeta potential measurements
Dynamic laser scattering (DLS) was used to measure the hydrodynamic diameter (nm), and Laser Doppler Anemometry (LDA) was used to determine zeta potential (mV). The DLS and LDA analyses were performed using Zetasizer Nano ZS (Malvern Instruments, Malvern4, UK). To determine the particle size and zeta potential, dilute suspension of void, quercetin-loaded liposome and the three drug-loaded liposome were prepared separately in double distilled water. All the measurements were performed thrice and the avarage with standard deviation is reported.
2.5. Differential scanning calorimetry
Differential Scanning Calorimeter (Pyris Diamond DSC, Perkin Elmer) was used to record the traces off thermal behavior of void and formulated liposomes. The samples were loaded onto standard aluminium pans and were scanned in a range from of 0 °C–200 °C with a scan rate of 10 °C/min. DSC traces of quercetin was obtained in the range of 0 °C–350 °C.
2.6. Determination of drug encapsulation efficiency
The lipid bilayer of drug loaded liposomes was disrupted with methanol and sonicated for 10 min followed centrifugation at 13000 rpm for 15 min and the released material is then quantified by fluorescence spectroscopy. EGCG, Quercetin and Doxorubicin were excited at 325, 365 and 480 nm with fluorescence emission maxima recorded at 458, 463 and 530 nm respectively. Percentage of drug encapsulation efficiency and drug loading efficiency was calculated as follows:
| Encapsulation efficiency weight % = (T−C)/T ×100 | (1) |
Where T is the total amount of drug in the supernatant and sediment, and C is the amount of drug in the supernatant [18].
| Loading capacity weight % = weight of drug in the vesicles / Total weight of vesicles tested ×100 | (2) |
2.7. In vitro release kinetics study
In vitro release of quercetin, EGCG and doxorubicin from liposomes was carried out by dissolving 2 mg of the formulated liposome in 1 ml of PBS (0.01 M, pH 7.4) containing 0.1% v/v of NaN3 to maintain a sink condition. The nano formulation suspension was equally divided in three tubes containing 1 ml each (as the experiment was performed in triplicate) and kept in a shaker at 37 °C at 150 rpm. At an interval of 24 h these tubes were taken out from shaker and centrifuged. Supernatant was collected and to the pellet obtained after centrifugation, 1 ml of fresh PBS containing NaN3 solutions were added to the centrifuge tube in the shaker for the next readings. The collected supernatants at each interval of time were lyophilized and dissolved in 1 ml of DMSO. The amount of Quercetin, EGCG and doxorubicin in the sample was measured fluorimetrically.
2.8. Cell line and culture
Leukemic cell line K562 was obtained from National Centre for Cell Sciences (NCCS), Pune, India and were maintained in RPMI medium supplemented with 10% FBS and 1% Pen-Step antibiotic in 25 cm2 culture flasks. All cells were maintained in 37 °C in a humified atmosphere of 5 % CO2 in air.
2.9. Measurement of apoptosis by annexin V FITC-PI staining
FITC Annexin V Apoptosis Detection Kit obtained from BD bioscience was used to quantify the percentage of cells undergoing apoptosis. K562 cells (1 × 105) were incubated overnight with 1 mg/ml of quercetin loaded liposome and doxorubicin, EGCG and quercetin formulated liposomes separately. K562 cells that were not subjected to incubation with liposomes were used as control. Drug treated and untreated cells were treated according to the protocol supplied with the kit. Percentage of apoptosis was assessed using a BD FACS Calibur (San Jose, CA, USA) equipped with 488 nm argon laser light source, 515 nm band-pass filters for FITC fluorescence and 623 nm band-pass filter for PI fluorescence and data were analysed using CellQuest software on flow cytometry. A total of 20,000 events were acquired and the cells were properly gated for analysis.
2.10. Measurement of ROS and MMP
The intracellular ROS and MMP were measured in K562 cells (5 × 105 cells/ml), fluorimetrically, using DCFDA (dichlorohydrofluorescin-di-acetate) and Rh123 (Rhodamine 123) respectively. DCFDA being non polar readily diffuses in the cell and gets hydrolyzed and deacetylated by cellular mechanism to non fluorescent compound which in the presence of ROS gets oxidised into highly fluorescent 2′7′-dichlorofluorescein (DCF). Quenching of Rh-123 fluorescence is induced by succinate and ADP and the rate of fluorescence decay is proportional to the mitochondrial membrane potential. Cells were treated with various concentrations of formulated liposomes (0.375–3 mg/ml) for 2 h at 37 °C. The cells were then washed twice and resuspended in PBS (pH 7.4). Subsequently cells were incubated in dark with 10 μM DCFDA/Rh123 at 37 °C for 30 min. The detected changes of ROS and MMP were then analyzed fluorimetrically by using an excitation wavelength of 488 nm. Both ROS and MMP measurements were carried out in triplicates.
2.11. Test microorganisms and growth media
Escherichia coli (MCC 2412), were chosen based on their clinical and pharmacological importance. The bacterial strains obtained from National Centre for Microbial resource, Pune, were used for evaluating antimicrobial activity. The bacterial stock cultures were incubated for 24 h at 37 °C on nutrient agar, following refrigeration storage at 4 °C.
2.12. Antimicrobial activity
The antibiotic sensitivity test was carried out by the method of Bauer et al (1966) on E. coli [19]. Sterile discs were obtained from Himedia Laboratories Limited, India. Separate sterile discs loaded with void, quercetin loaded and liposomal formulation were carefully dropped on to the surface of the agar plates using sterile forceps. The plates were incubated at 37 °C for 24 h. The agar plates were examined for circular clear area in the bacterial lawn around the disc.
Similarly minimum inhibitory concentration of the formulated liposome was obtained by disc diffusion method. The zone of inhibition around sterile discs loaded with different concentration of the formulated liposome was recorded.
3. Results
3.1. Atomic force microscopy
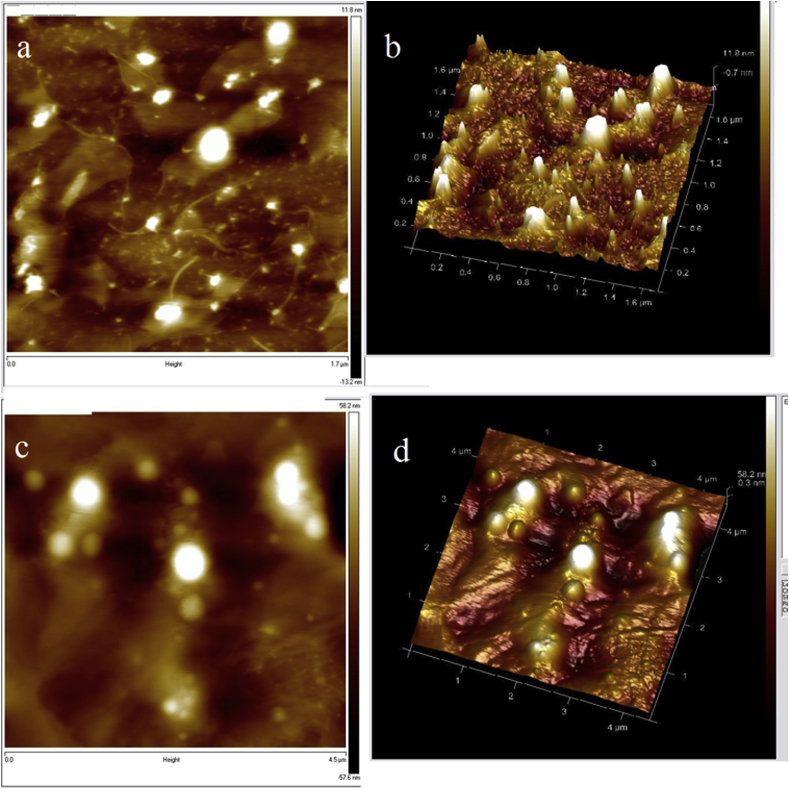
AFM in the tapping and noncontact mode approaches allows the observation of the liposomal morphology without any sample manipulation such as staining, labelling, or fixation. Particularly the intermittent contact motion of the tip (tapping) eliminates lateral or shear forces which would deform or scrape the sample. The main advantage of the technique is the possibility to operate with high resolution in air or in fluid in real-time and in the nanometre scale [20]. In corroboration with literature, the captured AFM image (Fig. 2), protrusion with spreading tails are assigned as the adsorbed liposome on the substrate [21]. The physical properties of void and formulated liposomes as calculated by AFM are tabulated in Table 1. The area of the formulation is more than that of void liposome signifying successful encapsulation of all the three drugs (Table 1). The diameters of the liposomes are in the same order as obtained by DLS. Flattening of liposome which is significant from their heights (18 & 25 nm) is because of their high surface density that prevents the tip from coming in proximity with the substrate surface as explained by Onyesom et al. 2013 [22]. From the images of liposome it is evident that histone coated quercetin, EGCG and doxorubicin incorporated stealth liposomes have more defined spherical form than the void liposome (Fig. 2). Quercetin being highly lipophilic enters the lamellae of the liposome lowering the phospholipid packing density (PPD) resulting in decreasing the stiffness of the liposome. Due to structural conformation of quercetin the intermolecular forces between lipids and quercetin is not high as the intermolecular forces between lipid-lipid (PC-PC, PC-cholesterol or cholestrol-cholesterol) interactions resulting in more flexible liposome. Also the repulsion of the individual positively charged histone coated liposomes leads to less aggregation of the lipid nanoparticles enabling clear visualization of individual liposomes (Fig. 2c and d).
Fig. 2.
AFM images of void (a & b) (scale: 1.7 μM) and formulated (quercetin, EGCG and doxorubicin loaded histone coated stealth liposome) (c & d) (scale: 4.5 μM) in 2D (a & c) and 3D (b & d) format.
Table 1.
Properties of liposomes as measured by AFM.
| Properties | Void liposome | Formulated liposome |
|---|---|---|
| Total Count | 15.00 | 17.00 |
| Density (/μm2) | 5.163 | 0.844 |
| Height (μm) | 0.018 ± 0.006 | 0.025 ± 0.021 |
| Area (μm2) | 0.006 ± 0.005 | 0.162 ± 0.268 |
| Diameter (μm) | 0.077 ± 0.032 | 0.342 ± 0.298 |
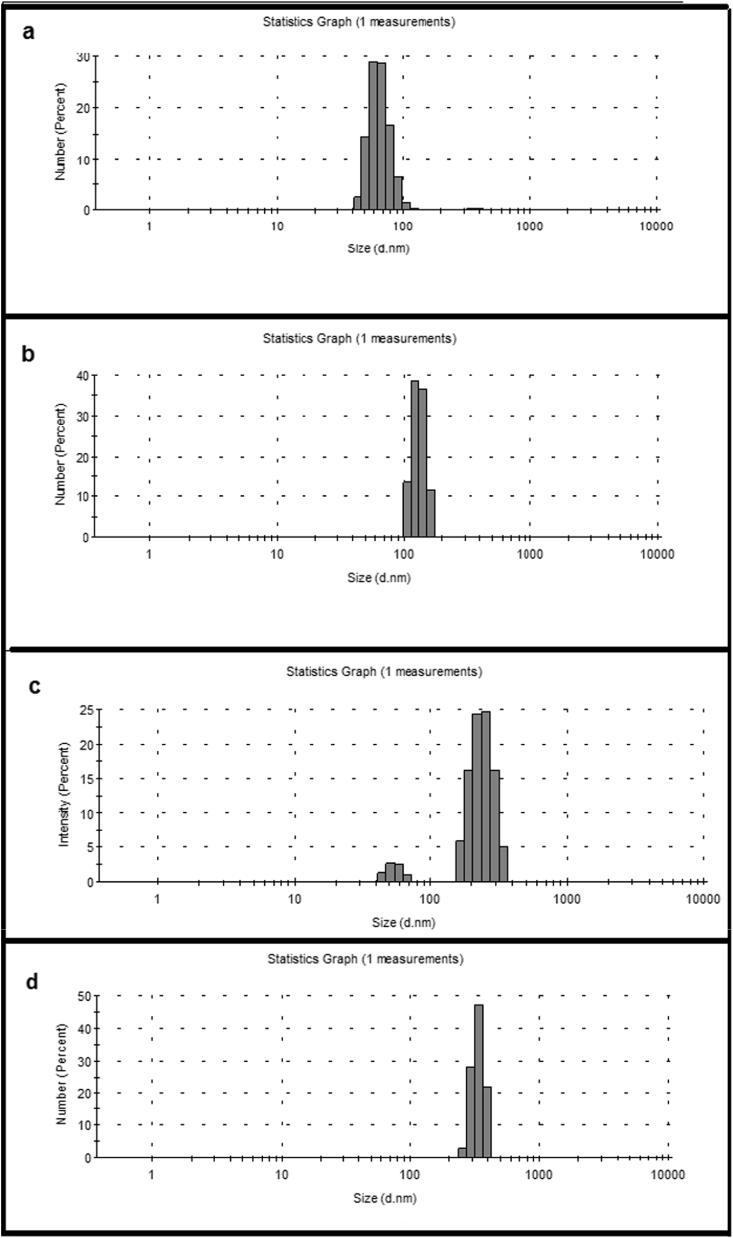
3.2. Size determination and zeta potential measurement
Size and zeta potential was measured using dynamic light scattering (DLS). Zeta potential values are recorded to be -11.9, -11.2, -26.3 and 2.98 mV for void, quercetin loaded, doxorubicin and flavonoids loaded liposome and histone coated PEGylated formulations respectively (Table 2). The negative charge imparted on the liposome by the flavonoids and PEG was negated and to some extent made positive by coating the liposome with histone. The measured sizes for void, quercetin loaded, formulated liposome and histone coated PEGylated formulation is 66, 132, 255 and 342 nm respectively (Fig. 3). Incorporation of quercetin within phosphatidylcholine and cholesterol resulted in increase in diameter of liposome. Further change was observed on encapsulation of doxorubicin and EGCG in the hydrophilic core. Addition of PEG to the membrane and coating it with histone increased the size further to 342 nm. The size of void, quercetin loaded, formulated, histone coated pegylated liposomes are tabulated in Table 2 with standard deviation calculated from three sets of experiments.
Table 2.
Size and ζ-potential of void liposomes, quercetin-loaded liposome, formulated liposome and histone coated PEGylated liposome as recorded by dynamic light scattering (DLS).
| Size (nm), ±SD | ζ-potential (mV), ±SD | |
|---|---|---|
| Void liposome | 66 ± 2.1 | -11.9 ± 3.2 |
| Quercetin-loaded liposome | 132 ± 0.9 | -11.2 ± 2.4 |
| Formulated liposome | 255 ± 3.3 | -26.3 ± 1.7 |
| Histone coated PEGylated liposome | 342 ± 3.2 | 2.98 ± 0.6 |
Fig. 3.
Size in nm (a) void liposomes (b) quercetin-loaded liposome (c) formulated liposome (d) histone coated PEGylated liposome as recorded by dynamic light scattering (DLS).
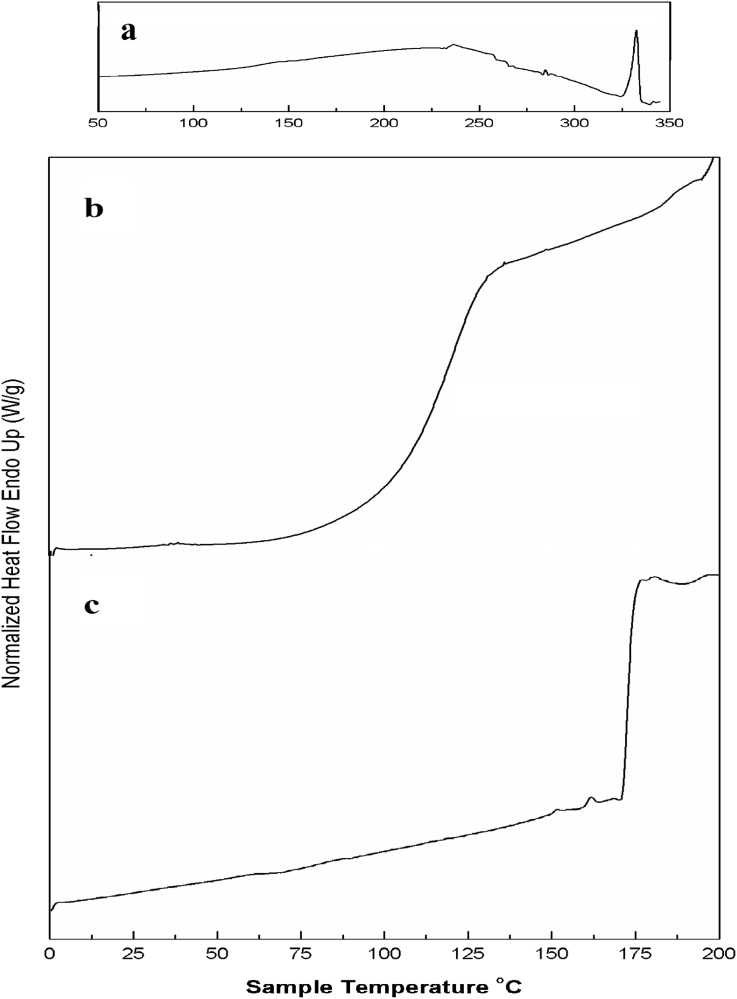
3.3. Differential scanning calorimetric (DSC) analysis
DSC thermograms of quercetin void liposome and drug loaded liposome with clear phase transition temperatures are represented in Fig. 4. From the figure it is observed that the phase transition temperature of the void liposome composed of phosphatidylcholine and cholesterol in the ratio 3:1, is at 115 °C in Fig. 4b, which is in accordance with the available literature [23]. On encapsulation of quercetin, EGCG and doxorubicin in the liposome a phase transition is observed at 175 °C evidencing changes in the chemical nature of the liposome (Fig. 4c). The DSC thermogram of pure quercetin as recorded earlier by us, shows melting temperature (tm) at 326 °C (Fig. 4a) [24]. The melting temperature of doxorubicin is reported around the same temperature as that of quercetin [24] and that of EGCG is reported at 209 °C [25]. Since liposome samples degraded at temperatures higher than 200 °C, the tm of the quercetin, EGCG and doxorubicin could not be observed in the DSC profiles of formulated liposomes. However the shift in phase transition of the liposome towards higher temperature suggests successful encapsulation of the drugs in the liposome.
Fig. 4.
Differential Scanning Calorimetry traces of (a) quercetin in the region of 0–350 °C and (b) void liposome (c) formulated liposome in the range of 0–200 °C.
3.4. Drug loading and entrapment efficiency
Quercetin, EGCG and doxorubicin were successfully encapsulated in the liposome as suggested by AFM, DLS and DSC results. The encapsulation efficiency of doxorubicin, quercetin and EGCG calculated using Eq. (1) in the methods section was found to be 65.8%, 96.8% and 98% respectively. Standard deviations are calculated from the results of three sets of experimental data. The results are tabulated in Table 3.
Table 3.
Entrapment efficiency of doxorubicin, quercetin and EGCG in formulated liposomes.
| Drugs | Entrapment efficiency (% ± SD) |
|---|---|
| Doxorubicin | 65.8 ± 2.3 |
| Quercetin | 96.8 ± 1.6 |
| EGCG | 98 ± 0.7 |
Loading efficiency was calculated in weight % by dividing the weight of drugs in the vesicle by the total weight of vesicles tested. All three drugs were successfully loaded in the liposome contributing to the total loading efficiency of 70.8% ± 5.25. Similar results were also reported in other published articles [26, 27].
3.5. In-vitro release-kinetics study
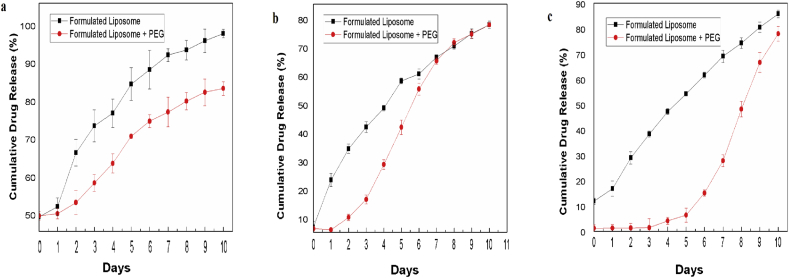
The in vitro release kinetics of the three encapsulated drugs in unPEGylated and PEGylated liposome are shown in Fig. 5. From the figure it is observed that on PEGylation the release of doxorubicin is slower compared to unPEGylated formulation (Fig. 5a) and also Quercetin release is delayed compared to doxorubicin due to its hydrophobicity (Fig. 5b). The EGCG release pattern displays that is takes more time to pass through the hydrophobic core and PEG coating (Fig. 5c). The release of the encapsulated drugs is more sustained in the PEGylated formulation and the histone coating renders the charge positive and saves the formulation from being degraded by phagosomes [28].
Fig. 5.
Release of (a) doxorubicin (b) quercetin (c) epigallocatechin gallate as measured fluorimetrically in the supernatant collected on regular intervals for 10 days and represented as cumulative drug release percentage.
3.6. Measurement of apoptosis by annexin V FITC-PI staining
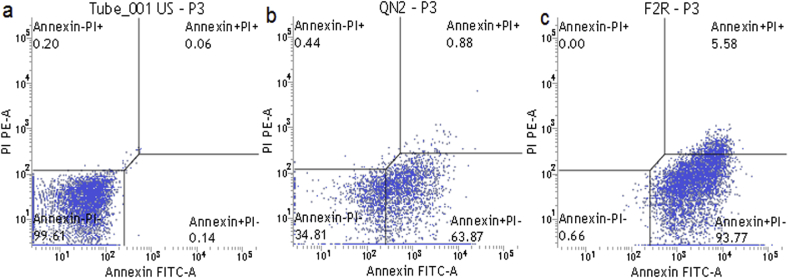
It is known that phosphatidylserine (PS) is flipped from the intra to extra plasma membrane leaflet during the early stage of apoptosis. Annexin V, with a high affinity for PS, can therefore be employed as a sensitive marker for early apoptosis [29]. By contrast, propidium iodide (PI) can conjugate to necrotic cells. Double staining of cells with Annexin V-FITC and PI was assayed with flow cytometry to follow apoptosis of each sample. A typical quadrant analysis of apoptotic and necrotic cells in displayed in Fig. 6. Sectors in the quadrant represent the Viable cells (bottom left) (PI-, annexin-V/FITC-), early apoptotic cells (bottom right) (PI-, annexin-V/FITC+), non-viable, late apoptotic/necrotic cells (top right) (PI+, annexin-V/FITC+), necrotic cells (top left) (PI+, annexin-V/FITC-). Cell death induced by quercetin-loaded liposome (b) and formulated liposome (c) is accompanied by PS exposure in the membrane. Unstained control represented in Fig. 6a. Numbers in the bottom left quadrants indicate the proportions of cells in the corresponding areas. Experiments were performed in triplicate, and data of one experiment is reported.
Fig. 6.
FACS analysis of K562 cells (a) unstained untreated control (b) cells treated with quercetin loaded liposomes (c) cells treated with formulated liposome loaded with doxorubicin, quercetin and EGCG.
Distinct changes are noted in the bottom right quadrant of the panel signifying change in population of apoptotic cells. Apoptotic population of the untreated cells were found to be 0.14 % which increased to 63.87% and 93.77% following treatment with quercetin loaded liposome and flavonoids and doxorubicin conjugated liposome.
3.7. Measurement of ROS and MMP
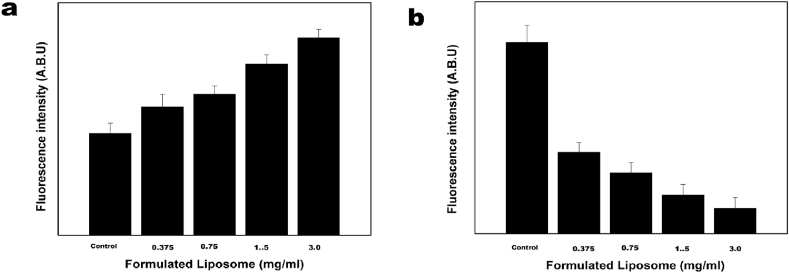
Chemotherapeutic drugs such as doxorubicin and flavonoids such as quercetin and EGCG are reported to generate ROS which in turn will induce oxidative damage in the nuclear materials such as DNA resulting in cell death. Increase in ROS was observed with the increase in concentration of the formulation which can be a contributory effect of doxorubicin and the flavonoids (Fig. 7a). Although there is no direct relation between ROS and MMP (Mitochondrial membrane permeabilization), some evidences suggest that ROS is a direct contributor to the decrease in MMP [30]. MMP is an early event of the apoptotic process which results in disruption of the inner transmembrane potential causing release of soluble inter membrane proteins [31]. Loss of MMP is also observed with the increased dose of liposomal formulation as illustrated in Fig. 7b. Standard deviations are calculated from the results obtained from three sets of experiments.
Fig. 7.
(a) ROS population and (b) MMP changes in K562 cells with and without exposure to formulated liposome.
3.8. Antimicrobial activity
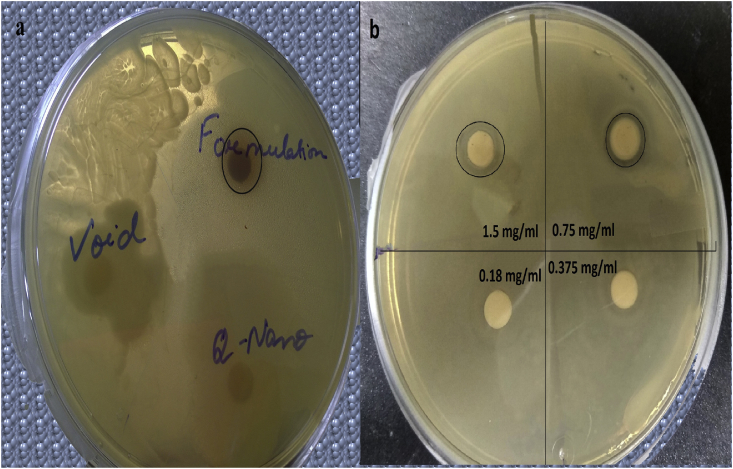
Zone of inhibition (ZOI) was observed around doxorubicin, quercetin and EGCG encapsulated liposome (Fig. 8a). Void and quercetin loaded liposomes had no inhibitory effects against E coli.
Fig. 8.
(a) Antimicrobial activity of void, quercetin-loaded and formulated liposomes on E. coli. (b) Minimum inhibitory concentration of quercetin, EGCG and doxorubicin loaded liposome on E. coli.
Minimum inhibitory concentration of formulated liposomes was determined to be 0.75 mg/ml by measuring the zone of inhibition (Fig. 8b).
4. Discussion
The need to formulate a drug which will also have the property of reversing MDR in cancer cells, protecting normal cells from the anticancer drugs, and evading secondary infection may be achieved through the proposed liposomal formulation as elucidated in the Fig. 1. The key feature of this formulation is size and composition which is responsible for its multifunctional capability. Anticancer drug doxorubicin along with flavonoids quercetin and EGCG having anticancer and antimicrobial activities are simultaneously loaded exploiting the amphiphilic property of the liposome. Co-encapsulation of quercetin and EGCG was considered by us in the present formulation as it is reported that combination of flavonoids has higher antioxidant efficiency than individual flavonoids [32]. This increased antioxidant efficacy will in turn increase the shelf life and stability of the liposome [33] and the pro-oxidant effects at higher flavonoids concentrations will render cancerous cells to apoptotic state [11, 34]. Entrapment into the liposomes will also increase the bioavailability of the polyphenols [35].
Formation of the void liposome and the formulation is displayed in the AFM images (Fig. 2). Similar images of flattened, spherically shaped vesicles that are assigned as liposomes that were reported in published literatures [21, 22]. The size of the formulation is greater than the void liposomes confirming encapsulation of doxorubicin, quercetin and EGCG (Table 1). The size of the liposomal formulation as determined by DLS is 342 nm (Fig. 3), which is small in comparison with the polymer (PLGA) nanomaterial synthesized by loading quercetin and doxorubicin as reported by us previously [24]. Particles of size around 400 nm have shown extravagation and accumulation in tumors [28, 36]. Liposome applied clinically ranges between 50 and 450 nm [28, 37]. Size of the polymeric nanoformulation is 400–500 nm and may not be able to reach out to all types of tumor cells as reported in our previous work [24] and is concluded that liposomes due to size may be better drug delivery systems than polymer nano particles. Changes in the DSC traces of void and formulated liposomes demonstrate encapsulation of the drugs (Fig. 4) in the liposome. The entrapment efficiency of quercetin and doxorubicin improved from 85% to 96.8% and from 23.2% to 65.8% in the liposomal formulation compared to the polymeric nano formulation [24].
PEGylation improved the stability of liposome and considerable amount of drugs were released upto 10 days (Fig. 5.). However controlled release of drugs is subjected to the amount of cholesterol in liposomes [38]. Earlier it has been reported that PEGylation increases circulation time of liposome in vivo, after intravenous administration can be achieved by rendering liposomes unidentifiable by the macrophages [28].
The anticancer activity tested on K562 cells as represented in Fig. 6 demonstrate that apoptosis and necrosis is more dominant in the cancer cells on treatment with the liposomal formulation compared to only quercetin loaded liposomes. Generation of ROS and decrease in MMP was observed as two of the probable mechanism of action of the formulations (Fig. 7). ROS mediated release of cytochrome C by mitochondria induces apoptosis in cancer cells has been reported elsewhere [31]. Decrease in MMP leads to disruption of the inner trans-membrane potential and release of soluble inter-membrane proteins.
The antimicrobial activity of the formulation tested on E. coli was confirmed by observing zone of inhibition around the sterile disc loaded with the liposomal formulation (Fig. 8). Both quercetin and EGCG have been reported earlier to have inhibitory effects on E. coli cell growth [39, 40]. Quercetin disrupts cell wall and membranes of E. coli [40]. Similar results were reported earlier where antimicrobial effect of flavonoids mixture were retained after liposome encapsulation [41]. Minimum inhibitory concentration was found to be 0.75 mg/ml which is very reasonable in amount.
As compared to polymeric nanoparticles, liposomes produce no antigenic reactions. The surface charge of the formulation was measured by DLS to be 2.98 mV. Surface charge of the liposome was made positive by incubating the stealth liposome formulations with histone. On incubation protein gets adsorbed on the surface of the liposome [42]. It is reported earlier that neutral and anionic liposomes clear extravasations from the tumor vasculature [43]. Cationic liposome on the other hand accumulates at the angiogenic vesicles due to negative charge on the tumor cells which results from over expression of glycoprotein and lactate secretion, a characteristic of metabolically active cancer cells [44]. Retention time for cationic liposome in the tumor site is more compared to negative or anionic liposome. Moreover presence of angiogenic vesicles with anionic sites such as phospholipids, proteoglycans, hyperglycosylated and hypersialylated membrane proteins etc. makes newly formed tumors more labile in the presence of cationic liposomes As mentioned elsewhere endocytic pathway is the preferential route of internalization for the positively charged liposomes [28]. This mode of interaction facilitates doxorubicin, quercetin and ECGC to have optimal chemotherapeutic activity. Positively charged liposomes also are reported to have deleterious effects on pathogenic microbes and hence aid in the purpose of dual therapy on cancer patients [45].
5. Conclusion
The formulation is considered to be innovative as it addresses both the anticancer and antimicrobial activity needed in cancer treatment. The advantage of loading doxorubicin, EGCG and quercetin in different compartments of liposome expose the cancerous cells to these drugs in tandem. Each drug has different mode of anticancer activity. ADR acts by intercalation and restriction of DNA replication whereas EGCG and quercetin is catalase inhibitor accounting for the increased oxidative stress leading to apoptosis in cancer cells. Quercetin can reverse MDR and induce apoptosis by modulation of signal transduction. The formulation will provide anticancer and antimicrobial activity with efficacy to evade secondary infection in cancer patients along with chemotherapy.
Declarations
Author contribution statement
Asmita Das: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Chabita Saha: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Pangwan M. Konyak, Argha Das: Performed the experiments.
Subrata K. Dey: Contributed reagents, materials, analysis tools or data.
Funding statement
Pangwan M Konyak received Ph.D thesis grant for M.Tech dissertation from Department of Biotechnology, India. Argha Das is supported by the Department of Biotechnology, India.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Pangwan M Konyak and Argha Das were supported by DBT, India. The authors are thankful to the Indian Association for the Cultivation of Science, Kolkata for providing DSC facility. Thanks are also extended to UGC-DAE Centre for Scientific Research, Kolkata for providing DLS facility. We are also thankful to S.N Bose Centre for Basic Sciences for providing us AFM facility. The Vice-Chancellor of MAKAUT, Prof. Saikat Maitra is acknowledged for his support and cooperation.
References
- 1.Bangham A.D., Standish M.M., Watkins J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965;13:238–252. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 2.Akbarzadeh A., Rezaei-sadabady R., Davaran S., Joo S.W., Zarghami N. Liposome: classification, preparation, and applications. Nanoscale Res. Lett. 2013;8(1):1. doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deshpande P.P., Biswas S., Torchilin V.P. Current trends in the use of liposomes for tumor targeting. Nanomedicine. 2013;8(9):1509–1528. doi: 10.2217/nnm.13.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lum H., Malik A.B. Regulation of vascular endothelial barrier function. Am. J. Physiol. 1994;267:223–241. doi: 10.1152/ajplung.1994.267.3.L223. [DOI] [PubMed] [Google Scholar]
- 5.Matsumura Y., Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumori tropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 6.Messerer C.L., Ramsay E.C., Waterhouse D., Ng R., Simms E.M., Harasym N., Tardi P., LDr Maye, Bally M.B. Liposomal irinotecan: formulation development and therapeutic assessment in murine xenograft models of colorectal cancer. Clin. Cancer Res. 2004;10(19):6638–6649. doi: 10.1158/1078-0432.CCR-04-0221. [DOI] [PubMed] [Google Scholar]
- 7.Çağdaş M., Sezer A.D., Bucak S. Liposomes as potential drug carrier systems for drug delivery. Appl. Nanotechnol. Drug Deliv. 1961;1–50 [Google Scholar]
- 8.Petre C.E. Liposomal daunorubicin as treatment for Kaposi’s sarcoma. Int. J. Nanomed. 2007;7960:277–288. [PMC free article] [PubMed] [Google Scholar]
- 9.Tardi P.G., Boman N.L., Cullis P.R. Liposomal doxorubicin. J. Drug Target. 1996;4(3):129–140. doi: 10.3109/10611869609015970. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y., Bachrach U. The specific anti-cancer activity of green tea (-) -epigallocatechin-3-gallate (EGCG) J. Amino Acids. 2002;22:131–143. doi: 10.1007/s007260200002. [DOI] [PubMed] [Google Scholar]
- 11.Pal S., Dey S.K., Saha C. Inhibition of catalase by tea catechins in free and cellular state: a biophysical approach. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0102460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forester S.C., Lambert J.D. The role of antioxidant versus pro-oxidant effects of green tea polyphenols in cancer prevention. Mol. Nutr. Food Res. 2011;55:844–854. doi: 10.1002/mnfr.201000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor P.W., Hamilton-miller J.M.T., Stapleton P.D. Antimicrobial properties of green tea catechins. Food Sci. Technol. Bull. 2009;2:71–81. doi: 10.1616/1476-2137.14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang M., Swarts S.G., Yin L., Liu C., Tian Y., Cao Y. Antioxidant properties of quercetin. Adv. Exp. Med. Biol. 2011;70:283–289. doi: 10.1007/978-1-4419-7756-4_38. [DOI] [PubMed] [Google Scholar]
- 15.Chen C., Zhou J., Ji C. Quercetin: a potential drug to reverse multidrug resistance. Life Sci. 2010;87:333–338. doi: 10.1016/j.lfs.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Kim S.H., Yeo G.S., Lim Y.S., Kang C.D., Kim C.M., Chung B.S. Suppression of multidrug resistance via inhibition of heat shock factors by Quercetin in MDR cells. Exp. Mol. Med. 1998;30:87–92. doi: 10.1038/emm.1998.13. [DOI] [PubMed] [Google Scholar]
- 17.Li Y., Wang E., Patten C.J., Chen L., Yang C.S. Effects of flavonoids on cytochrome P450-dependent acetaminophen metabolism in rats and human liver microsomes. Drug Metab. Dispos. 1994;22:566–571. [PubMed] [Google Scholar]
- 18.Fathalla D., Soliman G.M., Fouad E.A. Development and in vitro/in vivo evaluation of liposomal gels for the sustained ocular delivery of latanoprost. J. Clin. Exp. Ophthalmol. 2015;6:1. [Google Scholar]
- 19.Bauer A.W., Kirby W.M., Sherris J.C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966;45(4):493–496. [PubMed] [Google Scholar]
- 20.Ruozi B., Belletti D., Tombesi A., Tosi G. AFM, ESEM, TEM, and CLSM in liposomal characterization: a comparative study. Int. J. Nanomed. 2011;6:557–563. doi: 10.2147/IJN.S14615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muraji Y., Fujita T., Itoh H., Fujita D. Preparation procedure of liposome-absorbed substrate and tip shape correction of diameters of liposome measured by AFM. Microsc. Res. 2013;1:24–28. [Google Scholar]
- 22.Onyesom I., Lamprou D.A., Sygellou L., Owusu-Ware S.K., Antonijevic M., Chowdhry B.Z., Douroumis D. Sirolimus encapsulated liposomes for cancer therapy: physicochemical and mechanical characterization of sirolimus distribution within liposome bilayers. Mol. Pharm. 2013;10(11):4281–4293. doi: 10.1021/mp400362v. [DOI] [PubMed] [Google Scholar]
- 23.Hathout R.M., Mansour S., Mortada N.D., Guinedi A.S. Liposomes as an ocular delivery system for acetazolamide: in vitro and in vivo studies. AAPS PharmSciTech. 2007;8(1):1. doi: 10.1208/pt0804080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saha C., Kaushik A., Das A., Pal S., Majumder D. Anthracycline drugs on modified surface of quercetin-loaded polymer nanoparticles: a dual drug delivery model for cancer treatment. PLoS One. 2016;11(5):1–15. doi: 10.1371/journal.pone.0155710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang G., Zhang J. Enhanced oral bioavailability of EGCG using pH-sensitive polymeric nanoparticles: characterization and in vivo investigation on nephrotic syndrome rats. Drug Des. Dev. Ther. 2018;12:2509–2518. doi: 10.2147/DDDT.S172919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zucker D., Marcus D., Barenholz Y., Goldblum A. Liposome drugs' loading efficiency: a working model based on loading conditions and drug's physicochemical properties. J. Control. Release. 2009;139(1):73–80. doi: 10.1016/j.jconrel.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 27.J Shaji S. Iyer Double-loaded liposomes encapsulating Quercetin and Quercetin beta-cyclodextrin complexes: preparation, characterization and evaluation. Asian J. Pharm. 2012;6(3) [Google Scholar]
- 28.Bozzuto G., Molinari A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015;10:975–999. doi: 10.2147/IJN.S68861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koopman G., Reutelingsperger C.P., Kuijten G.A., Keehnen R.M., Pals S.T., van Oers M.H. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–1420. [PubMed] [Google Scholar]
- 30.Zamzami N., Kroemer G. Methods to measure membrane potential and permeability transition in the mitochondria during apoptosis. Methods Mol. Biol. 2004;282:103–115. doi: 10.1385/1-59259-812-9:103. [DOI] [PubMed] [Google Scholar]
- 31.Levraut J., Iwase H., Shao Z.H., Vanden Hoek T.L., Schumacker P. Cell death during ischemia: relationship to mitochondrial depolarization and ROS generation. J Physiol. Heart Circ. 2003;284(2):H549–H558. doi: 10.1152/ajpheart.00708.2002. [DOI] [PubMed] [Google Scholar]
- 32.Chen W., Zou M., Ma X., Lv R., Ding T., Liu D. Co-encapsulation of EGCG and quercetin in liposomes for optimum antioxidant activity. J. Food Sci. 2019;84(1):111–120. doi: 10.1111/1750-3841.14405. [DOI] [PubMed] [Google Scholar]
- 33.Yadav A.V., Murthy M.S., Shete A., Sfurti S. Stability aspects of liposomes. Ind. J. Pharm. Edu. Res. 2011;45(4):402–413. [Google Scholar]
- 34.Majumder D., Das A., Saha C. Catalase inhibition an anti cancer property of flavonoids: a kinetic and structural evaluation. Int. J. Biol. Macromol. 2017;104(Pt A):929–935. doi: 10.1016/j.ijbiomac.2017.06.100. [DOI] [PubMed] [Google Scholar]
- 35.Mignet N., Seguin J., Chabot G.G. Bioavailability of polyphenol liposomes: a challenge ahead. Pharmaceutics. 2013;5(3):457–471. doi: 10.3390/pharmaceutics5030457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaumet M., Vargas A., Gurny R., Delie F. Nanoparticles for drug delivery: the need for precision in reporting particle size parameters. Eur. J. Pharm. Biopharm. 2008;69:1–9. doi: 10.1016/j.ejpb.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Cho K., Wang X., Nie S., Chen Z.G., Shin D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008;14(5):1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 38.Briuglia M.L., Rotella C., Mc Farlane, Lamprou D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015;5(3):231–242. doi: 10.1007/s13346-015-0220-8. [DOI] [PubMed] [Google Scholar]
- 39.Wang S., Yao J., Zhou B., Yang J., Chaudry M.T., Wang M., Xiao, Li Y., Yin W. Bacteriostatic effect of quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro. J. Food Prot. 2018;81(1):68–78. doi: 10.4315/0362-028X.JFP-17-214. [DOI] [PubMed] [Google Scholar]
- 40.Serra D.O., Mika F., Richter A.M., Hengge R. The green tea polyphenol EGCG inhibits E. coli biofilm formation by impairing amyloid curli fibre assembly and downregulating the biofilm regulator CsgD via the σ (E) -dependent sRNA RybB. Mol. Microbiol. 2016;101(1):136–151. doi: 10.1111/mmi.13379. [DOI] [PubMed] [Google Scholar]
- 41.Matouskova P., Marova I., Bokrova J., Benesova P. Effect of encapsulation on antimicrobial activity of herbal extracts with lysozyme. Food Technol. Biotechnol. 2016;54(3):304–316. doi: 10.17113/ftb.54.03.16.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sangrà M., Estelrich J., Sabaté R., Espargaró A., Busquets M.A. Evidence of protein adsorption in pegylated liposomes: influence of liposomal decoration. Nanomaterials (Basel). 2017;7(2):37. doi: 10.3390/nano7020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krasnici S., Werner A. Effect of the surface charge of liposomes on their uptake by angiogenic tumor vessels. Int. J. Cancer. 2003;105(4):561–567. doi: 10.1002/ijc.11108. [DOI] [PubMed] [Google Scholar]
- 44.Chen B., Le W., Wang Y. Targeting negative surface charges of cancer cells by multifunctional nanoprobes. Theranostics. 2016;6(11):1887–1898. doi: 10.7150/thno.16358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mamizuka E.M., Carmona-Ribeiro A.M. Cationic liposomes as antimicrobial agents. Commun. Curr. Res. Edu. Topics Trends Appl. Microbiol. 2007 [Google Scholar]