Abstract
Purpose:
We characterized the early changes in cardiovascular biomarkers with contemporary thoracic radiation therapy (RT) and evaluated their associations with radiation dose-volume metrics including mean heart dose (MHD), V5, and V30.
Methods and Materials:
In a prospective longitudinal study of 87 patients with breast cancer, lung cancer, or mediastinal lymphoma treated with photon or proton thoracic RT, blood samples were obtained pre-RTand after completion of RT (median, 20 days; interquartile range [IQR], 1–35). High-sensitivity cardiac troponin T, N-terminal proB-type natriuretic peptide, placental growth factor (PIGF), and growth differentiation factor 15 (GDF-15) were measured. Associations between MHD, V5 and V30, and biomarker levels and associations between echocardiography-derived measures of cardiac function and biomarker levels were assessed in multivariable linear regression models. Analyses were performed according to the following subgroups: (1) breast cancer alone and (2) lung cancer and lymphoma combined.
Results:
The median (IQR) estimates of MHD ranged from 1.3Gy(0.9–2.4) inbreast cancer (n = 60) to 6.8Gy (5.4–10.2) inmediastinallymphoma (nZ14) and 8.4Gy(6.7–16.1) in lung cancer (n = 13) patients (P < .001). There were no significant increases in biomarker levels from pre-RT to post-RT in breast cancer. In lung cancer/lymphoma, PIGF increased from a median (IQR) of 20 ng/L (16–26) to 22 ng/L (16–30) (P = .005), and GDF-15 increased from 1171 ng/L (755–2493) to 1887 ng/L (9033763) (P = .006). MHD, V5, and V30 were significantly associated with post-RT PIGF and GDF-15 levels in multivariable models. Changes in biomarkers were not significantly associated with changes in echocardiography-derived measures of cardiac function.
Conclusion:
Contemporary thoracic RT induces acute abnormalities in vascular and inflammatory biomarkers that are associated with radiation dose-volume metrics, particularly in lung cancer and mediastinal lymphoma. Long-term follow-up studies are needed to determine the impact of these changes on the development of overt cardiac disease.
Summary
There is limited evidence on the acute effects of contemporary thoracic radiation therapy (RT) on cardiovascular biomarkers. In this study, we investigated the early changes in multiple cardiovascular biomarkers, reflective of diverse pathophysiologic processes, with RT. Our findings demonstrate that thoracic RT induces acute abnormalities in vascular and inflammatory biomarkers that are associated with radiation dosevolume metrics, particularly in lung cancer and mediastinal lymphoma.
Introduction
Cardiotoxicity is a well-recognized serious adverse effect of thoracic radiation therapy (RT). This can manifest in multiple ways, including restrictive cardiomyopathy, coronary heart disease, pericardial disease, and heart failure with preserved or reduced ejection fraction. Although overt disease most typically occurs 5 years or more after exposure, studies have indicated that subclinical structural and functional abnormalities may occur early after radiation exposure.1–5 Therefore, the period immediately after RT may be of particular interest, providing insight into both mechanisms and prognosis.6–8
Although the mechanisms underlying radiation-induced heart disease have not been fully elucidated, it has been suggested that radiation exposure induces multiple pathophysiologic processes including, but not limited to, direct cell damage, oxidative stress, endothelial dysfunction, inflammation, and fibrosis.4,9,10 Subsequently, there has been growing interest in the identification of diagnostic and/or predictive circulating biomarkers of cardiotoxicity in the field of radiation oncology. So far, few small studies have evaluated early changes in several biomarkers, particularly cardiac troponins and natriuretic peptides, in patients treated with thoracic RT.11–18
However, these studies have yet to provide conclusive evidence of the relevance of biomarkers; hence their potential utility as markers of radiation-induced cardiotoxicity remains uncertain. Further investigation is needed to ascertain the effects of contemporary thoracic RT, which minimizes the dose to the heart. In addition, newer biomarkers reflective of the diverse biologic processes associated with radiation exposure may provide important insight into the risk of cardiovascular complications.
In this study, our objectives were to (1) characterize the early changes in the levels of multiple biomarkers reflective of myocardial injury and stress (high-sensitivity cardiac troponin T [hs-cTnT] and N-terminal pro-B-type natriuretic peptide [NT-proBNP]), angiogenesis and vascular function (placental growth factor [PIGF]), and inflammation and oxidative stress (growth differentiation factor 15 [GDF15]); (2) evaluate the associations between mean heart dose (MHD), percent volume of the heart receiving 5 Gy ( V 5) and 30 Gy (V30), and early changes in biomarker levels; and (3) evaluate the associations between early changes in biomarker levels and changes in echocardiography-derived measures of cardiac function, including left ventricular ejection fraction (LVEF), longitudinal strain, and circumferential strain, in patients treated with thoracic RT.
Methods and Materials
Study population
Patients treated with photon or proton thoracic RT were enrolled in this prospective longitudinal cohort study from June 2015 until February 2018. Key inclusion criteria were as follows: (1) age 18 years or older; (2) left-sided breast cancer treated with fractionated whole breast/thoracic wall with or without regional nodal photon or proton RT; (3) right-sided breast cancer treated with fractionated photon or proton RT with nodal proton or photon RT; (4) lung cancer treated with definitive intent (≥50 Gy) using conventionally fractionated thoracic photon or proton RT (1.8,2.0 Gy per fraction); or (5) mediastinal lymphoma whose lowest extent of mediastinal disease was at or below the level of the carina, treated with consolidative radiation with definitive intent (≥20 Gy) using conventionally fractionated thoracic photon or proton RT; and (6) the ability to read and comprehend English. Patients with prior RT to the thorax that would have resulted in overlap of RT fields, those receiving stereotactic body RT, or those with life expectancy less than 12 months were excluded. The study was approved by the institutional review board. The current analysis included patients with available biomarker measurements both at pre-RT and after completion of RT.
Study procedures
Detailed clinical data, including demographic characteristics, cardiovascular risk factors, cardiovascular medications, and additional cancer therapy-related variables, were collected pre-RT and after completion of RT using standardized patient and physician questionnaires and were further verified by medical record review. Blood samples were collected pre-RT and after a median (interquartile range [IQR]) of 20 (1–35) days after completion of RT. Samples were stored at −80°C. In addition, transthoracic echocardiography was performed at both visits. Twodimensional images were acquired using Vivid E9 or E95 machines (GE Healthcare, Milwaukee, WI) and digitally archived for post hoc analyses.
Evaluation of cardiac radiation exposure
Computed tomography or positron emission tomography/computed tomography simulation was performed for each patient. Motion assessment was performed in each case using 4-dimensional technology. Contours for all organs at risk were performed on the average scan. The heart was contoured per the Radiation Therapy Oncology Group standard from the base to the apex, beginning from where the ascending aorta originates. Proton and photon treatment plans were generated using Eclipse 13.7 Treatment planning system (Palo Alto, CA) and dose calculations were performed using a 2.5 × 2.5 ×1.5-mm grid size. MHD, V5, and V30 were calculated using Eclipse.
Biomarker measurements
hs-cTnT was measured using the Elecsys® TnT-hs assay on a Cobas platform (Roche Diagnostics, Manheim, Germany), which has a limit of detection of 5 ng/L and a coefficient of variation (CV) of <10% at the 99th percentile upper reference limit of 14 ng/L. NT-proBNP was measured using the Elecsys® NT-proBNP assay on Cobas platform, which has a measurement range of 5 to 35,000 ng/L and CV of 2.9% to 6.1%. PIGF was measured using the Elecsys® PIGF immunoassay with a measurement range of 3 to 10,000 ng/L and CV of <5%. The Elecsys® GDF-15 immunoassay was used to measure GDF-15; the measurement range is 400 to 20,000 ng/L and CV is <10%.
Quantitative Echocardiography
Quantitative echocardiography was performed by a single blinded observer using the TomTec Imaging Systems platform (Unterschleissheim, Germany). Left ventricular end-diastolic and end-systolic volumes were calculated using the Simpson’s method of discs, and used to derive LVEF. In addition, longitudinal and circumferential strain measurements were performed from the digitally archived images. Estimates of intraobserver CV are 4.9% for LVEF, 10.9% for longitudinal strain, and 9.4% for circumferential strain.19
Statistical analysis
Baseline characteristics were summarized using proportions for categorical variables, and mean (standard deviation) and median (IQR) were used for normally and non-normally distributed continuous variables, respectively.
Given concerns over differences in median RT dose across the cancer types, the breast cancer subgroup was analyzed separately from the lung cancer and lymphoma subgroups. Differences in biomarker levels pre-RTand after completion of RT were tested with the Wilcoxon signedrank test. Proportions of patients with potentially clinically significant elevations in hs-cTnT and NT-proBNP from preRT to the completion of RT were calculated. Based on intra-assay CV and published values of intraindividual physiological variations for these biomarkers, elevations exceeding a prespecified threshold of 30% were considered to be clinically significant.20–22 Increases in hs-cTnT values to an abnormal range (i.e., >14 ng/L) after completion of RT were also considered clinically significant. In addition, the proportions of patients with >30% elevations in PIGF and GDF-15 values were explored.
Associations between MHD and biomarker levels after completion of RT were evaluated using linear regression models. Biomarker values were natural log-transformed and included as the dependent variable, and MHD was the independent variable. To account for differences in the magnitude and distribution of cardiac radiation exposure and chemotherapy-related factors across the different cancer types, we performed this analysis in breast cancer and lung cancer/lymphoma separately. Models were covariateadjusted for the pre-RT values of the biomarker under consideration, age, anthracycline or trastuzumab exposure before RT, hypertension, and diabetes. A sensitivity analysis was performed by additionally including pre-existing heart disease and the following cardiovascular medications: statins, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and beta-blockers. Apriori, we hypothesized that anthracycline or trastuzumab exposure in patients with breast cancer might influence the association between MHD and biomarker changes. Therefore, we explored for evidence of an interaction between MHD and anthracycline or trastuzumab exposure for each biomarker. We also evaluated the associations between V5 and V30 and biomarker changes using the same procedure described earlier.
The association between changes in biomarker levels and LVEF was explored using linear regression models in which the absolute change in biomarker levels from preRT to the completion of RT was included as the independent variable, and LVEF after completion of RT was the dependent variable. Each of the models was adjusted for baseline LVEF, cancer type, age, anthracycline or trastuzumab exposure, hypertension, and diabetes mellitus. A similar approach was implemented to evaluate the association between changes in biomarker levels and changes in longitudinal and circumferential strain. These analyses were not stratified given concerns over sample size.
A 2-sided alpha level of 0.05 was used to assess statistical significance. All analyses were performed using R 3.4.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study population
Baseline (pre-RT) characteristics of patients included in the study are summarized in Table 1. Briefly, 74 (85.1%) patients were female and 66 (75.9%) were white. The median (IQR) age of the study population was 52.5 (40.2 – 60.0). A total of 60 (69.0%) patients had breast cancer, and 13 (14.9%) and 14 (16.1%) had lung cancer and mediastinal lymphoma, respectively. Overall, 45 (51.7%) patients were treated with 3-dimensional conformal RT, 19 (21.8%) received intensity modulated RT, and 23 (26.5%) were treated with proton therapy. Twenty-six (43.3%) patients with breast cancer received internal mammary lymph node radiation. Among patients with lung cancer, 11 (84.6%) received mediastinal radiation.
Table 1.
Baseline characteristics of the analysis cohort
| Variable | Overall* (n = 87) | Breast cancer (n = 60) | Lung cancer/lymphoma (n = 27) |
|---|---|---|---|
| Age, y | 52.5 [40.2–60.0] | 53.0 [42.0–60.5] | 49.0 [27.5–60.0] |
| Sex | |||
| Female | 74 (85.1) | 60 (100) | 14 (51.9) |
| Male | 13 (14.9) | 0(0) | 13 (48.1) |
| Race | |||
| White | 66 (75.9) | 43 (71.7) | 23 (85.2) |
| Black or African American | 18 (20.7) | 14 (23.3) | 4 (14.8) |
| Asian/Pacific Islander | 3 (3.4) | 3 (5.0) | 0(0) |
| Anthracycline and/or trastuzumab as part of current treatment | 45 (51.7) | 34 (56.7) | 11 (40.7) |
| Past anthracycline exposure | 6 (6.9) | 2 (3.3) | 4 (14.8) |
| BMI (kg/m2) | 28.4 ± 6.7 | 28.5 ± 6.9 | 28.1 ± 6.3 |
| Current or past smoking | 34 (39.1) | 23 (38.3) | 11 (40.7) |
| Hypertension | 24 (27.6) | 20 (33.3) | 4 (14.8) |
| Diabetes mellitus | 9 (10.3) | 6 (10.0) | 3 (11.1) |
| Hypercholesterolemia or statin use | 26 (29.9) | 18 (30.0) | 8 (29.6) |
| ACEI/ARB or beta-blocker use | 24 (27.6) | 21 (35.0) | 3 (11.1) |
| Primary radiation technique | |||
| Protons (passive scattering) | 6 (7.0) | 4 (6.7) | 2 (7.4) |
| Protons (scanning) | 17 (19.5) | 4 (6.7) | 13 (48.2) |
| 3D Conformal | 45 (51.7) | 44 (73.3) | 1 (3.7) |
| IMRT | 19 (21.8) | 8 (13.3) | 11 (40.7) |
| Total radiation dose, Gy | 52.6 [50.4–60.0] | 52.6 [50.4–60.0] | 50.4 [30.6–63.3] |
| MHD, Gy | 2.1 [1.1–5.2] | 1.3 [0.9–2.4] | 7.3 [5.7–13.4] |
| V5, % | 6.8 [1.5–22.1] | 2.3 [1.1–7.9] | 34.5 [19.9–47.8] |
| V30, % | 0.3 [0–3.5] | 0.1 [0–0.9] | 7.9 [2.7–21.2] |
| hs-cTnT (ng/L) | 11 [6–21] | 11 [6–23] | 11 [8–18] |
| NT-proBNP (ng/L) | 72 [35–118] | 72 [42–118] | 70 [25–114] |
| PIGF (ng/L) | 18 [15–23] | 18 [15–23] | 20 [16–26] |
| GDF-15 (ng/L) | 1026 [727–1663] | 1012 [676–1512] | 1171 [756–2493] |
| LVEF, % | 52 [48–56] | 52 [48–56] | 52 [46–54] |
| Longitudinal strain, % | −14 [−16 to −12] | −13 [−16 to −12] | −14 [−16 to −12] |
| Circumferential strain, % | −23 [−25 to −19] | −22 [−25 to −18] | −24 [−25 to −22] |
Abbreviations: 3D = 3-dimensional; ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; BMI = body mass index; DBP = diastolic blood pressure; GDF-15 = growth differentiation factor 15; hs-cTnT = high-sensitivity cardiac troponin T; IMRT = intensity modulated radiation therapy; MHD = mean heart dose; NT-proBNP = N-terminal pro-B-type natriuretic peptide; PIGF = placental growth factor; RT = radiation therapy; SBP = systolic blood pressure.
Categorical variables were summarized with count (%); normally distributed continuous variables were summarized with the mean ± standard deviation, non-normally distributed continuous variables were summarized with the median [interquartile range]; V5 and V30 indicate the percent volume of heart receiving 5 Gy and 30 Gy, respectively.
Patients with available biomarker measurements at pre-RT and after completion of RT were included.
MHD
Overall, the median (IQR) MHD was 2.1 Gy (1.1–5.2). There was a significant difference in MHD across the different cancer types (P < .001). Median (IQR) estimates were low at 1.3 Gy (0.9–2.4) in breast cancer. In contrast, MHD estimates were greater and comparable in the lung cancer and lymphoma subgroups, with a median (IQR) of 8.4 Gy (6.7–16.1) in lung cancer and 6.8 Gy (5.4–10.2) in lymphoma.
Biomarker changes pre-RT to completion of RT
Individual changes in biomarkers from pre-RT to post-RT are presented in Figures 1A (breast cancer) and 1B ( lung cancer/lymphoma). Table 2 summarizes biomarker levels at the 2 time points for breast cancer and lung cancer/lymphoma.
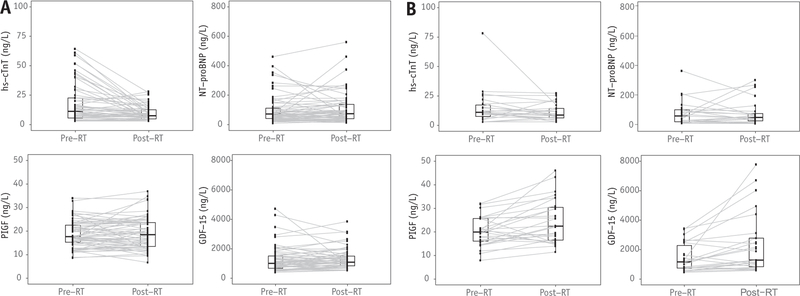
Fig. 1.
Changes in biomarker levels from pre-RT to the completion of RT in breast cancer (A) and lung cancer/mediastinal lymphoma (B). Box plots depict biomarker distributions at each time point; gray lines show individual changes in biomarker levels from pre-RT to the completion of RT. Abbreviations: GDF-15 = growth differentiation factor 15; hs-cTnT = high-sensitivity cardiac troponin T; NT-proBNP = N-terminal B-type natriuretic peptide; PIGF = placental growth factor; RT = radiation therapy.
Table 2.
Summary of biomarker levels at pre-RT and after completion of RT in breast cancer and lung cancer/lymphoma
| Breast cancer |
Lung cancer/Mediastinal lymphoma |
|||||
|---|---|---|---|---|---|---|
| Biomarker | Pre-RT | Post-RT | P value | Pre-RT | Post-RT | P value |
| hs-cTnT, ng/L | 11 [6–23] | 8 [5–13] | <.001 | 11 [8–18] | 9 [6–16] | .057 |
| NT-proBNP, ng/L | 77 [42–118] | 76 [40–139] | .430 | 70 [25–114] | 64 [38–168] | .860 |
| PIGF, ng/L | 18 [15–23] | 19 [13–24] | .746 | 20 [16–26] | 22 [16–30] | .005 |
| GDF-15, ng/L | 1012 [676–1513] | 1093 [841–1505] | .054 | 1171 [755–2493] | 1887 [903–3763] | .006 |
Abbreviations: GDF-15 = growth differentiation factor 15; hs-cTnT = high-sensitivity cardiac troponin T; NT-proBNP = N-terminal pro-B-type natriuretic peptide; PIGF = placental growth factor; RT = radiation therapy.
Median (interquartile range) was used to summarize biomarker levels. Differences in biomarker levels at the 2 time points were statistically tested with the Wilcoxon signed-rank test.
In breast cancer, hs-cTnT was significantly lower after completion of RT compared with pre-RT, likely secondary to anthracycline exposure before RT. There were no statistically significant differences in NT-proBNP, PIGF, and GDF-15 levels between the 2 time points. In patients with lung cancer or lymphoma, who received higher doses of cardiac radiation exposure compared with those with breast cancer, significant increases in PIGF and GDF-15 were observed from pre-RT to post-RT. Interestingly, consistent with the finding in breast cancer, hs-cTnT levels tended to decrease.
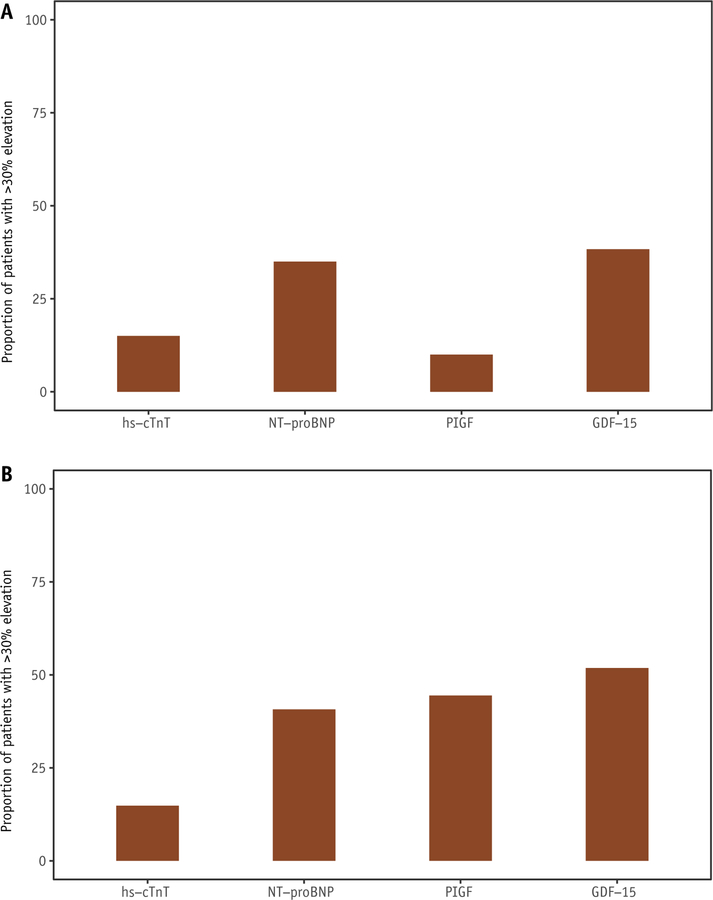
In breast cancer, 15.0% of patients developed >30% elevation in hs-cTnT or increase to >14 ng/L from pre-RT to post-RT. The proportions of patients who developed >30% elevation in NT-proBNP, PIGF, and GDF-15 were 35.0%, 10.0%, and 38.3%, respectively (Fig. 2A). Among those with lung cancer or lymphoma, the proportions were 14.8%, 40.7%, 44.4%, and 51.8% for hs-cTnT, NT-proBNP, PIGF, and GDF-15, respectively (Fig. 2B). Clinical characteristics of individual patients with potentially clinically significant elevations are presented for each biomarker in Tables E1 to E4 (available online at https://doi.org/10.1016/j.ijrobp.2018.11.013).
Fig. 2.
Proportion of patients with potentially clinically significant elevation in biomarker levels from pre-RT to completion of RT in breast cancer (A) and lung cancer/mediastinla lymphoma (B). The height of the bars represents the proportion of patients who developed potentially clinically significant elevations in biomarkers from pre-RT to the completion of RT (i.e., >30% elevation or increase to >14 ng/L for hs-cTnT, and >30% elevation for the other biomarkers). Abbreviations: GDF-15 = growth differention factor 15; hs-cTnT = high sensitivity cardiac troponin T; NT-proBNP = Nterminal B-type natriuretic peptide; PIGF = placental growth factor; RT = radiation therapy.
Associations between cardiac radiation exposure and biomarker changes
We examined the associations between cardiac dosimetry variables and early changes in biomarkers in breast cancer and lung cancer/lymphoma. In breast cancer, there were no consistent statistically significant associations between MHD, V5, and V30 and early biomarker changes (Table 3).
Table 3.
Associations between MHD, V5, and V30 and biomarker levels after completion of RT in breast cancer and lung cancer/lymphoma
| Biomarker | Breast cancer |
Lung cancer/Mediastinal lymphoma |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MHD |
V5 |
V30 |
MHD |
V5 |
V30 |
|||||||
| Beta (95% CI) | P value | Beta (95% CI) | P value | Beta (95% CI) | P value | Beta (95% CI) | P value | Beta (95% CI) | P value | Beta (95% CI) | P value | |
| hs-cTnT | 0.14 (0–0.28) | .052 | 0.12 (−0.01 to 0.25) | .061 | 0.09 (−0.01 to 0.19) | .071 | 0.07 (−0.22 to 0.37) | .606 | 0.05 (−0.25 to 0.35) | .737 | 0.10 (−0.26 to 0.46) | .561 |
| NT-proBNP | −0.01 (−0.20 to 0.19) | .956 | 0 (−0.18 to 0.18) | .997 | 0.05 (−0.09 to 0.19) | .444 | −0.14 (−0.56 to 0.27) | .476 | −0.18 (−0.61 to 0.24) | .374 | −0.09 (−0.60 to 0.41) | .703 |
| PIGF | 0.01 (−0.06 to 0.08) | .846 | 0.02 (−0.04 to 0.08) | .533 | 0.01 (−0.04 to 0.06) | .734 | 0.15 (0–0.30) | .050 | 0.21 (0.06–0.35) | .008 | 0.14 (−0.06 to 0.34) | .155 |
| GDF-15 | 0.07 (−0.03 to 0.16) | .176 | 0.07 (−0.01 to 0.16) | .091 | 0.01 (−0.06 to 0.08) | .808 | 0.40 (0.14–0.66) | .005 | 0.37 (0.10–0.65) | .011 | 0.46 (0.13–0.79) | .008 |
Abbreviations: CI = confidence interval; GDF-15 = growth differentiation factor 15; hs-cTnT = high-sensitivity cardiac troponin T; MHD = mean heart dose; NT-proBNP = N-terminal pro-B-type natriuretic peptide; PIGF = placental growth factor; RT = radiation therapy.
All biomarker variables were natural log-transformed. Per each interquartile range (i.e., 1.5 Gy for MHD, 6.8% for V5, and 0.9% for V30 in breast cancer; and 7.7 Gy for MHD, 27.9% for V5, and 18.4% for V30 in lung cancer/lymphoma) increase, the beta coefficient represents the percent change in biomarker value post-RT. Associations were adjusted for age, anthracycline or trastuzumab exposure, hypertension, diabetes mellitus, and pre-RT level of the biomarker under consideration.
In lung cancer/lymphoma, significant associations were seen in multivariable models between MHD and levels of PIGF and GDF-15 after completion of RT (Table 3). After adjustment for age, anthracycline or trastuzumab exposure, hypertension, diabetes mellitus, and pre-RT level of the biomarker under consideration, there was an average 15% increase in PIGF levels after completion of RT per each IQR (i.e., 7.7 Gy) increase in MHD (P = .050). For GDF-15, there was an average 40% increase after completion of RT per each IQR increase in MHD (P = .005). Significant associations were also seen with the other cardiac dosimetry (Table 3). Including pre-existing heart disease and cardiovascular medications in the multivariable models did not change these results.
Biomarker changes pre-RT to completion of RT: interaction with anthracycline or trastuzumab exposure
Biomarker distributions pre-RT and after completion of RT in patients with breast cancer stratified by anthracycline or trastuzumab exposure are presented in Figure E1 (available online at https://doi.org/10.1016/j.ijrobp.2018.11.013). No statistically significant interactions were observed between MHD and anthracycline or trastuzumab exposure in terms of associations with biomarker levels after completion of RT (Table E5; available online at https://doi.org/10.1016/j.ijrobp.2018.11.013).
Associations between biomarker changes and changes in LVEF and myocardial strain
Finally, we explored the associations between absolute biomarker changes from pre-RT to completion of RT and echocardiography-derived measures of cardiac function, including LVEF, longitudinal strain, and circumferential strain. Sixty patients had LVEF and strain assessments both pre-RT and after completion of RT. Given the limited sample size, these analyses were performed in the overall cohort. There were no statistically significant associations between absolute changes in biomarker values and levels of LVEF, longitudinal strain, or circumferential strain after completion of RT in multivariable models (Table E6; available online at https://doi.org/10.1016/j.ijrobp.2018.11.013).
Discussion
In this prospective study, we evaluated the early changes in a diverse panel of mechanistic cardiovascular biomarkers after contemporary thoracic RT. We found (1) significant increases in PIGF and GDF-15 levels after completion of RT compared to pre-RT, particularly in patients with lung cancer or mediastinal lymphoma; (2) significant associations between MHD and other cardiac dosimetry variables such as V5 and V30, and changes in PIGF and GDF-15 after completion of RT primarily in the lung cancer/lymphoma subgroup; (3) no significant associations between changes in biomarker levels from pre-RT to the completion of RT and changes in echocardiography-derived measures of cardiac function.
Early changes in circulating biomarkers after exposure to thoracic RT can provide important mechanistic insights that can advance our understanding of the pathophysiologic processes underlying radiation-induced cardiotoxicity. In addition, biomarkers can aid in the detection of subclinical cardiotoxicity and can play an important role in risk prediction. Our study is the first to report that newer markers PIGF and GDF-15 increased significantly with thoracic RT, particularly in lung cancer and lymphoma, and that MHD and other cardiac dosimetry variables, specifically V5 and V30, were significantly associated with these changes. Prior studies have indicated that PIGF and GDF-15 are predictive for the development and progression of cardiovascular disease in the general population.23–27 PIGF, which is a member of the vascular endothelial growth factor family, promotes angiogenesis, and studies suggest that it plays an important role in the development of early atherosclerotic lesions and predicts adverse outcomes in patients with chest pain.28,29 GDF-15 is a member of the transforming growth factor superfamily that is secreted by different cells including macrophages, vascular smooth muscle cells, and cardiomyocytes, and it is upregulated under conditions such as inflammation, oxidative stress, tissue injury, and hypoxia.27,30 We postulate that these processes are integral components of the pathophysiologic changes that occur early during the course of the pathogenesis of radiationinduced heart disease, and our findings suggest that additional translational research is needed to better understand these mechanisms.4,9,10 We propose that PIGF and GDF-15 show promise as new candidate biomarkers for the detection of subclinical cardiotoxicity. Further study is needed to define the value of early changes in these biomarkers in predicting the risk of overt heart disease in patients treated with thoracic RT.
A growing interest in conventional cardiac biomarkers including cardiac troponins and natriuretic peptides is evident in the radiation oncology literature. Multiple small studies have characterized early changes in these biomarkers after thoracic RT and evaluated associations with cardiac dosimetry variables.11–18 These studies mostly suggest that levels of cardiac troponins and natriuretic peptides are unaffected by thoracic RT. Consistent with this prior work, our study also demonstrated that levels of hscTnT and NT-proBNP, on average, did not significantly increase acutely from pre-RT to the completion of RT. In fact, hs-cTnT was significantly lower after the completion of RT compared to pre-RT levels, particularly in the breast cancer subgroup. We postulate that higher levels observed pre-RT with subsequent declines are secondary to anthracycline exposure and relatively low cardiac exposure in breast cancer.31,32 As shown in Figure E1 (available online at https://doi.org/10.1016/j.ijrobp.2018.11.013), levels of hs-cTnT generally decrease after completion of RT, most prominently in the subgroup of patients previously exposed to anthracycline or trastuzumab. We did not detect a statistically significant interaction by anthracycline or trastuzumab therapy on the associations between biomarkers and MHD, although we are limited by sample size.
These findings suggest that the conventional cardiac biomarkers, which primarily reflect direct myocardial damage, may not be optimal markers of subclinical radiation-induced cardiotoxicity in the acute phase. It may also be that the growing trend toward decreases in MHD and cardiac sparing in the current treatment era has resulted in less overall cardiomyocyte damage and stress; on population average, direct myocardial damage from RT may be increasingly difficult to quantify by these nonspecific biomarkers of cardiac injury and stress.33,34 However, it should be noted that subsets of patients, on the order of 15% to 40%, developed >30% elevations in hs-cTnT and NTproBNP. The impact of such elevations on long-term risk of cardiac events merits further investigation.
An increased focus on cardiac sparing has resulted in contemporary strategies with greater precision in dose distribution and lower cardiac radiation exposure.33 The low MHD observed in our study, particularly in breast cancer, is in line with this decreasing trend in cardiac exposure with thoracic RT. On average, there were no significant increases in cardiac, vascular, or inflammatory markers in breast cancer, although as noted there are subgroups with potentially clinically relevant elevations in these markers.
However, our findings suggest that acute biomarker changes, particularly those related to vascular and inflammatory processes, are more pronounced in lung cancer and lymphoma, where the average cardiac exposure is significantly higher compared to breast cancer. These findings indicate that vascular endothelial dysfunction and inflammation, rather than direct myocardial damage, may be more relevant in the early pathogenesis of contemporary RTinduced heart disease and motivate mechanistic studies to evaluate these pathways. Long-term follow-up studies are also needed to elucidate the relationship between these biomarker changes and the risk of overt heart disease in patients treated with contemporary thoracic RT.
An important limitation of our study arises from the heterogeneity of the patient population investigated. Differences in the magnitude and distribution of cardiac radiation exposure and chemotherapy-related factors across the different disease types can affect acute biomarker changes and possibly the association between cardiac dosimetry variables and biomarker changes. Therefore, we performed separate analyses in breast cancer and lung cancer/lymphoma. However, the sizes of the subgroups with lung cancer and lymphoma are relatively small, and given the higher cardiac radiation exposure and more pronounced biomarker changes, larger prospective studies are of critical importance to validate our findings.
Conclusions
In patients treated with contemporary thoracic RT, levels of PIGF and GDF-15 increased significantly after completion of RT compared to pre-RT, specifically in those with lung cancer or lymphoma, and were independently associated with MHD, V5, and V30. These findings suggest that mechanistic biomarkers indicative of vascular function and inflammation may be relevant and indicative of the cardiovascular effects of thoracic RT. Long-term follow-up studies are needed to determine the prognostic associations between biomarker changes and the future development of clinical cardiovascular disease.
Supplementary Material
Acknowledgments
This work was supported by Abramson Cancer Center and Radiation Oncology Pilot Grant Award (Ky, Simone, Lin), by R01 HL 118018 (Ky), and by an Investigator Initiated Award from Roche Diagnostics (Ky).
Footnotes
Conflict of interest: none.
Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2018.11.013.
References
- 1.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013; 368:987–998. [DOI] [PubMed] [Google Scholar]
- 2.Saiki H, Petersen IA, Scott CG, et al. Risk of heart failure with preserved ejection fraction in older women after contemporary radiotherapy for breast cancer. Circulation 2017;135:1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor C, Correa C, Duane FK, et al. Estimating the risks of breast cancer radiotherapy: Evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J Clin Oncol 2017;35:1641–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaworski C, Mariani JA, Wheeler G, Kaye DM. Cardiac complications of thoracic irradiation. J Am Coll Cardiol 2013;61:2319–2328. [DOI] [PubMed] [Google Scholar]
- 5.Filopei J, Frishman W. Radiation-induced heart disease. Cardiol Rev 2012;20:184–188. [DOI] [PubMed] [Google Scholar]
- 6.Erven K, Florian A, Slagmolen P, et al. Subclinical cardiotoxicity detected by strain rate imaging up to 14 months after breast radiation therapy. Int J Radiat Oncol Biol Phys 2013;85:1172–1178. [DOI] [PubMed] [Google Scholar]
- 7.Lind PA, Pagnanelli R, Marks LB, et al. Myocardial perfusion changes in patients irradiated for left-sided breast cancer and correlation with coronary artery distribution. Int J Radiat Oncol Biol Phys 2003;55: 914–920. [DOI] [PubMed] [Google Scholar]
- 8.Simone CB 2nd. New era in radiation oncology for lung cancer: Recognizing the importance of cardiac irradiation. J Clin Oncol 2017; 35:1381–1383. [DOI] [PubMed] [Google Scholar]
- 9.Spetz J, Moslehi J, Sarosiek K. Radiation-induced cardiovascular toxicity: Mechanisms, prevention, and treatment. Curr Treat Options Cardiovasc Med 2018;20:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yarnold J, Brotons MC. Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol 2010;97:149–161. [DOI] [PubMed] [Google Scholar]
- 11.Hughes-Davies L, Sacks D, Rescigno J, Howard S, Harris J. Serum cardiac troponin T levels during treatment of early-stage breast cancer. J Clin Oncol 1995;13:2582–2584. [DOI] [PubMed] [Google Scholar]
- 12.Gomez DR, Yusuf SW, Munsell MF, et al. Prospective exploratory analysis of cardiac biomarkers and electrocardiogram abnormalities in patients receiving thoracic radiation therapy with high-dose heart exposure. J Thorac Oncol 2014;9:1554–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skytta T, Tuohinen S, Boman E, Virtanen V, Raatikainen P, Kellokumpu-Lehtinen PL. Troponin T-release associates with cardiac radiation doses during adjuvant left-sided breast cancer radiotherapy. Radiat Oncol 2015;10:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozak KR, Hong TS, Sluss PM, et al. Cardiac blood biomarkers in patients receiving thoracic (chemo)radiation. Lung Cancer 2008;62: 351–355. [DOI] [PubMed] [Google Scholar]
- 15.Kuo AH, Ancukiewicz M, Kozak KR, Yock TI, Padera TP. Cardiac and inflammatory biomarkers do not correlate with volume of heart or lung receiving radiation. Radiat Oncol 2015;10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palumbo I, Palumbo B, Fravolini ML, et al. Brain natriuretic peptide as a cardiac marker of transient radiotherapy-related damage in left-sided breast cancer patients: A prospective study. Breast 2016;25: 45–50. [DOI] [PubMed] [Google Scholar]
- 17.Serrano NA, Mikkelsen R, Canada J, Mezzaroma E, Weiss E, Abbate A. Biomarkers of cardiac injury in patients undergoing thoracic radiation therapy. Int J Cardiol 2016;223:507–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wondergem J, Strootman EG, Frolich M, Leer JW, Noordijk EM. Circulating atrial natriuretic peptide plasma levels as a marker for cardiac damage after radiotherapy. Radiother Oncol 2001;58: 295–301. [DOI] [PubMed] [Google Scholar]
- 19.Narayan HK, French B, Khan AM, et al. Noninvasive measures of ventricular-arterial coupling and circumferential strain predict cancer therapeutics-related cardiac dysfunction. JACC Cardiovasc Imaging 2016;9:1131–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frankenstein L, Wu AH, Hallermayer K, Wians FH Jr., Giannitsis E, Katus HA. Biological variation and reference change value of high-sensitivity troponin T in healthy individuals during short and intermediate follow-up periods. Clin Chem 2011;57:1068–1071. [DOI] [PubMed] [Google Scholar]
- 21.Schou M, Gustafsson F, Kjaer A, Hildebrandt PR. Long-term clinical variation of NT-proBNP in stable chronic heart failure patients. Eur Heart J 2007;28:177–182. [DOI] [PubMed] [Google Scholar]
- 22.Meijers WC, van der Velde AR, Muller Kobold AC, et al. Variability of biomarkers in patients with chronic heart failure and healthy controls. Eur J Heart Fail 2017;19:357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cassidy A, Chiuve SE, Manson JE, Rexrode KM, Girman CJ, Rimm EB. Potential role for plasma placental growth factor in predicting coronary heart disease risk in women. Arterioscler Thromb Vasc Biol 2009;29:134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lenderink T, Heeschen C, Fichtlscherer S, et al. Elevated placental growth factor levels are associated with adverse outcomes at four-year follow-up in patients with acute coronary syndromes. J Am Coll Cardiol 2006;47:307–311. [DOI] [PubMed] [Google Scholar]
- 25.Rohatgi A, Patel P, Das SR, et al. Association of growth differentiation factor-15 with coronary atherosclerosis and mortality in a young, multiethnic population: Observations from the Dallas heart study. Clin Chem 2012;58:172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fluschnik N, Ojeda F, Zeller T, et al. Predictive value of long-term changes of growth differentiation factor-15 over a 27-year-period for heart failure and death due to coronary heart disease. PLoS One 2018; 13:e0197497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wollert KC, Kempf T, Wallentin L. Growth differentiation factor 15 as a biomarker in cardiovascular disease. Clin Chem 2017;63:140–151. [DOI] [PubMed] [Google Scholar]
- 28.Khurana R, Moons L, Shafi S, et al. Placental growth factor promotes atherosclerotic intimal thickening and macrophage accumulation. Circulation 2005;111:2828–2836. [DOI] [PubMed] [Google Scholar]
- 29.Heeschen C, Dimmeler S, Fichtlscherer S, et al. Prognostic value of placental growth factor in patients with acute chest pain. JAMA 2004; 291:435–441. [DOI] [PubMed] [Google Scholar]
- 30.Adela R, Banerjee SK. GDF-15 as a target and biomarker for diabetes and cardiovascular diseases: A translational prospective. J Diabetes Res 2015;2015:490842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cardinale D, Sandri MT, Colombo A, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation 2004;109:27492754. [DOI] [PubMed] [Google Scholar]
- 32.de Vries Schultink AHM, Boekhout AH, Gietema JA, et al. Pharmacodynamic modeling of cardiac biomarkers in breast cancer patients treated with anthracycline and trastuzumab regimens. J Pharmacokinet Pharmacodyn 2018;45:431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pierce LJ, Feng M, Griffith KA, et al. Recent time trends and predictors of heart dose from breast radiation therapy in a large quality consortium of radiation oncology practices. Int J Radiat Oncol Biol Phys 2017;99:1154–1161. [DOI] [PubMed] [Google Scholar]
- 34.Haque W, Verma V, Fakhreddine M, Butler EB, Teh BS, Simone CB 2nd. Trends in cardiac mortality in patients with locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2018;100:470–477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.