Abstract
There is growing awareness of the overlap between oncologic and cardiovascular (CV) diseases, including a wide range of CV effects of anticancer therapies. As novel anticancer therapeutics become available and cancer survival outcomes improve, the CV implications of cancer therapy become increasingly important. In addition to outlining the CV effects of commonly used cancer therapies and their consequences for long-term survivorship, this review highlights the recent efforts to improve the risk prediction and prevention of CV toxicity through the evaluation of sensitive measures for early toxicity detection and the implementation of cardioprotective strategies.
Keywords: cardiotoxicity, cardio-oncology, risk factors, heart failure, biomarkers, survivorship
INTRODUCTION
Although heart disease and cancer have long been recognized as the leading causes of death in the United States, more recent attention has focused on the potential interactions between cardiovascular (CV) risk and anticancer therapies (1). Indeed, treatment-related CV toxicity is now a widely recognized consequence of cancer therapies (2). The extent of this CV risk may depend on both treatment- and patient-related factors, including an exacerbation of underlying CV comorbidity in the setting of either an advanced malignancy or intensive cancer therapy. In addition, emerging evidence indicates significant biologic and epidemiologic overlap between cancer and CV disease, with the recognition of shared biologic mechanisms, modifiable risk factors, and potential genetic predispositions (3, 4).
Recent improvements in clinical outcomes for many cancer patients, often even in advanced oncologic stages, have raised the potential for long-term CV morbidity among cancer survivors. For example, in older women treated for breast cancer, the rates of CV mortality may ultimately exceed those from breast cancer recurrence (5, 6). Similarly, in survivors of testicular germ cell tumor, which is often viewed as the paradigm for a “curable advanced cancer,” the standardized incidence rates of late CV morbidity are significantly higher than those observed in the general population (7). Furthermore, the rapidly expanding variety of novel anticancer agents, which involve a multitude of therapeutic mechanisms and drug targets, has demonstrated a wide range of both early and late CV effects, including cardiomyopathy, heart failure with reduced or preserved ejection fraction, hypertension, arrhythmia, coronary artery disease, and thromboembolic events.
The field of cardio-oncology was established in response to this growing complexity of oncologic management, the improving cancer-specific survival outcomes, and the recognized hazards for significant CV morbidity. As a result, formal cardio-oncology clinical practice guidelines have been developed in a collaborative effort to detect, prevent, and manage CV toxicity among cancer patients (8). Moving forward, effective strategies for the prediction and prevention of CV toxicities will remain critically important, as improved CV care of oncologic patients may enable adequate treatment adherence and improved survivorship. This review highlights the current knowledge regarding the mechanisms and epidemiology of cardiotoxicity associated with commonly used anticancer therapies:
Anthracyclines
Anti-HER2 therapies (trastuzumab)
Hormonal therapies (selective estrogen receptor modulators, aromatase inhibitors, androgen deprivation therapy)
Radiation therapy
Antiangiogenic therapies
Immunotherapies
In addition, we review the long-term CV implications of cancer survivorship and recent efforts to develop novel methods to improve the early detection and management of CV complications in cancer patients.
CARDIOVASCULAR COMPLICATIONS OF CANCER THERAPEUTICS
Anthracycline Chemotherapy: CV Toxicities Since the 1970s
Anthracycline chemotherapy agents, including doxorubicin, are commonly used for a variety of solid tumor and hematologic malignancies, including breast cancer, sarcoma, leukemia, and lymphoma. They exert their cytotoxic effect through several proposed mechanisms, including topoisomerase II inhibition, DNA alkylation and cross-linking, and free radical formation causing DNA damage and direct membrane effects (9). The creation of reactive oxygen species in an iron-dependent manner following anthracycline exposure results in oxidative injury to the cardiomyocyte and mitochondrial membranes (2, 10). In addition, topoisomerase 2β-dependent generation of reactive oxygen species may contribute to cardiomyocyte injury (11). Thus, anthracyclines have been widely recognized to cause dose-dependent left ventricular dysfunction (LVD) that is both cumulative and irreversible (12, 13).
In addition to cumulative dose exposure, anthracycline-induced LVD is associated with several other clinical risk factors, including elderly age (>60 years), pediatric exposure, and prior or concurrent chest radiation therapy (RT) (14–16). Moreover, underlying CV disease or risk factors, such as coronary disease, diabetes, tobacco use, dyslipidemia, and a borderline normal baseline left ventricular function [LV ejection fraction (LVEF) of 50–55%] are also known to predispose to anthracycline-induced LVD, likely reflecting impaired cardiac reserve and an intolerance of additional myotoxic injury or stress (14, 16, 17). Detailed echocardiographic phenotyping of LV function in breast cancer patients has demonstrated that anthracycline-induced LVD occurs early, with sustained and modest deficits noted at three years following therapy completion (18). The CV structural and physiologic findings most strongly associated with anthracycline-induced LVD appear to be cardiac volumes, strain, and measures of cardiac afterload (18).
Various cardioprotective strategies have been studied in an effort to mitigate the risk of anthracycline-induced LVD. One such strategy is the use of dexrazoxane, which may provide cardioprotective effects in patients receiving anthracycline therapy through the scavenging of free radicals and inhibition of topoisomerase 2β (2). Multiple clinical trials and meta-analyses have demonstrated significant risk reductions in acute clinical and/or subclinical LVD in patients receiving dexrazoxane with anthracycline therapy (19–21). However, concerns about secondary malignancies and leukopenia have limited its widespread use in some malignancies. The current recommendations of the US Food and Drug Administration (FDA) advise dexrazoxane use only in patients who have previously received >300 mg/m2 of doxorubicin or 540 mg/m2 of epirubicin and who are perceived to derive oncologic benefit from additional doxorubicin therapy (22). Although recent clinical practice guidelines recommend consideration of dexrazoxane if ≥250 mg/m2 of doxorubicin therapy is planned, the potential CV risk reduction and clinical benefit should be considered within the context of the patient’s disease stage, comorbidities, and prior cumulative exposure to anthracyclines (8). Additional cardioprotective strategies that may be employed include the use of continuous infusion doxorubicin rather than a bolus treatment (in an attempt to limit peak plasma drug concentration) and liposomal formulations of doxorubicin (which demonstrate a prolonged plasma half-life) (23–25). Prior and ongoing studies, as discussed below, are investigating the potential role of prophylactic cardiac medications, including beta blockers, renin angiotensin aldosterone system antagonists, and statin therapy. However, to date, no definitive conclusions can be made regarding the use of routine cardioprotective prophylaxis. Finally, long-term follow-up of studies of non-anthracycline-containing chemotherapy regimens appears to demonstrate acceptable oncologic efficacy with improved cardiac safety profiles in select populations of patients with breast cancer overexpressing epidermal growth factor ErbB2 receptor tyrosine kinase (HER2) (26).
Targeted Monoclonal Antibodies: Trastuzumab and the CV Effects of ErbB2 Inhibition
Trastuzumab, a monoclonal antibody inhibiting HER2, is used in the treatment of the ∼20% of breast cancers that overexpress HER2. Notably, ErbB2 is also expressed in cardiac myocytes, and the neuregulin/ErbB2/4 system functions to activate several downstream signaling pathways involved in myocardial growth and cardioprotection (2). Thus, therapeutic inhibition of ErbB2 can result in significant cardiac dysfunction, including a dilated cardiomyopathic phenotype (27–29). However, unlike anthracycline-induced LVD, trastuzumab-related cardiac dysfunction has been observed to be largely reversible in the short term in most, though not all, cases (30).
In several randomized trials of adjuvant trastuzumab in combination with cytotoxic chemotherapy in HER2-overexpressing breast cancer, in which trastuzumab was administered following anthracycline therapy and with close cardiac monitoring, the rates of severe heart failure were relatively low (2–4%), although the incidence of declines in LVEF was greater, on the order of 4–20% (31, 32). More recently, long-term follow-up of several randomized studies has indicated relatively safe cardiac profiles; most observed events occurred while patients were on therapy, and rates of late CV events were not substantially increased (33, 34). Further, the observed reversibility of trastuzumab-induced CV toxicity indicates the potential for safe reintroduction of trastuzumab following a temporary treatment hold for detected LVD, often with the initiation of cardiac medications (30). One primary clinical risk factor for trastuzumab-induced LVD is prior anthracycline exposure, likely reflecting a vulnerable pool of injured cardiomyocytes that are dependent on intact ErbB2 signaling for effective cardiac recovery after anthracycline-related injury (2, 35). Indeed, several clinical trials evaluating trastuzumab therapy in patients treated with non-anthracycline-containing regimens demonstrate low risk for incident LVD (<1%) (26, 36). However, there are concerns over the generalizability of such clinical trial data, which often require strict eligibility criteria, including the exclusion of patients with CV disease, as well as strict criteria for early termination of therapy.
Hormonal Therapies: Used in the Most Common Cancers
Hormonal agents are commonly used in the therapy of the two most common female and male solid tumor malignancies—breast cancer and prostate cancer, respectively. Approximately 70% of breast cancers overexpress the estrogen receptor (ER) and/or progesterone receptor (PR), which are highly predictive of antitumor response to selective ER modulating (SERM) agents (such as tamoxifen) or aromatase inhibitor (AI) therapies (such as anastrazole, letrozole, and exemestane).
As a SERM with both agonist and antagonist activity on the ER, tamoxifen is believed to exert a cardioprotective effect, likely mediated through beneficial effects on known CV risk factors (37). An important exception, however, is that tamoxifen is associated with an increased risk of thrombotic events. In postmenopausal women with breast cancer, tamoxifen has demonstrated lowering of serum total and low-density lipoprotein cholesterol levels (38). Tamoxifen may additionally decrease C-reactive protein and homocysteine levels, indicating potential anti-inflammatory cardioprotective effects (37, 38). Indeed, in meta-analyses of the CV effects of tamoxifen compared with placebo, tamoxifen use was associated with a 33% relative risk reduction (39).
Conversely, because AIs reduce estrogen concentrations by impairing the conversion of androgens to estradiol in adipose tissue, concerns regarding CV toxicity have been raised. When compared to tamoxifen, AIs have demonstrated a significant association with ischemic heart disease in multiple clinical studies (40, 41), and breast cancer clinical guidelines recognize this potential CV risk (42). However, whether this observation reflects a true CV risk with AIs, or only an increased relative risk when compared to the cardioprotective effects of tamoxifen, remains unclear. A recent meta-analysis of 19 randomized clinical trials confirmed the cardioprotective effect of tamoxifen versus placebo and the increased CV risk with AIs compared to tamoxifen (39). However, AIs were not associated with increased CV risk when compared with placebo, thus suggesting that the observed increased risk with AIs relative to tamoxifen may be due to the latter’s cardioprotective effects (39).
In prostate cancer, systemic androgen deprivation therapy (ADT), most commonly via “medical castration” with gonadotropin-releasing hormone (GnRH) agonists, remains a core therapy for all men with metastatic disease and for many men treated with curative-intent RT. Prostate cancer often follows a prolonged disease course that generally affects an elderly male population with concurrent medical conditions, so competing health risks and the prospect for treatment-related morbidity are widely recognized. Indeed, in multiple reports evaluating the cause of death in men with prostate cancer, non-cancer-related events (in particular ischemic cardiac events) are the most common cause (43, 44).
ADT is associated with numerous metabolic alterations, including weight gain, visceral adiposity, insulin resistance, increased arterial stiffness, and less favorable lipid profiles (45). This medical symptom complex has significant overlap with the “metabolic syndrome” that is associated with an increased risk of CV death, even in the absence of known pre-existing coronary disease or diabetes (46). Consequently, several large retrospective studies have raised concern regarding treatment-related CV risk with the use of ADT in men with prostate cancer. The focus of such studies includes increased risks for nonfatal and fatal myocardial infarction and sudden cardiac death (47, 48). On the basis of these studies, a joint American Heart Association/American Cancer Society scientific advisory committee and the FDA both issued consensus statements recognizing a relationship between GnRH agonist ADT and CV events, including myocardial infarction and sudden cardiac death (49). However, this association has not been identified in all prostate cancer observational studies. In a subsequent meta-analysis of eight randomized clinical trials, ADT use was not found to be associated with an increased risk of CV death and was instead associated with lower risk of disease-related and all-cause mortality (50). Although these available data are conflicting, the current preponderance of evidence indicates that men with underlying medical comorbidity likely represent the population that is most at risk for worse CV outcomes on ADT (49, 51). Indeed, in secondary analyses of a randomized trial of prostate cancer patients receiving RT with or without six months of ADT, men with minimal or no baseline comorbidity achieved significant survival benefit with the addition of ADT. In contrast, men with moderate to severe underlying baseline comorbidity had a concerning increase in all-cause mortality with the use of ADT, presumably due to an increase in CV events (52). However, despite this awareness, consensus recommendations are currently lacking for the identification of the population most at risk for ADT-related CV toxicity, and current recommendations advise adherence to standard CV risk assessment and management guidelines as outlined by the American Heart Association or American Diabetic Association (49).
Radiation Therapy: A Myriad of Potential CV Complications
A broad array of adverse CV consequences can arise from RT. For example, RT used in the treatment of head and neck cancer may increase the risk of carotid disease, and this is an area of active study. Mediastinal ionizing RT, commonly used in the treatment of Hodgkin’s lymphoma and early-stage breast cancer, as well as in other thoracic malignancies, such as lung cancer, is known to increase the risk of CV disease. Due to the exposure of the heart and great vessels in mediastinal treatment fields, RT may result in both early and late CV complications, including pericarditis, premature atherosclerosis, valvular disease, conduction abnormalities, and cardiomyopathy (53). Acute radiation pericarditis is the earliest potential CV complication following mediastinal RT, typically occurring several weeks to a few months after treatment. Although this acute pericarditis is generally self-limited, a minority of patients may progress to develop a chronic constrictive pericarditis (54). Atherosclerotic coronary artery disease, particularly involving the left anterior descending artery, may manifest 10–15 years following RT and can lead to significant CV morbidity and mortality (15, 53). For example, mediastinal RT for Hodgkin’s lymphoma increases the risk for subsequent death from heart disease, and late CV complications account for 25% of mortality in Hodgkin’s lymphoma survivors (55). Similarly, RT for breast cancer is known to increase the subsequent risk of ischemic heart disease (56). Valvular dysfunction may also occur approximately one decade following RT as a result of valvular thickening, calcification, and fibrosis and may subsequently result in stenosis and/or regurgitation by 20 years post-treatment (53, 57).
The primary risk factor for radiation-related CV risk is the total RT dose affecting the heart. Indeed, numerous studies have demonstrated the dose-dependent nature of RT-related CV toxicity, with the most significant risk observed in patients receiving ≥30 Gy (55, 56, 58). As such, consensus guidelines advise that all patients who receive RT ≥30 Gy with treatment fields involving the heart are at risk for subsequent cardiac dysfunction (8). Fortunately, modern RT techniques, such as intensity-modulated RT, proton beam RT, and deep-inspiration breath holding, have enabled more precise contouring of the desired treatment field, thereby minimizing the dose to normal tissues (59, 60). Additionally, efforts have been made to reduce the total RT dose when oncologically feasible, such as in early-stage Hodgkin’s lymphoma (61).
Antiangiogenesis Agents: Risk of Hypertension and LV Dysfunction
Anticancer therapies that target tumor angiogenesis, including vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitors (TKIs), are approved for a variety of solid tumor malignancies, and the VEGFR-TKIs sunitinib and pazopanib represent the mainstay first-line treatment options for advanced renal cell carcinoma. Although these agents have significantly improved oncologic outcomes for renal cell carcinoma patients, they have also been associated with multiple CV toxicities, including hypertension, LV dysfunction, and symptomatic heart failure (62, 63). These CV effects may occur via multiple mechanisms. VEGF signaling is involved in the maintenance of cardiac function and homeostasis (64, 65). In addition, VEGFR-TKIs may have off-target effects on the AMP-activated protein kinase and the platelet-derived growth factor receptor, which are both implicated in cardiomyocyte function and survival (65). Finally, drug-induced vascular rarefaction and a reduction in vasodilatory nitric oxide production may contribute to an increase in systemic afterload (66).
The incidence of VEGFR-TKI CV toxicity varies widely, from approximately 3% to 30%, depending on the population studied and the diagnostic criteria that are utilized (67, 68). Recent prospective evidence with standardized quantitative echocardiography indicates an incidence of LV dysfunction, as defined by an absolute decline in LVEF by ≥10% to a resulting value <50%, of 9.7% with sunitinib exposure in advanced renal cell carcinoma patients (69). This CV toxicity appears to occur early in the treatment course, often within the first treatment cycle, and is reversible in most cases with careful CV management (69). Thus far, no patient- or treatment-related factors are clearly associated with CV risk on antiangiogenesis agents, and there are no consensus recommendations for LVD toxicity management (8, 68, 69). Controlled clinical trials are needed to inform the selection of appropriate antihypertensive agents for VEGFR-TKI-related hypertension. Some reports indicate benefit from dihydropyridine calcium channel blockers (given their direct vasodilatory effects) and angiotensin-converting enzyme inhibitors (given their potential effects on vascular rarefaction) (70, 71). Notably, the development of hypertension on VEGFR-TKI therapy is associated with improved oncologic outcomes in renal cell carcinoma patients, potentially reflecting either higher drug exposure levels or tumor- or host-related factors (72, 73).
Immuno-Oncology Agents: Rare Incidence of Fulminant Myocarditis
The most compelling recent addition to anticancer therapy has been immune checkpoint inhibitors, which can facilitate significant and durable antitumor immune responses in a variety of advanced malignancies (74). Although these agents generally exhibit favorable toxicity profiles, they may be associated with a unique profile of significant immune-related adverse effects, including an autoreactive T lymphocyte–mediated colitis, hepatitis, pneumonitis, dermatitis, or hypophysitis (75). In addition, an early onset of severe and potentially fatal myocarditis with rhabdomyolysis has been reported, particularly in patients receiving combination immune checkpoint inhibitor therapy (76). This can manifest as marked cardiac troponin and creatine phosphokinase elevations, with severe and refractory cardiac conduction system abnormalities (76). Although rare (observed in <1% of patients) (76), immune-mediated myocarditis is of clear importance, given its potential severity and the rapidly expanding oncologic use of immune checkpoint inhibitors. Moreover, the potential long-term toxicities of these therapies on additional aspects of the CV system remain unknown.
IMPROVING THE PREDICTION AND PREVENTION OF CANCER THERAPY–RELATED CARDIAC COMPLICATIONS
In nononcologic populations, the severity of subclinical LV compromise is strongly associated with the development of symptomatic heart failure. This suggests the possibility for clinical benefit from cardiac screening, early detection, and early intervention in oncology patients demonstrating subclinical cardiac injury during anticancer therapy (77, 78). Indeed, the prevention of cardiac dysfunction may be preferable to late attempts to treat overt cardiac injury, given the potential for irreversibility of CV disease, additional morbidity or mortality, and the risk of oncologic therapy dose delays or dose interruptions. Thus, novel tools for the more sensitive measurement of subclinical cardiac injury are highly desired. Moreover, ongoing research is focused on “deep phenotyping” strategies to better understand each individual patient’s risk and to enhance the personalized delivery of cardio-oncology care.
Novel Imaging and Blood-Based Biomarkers
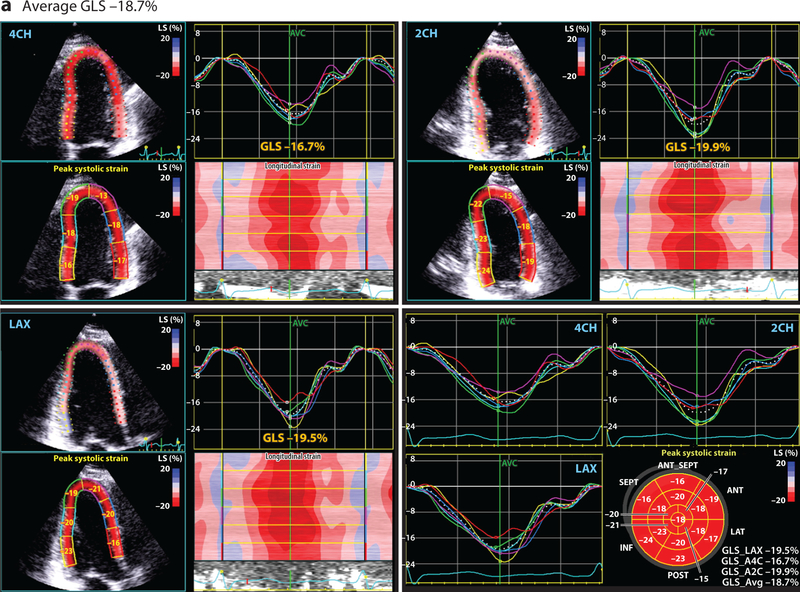
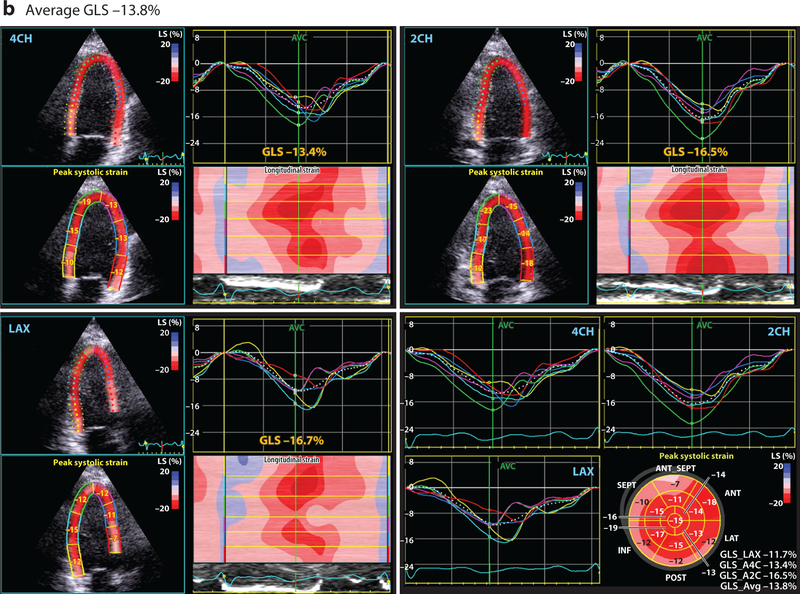
Conventional 2-dimensional (2D) echocardiography, with assessment of the LVEF, is the most common method for monitoring cardiac function in patients receiving potentially cardiotoxic anticancer agents (29). However, standard 2D LVEF assessment is associated with several technical limitations that hinder its clinical utility, including variability in both image acquisition and interpretation (79). Furthermore, the utility of LVEF measurement as a screening tool is limited by its relatively low sensitivity for the early detection of cardiac injury (80). One relatively recent imaging technology that seeks to overcome some of these limitations is quantitative echocardiography with evaluation of myocardial strain. Strain echocardiography can provide a global and regional assessment of cardiac function through the measurement of several myocardial deformation parameters (Figure 1) (81). Importantly, measures of myocardial strain, particularly systolic global longitudinal strain and circumferential strain, may represent an early subclinical change that precedes a significant LVEF decline (81–83). Ongoing cardio-oncology studies are aimed at validating and further defining the clinical utility and applicability of early myocardial strain changes during anticancer therapy.
Figure 1.
This four-paneled figure represents the global longitudinal strain (GLS) derived from a breast cancer patient, averaged from the following apical views: LAX (long axis), 2CH (two chamber), and 4CH (four chamber). The lower right panel demonstrates the summary values from each of these views and bullseye plot. Peak systolic GLS is a measure of cardiac deformation that can be readily derived by echocardiography using speckle tracking that provides insight into systolic function. At baseline (a), prior to any cardiotoxic cancer therapy, the average GLS is normal at –18.7%. However, (b) after 240 mg/m2 doxorubicin therapy (and prior to the initiation of trastuzumab), the GLS is reduced at –13.8%, suggestive of subclinical cardiac dysfunction. The GLS was reduced at this timepoint despite a normal estimated left ventricular ejection fraction.
Similarly, the use of blood-based biomarkers may represent an additional method for the sensitive, early detection of subclinical cardiac stress. Early changes in cardiac troponins, which are sensitive and specific markers for early myocardial injury, have been associated with subsequent CV toxicity in patients receiving anthracyclines, high-dose chemotherapy, and trastuzumab therapy (84, 85). Similarly, serum natriuretic peptides, such as N-terminal probrain natriuretic peptide, are commonly used for the detection of heart failure in nononcologic populations. Natriuretic peptides have demonstrated early elevations following cardiotoxic chemotherapy and may be associated with subsequent LVD (86). However, the clinical utility of these biomarkers in oncologic populations currently remains unclear, and their predictive abilities should be further evaluated in patients with advanced oncologic disease burdens and underlying medical comorbidities (87). In fact, a prospective study of advanced renal cell cancer patients found that a significant proportion of patients had significant baseline elevations of brain natriuretic peptide and high-sensitivity cardiac troponin even prior to the initiation of systemic therapy, potentially reflecting their underlying cancer burden and comorbid medical confounders (69).
Clinical Trials for CV Toxicity Prevention
Several recent clinical studies have sought to evaluate both pharmacologic and behavioral interventions in patients at risk for treatment-related CV injury. In a placebo-controlled randomized trial of 130 patients with early-stage breast cancer receiving adjuvant anthracycline-containing chemotherapy, concurrent use of the angiotensin receptor blocker candesartan was associated with a statistically significant, but very modest, attenuation of early LVEF declines as assessed by cardiac magnetic resonance imaging (MRI) (88). However, concurrent use of the beta blocker metoprolol did not have an observed effect on early LVEF change (88). Similarly, in a recently reported randomized placebo-controlled trial of either an angiotensin-converting enzyme inhibitor (perindopril) or a beta blocker (bisoprolol) in HER2-positive breast cancer patients receiving adjuvant trastuzumab, both interventions modestly attenuated trastuzumab-mediated declines in LVEF, as assessed by cardiac MRI, following 17 cycles (approximately one year) of trastuzumab therapy (89), but neither drug significantly affected measures of cardiac remodeling, as assessed by LV volumes (89). However, the largest randomized placebo-controlled study of candesartan use during and after adjuvant trastuzumab therapy did not identify a cardioprotective effect (90). In addition, the long-term clinical implications of any modest attenuation in early LVEF declines with regard to the subsequent development of symptomatic heart failure or significant asymptomatic late LVEF declines remain unclear. Ongoing studies will seek to prospectively evaluate the protective utility of other cardioprotective medications, such as statins, in breast cancer patients receiving anthracycline-based therapies (NCT01988571).
Exercise may be another promising cardioprotective strategy. In a large prospective study of nearly 3,000 women with nonmetastatic breast cancer, in which the majority received potentially cardiotoxic adjuvant therapies, recreational physical activity was associated with a substantial, graded reduction in the incidence of significant CV events over a median follow-up time of 8.6 years (91).
LONG-TERM CARDIOVASCULAR IMPLICATIONS OF CANCER SURVIVORSHIP
An estimated 14 million cancer survivors, including more than 400,000 childhood cancer survivors, are currently present in the United States, and these numbers are expected to increase over time as a result of rising pediatric cancer incidence and overall improvement in long-term cancer survival rates (92, 93). Such survivors are known to have late CV risks as a result of both prior cardiotoxic exposures and the accumulation of new CV risk factors with advancing age. Much of this evidence comes from long-term follow-up of pediatric cancer survivors, including the Childhood Cancer Survivor Study (94). For example, mortality rates from CV disease are up to 10 times higher among pediatric cancer survivors than among age-matched controls (95). Additionally, pediatric cancer survivors have increased rates of multiple CV risk factors consistent with the metabolic syndrome when compared to siblings, particularly those survivors following a sedentary lifestyle or with prior RT exposure (96). Even among asymptomatic childhood cancer survivors, dose-dependent, adverse LV echocardiographic changes are detectable >10 years after anthracycline exposure (97). Although fewer data are currently available to assess late CV risk among long-term survivors of adult-onset cancers, recent data also indicate significantly higher risk of CV disease in this population than in noncancer adult controls, particularly among those adult-onset cancer survivors with underlying CV risk factors (98).
The recognition of this potential for long-term CV toxicity in at-risk cancer survivors may enable the aggressive mitigation of CV risk factors in this population, as well as the early detection and treatment of incident CV dysfunction. Unfortunately, there is currently limited high-level evidence to guide specific CV screening strategies in asymptomatic cancer survivors who have received potentially cardiotoxic therapy (99). Furthermore, the optimal timing and nature of pharmacologic or behavioral cardioprotective interventions remain largely unknown. Nevertheless, recent consensus clinical practice guidelines counsel high clinical suspicion for CV disease in at-risk cancer survivors and recommend routine use of early cardiac imaging surveillance in certain higher-risk survivors (8).
CONCLUSIONS
We recommend the following research priorities for continued improvement in cardio-oncology clinical care:
Identifying methods to enhance risk prediction (imaging, biomarkers, CV clinical risk factors)
Optimizing cardioprotective strategies (pharmacologic, exercise)
Personalizing deep phenotyping strategies to individualize CV disease risk and prevention
Improving cancer survivorship (screening, cardioprotective interventions)
Evaluating modifications of oncologic therapy upon detection of CV toxicity
As novel anticancer therapeutics become available and cancer survival outcomes improve, there must be an increased awareness of potential cardiotoxic effects of cancer therapies and the significant overlap between cancer and CV disease. Within cardio-oncology, there is a growing emphasis on the identification of risk groups that are most susceptible to treatment-induced CV dysfunction to enhance the personalized delivery of therapy, as well as the evaluation of more sensitive measures for early toxicity detection and implementation of cardioprotective strategies. In fact, we have already begun to reap the benefits of this increased awareness and implementation of cardioprotective oncologic management, as highlighted by the decreasing late mortality rates among survivors of childhood cancers (100).
However, as the field of cardio-oncology expands, a critical balance must be achieved between the sensitive detection of CV toxicity and the possible unnecessary cessation of important oncologic therapy, given the potential risks for compromised treatment intensity and oncologic outcomes. Therefore, novel tools for early CV toxicity detection and mitigation require careful validation in treatment- and disease-specific contexts with attention to both cardiac and oncologic clinical outcomes. This critical balance clearly highlights the importance of continued cardio-oncology research and close clinical collaboration between cardiology and oncology providers.
Footnotes
DISCLOSURE STATEMENT
B.K. has received an investigator-initiated research award from Roche Diagnostics and Pfizer. She has served as a consultant to Bristol Myers Squibb, Mateon Therapeutics, and Gilead.
LITERATURE CITED
- 1.Siegel RL, Miller KD, Jemal A. 2016. Cancer statistics, 2016. CA Cancer J. Clin 66:7–30 [DOI] [PubMed] [Google Scholar]
- 2.Ewer MS, Ewer SM. 2015. Cardiotoxicity of anticancer treatments. Nat. Rev. Cardiol 12:547–58 [DOI] [PubMed] [Google Scholar]
- 3.Koene RJ, Prizment AE, Blaes A, et al. 2016. Shared risk factors in cardiovascular disease and cancer. Circulation 133:1104–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arts–de Jong M, Maas AH, Massuger LF, et al. 2014. BRCA½ mutation carriers are potentially at higher cardiovascular risk. Crit. Rev. Oncol. Hematol 91:159–71 [DOI] [PubMed] [Google Scholar]
- 5.Doyle JJ, Neugut AI, Jacobson JS, et al. 2005. Chemotherapy and cardiotoxicity in older breast cancer patients: a population-based study. J. Clin. Oncol 23:8597–605 [DOI] [PubMed] [Google Scholar]
- 6.Patnaik JL, Byers T, DiGuiseppi C, et al. 2011. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res 13:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Den Belt–Dusebout AW, De Wit R, Gietema JA, et al. 2007. Treatment-specific risks of second malignancies and cardiovascular disease in 5-year survivors of testicular cancer. J. Clin. Oncol 25:4370–78 [DOI] [PubMed] [Google Scholar]
- 8.Armenian SH, Lacchetti C, Barac A, et al. 2017. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol 35:893–911 [DOI] [PubMed] [Google Scholar]
- 9.Gewirtz DA. 1999. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol 57:727–41 [DOI] [PubMed] [Google Scholar]
- 10.Doroshow JH. 1983. Effect of anthracycline antibiotics on oxygen radical formation in rat heart. Cancer Res 43:460–72 [PubMed] [Google Scholar]
- 11.Zhang S, Liu X, Bawa-Khalfe T, et al. 2012. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med 18:1639–42 [DOI] [PubMed] [Google Scholar]
- 12.Lefrak EA, Pitha J, Rosenheim S, et al. 1973. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 32:302–14 [DOI] [PubMed] [Google Scholar]
- 13.Swain SM, Whaley FS, Ewer MS. 2003. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 97:2869–79 [DOI] [PubMed] [Google Scholar]
- 14.Harbeck N, Ewer MS, De Laurentiis M, et al. 2011. Cardiovascular complications of conventional and targeted adjuvant breast cancer therapy. Ann. Oncol 22:1250–58 [DOI] [PubMed] [Google Scholar]
- 15.Curigliano G, Cardinale D, Dent S, et al. 2016. Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA Cancer J. Clin 66:309–25 [DOI] [PubMed] [Google Scholar]
- 16.von Hoff DD, Layard MW, Basa P, et al. 1979. Risk factors for doxorubicin-induced congestive heart failure. Ann. Intern. Med 91:710–17 [DOI] [PubMed] [Google Scholar]
- 17.Ewer MS, Ewer SM. 2010. Cardiotoxicity of anticancer treatments: what the cardiologist needs to know. Nat. Rev. Cardiol 7:564–75 [DOI] [PubMed] [Google Scholar]
- 18.Narayan HK, Finkelman B, French B, et al. 2017. Detailed echocardiographic phenotyping in breast cancer patients: associations with ejection fraction decline, recovery, and heart failure symptoms over 3 years of follow-up. Circulation 135:1397–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swain SM, Whaley FS, Gerber MC, et al. 1997. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J. Clin. Oncol 15:1318–32 [DOI] [PubMed] [Google Scholar]
- 20.Lipshultz SE, Scully RE, Lipsitz SR, et al. 2010. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol 11:950–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Dalen EC, Caron HN, Dickinson HO, et al. 2011. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst. Rev 6:CD003917 [DOI] [PubMed] [Google Scholar]
- 22.Hensley ML, Hagerty KL, Kewalramani T, et al. 2009. American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J. Clin. Oncol 27:127–45 [DOI] [PubMed] [Google Scholar]
- 23.Casper ES, Gaynor JJ, Hajdu SI, et al. 1991. A prospective randomized trial of adjuvant chemotherapy with bolus versus continuous infusion of doxorubicin in patients with high-grade extremity soft tissue sarcoma and an analysis of prognostic factors. Cancer 68:1221–29 [DOI] [PubMed] [Google Scholar]
- 24.Lipshultz SE, Miller TL, Lipsitz SR, et al. 2012. Continuous versus bolus infusion of doxorubicin in children with ALL: long-term cardiac outcomes. Pediatrics 130:1003–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamaguchi N, Fujii T, Aoi S, et al. 2015. Comparison of cardiac events associated with liposomal doxorubicin, epirubicin and doxorubicin in breast cancer: a Bayesian network meta-analysis. Eur. J. Cancer 51:2314–20 [DOI] [PubMed] [Google Scholar]
- 26.Slamon DJ, Eiermann W, Robert NJ, et al. 2015. Ten-year follow-up of BCIRG-006 comparing doxorubicin plus cyclophosphamide followed by docetaxel with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab with docetaxel, carboplatin and trastuzumab in HER2-positive early breast cancer patients. Presented at San Antonio Breast Cancer Symp., San Antonio, TX, Dec. 11, Abstr. S5–04 [Google Scholar]
- 27.Crone SA, Zhao Y, Fan L, et al. 2002. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat. Med 8:459–65 [DOI] [PubMed] [Google Scholar]
- 28.Hahn VS, Lenihan DJ, Ky B. 2014. Cancer therapy-induced cardiotoxicity: basic mechanisms and potential cardioprotective therapies. J. Am. Heart Assoc 3:e000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slamon DJ, Leyland-Jones B, Shak S, et al. 2001. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med 344:783–92 [DOI] [PubMed] [Google Scholar]
- 30.Ewer MS, Vooletich MT, Durand J, et al. 2005. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J. Clin. Oncol 23:7820–26 [DOI] [PubMed] [Google Scholar]
- 31.Perez EA, Romond EH, Suman VJ, et al. 2011. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J. Clin. Oncol 29:3366–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slamon D, Eiermann W, Robert N, et al. 2011. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med 365:1273–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romond EH, Jeong JH, Rastogi P, et al. 2012. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J. Clin. Oncol 30:3792–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Azambuja E, Procter MJ, Van Veldhuisen DJ, et al. 2014. Trastuzumab-associated cardiac events at 8 years of median follow-up in the herceptin adjuvant trial (BIG 1–01). J. Clin. Oncol 32:2159–65 [DOI] [PubMed] [Google Scholar]
- 35.de Korte MA, de Vries EGE, Lub–de Hooge MN, et al. 2007. 111Indium-trastuzumab visualises myocardial human epidermal growth factor receptor 2 expression shortly after anthracycline treatment but not during heart failure: a clue to uncover the mechanisms of trastuzumab-related cardiotoxicity. Eur. J. Cancer 43:2046–51 [DOI] [PubMed] [Google Scholar]
- 36.Dang C, Guo H, Najita J, et al. 2016. Cardiac outcomes of patients receiving adjuvant weekly paclitaxel and trastuzumab for node-negative, ERBB2-positive breast cancer. JAMA Oncol 2:29–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foglietta J, Inno A, de Iuliis F, et al. 2017. Cardiotoxicity of aromatase inhibitors in breast cancer patients. Clin. Breast Cancer 17:11–17 [DOI] [PubMed] [Google Scholar]
- 38.Love RR, Wiebe DA, Feyzi JM, et al. 1994. Effects of tamoxifen on cardiovascular risk factors in postmenopausal women after 5 years of treatment. J. Natl. Cancer Inst 86:1534–39 [DOI] [PubMed] [Google Scholar]
- 39.Khosrow-Khavar F, Filion K, Al-Qurashi S, et al. 2017. Cardiotoxicity of aromatase inhibitors and tamoxifen in postmenopausal women with breast cancer: a systematic review and meta-analysis of randomized controlled trials. Ann. Oncol 28:487–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buzdar A, Howell A, Cuzick J, et al. 2006. Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol 7:633–43 [DOI] [PubMed] [Google Scholar]
- 41.Coates AS, Keshaviah A, Thürlimann B, et al. 2007. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J. Clin. Oncol 25:486–92 [DOI] [PubMed] [Google Scholar]
- 42.Burstein HJ, Temin S, Anderson H, et al. 2014. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J. Clin. Oncol 32:2255–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riihimäki M, Thomsen H, Brandt A, et al. 2011. What do prostate cancer patients die of? Oncologist 16:175–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Epstein MM, Edgren G, Rider JR, et al. 2012. Temporal trends in cause of death among Swedish and US men with prostate cancer. J. Natl. Cancer Inst 104:1335–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith JC, Bennett S, Evans LM, et al. 2001. The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J. Clin. Endocrinol. Metab 86:4261–67 [DOI] [PubMed] [Google Scholar]
- 46.Lakka H, Laaksonen DE, Lakka TA, et al. 2002. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 288:2709–16 [DOI] [PubMed] [Google Scholar]
- 47.Keating NL, O’Malley AJ, Smith MR. 2006. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J. Clin. Oncol 24:4448–56 [DOI] [PubMed] [Google Scholar]
- 48.D’Amico AV, Denham JW, Crook J, et al. 2007. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J. Clin. Oncol 25:2420–25 [DOI] [PubMed] [Google Scholar]
- 49.Nguyen PL, Alibhai SMH, Basaria S, et al. 2015. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur. Urol 67:825–36 [DOI] [PubMed] [Google Scholar]
- 50.Nguyen PL, Je Y, Schutz FAB, et al. 2011. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials. JAMA 306:2359–66 [DOI] [PubMed] [Google Scholar]
- 51.Bhatia N, Santos M, Jones LW, et al. 2016. Cardiovascular effects of androgen deprivation therapy for the treatment of prostate cancer: ABCDE steps to reduce cardiovascular disease in patients with prostate cancer. Circulation 133:537–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D’Amico AV, Chen M, Renshaw A, et al. 2015. Long-term follow-up of a randomized trial of radiation with or without androgen deprivation therapy for localized prostate cancer. JAMA 314:1291–93 [DOI] [PubMed] [Google Scholar]
- 53.Jaworski C, Mariani JA, Wheeler G, et al. 2013. Cardiac complications of thoracic irradiation. J. Am. Coll. Cardiol 61:2319–28 [DOI] [PubMed] [Google Scholar]
- 54.Gaya AM, Ashford RFU. 2005. Cardiac complications of radiation therapy. Clin. Oncol 17:153–59 [DOI] [PubMed] [Google Scholar]
- 55.Hancock SL, Hoppe RT, Tucker MA. 1993. Factors affecting late mortality from heart disease after treatment of Hodgkin’s disease. JAMA 270:1949–55 [PubMed] [Google Scholar]
- 56.Darby SC, Ewertz M, McGale P, et al. 2013. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med 368:987–98 [DOI] [PubMed] [Google Scholar]
- 57.Hull MC, Morris CG, Pepine CJ, et al. 2003. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of Hodgkin lymphoma treated with radiation therapy. JAMA 290:2831–37 [DOI] [PubMed] [Google Scholar]
- 58.Van Nimwegen FA, Schaapveld M, Cutter DJ, et al. 2016. Radiation dose-response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J. Clin. Oncol 34:235–43 [DOI] [PubMed] [Google Scholar]
- 59.Hoppe BS, Flampouri S, Su Z, et al. 2012. Effective dose reduction to cardiac structures using protons compared with 3DCRT and IMRT in mediastinal Hodgkin lymphoma. Int. J. Radiat. Oncol. Biol. Phys 84:449–55 [DOI] [PubMed] [Google Scholar]
- 60.Paumier A, Ghalibafian M, Gilmore J, et al. 2012. Dosimetric benefits of intensity-modulated radiotherapy combined with the deep-inspiration breath-hold technique in patients with mediastinal Hodgkin’s lymphoma. Int. J. Radiat. Oncol. Biol. Phys 82:1522–27 [DOI] [PubMed] [Google Scholar]
- 61.Engert A, Plütschow A, Eich HT, et al. 2010. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N. Engl. J. Med 363:640–52 [DOI] [PubMed] [Google Scholar]
- 62.Schmidinger M, Zielinski CC, Vogl UM, et al. 2008. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J. Clin. Oncol 26:5204–12 [DOI] [PubMed] [Google Scholar]
- 63.Chu TF, Rupnick MA, Kerkela R, et al. 2007. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 370:2011–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rainer PP, Doleschal B, Kirk JA, et al. 2012. Sunitinib causes dose-dependent negative functional effects on myocardium and cardiomyocytes. Br. J. Urol. Int 110:1455–62 [DOI] [PubMed] [Google Scholar]
- 65.Force T, Krause DS, Van Etten RA. 2007. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat. Rev. Cancer 7:332–44 [DOI] [PubMed] [Google Scholar]
- 66.Hamnvik OR, Choueiri TK, Turchin A, et al. 2015. Clinical risk factors for the development of hypertension in patients treated with inhibitors of the VEGF signaling pathway. Cancer 121:311–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Telli ML, Witteles RM, Fisher GA, et al. 2008. Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Ann. Oncol 19:1613–18 [DOI] [PubMed] [Google Scholar]
- 68.Hall PS, Harshman LC, Srinivas S, et al. 2013. The frequency and severity of cardiovascular toxicity from targeted therapy in advanced renal cell carcinoma patients. JACC Heart Fail 1:72–78 [DOI] [PubMed] [Google Scholar]
- 69.Narayan V, Keefe S, Haas N, et al. 2017. Prospective evaluation of sunitinib-induced cardiotoxicity in patients with metastatic renal cell carcinoma. Clin. Cancer Res 23:3601–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Copur MS, Obermiller A. 2011. An algorithm for the management of hypertension in the setting of vascular endothelial growth factor signaling inhibition. Clin. Colorectal Cancer 10:151–56 [DOI] [PubMed] [Google Scholar]
- 71.Narayan V, Haas NB. 2016. Axitinib in the treatment of renal cell carcinoma: patient selection and perspectives. Int. J. Nephrol. Renovascular Dis 9:65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rini BI, Schiller JH, Fruehauf JP, et al. 2011. Diastolic blood pressure as a biomarker of axitinib efficacy in solid tumors. Clin. Cancer Res 17:3841–49 [DOI] [PubMed] [Google Scholar]
- 73.Michaelson MD, Stadler WM. 2013. Predictive markers in advanced renal cell carcinoma. Semin. Oncol 40:459–64 [DOI] [PubMed] [Google Scholar]
- 74.Postow MA, Callahan MK, Wolchok JD. 2015. Immune checkpoint blockade in cancer therapy. J. Clin. Oncol 33:1974–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Naidoo J, Page DB, Li BT, et al. 2015. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol 26:2375–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnson DB, Balko JM, Compton ML, et al. 2016. Fulminant myocarditis with combination immune checkpoint blockade. N. Engl. J. Med 375:1749–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yancy CW, Jessup M, Bozkurt B, et al. 2013. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation 128:1810–52 [DOI] [PubMed] [Google Scholar]
- 78.Cardinale D, Colombo A, Bacchiani G, et al. 2015. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 131:1981–88 [DOI] [PubMed] [Google Scholar]
- 79.Plana JC, Galderisi M, Barac A, et al. 2014. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr 27:911–39 [DOI] [PubMed] [Google Scholar]
- 80.Ewer MS, Lenihan DJ. 2008. Left ventricular ejection fraction and cardiotoxicity: Is our ear really to the ground? J. Clin. Oncol 26:1201–3 [DOI] [PubMed] [Google Scholar]
- 81.Thavendiranathan P, Poulin F, Lim K, et al. 2014. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J. Am. Coll. Cardiol 63:2751–68 [DOI] [PubMed] [Google Scholar]
- 82.Narayan HK, French B, Khan AM, et al. 2016. Noninvasive measures of ventricular-arterial coupling and circumferential strain predict cancer therapeutics-related cardiac dysfunction. JACC Cardiovasc. Imaging 9:1131–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu AF, Ky B. 2016. Roadmap for biomarkers of cancer therapy cardiotoxicity. Heart 102:425–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cardinale D, Colombo A, Torrisi R, et al. 2010. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J. Clin. Oncol 28:3910–16 [DOI] [PubMed] [Google Scholar]
- 85.Cardinale D, Sandri MT, Colombo A, et al. 2004. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation 109:2749–54 [DOI] [PubMed] [Google Scholar]
- 86.Meinardi MT, Van Veldhuisen DJ, Gietema JA, et al. 2001. Prospective evaluation of early cardiac damage induced by epirubicin-containing adjuvant chemotherapy and locoregional radiotherapy in breast cancer patients. J. Clin. Oncol 19:2746–53 [DOI] [PubMed] [Google Scholar]
- 87.Daugaard G, Lassen U, Bie P, et al. 2005. Natriuretic peptides in the monitoring of anthracycline induced reduction in left ventricular ejection fraction. Eur. J. Heart Fail 7:87–93 [DOI] [PubMed] [Google Scholar]
- 88.Gulati G, Heck SL, Ree AH, et al. 2016. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur. Heart J 37:1671–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pituskin E, Mackey JR, Koshman S, et al. 2017. Multidisciplinary approach to novel therapies in cardio-oncology research (MANTICORE 101-breast): a randomized trial for the prevention of trastuzumab-associated cardiotoxicity. J. Clin. Oncol 35:870–77 [DOI] [PubMed] [Google Scholar]
- 90.Boekhout A, Gietema J, Kerklaan B, et al. 2016. Angiotensin II-receptor inhibition with candesartan to prevent trastuzumab-related cardiotoxic effects in patients with early breast cancer: a randomized clinical trial. JAMA Oncol 2:1030–37 [DOI] [PubMed] [Google Scholar]
- 91.Jones LW, Habel LA, Weltzien E, et al. 2016. Exercise and risk of cardiovascular events in women with nonmetastatic breast cancer. J. Clin. Oncol 34:2743–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.De Moor JS, Mariotto AB, Parry C, et al. 2013. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol. Biomarkers Prev 22:561–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lipshultz SE, Adams MJ, Colan SD, et al. 2013. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation 128:1927–55 [DOI] [PubMed] [Google Scholar]
- 94.Chow EJ, Chen Y, Kremer LC, et al. 2015. Individual prediction of heart failure among childhood cancer survivors. J. Clin. Oncol 33:394–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tukenova M, Guibout C, Oberlin O, et al. 2010. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J. Clin. Oncol 28:1308–15 [DOI] [PubMed] [Google Scholar]
- 96.Meacham LR, Chow EJ, Ness KK, et al. 2010. Cardiovascular risk factors in adult survivors of pediatric cancer—a report from the childhood cancer survivor study. Cancer Epidemiol. Biomark. Prev 19:170–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Armenian SH, Gelehrter SK, Vase T, et al. 2014. Screening for cardiac dysfunction in anthracycline-exposed childhood cancer survivors. Clin. Cancer Res 20:6314–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Armenian SH, Xu L, Ky B, et al. 2016. Cardiovascular disease among survivors of adult-onset cancer: a community-based retrospective cohort study. J. Clin. Oncol 34:1122–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carver JR, Shapiro CL, Ng A, et al. 2007. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J. Clin. Oncol 25:3991–4008 [DOI] [PubMed] [Google Scholar]
- 100.Armstrong GT, Chen Y, Yasui Y, et al. 2016. Reduction in late mortality among 5-year survivors of childhood cancer. N. Engl. J. Med 374:833–42 [DOI] [PMC free article] [PubMed] [Google Scholar]