ABSTRACT
Infliximab is a commonly used antitumor necrosis factor alpha agent, especially in patients with inflammatory bowel disease. It has been associated with drug-induced liver injury including immunologic reactions, with rare cases of acute liver failure. We describe a patient with chronic cholestasis and loss of intrahepatic bile ducts after therapy with infliximab for refractory ulcerative colitis consistent with a diagnosis of vanishing bile duct syndrome. About 3 months after the initial infusion, the patient developed subfulminant liver failure and required liver transplantation.
INTRODUCTION
Vanishing bile duct syndrome (VBDS) has been described as an immunologic drug-induced liver injury (DILI) with chronic cholestasis and histopathological evidence of loss of intrahepatic bile ducts.1 The initial presentation is often severe cholestatic hepatitis 1–6 months after exposure to previously reported medications including penicillins, fluoroquinolones, macrolides, anticonvulsants, antipsychotics, antineoplastic agents, and nonsteroidal anti-inflammatory drugs.2 Workup often reveals persistently elevated bilirubin and alkaline phosphatase despite improvement in liver inflammation. VBDS is a diagnosis of exclusion, with alternate diagnoses including primary biliary cholangitis and primary sclerosing cholangitis. Liver histology is required for the diagnosis of VBDS and typically shows a paucity of intralobular bile ducts in a sample taken at least 1 month after the onset of injury.1 VBDS has been shown to be reversible but may progress to cirrhosis or liver failure.3 Tumor necrosis factor alpha (anti-TNF alpha) inhibitors may slow progression; however, infliximab has been shown to cause DILI and induce autoimmune hepatitis possibly due to its immunomodulatory properties.4–7 Anti-TNF alpha-induced liver failure has been reported but with different characteristics when compared with anti-TNF alpha DILI.8 We describe a case of infliximab-induced VBDS leading to liver failure and an orthotopic liver transplant.
CASE REPORT
A 63-year-old African American man with ulcerative colitis presented with persistently elevated liver enzymes. The patient had been on mercaptopurine and mesalamine for many years, but because of flare-ups and prednisone dependence, he was started on infliximab 5 mg/kg (470 mg/dose). His first dose was administered 3 months before presentation, followed by a second and third dose 1 month apart, respectively. He had normal liver enzymes until 2 months before his first visit. His medications before elevation of liver enzymes included infliximab, mercaptopurine, omeprazole, and prednisone. His examination was pertinent for scleral icterus, a palpable liver edge at the costal margin, and jaundice. At that time, his bilirubin was 16 mg/dL, alkaline phosphatase 464 U/L, alanine aminotransferase 1,164 U/L, and aspartate aminotransferase 896 U/L.
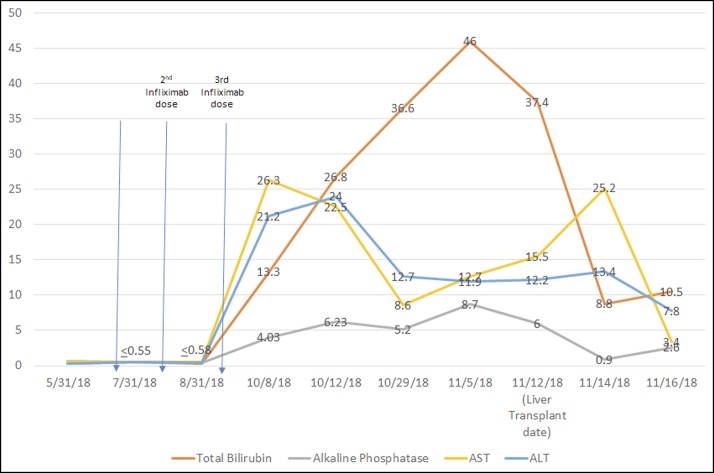
At follow-up, his transaminases continued to increase until 2 months after the last infliximab dose at which point they improved but remained above normal (Figure 1). His bilirubin, on the other hand, continued to increase. Workup was negative for viral hepatitis, smooth-muscle, anti-nuclear, and anti-mitochondrial antibodies (immunoglobulin G [IgG] 989 mg/dL). Serum ceruloplasmin and antitrypsin levels were also normal. An abdominal ultrasound showed increased hepatic parenchymal echogenicity and hepatomegaly. Bile ducts and spleen were normal. A liver biopsy 3 months after the initial infliximab dose showed cholestatic hepatitis with patchy lobular necrosis, ductopenia with marked duct injury, and mild steatosis without fibrosis (Figure 2). He was admitted to the hospital 2 weeks after presentation because of clinical and biochemical features of persistent cholestatic hepatitis with worsening bilirubin up to 55.3 mg/dL along with acute kidney injury, leukocytosis, and hyponatremia.
Figure 1.
Total bilirubin, alkaline phosphatase, and transaminases (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]) before and after infliximab treatment. The laboratory values are represented as multiples of the normal upper limit.
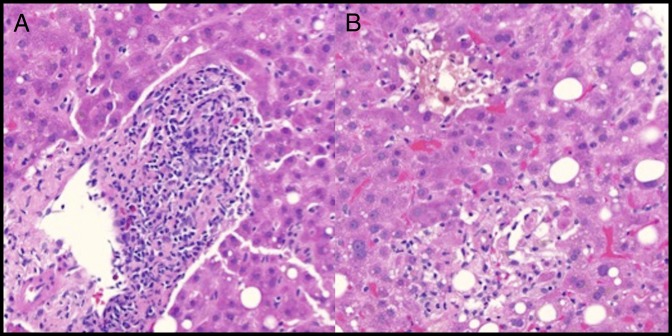
Figure 2.
(A) Hematoxylin and eosin (H&E) stain of the pretransplant liver biopsy showing mixed portal inflammation, including lymphocytes, plasma cells, and eosinophils (400×). Although present, plasma cells are not predominant. The portal vein and artery are readily identifiable, but the duct is heavily infiltrated by inflammatory cells. No significant interface activity. (B) H&E stain of the pretransplant liver biopsy showing marked lobular cholestasis with associated hepatocyte dropout and mild lobular lymphocytic inflammation (400×).
Given a Model for End-Stage Liver Disease (MELD) score of 38 along with subfulminant hepatic failure, he underwent expedited liver transplantation 3.5 months after his first dose of infliximab. He was started on tacrolimus and methylprednisolone for both immunosuppression and to treat his ulcerative colitis. The explanted liver pathology showed severe lobular cholestasis with patchy hepatocyte necrosis with severe bile duct injury and patchy bile duct loss (Figure 3). No fibrosis was identified. The extrahepatic and large bile ducts were sampled and did not show evidence of primary sclerosing cholangitis. No florid duct lesions or granulomas that would suggest primary biliary cholangitis were identified on parenchymal sampling. A diagnosis of autoimmune hepatitis was unlikely, given the lack of prominence of plasma cells, interface activity, and fibrosis along with the presence of marked cholestasis and duct injury/loss with minimal inflammation on the explanted liver. Instead, these findings are consistent with an infliximab-induced liver injury with subsequent VBDS that required a liver transplant.
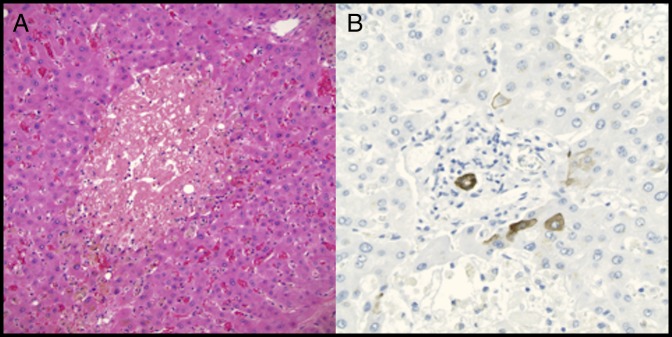
Figure 3.
(A) Hematoxylin and eosin stain of the explanted liver showing extensive hepatocyte dropout and marked cholestasis with minimal lobular lymphocytic inflammation and no fibrosis (200×). (B) Cytokeratin 7 immunostain of the explanted liver showing the ductal epithelium of a severely degenerative interlobular bile duct in a portal area (400×). There is minimal staining of periportal immature hepatocytes. No significant ductular reaction is present, which would also stain with the immunostain, indicating minimal reconstitution of the ducts.
DISCUSSION
Although the diagnosis of VBDS is one of exclusion, many features of this patient's presentation and histopathology are consistent with VBDS. Acute VBDS begins with persistent cholestatic hepatitis, which was noted 3 months after the first dose of infliximab and persisted until transplant. Although the explanted liver was not ductopenic, there was patchy duct loss with diffuse injury and degenerative changes of the remaining small ducts. Although VBDS is typically ductopenic, most cases are diagnosed after years of progressive injury. This case may have progressed more rapidly, as may be the case in DILI-associated cases of VBDS, with transplantation occurring before the complete disappearance of the injured bile ducts.
Such patterns have been described with infliximab-induced liver failure.8 A study of patients with drug-induced hepatitis that included 11 cases of infliximab-induced liver injury showed that 45% of patients had an elevated IgG and 91% had an elevated anti-nuclear antibody, but none had cholestatic liver damage.9 In addition, infliximab has been reported to elicit transient serum aminotransferase elevations and autoimmune features with positive serum markers that respond to steroids.8 This presentation is different from the usual DILI related to anti-TNF agents, given negative serologic and histological features of autoimmune hepatitis along with disease progression despite treatment with prednisone and discontinuation of infliximab, which suggests a different process. The timeframe of infliximab administration with the subsequent cholestasis and bile duct damage are consistent with VBDS, leading to liver failure. A Roussel Uclaf Causality Assessment Method (RUCAM) score of 6 was calculated, which suggests a probable causal relationship between infliximab and the observed liver damage. In addition, this patient developed liver injury after 3 infusions of infliximab over 12 weeks, which is consistent with previous reports of liver injury induced by infliximab.6 Furthermore, the initial biopsy showed portal and lobular inflammation, which was reduced on the explanted liver despite persistently worsening bilirubin, suggesting further loss of bile ducts despite improvement in inflammation, a pattern seen in drug-induced VBDS. The mechanism is unknown, but infliximab is the only chimeric mouse-human monoclonal antibody of its class, which may explain the pathophysiology.8 Although other medications have been implicated in VBDS, this case is believed to represent the first case of infliximab-induced VBDS and liver failure that required liver transplantation.
DISCLOSURES
Author contributions: All authors participated equally in manuscript creation. V. Sundaram is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
REFERENCES
- 1.Vanishing bile duct syndrome. National Institutes of Health. (https://livertox.nih.gov/Phenotypes_vbds.html) (2017). Accessed November 26, 2018.
- 2.Sundaram V, Björnsson ES. Drug-induced cholestasis. Hepatol Commun. 2017;1(8):726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vuppalanchi R, Chalasani N, Saxena R. Restoration of bile ducts in drug-induced vanishing bile duct syndrome due to zonisamide. Am J Surg Pathol. 2006;30(12):1619–23. [DOI] [PubMed] [Google Scholar]
- 4.White JC, Appleman S. Infliximab/plasmapheresis in vanishing bile duct syndrome secondary to toxic epidermal necrolysis. Pediatrics. 2014;134(4):194–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Björnsson ES, Gunnarsson BI, Gröndal G, et al. Risk of drug-induced liver injury from tumor necrosis factor antagonists. Clin Gastroenterol Hepatol. 2015;13(3):602–8. [DOI] [PubMed] [Google Scholar]
- 6.Miehsler W, Novacek G, Wenzl H, et al. A decade of infliximab: The Austrian evidence based consensus on the safe use of infliximab in inflammatory bowel disease. J Crohns Colitis. 2010;4(3):221–56. [DOI] [PubMed] [Google Scholar]
- 7.Ghabril M, Bonkovsky HL, Kum C, et al. Liver injury from tumor necrosis factor-α antagonists: Analysis of thirty-four cases. Clin Gastroenterol Hepatol. 2013;11(5):558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kok B, Lester ELW, Lee WM, et al. Acute liver failure from tumor necrosis factor-α antagonists: Report of four cases and literature review. Dig Dis Sci. 2018;63(6):1654–66. [DOI] [PubMed] [Google Scholar]
- 9.Björnsson ES, Bergmann O, Jonasson JG, Grondal G, Gudbjornsson B, Olafsson S. Drug-induced autoimmune hepatitis: Response to corticosteroids and lack of relapse after cessation of steroids. Clin Gastroenterol Hepatol. 2017;15(10):1635–6. [DOI] [PubMed] [Google Scholar]