ABSTRACT
A 61-year-old woman diagnosed with cervical cancer received systemic chemotherapy using paclitaxel and bevacizumab. Marked elevation of liver enzyme levels was observed. Ultrasonography and computed tomography showed wall thickening of the extrahepatic and intrahepatic bile ducts accompanied by stricture and dilatation. According to these, she was diagnosed as chemotherapy-induced sclerosing cholangitis (CISC), a form of secondary sclerosing cholangitis. Although CISC triggered by systemic chemotherapy is rare, CISC should be considered as a clinically important adverse event of chemotherapy because it causes rapid deterioration of liver function and necessitates interruption of chemotherapy.
INTRODUCTION
Sclerosing cholangitis is classified into primary sclerosing cholangitis, sclerosing cholangitis related to IgG4, and secondary sclerosing cholangitis. Secondary sclerosing cholangitis is induced by various factors. The pathologic processes of secondary sclerosing cholangitis include mechanical obstruction due to surgical manipulation of the biliary tract, tumors of the extrahepatic ducts, choledocholithiasis, ischemia from hepatic artery occlusion, drugs, and congenital abnormalities, such as cystic fibrosis.1 In addition, sclerosing cholangitis in critically ill patients that occurs during or after intensive care unit has recently been recognized as one of the relevant forms of somatic stem cell.2,3 Chemotherapy-induced sclerosing cholangitis (CISC), a form of secondary sclerosing cholangitis, has been reported to be occasionally caused by hepatic arterial infusion chemotherapy with fluoropyrimidines.4–6 In contrast, there have been only a few case reports of CISC triggered by systemic chemotherapy.7–10 We report a rare case of CISC caused by systemic chemotherapy.
CASE REPORT
A 61-year-old woman who complained of left groin pain and left leg edema was diagnosed with cervical cancer with paraaortic and left supraclavicular lymph node metastases. Her clinical course is shown in Figure 1. She received systemic chemotherapy: 135 mg/m2 of paclitaxel and 15 mg/kg of bevacizumab were administered for 63 and 36 days before the onset. Each treatment was followed by 50 mg/m2 of cisplatin on the next day. The chemotherapy led to the reduction of primary cancer and lymph node metastases and her leg symptoms improved. However, she needed to be admitted because of marked elevation of liver enzyme levels, that is, aspartate aminotransferase (AST) 173 U/L, alanine aminotransferase (ALT) 221 U/L, gamma-glutamyl transpeptidase (γ-GTP) 2,553 U/L, and alkaline phosphatase (ALP) 5,188 U/L. The onset of abnormal liver function corresponded to day 0.
Figure 1.

Clinical course of the patient who developed chemotherapy-induced sclerosing cholangitis after systemic chemotherapy for cervical cancer. ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BEV, bevacizumab; CDDP, cisplatin; ENBD, endoscopic nasobiliary drainage; ERBD, endoscopic retrograde biliary drainage; PTX, paclitaxel; UDCA, ursodeoxycholic acid; γ-GTP, gamma-glutamyl transpeptidase.
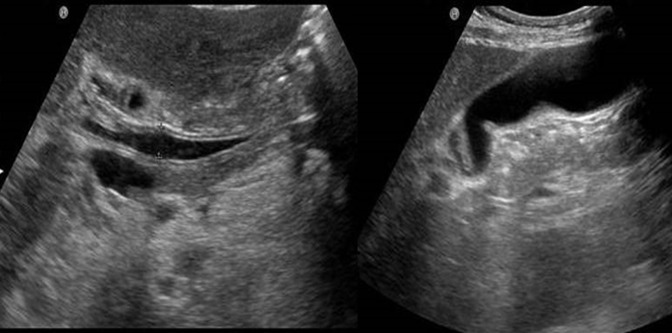
Ultrasonography and computed tomography showed wall thickening of the extrahepatic and intrahepatic bile ducts accompanied by stricture and dilatation (Figures 2 and 3). The gallbladder wall was also thickened. Endoscopic retrograde cholangiopancreatography, sphincterotomy, and nasobiliary drainage were carried out on day 2. Biopsy of the common bile duct showed no malignancies with modest infiltration of neutrophils (Figure 4). Cytology of bile collected from nasobiliary drainage tube also revealed no features of malignancies. Ursodeoxycholic acid treatment commenced on day 6. In the cholangiography, from the drainage tube on day 9, stricture and dilatation accompanied by wall irregularity were observed in the intrahepatic bile ducts of both liver lobes, although the change in the extrahepatic bile duct was modest (Figure 5).
Figure 2.
Saggital abdominal ultrasound showing wall thickening of the hepatic hilar bile duct, common bile duct, and gallbladder.
Figure 3.
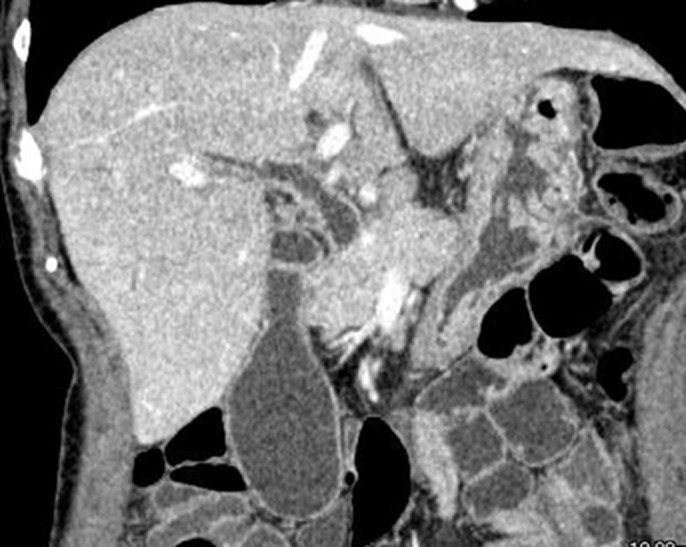
Computed tomography showing wall thickening of the bile duct in both extrahepatic and intrahepatic bile duct. The lumen of the bile duct became narrowed and dilated. The wall of the gallbladder was also thickened.
Figure 4.
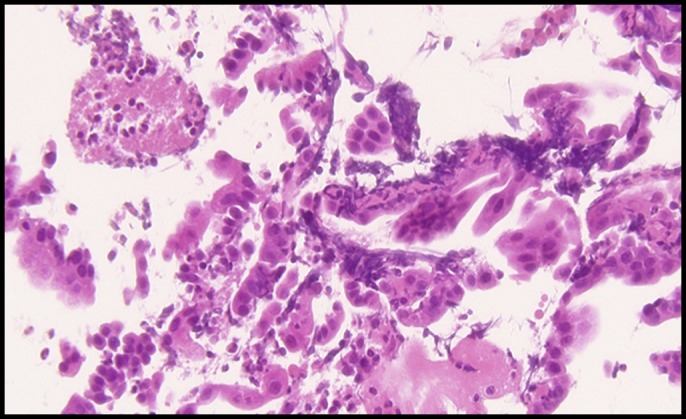
Biopsy of the common bile duct (hematoxylin and eosin stain 400×) showing no malignancies with modest infiltration of neutrophils.
Figure 5.
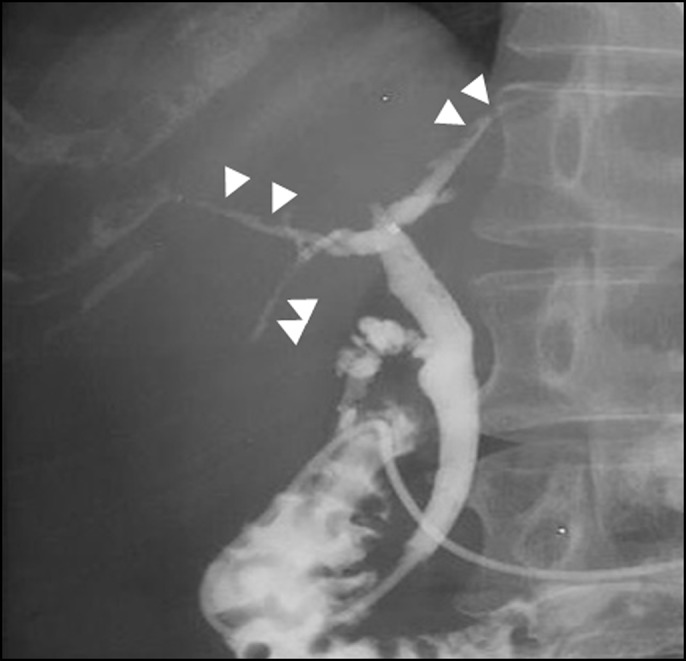
Cholangiography from the nasobiliary drainage showing stricture and dilatation accompanied by the wall irregularity in the intrahepatic bile ducts of both liver lobes (arrows), although the change in the extrahepatic bile duct was modest.
The patient became febrile with the inflammatory reaction and the re-elevation of the liver enzyme: for example, white blood cells 10,660/mm3, C-reactive protein 3.38 mg/dL, AST 29 U/L, ALT 65 U/L, γ-GTP 948 U/L, and ALP 1,790 U/L. She was diagnosed as acute cholangitis with bacteremia and made a full recovery after administration of tazobactam/piperacillin for 14 days. Liver biopsy on day 28 revealed mild fibrosis and moderate lymphocyte infiltration in the portal area. Plasma cells were not infiltrated and injury of intralobular bile ducts was not evident (Figure 6). Thus, the biopsy finding showed no features of primary biliary cholangitis or autoimmune hepatitis. Considering these findings, the patient was diagnosed as CISC. Endoscopic retrograde nasobiliary drainage was replaced by biliary drainage on day 34. The wall irregularity of the bile duct was improved by cholangiography at that time. Abnormal liver functions were ameliorated on day 49: AST 14 U/L, ALT 8 U/L, γ-GTP 259 U/L, and ALP 694 U/L. After discharge, the tube stent fell off naturally, but the patient's liver function was not worsened. Chemotherapy could not be resumed for fear of the relapse of CISC. The patient then received palliative treatment.
Figure 6.
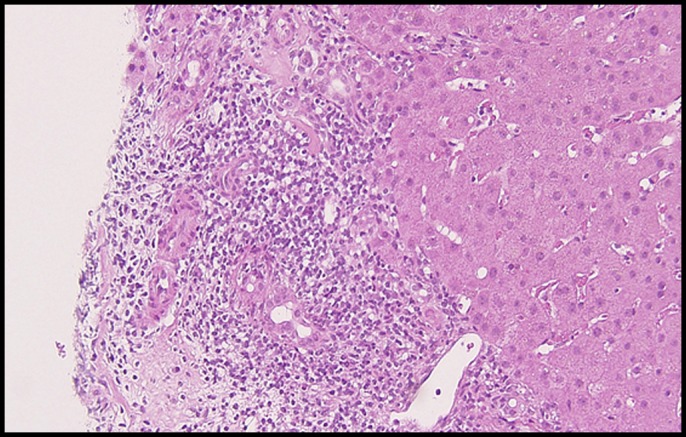
Hematoxylin and eosin stain (low power) of liver histology showing mild fibrosis and moderate lymphocyte infiltration were seen in the portal area. Plasma cells were not infiltrated. Injury of intralobular bile ducts was not evident.
DISCUSSION
This patient showed a marked liver function disorder of cholestatic type after systemic chemotherapy for cervical cancer. Wall thickening of the extrahepatic and intrahepatic bile ducts accompanied by stricture and dilatation were observed on ultrasonography, computed tomography, and cholangiography. Sclerosing cholangitis, eosinophilic cholangitis, and cholangiocarcinoma were raised as differential diagnoses. Among these diagnoses, eosinophilic cholangitis and cholangiocarcinoma were excluded because of the lack of eosinophilic infiltration and malignant cells in the bile duct biopsy. Cytology of bile also showed no features of cholangiocarcinoma. As for sclerosing cholangitis, cholangitis related to IgG4 was ruled out because of the normal serum IgG4 level and the absence of IgG4-expressing cells in the bile duct biopsy. Considering the sudden onset of the bile duct lesion during chemotherapy and improvement after cessation of chemotherapy, the patient was diagnosed with CISC instead of primary sclerosing cholangitis. The coexistence of primary biliary cholangitis needs to be examined because the patient was positive for antimitochondrial M2 antibody. Liver biopsy did not reveal the features of primary biliary cholangitis, such as chronic nonsuppurative destructive cholangitis. The distinctive findings of sclerosing cholangitis such as “onion skin” fibrosis around the bile duct were not found in the liver biopsy. This may be because the patient had a relatively short duration of CISC with the treatment for CISC being carried out promptly.
CISC is a form of secondary sclerosing cholangitis and has been shown to occur occasionally during hepatic arterial infusion chemotherapy with fluoropyrimidines.4–6 There have so far been 4 case reports of CISC triggered by systemic chemotherapy, 3 of which have been reported from Japan, although it remains unclear whether races and ethnic groups have some relationship with the development of CISC.7–10 In addition to fluoropyrimidines, other drugs, such as taxanes and bevacizumab, have been suggested to be causative drugs.7–10 In this patient, CISC is considered to have been triggered by taxane and/or bevacizumab. Although the precise pathogenic mechanism of CISC remains unclear, CISC is speculated to be induced via the direct toxicity of highly concentrated drug exposure or ischemic change as an indirect drug effect in peribiliary vascular plexuses. CISC is often treated by ursodeoxycholic acid, steroids, and biliary drainage.11 CISC is a disease of poor prognosis because it not only causes rapid deterioration of liver function but also necessitates interruption of chemotherapy. Indeed, 2 fatal cases due to CISC have been reported.9,10 In this patient, chemotherapy did considerably well before the onset of CISC, but the possible risk for recurrence of CISC led to hesitation about the recommencement of chemotherapy, resulting in palliative treatment. CISC should be considered as a rare, but clinically important, adverse event when abnormal liver function emerges during systemic chemotherapy. Great caution should be taken for early diagnosis of CISC.
DISCLOSURES
Author contributions: All authors participated in writing the manuscript. K. Ohkawa is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
REFERENCES
- 1.Gossard AA, Angulo P, Lindor KD. Secondary sclerosing cholangitis: A comparison to primary sclerosing cholangitis. Am J Gastroenterol. 2005;100:1330–3. [DOI] [PubMed] [Google Scholar]
- 2.Gelbmann CM, Rümmele P, Wimmer M, et al. Ischemic-like cholangiopathy with secondary sclerosing cholangitis in critically ill patients. Am J Gastroenterol. 2007;102(6):1221–9. [DOI] [PubMed] [Google Scholar]
- 3.Jüngst C, Stadlbauer V, Reichert MC, et al. NOD2 gene variants confer risk for secondary sclerosing cholangitis in critically ill patients. Sci Rep. 2017;7:7026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandrasegaran K, Alazmi WM, Tann M, et al. Chemotherapy-induced sclerosing cholangitis. Clin Radiol. 2006;61:670–8. [DOI] [PubMed] [Google Scholar]
- 5.Shea WJ, Jr, Demas BE, Goldberg HI, et al. Sclerosing cholangitis associated with hepatic arterial FUDR chemotherapy: Radiographic-histologic correlation. AJR Am J Roentgenol. 1986;146:717–21. [DOI] [PubMed] [Google Scholar]
- 6.Kemeny N, Daly J, Oderman P, et al. Hepatic artery pump infusion: Toxicity and results in patients with metastatic colorectal carcinoma. J Clin Oncol. 1984;2:595–600. [DOI] [PubMed] [Google Scholar]
- 7.Delis S, Triantopoulou C, Bakiyiannis A, et al. Sclerosing cholangitis in the era of target chemotherapy: A possible anti-VEGF effect. Dig Liver Dis. 2009;41:72–7. [DOI] [PubMed] [Google Scholar]
- 8.Matsuo T, Nakamura Y, Suzuki K. A case of secondary sclerosing cholangitis caused by chemotherapy with nab-paclitaxel. Nihon Shokakibyo Gakkai Zasshi. 2015;112:888–95. [Japanese.] [DOI] [PubMed] [Google Scholar]
- 9.Morisaki T, Ohnita K, Takeshima F, et al. A case of sclerosing cholangitis caused by oral chemotherapy with S-1. Nihon Shokakibyo Gakkai Zasshi. 2011;108:245–52. [Japanese.] [PubMed] [Google Scholar]
- 10.Nishimoto T, Kiriyama K, Yabu M, et al. A case of sclerosing cholangitis triggered by 5-FU/leucovorine combination therapy and rapidly deteriorated. Nihon Shokakibyo Gakkai Zasshi. 2005;102:1327–32. [Japanese.] [PubMed] [Google Scholar]
- 11.Alazmi WM, McHenry L, Watkins JL, et al. Chemotherapy-induced sclerosing cholangitis: Long-term response to endoscopic therapy. J Clin Gastroenterol. 2006;40:353–7. [DOI] [PubMed] [Google Scholar]