Abstract
Purpose
This study aimed to evaluate the clinical efficacy of a portable smartphone-based system for computer-assisted semen analysis (CASA) compared with the results of manual microscopic semen analysis (SA) and laboratory-based CASA for self-evaluation of semen parameters by a male partner.
Materials and Methods
From July 2017 to February 2018, a total of 28 samples were analyzed for concentration and motility with a smartphone-based CASA system and the results compared with those from laboratory-based CASA and manual microscopic SA with a Makler Counting Chamber (SEFI Medical Instruments, Israel).
Results
Sperm concentration and motility measured with the smartphone-based CASA system were positively correlated with the microscopic-based results. Likewise, sperm motility calculated with smartphone-based CASA was positively correlated with the laboratory-based CASA results. These results suggest that the smartphone-based CASA system can be used for clinical semen diagnosis.
Conclusions
A portable smartphone-based CASA system can play a role in motivating infertile males to visit clinics, thus resulting in early diagnosis and treatment with cost-effectiveness. The device can be used for easy follow-up on a screening basis by the male partner before visiting a clinic for fertility evaluation or by infertile males after receiving medical management. Additionally, future software advancements and post-marketing consumer surveys will make possible wider applications, including assessment of sperm morphology, in the coming future.
Keywords: Infertility, male; Semen analysis; Smartphone
INTRODUCTION
There are more than 45 million infertile couples worldwide, and in more than 30% to 50% of cases, the infertility in couples is caused by a male factor. The worldwide prevalence of male infertility has increased to up to 12% of the world male population, and it is estimated that at least 30 million males worldwide are infertile [1]. The global decline in sperm count in adult males is a contemporary hot issue, and various environmental and medical conditions have been suggested as causes [1]. Semen analysis (SA) is mandatory as the first diagnostic test for potential infertility [2]. Nevertheless, the lower likelihood of the male partner to seek traditional medical advice about fecundity than the female partner, the hesitancy to seek medical evaluation because of embarrassment, and the uncomfortable clinic system can lead to delays in diagnosis of male infertility. As a consequence, the treatment of infertility can be prolonged for couples, who may undergo unnecessary interventions or experience unsuccessful pregnancy results. The World Health Organization (WHO) manual (2010) proposed sperm parameters for healthy successful pregnancy as an important tool of infertility assessment [3]. Nevertheless, 15% of males with normal results on semen analyses have infertility, whereas almost half of males with abnormal results on semen analyses are able to have children even though a male infertility problem [4].
The global sperm bank market is expected to reach a value of United States dollar (USD) 4.96 billion by 2025, according to 2017 report by Grand View Research, Inc. In various countries, technological advances related to assistive reproduction, an increase in the number of countries legalizing same-sex marriage, and growing government support for sperm donation and donor offspring are expected to be crucial factors boosting the market during the forecast period. Within business service types for the sperm bank market, SA held the majority, with close to 50% of the revenue share due to growing research and development, academic research, and assisted reproductive procedures. In response to the growing awareness of new approaches to sperm analysis, devices for at-home sperm analysis have been introduced to the consumer market as a valuable tool for determining fertility potential [2].
Current popularly used laboratory-based systems for SA, including both manual microscopic analysis and computer-assisted semen analysis (CASA), have problems such as being time-limited, space-limited, inconvenient, expensive, and labor-intensive [2]. Manual microscopic SA especially is very subjective. Results can vary according to the test procedure used and the technician's decision-making. Owing to cultural and social backgrounds and the technical limitations of the traditional SA systems, at-home sperm analysis for male factor infertility could overcome the limitations of conventional clinical sperm methods. At-home analysis has great potential for point-of-care fertility diagnostic analysis by allowing the male partner to privately perform a screening test. At home, a smartphone camera-based sperm test may be beneficial to males who are hesitant to seek medical evaluation and can provide effective diagnostic analysis for determining fertility potential [5,6].
In the present study, we present a novel smartphone-based SA device called the SEEM® (Recruit Lifestyle Co., Ltd., Tokyo, Japan), which provides convenient, easy-to-use, and rapid measurement of sperm concentration and motility. We evaluated the smartphone-based CASA using fresh, unwashed semen samples from patients and compared the results with those from manual microscopic and automated CASA systems.
MATERIALS AND METHODS
1. Study design and participants
After approval by the Institutional Review Board of Pusan National University Hospital (approval number: 1805-033-068), a total of 28 semen samples were obtained from patients with male infertility who had attended the male infertility clinic from July 2017 to February 2018. All subjects provided written consent. All samples were tested, irrespective of semen quality. Our evaluation was performed using undiluted, unprocessed, and fresh semen samples.
2. Semen preparation and procedures
Semen samples were obtained by masturbation into a sterile cup. After the samples were liquefied for approximately 15 to 30 minutes, they were mixed well and transferred to a built-in sperm-counting chamber with an objective lens and cover glass. The sperm concentration and motility results of the smartphone-based CASA system (SEEM®) were compared with the results calculated by laboratory-based CASA (SAIS plus Sperm Analysis Imaging System, Medical Supply, Seoul, Korea) and manual microscopic SA with the Makler Counting Chamber (SEFI Medical Instruments, Tel Aviv, Israel). A total of 28 samples were split into equal aliquots to evaluate SA results simultaneously on the three devices in a blinded manner; SA was performed in duplicate on each semen sample. SA was performed by one person who was a trained and licensed clinical pathology technician in the outpatient andrology laboratory. Two independent sperm counts were performed. Spermatozoa with progressive motility as well as those with nonprogressive motility were considered as motile sperm. Motility was calculated as the motile sperm count divided by the total sperm count. We tested semen samples that were laboratory-based CASA results with more than 5×106/mL to minimize the wide data variation.
3. Components of the smartphone-based CASA system
The portable smartphone-based CASA system used was the SEEM® kit (Recruit Lifestyle Co., Ltd). The SEEM® kit consisted of a disposable glass slide with an integrated prototype specimen chamber with a hemispherical microscopic lens with a built-in type cover glass and QR code to download the result to the accompanying application software. The application for operating the CASA system worked only on an iPhone (Apple Inc, Cupertino, CA, USA) because of that phone's high-quality digital camera (Fig. 1A). To begin testing, the application software was initially downloaded and installed on the smartphone. Semen samples, which were harvested under audiovisual stimulation in the semen collection room of the outpatient clinic, were allowed to sit for 15 to 30 minutes for liquefaction of semen. One drop (10 µL) of liquefied semen was loaded into the covered glass of the built-in sperm-counting chamber using a collection pole/stick and allowed to spread onto the lens by capillary effect (Fig. 1B). The black-colored lens portion of the chamber was put on the front lens of the smartphone's camera. The smartphone's autofocus automatically observed sperm on the high-quality screen field and captured the image for analysis (Fig. 1C). Sperm concentration and motility were shown automatically on the screen of the smartphone, in addition to normal WHO seminal parameters.
Fig. 1. Devices and components for the smartphone-based computer-assisted semen analysis (CASA) system. (A) Photo of the SEEM® kit with instructions for use, sperm analysis device, semen transfer device, QR code sheet to download the application for operating, and disposable built-in counting chamber with objective lens and cover glass. (B) The technique for observing the semen sample using smartphone-based CASA. After a drop of semen (black arrow) is placed under the covered glass of the built-in sperm-counting chamber, the black-colored lens portion of the chamber is put on the front lens of the smartphone. (C) Sample screenshot of the captured test results of a semen sample with concentration and motility.
4. Manual microscopic and laboratory-based CASA
To measure both sperm concentration and motility, one drop (10 µL) of semen from each sample was placed into a counting chamber (SEFI Medical Instruments) and analyzed using a CASA system (SAIS plus Sperm Analysis Imaging System). Manual and automated SA was performed according to a standard protocol in the WHO 2010 manual [3] and according to the protocol of the manufacturing company. In brief, five fields per chamber were counted and averaged for each sperm count and motility data according to a conventional SA method; this procedure was then repeated two independent times for each specimen. For CASA counts, samples were well mixed and transferred with a glass capillary into a chamber. All chambers were allowed to settle, and sperm cells were counted by use of phase contrast light microscopy. CASA counts were obtained by continuously repeating a 1-s video of a random single field of view. All CASA counts were performed at 200× magnification. The samples were loaded into the counting chamber and tested using the smartphone-based semen analyzer in parallel to the laboratory-based CASA system.
5. Statistical analysis
Descriptive statistics were used to explore characteristics of the sample. Continuous data are expressed as means. Statistical analyses were performed using SAS version 9.3, R 3.3.2 (SAS Institute Inc., Cary, NC, USA), with the calculation of Pearson correlation coefficients between two differently analyzed data sets. Significance for all tests was two-tailed, and p-values less than 0.05 were considered to indicate statistical significance.
RESULTS
1. Characteristics of semen samples
In all, 28 males were recruited for this study. The underlying causes of male infertility were 21 cases (75.0%) of post-varicocelectomy, 4 cases (14.3%) of infertility screening, and 3 cases (10.7%) of post-vasectomy reversal. Subjects were aged 31.8±7.9 years on average (range, 21.0–51.0 years) (Table 1). We analyzed sperm concentration and motility by use of three different methods: manual microscopic analysis, laboratory-based CASA, and smartphone-based CASA.
Table 1. Summary of patient demographics in the consumer clinical study (n=28).
| Parameter | Results |
|---|---|
| Age (y) | 31.8±7.9 (21.0–51.0) |
| Cases | |
| Post-varicocelectomy | 21 (75.0) |
| Infertility screening | 4 (14.3) |
| Post-vasectomy reversal | 3 (10.7) |
Values are presented as mean±standard deviation (range) or number (%).
2. Methods comparison of sperm concentration and motility
Comparison of values of sperm concentration and motility obtained by using the manual microscopic (Makler) method with those obtained by using laboratory-based CASA (L-CASA) resulted in highly statistically significant (p<0.001) regression lines, with Pearson and concordance correlation coefficients of 0.998 and 0.968, respectively (Fig. 2). Agreement between the Makler and L-CASA methods in measuring concentration and motility indicated overall accuracy (Tables 2, 3). Comparison of the results of sperm concentration and motility with use of the Makler method with the results obtained by use of smartphone-based CASA (S-CASA) was also statistically significant and showed positive correlation in the regression, with Pearson and concordance correlation coefficients of 0.382 and 0.594, respectively (p<0.05) (Fig. 3).
Fig. 2. Scatter diagram and regression analysis comparing manual microscopic semen analysis with the Makler Counting Chamber and laboratory-based computer-assisted semen analysis (L-CASA) for sperm concentration (A) and sperm motility (B).
Table 2. Comparison of data for sperm concentration among manual microscopic semen analysis, laboratory-based CASA, and smartphone-based CASA.
| Devices | Concentration (×106/mL) | Subgroups | |||||
|---|---|---|---|---|---|---|---|
| 5–10 | 11–14 | 15–29 | 30–50 | 51–99 | ≥100 | ||
| Manual microscopic semen analysisa | 67.1±35.8 (17–165) | 0 | 0 | 3 | 8 | 12 | 5 |
| Laboratory-based CASA | 68.8±36.8 (19.2–139.4) | 0 | 0 | 3 | 8 | 12 | 5 |
| Smartphone-based CASA | 43.9±27.2 (7.1–99.1) | 2 | 3 | 2 | 12 | 9 | 0 |
Values are presented as mean±standard deviation (range) or number only.
CASA, computer-assisted semen analysis.
a:Used with Makler Counting Chamber.
Table 3. Comparison of data for sperm motility among manual microscopic semen analysis, laboratory-based CASA, and smartphone-based CASA.
| Devices | Motility (%) | Subgroups | |||
|---|---|---|---|---|---|
| 0–19 | 20–39 | 40–59 | ≥60 | ||
| Manual microscopic semen analysisa | 41.8±15.7 (10–72) | 2 | 11 | 11 | 4 |
| Laboratory-based CASA | 42.6. ±15.5 (10.6–68.0) | 2 | 12 | 9 | 5 |
| Smartphone-based CASA | 58.3±25.8 (0–93.8) | 2 | 4 | 6 | 16 |
Values are presented as mean±standard deviation (range) or number only.
CASA, computer-assisted semen analysis.
a:Used with Makler Counting Chamber.
Fig. 3. Scatter diagram and regression analysis comparing manual microscopic semen analysis with the Makler Counting Chamber and smartphone-based computer-assisted semen analysis (S-CASA) for sperm concentration (A) and sperm motility (B).
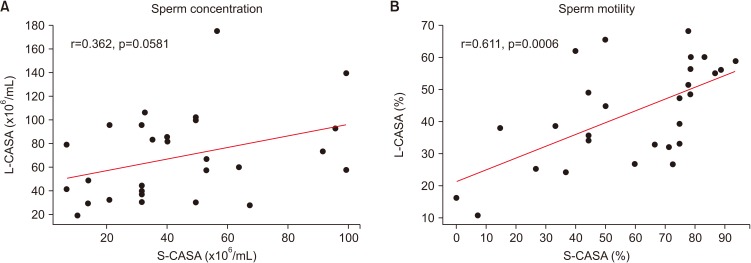
There was statistically significant and positive correlation between L-CASA and S-CASA for semen motility (p<0.001). No statistically significant differences were found between L-CASA and S-CASA for sperm concentration (Fig. 4) (p>0.05). However, there were no particular problems with clinical use of the smartphone-based CASA system for evaluating indicators of sperm quality such as concentration and motility.
Fig. 4. Scatter diagram and regression in comparing between manual microscopic semen analysis with laboratory-based computer-assisted semen analysis (L-CASA) and smartphone-based CASA (S-CASA) in the sperm concentration (A) and sperm motility (B).
DISCUSSION
We have demonstrated that smartphone-based CASA can accurately measure sperm concentration and motility using a small-volume semen sample loaded into a disposable, built-in counting chamber with an objective lens and cover glass. Our results suggest that the smartphone-based diagnostic assay will be of use as a rapid, accessible, and easy-to-use method for male fertility testing of sperm concentration and motility using unwashed and unprocessed clinical semen samples.
In the evaluation of male infertility, the analysis of at least one semen parameter is always mandatory [4]. Recently, home kits have become available to substitute for clinical laboratory methods [5]. It is helpful to evaluate male fertility for infertile couples trying to become pregnant or to verify the success of various therapies, such as for health and stamina, in infertile males. However, conventional laboratory-based SA is regularly performed even though it has disadvantages like the requirement for skilled technicians, the need for centralized laboratories, and variability in results caused by a diversity of practices [5,6]. In addition, many males are not comfortable with clinical diagnostic SA or often consider it embarrassing or a waste of time [2,6]. Thus, new techniques such as at-home semen testing have recently been developed as solutions to these problems.
As the first commercial home-based methods to measure male fertility, SpermCheck® Fertility (Princeton BioMeditech Corp., Monmouth Junction, NJ, USA), SpermCheck® Vasectomy Fertility (Princeton BioMeditech Corp.), SwimCount Sperm Quality Test (MotilityCount ApS., Copenhagen, Denmark), FertilitySCORE® kit (FertiPro N.V., Beernem, Belgium), Trak® Male Fertility Testing (Sandstone Diagnostics Inc., Pleasanton, CA, USA) and FertileMARQ™ Male Fertility Test (Princeton BioMeditech Corp.) are currently available on the market, all of which provide indirect measures of sperm concentration [5]. Among them, SpermCheck® Fertility and Trak® Male Fertility Testing, which are approved by the U.S. Food and Drug Administration, provide the results of sperm concentration using a chemical labeling approach that detects sperm-specific proteins on the sperm head or a centrifugal microfluidic device [5,7,8,9]. Especially, microfluidics-combined biotechnology has shown great promise in the development of biomedical devices with broad applications in medicine and biology [7]. Although various devices for home semen testing are becoming available and provide easy-to-understand results using cutoff thresholds, these tests report only sperm concentration and may result in errors or biased judgments as the result of handling by users who are not experts [5,10,11].
CASA is a catch-all phrase for automatic or semi-automatic SA techniques. Most systems are based on image analysis, but alternative methods exist such as tracking cell movement on a digitized tablet [6,12]. Computer-assisted techniques are most often used to assess sperm concentration and mobility characteristics, such as velocity and linear velocity in a regular laboratory. Nowadays, there are CASA systems based on image analysis and new techniques, with near perfect results which can perform full analysis in a few seconds. With some techniques, sperm concentration and motility measurements are at least as reliable as current manual methods [12]. On the developmental history of SA modalities, sperm-counting chambers and information and communications technology (ICT) have evolved successively to promote the diagnostic accuracy of SA over the last 40 years. Of these technologies, a high-resolution camera, a built-in sperm-counting chamber with an objective lens, and an operation application are prerequisites for the smartphone-based CASA.
Global smartphone usage and mobile network coverage have been expanding rapidly during the last 10 years, and global smartphone subscription was 96.8% in 2015 [6]. Smartphones have created interesting opportunities for promoting wellness and are already used as part of point-of-care devices for diabetes care, mobile thermography, and perioperative management as well as to communicate laboratory data for blood tests and bacterial, viral, and biomarker detection [13,14,15]. The emerging use of smartphones equipped with cameras has led to the development of various sperm-counting chambers, which provide test accuracy as well as test simplicity without an appointment for conventional laboratory-based SA [2,6,10,11]. Self-examination of semen with a smartphone at home has several advantages. It is a good way to collect preliminary data for fertility screening or post-treatment data during a long follow-up period. Especially in the young generation, smartphones have great potential to support home sperm testing because they are small, portable devices with excellent digital cameras and can easily be attached to a microscope. Therefore, smartphone-based CASA is expected to be of use to easily evaluate male fecundity in a young generation familiar with the ICT environment and without needing training [16].
Kanakasabapathy et al. [11] presented the first novel device for automated smartphone-based diagnostic assay. Their device uses a small volume of semen (<35 µL) loaded into a disposable microfluidic device to measure sperm concentration and motility together. A compact and lightweight automated SA platform using lens-free, on-chip microscopy at home was introduced by Su et al. [17], and a single-ball lens microscope was introduced by Kobori et al. [6] for smartphone use. These technologies will also be available to various medical fields in addition to SA. The current new approaches in smartphone-based sperm analysis with low-cost, convenient devices may overcome the technology of home semen testing, such as immunochromatographic test strips and microfluidic devices [5].
Until now, three different at-home smartphone-based devices for semen testing had been introduced. Three types of smartphones (iOS 8, iOS 9, and Android 4.4) combined with a single-ball lens microscope have been evaluated for determining sperm concentration and motility compared with a CASA system [6]. Also, two types of smartphones (Galaxy S7 [Samsung, Korea] and iPhone 7) combined with a YO device (Medical Electronic Systems Ltd., Los Angeles, CA, USA) have been evaluated for determining motile sperm motility compared with SQA-Vision (Medical Electronic Systems Ltd.), an FDA-cleared automated laboratory semen quality analyzer [2]. In this study, the commercially available smartphone-based CASA was produced by Tenga Co., Ltd. (Tokyo, Japan) in late 2000 and was developed initially as a sperm imaging viewer only without the automatic counting function of sperm concentration and motility. In this system, it is possible to count sperm concentration and motility manually with a magnified viewing smartphone screen. A subsequently developed smartphone-based CASA system was SEEM®, which has automatic counting and a display function for sperm concentration and motility that provides a real-time video display of sperm motility. Although SEEM® can measure sperm concentration and motility, a particularly notable limitation of the technology is that the system does not measure sperm morphology, oxidation reduction potential, or DNA integrity for successful reproductive outcome. Another limitation is that these smartphone-based CASA systems need a high-resolution camera at the periphery of the field of view of the captured image.
In this study, we could confirm the technical advancements of the smartphone-based CASA system. The results obtained with SEEM® were positively correlated with the results obtained with the manual microscopic and laboratory-based CASA system for self-evaluation of semen parameters. The limitations of this study had to do with the small number of participants and that the smartphone-based CASA system is currently available only for iOS. An additional limitation of our study was that consumers did not examine the smartphone-based CASA system.
The results of our study indicate that a smartphone-based diagnostic CASA system can be useful for automated SA for male infertility screening for infertile couples before medical intervention or for a man who wants to evaluate male fecundity premaritally. Therefore, smartphone-based CASA, which is rapid, automated, inexpensive, and easy to use, is expected to be an integral part of the ubiquitous global health care system.
CONCLUSIONS
In conclusion, this study shows that a portable, smartphone-based CASA system can play a role not only in motivating infertile males to visit clinics for early diagnosis and treatment, but also in allowing easy follow-up on a screening basis by the patient himself after receiving specific medical management. This system is expected to contribute to a decrease in the mental and physical burden of infertile couples before infertility management, shortening the time to achieve pregnancy and decreasing medical expense. Further post-marketing consumer surveys and future technical advancements will allow wider clinical application, including assessment of sperm morphology, in the coming future.
ACKNOWLEDGMENTS
This study work was funded by a research grant from the BioMedical Research Institute, Pusan National University Hospital (Grant No. 201709390001). This study was also supported by the free supply of SEEM® kit from Recruit Lifestyle Co., Ltd. (Tokyo, Japan). Research findings were not approved by Recruit Lifestyle Co., Ltd. or other sponsors prior to submission or publication. Statistical analyses in this work were supported by the Department of Biostatistics, Clinical Trial Center, BioMedical Research Institute, Pusan National University Hospital.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37. doi: 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal A, Panner Selvam MK, Sharma R, Master K, Sharma A, Gupta S, et al. Home sperm testing device versus laboratory sperm quality analyzer: comparison of motile sperm concentration. Fertil Steril. 2018;110:1277–1284. doi: 10.1016/j.fertnstert.2018.08.049. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: World Health Organization; 2010. [Google Scholar]
- 4.Nallella KP, Sharma RK, Aziz N, Agarwal A. Significance of sperm characteristics in the evaluation of male infertility. Fertil Steril. 2006;85:629–634. doi: 10.1016/j.fertnstert.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 5.Kobori Y. Home testing for male factor infertility: a review of current options. Fertil Steril. 2019;111:864–870. doi: 10.1016/j.fertnstert.2019.01.032. [DOI] [PubMed] [Google Scholar]
- 6.Kobori Y, Pfanner P, Prins GS, Niederberger C. Novel device for male infertility screening with single-ball lens microscope and smartphone. Fertil Steril. 2016;106:574–578. doi: 10.1016/j.fertnstert.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 7.Coppola MA, Klotz KL, Kim KA, Cho HY, Kang J, Shetty J, et al. SpermCheck Fertility, an immunodiagnostic home test that detects normozoospermia and severe oligozoospermia. Hum Reprod. 2010;25:853–861. doi: 10.1093/humrep/dep413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klotz KL, Coppola MA, Labrecque M, Brugh VM, 3rd, Ramsey K, Kim KA, et al. Clinical and consumer trial performance of a sensitive immunodiagnostic home test that qualitatively detects low concentrations of sperm following vasectomy. J Urol. 2008;180:2569–2576. doi: 10.1016/j.juro.2008.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaff UY, Fredriksen LL, Epperson JG, Quebral TR, Naab S, Sarno MJ, et al. Novel centrifugal technology for measuring sperm concentration in the home. Fertil Steril. 2017;107:358–364.e4. doi: 10.1016/j.fertnstert.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 10.Kricka LJ, Heyner S. Smartphones and semen analysis. Clin Chem. 2018;64:257–258. doi: 10.1373/clinchem.2017.274621. [DOI] [PubMed] [Google Scholar]
- 11.Kanakasabapathy MK, Sadasivam M, Singh A, Preston C, Thirumalaraju P, Venkataraman M, et al. An automated smartphone-based diagnostic assay for point-of-care semen analysis. Sci Transl Med. 2017;9:eaai7863. doi: 10.1126/scitranslmed.aai7863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomlinson MJ, Pooley K, Simpson T, Newton T, Hopkisson J, Jayaprakasan K, et al. Validation of a novel computer-assisted sperm analysis (CASA) system using multitarget-tracking algorithms. Fertil Steril. 2010;93:1911–1920. doi: 10.1016/j.fertnstert.2008.12.064. [DOI] [PubMed] [Google Scholar]
- 13.Roy S, Pantanowitz L, Amin M, Seethala RR, Ishtiaque A, Yousem SA, et al. Smartphone adapters for digital photomicrography. J Pathol Inform. 2014;5:24. doi: 10.4103/2153-3539.137728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallegos D, Long KD, Yu H, Clark PP, Lin Y, George S, et al. Label-free biodetection using a smartphone. Lab Chip. 2013;13:2124–2132. doi: 10.1039/c3lc40991k. [DOI] [PubMed] [Google Scholar]
- 15.Zhu H, Sencan I, Wong J, Dimitrov S, Tseng D, Nagashima K, et al. Cost-effective and rapid blood analysis on a cell-phone. Lab Chip. 2013;13:1282–1288. doi: 10.1039/c3lc41408f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vij SC, Agarwal A. Editorial on “An automated smartphone-based diagnostic assay for point-of-care semen analysis”. Ann Transl Med. 2017;5:507. doi: 10.21037/atm.2017.09.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su TW, Erlinger A, Tseng D, Ozcan A. Compact and light-weight automated semen analysis platform using lensfree on-chip microscopy. Anal Chem. 2010;82:8307–8312. doi: 10.1021/ac101845q. [DOI] [PMC free article] [PubMed] [Google Scholar]