Abstract
Purpose
To investigate whether treatment with low-energy shock wave (LESW) alleviates pain and bladder dysfunction in a mouse model of uroplakin 3A (UPK3A)-induced interstitial cystitis/painful bladder syndrome (IC/PBS).
Materials and Methods
Forty female BALB/c mice were divided into four groups (n=10/group): Sham, Sham+LESW, UPK3A, and UPK3A+LESW. At 6 weeks of age, mice were injected with an emulsion containing water and complete Freund's adjuvant with (UPK3A and UPK3A+LESW groups) or without (Sham and Sham+LESW groups) 200 µg of UPK3A. At 10 weeks, mice received a second dose of Freund's adjuvant to booster immunization. At 12 weeks, mice underwent pain assessment and a frequency volume chart (FVC) test as the pretreatment assessment. LESW treatment and pain assessment were conducted from 13 to 15 weeks. One week after the final treatment, pain assessment and the FVC were conducted again as the post-treatment assessment. Mice were euthanized and sacrificed at 17 weeks.
Results
The presence of tactile allodynia and bladder dysfunction was significant in the UPK3A-injected mice. LESW raised the pain threshold and improved bladder function with decreased urinary frequency and increased mean urine output. Expression and secretion of local and systemic inflammatory markers, including tumor necrosis factor-α (TNF-α) and nerve growth factor (NGF), increased after UPK3A immunization. These markers were significantly decreased after LESW treatment (p<0.05).
Conclusions
LESW treatment attenuated pain and bladder dysfunction in a UPK3A-induced model of IC/PBS. Local and systemic inflammation was partially controlled, with a reduced number of infiltrated inflammatory cells and reduced levels of TNF-α and NGF.
Keywords: Cystitis, interstitial; Inflammation; Pain; Shockwave, ultrasonic
INTRODUCTION
Interstitial cystitis/painful bladder syndrome (IC/PBS) is a challenging chronic bladder syndrome characterized by symptoms of urinary frequency, urgency, and pelvic pain and hypersensitivity [1,2]. The morbidity of IC/PBS is two to five times higher in women than in men with an estimated prevalence of 2.7% to 6.5% according to recent epidemiologic studies from the National Institutes of Health [3,4,5]. IC/PBS is associated with negative impacts on quality of life, increased risk of mental health disorders and suicidal ideation, and increased health care costs [6].
The etiopathogenesis of IC/PBS remains unclear. Possible theories include increased urothelial permeability; disorganized neurogenic, angiogenic, inflammatory, or mast cell factors [1]; chronic subclinical infection; and psychological distress [7]. IC/PBS is also associated with several conditions such as chronic fatigue syndrome, fibromyalgia, irritable bowel syndrome, and migraine [2]. Uroplakins (UPK 1/2/3) belong to a family of integral membrane proteins of the urothelium that are highly expressed in bladder tissue [8]. Injection of UPK3A (uroplakin 65–84) has been proven to induce T cells to attack the bladder epithelium in animal models of experimental autoimmune cystitis (EAC), which leads to chronic suprapubic tactile allodynia and other symptoms, mimicking human IC/PBS disease [1].
Treatments for IC/PBS are limited because of the unclear etiology of the disease. Both American and European Urological Association guidelines recommend multimodal behavioral techniques alongside oral drugs (e.g., amitriptyline and pentosan poly-sulfate sodium) or minimally invasive treatments (e.g., botulinum toxin, dimethyl sulfoxide, chondroitin sulfate, triamcinolone, hyaluronic acid, and lidocaine). Novel treatment modalities, including immunomodulating drugs, stem cell therapy, nerve growth factor (NGF), and ASP6294, have been evaluated but with conflicting results [6,7].
Treatment with low-energy shock wave (LESW) has been shown to promote local tissue recovery and relieve skeletal muscle pain in various preclinical and clinical settings [9,10,11,12,13]. In our previous research, we showed that LESW has beneficial effects for urogenital tissue injury recovery and local neural axon regeneration in animal-based studies of erectile dysfunction and stress urinary incontinence [9].
In the present study, we explored the therapeutic effects of LESW in a mouse model of EAC induced by UPK3A. Functional and histologic studies were conducted to evaluate the severity of IC/PBS induced by UPK3A and influenced by LESW. We also examined changes in local and systemic inflammatory factors, including tumor necrosis factor-α (TNF-α) and NGF, to explore the possible underlying mechanisms.
MATERIALS AND METHODS
1. Immunization of mice and experimental design
All animal protocols were approved by the Institutional Animal Care and Use Committee of Peking University First Hospital (permit no. 201734). Forty 5-week-old female BALB/c mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China) and were divided into four groups with 10 mice per group: Sham, Sham+LESW, UPK3A, and UPK3A+LESW. UPK3A (65–84, sequence AMVDSAMSRNVSVQDSAGVP, purity: 98%) was manufactured by Beijing SciLight Biotechnology Ltd., Co. (Beijing, China). At 6 weeks of age, the mice were injected subcutaneously in the abdominal flank with 200 µL of an emulsion containing equal volumes of water and complete Freund's adjuvant (CFA with mycobacteria tuberculosis) with (UPK3A and UPK3A+LESW groups) or without (Sham and Sham+LESW groups) 200 µg of UPK3A (first dose). At 10 weeks of age, the mice received a second dose of a booster immunization in exactly the same procedure as the first dose except that CFA was replaced by Freund's adjuvant (FA without mycobacteria tuberculosis). At 12 weeks of age, the mice underwent pain assessment (von Frey filaments test) and the frequency volume chart (FVC) test (micturition recording) as the pretreatment baseline assessment. LESW treatment and pain assessment were conducted from 13 to 15 weeks of age. One week after the final treatment (16 weeks of age), pain assessment and the FVC were conducted again as the post-treatment assessment. Mice were euthanized with an overdose of ketamine xylazine and sacrificed at 17 weeks of age. Serum and bladder tissue were processed for hematoxylin and eosin (H&E) staining, enzyme-linked immunosorbent assay (ELISA), polymerase chain reaction (PCR) testing, and Western blot.
2. LESW treatment
A shock wave was delivered to the pelvic region with a special probe that was attached to a compact electrohydraulic unit with a focused shock wave source (HaiBing Medical Equipment Limited Corporation, Zhanjiang Economic and Technological Development Zone, Guangdong, China). Each mouse was anesthetized with isoflurane and then placed in the supine position with its lower abdomen shaved and sterilized with 75% alcohol. Standard commercial ultrasound gel (Aquasonic; Parker Laboratories Inc., Fairfield, NJ, USA) was applied between the probe and the skin of the pelvic region for optimal coupling. The treatment was applied at the energy flux density (maximum amount of acoustical energy that is transmitted through an area of 1 mm2 per pulse) of 0.00 mJ/mm2 (Sham group and UPK3A group) or 0.09 mJ/mm2 (Sham+LESW group and UPK3A+LESW group) at 3 Hz (3 shocks per second), 400 shocks each time and three times a week. The course of treatment was designed on the basis of the clinical application of LESW, which patients with erectile dysfunction receive 2 to 3 times per week. This was also the course we used in our previous research [9].
3. Tactile allodynia assessment
Tactile sensitivities of the suprapubic region of mice were measured by using a series of 14 von Frey filaments with increasing calibrated forces from 0.008 to 8.0 g (Stoelting, Wood Dale, IL, USA). Beginning with the smallest filament, each filament was applied a total of five times for 3 seconds, with intervals of 8 seconds between each stimulus. The following behaviors were considered to be positive responses: 1) sharp retraction of the suprapubic abdominal region, 2) instant licking or scratching of the suprapubic abdominal region, and 3) jumping. The mouse was counted as positive if the response was positive more than 3 of 5 times (≥3/5). The whole procedure was repeated three times at each time point from 12 to 16 weeks of age weekly, and the number of mice with a positive response was recorded. In addition, the von Frey force that would elicit a positive response in half of the mice in each group (50% threshold) was also calculated.
4. Histologic analysis
Bladders were removed and fixed in 4% paraformaldehyde overnight. Paraffin-embedded tissue was cut into 5-µm sections and then stained with H&E. Gross histologic observations were performed using light microscopy.
5. ELISA for serum inflammatory markers
Blood samples were collected and serum was separated and stored at −80℃ for ELISA. Concentrations of TNF-α (575209; Biolegend, San Diego, CA, USA), NGF (E-EL-M0815c; Elabscience, Houston, TX, USA), interleukin-2 (IL-2, 575409; Biolegend), and IL-6 (575709; Biolegend) were measured using ELISA kits according to the manufacturers' instructions. Absorbance at 450 nm was read in a Versamax ELISA microplate reader (Molecular Devices, Sunnyvale, CA, USA).
6. Real-time quantitative reverse transcription PCR
Fresh bladder tissue was immediately homogenized in TRIzol reagent (Invitrogen, Carlsbad, CA, USA) followed by isolation of total RNA. cDNA was synthesized from the RNA using a Super Script III cDNA synthesis kit with random hexamer primers (Invitrogen). Expression of the following genes was analyzed: NGF (ACAGATAGCAATGTCCCAGAGGATCCAGAGTGTCCGAAGAGGTG), interferon-γ (IFN-γ) (ATGAACGCTACACACTGCATCCCATCCTTTTGCCAGTTCCTC), TNF-α (CAGGCGGTGCCTATGTCTCCGATCACCCCGAAGTTCAGTAG), IL-2 (GTCACAAACAGTGCACCTACCCCTGGGTCTTAAGTGAAAG), IL-4 (TCCATGCACCGAGATGTTTGTACCCGTAGGATGCTCCCTTTATGAACG), IL-6 (TAGTCCTTCCTACCCCAATTTCCTTGGTCCTTAGCCACTCCTTC), IL-8 (TTGCCAAGGAGTGCTAAAGAAGCCCTCTTCAAAAACTTCTCC), and GAPDH (AGGTCGGTGTGAACGGATTTGTGTAGACCATGTAGTTGAGGTCA). Quantitative PCR was performed using the SYBR Green PCR Master kit with an ABI Prism 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA).
7. Western blot
Protein isolation and Western blot were conducted as previously reported [9]; a total of 20 mg protein was loaded for each sample. The primary antibodies used in Western blot were nuclear factor-κB (NF-κB, 1:500, ab16502; Abcam, Cambridge, MA, USA), NGF (1:300, ab6199; Abcam), TNF-α (1:500, ab6671; Abcam), and β-actin (1:1,000, ab8227; Abcam). After incubation with the secondary antibody, the resulting images were analyzed with ChemiImager 4000 (Alpha Innotech Corp., San Leandro, CA, USA) to determine the integrated density value of each protein band.
8. Statistical analysis
Results were analyzed using Prism 5 (GraphPad Software, San Diego, CA, USA) and are expressed as means±standard deviations. Multiple groups were compared by using one-way analysis of variance followed by the Tukey Kramer test for post-hoc comparisons (four variables). Statistical significance was set at p<0.05.
RESULTS
1. Presence of tactile allodynia in EAC mice and pain-alleviating effect of LESW treatment
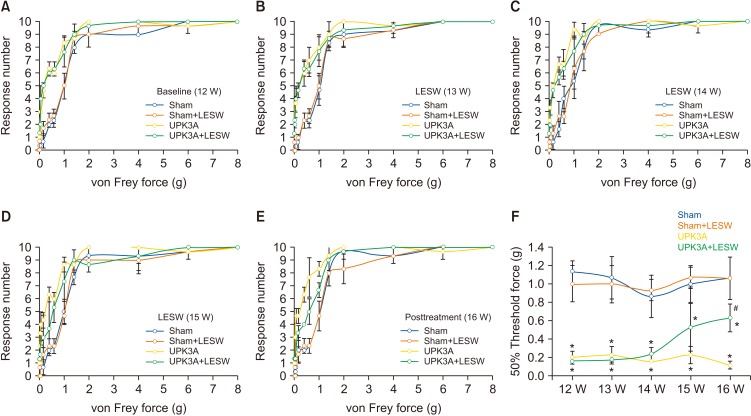
Local pain or allodynia is the most prominent feature of IC/PBS. Injection of UPK3A induced persistent referred pelvic pain responses after 2 weeks. Mechanical stimulation with von Frey filaments of increasing size on the suprapubic region resulted in significantly greater tactile sensitivity in the EAC mice than in uninjected mice (Fig. 1A–E). Calculation of 50% threshold forces revealed significantly a lower tactile response threshold in UPK3A-immunized mice than in control mice (Fig. 1F). These results demonstrate the persistence of allodynia in the EAC mouse model. Three weeks of LESW treatment lowered the tactile sensitivity in UPK3A-induced EAC mice, and the 50% threshold forces increased significantly in the UPK3A+LESW group compared with the UPK3A group (p=0.005).
Fig. 1. Presence of tactile allodynia in experimental autoimmune cystitis (EAC) mice and pain-alleviating effect of low-energy shock wave (LESW) treatment. Mechanical stimulation with von Frey filaments of increasing size on the suprapubic region resulted in significantly greater tactile sensitivity in the EAC mouse model than in uninjected mice (A–E). Fifty percent threshold forces were significantly decreased after uroplakin 3A (UPK3A) immunization (F). LESW treatment of UPK3A-immunized mice significantly alleviated the local pain and increased 50% threshold forces (A–F). *p<0.05 compared to Sham. #p<0.05 compared to UPK3A.
2. LESW treatment decreases urinary frequency and increases mean urinary output in an EAC mouse model
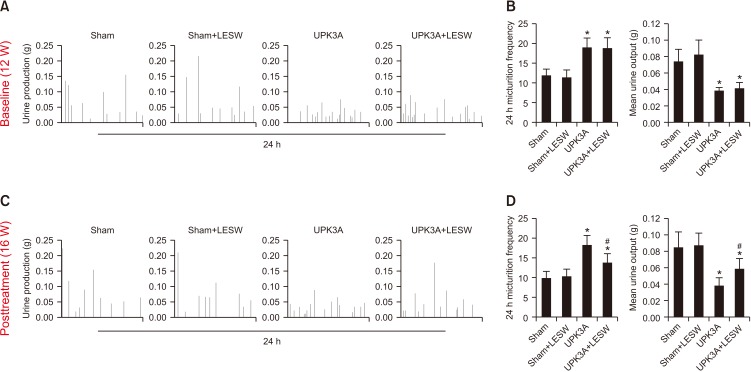
Urinary FVCs were used to determine whether LESW treatment could influence bladder function. Mice immunized with UPK3A showed significantly increased micturition frequency and significantly decreased mean urine outputs (Fig. 2A, B, p=0.001 and 0.001, respectively). After treatment, FVC measurement at 16 weeks showed that LESW decreased the urinary frequency and increased the mean urine output in the UPK3A+LESW group compared with that in the UPK3A group (Fig. 2C, D, p=0.011 and 0.049, respectively).
Fig. 2. Low-energy shock wave (LESW) treatment decreases urinary frequency and increases mean urine output in an experimental autoimmune cystitis (EAC) mouse model. Mice immunized with uroplakin 3A (UPK3A) showed significantly increased micturition frequency and significantly decreased mean urine outputs (A, B). LESW treatment decreased the urinary frequency and increased the mean urine output in the UPK3A+LESW group compared with the UPK3A group (C, D). *p<0.05 compared to Sham. #p<0.05 compared to UPK3A.
3. Bladder wall thickness and inflammatory cell infiltration are influenced by UPK3A injection and LESW treatment
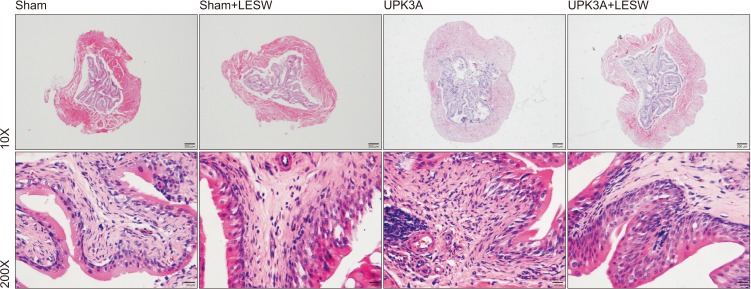
To determine whether functional changes in the bladder are associated with potential bladder tissue changes, H&E staining was done. The number of infiltrated inflammatory cells was higher in UPK3A-injected groups. The thickness of the bladder wall was also greater after UPK3A immunization, which was associated with impaired bladder compliance. LESW treatment attenuated these pathologic changes. Infiltrated inflammatory cells were especially decreased in the smooth muscle layers (Fig. 3).
Fig. 3. Hematoxylin & eosin staining (10× and 200×) to show bladder wall thickness and inflammatory cell infiltration influenced by uroplakin 3A (UPK3A) injection and low-energy shock wave (LESW) treatment.
4. LESW treatment relieves the systemic inflammatory response
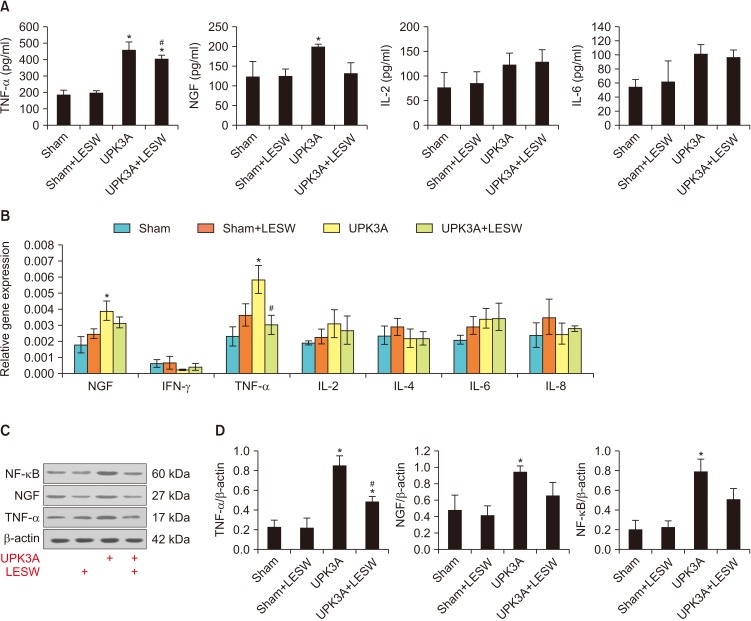
The systemic inflammatory response was measured as represented by the levels of inflammatory cytokines in serum. Levels of TNF-α, NGF, IL-2, and IL-6 were calculated by using an ELISA kit. Serum levels of TNF-α and NGF increased significantly after UPK3A injection, which corresponded to the local inflammatory reaction in bladder tissue. LESW treatment also relieved the systemic inflammatory response represented by decreased TNF-α and NGF levels in serum. The decrease in the TNF-α level was significant after LESW treatment (Fig. 4A, p=0.004).
Fig. 4. Systemic and local inflammation in bladder tissue was induced by uroplakin 3A (UPK3A) immunization, and low-energy shock wave (LESW) treatment decreased local inflammatory reactions. (A) Serum levels of tumor necrosis factor-α (TNF-α), nerve growth factor (NGF), interleukin (IL)-2, and IL-6, respectively, influenced by UPK3A immunization and LESW treatment. (B) Reverse transcription polymerase chain reaction examination of expression levels of NGF, interferon-γ (IFN-γ), TNF-α, and IL-2, -4, -6, and -8. (C) Western blot examination of protein levels of TNF-α, NGF, and downstream inflammatory marker nuclear factor-κB (NF-κB). (D) Calculation of Western blot results based on bands' grey scale. *p<0.05 compared to Sham. #p<0.05 compared to UPK3A.
5. Local inflammation in bladder tissue is induced by UPK3A immunization and LESW treatment decreases local inflammatory reactions
To assess the influence of UPK3A and LESW treatment on the expression of inflammatory markers in the bladder, bladders were harvested and processed for quantitative reverse transcription (RT)-PCR and Western blot. Common inflammatory markers including NGF, IFN-γ, TNF-α, and IL-2, -4, -6, and -8 were measured by use of RT-PCR. The results demonstrated that mRNA levels of TNF-α and NGF increased significantly after UPK3A immunization, and LESW treatment decreased the expression levels of TNF-α and NGF (Fig. 4B). These changes were further confirmed using Western blot, in which the protein levels of TNF-α, NGF, and the downstream inflammatory marker NF-κB were measured. Changes in the levels of TNF-α and NGF corresponded to the changes in mRNA expression levels. LESW treatment induced significantly decreased protein levels of TNF-α in the UPK3A+LESW group compared with the UPK3A group (Fig. 4C, D, p=0.003).
DISCUSSION
Pelvic pain is a hallmark of IC/PBS. Patients suffer from diffuse pelvic pain and hyperalgesia over the course of their lives [4]. The culprit of chronic pelvic pain in IC/PBS remains unclear, and progression in the management of pain from this disease has been slow. Pelvic pain in IC/PBS is generally diffuse on the body surface referred from the bladder as a result of the convergence of visceral and somatic sensory fibers in the central nervous system. In the present study, we focused on the allodynia phenotype in the UPK3A-induced mouse model of EAC. By assessing tactile allodynia at multiple time points, we showed that EAC mice develop early and chronic pain similar to the behavior in the pelvic region previously reported [1]. The persistent increase in the number of inflammatory cells in the bladder tissue is consistent with findings of increased inflammatory cells or markers in many preclinical and clinical studies involving IC/PBS [2,14,15]. It is still not clear why inflammatory cells migrate to, proliferate, and are activated in the bladder in patients with IC/PBS. However, our results suggest that chronic tactile allodynia in the EAC mouse model may be mediated through recruitment of inflammatory cells and secreted cytokines, including TNF-α and NGF.
Opioids are the most widely used medication for pain-related symptoms in IC/PBS worldwide. However, the potential problems of drug abuse and drug safety are serious issues for health care providers. According to a previous report, opioids kill more than 42,000 people each year in the United States, which accounts for about two-thirds of all overdose deaths involving opioids, cocaine, and psychostimulants [16]. It is important to recognize potential nondrug therapies for treating chronic pain, including IC/PBS. Our results show clear pain-alleviating effects and recovery of bladder function by LESW in an EAC animal model. These effects are concomitant with the control of local and systemic inflammation and reduced synthesis and secretion of TNF-α and NGF. As a physical therapy, LESW could exert a “cavitation effect,” which induces lots of “micro-bubbles” in local tissue that might generate “shear stress” on the cell membrane, neural axon, extracellular matrix, and cell skeleton system or related signaling pathways, such as the actin-integrin-fibronectin-collagen signaling pathway, SDF-1-CXCR4 signaling pathway, and NGF-p75NTR signaling pathway [9,17,18,19]. Among these, NGF is the most widely studied factor in IC/PBS. NGF was thought to be responsible for hyperalgesia and was assumed to act via a direct effect on sensorial nerve fiber endings or increasing sensorial neuropeptides, substance-P, or calcitonin-gene-related peptide [2]. A decrease in the NGF level could be correlated with treatment response in some patients with IC/PBS [20], and in this study, the therapeutic effects of LESW were also accompanied by a decreased level of NGF.
In fact, neuromodulation by physical therapy has shown tremendous advancements since 1967, when researchers reported pain relief following electrical stimulation in patients with chronic neuropathic pain [21]. The first study focused on nerve fibers and neuronal function in IC/PBS was conducted by Hand in 1949 [22]. An increase in the amount of nerve fibers and neural factors such as substance-P in IC/PBS has been reported by several researchers [23,24,25]. Nociceptive input is believed to be carried via small-diameter fibers. According to the most famous “gate control theory” [26], we believe that LESW causes stimulation of non-nociceptive fibers, which close the “gates” to nociceptive input that is competitively regulated at the spinal cord dorsal horn laminae. This mechanism is similar to that underling the therapeutic effect of peripheral nerve stimulation, electroacupuncture, and rubbing or massage for the management of chronic pain [27]. Besides, LESW might also induce blockade of cell membrane depolarization that prevents the propagation of axon conduction for nociceptive input in IC/PBS. It should also be noted that pain perception is a product of the brain's analyzing of afferent inputs, which is a complex process that involves local and systemic inflammation and numerous sensory and affective/cognitive components [28,29,30]. The specific mechanism underling the pain relief provided by LESW for the management of IC/PBS require further study.
CONCLUSIONS
LESW treatment attenuated pain and bladder dysfunction in a UPK3A-induced experimental mouse model of autoimmune cystitis. Local and systemic inflammatory responses were partially controlled with a reduced number of infiltrated inflammatory cells and reduced levels of inflammatory cytokines, including TNF-α and NGF.
ACKNOWLEDGMENTS
We thank Dr. Liguo Zhang from the Institute of Biophysics, Chinese Academy of Sciences, for help in establishing the IC/PBS animal model.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Bicer F, Altuntas CZ, Izgi K, Ozer A, Kavran M, Tuohy VK, et al. Chronic pelvic allodynia is mediated by CCL2 through mast cells in an experimental autoimmune cystitis model. Am J Physiol Renal Physiol. 2015;308:F103–F113. doi: 10.1152/ajprenal.00202.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tonyali S, Ates D, Akbiyik F, Kankaya D, Baydar D, Ergen A. Urine nerve growth factor (NGF) level, bladder nerve staining and symptom/problem scores in patients with interstitial cystitis. Adv Clin Exp Med. 2018;27:159–163. doi: 10.17219/acem/69231. [DOI] [PubMed] [Google Scholar]
- 3.Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186:540–544. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Link CL, Pulliam SJ, Hanno PM, Hall SA, Eggers PW, Kusek JW, et al. Prevalence and psychosocial correlates of symptoms suggestive of painful bladder syndrome: results from the Boston area community health survey. J Urol. 2008;180:599–606. doi: 10.1016/j.juro.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altuntas CZ, Daneshgari F, Sakalar C, Goksoy E, Gulen MF, Kavran M, et al. Autoimmunity to uroplakin II causes cystitis in mice: a novel model of interstitial cystitis. Eur Urol. 2012;61:193–200. doi: 10.1016/j.eururo.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nickel JC, Egerdie B, Davis E, Evans R, Mackenzie L, Shrewsbury SB. A phase II study of the efficacy and safety of the novel oral SHIP1 activator AQX-1125 in subjects with moderate to severe interstitial cystitis/bladder pain syndrome. J Urol. 2016;196:747–754. doi: 10.1016/j.juro.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Dellis AE, Papatsoris AG. Bridging pharmacotherapy and minimally invasive surgery in interstitial cystitis/bladder pain syndrome treatment. Expert Opin Pharmacother. 2018;19:1369–1373. doi: 10.1080/14656566.2018.1505865. [DOI] [PubMed] [Google Scholar]
- 8.Kong XT, Deng FM, Hu P, Liang FX, Zhou G, Auerbach AB, et al. Roles of uroplakins in plaque formation, umbrella cell enlargement, and urinary tract diseases. J Cell Biol. 2004;167:1195–1204. doi: 10.1083/jcb.200406025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Matheu MP, Sun F, Wang L, Sanford MT, Ning H, et al. Low-energy shock wave therapy ameliorates erectile dysfunction in a pelvic neurovascular injuries rat model. J Sex Med. 2016;13:22–32. doi: 10.1016/j.jsxm.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Abe Y, Ito K, Hao K, Shindo T, Ogata T, Kagaya Y, et al. Extracorporeal low-energy shock-wave therapy exerts anti-inflammatory effects in a rat model of acute myocardial infarction. Circ J. 2014;78:2915–2925. doi: 10.1253/circj.cj-14-0230. [DOI] [PubMed] [Google Scholar]
- 11.Al-Abbad H, Simon JV. The effectiveness of extracorporeal shock wave therapy on chronic achilles tendinopathy: a systematic review. Foot Ankle Int. 2013;34:33–41. doi: 10.1177/1071100712464354. [DOI] [PubMed] [Google Scholar]
- 12.Angehrn F, Kuhn C, Voss A. Can cellulite be treated with low-energy extracorporeal shock wave therapy? Clin Interv Aging. 2007;2:623–630. doi: 10.2147/cia.s1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paoloni M, Tavernese E, Cacchio A, D'orazi V, Ioppolo F, Fini M, et al. Extracorporeal shock wave therapy and ultrasound therapy improve pain and function in patients with carpal tunnel syndrome. A randomized controlled trial. Eur J Phys Rehabil Med. 2015;51:521–528. [PubMed] [Google Scholar]
- 14.Done JD, Rudick CN, Quick ML, Schaeffer AJ, Thumbikat P. Role of mast cells in male chronic pelvic pain. J Urol. 2012;187:1473–1482. doi: 10.1016/j.juro.2011.11.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theoharides TC, Kempuraj D, Sant GR. Mast cell involvement in interstitial cystitis: a review of human and experimental evidence. Urology. 2001;57(6 Suppl 1):47–55. doi: 10.1016/s0090-4295(01)01129-3. [DOI] [PubMed] [Google Scholar]
- 16.Seth P, Scholl L, Rudd RA, Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants-United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 2018;67:349–358. doi: 10.15585/mmwr.mm6712a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao F, Chen N, Sun W, Wang B, Shi Z, Cheng L, et al. Combined therapy with shock wave and retrograde bone marrow-derived cell transplantation for osteochondral lesions of the talus. Sci Rep. 2017;7:2106. doi: 10.1038/s41598-017-02378-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang W, He Y, Gan L, Zhang F, Hua B, Yang P, et al. Cardiac shock wave therapy promotes arteriogenesis of coronary micrangium, and ILK is involved in the biomechanical effects by proteomic analysis. Sci Rep. 2018;8:1814. doi: 10.1038/s41598-018-19393-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu JK, Chen HJ, Li XD, Huang ZL, Xu H, Yang HL, et al. Optimal intensity shock wave promotes the adhesion and migration of rat osteoblasts via integrin β1-mediated expression of phosphorylated focal adhesion kinase. J Biol Chem. 2012;287:26200–26212. doi: 10.1074/jbc.M112.349811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu HT, Tyagi P, Chancellor MB, Kuo HC. Urinary nerve growth factor level is increased in patients with interstitial cystitis/bladder pain syndrome and decreased in responders to treatment. BJU Int. 2009;104:1476–1481. doi: 10.1111/j.1464-410X.2009.08675.x. [DOI] [PubMed] [Google Scholar]
- 21.Nayak R, Banik RK. Current innovations in peripheral nerve stimulation. Pain Res Treat. 2018;2018:9091216. doi: 10.1155/2018/9091216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hand JR. Interstitial cystitis; report of 223 cases (204 women and 19 men) J Urol. 1949;61:291–310. doi: 10.1016/S0022-5347(17)69067-0. [DOI] [PubMed] [Google Scholar]
- 23.Christmas TJ, Rode J, Chapple CR, Milroy EJ, Turner-Warwick RT. Nerve fibre proliferation in interstitial cystitis. Virchows Arch A Pathol Anat Histopathol. 1990;416:447–451. doi: 10.1007/BF01605152. [DOI] [PubMed] [Google Scholar]
- 24.Pang X, Marchand J, Sant GR, Kream RM, Theoharides TC. Increased number of substance P positive nerve fibres in interstitial cystitis. Br J Urol. 1995;75:744–750. doi: 10.1111/j.1464-410x.1995.tb07384.x. [DOI] [PubMed] [Google Scholar]
- 25.Hohenfellner M, Nunes L, Schmidt RA, Lampel A, Thüroff JW, Tanagho EA. Interstitial cystitis: increased sympathetic innervation and related neuropeptide synthesis. J Urol. 1992;147:587–591. doi: 10.1016/s0022-5347(17)37314-7. [DOI] [PubMed] [Google Scholar]
- 26.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 27.Ulloa L, Quiroz-Gonzalez S, Torres-Rosas R. Nerve stimulation: immunomodulation and control of inflammation. Trends Mol Med. 2017;23:1103–1120. doi: 10.1016/j.molmed.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Research Council (US) Committee on Recognition and Alleviation of Pain in Laboratory Animals; National Academy of Sciences (US), editors The National Academies collection: reports funded by National Institutes of Health. Washington, DC: National Academies Press; 2009. Recognition and alleviation of pain in laboratory animals. [PubMed] [Google Scholar]
- 29.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Almeida TCDC, Figueiredo FWDS, Barbosa Filho VC, de Abreu LC, Fonseca FLA, Adami F. Effects of transcutaneous electrical nerve stimulation (TENS) on proinflammatory cytokines: protocol for systematic review. Syst Rev. 2017;6:139. doi: 10.1186/s13643-017-0532-5. [DOI] [PMC free article] [PubMed] [Google Scholar]