Abstract
Objectives
Delirium is a common acute cognitive impairment syndrome among intensive care unit (ICU) patients. This study was aimed to investigate the incidence, risk factors, and cumulative risk of delirium among ICU patients.
Methods
A case-control study including clinical records of 452 patients were retrospectively analyzed. Delirium was assessed using the Confusion Assessment Method for the ICU and Richmond Agitation–Sedation Scale.
Results
We found that 163 out of the 452 patients (36.1%) had delirium. Multivariate analysis showed that use of sedatives, length of ICU hospitalization, and physical restraint were independent risk factors for delirium. The additive effect of all three factors resulted to an odds ratio of 30.950.
Conclusion
The incidence of delirium remained high. Thus, nurses shall strengthen the monitoring of delirium, regularly access the patient's level of calmness, and limit the use of physical restraint.
Keywords: Delirium, Intensive care units, Risk factors, Cumulative risk, Case-control studies
What is known?
-
●
Delirium is a common acute cognitive impairment syndrome among intensive care unit (ICU) patients. Clarifying the incidence of delirium and its major risk factors would help its prevention.
-
●
Few studies differentiated the factors of delirium into modifiable and unmodifiable ones. The cumulative effect of the factors has not been well established.
What is new?
-
●
Despite the increased concern on delirium and the implementation of prevention strategies, the incidence of ICU delirium remained high.
-
●
Physical restraint, use of sedative drugs, and the length of ICU hospitalization for ≥7 days would lead to a cumulative odds ratio value of up to 30.950 for delirium.
-
●
Nurses shall strengthen delirium monitoring, regularly access the patient's calm level, and limit the use of physical restraint to reduce the occurrence of delirium.
1. Introduction
Delirium is a group of acute cognitive impairment syndromes, mainly manifested as acute changes in mental state or repeated fluctuations, inattention, and disorganized thinking [1]. It is most commonly witnessed in intensive care unit (ICU). Thus, it is also called ICU delirium. Delirium not only causes immediate adverse effects, such as prolonged ICU hospitalization and increased medical costs [2,3], but also adversely affects the patient's health and quality of life in the long term. A multicenter study of 360 ICU patients showed remarkable memory impairment in the delirium group compared with the non-delirium group 6 months after discharge [4]. A recent prospective cohort study showed that 80% and 78% of the delirium patients were readmitted 30 and 180 days after discharge, respectively; 6 out of the 8 patients that died in 180 days had delirium [5].
Health care staff had greatly deepened their understanding of delirium over the last decade. Most ICU staff regard delirium as a common and serious ICU problem [6,7], and the evaluation tools, risk factors, clinical outcome, and prevention strategies were investigated to varying degrees. The Pain, Agitation, and Delirium Clinical Practice Guidelines released by the American College of Critical Care Medicine emphasized the early prevention of delirium [8]. Although the necessity to assess, prevent, and treat delirium has been well recognized by health care workers, their implementation proves problematic, and the incidence of delirium in ICU remains high [9,10]. This study seeks to explore the modifiable risk factors to provide basis for the effort to reduce the incidence of delirium and the development of prevention strategies to improve the clinical outcome of delirium patients and reduce the economic burden of patients and medical costs. A single-center prospective cohort study demonstrated that ICU stay and mechanical ventilation were independent predictors of delirium [11]. Mori et al. [12] found that age and sedative–analgesic medication could induce delirium. A prospective observational study of 120 patients also demonstrated that history of hypertension, carotid artery disease, length of ICU hospitalization, and postoperative pain were independent risk factors for delirium [13]. Most previous studies have investigated the risk factors of delirium based on patients’ baseline data, treatments, and drugs [[14], [15], [16]], whereas few had differentiated the factors into modifiable and unmodifiable ones, and fewer focused on modifiable environmental factors, such as ICU settings, isolation, and physical restraint. Yet, only strategies that aims at modifiable factors are effective in preventing delirium. Delirium can be caused by a single factor, but more commonly for the cumulative effect of multiple factors [17]. Previous studies used multivariate regression to obtain the independent risk factors of delirium. However, few studies have investigated the cumulative risk of these factors.
Therefore, this study retrospectively explored the incidence of ICU delirium. Moreover, we differentiated the risk factors into modifiable and unmodifiable ones and analyzed the cumulative risk of several combination of factors for the targeted prevention of delirium and to provide reference for the development of prevention strategies.
2. Methods
2.1. Study design
Single-center case-control study.
2.2. Setting and participants
The patients included in this study were admitted to the comprehensive ICU of the Affiliated Hospital of Zunyi Medical University, which serves as a teaching hospital and medical center of a province from Jun. 2016 to Apr. 2017. The inclusion criteria were: age ≥18 years and length of ICU hospitalization ≥24 hours. Exclusion criteria were: deep coma, history of mental illness, nervous system diseases, and brain injury. The subjects were divided into a delirium group that had delirium during ICU hospitalization and a non-delirium group that did not suffer from delirium.
2.3. Delirium assessment
Richmond Agitation-Sedation Scale (RASS) was used to evaluate once every 8 hours, and the Confusion Assessment Method for the ICU (CAM-ICU) was used to examine once a day by a trained nurse until the death or transfer of the patient. CAM-ICU assesses four characteristics: (1) acute changes or fluctuations in the state of consciousness, (2) inattention, (3) changes in consciousness level, and (4) thinking disorder. Patients with (1), (2), plus (3) or (4) were diagnosed with delirium. RASS was a 10-level scale that ranges from unarousable (−5) to combativeness (+4), which assesses the subclass of delirium. The 10-level scale represented coma, severe sedation, moderate sedation, light sedation, lethargy, wakefulness and calmness, restlessness, agitation, extreme agitation, and aggressiveness, respectively. Patients with a RASS between +1 and +4 were diagnosed with hyperactive delirium, those between −3 and 0 were diagnosed with hypoactive delirium, and those whose scores fluctuated between the positive and negative scores were identified as mixed delirium [18].
2.4. Data collection
The study has been approved by the Hospital Medical Ethics Committee (Number: 2016 Ethical Review No. 6). Data were collected by referring to electronic medical and nursing records. To enable medical workers to promote a general understanding of the risk factors for delirium and focus on intervenable, the collected data were divided into 2 categories Unmodifiable factors, include (1) basic characteristics, such as age, gender, Acute Physiology and Chronic Health Evaluation Ⅱ (APACHE Ⅱ) within 24 hours after admission, smoking, drinking, and living alone; and (2) chronic pathology, such as hypertension, diabetes, and cardiovascular history. Modifiable factors include (1) acute disease conditions, such as mechanical ventilation, sedative–analgesic medication, fever, length of ICU hospitalization, and the number of intubations; and (2) environmental factors, such as ICU setting, isolation, and physical restraint.
2.5. Statistical analysis
SPSS18.0 was used for statistical analysis. Quantitative variables were represented as mean and standard deviation (SD) or median and inter-quartile range (IQR). Categorical variables were represented as frequency and percentage. The data were analyzed using t-test, Mann–Whitney U test, test, receiver operating characteristic (ROC) curve, and forward multivariate logistic regression. P < 0.05 was considered statistically significant.
3. Results
A total of 452 patients were included in the study, in which 163 patients were confirmed to have delirium, and the corresponding incidence rate was 36.1%. The incidence of hyperactive, hypoactive, and mixed delirium was 44.2%, 43.5%, and 12.3%, respectively. During hospitalization in ICU, 163 delirium patients (without the presence of delirium upon ICU admission) were included in the delirium group, and 289 patients without the occurrence of delirium were included in the non-delirium group.
Table 1 shows the unmodifiable and modifiable factors of the delirium and non-delirium groups. The APACHE II score of the unmodifiable factors in the delirium group was greater than that in the non-delirium group (t = 2.12, P = 0.034). The rates of mechanical ventilation (= 18.34, P < 0.001), sedative (= 28.04, P < 0.001), analgesic (= 12.18, P < 0.001), and physical restraint (= 32.98, P < 0.001) of the modifiable factors in the delirium group were all higher than those in the non-delirium group, length of ICU hospitalization (Z = −6.77, P < 0.001) was longer, and more intubations were applied (t = −2.40, P = 0.017).
Table 1.
Unmodifiable and modifiable risk factors of delirium group and non-delirium group (n = 452).
| Variable | Delirium group (n = 163) |
Non-delirium group (n = 289) |
/t/Z | P | ||
|---|---|---|---|---|---|---|
| n (%) | MeanSD/Median (IQR) | n (%) | MeanSD/Median (IQR) | |||
| Unmodifiable factors | ||||||
| Age≥65 years | 70 (42.9) | 128 (44.3) | 0.08 | 0.782 | ||
| Male | 116 (71.2) | 184 (63.7) | 2.63 | 0.105 | ||
| Drinking | 44 (27.0) | 72 (24.9) | 0.24 | 0.627 | ||
| Smoking | 55 (33.7) | 91 (31.5) | 0.24 | 0.623 | ||
| Living alone | 10 (6.1) | 16 (5.5) | 0.07 | 0.793 | ||
| Transferred in after surgery | 42 (32.8) | 86 (29.8) | 0.82 | 0.366 | ||
| APACHE Ⅱ score | 16.66.2 | 15.27.0 | 2.12 | 0.034 | ||
| Hypertension | 29 (17.8) | 55 (19.0) | 0.11 | 0.745 | ||
| Diabetes | 13 (8.0) | 23 (8.0) | 0.01 | 0.995 | ||
| Cardiovascular disease | 5 (3.1) | 17 (5.9) | 1.78 | 0.182 | ||
| Modifiable factors | ||||||
| Mechanical ventilation | 148 (90.8) | 214 (74.0) | 18.34 | <0.001 | ||
| Sedative | 143 (87.7) | 187 (64.7) | 28.04 | <0.001 | ||
| Analgesic | 128 (78.5) | 181 (62.6) | 12.18 | <0.001 | ||
| Fever | 97 (59.5) | 147 (50.9) | 3.14 | 0.077 | ||
| Length of ICU hospitalization (day) | 9 (6–15) | 6 (4–10) | −6.77 | <0.001 | ||
| Number of intubations | 5.31.5 | 4.91.7 | −2.40 | 0.017 | ||
| Isolation | 8 (4.9) | 20 (6.9) | 0.73 | 0.394 | ||
| Physical restraint | 155 (95.1) | 211 (73.0) | 32.98 | <0.001 | ||
| Open environment | 29 (17.8) | 66 (22.8) | 1.60 | 0.206 | ||
| Visibility of clock | 28 (17.2) | 43 (14.9) | 0.42 | 0.519 | ||
| Visibility of sun light | 51 (31.3) | 104 (36.0) | 1.02 | 0.312 | ||
Note: IQR, inter-quartile range; APACHE Ⅱ, acute physiology and chronic health evaluation Ⅱ; ICU, intensive care unit.
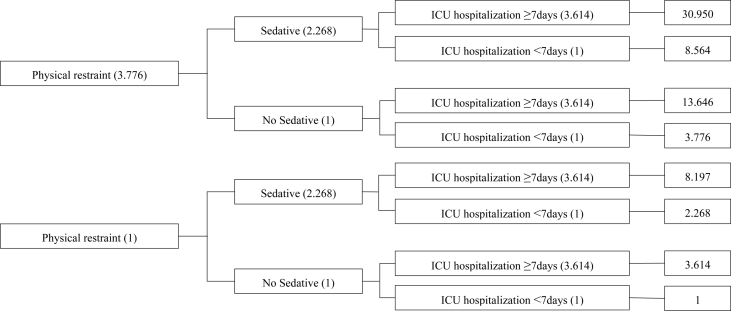
Forward logistic regression analysis was used to screen out the risk factors for delirium. With occurrence of delirium as the independent variable, potential risk factors, including APACHE Ⅱ score, mechanical ventilation, sedative, analgesic, length of ICU hospitalization, number of intubations, and physical restraint, were tested in the regression model. The variables were screened in the order of length of ICU hospitalization, physical restraint, and use of sedative. ROC curve analysis was performed to determine the optimum cutoff point of the length of stay in ICU during hospitalization. The optimum cutoff point with respect to the length of ICU hospitalization was estimated to be 7 days, and the corresponding sensitivity and specificity were 71.2% and 60.2%, respectively. Physical restraint, sedative, and the length of ICU hospitalization for 7 days entered the logistic regression model again, and the results were presented in Table 2. The overall accuracy of the model was 71.5%. From the result of the model, the odds ratio (OR) of delirium when the physical restraint was used was 3.776. OR was increased to 8.564 (3.776 × 2.268) when the patient used sedative again, and it surged to 30.950 (3.776 × 2.268 × 3.614) when the patient stayed in ICU for over 7 days. The risk of delirium was illustrated in Fig. 1 by means of cumulative multiple factor analysis.
Table 2.
Multivariate regression analysis of risk factors for delirium.
| Step | Risk factor | SE | Wald | P - value | Exp () | 95%CI | |
|---|---|---|---|---|---|---|---|
| 1 | Length of ICU hospitalization ≥ 7days | 1.318 | 0.211 | 39.151 | <0.001 | 3.374 | 2.472–5.642 |
| Constant | −1.309 | 0.164 | 63.398 | <0.001 | 0.270 | ||
| 2 | Physical restraint | 1.894 | 0.394 | 23.147 | <0.001 | 6.644 | 3.072–14.369 |
| Length of ICU hospitalization ≥ 7days | 1.265 | 0.217 | 33.883 | <0.001 | 3.543 | 2.314–5.424 | |
| Constant | −2.921 | 0.402 | 52.818 | <0.001 | 0.054 | ||
| 3 | Sedative | 0.819 | 0.324 | 34.282 | 0.012 | 2.268 | 1.201–4.284 |
| Physical restraint | 1.329 | 0.447 | 6.372 | 0.003 | 3.776 | 1.571–9.074 | |
| Length of ICU hospitalization ≥ 7days | 1.285 | 0.219 | 8.824 | <0.001 | 3.614 | 2.351–5.556 | |
| Constant | −3.080 | 0.408 | 56.992 | <0.001 | 0.046 |
Note: ICU, intensive care unit; CI, confidence interval.
Forward logistic regression model: accuracy of model 71.5%; Cox and Snell R2, 0.165; Nagelkerk R2, 0.227.
In ([P of delirium]/[P of non-delirium]) = (constant) + (physical restraint) + (sedative) + (length of ICU hospitalization).
That is, OR (delirium) = e β(constant) × e β(physical restraint) × e β(sedative) × e β(length of ICU hospitalization) = e −3.080 × e 1.329 (physical restraint) × e 0.819 (sedative) × e 1.285 (length of ICU hospitalization). In logistic regression model, 0 for length of ICU hospitalization less than 7 days, no physical restraint, and without sedative; 1 for length of ICU hospitalization of 7 days or greater, with physical restraint, and with sedative.
Fig. 1.
Cumulative multiple factor analysis of delirium. Note: ICU, intensive care unit.
4. Discussion
In this study, the incidence rate of delirium was 36.1%, which is higher than that in Shaughnessy's study (21%) [19] but lower than that by Mori et al. (46.3%) [12]. The low incidence of delirium in Shaughnessy [19] may be partially due to the short duration of the study (only 6 weeks; thus, the number of patients enrolled were limited) and low rate of delirium assessment conducted by the medical staff. Notably, in Mori et al. [12], only patients with previous history of cognitive impairment were excluded in the process of inclusion and exclusion, patients with neurological diseases and traumatic brain injuries were involved in their study, both of which were liable to induce the occurrence of delirium. A possible reason might be that patients with neurological diseases and traumatic brain injuries were excluded from our study, thereby resulting in relatively lower incidence of delirium when compared with that in the investigation of Mori et al. [12]. In addition, in their study, delirium was assessed in patients every 12 h within the first 5 days from entering ICU and every 24 h for patients who developed delirium after 5 days of stay in ICU. Patients without the onset of delirium in ICU were assessed with a 24-h interval, and the assessment was finished until the transfer or death of the patient. By comparison, our study evaluates per day, thereby also contributing to the underestimation of delirium.
The incidence of hyperactive delirium (44.2%) was the highest, followed by hypoactive delirium and mixed delirium, which were consistent with previous studies [13,20]. The most obvious reason for this result was caused by patients with hyperactive delirium, who were characterized by increased activity, restlessness, alertness, or combined with aggressive behavior, indicating relatively high clinical recognition. By contrast, patients with hypoactive delirium were less detectable due to the clinical manifestations of lethargy, drowsiness, and decreased reactivity [18].
Furthermore, APACHE II score, which is an unmodifiable factor, was higher in the delirium group than in the non-delirium group, indicating that delirium was associated with serious conditions. Among the modifiable factors, the use of mechanical ventilation, use of sedative-analgesic medication, length of ICU hospitalization, physical restraint, and number of intubations were significantly increased in the delirium group compared with the non-delirium group (Table 1). No statistically significant differences were detected between the delirium and non-delirium patients in fever, smoking, and history of hypertension, which were commonly recognized as risk factors for delirium. This result may be associated with the characteristics of the population, the department from which the patient was transferred in, memory bias, and the limited strength of a single-center case-control study.
Multivariate analysis showed that the use of sedatives was an independent risk factor for delirium (Table 2), which was consistent with previous findings [21,22]. The clinical practice guidelines for the pain management, agitation, and delirium recommended dexmedetomidine in place of benzodiazepines for sedating ICU patients [8]. The sedatives used in this study included midazolam, dexmedetomidine, and propofol, and approximately 2/3 of the patients used midazolam and benzodiazepine, which induce delirium [23]. This study found that sedatives are high-risk factors for delirium, possibly due to the extensive use of benzodiazepines for sedation. Currently, the mechanism of sedation-induced delirium has not been clarified. However, excessive use of sedative drugs is speculated to cause prolonged over-inhibition of the central nervous system, which disrupts the neurotransmitter system and leads to delirium. Thus, medical workers should try to avoid benzodiazepines and reduce the dose of sedatives to achieve mild sedation as long as safety of the patient is ensured [24]. In addition, ICU patients on sedative drugs shall be woken up daily to reduce the incidence of delirium.
According to the regression model, physical restraint was the strongest independent risk factor for delirium with an OR of 3.776 (Table 2). Previous studies have also identified physical restraint as a risk factor for delirium. A cohort study of 523 patients enrolled in four hospitals found that the physical restraint was a risk factor for delirium (OR, 33.84; 95%CI, 11.19–102.36; P < 0.001) [25]. A multicenter study of 420 patients also found that physical restraint was an independent predictor of delirium (OR, 1.87; 95%CI, 1.33–2.63; P < 0.001) [26]. Physical restraint places the patient in the same posture for a long time, thereby causing sleep–wake cycle disorders and melatonin secretion abnormalities and lead to the occurrence of delirium. Restraint also causes anger, irritability, and other negative emotions, thereby leading to neurotransmitter balance disorders and increased risk of delirium. Therefore, nurses need to minimize the use of physical restraint, even after a full assessment of physical, psychological condition of the patient, medical equipment, and the environment when necessary [27].
This study found that length of ICU hospitalization is an independent risk factor for delirium, and the length of ICU hospitalization ≥7 days is the best cut-off value for delirium. A previous single-center cohort study also concluded that the length of ICU hospitalization was longer in the delirium group than in the non-delirium group (OR, 1.91; 95%CI, 1.22–3.00; P < 0.05) [11].
The condition of ICU patients is generally critical, and the occurrence of delirium is often multifactorial. Many studies had investigated the incidence, risk factors, and clinical outcomes of delirium in ICU patients, but few had probed into the cumulative risk of delirium after investigating the independent risk factors. Cumulative risk analysis suggested that if a patient was physically restrained, took sedative drugs, and stayed in ICU for ≥7 days, then the OR value for delirium would reach up to 30.950 (Fig. 1). Therefore, medical personnel shall carry out routine assessment; remember the risk factors and the synergy of multiple factors; and take effective interventions, such as prudent use of physical restraints, reducing the frequency and duration of restraints, adjusting the sedation plan, and shortening ICU duration, as early as possible, thereby reducing the incidence of delirium.
5. Limitation of the study
This study has several limitations. First, delirium is mainly assessed by the nurses without the participation of clinicians, and the assessment was conducted only once a day, which may lead to underestimation of the incidence of delirium. Second, the study only involved risk factors that were considered important, and other potential factors might have been overlooked. Third, the patients were not followed up for long-term survival, re-hospitalization, and mortality after discharge.
6. Conclusion and recommendations
In ICU, 36.1% of the patients had delirium. If a patient was physically restrained, took sedative drugs, and stayed in ICU for ≥7 days, then the cumulative effect would lead to an OR of up to 30.950. Nurses shall strengthen the monitoring of delirium, regularly access the patient's calm level, and limit the use of physical restraint after the patient is transferred to ICU to reduce the occurrence of delirium.
Funding
This research was supported by the Education Humanities and Social Sciences Project of Guizhou Province (2015SSD19).
Conflicts of interest
The authors have no conflict of interest to declare.
Authors’ contribution
Jiang ZX and Pan YB conceived and designed the study; Pan YB, Zhang JJ and Yang KH performed the progress; Pan YB and Yan JL analyzed the data; Pan YB, Yan JL and Luo JY wrote the manuscript.
Footnotes
Peer review under responsibility of Chinese Nursing Association.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijnss.2019.05.008.
Contributor Information
Yanbin Pan, Email: 1114631714@qq.com.
Jianlong Yan, Email: 935428653@qq.com.
Zhixia Jiang, Email: jzxhl@126.com.
Jianying Luo, Email: hdryhIb@163.com.
Jingjing Zhang, Email: 919736053@qq.com.
Kaihan Yang, Email: 124504446@qq.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Malik A., Harlan T., Cobb J. Stop. Think. Delirium! A quality improvement initiative to explore utilising a validated cognitive assessment tool in the acute inpatient medical setting to detect delirium and prompt early intervention. J Clin Nurs. 2016;25(21–22):3400–3408. doi: 10.1111/jocn.13166. [DOI] [PubMed] [Google Scholar]
- 2.Abelha F.J., Luis C., Veiga D., Parente D., Fernandes V., Santos P. Outcome and quality of life in patients with postoperative delirium during an ICU stay following major surgery. Crit Care. 2013;17(5):R257. doi: 10.1186/cc13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang W.Y., Wu W.L., Gu J.J., Sun Y., Ye X.F., Qiu W.J. Risk factors for postoperative delirium in patients after coronary artery bypass grafting: a prospective cohort study. J Crit Care. 2015;30(3):606–612. doi: 10.1016/j.jcrc.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Svenningsen H., Tonnesen E.K., Videbech P., Frydenberg M., Christensen D., Egerod I. Intensive care delirium - effect on memories and health-related quality of life - a follow-up study. J Clin Nurs. 2014;23:634–644. doi: 10.1111/jocn.12250. [DOI] [PubMed] [Google Scholar]
- 5.Eide L.S., Ranhoff A.H., Fridlund B., Haaverstad R., Hufthammer K.O., AUID- Oho Readmissions and mortality in delirious versus non-delirious octogenarian patients after aortic valve therapy: a prospective cohort study. BMJ open. 2016;6(10) doi: 10.1136/bmjopen-2016-012683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glynn L., Corry M. Intensive care nurses' opinions and current practice in relation to delirium in the intensive care setting. Intensive Crit Care Nurs. 2015;31(5):269–275. doi: 10.1016/j.iccn.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Trogrlic Z., Ista E., Ponssen H.H., Schoonderbeek J.F., Schreiner F., Verbrugge S.J. Attitudes, knowledge and practices concerning delirium: a survey among intensive care unit professionals. Nurs Crit Care. 2017;22(3):133–140. doi: 10.1111/nicc.12239. [DOI] [PubMed] [Google Scholar]
- 8.Barr J., Fraser G.L., Puntillo K., Ely E.W., Gelinas C., Dasta J.F. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 9.Ozsaban A., Acaroglu R. Delirium assessment in intensive care units: practices and perceptions of Turkish nurses. Nurs Crit Care. 2016;21(5):271–278. doi: 10.1111/nicc.12127. [DOI] [PubMed] [Google Scholar]
- 10.Saller T., V.D.V., Hofmann-Kiefer K. [Knowledge and implementation of the S3 guideline on delirium management in Germany] Anaesthesist. 2016;65(10):755–762. doi: 10.1007/s00101-016-0218-8. [DOI] [PubMed] [Google Scholar]
- 11.Norkiene I., Ringaitiene D., Kuzminskaite V., Sipylaite J. Incidence and risk factors of early delirium after cardiac surgery. BioMed Res Int. 2013:2013323491. doi: 10.1155/2013/323491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mori S., Takeda J.R., Carrara F.S., Cohrs C.R., Zanei S.S., Whitaker I.Y. Incidence and factors related to delirium in an intensive care unit. Rev Esc Enferm USP. 2016;50(4):587–593. doi: 10.1590/S0080-623420160000500007. [DOI] [PubMed] [Google Scholar]
- 13.Kumar A.K., Jayant A., Arya V.K., Magoon R., Sharma R. Delirium after cardiac surgery: a pilot study from a single tertiary referral center. Ann Card Anaesth. 2017;20(1):76–82. doi: 10.4103/0971-9784.197841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pipanmekaporn T., Chittawatanarat K., Chaiwat O., Thawitsri T., Wacharasint P., Kongsayreepong S. Incidence and risk factors of delirium in multi-center Thai surgical intensive care units: a prospective cohort study. J Intensive Care. 2015;3(1):53. doi: 10.1186/s40560-015-0118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huai J., Ye X. A meta-analysis of critically ill patients reveals several potential risk factors for delirium. Gen Hosp Psychiatry. 2014;36(5):488–496. doi: 10.1016/j.genhosppsych.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Kubota K., Suzuki A., Ohde S., Yamada U., Hosaka T., Okuno F. Age is the most significantly associated risk factor with the development of delirium in patients hospitalized for more than five days in surgical wards: retrospective cohort study. Ann Surg. 2018;267(5):874–877. doi: 10.1097/SLA.0000000000002347. [DOI] [PubMed] [Google Scholar]
- 17.Inouye S.K., Westendorp R.G., Saczynski J.S. Delirium in elderly people. Lancet. 2014;383(9920):911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McPherson J.A., Wagner C.E., Boehm L.M., Hall J.D., Johnson D.C., Miller L.R. Delirium in the cardiovascular ICU: exploring modifiable risk factors. Crit Care Med. 2013;41(2):405–413. doi: 10.1097/CCM.0b013e31826ab49b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaughnessy L. Introducing delirium screening in a cardiothoracic critical care unit. Nurs Crit Care. 2013;18(1):8–13. doi: 10.1111/j.1478-5153.2012.00514.x. [DOI] [PubMed] [Google Scholar]
- 20.Lahariya S., Grover S., Bagga S., Sharma A. Phenomenology of delirium among patients admitted to a coronary care unit. Nord J Psychiatr. 2016;70(8):626–632. doi: 10.1080/08039488.2016.1194467. [DOI] [PubMed] [Google Scholar]
- 21.Pisani M.A., Murphy T.E., Araujo K.L., Slattum P., Van Ness P.H., Inouye S.K. Benzodiazepine and opioid use and the duration of intensive care unit delirium in an older population. Crit Care Med. 2009;37(1):177–183. doi: 10.1097/CCM.0b013e318192fcf9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svenningsen H., Tonnesen E. Delirium incidents in three Danish intensive care units. Nurs Crit Care. 2011;16(4):186–192. doi: 10.1111/j.1478-5153.2011.00421.x. [DOI] [PubMed] [Google Scholar]
- 23.Zaal I.J., Devlin J.W., Hazelbag M., Klein K.P.M., van der Kooi A.W., Ong D.S. Benzodiazepine-associated delirium in critically ill adults. Intensive Care Med. 2015;41(12):2130–2137. doi: 10.1007/s00134-015-4063-z. [DOI] [PubMed] [Google Scholar]
- 24.Kanova M., Sklienka P., Roman K., Burda M., Janoutova J. Incidence and risk factors for delirium development in ICU patients - a prospective observational study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2017;161(2):187–196. doi: 10.5507/bp.2017.004. [DOI] [PubMed] [Google Scholar]
- 25.Van Rompaey B., Elseviers M.M., Schuurmans M.J., Shortridge-Baggett L.M., Truijen S., Bossaert L. Risk factors for delirium in intensive care patients: a prospective cohort study. Crit Care. 2009;13(3):R77. doi: 10.1186/cc7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta S., Cook D., Devlin J.W., Skrobik Y., Meade M., Fergusson D. Prevalence, risk factors, and outcomes of delirium in mechanically ventilated adults. Crit Care Med. 2015;43(3):557–566. doi: 10.1097/CCM.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 27.Park M., Tang J.H. Changing the practice of physical restraint use in acute care. J Gerontol Nurs. 2007;33(2):9–16. doi: 10.3928/00989134-20070201-04. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.