Graphical abstract
Abbreviations: FOSHU, Foods for Specified Health Uses; FNFC, Foods with Nutrient Functional Claim; CAA, Consumer Affairs Agency; FFC, Foods with Function Claims; E2, 17β-estradiol; HPLC, high-performance liquid chromatography; ORAC, oxygen radical absorption capacity; CYP, cytochrome P-450; BMD, bone mineral density; TE, Trolox equivalent; DGL, deglycyrrhizin
Keywords: Licorice, Herbal medicines, Health foods, Safety assessment, Estrogenic activity, Cytochrome P-450 (CYP)
Highlights
-
•
The contents of glabridin and medical component glycyrrhizin were analyzed in herbal medicines and health foods containing licorice.
-
•
A small amount of glycyrrhizin was detected in some of health foods.
-
•
Estrogenic activity was detected in some of health food ingredients and health foods.
-
•
Antioxidant activity was detected in all the materials.
-
•
Assessment of hepatic CYP activity, liver and uterine weights in estrogen deficient mice was useful for evaluating the safety of estrogenic ingredients.
Abstract
Focusing on licorice, a highly used raw material in health foods, quantitative analysis of functional/medicinal components and a safety and functional evaluation was carried out for herbal medicines, health food ingredients, and so-called health foods. A functional component, glabridin, was detected in herbal medicines from Glycyrrhiza glabra and G. inflata, health food ingredients, and in commercially available health foods that contain licorice. Likewise, glycyrrhizin, a medicinal component, was detected in these sources, except in licorice oil extract. Estrogen activity in vitro was detected in some of the herbal medicines, health food ingredients, and in health foods containing licorice. In the in vivo study, liver weight in ovariectomized (OVX) mice treated with licorice oil extract was significantly higher than that in OVX and sham mice in a dose dependent manner. These results suggest that excessive intake of licorice oil extract from health foods should be avoided, even though these ingredients might be beneficial for medical use in order to maintain bone health in postmenopausal women. Measurement of hepatic cytochrome P-450 (CYP) activity, reproductive organ weight, and fat and bone mass in OVX mice was considered useful for evaluating the safety and efficacy of estrogenic health food ingredients derived from herbal medicines.
1. Introduction
In recent years, many functional foods with indicated health claims such as Foods for Specified Health Uses (FOSHU) and Foods with Nutrient Function Claim (FNFC) have become available in Japan. FOSHU is the functional foods that have been approved by the Consumer Affairs Agency (CAA) and are labeled with the physiological effects of the principal ingredients on the human body 1. FNFC are used as supplements or to complement the daily nutrient requirements such as vitamins and minerals, which tend to be insufficient in daily diet 2. Furthermore, in line with the enforcement of Food Labeling Standards, based on the Food Labeling Act of 2015, Foods with Function Claims (FFC) was released by the CAA, which permits the labeling of structure and function claims under the industry’s own responsibility 3. However, there are many so-called health foods other than FOSHU, FNFC, and FFC, in the Japanese market, which have still not undergone proper efficacy and safety evaluations. In particular, products containing herbal ingredients may be inappropriate for use and labeling. Therefore, the safety evaluation of these herbal ingredients and establishment of an appropriate quality evaluation method are considered urgent for the proper verification of the safety and efficacy of these so-called health foods. Based on this background, we aimed to assemble the quality evaluation methods necessary for verifying the safety and efficacy of functional foods, a food category that is only expected to increase in the future.
Licorice (Glycyrrhiza uralensis, G. glabra, and G. inflata) is a perennial of the legume family, distributed from East Asia to Europe. It contains glycyrrhizin and other functional components and is used in both pharmaceuticals and health foods. In the Japanese Pharmacopoeia, products containing not less than 2% of glycyrrhizin as dried matter from the roots and stolons of G. uralensis and G. glabra are used as crude drugs 4. In the Chinese Pharmacopoeia, the underground part of G. inflata is also included as a drug 5. As for the medicinal effects, licorice is reported to show anti-tussive, anti-digestive gastric ulcer, anti-allergic, and estrogenic activity, among others. Meanwhile, the Ministry of Health, Labor, and Welfare prescribes the food category, "Borderline of pharmaceuticals to non-pharmaceuticals ", indicating that the root and stolon of licorice are of the "nature of raw materials", which are not judged as pharmaceuticals, unless they are described with medical claims 6. For this reason, licorice and licorice oil extract are widely used as health food ingredients that have an indirectly proposed health claim. In recent years, FFC containing licorice oil extract are commercially available as functional foods to reduce visceral fat 7. In addition, there are many imported so-called health foods that contain licorice on the Japanese market.
Therefore, to confirm the safety and efficacy of these health foods and to establish a quality evaluation method, components of the medicinal and functional ingredients, estrogenic activity, and antioxidant activity were analyzed for licorice herbal medicines, health food ingredients, and health foods. Furthermore, for in vivo safety and efficacy evaluation, the effects of treatment with licorice oil extract on hepatic drug metabolizing enzymes, cytochrome P-450 (CYP), and fat and bone mass were evaluated in estrogen-deficient mice.
2. Materials and methods
2.1. Materials
Herbal medicines of licorice derived from G. glabra, G. inflata, and G. uralensis were kindly provided by Maruzen Pharmaceuticals Co., Ltd. (Hiroshima, Japan) and were deposited at the Research Center for Medicinal Plant Resources, NIBIOHN. Some of the ingredients for health foods, such as licorice root powder and extracts, were obtained from Nippon Powder KK. (Osaka, Japan), Fukuda Ryu KK (Osaka, Japan), Matsuura Pharmaceuticals (Nagoya, Japan), and Tokiwa Phytochemical Co., Ltd (Tokyo, Japan). The licorice oil extract, Glavonoid™, was obtained from Kaneka healthcare. Co., Ltd. (Osaka, Japan), and consisted of 30% licorice polyphenol with 3% glabridin from G. glabra in 70% edible oil, without the medicinal component glycyrrhizin. The so-called heath foods were purchased from local drug stores in the Tokyo area. The list of samples used in this study is shown in Table 1.
Table 1.
List of the samples used in this study.
| Categories | # | Origin† | Form | Remarks |
|---|---|---|---|---|
| Herbal crude drugs | 1 | G. glabra | Dried root or stolon | |
| 2 | G. inflata | Dried root or stolon | ||
| 3 | G. glabra | Dried root or stolon | ||
| 4 | G. uralensis | Dried root or stolon | ||
| Health food ingredients | 1 | N/A | Dry extract | |
| 2 | N/A | Viscous extract | ||
| 3 | G. uralensis | Bulk powder | ||
| 4 | G. uralensis or G. glabra | Bulk powder | ||
| 5 | G. glabra | Dry extract | ||
| 6 | G. glabra | Oil extract | ||
| Health foods containing licorice oil extract (health food ingredient #6, domestic) | 1 | G. glabra | Soft capsule | |
| 2 | G. glabra | Soft capsule | ||
| 3 | G. glabra | Soft capsule | FFC | |
| 4 | G. glabra | Soft capsule | FFC | |
| 5 | G. glabra | Soft capsule | ||
| 6 | G. glabra | Soft capsule | FFC | |
| 7 | G. glabra | Soft capsule | FFC | |
| 8 | G. glabra | Soft capsule | FFC | |
| 9 | G. glabra | Soft capsule | FFC | |
| 10 | G. glabra | Soft capsule | ||
| Health foods (others, domestic) | 11 | N/A | Capsule | |
| 12 | N/A | Powder | ||
| 13 | N/A | Liquid | ||
| Health foods (imported) | 14 | G. glabra | Capsule | |
| 15 | N/A | Tablet | DGL | |
| 16 | G. glabra | Capsule | ||
| 17 | N/A | Tablet | DGL | |
| 18 | G. glabra | Tablet | DGL | |
| 19 | N/A | Tablet | DGL | |
| 20 | G. glabra | Tablet | DGL | |
| 21 | N/A | Liquid |
DGL = deglycyrrhizinated licorice. FFC = Food with Function Claim. N/A = not available.
Origin is based on the information of the food-label or the appended paper of each product.
2.2. Sample preparation
The dried samples were pulverized as necessary, using an Oster Blender (Osaka Chemical, Osaka, Japan) or a Hikikko 300 cc (Tokyo Unicom, Tokyo, Japan). The liquid samples were used directly. The samples (0.5 g) were placed in 50 mL conical centrifuge tubes (Falcon, NY, USA), ethanol/water (1:1, 10 mL) was added, and the samples were vortexed (30 s). The samples were heated at 90 °C for 1 h in a heating oven DRM420DA (Advantec, Tokyo, Japan), with manual agitation every 15 min. The tube was centrifuged at 1580 × g for 10 min in an LC-200 (Tomy Seiko, Tokyo, Japan), and the supernatant was transferred to a 50-mL volumetric flask. The samples were extracted 2 more times in the same way and filled up to 50 mL with ethanol/water (1:1). This extract solution was then diluted as appropriate with ethanol/water (1:1), and used for measuring glabridin, oxygen radical absorption capacity (ORAC), and estrogenic activity.
For all samples other than herbal medicines, glycyrrhizin content was measured as described in the Japanese Pharmacopoeia (JP) 17th edition 4. Method A (applied to health food ingredients Nos. 3 and 4 in Table 1): To an accurately weighed sample (0.5 g) in a centrifuge tube, 70 mL of diluted ethanol (ethanol/water = 1:1) was added. The sample was then shaken for 15 min, centrifuged, and the supernatant was removed. To the residue, 25 mL of diluted ethanol was added and the mixture was shaken for 15 min, centrifuged, and the supernatant was removed. The supernatants were combined and diluted ethanol was added to make exactly 100 ml. This solution was used as the sample solution. Method B (applied to health food ingredients Nos. 1, 2, 5, 6 and health foods Nos 4, 8, 11, 12, 14, and 16 in Table 1): To an accurately weighed sample (0.15 g) in a centrifuge tube, 25 mL of diluted ethanol (ethanol/water = 1:1) was added. The sample was then heated at 50 °C for 30 min. After cooling, the solution was centrifuged, and the supernatant was removed. To the residue, 20 mL of diluted ethanol was added. The sample was heated at 50 °C for 30 min and the supernatant was removed. The supernatants were combined, and diluted ethanol was added to make exactly 100 ml. This solution was used as the sample solution. Separately, an accurately weighed GL standard sample (25 mg) was dissolved in 100 mL of diluted ethanol, and this solution was used as the standard solution.
2.3. Analysis of the medicinal and functional ingredients
2.3.1. Determination of glabridin
Glabridin concentration was measured using high-performance liquid chromatography (HPLC). The extract solution was filtered using a 0.45-μm membrane filter DISMIC-13HP (Advantec) and injected (10 μL) into an HPLC system (Class-VP, Shimadzu, Kyoto, Japan). Chromatographic analyses were carried out on an InertSustain C18 column (4.6 i.d. × 150 mm, 3 μm, GL Sciences, Tokyo, Japan) with an InertSustain C18 (4.0 i.d. × 10 mm, 3 μm, GL Sciences) guard column kept at 40 °C, using water/acetonitrile (50:50, v/v), containing 0.5% (v/v) acetic acid, as the mobile phase. The flow rate was 1.0 mL/min and absorbance at 280 nm was monitored. The glabridin content in each sample was calculated based on standard curves.
2.3.2. Determination of glycyrrhizin 8
The HPLC system and analytical conditions for glycyrrhizin in health food ingredients and health foods were as follows: Waters HPLC system (1527 binary pump, 2487 UV detector, 717plus autosampler), Column for HPLC: Waters XBridge C18 (4.6 × 150 mm, 5μm). Mobile phase: 3.85 g of ammonium acetate dissolved in 720 mL of water, followed by adding 5 mL of acetic acid (100) and 280 mL of acetonitrile. Flow rate: 0.8 mL/min. Detector: UV 254 nm. Glycyrrhizin content was calculated based on the calibration curve obtained from the standard solution.
Glycyrrhizin contents of herbal medicines Nos. 1–4 (Table 1), were measured as follows. Fifty milligrams of each powdered sample were extracted by sonication with 7.0 mL of 50% ethanol for 30 min. The extract solution was filtered through an Ultrafree-MC (0.45 μm filter unit; Millipore, Bedford, MA, USA) and a 5–20 μL aliquot was analyzed by HPLC using a Waters Alliance HT HPLC system (2795separation module and 2996 photodiode array detector; Waters, Milford, MA, USA). The HPLC column was a TSKgel ODS-100 V column (4.6 mm i.d. × 250 mm, 5 μm, TOSOH, Tokyo, Japan). The mobile phase consisted of acetonitrile (solvent A) and 1% acetic acid (solvent B). GL was resolved using the following gradient conditions with a flow rate of 1.0 mL/min at 40 °C (0–21 min 20–76% A, 21–22 min 76–100% A, 22–24 min 100% A, 24–35 min 100-20% A). Glycyrrhizin content was quantified by the peak area at 254 nm.
2.4. Antioxidant activity, ORAC assay
The ORAC assay is one of the methods used to evaluate the antioxidant activity of samples, and H-ORAC values, which reflect the sum of the hydrophilic antioxidants in the samples, were measured, as described previously 9. Briefly, a diluted extract solution (35 μL), fluorescein (115 μL, 110.7 nM, Wako Pure Chemicals) and 2,2′-azobis (2-methylpropionamidine) dihydrochloride (50 μL, 31.7 mM, Wako Pure Chemicals, Osaka, Japan) were incubated in 75 mM KH2PO4-K2HPO4 buffer at 37 °C in a 96-well plate. Fluorescence (Ex: 485 nm, Em: 520 nm) was monitored every 2 min for 90 min using Powerscan HT (DS Pharma Biomedical, Osaka, Japan). The H-ORAC value for each sample was calculated based on the standard curves for Trolox (Aldrich Chemicals, Tokyo, Japan). The result was expressed as the Trolox equivalent (TE), that is, the amount of Trolox necessary to obtain the same antioxidant capacity of the sample (μmol-TE/g).
2.5. Estrogen activity assay
2.5.1. Cell culture and DNA transfection
Estradiol (E2) was purchased from Sigma (USA). Premarin® tablets (0.625 mg) was purchased from Pfizer Inc. (NY, USA). MCF-7 cells (ATCC) were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Thermo Fisher, USA), supplemented with 10% fetal calf serum (Biological Industries, SC, USA) and 100 μg/ml penicillin/streptomycin (Thermo Fisher, Yokohama, Japan), as described previously 10. For estrogen-free experiments, the cells were seeded in phenol-red free DMEM (Thermo Fisher), and supplemented with 10% charcoal-dextran-treated fetal calf serum (Thermo Fisher) prior to treatment with the extracts. MCF-7 cells were transfected with 3XERE-TATA-luc and tk-Rluc, followed by incubation with the vehicle control, 10−10 M to 10-8 M E2, 1/1000 to 1/1 Premarin, 10-8 M to 10-6 M glabridin, or 50% ethanol extract licorice-based products. To minimize the effect on cells, 0.5 μL of the extract was added in 0.5 mL of the culture media (1/1000). A luciferase reporter vector containing and estrogen responsive element was transiently transfected into the estrogen receptor (ER)-alpha positive MCF-7 cells. DNA transfection and luciferase assay were performed as described previously 10.
2.6. CYP mRNA expression and activity in the liver
2.6.1. Animals, diet, and experimental design
Female ddY strain mice, aged 8 weeks, were purchased from the Shizuoka Laboratory Animal Center (Shizuoka, Japan). The mice were sham-operated (Sham group, n = 8) or underwent ovariectomy on the same day. Ovariectomized (OVX) mice were randomly divided into three groups (n = 8 each): the OVX control mice (OVX), the OVX mice fed a 0.39% licorice oil extract-supplemented diet (OVX + 10 L), and the OVX mice fed a 1.95% licorice oil extract-supplemented diet (OVX + 50 L). The mice were pair-fed their respective diets for 28 days, with free access to distilled water during this period. Table 2 shows the composition of the experimental diets, which were prepared according to the AIN-93 G formulation [11]. Triglyceride (glyceryl trioctanoate) was added to the control and 0.39% licorice oil extract diets, in accordance with the triglyceride content in the 1.95% licorice oil extract diet. The licorice oil extract used in the diets was Kaneka Glavonoid™.
Table 2.
Composition of the experimental diets (g/kg diet)*.
| Ingredients | Control | 0.39% licorice oil extract (10 L) | 1.95% licorice oil extract (50 L) |
|---|---|---|---|
| Corn starch | 529.5 | 528.3 | 523.7 |
| Casein milk | 200 | 200 | 200 |
| Sucrose | 100 | 100 | 100 |
| Corn oil | 56.3 | 56.3 | 56.3 |
| Cellulose | 50 | 50 | 50 |
| Mineral mixture | 35 | 35 | 35 |
| Vitamin mixture | 10 | 10 | 10 |
| L-Cystine | 3.0 | 3.0 | 3.0 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 |
| Tert-butylhydroquinone | 0.014 | 0.014 | 0.014 |
| Licorice oil extract # | 0.00 | 3.90 | 19.5 |
| Triglyceride (Glyceryl trioctanoate) ** | 13.7 | 11.0 | 0.0 |
Control, control diet; 0.39% licorice oil extract (10 L), 0.39% licorice oil extract -supplemented diet, whose licorice oil extract dose was 10 times of recommended human doses; 1.95% licorice oil extract (50 L), 1.95% licorice oil extract -supplemented diets, whose licorice oil extract dose was 50 times of recommended human doses.
The licorice oil extract (Kaneka Glavonoid™; Kaneka, Osaka, Japan) contained licorice (Glycyrrhiza glabra) ethanolic extract at approximately 30% polyphenol with 3% glabridin, triglycerides (Glyceryl trioctanoate) at approximately 70%, and glycyrrhizex acid at below 0.005%.
Control and 0.39% licorice oil extract diet were added tiglyceride (glyceryl trioctanoate), in accordance with triglyceride (glyceryl trioctanoate) content in the 1.95% licorice oil extract diet.
After 28 days of treatment, the mice were euthanized by exsanguination under anesthesia. Subsequently, blood was collected in vacutainers. The plasma was removed and stored at −80 °C until assayed. The liver was excised, washed, weighed, and then immediately frozen in dry ice for CYP analysis, or submerged completely in RNAlater® (Qiagen, Hilden, Germany) in a tube for RNA extraction and stored at −80 °C until assayed. The uterus was also removed, and its wet weight was measured. The left femur was removed to measure the bone mineral density (BMD). All procedures involving animals were conducted in accordance with the National Institutes of Biomedical Innovation, Health and Nutrition Guidelines for the Care and Use of Laboratory Animals (Tokyo, Japan).
2.6.2. Determination of licorice oil extract doses in the diets
The diets used in this experiment were AIN-93 G diets, containing 0.39 or 1.95% licorice oil extract (health food ingredient No.6 in Table 1). The recommended daily dose of licorice oil extract for humans through FFC in Japan is 300 mg/day (approximately 5.66 mg/kg body weight (BW)). This dose translated to 69.8 mg/kg BW of licorice oil extract, Glavonoid ™, for mice, using the body surface area normalization method 12. Because mice (25 g BW) consume approximately 4.5 g of diet/day, 0.039% in the diet is similar to a 69.8 mg/kg BW intake. Therefore, we examined the effect of 0.39% and 1.95% licorice oil extract in diet; these doses were 10 or 50 times the recommended human doses, respectively.
2.6.3. Preparation of the liver microsomal fraction
Livers were homogenized in 50 mM Tris−HCl buffer, containing 0.25 M sucrose (pH 7.4) with a polytron homogenizer. The homogenate was centrifuged at 10,000 × g for 30 min at 4 °C, and the supernatant was collected. The supernatant was centrifuged again at 105,000 × g for 60 min at 4 °C, and the supernatant was discarded. The pellet was resuspended in 50 mM Tris−HCl buffer (pH 7.4) and used as the liver microsomal fraction. Protein concentrations were determined using the BCA protein assay kit (Pierce, Rockford, IL, USA).
2.6.4. Measurement of CYP activity
The activity of each CYP subtype in the liver microsomal fraction was measured using a luminescence method with the P450-Glo™ CYP1A1 System (Luciferin-CEE) Assay, CYP1A2 System (Luciferin-1A2) Assay, CYP2C9 System (Luciferin-H) Assay, CYP 3A4 system (Luciferin-PPXE) Assay, and Detection System (Promega Co., Madison, WI, USA). CYP activity was adjusted to the protein concentration, and the results were represented as a percentage of the Sham group.
2.6.5. RNA extraction from liver and quantitative real-time PCR
Total RNA was extracted from the liver using the RiboPure™ RNA Purification Kit (Ambion by Life Technologies, Carlsbad, CA, USA), according to manufacturer’s instructions. Complementary DNA (cDNA) was synthesized from 1 μg of total RNA, using PrimeScript RT Master Mix (TaKaRa Bio, Shiga, Japan). cDNA was quantified by real-time PCR using SYBR Premix Ex Taq II (Takara Bio, Shiga, Japan). The PCR conditions were 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. The primer sequences are shown in Table 3. The results are expressed as the fold-change relative to the controls, after normalization to the expression of the β-actin gene.
Table 3.
Sequence of primers used for real-time PCR for CYP family.
| Gene | Forward primer (5' to 3') | Reverse primer (5' to 3') | |
|---|---|---|---|
| β-actin | CCACAGCTGAGAGGGAAATC | AAGGAAGGCTGGAAAAGAGC | |
| CYP1A2 | ACAGCAAGGACTTTGTGGAGAA | GTGATGTCTTGGATACTGTTCTTGT | |
| CYP2A4 | GGCCTGGAGGACTTTATAACCA | TTCTTCTCCTCCAGCATTCGG | |
| CYP2B9 | GAAGACCCTTCGGCGATTCT | AGGGGCACTCCCTGGTATTT | |
| CYP2B10 | TCAAGTCTTTTATTCAGCTTCGAGA | TTGGCTCAACGACAGCAACT | |
| CYP2C29 | TGTCACAGCTAAAGTCCAGG | CTAGTGGGGAGGAGGTCGAT | |
| CYP2D10 | TCAGCAGGCCGCAGATCAT | GCAACCGGAAAAGGAAAGACA | |
| CYP2E1 | CAGAGACCACCAGCACAACT | ATGCACTACAGCGTCCATGT | |
| CYP3A11 | CTCAATGGTGTGTATATCCCC | CCGATGTTCTTAGACACTGCC | |
| CYP3A41 | CTCTACCGATATGGGACCCG | GCACAGTGCCTAAAAATGGCA |
2.7. Measurement of plasma aspartate aminotransferase and alanine aminotransferase concentrations
The activities of plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT), which are markers for liver function, were determined using respective enzymatic methods. These analyses were conducted by Oriental Yeast (Tokyo, Japan).
2.8. Bone analysis
2.8.1. Radiographic analysis of the femur
The BMD of the femur was quantified by dual-energy X-ray absorptiometry (DEXA, DCS-600EX-RIII; Aloka, Tokyo, Japan) and calculated using the bone mineral content of the measured area. The scanned area of the mouse femur was divided into three equal parts: proximal, midshaft, and distal.
2.9. Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM). The data were analyzed using one-way ANOVA. Differences among the groups were assessed by Tukey’s post hoc test. Differences were considered significant if P < 0.05. Statistical analyses were conducted using SPSS Statistics, version 19 (IBM, Armonk, NY, USA).
2.10. Differentiation of raw plant species by genetic analysis
Genomic DNA was extracted using the DNeasy Plant Mini Kit (Qiagen; health food ingredients Nos. 3 and 4, health foods Nos. 12 and 16) or the alkaline PVPP buffer 13 (health food No. 14). The partial DNA fragments of the ITS (379 bp) and matK (143 bp) region were amplified by touchdown PCR using GoTaq Green master mix (Promega, Tokyo, Japan). The amplifying and sequencing primers for ITS were 5′-GCCACGCACTGTGTTCTCTCCT-3′ and 5′-GCAATGCTCACGGGAAGCCAACA-3′ 14, and those for matK were 5′-CTTCGACACTGGGTGAAAGATG-3′ and 5′-AGGAACAAGAATAATCTTGG-3′ 15. The PCR products were purified using Illustra ExoProSTAR (GE Healthcare, Chicago, USA) and sequenced directly with a Big Dye Terminator Cycle Sequencing Kit 3.1 on a 3130 Genetic Analyzer (Applied Biosystems, Tokyo, Japan) following the manufacturer’s instructions.
3. Results
3.1. Analysis of functional and medicinal components
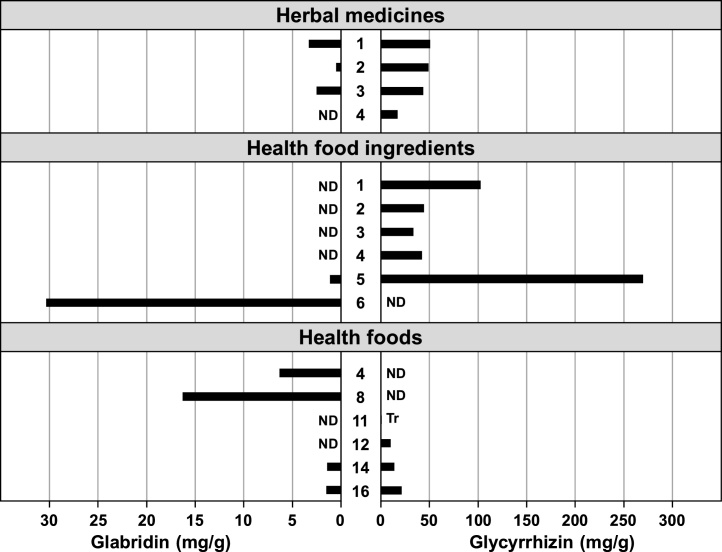
The contents of the functional and medicinal components in licorice were analyzed for 4 licorice herbal medicines, 6 licorice-based health food ingredients (here after referred to as "health food ingredients"), and 6 licorice-containing health foods (here after referred to as "health foods") (Fig. 1). A functional component, glabridin, of the herbal medicines was detected in G. glabra (Nos.1 and 3) and G. inflata (No. 2) but was not detected in G. uralensis (No.4). In addition, glabridin was detected at high levels in licorice oil extract, a health food ingredient (No.6), and in health foods including FFC containing the oil extract (Nos. 4 and 8 in Fig. 1 and Nos. 1–10 in Fig. 2).
Fig. 1.
Contents of glabridin and glycyrrhizin in licorice based products available in Japan were measured by HPLC with ultraviolet detection. Each bar represents the mean of the results (n = 2–3). Details of the samples are shown in Table 1. ND = not detected. Tr = trace.
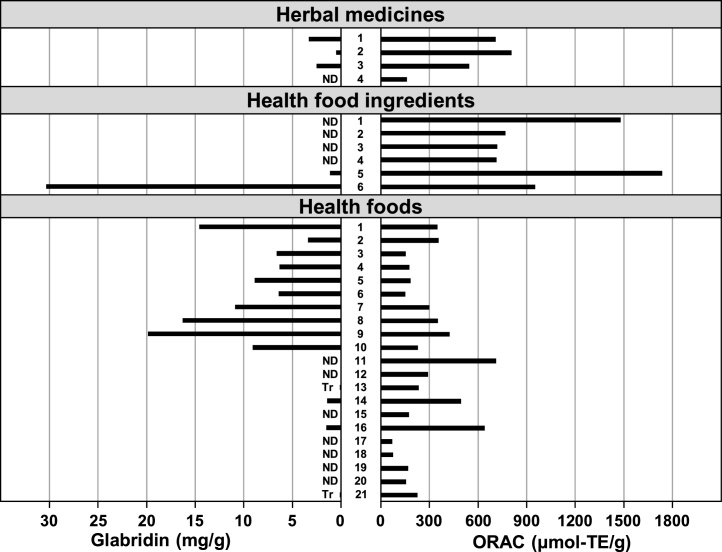
Fig. 2.
Contents of glabridin in licorice based products available in Japan were measured by HPLC with ultraviolet detection, and their antioxidant activities were measured by ORAC method. Each bar represents the mean of the results (n = 2). Details of the samples are shown in Table 1. ND = not detected. Tr = trace.
Four licorice herbal medicines, including a Chinese-marketed product, contained 1.7 to 5.1% of the medical component glycyrrhizin. Glycyrrhizin was also detected in health food ingredients (Nos. 1–5) except the oil extract (No. 6), at 3 to 27%. Especially, dried extract from G. glabra contained a high dose of glycyrrhizin at 27% (No.5). Regarding health foods, 0.1 to 2.2% of glycyrrhizin was present in foods labeled with licorice powder or its extract as raw materials (Nos.11, 12, 14, 16).
3.2. Antioxidant activity
For the 4 herbal medicines, 6 health food ingredients, and 21 health foods mentioned above, antioxidant capacity was measured using the ORAC method (Fig. 2). Antioxidant activity was detected in all the materials but was not necessarily proportional to the amount of the functional component, glabridin.
3.3. Estrogen activity
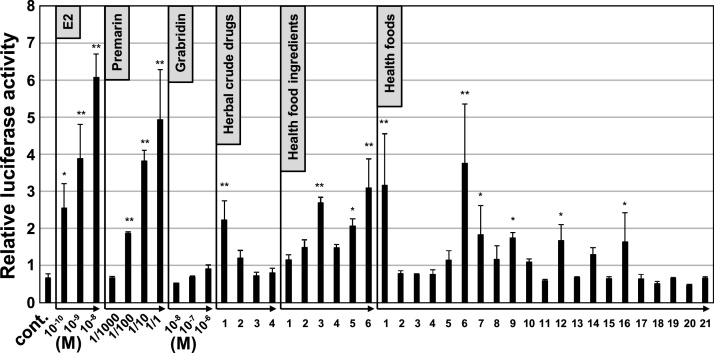
The estrogenic activity of the licorice-based products was evaluated using a luciferase reporter assay. A luciferase reporter vector containing an estrogen responsive element was transiently transfected into estrogen receptor (ER)-alpha positive MCF-7 cells. Premarin is an estrogen medication that conjugates estrogens. Since Premarin is orally used for hormone therapy in women, we used Premarin as oral absorption control of estrogenic activity. As shown in Fig. 3, we found that estrogenic activity exists in several licorice products (for example, herbal medicine-1, health food ingredient-3, 5, 6, health foods-1, 6, 7, 9, 12, 16). However, there is no estrogenic activity in glabridin per se (see Glabridin lanes).
Fig. 3.
Estrogenic activities of Premarin, glabridin and 50% ethanol extract of licorice based products. MCF-7 cells were transfected with 3XERE-TATA-luc and tk-Rluc, followed by incubation with control vehicle (Cont.), 10−10 M to 10-8 M estrogen (E2), 1/1000 to 1/1 Premarin, 10-8 M to 10-6 M Grabridin or licorice based products. Relative luciferase activity was shown by mean ± S.E. The data were analyzed using one-way ANOVA followed by Turkey’s post hoc test, *, p < 0.05, **, p < 0.001, compared by cont.
3.4. Effect of licorice oil extract on the liver, and fat and bone mass in OVX mice
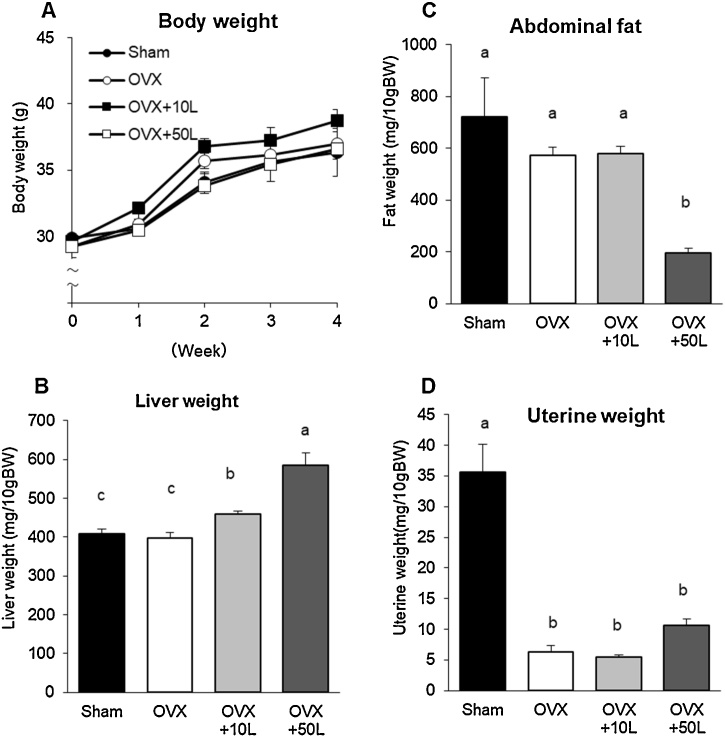
In order to evaluate the effects of licorice oil extract on liver weight, the liver drug metabolizing enzyme CYP was used for safety assessment along with the fat and the bone mass in estrogen-deficient conditions. OVX mice were fed 0.39% or 1.95% licorice oil extract in their diet for 4 weeks, which corresponds to 10 or 50 times the recommended human doses, respectively. There was no difference in body weight among all groups for 4 weeks but the liver weight in mice treated with the extract was significantly higher than that in the OVX and sham mice (Fig. 4A, B). The abdominal fat mass was decreased by treatment with the 50-fold dose of licorice oil extract in estrogen-deficient mice (Fig. 4C). Although the uterine weight in OVX mice treated with licorice oil extract at the 50-fold dose was not significantly higher compared with that of the OVX mice (Fig. 4D), it was statistically different from that of OVX mice by ANOVA with Dunnett’s post hoc test among the 3 OVX groups (data not shown).
Fig. 4.
Body weight, abdominal fat, liver weight and uterine weight in ovariectomized mice. Values are expressed as mean ± SEM, n = 8; means with different letters differ, p < 0.05. The data were analyzed using one-way ANOVA, and differences among the groups were assessed by Tukey’s post hoc test. Sham-operated mice fed a control diet (Sham), ovariectomized mice fed a control diet (OVX), OVX mice fed a 0.39% licorice oil extract-supplemented diet (OVX+10 L), and OVX mice fed a 1.95% licorice oil extract-supplemented diet (OVX + 50 L).
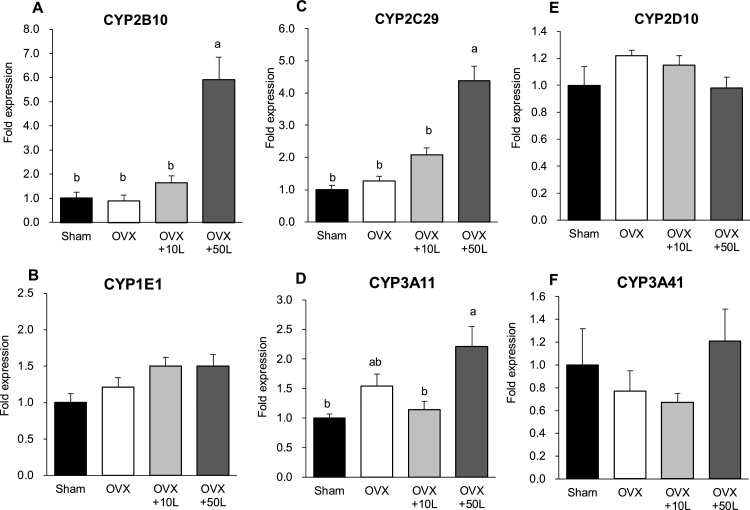
Evaluating the influence of the extract on liver metabolic enzyme gene expression revealed that CYP 2B10, CYP 2C29, and CYP 3A11 expression levels were significantly enhanced by ingestion of the licorice oil extract at the 50-fold dose (Fig. 5A, C, D). However, CYP 1A2, CYP 2A4, CYP 2B9, CYP 2D10, CYP 1E1, and CYP 3A41 were not affected by the licorice oil extract treatment (Fig.5B, E, F, data of CYP1A2, 2A4 and CYP2B9 were not shown). The enzymatic activity of CYP 1A, CYP 2D, and CYP 3A in the liver was also enhanced by the same dose of licorice oil extract (data not shown).
Fig. 5.
CYP mRNA expression in liver in ovariectomized mice. Values are expressed as mean ± SEM, n = 8; means with different letters differ, p < 0.05. The data were analyzed using one-way ANOVA, and differences among the groups were assessed by Tukey’s post hoc test. Sham-operated mice fed a control diet (Sham), ovariectomized mice fed a control diet (OVX), OVX mice fed a 0.39% licorice oil extract-supplemented diet (OVX+10 L), and OVX mice fed a 1.95% licorice oil extract-supplemented diet (OVX + 50 L).
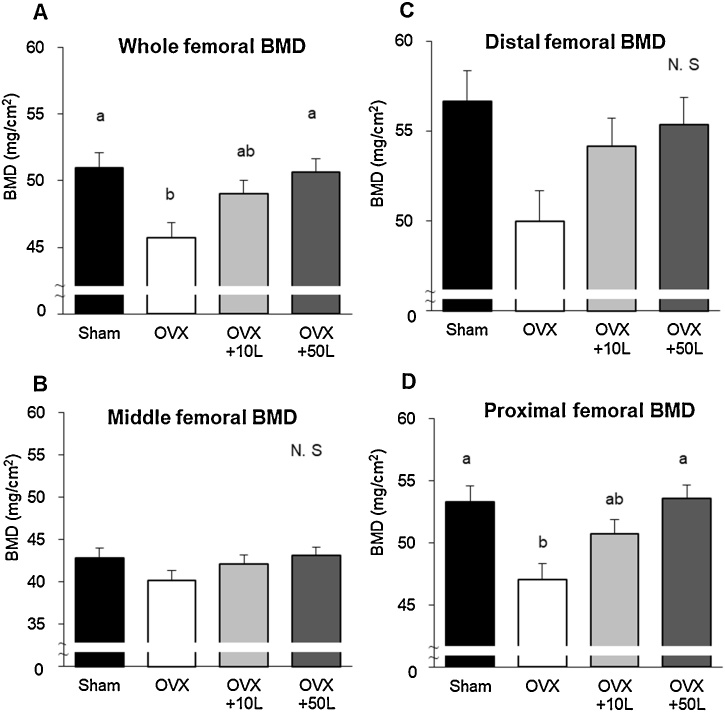
Bone mineral density (BMD) in the whole, distal, and proximal femora was decreased in the OVX mice; however, the licorice oil extract at 50 times of the recommended doses suppressed the reduction of whole and proximal BMD in OVX mice (Fig. 6).
Fig. 6.
Bone mineral density of ovariectomized mice. Values are expressed as mean ± SEM, n = 8; means with different letters differ, p < 0.05. The data were analyzed using one-way ANOVA, and differences between groups were assessed by Tukey’s post hoc test. Sham-operated mice fed a control diet (Sham), ovariectomized mice fed a control diet (OVX), OVX mice fed a 0.39% licorice oil extract-supplemented diet (OVX+10 L), and OVX mice fed a 1.95% licorice oil extract-supplemented diet (OVX + 50 L).
3.5. Differentiation of raw plant species by genetic analysis
Genomic DNA was extracted from 2 health food ingredients and 3 health foods, and the sequences of their ITS and matK regions was analyzed by PCR in order to differentiate the raw plant species. As a result, in Table 4, 2 health food ingredients in powder (Nos. 3 and 4 in Table1) corresponded to the sequence of G. uralensis in the registration database, in both regions, and seemed to be of G. uralensis origin. However, the health foods in the capsule were consistent with the sequences of G. uralensis (No.12) and G. glabra (Nos.14 and 16), respectively.
Table 4.
Comparison of ITS and matK sequences among health foods and their ingredients.
| GenBank | ITS | matK | ||||
|---|---|---|---|---|---|---|
| species | accession # | 187 | 411-413 | accession # | 568-573 | genotype |
| G. uralensis | AB280738 | C | TGC | AB280741 | CTTATT | Gu |
| G. glabra | AB280739 | T | CAA | AB280742 | CTTATT | Gg |
| G. inflata | AB280740 | T | CAA | AB280743 | deletion | Gi |
| samples | ITS | matK | ||||
| categories | # | 187 | 411-413 | # | 568-573 | genotype |
| Health food ingredients | 3 | C | TGC | 3 | CTTATT | Gu |
| 4 | C | TGC | 4 | CTTATT | Gu | |
| Health foods | 12 | C | TGC | 12 | CTTATT | Gu |
| 14 | T | CAA | 14 | CTTATT | Gg | |
| 16 | T | CAA | 16 | CTTATT | Gg | |
Accession # indicates GenBank accession numbers of internal transcribed spacer (ITS) and matK sequences of Glycyrrhiza species. # of health food ingredients and health foods corresponds to Table 1.
4. Discussion
We assessed the safety of health food ingredients using licorice herbal medicines, health food ingredients, and marketed health foods, and established methods for their safety evaluation. Quantitative analysis of the medicinal and functional components provided useful information for assessing the safety of these products. The ORAC assay was considered useful for estimating the overall antioxidant capacity of the materials [9]. Furthermore, for evaluating the safety of estrogenic ingredients, an in vitro reporter gene assay, using human breast cancer cells 10, was found to be a simple and appropriate strategy. It was also demonstrated that these activities could be evaluated using a 50% ethanol extract for all the materials. Evaluating the effects of health food ingredients on hepatic CYP activity, uterine weight, and bone mass using OVX mice appeared to be valuable for the safety evaluation of these estrogenic ingredients in vivo. Differentiation of raw plant species by genetic analysis seems to be useful for quality management of plant-derived health foods.
4.1. Comparison of the glabridin and glycyrrhizin content in herbal medicines, health food ingredients, and health foods containing licorice
The present study is the first to examine the contents of both the functional food component glabridin and the medicinal component glycyrrhizin in health food-related materials (Fig. 1). Glabridin has been detected in crude drugs from G. glabra and G. inflata, but not from G. uralensis 16. Glabridin was also detected in health food ingredients derived from G. glabra. In particular, a high concentration (3%) of glabridin was found in licorice oil extract (health food ingredient No.6 in Fig. 1, Fig. 2), which consists of 30% licorice polyphenol, extracted from G. glabra, and 70% edible oil. It thus appears that glabridin was detected at high concentrations in health foods, including FFC-formulated with this licorice oil extract (health food Nos. 1–10 in Fig. 2). Glabridin was also detected in imported health foods containing licorice powder or extract (health food Nos. 14 and 16 in Fig. 1, Fig. 2), but not in foods labeled with deglycyrrhizinated licorice (Nos. 17–20 in Fig. 2). However, the medical property glycyrrhizin was found in all health food ingredients except the oil extract (No.6) (Fig. 1). Especially, a high concentration of glycyrrhizin was detected in the G. glabra-originated dry extract (27%) (health food ingredient No.5 in Fig. 1). Glycyrrhizin was not detected in health foods including FFC formulated with licorice oil extract. These results revealed that glabridin is mainly present in health foods and in FFC that contain licorice oil extract as a low material, and that these foods did not include the medicinal component glycyrrhizin. However, some of the health foods with dried licorice powder or extract (Nos. 14, 16 in Fig. 1) contained glycyrrhizin at the same level as that in the herbal medicines. This fact provides important basic data considering the possibility of unfavorable effects in humans.
4.2. Antioxidant activity
In recent years, the antioxidant activity of foods and crude drugs has been well evaluated [17,18]. Antioxidant activity can thus be used as one of the indices for evaluating food functionality. In this study, the antioxidant capacity of licorice herbal medicines, health food ingredients, and health foods was not necessarily proportional to the amount of the functional component glabridin (Fig. 2). From these results, it was suggested that licorice and related ingredients include some other polyphenolic compounds in addition to the known functional component glabridin. Further studies are thus necessary to clarify the compounds with antioxidant activity in licorice.
4.3. Estrogen activity
Licorice extracts are used in dietary supplements and are believed to contain estrogenic components 19. Recent studies have characterized the estrogenic activity of a major licorice compound, liquiritigenin [20,21]. We supposed that the differences in estrogenic activity across several licorice products depends on the difference in the amount of the liquiritigenin and its derivatives. The contents of those components in the herbal crude drugs and health food ingredients showing in Table S1 were correlated to the antioxidant activity in Fig. 2, but not always correlated to the estrogen activity in Fig. 3, suggesting that some other estrogenic compounds also might be existed in these products. In this experiment, there was no estrogenic activity in glabridin, which is one of the major components of licorice, as described previously 22. Attention should thus be paid to overdosing on these health foods that contain licorice dry extract as well as phytoestrogen [23,24], especially by young women.
4.4. Effect of licorice oil extract on the liver, and fat and bone mass in OVX mice
To evaluate the safety of licorice oil extract in vivo, mRNA expression and the activity of the liver drug metabolizing enzyme CYP, was examined. Hepatic CYP activity is used for assessments concerning drug metabolism and interactions between drugs and dietary components. However, as an evaluation for the safety and efficacy of licorice ingredients, an examination of the effects on the uterine weight and fat and bone mass in an osteoporotic animal model was considered useful.
Notably, the liver weight in OVX mice treated with licorice oil extract at 10 and 50 times the recommended human doses was significantly higher than that in OVX and sham mice in a dose dependent manner. Furthermore, overdose of licorice oil extract enhanced 3 of the 10 types of hepatic CYP mRNA expression and activity in OVX mice. These results are in agreement with those of previous reports that evaluated the safety of licorice in rodents [25,26]. On the other hand, although the enzymatic activity of CYP1A, CYP2D, and CYP3A in the liver was enhanced by the same dose of licorice oil extract, the mRNA expression of these respective CYPs was not necessarily increased. Two CYP1A, nine CYP2D, and six CYP3A isoforms are identified in mice; however, we have not analyzed all the CYP isoforms in this study. Therefore, the contribution of respective CYP mRNA expression to their enzymatic activities has not been revealed in our study. The uterine weight was higher in OVX mice treated with licorice oil extract at the 50-fold dose compared to that in the estrogen-deficient mice, indicating that the high dose of the licorice oil extract shows estrogenic activity in vivo. Thus, together with the results from the estrogenic activity in vitro and the effects on the liver in vivo, excessive intake of licorice oil extract from health foods should be avoided especially by young women.
It has been reported that dietary licorice root or licorice oil extract treatment reduced body weight and fat mass in OVX 27 or obese mice 28, respectively. Our results showing that the body fat mass was lower in OVX mice treated with the high dose of licorice oil extract, compared to untreated OVX mice, were consistent with these previous studies. The effects of licorice on fat mass seem to be induced by the functional components glabridin and glycyrrhizin, through their anti-inflammatory activity [29,30].
In this study, the BMD of the femur was higher in OVX mice treated with the high dose of the licorice oil extract than in untreated OVX mice. Although there has been in vitro research examining the effects of licorice oil extract on osteoblastic 31 and osteoclastic [32] cells, there have been few in vivo studies examining the BMD in OVX mice. As for the mechanism of the reduction of bone loss by licorice, Zhu et al. have reported that another functional component, isoliquiritigenin, suppresses RANKL-induced osteoclastogenesis 33. Considering these results, licorice oil extract expresses beneficial bone effects viaestrogenic activity, as well as an anti-inflammatory property from polyphenols, which include glabridin and isoliquiritigenin in estrogen-deficient osteopenia. They may also suggest that a high dose of licorice oil extract is beneficial as an herbal medicine for postmenopausal women’s health, under medical use conditions.
5. Conclusions
Upon evaluating the safety and effectiveness of licorice, glabridin and a small amount of glycyrrhizin were detected in health foods. Because estrogenic activity was detected in some of the related compounds and products, overdose of these products should be avoided especially by young women, although these ingredients may be beneficial for medical use in order to maintain bone health in postmenopausal women. The methods used in this study were considered useful for evaluating the safety and efficacy of estrogenic plant-derived ingredients.
Transparency document
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
We are grateful for the support provided by Dr Keizo Umegaki regarding the use of the “Information System on Safety and Effectiveness for Health Foods” of the National Institute of Health and Nutrition (Tokyo, Japan). This study was partially supported by the Research on Development of New Drugs from Japan Agency for Medicinal Research and Development, AMED, JP16ak0101034h0002.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.toxrep.2019.08.013.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Shibano M. Determination of flavonoids in licorice using acid hydrolysis and reversed-phase HPLC and evaluation of the chemical quality of cultivated licorice. Planta Med. 2010;76(07):729–733. doi: 10.1055/s-0029-1240690. [DOI] [PubMed] [Google Scholar]
- 2.Foods with Nutrient Function Claim, Consumer Affairs Agency. https://www.caa.go.jp/policies/policy/food_labeling/health_promotion/pdf/health_promotion_170606_0001.pdf. (Jul. 15, 2019).
- 3.Foods with Function Claims, Consumer Affairs Agency. https://www.caa.go.jp/policies/policy/food_labeling/about_foods_with_function_claims/. (Jul. 15, 2019).
- 4.The Japanese Pharmacopoeia, Seventeenth Edition, Mistry of Health, Labour and Welfare. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000066597.html (Jul. 15, 2019).
- 5.I. 2010. pp. 214–215. (Pharmacopoeia of The People’S Republic of China). 2010. [Google Scholar]
- 6.Borderline of pharmaceuticals to non-pharmaceuticals, Ministry of Health, Labour and Welfare. http://www.mhlw.go.jp/file/06-Seisakujouhou-11130500-Shokuhinanzenbu/0000086063_1.pdf (Jul. 15, 2019).
- 7.Tominaga Y., Nakagawa K., Mae T., Kitano M., Yokota S., Arai T., Ikematsu H., Inoue S. Licorice flavonoid oil reduces total body fat and visceral fat in overweight subjects: a randomized, double-blind, placebo-controlled study. Obes. Res. Clin. Pract. 2009;3(3):I–IV. doi: 10.1016/j.orcp.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Kondo K., Shiba M., Nakamura R., Morota T., Shoyama K. Constituent properties of licorices derived from Glycyrrhiza uralensis, G. glabra, or G. Inflata identified by genetic information. Biol. Pharm. Bull. 2007;30:1271–1277. doi: 10.1248/bpb.30.1271. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe J., Oki T., Takebayashi J., Yamasaki K., Takano-Ishikawa Y., Hino A., Yasui A. Method validation by interlaboratory studies of improved hydrophilic oxygen radical absorbance capacity methods for determination of antioxidant capacities of antioxidant solutions and food extracts. Anal. Sci. 2012;28:159–165. doi: 10.2116/analsci.28.159. [DOI] [PubMed] [Google Scholar]
- 10.Inoue E., Yamashita A., Inoue H., Sekiguchi M., Shiratori A., Yamamoto Y., Tadokoro T., Ishimi Y., Yamauchi J. Identification of glucose transporter 4 knockdown-dependent transcriptional activation element on the retinol binding protein 4 gene promoter and requirement of the 20 S proteasome subunit for transcriptional activity. J. Biol. Chem. 2010;285(33):25545–25553. doi: 10.1074/jbc.M109.079152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reeves P.G., Nielsen F.H., Fahey G.C., Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 12.Reagan-Shaw S., Nihal M., Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 13.Kasajima I. Protocol for DNA extraction from any plant species (alkaline PVPP method) Protoc. Exch. 2017 [Google Scholar]
- 14.Shibata S., Kondo K., Terabayashi S. Identification of the licorice root stored in Shosoin based on the sequences of internal transcribed spacer (ITS) on nr DNA and the chemotaxonomic consideration. Proc. Japan Acad. 2003;79(Ser. B):176–180. [Google Scholar]
- 15.Kondo K., Shiba M., Yamaji H., Morota T., Cheng Z., Pan H., Shoyama Y. Species identification of licorice using nrDNA and cpDNA genetic Markers. Biol. Pharm. Bull. 2007;30:1497–1502. doi: 10.1248/bpb.30.1497. [DOI] [PubMed] [Google Scholar]
- 16.Nomura T., Fukai T. Phenolic constituents if licorice (Glycyrrhiza species) In: Herz W., Kirby G.W., Moore R.E., Steglich W., Tamm C., editors. Vol. 73. Springer; Vienna: 1998. pp. 1–140. (Progress in the Chemistry of Organic Natural Products). Chap. 5. [DOI] [PubMed] [Google Scholar]
- 17.Takebayashi J., Oki T., Chen J., Sato M., Matsumoto T., Taku K., Tsubota-Utsugi M., Watanabe J., Ishimi Y. Estimated average daily intake of antioxidants from typical vegetables consumed in Japan: a preliminary study. Biosci. Biotechnol. Biochem. 2010;74(10):2137–2140. doi: 10.1271/bbb.100430. [DOI] [PubMed] [Google Scholar]
- 18.Fu Y., Chen J., Li Y.J., Zheng Y.F., Li P. Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem. 2013;141(2):1063–1071. doi: 10.1016/j.foodchem.2013.03.089. [DOI] [PubMed] [Google Scholar]
- 19.Hu C., Liu H., Du J., Mo B., Qi H., Wang X., Ye S., Li Z. Estrogenic activities of extracts of Chinese licorice (Glycyrrhiza uralensis) root in MCF-7 breast cancer cells. J. Steroid Biochem. Mol. Biol. 2009;113(3-5):209–216. doi: 10.1016/j.jsbmb.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 20.Hajirahimkhan A., Dietz B.M., Bolton J.L. Botanical modulation of menopausal symptoms: mechanisms of action? Planta Med. 2013;79(7):538–553. doi: 10.1055/s-0032-1328187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang Y., Gong P., Madak-Erdogan Z., Martin T., Jeyakumar M., Carlson K., Khan I., Smillie T.J., Chittiboyina A.G., Rotte S.C., Helferich W.G., Katzenellenbogen J.A., Katzenellenbogen B.S. Mechanisms enforcing the estrogen receptor β selectivity of botanical estrogens. FASEB J. 2013;27(11):4406–4418. doi: 10.1096/fj.13-234617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boonmuen N., Gong P., Ali Z., Chittiboyina A.G., Khan I., Doerge D.R., Helferich W.G., Carlson K.E., Martin T., Piyachaturawat P., Katzenellenbogen J.A., Katzenellenbogen B.S. Licorice root components in dietary supplements are selective estrogen receptor modulators with a spectrum of estrogenic and anti-estrogenic activities. Steroids. 2016;105:42–49. doi: 10.1016/j.steroids.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solak K.A., Wijnolts F.M.J., Nijmeijer S.M., Blaauboer B.J., Berg M., Duursen M.B.M. Excessive levels of diverse phytoestrogens can modulate steroidogenesis and cell migration of KGN human granulosa-derived tumor cells. Toxicol. Rep. 2014;1:360–372. doi: 10.1016/j.toxrep.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tousen Y., Ishiwata H., Takeda K., Ishimi Y. Assessment of safety and efficacy of perinatal or peripubertal exposure to daidzein on bone development in rats. Toxicol. Rep. 2015;2:429–436. doi: 10.1016/j.toxrep.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paolini M., Pozzetti L., Sapone A., Cantelli-Forti G. Effect of licorice and glycyrrhizin on murine liver CYP-dependent monooxygenases. Life Sci. 1998;62(6):571–582. doi: 10.1016/s0024-3205(97)01154-5. [DOI] [PubMed] [Google Scholar]
- 26.Hou Y.C., Lin S.P., Chao P.D. Liquorice reduced cyclosporine bioavailability by activating P-glycoprotein and CYP 3A. Food Chem. 2012;135(4):2307–2312. doi: 10.1016/j.foodchem.2012.07.061. [DOI] [PubMed] [Google Scholar]
- 27.Madak-Erdogan Z., Gong P., Zhao Y.C., Xu L., Wrobel K.U., Hartman J.A., Wang M., Cam A., Iwaniec U.T., Turner R.T., Twaddle N.C., Doerge D.R., Khan I.A., Katzenellenbogen J.A., Katzenellenbogen B.S., Helferich W.G. Dietary licorice root supplementation reduces diet-induced weight gain, lipid deposition, and hepatic steatosis in ovariectomized mice without stimulating reproductive tissues and mammary gland. Mol. Nutr. Food Res. 2016;60(2):369–380. doi: 10.1002/mnfr.201500445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aoki F., Honda S., Kishida H., Kitano M., Arai N., Tanaka H., Yokota S., Nakagawa K., Asakura T., Nakai Y., Mae T. Suppression by licorice flavonoids of abdominal fat accumulation and body weight gain in high-fat diet-induced obese C57BL/6J mice. Biosci. Biotechnol. Biochem. 2007;71(1):206–214. doi: 10.1271/bbb.60463. [DOI] [PubMed] [Google Scholar]
- 29.Yehuda I., Madar Z., Leikin-Frenkel A., Tamir S. Glabridin, an isoflavan from licorice root, downregulates iNOS expression and activity under high-glucose stress and inflammation. Mol. Nutr. Food Res. 2015;59(6):1041–1052. doi: 10.1002/mnfr.201400876. [DOI] [PubMed] [Google Scholar]
- 30.Park M., Lee J.H., Choi J.K., Hong Y.D., Bae I.H., Lim K.M., Park Y.H., Ha H. 18β-glycyrrhetinic acid attenuates anandamide-induced adiposity and high-fat diet induced obesity. Mol. Nutr. Food Res. 2014;58(7):1436–1446. doi: 10.1002/mnfr.201300763. [DOI] [PubMed] [Google Scholar]
- 31.Choi E.M. The licorice root derived isoflavan glabridin increases the function of osteoblastic MC3T3-E1 cells. Biochem. Pharmacol. 2005;70(3):363–368. doi: 10.1016/j.bcp.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 32.Kim H.S., Suh K.S., Sul D., Kim B.J., Lee S.K., Jung W.W. The inhibitory effect and the molecular mechanism of glabridin on RANKL-induced osteoclastogenesis in RAW264.7 cells. Int. J. Mol. Med. 2012;29(2):169–177. doi: 10.3892/ijmm.2011.822. [DOI] [PubMed] [Google Scholar]
- 33.Zhu L., Wei H., Wu Y., Yang S., Xiao L., Zhang J., Peng B. Licorice isoliquiritigenin suppresses RANKL-induced osteoclastogenesis in vitro and prevents inflammatory bone loss in vivo. Int. J. Biochem. Cell Biol. 2012;44(7):1139–1152. doi: 10.1016/j.biocel.2012.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.