Abstract
Two new capnosane-based diterpenoids, flaccidenol A (1) and 7-epi-pavidolide D (2), two new cembranoids, flaccidodioxide (3) and flaccidodiol (4), and three known compounds 5 to 7 were characterized from the marine soft coral Klyxum flaccidum, collected off the coast of the island of Pratas. The structures of the new compounds were determined by extensive spectroscopic analyses, including 1D and 2D nuclear magnetic resonance (NMR) spectroscopy, and spectroscopic data comparison with related structures. The rare capnosane diterpenoids were isolated herein from the genus Klyxum for the first time. The cytotoxicity of compounds 1 to 7 against the proliferation of a limited panel of cancer cell lines was assayed. The isolated diterpenoids also exhibited anti-inflammatory activity through suppression of superoxide anion generation and elastase release in the N-formyl-methionyl-leucyl-phenylalanine/cytochalasin B (fMLF/CB)-stimulated human neutrophils. Furthermore, 1 and 7 also exhibited cytotoxicity toward the tested cancer cells, and 7 could effectively inhibit elastase release. It is worth noting that the biological activities of 7 are reported for the first time in this paper.
Keywords: soft coral, Klyxum flaccidum, capnosane, cembrane, cytotoxic activity, anti-inflammatory activity
1. Introduction
Chemical investigations on marine invertebrates have disclosed structural diversity in the terpenoid and steroid constituents of octocorals [1]. Cembranoid diterpenes, which contain a 14-membered macro cyclic skeleton, have been shown to exhibit a wide range of biological activities, including cytotoxic [2,3,4,5], antibacterial [6,7,8], anti-inflammatory [3,4,9,10,11], and antiviral [12,13,14] activities. The rarely found cembranoids with 3,7-fused carbobicyclic (capnosane) skeleton have also been found to possess inhibitory effects on protein tyrosine phosphatase 1B, a significant target in treating obesity and type II diabetes [15]; and against A2780 human ovarian tumor cells [16], human disease-related bacteria, and phytopathogens [17]. Additionally, cembranoid-derived diterpenoids are considered to be the main chemical defense of coral against natural predators [18]. Our previous investigation on the chemical constituents of soft corals belonging to the genus Klyxum [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33] has shown a wealth of unique secondary metabolites, including cembrane- and eunicellin-based diterpenoids, and steroids. In this context, we isolated a series of cembrane-diterpenoids [32] and steroids [29,31,33] from the Formosan soft corals Klyxum flaccidum, some of which have been found to possess cytotoxic and anti-inflammatory activities. A further investigation on the secondary metabolites of the same coral has thus been conducted to discover new metabolites and their bioactivity. In this paper we report the isolation, structure determination, and bioactivity of four new cembrane-derived diterpenoids, flaccidenol A (1) and 7-epi-pavidolide D (2), flaccidodioxide (3), and flaccidodiol (4), and three known compounds, sarcophytol T (5) [34], flaccidoxide-13-acetate (6) [35], and 14-O-acetylsarcophytol B (7) [36]. The structures of compounds were established by extensive spectroscopic analyses (Supplementary Materials, Figures S1 to S28). The cytotoxicities of compounds 1 to 7 were assayed against the cancer cell lines; human lung adenocarcinoma (A549), human colorectal adenocarcinoma (DLD-1), and mouse lymphocytic leukemia (P388D1). Furthermore, the anti-inflammatory activities that occur due to the inhibition of superoxide anion (O2−•) generation and elastase release in N-formyl-methionyl-leucyl-phenylalanine/cytochalasin B (fMLF/CB)-challenged human neutrophils, were evaluated.
2. Results and Discussion
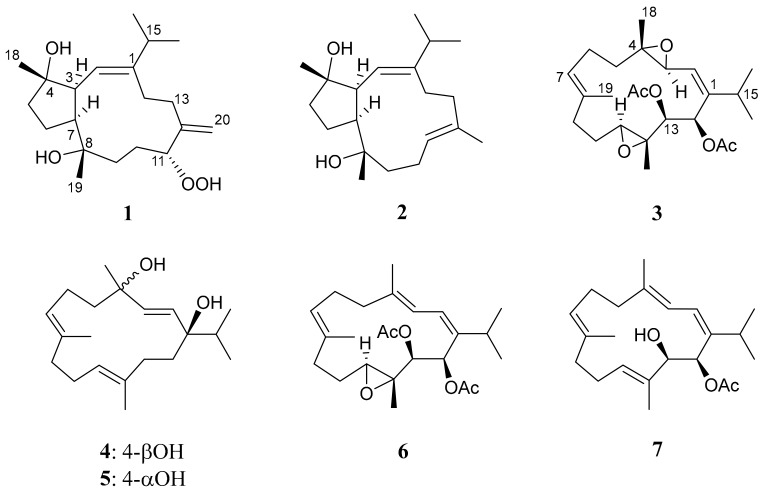
The ethyl acetate (EtOAc) extract of K. flaccidum was initially fractionated over a silica gel column, and the eluted fractions displaying terpenoidal methyl, olefinic, and oxymethine proton signals in the 1H NMR spectra were selected for further purification. One of these fractions was purified by repeated column chromatography to yield diterpenoids 1 to 7 (Figure 1), the structures were established on the basis of spectroscopic analyses (Supplementary Materials, Figures S1 to S28).
Figure 1.
Structures of compounds 1 to 7.
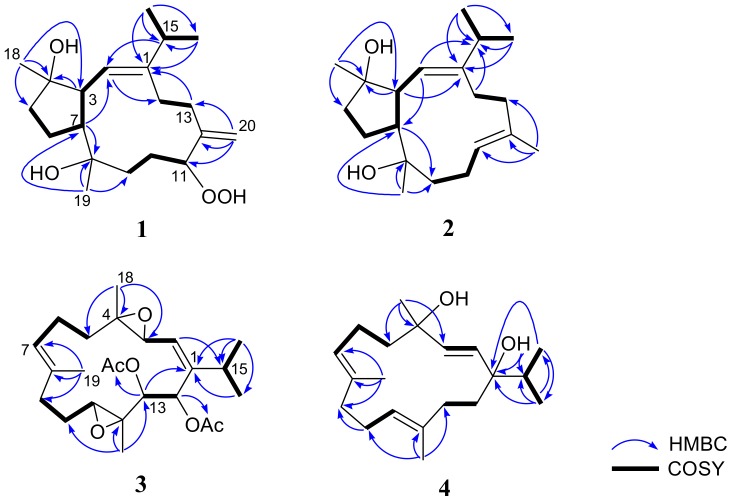
Flaccidenol A (1) was isolated as a colorless oil and has a molecular formula of C20H34O4 with four unsaturations, based on the sodium adduct ion peak [M + Na]+ obtained by positive high-resolution electrospray ionization mass spectroscopy (HRESIMS). The infrared (IR) spectrum revealed the presence of hydroxy and olefinic functionalities (νmax 3391 and 1647 cm−1). This was evidenced from the carbon signals of a trisubstituted and one 1,1-disubstituted carbon–carbon double bonds [δC 148.1 (C), 146.6 (C), 122.3 (CH), and 117.4 (CH2)] and three sp3 oxygenated carbons [δC 90.7 (CH), 82.8 (C), and 74.5 (C)] (Table 1), respectively, out of the 20 carbon signals in 13C NMR spectrum of 1. Moreover, the remaining two unsaturations indicated that compound 1 was a bicyclic diterpenoid. Similar to cembranoids [32], the 1H NMR of 1, in conjunction with the heteronuclear single quantum coherence (HSQC) spectrum, revealed the presence of six exocyclic carbons of isopropyl [δH 0.97 (3H, d, J = 7.0 Hz), 0.90 (3H, d, J = 7.0 Hz), and 2.21 (1H, sept, J = 7.0 Hz)], two tertiary methyl [δH 1.05 and 0.99 (each 3H, s), and α-methylene [δH 4.95 and 4.93 (each 1H, s)] groups (Table 2). The analysis of correlation spectroscopy (COSY) of 1 indicated four partial structures bearing consecutive proton systems (Figure 2). One of the partial structures included two ring-juncture methines [δH/δC 2.89/51.0 (CH) and 2.63/50.5 (CH)] and were positioned at C-3 and C-7 from the analysis of heteronuclear multiple-bond correlation (HMBC) spectra (Figure 2), similar to those of 3,7-cyclized cembranoid (capnosane) diterpenoids [15,17,37,38]. Key HMBC correlations observed from H3-18 to C-3 and C-5; H3-19 to C-7 and C-9; H2-20 to C-11 and C-13; H-15 to C-2 were used to connect the four COSY correlated partial structures and to further establish the 3,7-linkage in the capnosane molecular skeleton. Moreover, the NMR signals at δH 7.64 (1H, br s) and δC 90.7 (CH) pointed out the presence of an allylic hydroperoxy group [39,40,41]. Finally, the detailed HMBC correlation analysis confirmed the hydroperoxyl, the two hydroxyls, the exomethylene, and the trisubstituted double bond to be at C-11, C-4, C-8, C-12, and C-1/C-2, respectively, and consequently the planar structure of 1 as shown in Figure 2.
Table 1.
13C nuclear magnetic resonance (NMR) data of compounds 1 to 4.
| Position | 1 a | 2 b | 3 b | 4 c |
|---|---|---|---|---|
| 1 | 146.6, C | 144.4, C | 149.6, C | 78.6, C |
| 2 | 122.3, CH d | 121.8, CH | 124.8, CH | 131.5, CH |
| 3 | 51.0, CH | 51.1, CH | 58.1, CH | 136.5, CH |
| 4 | 82.8, C | 82.8, C | 62.1, C | 72.3, C |
| 5 | 40.3, CH2 | 37.7, CH2 | 37.8, CH2 | 43.9, CH2 |
| 6 | 25.5, CH2 | 23.0, CH2 | 23.5, CH2 | 22.3, CH2 |
| 7 | 50.5, CH | 54.3, CH | 126.4, CH | 128.4, CH |
| 8 | 74.5, C | 75.2, C | 133.7, C | 132.8, C |
| 9 | 37.9, CH2 | 44.1, CH2 | 35.9, CH2 | 39.0, CH2 |
| 10 | 25.4, CH2 | 23.1, CH2 | 24.0, CH2 | 23.9, CH2 |
| 11 | 90.7, CH | 127.1, CH | 59.1, CH | 126.5, CH |
| 12 | 148.1, C | 132.3, C | 60.4, C | 136.3, C |
| 13 | 30.3, CH2 | 36.0, CH2 | 73.2, CH | 36.2, CH2 |
| 14 | 28.6, CH2 | 28.3, CH2 | 67.8, CH | 32.7, CH2 |
| 15 | 32.7, CH | 32.9, CH | 28.9, CH | 38.7, CH |
| 16 | 21.1, CH3 | 21.2, CH3 | 24.2, CH3 | 16.7, CH3 |
| 17 | 23.7, CH3 | 23.8, CH3 | 24.0, CH3 | 17.6, CH3 |
| 18 | 25.8, CH3 | 25.2, CH3 | 17.5, CH3 | 27.8, CH3 |
| 19 | 27.1, CH3 | 22.1, CH3 | 15.9, CH3 | 14.8, CH3 |
| 20 | 117.4, CH2 | 17.3, CH3 | 16.2, CH3 | 14.7, CH3 |
| 13-OAc | 20.7, CH3 | |||
| 170.4, C | ||||
| 14-OAc | 20.7, CH3 | |||
| 169.0, C |
a Spectrum recorded in C6D6 at 125 MHz at 25 °C, b Spectra recorded in CDCl3 at 100 MHz at 25 °C, c Spectrum recorded in CDCl3 at 125 MHz at 25 °C, d Attached protons were determined by distortionless enhancement by polarization transfer (DEPT) experiments.
Table 2.
1H NMR spectral data for compounds 1 to 4.
| Position | 1 a | 2 b | 3 b | 4 c |
|---|---|---|---|---|
| 2 | 4.96 d (9.5) d | 5.05 d (10.8) | 5.25 m | 5.59 d (16.0) |
| 3 | 2.89 dd (10.0, 10.0) | 2.37 dd (7.8, 9.6) | 3.56 d (9.6) | 6.05 d (16.0) |
| 5 | 1.52 2H, m | 1.72 m; 1.65 m | 1.48 m; 2.11 m | 1.52 m; 1.99 m |
| 6 | 1.94 m; 1.64 m | 1.88 m; 1.76 m | 2.13 m; 2.23 m | 2.22 m; 2.35 m |
| 7 | 2.63 m | 2.65 ddd (6.6, 7.8, 9.6) | 5.22 m | 5.34 dd (7.5, 7.5) |
| 9 | 1.85 ddd (13.0, 13.0, 3.0); 1.20 ddd (13.0, 13.0, 4.5) | 1.88 m; 2.00 m | 2.14 m; 2.24 m | 1.96 m; 2.18 m |
| 10 | 1.94 m; 1.68 ddd (13.0, 10.5, 3.0) | 2.14 m | 1.09 m; 1.61 m | 2.04 m; 2.23 m |
| 11 | 4.27 dd (11.0, 4.5) | 4.99 dd (7.6, 7.6) | 3.12 dd (5.6, 5.6) | 5.18 dd (9.0, 3.5) |
| 13 | 2.66 m; 2.20 dd (11.5, 5) | 2.03 m; 1.92 m | 5.49 d (7.6) | 2.10 m; 2.15 d (3.0) |
| 14 | 2.79 ddd (11.5, 11.5, 4.5); 2.00 m | 2.40 dd (14.4, 7.2); 1.94 ddd (14.4, 12.0, 3.0) |
5.89 d (7.6) | 1.86 td (12.5, 3.5); 1.60 m |
| 15 | 2.22 sept (7.0) | |||
| 16 | 0.97 3 H, d (7.0) | 1.06 3H, d (7.2) | 1.02 3H, d (6.4) | 0.87 3H, d (7.0) |
| 17 | 0.90 3 H, d (7.0) | 1.02 3H, d (6.8) | 1.02 3H, d (6.4) | 0.87 3H, d (7.0) |
| 18 | 0.99 3 H, s | 1.14 3H, s | 1.33 3H, s | 1.40 3H, s |
| 19 | 1.05 3 H, s | 1.25 3H, s | 1.64 3H, s | 1.60 3H, s |
| 20 | 4.93 s; 4.97 s | 1.67 3H, s | 1.26 3H, s | 1.67 3H, s |
| 13-OAc | 2.14 3H, s | |||
| 14-OAc | 2.01 3H, s | |||
| 1-OH | 2.39 s | |||
| 11-OOH | 7.64 br s |
a Spectrum recorded in C6D6 at 500 MHz at 25 °C, b Spectra recorded in CDCl3 at 400 MHz at 25 °C, c Spectrum recorded in CDCl3 at 500 MHz at 25 °C, d J values (Hz) in parentheses.
Figure 2.
Selected correlation spectroscopy (COSY) and heteronuclear multiple-bond correlation (HMBC) correlations of 1 to 4.
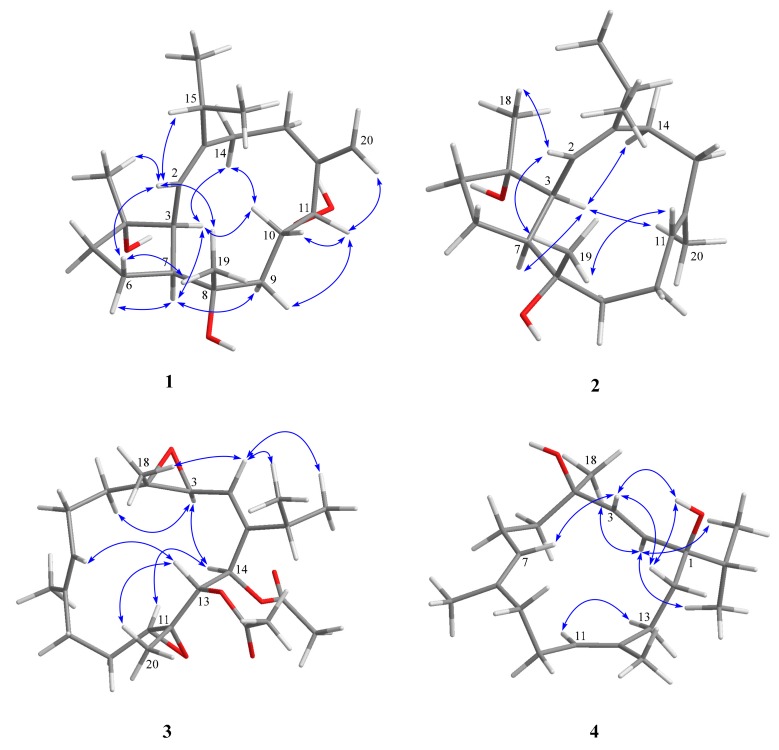
The relative configurations of 1 at C-3, C-4, C-7, C-8, and C-11 were proposed from the analysis of nuclear Overhauser effect spectroscopy (NOESY) correlations in combination with molecular modeling using molecular mechanical parameters (MM2 force field) calculations (Figure 3). A strong NOE interaction of H-3 (δH 2.89, dd, J = 10.0, 10.0 Hz) with H-7 (δH 2.63, m) indicated the syn-orientation of both protons which were arbitrarily assigned as α-oriented. The olefinic proton H-2 (δH 4.96, d, J = 9.5 Hz) exhibited NOESY correlations with both of H3-18 and H3-19, but not correlated with H-3. Therefore, H3-18 and H3-19 should be placed on the β-face of the molecule. One of the methylene protons at C-10 (δH 1.68, ddd, J = 13.0, 10.5, 3.0 Hz, H-10α) displayed NOE interaction with H-3, while the other one (δH 1.94, m, H-10β) exhibited NOE correlation with H-11. H-11 is thus β-oriented, and the 11-hydroperoxy group is α-oriented accordingly. The NOE interaction shown by H-11 with H-9β (δH 1.20 ddd, J = 13.0, 13.0, 4.5 Hz) and by H-9α (δH 1.85 ddd, J = 13.0, 13.0, 3.0 Hz) with H-7 further confirmed the α-orientation of 11-OOH. The E-geometry of the 1,2-double bond was assigned from the NOE correlation of H-2 with H-15 of the isopropyl moiety. From the above findings and other detailed NOEs analysis (Figure 3), the relative configuration of 1 was determined as 1E, 3S *, 4S *, 7S *, 8S *, 11R *.
Figure 3.
Selected nuclear Overhauser effect (NOE) correlations for 1 to 4.
The molecular formula of compound 2 was found to be C20H34O2 as deduced from HRESIMS and 13C NMR data, appropriate for four degrees of unsaturation. The IR spectrum showed the presence of the hydroxy group (νmax 3391 cm−1) and olefinic functionality (νmax 1656 cm−1). The NMR spectroscopic data were found to be similar to those of pavidolide D [37], while detailed analyses of 2D NMR correlations (COSY and HMBC) revealed that both two compounds possessed the same molecular skeleton (Figure 2). On careful comparison of the 1H and 13C NMR spectroscopic data (Table 1 and Table 2, respectively) of 2 with those of pavidolide D, it was found that the ring-juncture protons H-3 (δH 2.37, dd, J = 9.6, 7.8 Hz) and H-7 (δH 2.65, ddd, J = 9.6, 7.8, 6.6 Hz) showed differentiable NMR data with the corresponding H-3 (δH 2.55, t, J =10.3 Hz) and H-7 (δH 1.98, m) of the known compound. Further NOE correlations analysis of 2, confirmed compound 2 to be a new capnosane diterpenoid that should be the 7-epimer of pavidolide D. The assignment of the cis ring juncture protons H-3 and H-7 in 2 was further supported by the similar upfield chemical shift C-7 in 2 (δC 54.3) with that of trocheliophol F (δC 54.2) [17] relative to those of the related capnosenes with trans fused rings (δC ≥ 55.5) [16,17,37]. Furthermore, the E-geometry of C-11/C-12 double bond was determined from the NOE correlations observed between H3-20 and H-3 and between H-11 and H3-19, and from the chemical shift of C-20 (δC < 20 ppm) [42].
Flaccidodioxide (3) was obtained as a colorless oil and had a molecular formula of C24H36O6 as established by HRESIMS (m/z 443.2406 [M + Na]+, calcd 443.2404), corresponding to seven degrees of unsaturation. IR absorption at 1746 cm−1 showed the presence of ester carbonyl group. Comparison of the NMR spectroscopic data of 3 (Table 1 and Table 2), with those of a known metabolite gibberosene C [43], revealed that both compounds possessed the same 3(4),11(12)-diepoxyl groups in the cembrane ring-structure. The hydroxyl group at C-13 of gibbrosene C was acetylated, and the methylene group of C-14 was converted to acetoxyl-bearing methine, as support by the downfield chemical shifts of H-13 (δH 5.49) and H-14 (δH 5.89) of 3, and the three-bond correlations of both protons to two acetate carbonyl carbons unveiled from HMBC spectrum of 3. The planar structure of 3 was further confirmed by the detailed analyses of 1H-1H COSY and HMBC spectra (Figure 2). These results, along with extensive analysis of NOE correlations of 3 (Figure 3), determined the structure of 3 unambiguously.
Flaccidodiol (4) was isolated as a gum. HRESIMS of 4 exhibited a sodiated molecular ion peak at m/z 329.2448, corresponding to the molecular formula C20H34O2. IR absorptions at 3457 cm−1 indicated the presence of a hydroxyl group in 4. The NMR data (Table 1 and Table 2) assigned the presence of an isopropyl group and three methyls, including two olefinic ones and one attaching to an oxygenated sp3 carbon atom. It was found that the proton and carbon chemical shifts of 4 were very similar to those of a known dihydroxycembrane, sarcophytol T [34]. Thus, both compounds should be diastereomers. Furthermore, the proton signal of 1-OH exhibited NOE correlation with H-3, while H3-18 showed NOE correlation with H-2, revealing the position of both 1-OH and 4-OH to be in the same face of 4. Thus, the relative configuration for 4 was established.
Cytotoxicities of metabolites 1 to 7 against the growth of human lung adenocarcinoma (A549), human colorectal adenocarcinoma (DLD-1), and mouse lymphocytic leukemia (P388D1) cell lines were screened. Compounds 1, 2, and 7 exhibited inhibitory activity against the growth of the tested cancer cells (Table 3). The hydroperoxyl (as in 1) seem to potentiate the cytotoxic effect of the diterpenoid molecules. However, the diepoxide compound 3 could selectively inhibit the growth of P388D1 cancer cells relative to the inactive monoepoxide derivative (6). These compounds also exhibited the potent anti-inflammatory activity by suppressing O2−• generation and elastase release in the fMLF/CB-stimulated human neutrophils (Table 4). Compound 7 could effectively inhibit elastase release (IC50 = 7.22 ± 0.85 µM), reducing the level of elastase release to 59.66 ± 0.83% at a concentration of 10 µM relative to the control group.
Table 3.
Cytotoxicity (IC50 μg/mL) of compounds 1 to 7.
| Compound | Cell Lines IC50 (μg/mL) | ||
|---|---|---|---|
| A549 a | DLD-1 b | P388D1 c | |
| 1 | 9.7 ± 1.2 | 6.0 ± 0.4 | 7.2 ± 1.8 |
| 2 | 28.6 ± 3.8 | 31.6 ± 3.7 | 30.4 ± 4.8 |
| 3 | − d | − | 19.6 ± 8.3 |
| 4 | − | − | − |
| 5 | − | − | − |
| 6 | − | − | − |
| 7 | 10.8 ± 4.9 | 11.7 ± 4.8 | 8.9 ± 2.2 |
| Doxorubicin e | 0.3 ± 0.1 | 1.5 ± 0.2 | 0.9 ± 0.2 |
a A549: Human lung adenocarcinoma. b DLD-1: Human colorectal adenocarcinoma. c P388D1: Mouse lymphocytic leukemia cell line. d IC50 > 40 μg/mL. e Clinical anticancer drug used as a positive control.
Table 4.
Inhibitory effects of compounds 2 to 7 on superoxide anion generation and elastase release in N-formyl-methionyl-leucyl-phenylalanine/cytochalasin B (fMLF/CB)-induced human neutrophils at 10 μM.
| Compounds | Superoxide Anion | Elastase Release | ||||
|---|---|---|---|---|---|---|
| IC50 (μM) a | Inh % b | IC50 (μM) a | Inh % b | |||
| 2 | >10 | 24.46 ± 6.99 | * | >10 | 29.96 ± 6.14 | ** |
| 3 | >10 | 8.88 ± 3.33 | >10 | 27.18 ± 4.05 | ** | |
| 4 | >10 | 3.29 ± 0.88 | * | >10 | 14.43 ± 3.75 | * |
| 5 | >10 | 8.89 ± 4.28 | >10 | 14.89 ± 3.62 | * | |
| 6 | >10 | 4.73 ± 1.53 | * | >10 | 3.09 ± 3.88 | |
| 7 | >10 | 11.95 ± 2.53 | ** | 7.22 ± 0.85 | 59.66 ± 0.83 | *** |
| Idelalisib | 0.07 ± 0.01 | 102.8 ± 2.2 | *** | 0.3 ± 0.1 | 99.6 ± 4.2 | *** |
a Concentration necessary for 50% inhibition (IC50). b Percentage of inhibition (Inh %) at 10 μM. Results are shown as mean ± S.E.M. (n = 3–4). * p < 0.05, ** p < 0.01, *** p < 0.001 compared with the control value.
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations and IR spectra were measured on a JASCO P-1020 polarimeter and JASCO FT/IR-4100 spectrophotometer (JASCO Corporation, Tokyo, Japan), respectively. NMR spectra were recorded on a Varian 400 FT-NMR (Bruker, Bremen, Germany) at 400 and 100 MHz for 1H and 13C, respectively; and on a Varian Unity INOVA 500 FT-NMR (Varian Inc., Palo Alto, CA, USA) at 500 and 125 MHz for 1H and 13C, respectively, in C6D6 or CDCl3 (δ in ppm, J in Hz). Silica and RP-18 silica gels (230 to 400 mesh), and precoated silica gel 60 F-254 (0.2 mm) plates (Merck, Darmstadt, Germany) were used for column and thin-layer chromatography, respectively. Preparative high-performance liquid chromatography (HPLC) was performed on a Hitachi L-2455 HPLC apparatus (Hitachi Ltd., Tokyo, Japan) with a Supelco C18 (250 × 21.2 mm, 5 μm) column (Supelco, Bellefonte, PA, USA).
3.2. Animal Material
The soft coral Klyxum flaccidum Tixier-Durivault was collected along the coast of Pratas island, Taiwan, and stored until extraction as described before [32]. The organism was identified by one of the co-authors (C.-F.D.). A voucher sample (No. LI6) was deposited at the Department of Marine Biotechnology and Resources, National Sun Yat-sen University.
3.3. Extraction and Isolation
The frozen bodies of K. flaccidum (8.0 kg, wet weight) were sliced and worked up as described previously to yield EtOAc extract (120 g) [32]. Twenty-six fractions were eluted by silica gel column chromatography, using n-hexane, EtOAc–n-hexane (0:100 to 100:0, gradient), and subsequently MeOH–EtOAc (1:100 to 100:1, gradient). Based on the analysis of 1H NMR spectra, the terpenoids-containing fractions were selected for further chromatographic separation. Fraction 7 eluted with EtOAc–n-hexane (1:3) was purified by a Sephadex LH-20 column using acetone as eluent to obtain a mixture (7a to 7c), Subfraction 7a was further separated by reversed-phase RP-18 silica gel column using MeOH–H2O (3:1) to give 3 (4.8 mg) and 5 (34.6 mg), and subfraction 7c using MeOH–H2O (4:1) to give 4 (1.3 mg). Subfraction 7b was further separated by RP-18 silica gel column using MeOH–H2O (4:1), and further semipreparative RP-18 HPLC (MeOH–H2O, 3:1) to yield compounds 6 (3.4 mg) and 7 (2.1 mg). Fraction 13, eluted with EtOAc–n-hexane (3:1), was also rechromatographed by a Sephadex LH-20 column, using acetone as the mobile phase, and then was further purified by RP-18 HPLC using MeOH–H2O (5:1) to afford 1 (1.5 mg) and 2 (9.7 mg).
Flaccidenol A (1): Colorless oil; −10.3 (c 0.43, CHCl3); IR (neat) νmax 3391, 2957, 2870, 1647, 1455, 1376, 1109, 912, 754, and 614 cm–1; 13C NMR data (125 MHz; C6D6), see Table 1; 1H NMR data (500 MHz; C6D6), see Table 2; ESIMS m/z 361 [M + Na]+; HRESIMS m/z 361.2349 [M + Na]+ (calcd for C20H34O4Na, 361.2349).
7-epi-Pavidolide D (2): Colorless oil; −3.8 (c 2.77, CHCl3); IR (neat) νmax 3391, 2957, 2865, 1656, 1451, 1372, 1127, 908, and 754 cm–1; 13C NMR data (100 MHz; CDCl3), see Table 1; 1H NMR data (400 MHz; CDCl3), see Table 2; ESIMS m/z 329 [M + Na]+; HRESIMS m/z 329.2453 [M + Na]+ (calcd for C20H34O2Na, 329.2452).
Flaccidodioxide (3): Colorless oil; +13.0 (c 0.50, CHCl3); IR (neat) νmax 2962, 2930, 2872, 1746, 1455, 1373, 1223, 1029, 966, 877, and 757 cm–1; 13C NMR data (100 MHz; CDCl3), see Table 1; 1H NMR data (400 MHz; CDCl3), see Table 2; ESIMS m/z 443 [M + Na]+; HRESIMS m/z 443.2406 [M + Na]+ (calcd for C24H36O6Na, 443.2404).
Flaccidodiol (4): Colorless oil; −65.0 (c 0.25, CHCl3); IR (neat) νmax3457, 2925, 1450, 1368, 1095, and 986 cm–1; 13C NMR data (125 MHz; CDCl3), see Table 1; 1H NMR data (500 MHz; CDCl3), see Table 2; ESIMS m/z 329 [M + Na]+; HRESIMS m/z 329.2448 [M + Na]+ (calcd for C20H34O2Na, 329.2451).
3.4. Cytotoxicity Assay
The cancer cell lines were purchased from American Type Culture Collection (ATCC). Cytotoxicity of the compounds were measured using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric method [44,45]. The positive control used was doxorubicin. The compound was considered inactive if IC50 > 20 μg/mL.
3.5. In Vitro Anti-Inflammatory Assay
Human neutrophils were obtained from blood by dextran sedimentation, Ficoll-Hypaque centrifugation, and hypotonic lysis and then incubated as previously described [46]. Neutrophils (6 × 105 cells mL−1) incubated in Hank’s Balanced Salt Solution (HBSS) with MeO-Suc-Ala-Ala-Pro-Val-p-nitroanilide (100 μM), and Ca2+ (1 mM) at 37 °C were treated with the tested compound or Dimethyl sulfoxide (DMSO) for 5 min. The activation of neutrophils was challenged for 10 min with fMLF (100 nM)/CB (0.6 and 0.5 μg/mL‒1 for O2−• generation and elastase release, respectively). The anti-inflammatory activity of compounds 2 to 7 were assessed by measuring the inhibition of fMLF/CB-stimulated cells producing O2−• and elastase by using UV spectrometer detection at wavelength 550 nm and 405 nm, respectively [46,47].
4. Conclusions
Further chemical investigation on Formosan soft coral Klyxum flaccidum has led to the isolation and characterization of two new capnosanoids, flaccidenols A (1) and B (2); two new cembranoids, flaccidodioxide (3) and flaccidodiol (4); along with three known cembranoids. This is the first study to discover the rarely found capnosane diterpenoids from genus Klyxum. Diterpenoids 1, 2, and 7 exhibited significant cytotoxicity against a limited panel of cancer cell lines. The hydroperoxy group might be required to potentiate the cytotoxic effect, as shown by the capnosanoid 1. Moreover, 14-O-acetylsarcophytol B (7) could inhibit the elastase release in the fMLF/CB-stimulated human neutrophils effectively. This is the first time to disclose bioactivity for this known compound, which might be considered as an anti-inflammatory candidate.
Acknowledgments
Financial supported was mainly provided by the Ministry of Science and Technology of Taiwan (MOST 104-2113-M-110-006, 104-2320-B-110-001-MY2, and 107-2320-B-110-001-MY3) to J.-H.S., A.F.A. and R.S.O. extend their appreciation to the Deanship of Scientific Research at King Saud University for partial funding of this work through research group RG-1440-127.
Supplementary Materials
HRESIMS and NMR spectra of new compounds 1 to 4 are available online at https://www.mdpi.com/1660-3397/17/8/461/s1. Figure S1: HRESIMS spectrum of 1, Figure S2: 1H NMR spectrum of 1 in C6D6 at 500 MHz, Figure S3: 13C NMR spectrum of 1 in C6D6 at 125 MHz, Figure S4: 1H-1H COSY spectrum of 1 in C6D6, Figure S5: HSQC spectrum of 1 in C6D6, Figure S6: HMBC spectrum of 1 in C6D6, Figure S7: NOESY spectrum of 1 in C6D6, Figure S8: HRESIMS spectrum of 2, Figure S9: 1H NMR spectrum of 2 in CDCl3 at 400 MHz, Figure S10: 13C NMR spectrum of 2 in CDCl3 at 100 MHz, Figure S11: 1H-1H COSY spectrum of 2 in CDCl3, Figure S12: HSQC spectrum of 2 in CDCl3, Figure S13: HMBC spectrum of 2 in CDCl3, Figure S14: NOESY spectrum of 2 in CDCl3, Figure S15: HRESIMS spectrum of 3, Figure S16: 1H NMR spectrum of 3 in CDCl3 at 400 MHz, Figure S17: 13C NMR spectrum of 3 in CDCl3 at 100 MHz, Figure S18: 1H-1H COSY spectrum of 3 in CDCl3, Figure S19: HSQC spectrum of 3 in CDCl3, Figure S20: HMBC spectrum of 3 in CDCl3, Figure S21: NOESY spectrum of 3 in CDCl3, Figure S22: HRESIMS spectrum of 4, Figure S23: 1H NMR spectrum of 4 in CDCl3 at 500 MHz, Figure S24: 13C NMR spectrum of 4 in CDCl3 at 125 MHz, Figure S25: 1H-1H COSY spectrum of 4 in CDCl3, Figure S26: HSQC spectrum of 4 in CDCl3, Figure S27: HMBC spectrum of 3 in CDCl3, Figure S28: NOESY spectrum of 3 in CDCl3.
Author Contributions
J.-H.S. conceived the experiment. Y.-Y.T. and W.-R.T. isolated the compounds, performed spectroscopic data measurement and the initial interpretation. C.-Y.H., W.-R.T., A.F.A., and Y.-Y.T. performed the structure interpretation. C.-Y.H., A.F.A., C.-J.T., R.S.O., and J.-H.S. performed the spectroscopic data analyses, final structure determination, and preparation of the manuscript. C.-Y.H., T.-L.H., and Y.-H.W. contributed to the biological activity analyses. C.-F.D. contributed to species identification of the soft coral.
Funding
This research was mainly funded by the Ministry of Science and Technology of Taiwan (MOST 104-2113-M-110-006, 104-2320-B-110-001-MY2, and 107-2320-B-110-001-MY3) and further by Deanship of Scientific Research at King Saud University for funding this work through research group RG-1440-127.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Blunt J.W., Carroll A.R., Copp B.R., Davis R.A., Keyzers R.A., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2018;35:8–53. doi: 10.1039/C7NP00052A. [DOI] [PubMed] [Google Scholar]
- 2.Farag M.A., Fekry M.I., Al-Hammady M.A., Khalil M.N., El-Seedi H.R., Meyer A., Porzel A., Westphal H., Wessjohann L.A. Cytotoxic Effects of Sarcophyton sp. Soft Corals-Is there a correlation to their NMR fingerprints? Mar. Drugs. 2017;15:211. doi: 10.3390/md15070211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C.Y., Tseng Y.J., Chokkalingam U., Hwang T.L., Hsu C.H., Dai C.F., Sung P.J., Sheu J.H. Bioactive Isoprenoid-Derived Natural Products from a Dongsha Atoll Soft Coral Sinularia Erecta. J. Nat. Prod. 2016;79:1339–1346. doi: 10.1021/acs.jnatprod.5b01142. [DOI] [PubMed] [Google Scholar]
- 4.Huang C.Y., Sung P.J., Uvarani C., Su J.H., Lu M.C., Hwang T.L., Dai C.F., Wu S.L., Sheu J.H. Glaucumolides A and B biscembranoids with new structural type from a cultured soft coral Sarcophyton Glaucum. Sci. Rep. 2015;5:15624. doi: 10.1038/srep15624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eltahawy N.A., Ibrahim A.K., Radwan M.M., ElSohly M.A., Hassanean H.A., Ahmed S.A. Cytotoxic cembranoids from the Red Sea soft coral Sarcophyton Auritum. Tetrahedron Lett. 2014;55:3984–3988. doi: 10.1016/j.tetlet.2014.05.013. [DOI] [Google Scholar]
- 6.Liang L.F., Kurtan T., Mandi A., Yao L.G., Li J., Lan L.F., Guo Y.W. Structural, stereochemical and bioactive studies of cembranoids from Chinese soft coral Sarcophyton Trocheliophorum. Tetrahedron. 2018;74:1933–1941. doi: 10.1016/j.tet.2018.02.059. [DOI] [Google Scholar]
- 7.Cheng S.Y., Wen Z.H., Chiou S.F., Hsu C.H., Wang S.K., Dai C.F., Chiang M.Y., Duh C.Y. Durumolides A−E, anti-inflammatory and antibacterial cembranolides from the soft coral Lobophytum durum. Tetrahedron. 2008;64:9698–9704. doi: 10.1016/j.tet.2008.07.104. [DOI] [Google Scholar]
- 8.Bishara A., Rudi A., Benayahu Y., Kashman Y. Three biscembranoids and their monomeric counterpart cembranoid, a biogenetic Diels-Alder precursor, from the soft coral Sarcophyton elegans. J. Nat. Prod. 2007;70:1951–1954. doi: 10.1021/np070129n. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed A.F., Chen Y.W., Huang C.Y., Tseng Y.J., Lin C.C., Dai C.F., Wu Y.C., Sheu J.H. Isolation and structure elucidation of cembranoids from a Dongsha Atoll soft coral Sarcophyton stellatum. Mar. Drugs. 2018;16:210. doi: 10.3390/md16060210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai T.C., Chen H.Y., Sheu J.H., Chiang M.Y., Wen Z.H., Dai C.F., Su J.H. Structural elucidation and structure-anti-inflammatory activity relationships of cembranoids from cultured soft corals Sinularia sandensis and Sinularia flexibilis. J. Agric. Food Chem. 2015;63:7211–7218. doi: 10.1021/acs.jafc.5b01931. [DOI] [PubMed] [Google Scholar]
- 11.Thao N.P., Luyen B.T., Ngan N.T., Song S.B., Cuong N.X., Nam N.H., Kiem P.V., Kim Y.H., Minh C.V. New anti-inflammatory cembranoid diterpenoids from the Vietnamese soft coral Lobophytum crassum. Bioorg. Med. Chem. Lett. 2014;24:228–232. doi: 10.1016/j.bmcl.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 12.Qin G.F., Tang X.L., Sun Y.T., Luo X.C., Zhang J., van Ofwegen L., Sung P.J., Li P.L., Li G.Q. Terpenoids from the soft coral Sinularia sp. Collected in Yongxing Island. Mar. Drugs. 2018;16:127. doi: 10.3390/md16040127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng S.Y., Wang S.K., Duh C.Y. Secocrassumol, a seco-cembranoid from the Dongsha Atoll soft coral Lobophytum crassum. Mar. Drugs. 2014;12:6028–6037. doi: 10.3390/md12126028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng S.Y., Lin S.T., Wang S.K., Duh C.Y. α-Tocopherols from the Formosan soft coral Lobophytum crassum. Bull. Chem. Soc. Jpn. 2011;84:783–787. doi: 10.1246/bcsj.20110051. [DOI] [Google Scholar]
- 15.Ye F., Zhu Z.D., Gu Y.C., Li J., Zhu W.L., Guo Y.W. Further new diterpenoids as PTP1B inhibitors from the Xisha soft coral Sinularia polydactyla. Mar. Drugs. 2018;16:103. doi: 10.3390/md16040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xi Z., Bie W., Chen W., Liu D., van Ofwegen L., Proksch P., Lin W. Sarcophyolides B-E, new cembranoids from the soft coral Sarcophyton elegans. Mar. Drugs. 2013;11:3186–3196. doi: 10.3390/md11093186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Z., Cheng W., Liu D., van Ofwegen L., Proksch P., Lin W. Capnosane-type cembranoids from the soft coral Sarcophyton trocheliophorum with antibacterial effects. Tetrahedron. 2014;70:8703–8713. doi: 10.1016/j.tet.2014.09.034. [DOI] [Google Scholar]
- 18.Roethle P.A., Trauner D. The chemistry of marine furanocembranoids, pseudopteranes, gersolanes and related natural products. Nat. Prod. Rep. 2008;25:298–317. doi: 10.1039/b705660p. [DOI] [PubMed] [Google Scholar]
- 19.Liang C.H., Wang G.H., Liaw C.C., Lee M.F., Wang S.H., Cheng D.L., Chou T.H. Extracts from Cladiella australis, Clavularia viridis and Klyxum simplex (soft corals) are capable of inhibiting the growth of human oral squamous cell carcinoma cells. Mar. Drugs. 2008;6:95–606. doi: 10.3390/md6040595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen B.W., Wu Y.C., Chiang M.Y., Su J.H., Wang W.H., Fan T.Y., Sheu J.H. Eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Tetrahedron. 2009;65:7016–7022. doi: 10.1016/j.tet.2009.06.047. [DOI] [Google Scholar]
- 21.Wu S.L., Su J.H., Wen Z.H., Hsu C.H., Chen B.W., Dai C.F., Kuo Y.H., Sheu J.H. Simplexins A-I, eunicellin-based diterpenoids from the soft coral Klyxum simplex. J. Nat. Prod. 2009;72:994–1000. doi: 10.1021/np900064a. [DOI] [PubMed] [Google Scholar]
- 22.Chen B.W., Chao C.H., Su J.H., Wen Z.H., Sung P.J., Sheu J.H. Anti-inflammatory eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Org. Biomol. Chem. 2010;8:2363–2366. doi: 10.1039/b926353e. [DOI] [PubMed] [Google Scholar]
- 23.Chen B.W., Chao C.H., Su J.H., Tsai C.W., Wang W.H., Wen Z.H., Huang C.Y., Sung P.J., Wu Y.C., Sheu J.H. Klysimplexins I-T, eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Org. Biomol. Chem. 2011;9:834–844. doi: 10.1039/C0OB00351D. [DOI] [PubMed] [Google Scholar]
- 24.Chen B.W., Huang C.Y., Wen Z.H., Su J.H., Wang W.H., Sung P.J., Wu Y.C., Sheu J.H. Klysimplexins U-X, eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Bull. Chem. Soc. Jpn. 2011;84:1237–1242. doi: 10.1246/bcsj.20110156. [DOI] [PubMed] [Google Scholar]
- 25.Hsu F.J., Chen B.W., Wen Z.H., Huang C.Y., Dai C.F., Su J.H., Wu Y.C., Sheu J.H. Klymollins A-H, bioactive eunicellin-based diterpenoids from the formosan soft coral Klyxum molle. J. Nat. Prod. 2011;74:2467–2471. doi: 10.1021/np200589n. [DOI] [PubMed] [Google Scholar]
- 26.Wu S.L., Su J.H., Lu Y., Chen B.W., Huang C.Y., Wen Z.H., Kuo Y.H., Sheu J.H. Simplexins J-O, eunicellin-based diterpenoids from a Dongsha Atoll soft coral Klyxum simplex. Bull. Chem. Soc. Jpn. 2011;84:626–632. doi: 10.1246/bcsj.20110013. [DOI] [Google Scholar]
- 27.Lin M.C., Chen B.W., Huang C.Y., Dai C.F., Hwang T.L., Sheu J.H. Eunicellin-based diterpenoids from the Formosan soft coral Klyxum molle with inhibitory activity on superoxide generation and elastase release by neutrophils. J. Nat. Prod. 2013;76:1661–1667. doi: 10.1021/np400372v. [DOI] [PubMed] [Google Scholar]
- 28.Chang F.Y., Hsu F.J., Tai C.J., Wei W.C., Yang N.S., Sheu J.H. Klymollins T-X, bioactive eunicellin-based diterpenoids from the soft coral Klyxum molle. Mar. Drugs. 2014;12:3060–3071. doi: 10.3390/md12053060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai C.R., Huang C.Y., Chen B.W., Tsai Y.Y., Shih S.P., Hwang T.L., Dai C.F., Wang S.Y., Sheu J.H. New bioactive steroids from the soft coral Klyxum flaccidum. Rsc. Adv. 2015;5:12546–12554. doi: 10.1039/C4RA13977A. [DOI] [Google Scholar]
- 30.Chang F.Y., Chokkalingam U., Tai C.J., Huang C.Y., Wei W.C., Yang N.S., Su J.H., Sung P.J., Sheu J.H. New eunicellin-derived diterpenoids from a Taiwanese soft coral Klyxum molle. Tetrahedron. 2016;72:192–198. doi: 10.1016/j.tet.2015.11.025. [DOI] [Google Scholar]
- 31.Tseng W.R., Huang C.Y., Tsai Y.Y., Lin Y.S., Hwang T.L., Su J.H., Sung P.J., Dai C.F., Sheu J.H. New cytotoxic and anti-inflammatory steroids from the soft coral Klyxum flaccidum. Bioorg. Med. Chem. Lett. 2016;26:3253–3257. doi: 10.1016/j.bmcl.2016.05.060. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed A.F., Tsai C.R., Huang C.Y., Wang S.Y., Sheu J.H. Klyflaccicembranols A-I, new cmbranoids from thes soft coral Klyxum flaccidum. Mar. Drugs. 2017;15:23. doi: 10.3390/md15010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai Y.Y., Huang C.Y., Tseng W.R., Chiang P.L., Hwang T.L., Su J.H., Sung P.J., Dai C.F., Sheu J.H. Klyflaccisteroids K-M, bioactive steroidal derivatives from a soft coral Klyxum flaccidum. Bioorg. Med. Chem. Lett. 2017;27:1220–1224. doi: 10.1016/j.bmcl.2017.01.060. [DOI] [PubMed] [Google Scholar]
- 34.König G.M., Wright A.D. New cembranoid Diterpenes from the Soft Coral Sarcophyton ehrenbergi. J. Nat. Prod. 1998;61:494–496. doi: 10.1021/np9704112. [DOI] [Google Scholar]
- 35.Gray C.A., Davies-Coleman M.T., Schleyer M.H. Cembrane diterpenes from the southern African soft coral Cladiella kashmani. J. Nat. Prod. 2000;63:1551–1553. doi: 10.1021/np000179r. [DOI] [PubMed] [Google Scholar]
- 36.Iguchi K., Shimura H., Yamada Y. New Cembrane-type diterpenoids from the Okinawan soft coral Sinularia sp. J. Nat. Prod. 1992;55:1779–1782. doi: 10.1021/np50090a012. [DOI] [Google Scholar]
- 37.Shen S., Zhu H., Chen D., Liu D., van Ofwegen L., Proksch P., Lin W. Pavidolides A−E, new cembranoids from the soft coral Sinularia pavida. Tetrahedron Lett. 2012;53:5759–5762. doi: 10.1016/j.tetlet.2012.08.049. [DOI] [Google Scholar]
- 38.Chen W.T., Yao L.G., Li X.W., Guo Y.W. Sarcophytrols A–C, new capnosane diterpenoids from the South China Sea soft coral Sarcophyton trocheliophorum. Tetrahedron Lett. 2015;56:1348–1352. doi: 10.1016/j.tetlet.2015.01.157. [DOI] [Google Scholar]
- 39.Hegazy M.E., Gamal Eldeen A.M., Shahat A.A., Abdel-Latif F.F., Mohamed T.A., Whittlesey B.R., Pare P.W. Bioactive hydroperoxyl cembranoids from the Red Sea soft coral Sarcophyton glaucum. Mar. Drugs. 2012;10:209–222. doi: 10.3390/md10010209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng C.C., Huang C.Y., Ahmed A.F., Hwang T.L., Dai C.F., Sheu J.H. New Cembranoids and a biscembranoid peroxide from the soft coral Sarcophyton cherbonnieri. Mar. Drugs. 2018;16:276. doi: 10.3390/md16080276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su J.H., Ahmed A.F., Sung P.J., Chao C.H., Kuo Y.H., Sheu J.H. Manaarenolides A-I, diterpenoids from the soft coral Sinularia manaarensis. J. Nat. Prod. 2006;69:1134–1139. doi: 10.1021/np050483q. [DOI] [PubMed] [Google Scholar]
- 42.Kalinowski H.O., Berger S., Braun S. Carbon13 NMR Spectroscopy. John Wiley & Sons; Chichester, UK: 1988. [Google Scholar]
- 43.Ahmed A.F., Wen Z.H., Su J.H., Hsieh Y.T., Wu Y.C., Hu W.P., Sheu J.H. Oxygenated cembranoids from a Formosan soft coral Sinularia gibberosa. J. Nat. Prod. 2008;71:179–185. doi: 10.1021/np070356p. [DOI] [PubMed] [Google Scholar]
- 44.O’Brien J., Wilson I., Orton T., Pognan F. Investigation of the Alamar blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. FEBS. 2000;267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 45.Nakayama G.R., Caton M.C., Nova M.P., Parandoosh Z. Assessment of the Alamar Blue assay for cellular growth and viability in vitro. J. Immunol. Methods. 1997;204:205–208. doi: 10.1016/S0022-1759(97)00043-4. [DOI] [PubMed] [Google Scholar]
- 46.Hwang T.L., Li G.L., Lan Y.H., Chia Y.C., Hsieh P.W., Wu Y.H., Wu Y.C. Potent inhibition of superoxide anion production in activated human neutrophils by isopedicin, a bioactive component of the Chinese medicinal herb Fissistigma oldhamii. Free Radic. Biol. Med. 2009;46:520–528. doi: 10.1016/j.freeradbiomed.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 47.Yang S.C., Chung P.J., Ho C.M., Kuo C.Y., Hung M.F., Huang Y.T., Chang W.Y., Chang Y.W., Chan K.H., Hwang T.L. Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1. J. Immunol. 2013;190:6511–6519. doi: 10.4049/jimmunol.1202215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.