Abstract
Calendula officinalis, Ribes nigrum, and Vaccinium myrtillus (CRV) possess a high phenolic compound content with excellent antioxidant activity. Dietary antioxidants can reduce exercise-induced oxidative stress. Consumption of large amounts of phenolic compounds is positively correlated with reduction in exercise-induced muscle damage. Research for natural products to improve exercise capacity, relieve fatigue, and accelerate fatigue alleviation is ongoing. Here, CRV containing a large total phenolic content (13.4 mg/g of CRV) demonstrated antioxidant activity. Ultra-performance liquid chromatography quantification revealed 1.95 ± 0.02 mg of salidroside in 1 g of CRV. In the current study, CRV were administered to mice for five weeks, and the antifatigue effect of CRV was evaluated using the forelimb grip strength test; weight-loaded swimming test; and measurement of fatigue-related biochemical indicators, such as blood lactate, ammonia, glucose, blood urea nitrogen (BUN), and creatine kinase (CK) activity; and muscle and liver glycogen content. The results indicated that in CRV-treated mice, the forelimb grip strength significantly increased; weight-loaded swimming time prolonged; their lactate, ammonia, BUN, and CK activity decreased, and muscle and liver glucose and glycogen content increased compared with the vehicle group. Thus, CRV have antifatigue activity and can increase exercise tolerance.
Keywords: antifatigue activity, antioxidant, polyphenol, salidroside, exercise adaption
1. Introduction
Fatigue is one of the most common physiological reactions. The main physiological effect of fatigue is on the energy metabolism during muscle activity [1]. Endurance exercise has a positive effect on antifatigue activity [1]. Currently, scientists are seeking natural products that can not only improve exercise capacity, but also relieve fatigue and accelerate fatigue alleviation without side effects [2]. Fatigue is the loss of normal performance during an athletic activity or exercise and is often associated with tissue damage and energy imbalance. Recovery from fatigue depends on various modes of challenge and treatment. However, few studies have investigated the effects of functional plant extracts on exercise performance and antifatigue activity.
Calendula officinalis is a popular medicinal plant rich in bioactive phytochemicals such as terpenoids, flavonoids, coumarins, quinones, volatile oil, and carotenoids [3]. Ribes nigrum contains vitamin C and a large amount of phenolic compounds [4,5,6,7]. Vaccinium myrtillus has numerous health-promoting properties attributable to proanthocyanidins and anthocyanins [8,9,10,11]. In addition, phenolic compounds with high antioxidant capacity are commonly present in edible plants [12]. In this study, C. officinalis, R. nigrum, and V. myrtillus (CRV), all of which contain high levels of phenolic compounds, were used. Moreover, dietary antioxidants can reduce the effects of exercise-induced oxidative stress and improve physiological conditions [13,14,15]. Recent epidemiological studies have demonstrated that the consumption of large amounts of phenolic compounds is positively correlated with the prevention of exercise-induced muscle damage [16]. Therefore, here, we evaluated the effects of CRV on exercise performance fatigue–associated biochemical indicators in mice.
2. Materials and Methods
2.1. Materials
The supplement was extracted from Calendula officinalis, Ribes nigrum and Vaccinium myrtillus and provided from HOCKSHENG Trading Co., LTD (Taipei City, Taiwan).
2.2. Extraction and Isolation
CRV was cut into small pieces and soaked in 40% ethanol at ambient temperature for 2 h for 3 times. The extract was decanted, filtered under vacuum, concentrated in a rotary evaporator, and then lyophilized. The extract was isolated using a Waters Acquity UPLC system (Waters, Prague, Czech Republic) equipped with a photodiode array detector and a 2.1 × 50.0 mm2, 1.7-µm-internal-diameter Waters Acquity BEH C18 column. The mobile phase comprised solvent A, ultrapure water and solvent B, methanol. Elution conditions were 0–5 min of 98–98% A to B; 5–8 min of 98–10% A to B (linear gradient); 8–10 min of 10%–10% A to B; 10–11 min of 10–98% A to B (linear gradient); 11–15 min of 98–98% A to B at a flow rate of 0.8 mL/min. The individual peak area corresponding to salidroside set as the index compound in the UPLC profile was determined at the observed maximal absorbance at 223 nm. A standard calibration curve of salidroside was obtained with a series of standard compound concentrations. Quantification of the index compound in CRV was subsequently performed through UPLC analysis. The peak area of the candidate compound in the chromatogram of CRV was defined, and its content in the sample was obtained on the basis of the quantity calculated from the standard calibration curve.
2.3. Determination of Total Phenolics
This assay was determined according to the method described by Tung et al. [17]. The total phenolic content was determined according to the Folin–Ciocalteu method, with gallic acid as the standard. CRV were dissolved in methanol mixed with water (50:50, v/v). The solution (500 µL) was mixed with 500 µL of 50% Folin–Ciocalteu reagent. The mixture was kept for 5 min before 1.0 mL of 20% Na2CO3 was added. After 10 min of incubation at room temperature, the mixture was centrifuged for 8 min (12,000× g), and the absorbance of the supernatant was measured at 730 nm. The total phenolic content was expressed as gallic acid equivalent in milligrams per gram sample. Three replicates were made for each test sample.
2.4. Antioxidant Activity
The scavenging activity of CRV DPPH radicals was determined. A dose of 10 μL of CRV in DMSO (1, 5, 10, 50, and 100 μg/mL) was mixed with 200 μL of 0.1 mM DPPH–ethanol solution and 90 μL of 50 mM Tris-HCl buffer (pH 7.4). DMSO and (+)-catechin were used as control and positive control, respectively, for this experiment. After 30 min of incubation at room temperature, the reduction of DPPH radicals was measured by reading the absorbance at 517 nm. The inhibition ratio (%) was calculated as follows: % inhibition = [(absorbance of control − absorbance of test sample)/absorbance of control] × 100. Three replicates were made for each test sample.
2.5. Animals and Experimental Designs
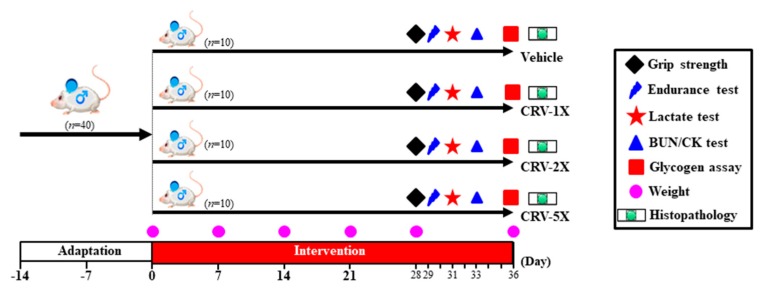
Male ICR mice (aged 8 weeks) from BioLASCO Taiwan (Yi-Lan, Taiwan) with AAALAC accreditation were used in this study. The standard laboratory diet #5001 (PMI Nutrition International, Brentwood, MO, USA) and distilled water were provided to all animals ad libitum, and the environmental conditions were maintained at constant photoperiod, temperature, and humidity (12-h light–12-h dark cycle, 24 °C ± 2 °C and 55–65%, respectively). Routine bedding cleaning was conducted twice per week, and a veterinarian observed and monitored the behavior and health of the animals daily. The Institutional Animal Care and Use Committee (IACUC) of National Taiwan Sport University approved all protocols in the current study with regard to animal welfare considerations, and the study conformed to the guidelines of protocol IACUC-10606 approved by the IACUC Ethics Committee. The starting dosage of CRV supplement recommended was 80 mg/kg, 1×. Furthermore, distinct dependence dosages (vehicle, CRV-1X, CRV-2X, and CRV-5X groups) were designed to validate the possible physiological activities. The detailed experimental procedure is illustrated in Figure 1. All animals were provided with an acclimation period of 2 weeks to adapt to the environment and diet prior to the intervention. The body weight, diet, and social behavior were monitored during supplementation, and daily supplements were administered regularly with fresh CRV sample preparations. Treatment dosages of 0, 80, 160, and 400 mg/kg/day were designated for the vehicle, CRV-1X, CRV-2X, and CRV-5X groups and administered by oral gavage with a volume of 10 mL/kg of the body weight. During 5-weeks CRV treatment, the mice were not subjected to any physical efforts. The grip strength and aerobic endurance capacities were measured to evaluate physical fitness, and the exercise-related biochemistries were immediately assessed during an acute exercise intervention.
Figure 1.
Experimental designs for the effect of Calendula officinalis, Ribes nigrum, and Vaccinium myrtillus (CRV) on antifatigue activity. The animals were randomly assigned to the indicated four groups (vehicle, CRV-1X, CRV-2X, and CRV-5X), and CRV were continual supplemented until the end of the experiment. The physical capacities, related biochemistries, glycogen, and histopathology were assessed in the study.
2.6. Exercise Endurance Performance Test
Exercise performance was based on the survival motives to assess the aerobic endurance capacities. The animals were loaded with a weight equivalent to 5% of their individual body weight and forced to swim in a tank until exhaustion. The persistent time from beginning to exhaustion was recorded as the endurance index. The detailed procedure and protocol were described in our previous study [18].
2.7. Forelimb Grip Strength
The grip strength was assessed using a low-force testing system (Model-RX-5, Aikoh Engineering, Nagoya, Japan) for forelimb muscle strength. The details of the method were described in our previous study [19].
2.8. Determination of Fatigue-Associated Biochemical Variables
According to our previous report [20], the effect of CRV supplementation on fatigue-related biochemical indicators has been slightly modified to accurately display the physiological status. The animals were fasting for at least 10 h to maintain basal physiological levels before the test. For the lactate metabolite profile, the blood sampling time points were before exercise, immediately after 10 min of acute exercise, and after 20 min of rest. In addition, the NH3 and glucose concentrations were analyzed immediately after 10 min of acute exercise. Blood urea nitrogen (BUN) concentration and creatine kinase (CK) activity were subsequently assessed immediately at the 60-min rest time point after 90 min of acute exercise. The blood samples were kept at room temperature for 40 min to enable clotting, after which the samples were centrifuged at 1000× g at 4 °C for 15 min. Subsequently, the serum was determined using an AutoAnalyzer (Hitachi 7060, Hitachi, Tokyo, Japan). Besides, the lactate production rate was calculated as the post-exercise rate divided by the before exercise rate (B/A), and the lactate difference between the post-exercise rate and the post-rest rate divided by the post-rest rate was defined as the clearance rate.
2.9. Clinical Biochemical Profiles
CRV supplementation was administered continuously until the animals were sacrificed. All mice were euthanatized using 95% CO2 asphyxiation 1 h after the last treatment, and blood was immediately sampled by cardiac puncture. Serum was separated through centrifugation, and clinical biochemical variables, including aspartate aminotransferase, alanine transaminase, CK, glucose, creatinine, BUN, UA, total cholesterol, triglyceride, albumin, and total protein levels, were measured using an AutoAnalyzer (Hitachi 7060).
2.10. Body Composition and Glycogen Content Analysis
After the mice were sacrificed, the main visceral organs, namely the liver, muscles (gastrocnemius and soleus), kidney, heart, lung, EFP, and BAT, were accurately excised and weighed. The organs were then saved in 10% formalin for further histopathological analysis. Portions of the liver and muscles were stored in liquid nitrogen for glycogen content analysis, as described previously [21].
2.11. Histopathology
The visceral organs preserved in 10% formalin were trimmed and embedded in paraffin for tissue sections of 4-μm thickness. Tissue sections were further stained with hematoxylin and eosin and examined under a light microscope equipped with a CCD camera (BX-51, Olympus, Tokyo, Japan) by a veterinary pathologist.
2.12. Statistical Analysis
The data were represented as means ± standard errors of the mean (n = 10), and the physical activities, biochemistries, body composition, diet, and glycogen content were analyzed through one-way ANOVA for the statistical difference among groups. The Cochran–Armitage test was used to evaluate the dose-effect trend using SPSS (version 19.0). A mixed design two-way ANOVA (supplementation × time) was also applied to the supplementation effects on lactate metabolite profiles and growth curve within repeated time points. Data were considered statistically significant when the probability of a type I error was < 0.05.
3. Results
3.1. Effect of CRV Supplementation on Grip Strength
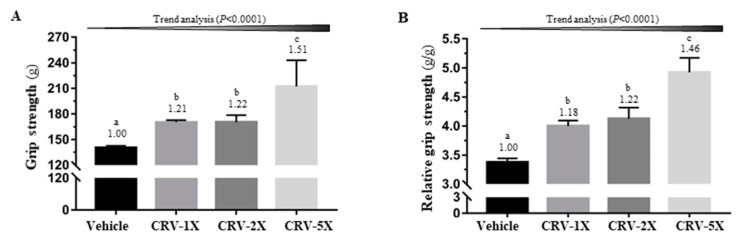
Figure 2A,B illustrates the difference in absolute and relative grip-strength among groups [F(3, 36) = 20.55 and 14.28, respectively; both p < 0.001]. For the supplementation groups, namely CRV-1X, CRV-2X, and CRV-5X, absolute strength was respectively 1.21-, 1.22-, and 1.51-fold and relative strength was 1.18-, 1.22-, and 1.46-fold higher than those of the vehicle group (all p < 0.05). In the trend analysis, the absolute grip force and relative grip force of the CRV-treated groups appeared to have significantly increased in a dose-dependent manner (p < 0.001).
Figure 2.
Effect of five-week CRV supplementation on the absolute forelimb grip strength (A) and forelimb grip strength relative to the body weight (B). Data are expressed as means ± standard errors of the mean for n = 10 mice per group. Columns with distinct superscript letters (a, b, and c) differ significantly at p < 0.05. Values above the columns are the fold changes compared with the vehicle group.
3.2. Effect of CRV Supplementation on Endurance Capacity
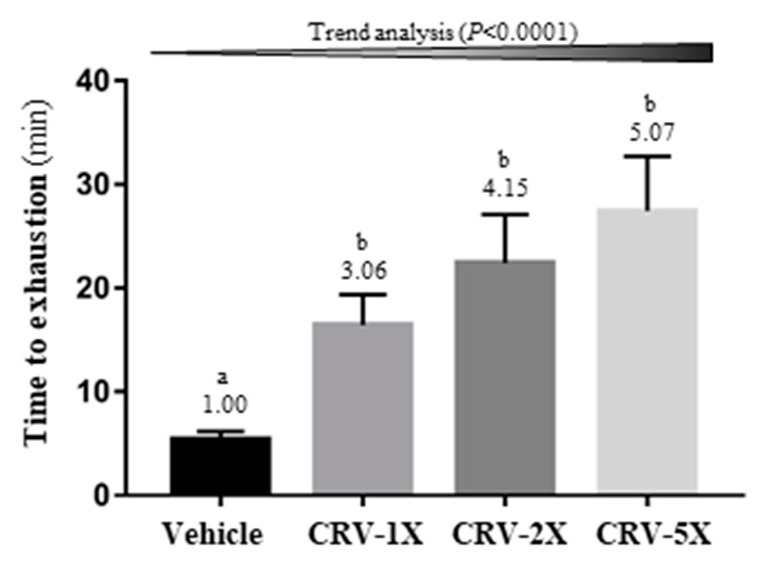
Endurance capacity was measured using the exhaustive swimming test, and the results revealed a significant difference among groups [F(3, 36) = 6.11, p = 0.002] (Figure 3). The CRV supplementation groups, namely CRV-1X, CRV-2X, and CRV-5X, had 3.06-, 4.15-, and 5.07-fold higher endurance capacity than the vehicle group; however, endurance capacity did not differ significantly among the CRV supplementation groups. Furthermore, the effects of endurance capacity in the CRV-treated groups significantly increased in a dose-dependent manner (p < 0.001).
Figure 3.
Effect of a five-week CRV supplementation on the exhaustive swimming time. Data are expressed as means ± standard errors of the mean for n = 10 mice per group. Columns with distinct superscript letters (a and b) differ significantly at p < 0.05. Values above the columns are the fold changes compared with the vehicle group.
3.3. Effect of CRV Supplementation on Exercise-Related Biochemical Indexes after Exercise Challenge
The lactate metabolite was assessed through repeated measurements (pre-exercise, immediately post-exercise, and after a rest) with various CRV treatments (Table 1). The results indicated significant differences in the supplement main and time effects [F(3, 36) = 27.32 and F(2, 72) = 2932.4, respectively; both p < 0.001]. In addition, the difference in the interaction between the supplement main and time effects was significant [F(6, 72) = 174.3, p < 0.001]. At the post-exercise and post-rest time points, the lactate concentration among the four groups exhibited significant differences [F(3, 36) = 45.53 and 66.50, respectively; p < 0.001]; moreover, the vehicle group had significantly higher lactate concentration than did the CRV-treated groups based on the one-way analysis of variance (ANOVA). In general, CRV supplementation reduced the lactate production rate and increased the clearance rate (p < 0.05).
Table 1.
Effects of Calendula officinalis, Ribes nigrum, and Vaccinium myrtillus (CRV) on lactate metabolite profiles during acute exercise challenge.
| Time Point | Vehicle | CRV-1X | CRV-2X | CRV-5X |
|---|---|---|---|---|
| Lactate (mmol/L) | ||||
| Before swimming (A) | 2.9 ± 0.2 a | 2.9 ± 0.2 a | 2.9 ± 0.2 a | 2.9 ± 0.1 a |
| After swimming (B) | 8.5 ± 0.3 b | 5.7 ± 0.2 a | 5.5 ± 0.2 a | 5.4 ± 0.2 a |
| After a 20-min rest (C) | 6.4 ± 0.2 b | 4.0 ± 0.2 a | 3.7 ± 0.2 a | 3.5 ± 0.2 a |
| Rate of lactate production and clearance | ||||
| Production rate = B/A | 2.96 ± 0.1 b | 2.03 ± 0.1 a | 1.92 ± 0.07 a | 1.85 ± 0.04 a |
| Clearance rate = (B − C)/B | 0.25 ± 0.01 b | 0.31 ± 0.02 a | 0.32 ± 0.01 a | 0.34 ± 0.02 a |
The lactate metabolites were assessed for the four groups, namely vehicle, CRV-1X, CRV-2X, and CRV-5X, at three repeated time points within each group. Data are expressed as means ± standard errors of the mean for n = 10 mice per group. Values in the same row with distinct superscript letters (a and b) differ significantly at p < 0.05.
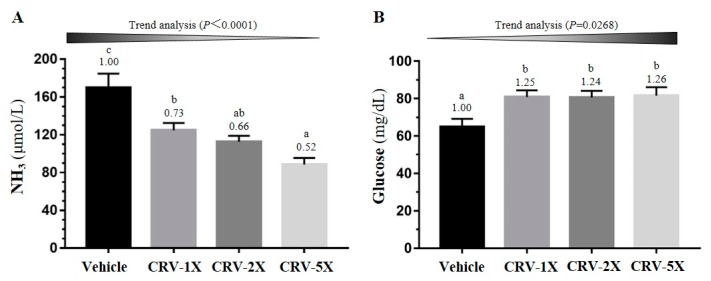
The energy homeostasis indexes, ammonia (NH3) and glucose concentrations, were measured immediately after the exercise (post-exercise point). As illustrated in Figure 4A, there was a significant difference between the four groups in NH3 [F(3, 36) = 12.92, p < 0.0001]; moreover, the NH3 concentration in the CRV-treated groups significantly decreased by approximately 30–50% compared with that in the vehicle group, all in a dose-dependent manner (p < 0.0001). In addition, the difference in glucose between the groups was significant [F(3, 36) = 3.99, p = 0.015]; the glucose concentration in the CRV-treated groups significantly increased by approximately 25% (p < 0.05; Figure 4B).
Figure 4.
Effect of a five-week CRV supplementation on the serum NH3 (A) and glucose (B) concentrations after acute 10-min swimming exercise challenge. Data are expressed as means ± standard errors of the mean for n = 10 mice per group. Columns with distinct superscript letters (a, b and c) differ significantly at p < 0.05. Values above the columns are the fold changes compared with the vehicle group.
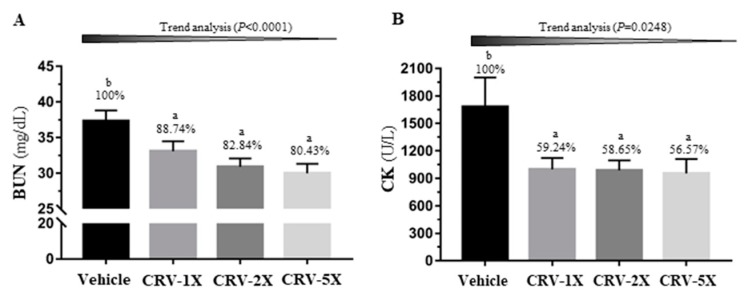
Figure 5A illustrates a significant difference in the other metabolic indicator, blood urea nitrogen (BUN), among groups after acute exercise [F(3, 36) = 5.63, p = 0.003]. CRV supplementations significantly decreased the exercise-induced BUN concentration by 12–20% (p < 0.05) in a dose-dependent manner (p < 0.0001). In addition, the other major injury index creatine kinase (CK) demonstrated significant differences among groups [F(3, 36) = 3.19, p = 0.035] (Figure 5B). CRV supplementation alleviated approximately 40% of the increase in CK and exhibited a dose-dependent trend (p = 0.0248).
Figure 5.
Effect of a five-week CRV supplementation on the serum blood urea nitrogen (BUN) concentration (A) and creatine kinase (CK) activity (B) after acute exercise challenge. The indicated four groups underwent 90 min of swimming exercise, and blood was sampled after 60 min of rest. Data are expressed as means ± standard errors of the mean for n = 10 mice per group. Columns with distinct superscript letters (a and b) differ significantly at p < 0.05. Values above the columns are the relative percentage (%) of the vehicle group.
3.4. Effect of CRV Supplementation on Glycogen Content
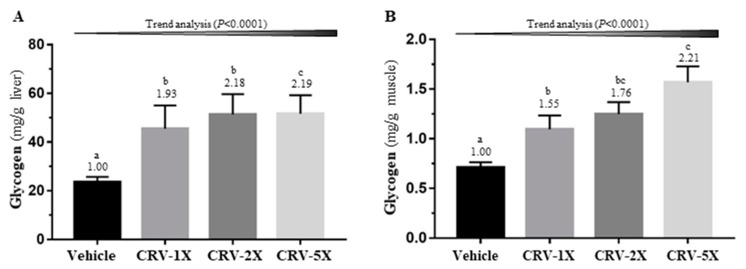
Glycogen stored in the liver and muscle tissue is used for energy regulation and homeostasis. Figure 6A illustrates that CRV supplementation significantly modulated the glycogen content in the liver [F(3, 36) = 3.16, p = 0.036]. Compared with the vehicle group, the liver glycogen in the CRV-1X, CRV-2X, and CRV-5X groups increased by 1.93-, 2.18-, and 2.19-fold, respectively, in a dose-dependent manner (p < 0.0001). In addition, muscle glycogen concentration exhibited a significant difference among the groups [F(3, 36) = 8.10, p < 0.0001]. The muscle glycogen of the CRV-1X, CRV-2X, and CRV-5X groups increased in a dose-dependent manner (p < 0.0001) and significantly increased by 1.55-, 1.76-, and 2.21-fold, respectively, compared with that of the vehicle group (Figure 6B).
Figure 6.
Effect of a five-week CRV supplementation on the liver (A) and muscle (B) glycogen concentration. Data are expressed as means ± standard errors of the mean for n = 10 mice per group. Columns with distinct superscript letters (a, b and c) differ significantly at p < 0.05. Values above the columns are the fold changes compared with the vehicle group.
3.5. Subacute Oral Toxicity Evaluation after CRV Supplementation
According to Organization for Economic Co-operation and Development (OECD) Test Guideline 407, subacute oral toxicity evaluation is used to assess the safety of a supplementation. Several indexes including behavior, diet, growth curve, organ weight, biochemistries, and histopathology were evaluated for the subacute toxic effects of CRV supplementation. Behavior was monitored daily during CRV administration, and the behavior was normal among the groups. As presented in Table 2, the treatment main effect did not demonstrate a significant difference in terms of body weight [F(3, 36) = 0.004, p = 1.00], but the time main effect exhibited a significant difference in body weight [F(5, 180) = 362.1, p < 0.0001]. Therefore, the results indicated that the increase in body weight occurred in a time-dependent manner. However, no significant difference was noted in the interaction effect (supplement × time) [F(15, 180) = 0.279, p = 0.997], and the one-way ANOVA at various time points revealed no significant difference between groups (p > 0.05). Moreover, no significant differences was noted in the diet and energy intake among CRV-treated groups.
Table 2.
Growth curve and dietary profiles during the experiment.
| Time Point | Vehicle | CRV-1X | CRV-2X | CRV-5X |
|---|---|---|---|---|
| Initial BW (g) | 38.2 ± 0.4 | 38.1 ± 0.8 | 28.1 ± 0.7 | 38.2 ± 0.5 |
| 1st wk BW (g) | 40.0 ± 0.7 | 40.0 ± 1.0 | 40.0 ± 0.8 | 40.0 ± 0.3 |
| 2nd wk BW (g) | 40.5 ± 0.6 | 40.8 ± 0.8 | 40.5 ± 0.8 | 40.8 ± 0.4 |
| 3rd wk BW (g) | 41.0 ± 0.7 | 41.0 ± 1.0 | 41.0 ± 0.8 | 41.0 ± 0.3 |
| 4th wk BW (g) | 41.7 ± 0.6 | 41.7 ± 1.1 | 41.7 ± 0.8 | 41.9 ± 0.2 |
| Final BW (g) | 42.7 ± 0.7 | 42.7 ± 1.1 | 42.7 ± 1.0 | 42.7 ± 0.3 |
| Water intake (mL/mouse/day) | 9.7 ± 0.2 | 9.6 ± 0.2 | 9.7 ± 0.2 | 9.6 ± 0.1 |
| Chow 5001 (g/mouse/day) | 7.1 ± 0.1 | 7.0 ± 0.1 | 7.0 ± 0.1 | 7.1 ± 0.1 |
| Energy intake (Kcal/mouse/day) | 23.8 ± 0.3 | 23.6 ± 0.3 | 23.5 ± 0.3 | 23.8 ± 0.5 |
The weight and diet were measured regularly for the four groups namely the vehicle, CRV-1X, CRV-2X, and CRV-5X groups. Data are expressed as means ± standard errors of the mean for n = 10 mice per group.
Body composition may also reflect the effect of supplementation on various organs (Table 3). The results indicated that there were no significant differences in the liver, muscle, kidney, heart, lung, epididymal fat pad (EFP), and brown adipose tissue (BAT) among the vehicle and CRV-treated groups (p > 0.05), and the relative organ weight adjusted by individual weight revealed similar results. Clinical biochemistry was used to assess the effect of supplementation on physiological conditions. As presented in Table 4, these indicators are related to the liver function, blood lipid, renal function and injury, and metabolic indicators applicable to current biochemical assessments. The uric acid (UA) indexes differed significantly among the groups [F(3, 36) = 11.88, p < 0.0001], and they were significantly lower in the CRV-treated groups than in the vehicle group (p < 0.05).
Table 3.
Effects of CRV on body composition.
| Characteristic | Vehicle | CRV-1X | CRV-2X | CRV-5X |
|---|---|---|---|---|
| Liver (g) | 2.27 ± 0.07 | 2.29 ± 0.10 | 2.26 ± 0.08 | 2.25 ± 0.05 |
| Muscle (g) | 0.41 ± 0.01 | 0.41 ± 0.01 | 0.41 ± 0.01 | 0.41 ± 0.01 |
| Kidney (g) | 0.62 ± 0.02 | 0.62 ± 0.02 | 0.62 ± 0.02 | 0.63 ± 0.02 |
| Heart (g) | 0.25 ± 0.01 | 0.24 ± 0.01 | 0.24 ± 0.01 | 0.23 ± 0.01 |
| Lung (g) | 0.23 ± 0.01 | 0.23 ± 0.01 | 0.23 ± 0.01 | 0.23 ± 0.01 |
| EFP (g) | 0.29 ± 0.04 | 0.29 ± 0.03 | 0.29 ± 0.04 | 0.29 ± 0.02 |
| BAT (g) | 0.12 ± 0.003 | 0.12 ± 0.002 | 0.12 ± 0.01 | 0.12 ± 0.01 |
| Relative liver weight (%) | 5.30 ± 0.08 | 5.34 ± 0.12 | 5.27 ± 0.09 | 5.28 ± 0.07 |
| Relative muscle weight (%) | 0.95 ± 0.01 | 0.97 ± 0.01 | 0.97 ± 0.01 | 0.95 ± 0.01 |
| Relative kidney weight (%) | 1.44 ± 0.02 | 1.45 ± 0.03 | 1.46 ± 0.02 | 1.47 ± 0.03 |
| Relative heart weight (%) | 0.58 ± 0.01 | 0.57 ± 0.01 | 0.56 ± 0.02 | 0.52 ± 0.01 |
| Relative lung weight (%) | 0.54 ± 0.003 | 0.54 ± 0.01 | 0.54 ± 0.003 | 0.53 ± 0.01 |
| Relative EFP weight (%) | 0.67 ± 0.08 | 0.67 ± 0.06 | 0.67 ± 0.08 | 0.67 ± 0.05 |
| Relative BAT weight (%) | 0.28 ± 0.003 | 0.29 ± 0.003 | 0.28 ± 0.01 | 0.28 ± 0.01 |
Data are expressed as means ± standard errors of the mean for n = 10 mice per group. EFP: epididymal fat pad; BAT: brown adipose tissue.
Table 4.
Effects of CRV on clinical biochemical analysis at the end of the experiment.
| Parameter | Vehicle | CRV-1X | CRV-2X | CRV-5X |
|---|---|---|---|---|
| AST (U/L) | 73 ± 4 | 68 ± 3 | 69 ± 5 | 69 ± 2 |
| ALT (U/L) | 49 ± 3 | 41 ± 2 | 41 ± 2 | 42 ± 3 |
| CK (U/L) | 199 ± 25 | 173 ± 23 | 176 ± 26 | 176 ± 26 |
| GLU (mg/dL) | 149 ± 4 | 144 ± 3 | 145 ± 3 | 144 ± 4 |
| CREA (mg/dL) | 0.25 ± 0.01 | 0.24 ± 0.01 | 0.23 ± 0.01 | 0.24 ± 0.01 |
| BUN (mg/dL) | 20.5 ± 0.6 | 20.0 ± 0.5 | 20.4 ± 0.7 | 20.1 ± 0.7 |
| UA (mg/dL) | 1.2 ± 0.1 b | 0.8 ± 0.04 a | 0.8 ± 0.1 a | 0.7 ± 0.03 a |
| TC (mg/dL) | 145 ± 4 | 141 ± 5 | 142 ± 6 | 135 ± 4 |
| TG (mg/dL) | 174 ± 6 | 157 ± 5 | 157 ± 8 | 158 ± 6 |
| ALB (g/dL) | 2.9 ± 0.03 | 2.9 ± 0.03 | 2.9 ± 0.04 | 3.0 ± 0.05 |
| TP (g/dL) | 4.9 ± 0.1 | 4.9 ± 0.05 | 4.9 ± 0.04 | 4.9 ± 0.04 |
Data are expressed as means ± standard errors of the mean for n = 10 mice per group. Values in the same row with distinct superscript letters (a and b) differ significantly at p < 0.05. AST: aspartate aminotransferase; ALT: alanine transaminase; CK: creatine kinase; GLU: glucose; CREA: creatinine; BUN: blood urea nitrogen; UA: uric acid; TC: total cholesterol; TG: triacylglycerol; ALB: albumin; TP: total protein.
Except for UA, no significant differences in biochemical indicators were noted among groups. In addition, the histopathological results did not exhibit pathological abnormalities caused by long-term CRV supplementation, as observed by a clinical veterinarian (Figure 7).
Figure 7.
Effect of CRV supplementation on the histomorphological features of the liver (A), muscle (B), heart (C), lung (D), kidney (E), and adipocyte (F) tissue in mice. Specimens were photographed under a light microscope (hematoxylin and eosin staining, magnification: 200×; scale bar, 40 or 80 μm).
4. Discussion
Physical exercise and nutritional behavior are now widely considered major parts of a healthy lifestyle. In addition, moderate exercise and active lifestyle are useful to prevent cardiovascular diseases [22], type 2 diabetes mellitus [23], metabolic syndrome [24], and neurodegenerative diseases [25,26]. Regular and moderate exercise represents a mild source of stress that can induce adaptive responses, and most organisms have the ability to adapt to stress. However, overexercising or overtraining can also lead to muscle damage, oxidative stress, and inflammation. Exercise induces the production of reactive oxygen species, which leads to oxidative stress, such as that by inducing lipid peroxidation [27,28,29,30], activating xanthine oxidase to produce superoxide anion, and increasing the oxidized/reduced glutathione (GSSG/GSH) ratio [31,32]. Moreover, exhaustive exercise can lead to oxidative stress, inflammation, and structural damage to muscle cells, as evidenced by the increased lactate dehydrogenase (LDH) and CK activities [29,33,34]. Therefore, several studies have investigated the possibility of preventing exercise-induced oxidative stress and muscle damage through nutritional interventions [35,36,37,38,39,40,41]. The current study systematically investigated the potential effects of CRV on exercise performance to prevent exercise-induced muscle damage.
C. officinalis is a popular medicinal plant and herb used in cosmetics in Europe and the United States. In traditional medicine, its flowers are used in the treatment of various skin conditions such as ulcers, eczema, burns, bruises, rashes, varicose veins, and acne in the form of ointments [42]. C. officinalis flower has been reported to possess a wide range of biological activities, including choleretic, anti-inflammatory, analgesic, anticancer, bactericidal, diuretic, tonic [43], anti-HIV, hepatoprotective, spasmolytic, and spasmogenic [3] actions. C. officinalis flower extracts are rich in secondary bioactive metabolites such as terpenoids, flavonoids, coumarins, quinones, volatile oil, and carotenoids [3]. Blackcurrant (R. nigrum) fruits are a rich source of vitamin C [7], and they contain a large amount of phenolic compounds, including phenolic acids, flavonoids, and most notably anthocyanins (250 mg/100 g of fresh fruit), and have antioxidant activity [4,5,6]. R. nigrum has therapeutic benefits in cardiovascular disorder treatment and is commonly reported as a free radical scavenger [44]. Bilberries (V. myrtillus) have numerous health-promoting properties attributed mostly to pharmacologically active ingredients (i.e., proanthocyanidins and anthocyanins) [8,9,10,11]. The content of anthocyanins in bilberries represents 60–70% [45]. Bilberries have demonstrated a wide range of biological activities, including antioxidant capacity, astringent and antiseptic properties, ability to reduce the permeability and fragility of capillaries, inhibition of platelet aggregation and urinary tract infection, and strengthening of collagen matrices through cross linkages [46,47,48,49,50].
Recently, plant phenolic compounds have received increased attention because epidemiological studies have revealed that a high phenol intake may positively correlate with the reduction of exercise-induced muscle damage [16]. Therefore, phenolic compounds play a biological and physiological role in the improvement of physical properties. In the current study (data not presented), CRV possessed a large total phenolic content (13.4 mg/g of CRV) and significant DPPH radical scavenging activity (antioxidant activity), with IC50 = 38.7 μg/mL. Preethi et al. [51] found that C. officinalis flower extract exhibited significant inhibition in DPPH free radical formation with IC50 values of 100 μg/mL. Olennikov et al. [52] reported that the basic phenolic groups of total extracts of C. officinalis flowers were flavonoids and phenylpropanoids with content values of 10.5 to 46.9 mg/g and 6.1 to 33.5 mg/g, respectively. Bryan-Thomas [53] showed that R. nigrum possessed a large total phenolic content (5.7 mg/g) and significant DPPH radical scavenging activity (IC50 = 40.8 μg/mL). Saral et al. [54] revealed that V. myrtillus had high total polyphenols (11.5-20.7 mg GAE/g dry sample), flavonoids (1. 2-2.7 mg QE/g dry sample) and anthocyanins (3.3-11.5 mg Cyn/g dry sample). Matsunaga et al. [55] found that V. myrtillus exhibited radical scavenging ability against DPPH radical, the IC50 value being 9.1 μg/mL. CRV is consist of C. officinalis, R. nigrum, and V. myrtillus. Taken together, these results indicate that CRV has better DPPH free radical scavenging ability than C. officinalis and R. nigrum, but is lower than V. myrtillus. In addition, total phenolic compounds of CRV are mainly derived from C. officinalis and V. myrtillus.
Among three plants of CRV, the major phytocompounds of C. officinalis are rutin, quercetin-3-O-glucoside, scopoletin-7-O-glucoside, isorhamnetin-3-O-glucoside and gallic acid [56]. R. nigrum is enriched in anthocyanins, delphinidin-3-O-glucoside, delphinidin-3-O-rutinoside, cyanidin-3-O-glucoside, cyanidin-3-O-rutinoside, petunidin-3-O-rutinoside [57]. In addition, V. myrtillus contains delphinidin (the major anthocyanin moiety), sinapic acid (the major phenolic acid in the free form), and p-coumaric acid in the ester, glycoside and ester-bound forms [58]. In this study, 1.95 ± 0.02 mg of salidroside was quantified in 1 g of CRV through ultra-performance liquid chromatography (UPLC). Salidroside has various biological effects, including liver protection [59], antihypoxia activity [60,61], suppression of leukemia cell growth [62], and reduction in the differentiation of 3T3-L1 adipocytes [63]. Salidroside can also stimulate muscle glucose uptake through AMPK activation [64] and alleviate the apoptosis caused by oxidative stress through AKT/mTOR/p70S6K and MAPK pathway regulation [65]. Therefore, these activities may be beneficial to exercise physiological adaption and performance; this result is supported by other experimental validations [66]. Regarding the dose, studies have indicated that relatively high doses (approximately 180 mg/kg) of salidroside can ameliorate exercise fatigue in 1 [67] or 15 [68] days. However, safety remains a major preliminary concern, particularly for single phytocompounds at such high doses. Therefore, the effective dose selected for the antifatigue effect of CRV is 80 mg/kg in the current study, and the safety of the supplementation is defined based on body composition as well as biochemical and pathological observations.
The grip strength and weight-loaded swimming test are commonly used to evaluate muscle fitness and endurance capacities [2,69]. Our results indicated that CRV-treated groups had significantly increased grip strength and prolonged swimming time (Figure 2; Figure 3), suggesting that CRV can improve exercise tolerance in mice. C. officinalis-flower and R. nigrum-fruit extracts are rich in flavonoids, and studies have proposed that some flavonoids are beneficial to aid exercise and exercise performance [16]. In addition, R. nigrum is a rich source of vitamin C. Askari et al. [70] conducted a double-blind clinical trial of 60 male students with an athletic history of at least 3 years and noted that daily treatment with 500 mg of quercetin plus 250 mg of vitamin C for 8 weeks improved some markers, including the lean body mass, basal metabolic rate, and total energy expenditure. Askari et al. [71] also reported that quercetin combined with vitamin C could reduce plasma CK after treadmill exercise. In addition, a study demonstrated that salidroside could significantly upregulate the expression of mTOR, p-mTOR, and myosin heavy chain (MyHC) in muscles and rescue their downregulation induced by inflammatory cytokines [72].
When muscles receive sufficient energy from anaerobic glycolysis during high-intensity exercise, muscles produce large amounts of lactate. Table 1 presents the lactate metabolite of the CRV-treated groups; it significantly decreased after swimming compared with the vehicle group. The results suggest that CRV can decrease the metabolite of blood lactate during exercise. BUN and NH3 are key blood biochemical parameters related to fatigue [73]. BUN is the product of energy metabolism during exercise, and it is a sensitive indicator to assess the bearing capability when the human body experiences physical loads. The BUN in vivo and exercise tolerance are positively correlated [74,75,76]. In other words, the body adapts poorly to exercise, the higher is the BUN concentration [77]. Urea is the final product of protein metabolism, mainly formed in the liver. During digestion, proteins are broken down into amino acids. Amino acids contain nitrogen and thus are used to generate energy or other substances needed by the cells; the remaining molecules are excreted as NH3. Figure 4A and Figure 5A indicates that the NH3 and BUN concentrations of the CRV-treated groups were lower than those of the vehicle group. In addition, high-intensity exercise challenges can cause physical or chemical tissue damage such as sacromeric damage, muscle cell necrosis, and oxidative stress [78]. Therefore, cells release specific proteins, such as CK and myoglobin, into the blood, and these can be used as muscle damage indexes. The CK activity increases with exercise loading. However, the serum CK activity of the CRV-treated groups was significantly lower than that of the vehicle group (Figure 6B). CRV extracts contain not only phenolic compounds, but also salidroside that exerts protective effects against the oxidative stress induced by free radicals [79]. Therefore, CRV also ameliorates the stress and fatigue. Energy during exercise is originally derived from the breakdown of glycogen, followed by the circulation of glucose released from the liver [80]. Therefore, liver and muscle glycogen are sensitive parameters related to fatigue. Figure 7A,B illustrates that the liver and muscle glycogen concentration of the CRV-treated groups were higher than those of the vehicle group after swimming. The glycogen is an important energy source to maintain the energy metabolism during physical activity and the exercise training or nutritional supplement could also modulate the glycogenolysis during exercise [81,82]. Besides, the other study also showed the glycogen content could be elevated by nutritional supplement for better performance [83,84]. Thus, the CRV could also positively regulate the glycogen metabolism for the higher exercise performance in current study. The effects of the animal experiment may not be extrapolated to clinical trials because of diversity on physiological and genomic backgrounds. However, the animal studies could not only explore the physiological effects from basic research point view, but also reveal the potential side effects regarding to toxicological and pathological evaluation and observation.
5. Conclusions
CRV has significant antifatigue effects, and these effects occur in a dose-dependent manner. Moreover, the possible antifatigue mechanisms reduced the concentrations of NH3 and BUN, and CK activity; increased glucose concentration; increased liver and muscle glycogen concentrations by improving the energy storage; elevated lactate metabolite concentration; and protected the muscle tissue. Therefore, CRV can improve the aerobic and anaerobic exercise capacity and physiological adaption in practical applications. CRV, which are rich in phenolic compounds and salidroside, can be potential supplementation alternatives for fatigue improvement; In addition, we also elucidated the doses of CRV with long-term supplementation were safe based on the observation of pathology, body composition, growth curve, and biochemistry indexes. However, further research for evaluating the antifatigue activity at the molecular levels and clinical trial is warranted.
Acknowledgments
The authors are grateful to the graduate students at the Sport Nutrition Laboratory of National Taiwan Sport University for their technical assistance in conducting animal experiments.
Author Contributions
W.-C.H., M.-F.W., and C.-C.H. designed the experiments. Y.-T.T., M.-F.W., M.-C.L., and J.-H.W. performed the laboratory experiments. M.-F.W. and C.-C.H. contributed the reagents, materials, and analysis platforms. Y.-T.T. and W.-C.H. analyzed the data. Y.-T.T., W.-C.H., and C.-C.H. interpreted the results, prepared the figures, wrote the manuscript, and revised the manuscript.
Funding
This study was funded in part by the University-Industry Cooperation Fund of National Taiwan Sport University, Taoyuan, Taiwan (No.1061010) and in part by the Ministry of Science and Technology (MOST) of Taiwan (grant no. MOST 107-2410-H-227-007).
Conflicts of Interest
The authors declare no conflict of interest and HOCKSHENG Trading Co., LTD (Taipei City, Taiwan) had no role in the design, analysis, or writing of this article.
References
- 1.Belluardo N., Westerblad H., Mudó G., Casabona A., Bruton J., Caniglia G., Pastoris O., Grassi F., Ibáñez C.F. Neuromuscular junction disassembly and muscle fatigue in mice lacking neurotrophin-4. Mol. Cell Neurosci. 2001;18:56–67. doi: 10.1006/mcne.2001.1001. [DOI] [PubMed] [Google Scholar]
- 2.Kim K.M., Yu K.W., Kang D.H., Suh H.J. Anti-stress and anti-fatigue effect of fermented rice bran. Phytother. Res. 2002;16:700–702. doi: 10.1002/ptr.1019. [DOI] [PubMed] [Google Scholar]
- 3.Preethi K.C., Kuttan R. Hepato and reno protective action of Calendula officinalis L. flower extract. Indian J. Exp. Biol. 2009;47:163–168. [PubMed] [Google Scholar]
- 4.Koeppen B.H., Herrmann K. Flavonoid glycosides and hydroxycinnamic acid esters of blackcurrants (Ribes nigrum). Phenolics of fruits 9. Z. Lebensm. Unters. 1977;164:263–268. doi: 10.1007/BF01147302. [DOI] [PubMed] [Google Scholar]
- 5.Macheix J.J., Fleuriet A., Billot J. Fruit Phenolics. CRC Press; Boca Raton, FL, USA: 1990. pp. 1–103. [Google Scholar]
- 6.Schuster B., Herrmann K. Hydroxybenzoic and hydroxycinnamic acid derivatives in soft fruits. Phytochemistry. 1985;24:2761–2764. doi: 10.1016/S0031-9422(00)80722-0. [DOI] [Google Scholar]
- 7.Anttonen M.J., Karjalainen R.O. High-performance liquid chromatography analysis of black currant (Ribes nigrum L.) fruit phenolics grown either conventionally or organically. J. Agric. Food Chem. 2006;54:7530–7538. doi: 10.1021/jf0615350. [DOI] [PubMed] [Google Scholar]
- 8.Katsube N., Iwashita K., Tsushida T., Yamaki K., Kobori M. Induction of apoptosis in cancer cells by Bilberry (Vaccinium myrtillus) and the anthocyanins. J. Agric. Food Chem. 2003;51:68–75. doi: 10.1021/jf025781x. [DOI] [PubMed] [Google Scholar]
- 9.Faria A., Oliveira J., Neves P., Gameiro P., Santos-Buelga C., de Freitas V., Mateus N. Antioxidant properties of prepared blueberry (Vaccinium myrtillus) extracts. J. Agric. Food Chem. 2005;53:6896–6902. doi: 10.1021/jf0511300. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Moreno C., Cao G., Ou B., Prior R. Anthocyanin and proanthocyanidin content in selected white and red wines. Oxygen radical absorbance capacity comparison with nontraditional wines obtained from highbush blueberry. J. Agric. Food Chem. 2003;51:4889–4896. doi: 10.1021/jf030081t. [DOI] [PubMed] [Google Scholar]
- 11.Santos-Buelga C., Scalbert A. Proanthocyanidins and tannin-like compounds snature, occurrence, dietary intake, and effects on nutrition and health. J. Sci. Food Agric. 2000;80:1094–1117. doi: 10.1002/(SICI)1097-0010(20000515)80:7<1094::AID-JSFA569>3.0.CO;2-1. [DOI] [Google Scholar]
- 12.Salta J., Martins A., Santos R.S., Neng N.R., Nogueira J.M.F., Justino J., Rauter A.P. Phenolic composition and antioxidant activity of Rocha pear and other pear cultivars—A comparative study. J. Funct. Foods. 2010;2:153–157. doi: 10.1016/j.jff.2010.02.002. [DOI] [Google Scholar]
- 13.Huang S.C., Lee F.T., Kuo T.Y., Yang J.H., Chien C.T. Attenuation of long-term Rhodiola rosea supplementation on exhaustive swimming-evoked oxidative stress in the rat tissues. Chin. J. Physiol. 2009;52:316–324. doi: 10.4077/CJP.2009.AMH029. [DOI] [PubMed] [Google Scholar]
- 14.Abbey E.L., Rankin J.W. Effect of quercetin supplementation on repeated-sprint performance, xanthine oxidase activity, and inflammation. Int. J. Sport Nutr. Exerc. Metab. 2011;21:91–96. doi: 10.1123/ijsnem.21.2.91. [DOI] [PubMed] [Google Scholar]
- 15.König D., Wagner K.H., Elmadfa I., Berg A. Exercise and oxidative stress: Significance of antioxidants with reference to inflammatory, muscular, and systemic stress. Exerc. Immunol. Rev. 2001;7:108–133. [PubMed] [Google Scholar]
- 16.Malaguti M., Angeloni C., Hrelia S. Polyphenols in exercise performance and prevention of exercise-induced muscle damage. Oxid. Med. Cell Longev. 2013;2013:825928. doi: 10.1155/2013/825928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tung Y.T., Lin L.C., Liu Y.L., Ho S.T., Lin C.Y., Chuang H.L., Chiu C.C., Huang C.C., Wu J.H. Antioxidative phytochemicals from Rhododendron oldhamii Maxim. leaf extracts reduce serum uric acid levels in potassium oxonate-induced hyperuricemic mice. BMC Complement. Altern. Med. 2015;15:423. doi: 10.1186/s12906-015-0950-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang W.C., Hsu Y.J., Wei L., Chen Y.J., Huang C.C. Association of physical performance and biochemical profile of mice with intrinsic endurance swimming. Int. J. Med. Sci. 2016;13:892–901. doi: 10.7150/ijms.16421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho C.S., Tung Y.T., Kung W.M., Huang W.C., Leung W.K., Huang C.C., Wu J.H. Effect of Coriolus versicolor mycelia extract on exercise performance and physical fatigue in mice. Int. J. Med. Sci. 2017;14:1110–1117. doi: 10.7150/ijms.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsiao C.Y., Hsu Y.J., Tung Y.T., Lee M.C., Huang C.C., Hsieh C.C. Effects of Antrodia camphorata and Panax ginseng supplementation on anti-fatigue properties in mice. J. Vet. Med. Sci. 2018;80:284–291. doi: 10.1292/jvms.17-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu Y.J., Huang W.C., Chiu C.C., Liu Y.L., Chiu W.C., Chiu C.H., Chiu Y.S., Huang C.C. Capsaicin supplementation reduces physical fatigue and improves exercise performance in mice. Nutrients. 2016;8:648. doi: 10.3390/nu8100648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daviglus M.L., Lloyd-Jones D.M., Pirzada A. Preventing cardiovascular disease in the 21st century: Therapeutic and preventive implications of current evidence. Am. J. Cardiovasc. Drugs. 2006;6:87–101. doi: 10.2165/00129784-200606020-00003. [DOI] [PubMed] [Google Scholar]
- 23.Bertram M.Y., Lim S.S., Barendregt J.J., Vos T. Assessing the cost-effectiveness of drug and lifestyle intervention following opportunistic screening for pre-diabetes in primary care. Diabetologia. 2010;53:875–881. doi: 10.1007/s00125-010-1661-8. [DOI] [PubMed] [Google Scholar]
- 24.Church T. Exercise in obesity, metabolic syndrome, and diabetes. Prog. Cardiovasc. Dis. 2011;53:412–418. doi: 10.1016/j.pcad.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Deweerdt S. Prevention: Activity is the best medicine. Nature. 2011;475:S16–S17. doi: 10.1038/475S16a. [DOI] [PubMed] [Google Scholar]
- 26.Hurley B.F., Hanson E.D., Sheaff A.K. Strength training as a countermeasure to aging muscle and chronic disease. Sports Med. 2011;41:289–306. doi: 10.2165/11585920-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Mastaloudis A., Leonard S.W., Traber M.G. Oxidative stress in athletes during extreme endurance exercise. Free Radic Biol. Med. 2001;31:911–922. doi: 10.1016/S0891-5849(01)00667-0. [DOI] [PubMed] [Google Scholar]
- 28.Steensberg A., Morrow J., Toft A.D., Bruunsgaard H., Pedersen B.K. Prolonged exercise, lymphocyte apoptosis and F2-isoprostanes. Eur. J. Appl. Physiol. 2002;87:38–42. doi: 10.1007/s00421-002-0584-6. [DOI] [PubMed] [Google Scholar]
- 29.Malaguti M., Angeloni C., Garatachea N., Baldini M., Leoncini E., Collado P.S., Teti G., Falconi M., Gonzalez-Gallego J., Hrelia S. Sulforaphane treatment protects skeletal muscle against damage induced by exhaustive exercise in rats. J. Appl. Physiol. 2009;107:1028–1036. doi: 10.1152/japplphysiol.00293.2009. [DOI] [PubMed] [Google Scholar]
- 30.Navalta J.W., McFarlin B.K., Lyons T.S. Does exercise really induce lymphocyte apoptosis? Front. Biosci. 2010;2:478–488. doi: 10.2741/e106. [DOI] [PubMed] [Google Scholar]
- 31.Sastre J., Asensi M., Gascó E., Pallardó F.V., Ferrero J.A., Furukawa T., Viña J. Exhaustive physical exercise causes oxidation of glutathione status in blood: Prevention by antioxidant administration. Am. J. Physiol. 1992;263:992–995. doi: 10.1152/ajpregu.1992.263.5.R992. [DOI] [PubMed] [Google Scholar]
- 32.Viña J., Gimeno A., Sastre J., Desco C., Asensi M., Pallardó F.V., Cuesta A., Ferrero J.A., Terada L.S., Repine J.E. Mechanism of free radical production in exhaustive exercise in humans and rats; role of xanthine oxidase and protection by allopurinol. Iubmb Life. 2000;49:539–544. doi: 10.1080/15216540050167098. [DOI] [PubMed] [Google Scholar]
- 33.Armstrong R.B., Ogilvie R.W., Schwane J.A. Eccentric exercise-induced injury to rat skeletal muscle. J. Appl. Physiol. Respir. Env. Exerc. Physiol. 1983;54:80–93. doi: 10.1152/jappl.1983.54.1.80. [DOI] [PubMed] [Google Scholar]
- 34.Popovic L.M., Mitic N.R., Radic I., Miric D., Kisic B., Krdzic B., Djokic T. The effect of exhaustive exercise on oxidative stress generation and antioxidant defense in guinea pigs. Adv. Clin. Exp. Med. 2012;21:313–320. [PubMed] [Google Scholar]
- 35.McGinley C., Shafat A., Donnelly A.E. Does antioxidant vitamin supplementation protect against muscle damage? Sports Med. 2009;39:1011–1032. doi: 10.2165/11317890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 36.Davison G., Gleeson M. The effect of 2 weeks vitamin C supplementation on immunoendocrine responses to 2.5 h cycling exercise in man. Eur. J. Appl. Physiol. 2006;97:454–461. doi: 10.1007/s00421-006-0196-7. [DOI] [PubMed] [Google Scholar]
- 37.Thompson D., Williams C., McGregor S.J., Nicholas C.W., McArdle F., Jackson M.J., Powell J.R. Prolonged vitamin C supplementation and recovery from demanding exercise. Int. J. Sport Nutr. Exerc. Metab. 2001;11:466–481. doi: 10.1123/ijsnem.11.4.466. [DOI] [PubMed] [Google Scholar]
- 38.Beaton L.J., Allan D.A., Tarnopolsky M.A., Tiidus P.M., Phillips S.M. Contraction-induced muscle damage is unaffected by vitamin E supplementation. Med. Sci. Sports Exerc. 2002;34:798–805. doi: 10.1097/00005768-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Filaire E., Massart A., Rouveix M., Portier H., Rosado F., Durand D. Effects of 6 weeks of n-3 fatty acids and antioxidant mixture on lipid peroxidation at rest and postexercise. Eur. J. Appl. Physiol. 2011;111:1829–1839. doi: 10.1007/s00421-010-1807-x. [DOI] [PubMed] [Google Scholar]
- 40.Malaguti M., Baldini M., Angeloni C., Biagi P., Hrelia S. High-protein-PUFA supplementation, red blood cell membranes, and plasma antioxidant activity in volleyball athletes. Int. J. Sport Nutr. Exerc. Metab. 2008;18:301–312. doi: 10.1123/ijsnem.18.3.301. [DOI] [PubMed] [Google Scholar]
- 41.Phillips T., Childs A.C., Dreon D.M., Phinney S., Leeuwenburgh C. A dietary supplement attenuates IL-6 and CRP after eccentric exercise in untrained males. Med. Sci. Sports Exerc. 2003;35:2032–2037. doi: 10.1249/01.MSS.0000099112.32342.10. [DOI] [PubMed] [Google Scholar]
- 42.Zitterl-Eglseer K., Sosa S., Jurenitsch J., Schubert-Zsilavecz M., Della Loggia R., Tubaro A., Bertoldi M., Franz C. Anti-oedematous activities of the main triterpendiol esters of marigold (Calendula officinalis L.) J. Ethnopharmacol. 1997;57:139–144. doi: 10.1016/S0378-8741(97)00061-5. [DOI] [PubMed] [Google Scholar]
- 43.Cordova C.A., Siqueira I.R., Netto C.A., Yunes R.A., Volpato A.M., Cechinel Filho V., Curi-Pedrosa R., Creczynski-Pasa T.B. Protective properties of butanolic extract of the Calendula officinalis L. (marigold) against lipid peroxidation of rat liver microsomes and action as free radical scavenger. Redox Rep. 2002;7:95–102. doi: 10.1179/135100002125000325. [DOI] [PubMed] [Google Scholar]
- 44.Bruneton J. In Pharmacognosy, Phytochemistry, Medical Plants. Intercept Limited; Andover, MA, USA: 1995. pp. 309–310. [Google Scholar]
- 45.Kalt W., McDonald J.E., Ricker R.D., Lu X. Anthocyanin content and profile within and among blueberry species. Can. J. Plant Sci. 1999;79:617–623. doi: 10.4141/P99-009. [DOI] [Google Scholar]
- 46.Bettini V., Aragno R., Bettini M., Braggion G., Calore L., Concolato M. Vasodilator and inhibitory effects of Vaccinium myrtillus anthocyanosides on the contractile responses of coronary artery segments to acetylcholine: Role of the prostacyclins and of the endothelium-derived relaxing factor. Fitoterapia. 1991;62:15–28. [Google Scholar]
- 47.Laplaud P.M., Lelubre A., Chapman M.J. Antioxidant action of Vaccinium myrtillus extract on human low density lipoproteins in vitro: Initial observations. Fundam. Clin. Pharm. 1997;11:35–40. doi: 10.1111/j.1472-8206.1997.tb00166.x. [DOI] [PubMed] [Google Scholar]
- 48.Morazzoni P., Livio S., Scilingo A., Malandrino S. Vaccinium myrtillus anthocyanosides pharmacokinetics in rats. Arzneimittelforschung. 1991;41:128–131. [PubMed] [Google Scholar]
- 49.Morazzoni P., Bombardelli E. Vaccinium myrtillus. Fitoterapia. 1996;67:3–30. [Google Scholar]
- 50.Morazzoni P., Magistretti M.J. Activity of myrtocyan, anthocyanoside complex from Vaccinium myrtillus (VMA), on platelet aggregation and adhesiveness. Fitoterapia. 1990;61:13–21. [Google Scholar]
- 51.Preethi K.C., Kuttan G., Kuttan R. Antioxidant potential of an extract of Calendula officinalis flowers in vitro. and in vivo. Pharm. Biol. 2006;44:691–697. doi: 10.1080/13880200601009149. [DOI] [Google Scholar]
- 52.Olennikov D.N., Kashchenko N.I., Chirikova N.K., Akobirshoeva A., Zilfikarov I.N., Vennos C. Isorhamnetin and quercetin derivatives as anti-acetylcholinesterase principles of marigold (Calendula officinalis) flowers and preparations. Int. J. Mol. Sci. 2017;18:1685. doi: 10.3390/ijms18081685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bryan-Thomas J. A comparative study of the antioxidant activity (DPPH), total flavonoid, total tannin, total polyphenol levels in plant extracts of the Annona muricata, Ribes nigrum and Manilkara zapota. Int. J. Sci. Res. Public. 2016;6:490–494. [Google Scholar]
- 54.Saral Ö., Ölmez Z., Şahin H., Turkish J. Comparison of antioxidant properties of wild blueberries (Vaccinium arctostaphylos L. and Vaccinium myrtillus L.) with cultivated blueberry varieties (Vaccinium corymbosum L.) in Artvin region of Turkey. Turk. J. Agric. Food Sci. Technol. 2014;3:40–44. doi: 10.24925/turjaf.v3i1.40-44.166. [DOI] [Google Scholar]
- 55.Matsunaga N., Chikaraishi Y., Shimazawa M., Yokota S., Hara H. Vaccinium myrtillus (bilberry) extracts reduce angiogenesis in vitro and in vivo. Evid. Based Complement. Altern. Med. 2010;7:47–56. doi: 10.1093/ecam/nem151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rigane G., Ben Younes S., Ghazghazi H., Ben Salem R. Investigation into the biological activities and chemical composition of Calendula officinalis L. growing in Tunisia. Int. Food Res. J. 2013;20:3001–3007. [Google Scholar]
- 57.Bonarska-Kujawa D., Cyboran S., Żyłka R., Oszmiański J., Kleszczyńska H. Biological activity of blackcurrant extracts (Ribes nigrum L.) in relation to erythrocyte membranes. Biomed. Res. Int. 2014;2014:783059. doi: 10.1155/2014/783059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Colak N., Primetta A.K., Riihinen K.R., Jaakola L., Grúz J., Strnad M., Torun H., Ayaza F.A. Phenolic compounds and antioxidant capacity in different-colored and non-pigmented berries of bilberry (Vaccinium myrtillus L.) Food Biosci. 2017;20:67–78. doi: 10.1016/j.fbio.2017.06.004. [DOI] [Google Scholar]
- 59.Song E.K., Kim J.H., Kim J.S., Cho H., Nan J.X., Sohn D.H., Ko G.I., Oh H., Kim Y.C. Hepatoprotective phenolic constituents of Rhodiola sachalinensis on tacrine-induced cytotoxicity in Hep G2 cells. Phytother. Res. 2003;17:563–565. doi: 10.1002/ptr.1166. [DOI] [PubMed] [Google Scholar]
- 60.Zhao H.L., Lin S.X., Jia B., Li J., Zhang L., Zhang S. Inhibitory effects of salidroside on hypoxia-induced proliferation of rabbit pulmonary arterial smooth muscle cells. J. Fourth Mil. Med Univ. 2000;21:186–189. [Google Scholar]
- 61.Zhang W.S., Zhu L.Q., Niu F.L., Deng R.C., Ma C.X. Protective effects salidroside on injury induced by hypoxia/hypoglycemia in cultured neurons. Zhongguo Zhong Yao Za Zhi. 2004;29:462–465. [PubMed] [Google Scholar]
- 62.Kucinskaite A., Briedis V., Savickkas A. Experimental analysis of therapeutic properties of Rhodiola rosea L. and its possible application in medicine. Medicina. 2004;40:614–619. [PubMed] [Google Scholar]
- 63.Wang S.H., Wang W.J., Wang X.F., Chen W.H. Effects of salidroside on carbohydrate metabolism and differentiation 3T3-L1 adipocytes. Zhong Xi Yi Jie He Za Zhi. 2004;2:193–195. doi: 10.3736/jcim20040312. [DOI] [PubMed] [Google Scholar]
- 64.Li H.B., Ge Y.K., Zheng X.X., Zhang L. Salidroside stimulated glucose uptake in skeletal muscle cells by activating AMP-activated protein kinase. Eur. J. Pharm. 2008;588:165–169. doi: 10.1016/j.ejphar.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 65.Tang Y., Vater C., Jacobi A., Liebers C., Zou X., Stiehler M. Salidroside exerts angiogenic and cytoprotective effects on human bone marrow-derived endothelial progenitor cells via Akt/mTOR/p70S6K and MAPK signalling pathways. Br. J. Pharm. 2014;171:2440–2456. doi: 10.1111/bph.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li M., Donglian C., Huaixing L., Bende T., Lihua S., Ying W. Anti-fatigue effects of salidroside in mice. J. Med Coll. PLA. 2008;23:88–93. doi: 10.1016/S1000-1948(08)60028-3. [DOI] [Google Scholar]
- 67.Ma C., Hu L., Tao G., Lv W., Wang H. An UPLC-MS-based metabolomics investigation on the anti-fatigue effect of salidroside in mice. J. Pharm. Biomed. Anal. 2015;105:84–90. doi: 10.1016/j.jpba.2014.11.036. [DOI] [PubMed] [Google Scholar]
- 68.Ma L., Cai D.L., Li H.X., Tong B.D., Wang Y., Pei S.P. Protective effects of salidroside on oxidative damage in fatigue mice. Zhong Xi Yi Jie He Xue Bao. 2009;7:237–241. doi: 10.3736/jcim20090308. [DOI] [PubMed] [Google Scholar]
- 69.Deyama T., Nishibe S., Nakazawa Y. Constituents and pharmacological effects of Eucommia and Siberian ginseng. Acta Pharm. Sin. 2001;12:1057–1070. [PubMed] [Google Scholar]
- 70.Askari G., Ghiasvand R., Paknahad Z., Karimian J., Rabiee K., Sharifirad G., Feizi A. The effects of quercetin supplementation on body composition, exercise performance and muscle damage indices in athletes. Int. J. Prev. Med. 2013;4:21–26. [PMC free article] [PubMed] [Google Scholar]
- 71.Askari G., Ghiasvand R., Karimian J., Feizi A., Paknahad Z., Sharifirad G., Hajishafiei M. Does quercetin and vitamin C improve exercise performance, muscle damage, and body composition in male athletes? J. Res. Med. Sci. 2012;17:328–331. [PMC free article] [PubMed] [Google Scholar]
- 72.Chen X., Wu Y., Yang T., Wei M., Wang Y., Deng X., Shen C., Li W., Zhang H., Xu W., et al. Salidroside alleviates cachexia symptoms in mouse models of cancer cachexia via activating mTOR signalling. J. Cachexia Sarcopenia Muscle. 2016;7:225–232. doi: 10.1002/jcsm.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang C.C., Hsu M.C., Huang W.C., Yang H.R., Hou C.C. Triterpenoid-rich extract from Antrodia camphorata improves physical fatigue and exercise performance in mice. Evid. Based Complement. Altern. Med. 2012;2012:364741. doi: 10.1155/2012/364741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu J.L., Wu Q.P., Huang J.M., Chen R., Cai M., Tan J.B. Effects of L-malate on physical stamina and activities of enzymes related to the malate-aspartate shuttle in liver of mice. Physiol. Res. 2007;56:213–220. doi: 10.33549/physiolres.930937. [DOI] [PubMed] [Google Scholar]
- 75.Shang H.P., Cao S.H., Wang J.H., Zheng H., Putheti R. Glabridin from Chinese herb licorice inhibits fatigue in mice. Afr. J. Trad. Complementary Altern. Med. 2010;7:17–23. doi: 10.4314/ajtcam.v7i1.57225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koo H.N., Lee J.K., Hong S.H., Kim H.M. Herbkines increases physical stamina in mice. Biol. Pharm. Bull. 2004;27:117–119. doi: 10.1248/bpb.27.117. [DOI] [PubMed] [Google Scholar]
- 77.Tsopanakis A. Stress hormonal factors, fatigue, and antioxidant responses to prolonged speed driving. Pharm. Biochem. Behav. 1998;60:747–751. doi: 10.1016/S0091-3057(98)00037-9. [DOI] [PubMed] [Google Scholar]
- 78.Sahlin K., Tonkonogi M., Söderlund K. Energy supply and muscle fatigue in humans. Acta Physiol Scand. 1998;162:261–266. doi: 10.1046/j.1365-201X.1998.0298f.x. [DOI] [PubMed] [Google Scholar]
- 79.Qian E.W., Ge D.T., Kong S.K. Salidroside protects human erythrocytes against hydrogen peroxide-induced apoptosis. J. Nat. Prod. 2012;75:531–537. doi: 10.1021/np200555s. [DOI] [PubMed] [Google Scholar]
- 80.Suh S.H., Paik I.Y., Jacobs K. Regulation of blood glucose homeostasis during prolonged exercise. Mol. Cells. 2007;23:272–279. [PubMed] [Google Scholar]
- 81.Gonzalez J.T., Fuchs C.J., Betts J.A., van Loon L.J. Liver glycogen metabolism during and after prolonged endurance-type exercise. Am. J. Physiol. Endocrinol. Metab. 2016;311:E543–E553. doi: 10.1152/ajpendo.00232.2016. [DOI] [PubMed] [Google Scholar]
- 82.Kim J.H., Pan J.H., Lee E.S., Kim Y.J. L-Carnitine enhances exercise endurance capacity by promoting muscle oxidative metabolism in mice. Biochem. Biophys. Res. Commun. 2015;464:568–573. doi: 10.1016/j.bbrc.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 83.Huang W.C., Huang H.Y., Hsu Y.J., Su W.H., Shen S.Y., Lee M.C., Lin C.L., Huang C.C. The effects of thiamine tetrahydrofurfuryl disulfide on physiological adaption and exercise performance improvement. Nutrients. 2018;10:851. doi: 10.3390/nu10070851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li H., Chen Y.J., Hsu Y.J., Wu M.F., Chiu C.C., Tung Y.T., Tsai W.J., Huang W.C., Huang C.C. Effects of Ganoderma lucidum and ‘essence of chicken’ on physical fatigue recovery and exercise performance improvement. Chin. J. Physiol. 2018;61:372–383. doi: 10.4077/CJP.2018.BAH646. [DOI] [PubMed] [Google Scholar]