Abstract
Background: Ventilator-associated pneumonia (VAP) is associated to longer stay and poor outcomes. Lacking definitive diagnostic criteria, worsening gas exchange assessed by PaO2/FIO2 ≤ 240 in mmHg has been proposed as one of the diagnostic criteria for VAP. We aim to assess the adequacy of PaO2/FIO2 ≤ 240 to diagnose VAP. Methods: Prospective observational study in 255 consecutive patients with suspected VAP, clustered according to PaO2/FIO2 ≤ 240 vs. > 240 at pneumonia onset. The primary analysis was the association between PaO2/FIO2 ≤ 240 and quantitative microbiologic confirmation of pneumonia, the most reliable diagnostic gold-standard. Results: Mean PaO2/FIO2 at VAP onset was 195 ± 82; 171 (67%) cases had PaO2/FIO2 ≤ 240. Patients with PaO2/FIO2 ≤ 240 had a lower APACHE-II score at ICU admission; however, at pneumonia onset they had higher CPIS, SOFA score, acute respiratory distress syndrome criteria and incidence of shock, and less microbiological confirmation of pneumonia (117, 69% vs. 71, 85%, p = 0.008), compared to patients with PaO2/FIO2 > 240. In multivariate logistic regression, PaO2/FIO2 ≤ 240 was independently associated with less microbiological confirmation (adjusted odds-ratio 0.37, 95% confidence interval 0.15–0.89, p = 0.027). The association between PaO2/FIO2 and microbiological confirmation of VAP was poor, with an area under the ROC curve 0.645. Initial non-response to treatment and length of stay were similar between both groups, while hospital mortality was higher in patients with PaO2/FIO2 ≤ 240. Conclusion: Adding PaO2/FIO2 ratio ≤ 240 to the clinical and radiographic criteria does not help in the diagnosis of VAP. PaO2/FIO2 ratio > 240 does not exclude this infection. Using this threshold may underestimate the incidence of VAP.
Keywords: intensive care unit, ventilator-associated pneumonia, nosocomial infection, PaO2/FIO2
1. Introduction
Ventilator-associated pneumonia (VAP) remains the most frequent intensive care unit (ICU)-acquired infection [1,2]. Its incidence ranges from 9% to 27% of ventilated patients depending on diagnostic criteria [3]. VAP is associated with an increased length of stay, mortality and health care costs [4,5].
The diagnosis of VAP may be complex. Therefore, multiple efforts have been directed to overcome these difficulties and to identify the most appropriate diagnostic strategies [6], including integrative scoring systems that take into account multiple clinical data and microbiological findings.
The Clinical Pulmonary Infection Score (CPIS) score was developed to objectively diagnose VAP [6]. The score combines six variables: body temperature, white blood cell count, quantity and purulence of tracheal secretions, chest radiograph, bacterial growth in tracheal secretions and oxygenation (PaO2/FIO2 ≤ 240 in mmHg). However, the reported poor sensitivity and specificity of CPIS score in most studies preclude its use as an accurate diagnostic strategy [7,8]. Similarly, the Centers for Disease Control (CDC) and Prevention have proposed the inclusion of a decline in oxygenation, assessed by a PaO2/FIO2 ratio ≤ 240, in the diagnostic criteria for VAP [9]. Current American guidelines define pneumonia as the presence of a new or progressive radiological lung infiltrate plus the clinical evidence that the infiltrate is of an infectious origin and a decline in oxygenation [2]. Indeed, the most reliable evidence of an infectious origin in patients suspected of having VAP combines these criteria with the microbiological identification of the potentially-pathogenic microorganisms (PPM) that cause pneumonia [10].
To our knowledge, no studies have comprehensively evaluated the relationship between microbiological diagnosis in populations with clinical suspicion of VAP and better or worse oxygenation, as assessed by the PaO2/FIO2 ratio.
We hypothesized that patients with worse oxygenation would have more frequent microbiological confirmation of VAP. In order to assess the adequacy of PaO2/FIO2 ≤ 240 to help in the diagnosis of VAP, we compared the characteristics and outcomes of a real-life ICU population with suspected VAP and PaO2/FIO2 ≤ or > 240, with special emphasis on microbiological confirmation.
2. Methods (Extended Information is Provided in Supplementary Materials)
2.1. Study Population
The study was conducted in six medical and surgical ICUs, overall comprising of 45 beds, at an 800-bed university hospital. Data were prospectively collected from 2007 to 2017. Investigators made daily rounds of each ICU. Patients older than 18 years, mechanically-ventilated for 48 h or more, with suspected VAP, were consecutively included into the study and only the first episode was analyzed. We excluded patients with severe immune-suppression [11]. The institution’s Internal Review Board approved the study (Comitè Ètic d’Investigació Clínica, registry number 2009/5427) and written informed consent was obtained from patients or their next-of-kin.
2.2. Definition of Pneumonia, Microbiologic Processing, and Antimicrobial Treatment
The clinical suspicion of pneumonia was based on clinical criteria (new or progressive radiological pulmonary infiltrate together with at least two of the following: (1) temperature >38 °C or < 36 °C; (2) leukocytosis > 12,000/mm3 or leucopoenia < 4000/mm3; or (3) purulent respiratory secretions) [12,13].
The microbiologic evaluation included the collection of at least one lower respiratory airways sample: tracheobronchial aspirates (TBAS) and/or bronchoscopic [14] or blind bronchoalveolar lavage (BAL) [15] if possible, within the first 24 hours of inclusion [16]. The same sampling method was performed on the third day if clinically indicated. Blood cultures and cultures from the pleural fluid were also collected if clinically justified.
Microbiologic confirmation of pneumonia was defined by the presence of at least one PPM [17] in the respiratory samples above pre-defined values (BAL > 104 and TBAS > 105 colony-forming units/mL, respectively, or any value if the patient was under antibiotic treatment), in pleural fluid or in blood cultures if an alternative cause of bacteremia was ruled out [18,19]. Microbiologic identification and susceptibility testing were performed by standard methods [20].
The initial empiric antimicrobial treatment was administered in all patients according to the local adaptation of guidelines [1,21], and subsequently revised according to the microbiologic results.
The empiric antimicrobial treatment was considered appropriate when the isolated pathogens were susceptible in vitro to at least one of the antimicrobials administrated at adequate dose [22].
The initial response to treatment was evaluated after 72 hours of antimicrobial treatment. In patients with initial non-response to treatment [23,24], respiratory samples and blood cultures were obtained again, and the empiric antimicrobial treatment was revised.
2.3. Assessment of the Systemic Inflammatory Response
We evaluated the serum levels of interleukin (IL)-6, IL-8, IL-10, tumour necrosis factor-alpha (TNF-alpha), C-reactive protein (CRP), Procalcitonin (PCT), and mid-regional pro-adrenomedullin (MR-proADM) within the first 24 hours and the third day after the diagnosis of pneumonia. All methods for this analysis have been described in detail elsewhere [25,26].
2.4. Data Collection
All relevant data were collected at admission and at onset of pneumonia from the medical records and bedside flow charts. Patients were followed until hospital discharge, death or up to 90-days after the diagnosis of pneumonia. Septic shock [27] and acute respiratory distress syndrome (ARDS) [28] were defined according to the previously described criteria.
2.5. Outcomes Variables
The rate of quantitative microbiological confirmation of patients with PaO2/FIO2 ≤ 240 was compared with that of patients with PaO2/FIO2 > 240. The lowest value of PaO2/FiO2 on the day of VAP diagnosis was considered for patients’ group allocation. Secondary outcomes were length of stay and mortality, and 90-day survival after VAP diagnosis.
2.6. Statistical Analysis
Categorical and continuous data are presented as number (percentage) and as mean ± SD (or median, inter-quartile range), respectively. Categorical variables were compared with the Chi-square or Fisher’s exact tests. Quantitative continuous variables were compared using the unpaired Student’s t-test or the Mann-Whitney test for normally and not normally distributed variables, respectively. The Kaplan-Meier curves were used to compare survival in the two groups.
Univariate and multivariate logistic regression analyses were performed to assess the association of PaO2/FIO2 ≤ 240 or > 240 with positive microbiology. Variables that showed a significant result univariately (p < 0.10) were included in the corresponding multivariate logistic regression backward stepwise model. Adjusted odds-ratio (OR) and 95% confidence intervals (CI) were calculated.
In addition, the association between PaO2/FIO2 at VAP onset as continuous variable and quantitative microbiological confirmation was assessed using receiver-operator-characteristics (ROC) curve analysis. The area under the ROC curve (AUC) with 95% confidence interval (CI), and the optimal cut-off value of PaO2/FIO2 with sensitivity and specificity were calculated.
A two-sided p-value ≤ 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 22.0.0.0 (Chicago, IL, USA).
3. Results
3.1. Patients’ Characteristics
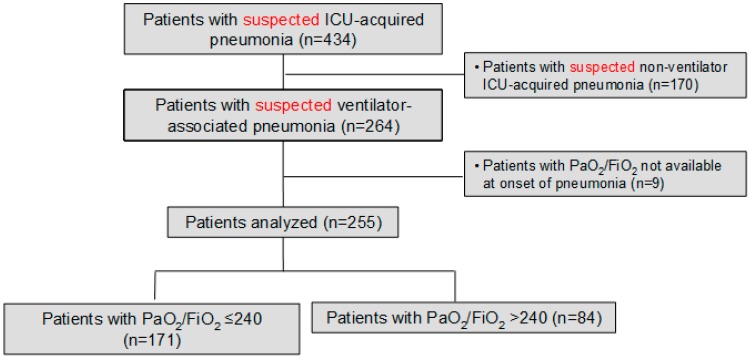
We prospectively identified 264 consecutive patients with suspected VAP during the study period; we excluded nine cases because PaO2/FiO2 at onset of pneumonia was not available. Therefore, we included 255; PaO2/FiO2 was ≤240 in 171 (67%) patients, and higher than this value in 84 (33%) patients (Figure 1).
Figure 1.
Flow chart of the study population.
The characteristics of patients at ICU admission and pneumonia onset according PaO2/FIO2 are summarized in Table 1 and Table 2.
Table 1.
Baseline characteristics of patients at ICU admission.
| PaO2/FIO2 ≤ 240 n = 171 |
PaO2/FIO2 > 240 n = 84 |
p Value | |
|---|---|---|---|
| Age, year | 62 ± 16 | 61 ± 15 | 0.57 |
| Sex, male/female, n | 116/55 | 60/24 | 0.56 |
| Alcohol abuse (current or former), n (%) | 41 (24) | 18 (21) | 0.63 |
| Smoking habit (current or former), n (%) | 90 (53) | 40 (48) | 0.45 |
| APACHE-II score | 17 ± 6 | 19 ± 8 | 0.044 |
| SAPS score | 42 ± 15 | 45 ± 16 | 0.24 |
| SOFA score | 8 ± 3 | 8 ± 3 | 0.71 |
| Co-morbidities, n (%) | |||
| Diabetes mellitus | 41 (24) | 19 (23) | 0.81 |
| Chronic renal failure | 15 (9) | 5 (6) | 0.43 |
| Solid cancer | 19 (11) | 10 (12) | 0.85 |
| Chronic heart disorders | 54 (32) | 28 (33) | 0.78 |
| Chronic lung disease | 58 (34) | 30 (24) | 0.10 |
| Chronic liver disease | 27 (16) | 9 (11) | 0.27 |
| Recent surgery, n (%) | 81 (47) | 43 (51) | 0.57 |
| Tracheotomy at admission, n (%) | 16 (9) | 11 (13) | 0.37 |
| Causes of ICU admission, n (%) | 0.051 | ||
| Post-operative | 40 (23) | 13 (16) | |
| Hypoxemic respiratory failure | 20 (12) | 5 (6) | |
| Decreased consciousness | 31 (18) | 17 (20) | |
| Hypercapnic respiratory failure | 16 (9) | 5 (6) | |
| Septic shock | 12 (7) | 10 (12) | |
| Multiple trauma | 12 (7) | 16 (19) | |
| Non-surgical abdominal disease | 5 (3) | 3 (4) | |
| Acute coronary syndrome | 10 (6) | 2 (2) | |
| Cardiac arrest | 12 (7) | 9 (11) | |
| Other | 6 (4) | 4 (5) |
Definition of abbreviations: APACHE: Acute Physiology and Chronic Health Evaluation; ICU: Intensive Care Unit; SAPS: Simplified Acute Physiology Score; SOFA: Sepsis-related Organ Failure Assessment.
Table 2.
Characteristics of patients at pneumonia onset.
| PaO2/FIO2 ≤ 240 n = 171 |
PaO2/FIO2 > 240 n = 84 |
p Value | |
|---|---|---|---|
| Previous antibiotics, n (%) | 139 (81) | 66 (79) | 0.61 |
| Most frequent groups, n (%): | |||
| Penicillins | 78 (46) | 26 (31) | |
| Cephalosporins | 50 (29) | 26 (31) | |
| Quinolones | 48 (28) | 20 (24) | |
| Carbapenems | 23 (13) | 14 (17) | |
| Glycopeptides | 28 (16) | 9 (11) | |
| Aminoglycosides | 15 (9) | 11 (13) | |
| Clindamycin | 14 (8) | 11 (13) | |
| Antifungals | 6 (4) | 2 (2) | |
| Hospital stay before pneumonia, days * | 7 (4–13) | 7 (5–15) | 0.34 |
| ICU stay before pneumonia, days * | 5 (3–9) | 6 (4–10) | 0.12 |
| SOFA score | 8 ± 3 | 7 ± 3 | 0.004 |
| Bilateral pulmonary infiltrates, n (%) | 55 (32) | 19 (23) | 0.11 |
| ARDS criteria, n (%) | 32 (19) | 1 (1) | <0.001 |
| Pleural effusion, n (%) | 56 (33) | 18 (22) | 0.10 |
| PaO2/FiO2 | 161 ± 47 | 301 ± 49 | <0.001 |
| FiO2 | 0.55 ± 0.18 | 0.41 ± 11 | <0.001 |
| PEEP, cmH2O | 7.4 ±3.4 | 7.8 ± 3.0 | 0.36 |
| PaCO2, mmHg | 41 ± 9 | 39 ± 7 | 0.066 |
| pHa | 7.40 ± 0.09 | 7.41 ± 0.07 | 0.33 |
| Shock at onset of pneumonia, n (%) | 96 (56) | 32 (38) | 0.007 |
| Temperature | 36.9 ± 1.4 | 37.0 ± 1.3 | 0.37 |
| Serum creatinine, mg/dL | 1.3 ± 1.0 | 1.2 ± 1.2 | 0.85 |
| Blood haemoglobin, g/L | 11 ± 2 | 10 ± 2 | 0.67 |
| White blood cell count, L−9 | 14 ± 7 | 13 ± 6 | 0.11 |
| Sodium, mEq/L | 139 ± 6 | 141 ± 8 | 0.042 |
| Potassium, mEq/L | 4.0 ± 0.7 | 4.1 ± 0.5 | 0.75 |
| CPIS day 1 | 7 ± 1 | 5 ± 1 | <0.001 |
| CPIS day 3 | 6 ± 2 | 5 ± 2 | <0.001 |
* Results given as median (inter-quartile range). Definition of abbreviations: ICU: Intensive Care Unit; SOFA: Sepsis-related Organ Failure Assessment; ARDS: Acute Respiratory Distress Syndrome; PaO2/FiO2: ratio of arterial oxygen tension to inspired oxygen fraction; CPIS: Clinical Pulmonary Infection Score.
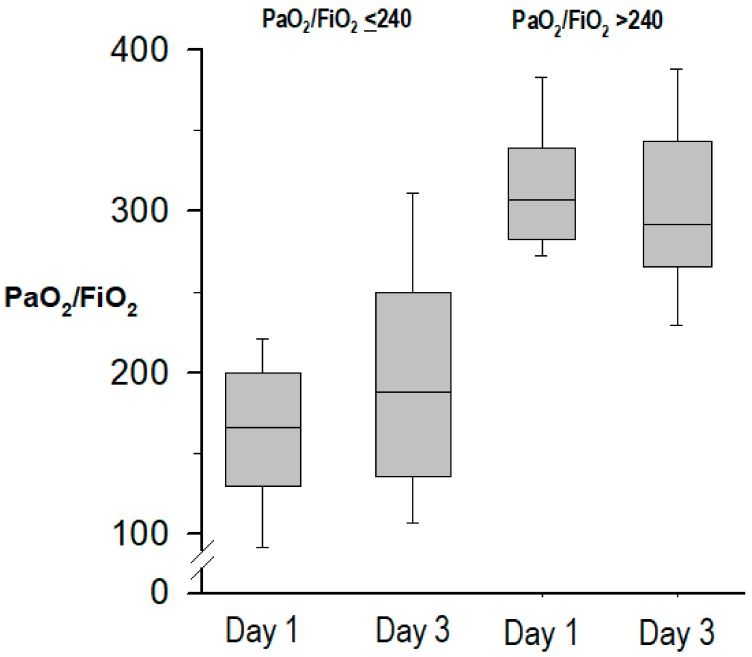
Compared to patients with PaO2/FIO2 > 240, those with PaO2/FIO2 ≤ 240 had a lower APACHE-II score at ICU admission. However, at onset of pneumonia, patients with PaO2/FIO2 ≤ 240 had a higher SOFA score and CPIS, in both cases due to the lower values of PaO2/FIO2, presented ARDS criteria and shock more frequently, and had lower serum levels of sodium. Reasons for ICU admission were slightly different between groups (p = 0.051), with higher proportions of multiple trauma and septic shock among patients with PaO2/FIO2 > 240, and higher proportions of postoperative care and hypoxemic respiratory failure in patients with PaO2/FIO2 ≤ 240. The evolution of PaO2/FiO2 at pneumonia onset and at day 3 in both groups is shown in Figure 2.
Figure 2.
Evolution of PaO2/FiO2 from pneumonia onset (day 1) to day 3.
3.2. Microbiological Aetiology
The etiologic diagnosis of patients is shown in Table 3. The number of samples processed for microbiology was similar between both groups. Pneumonia was microbiologically confirmed in 188 (74%) cases, with a lower rate of microbiological confirmation in patients with PaO2/FIO2 ≤ 240, compared with those with PaO2/FIO2 > 240 (117, 69% vs. 71, 85%, p = 0.007; odds-ratio 0.40, 95% CI 0.21 to 0.79, p = 0.008). The multivariate logistic regression analysis showed that PaO2/FIO2 ≤ 240 mmHg was independently associated with less microbiological confirmation of pneumonia (adjusted OR 0.37, 95% CI 0.15–0.89, p = 0.027). The association between PaO2/FIO2 and microbiological confirmation of VAP was poor, although statistically significant, with an AUC 0.645 (95% CI 0.568 to 0.725, p = 0.040). The optimal cut-off value of PaO2/FIO2 associated with microbiological confirmation of VAP was 223, with sensitivity 45% and specificity 79%.
Table 3.
Etiologic diagnosis of pneumonia according to PaO2/FIO2.
| Pathogen | PaO2/FIO2 ≤ 240 n = 171 |
PaO2/FIO2 > 240 n = 84 |
p Value |
|---|---|---|---|
| Sample processed for microbiology, n (%) | |||
| Endotracheal aspirates | 157 (92) | 79 (94) | 0.52 |
| Bonchoalveolar lavage | 38 (23) | 21 (25) | 0.62 |
| Blood | 126 (74) | 58 (69) | 0.44 |
| Pleural fluid | 18 (11) | 5 (6) | 0.23 |
| Positive microbiology, n (%) | 117 (69) | 71 (85) | 0.007 |
| Gram-positive bacteria, n (%) | 37 (32) | 23 (32) | 0.96 |
| MS Staphylococcus aureus | 18 (15) | 17 (24) | 0.20 |
| MR Staphylococcus aureus | 12 (10) | 4 (6) | 0.41 |
| Streptococcus pneumoniae | 8 (7) | 2 (3) | 0.39 |
| Gram-negative enteric bacteria, n (%) | 39 (33) | 16 (23) | 0.16 |
| Enterobacter spp | 7 (6) | 2 (3) | 0.53 |
| Klebsiella spp | 11 (9) | 6 (9) | 0.78 |
| Escherichia coli | 9 (8) | 2 (3) | 0.62 |
| Proteus spp | 2 (2) | 2 (3) | 0.99 |
| Citrobacter spp | 3 (3) | 3 (4) | 0.84 |
| Serratia spp | 8 (7) | 4 (6) | 0.97 |
| Morganella morganii | 1 (1) | 0 (0) | 0.80 |
| Non-fermentating gram-negative bacilli | 38 (32) | 29 (41) | 0.32 |
| Stenotrophomonas maltophilia | 7 (6) | 3 (4) | 0.85 |
| Pseudomonas aeruginosa | 31 (27) | 26 (37) | 0.19 |
| Other gram-negative bacteria | |||
| Moraxella catarrhalis | 2 (2) | 0 (0) | 0.71 |
| Haemophilus influenzae | 3 (3) | 4 (6) | 0.50 |
| Fungi, n (%) | 2 (2) | 1 (1) | 0.66 |
| Aspergillus spp | 2 (2) | 1 (1) | 0.66 |
| Others, n (%) | 0 (0) | 1 (5) | - |
| Polymicrobial aetiology * | 19 (11) | 9 (11) | 0.91 |
* Polymicrobial pneumonia was defined when more than one potentially-pathogenic microorganism was identified as causative agents.
The most frequent pathogens identified were Pseudomonas aeruginosa, Gram-negative enteric bacteria and Staphylococcus aureus, with no significant differences in the relative proportion of any pathogen among patients with microbiological diagnosis of ICUAP.
3.3. Systemic Inflammatory Response
The serum levels of all inflammatory biomarkers at pneumonia onset and at day 3 are shown in Table 4. Patients with PaO2/FIO2 ≤ 240 had higher serum levels of Procalcitonin at pneumonia onset, without any significant difference in the remaining biomarkers.
Table 4.
Serum levels of inflammatory biomarkers *.
| n † | PaO2/FIO2 ≤ 240 n = 171 |
n † | PaO2/FIO2 > 240 n = 84 |
p Value | |
|---|---|---|---|---|---|
| C-reactive protein day 1, mg/dL | 164 | 13 (6–21) | 81 | 11 (4–19) | 0.13 |
| C-reactive protein day 3, mg/dL | 149 | 11 (4–19) | 78 | 9 (5–15) | 0.078 |
| IL-6 day 1, pg/mL | 89 | 168 (72–431) | 36 | 109 (41–229) | 0.077 |
| IL-6 day 3, pg/mL | 75 | 91 (19–204) | 32 | 78 (33–183) | 0.98 |
| IL-8 day 1, pg/mL | 89 | 108 (64–214) | 36 | 94 (57–137) | 0.17 |
| IL-8 day 3, pg/mL | 75 | 76 (41–145) | 32 | 99 (63–170) | 0.15 |
| TNF-alpha day 1, pg/mL | 89 | 8 (5–17) | 36 | 7 (5–14) | 0.73 |
| TNF-alpha day 3, pg/mL | 75 | 7 (4–14) | 32 | 8 (5–15) | 0.61 |
| Procalcitonin day 1, ng/mL | 90 | 0.45 (0.14–1.37) | 37 | 0.19 (0.07–0.59) | 0.037 |
| Procalcitonin day 3, ng/mL | 78 | 0.30 (0.10–1.06) | 34 | 0.20 (0.08–0.74) | 0.57 |
| MR-proADM day 1, nmol/L | 98 | 1.27 (0.33–2.22) | 40 | 1.16 (0.65–2.23) | 0.84 |
| MR-proADM day 3, nmol/L | 87 | 1.36 (0.38–2.37) | 37 | 1.08 (0.61–2.04) | 0.92 |
* Results are given as median (inter-quartile range). † Number of cases with blood samples processed for each inflammatory biomarker and group. Definition of abbreviations: IL: interleukin; TNF: tumor necrosis factor; MR-proADM: mid-regional pro-adrenomedullin.
3.4. Empiric Antibiotic Treatment and Outcome Variables
Initial non-response to treatment, length of stay and ICU mortality were similar between both groups (Table 5).
Table 5.
Outcome variables.
| PaO2/FIO2 ≤ 240 n = 171 |
PaO2/FIO2 > 240 n = 84 |
p Value | |
|---|---|---|---|
| ICU stay, days * | 19 (12–29) | 18 (13–32) | 0.75 |
| Hospital stay, days * | 34 (19–56) | 43 (21–56) | 0.34 |
| Non-response to treatment, n (%) | 94 (55) | 48 (57) | 0.74 |
| ICU mortality, n (%) | 53 (31) | 18 (24) | 0.11 |
| Hospital mortality, n (%) | 71 (42) | 24 (29) | 0.044 |
| Ventilator-free days at day 28 * | 3 (0–21) | 17 (0–24) | 0.022 |
| Causes of death within 90 days: | 0.28 | ||
| Shock, n (%) | 52 (74) | 14 (58) | |
| Refractory hypoxemia, n (%) | 4 (6) | 2 (8) | |
| Order do-not-resuscitate, n (%) | 1 (1) | 2 (8) | |
| Brain anoxia, n (%) | 11 (16) | 6 (25) | |
| Others, n (%) | 2 (3) | 0 (0) |
* Results given as median (inter-quartile range). Definition of abbreviations: ICU: Intensive Care Unit.
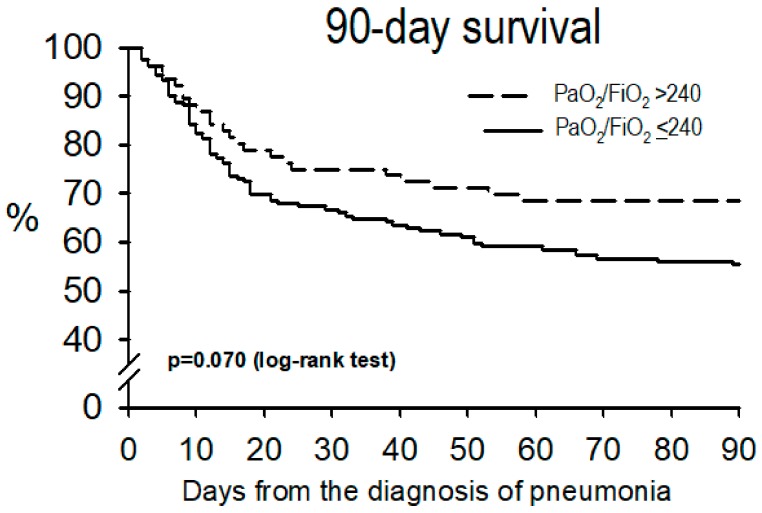
Hospital mortality was higher, and the ventilator-free days were lower, in patients with PaO2/FIO2 ≤ 240, while 90-day survival tended to be lower in this group (p = 0.070, Figure 3).
Figure 3.
Survival curves at 90 days.
4. Discussion
To the best of our knowledge, this is the first study investigating the relationship between quantitative microbiological confirmation and oxygenation in a prospective cohort of patients mechanically ventilated and suspected of having VAP. We found that PaO2/FIO2 ≤ 240 was associated with less microbiological confirmation in this subgroup of patients. In addition, patients with worse oxygenation had higher hospital mortality.
The diagnosis of VAP yields on clinical criteria; however, due to their potential subjectivity, other parameters has been proposed in order to better define VAP episodes [29]. Over the last years, due to an excess of antibiotic treatment, the United States Critical Care Collaborative Societies and the Department of Health and Human Services have proposed the implementation of “ventilator-associated complications” and “ventilator-associated infections” [30]. However, the use of “more objective” criteria such as chest X-ray and oxygenation has important limitations. Chest X-rays may be difficult to interpret in ICU patients due to multiple non-infectious causes such as congestive heart failure, atelectasis, prior chronic lung disease, or ARDS.
Microbiological confirmation is probably the best matching to assess VAP diagnosis quality [10]. We hypothesized that patients with more impaired oxygenation would have more frequently etiologic diagnosis and therefore they would be considered as definitive VAP episodes. Surprisingly our findings demonstrated the opposite. We previously reported that negative microbiology in patients with both community-acquired and ICU-acquired pneumonia was associated with renal and cardiac co-morbidities [31,32]. These studies suggested that some of these cases might also represent, at least in part, fluid overload because of renal failure or congestive heart failure added to the underlying inflammatory process potentially mimicking pneumonia. These conditions often exhibit more severe deterioration of oxygenation, thus possibly explaining the association of lower PaO2/FIO2 with less microbiological confirmation of VAP. However, lack of detection of a PPM would not preclude existence of an infection since many patients are previously treated with antibiotics. Therefore, until more specific diagnostic criteria are available, initiation of antimicrobial treatment should still be based on the clinical signs of infection together with radiographic criteria.
The use of oxygenation has been proposed in many diseases as a criterion for diagnosis. Indeed, in case of ARDS PaO2/FIO2 ratio is both a diagnostic and stratification tool [28,33]. Therefore, since pneumonia is the most frequent cause of ARDS [33], PaO2/FIO2 ratio has also been considered for pneumonia diagnosis too. Unfortunately, although PaO2/FIO2 might be a good parameter of severity, it might lead clinicians to over treat patients. In many situations of daily clinical practice, the use of PaO2/FIO2 ratio might trigger antibiotics start when they are probably not needed, and conversely patients with clear infiltrates in the X-rays but without major oxygenation derangements might preclude that the patient is not developing a VAP. In our population, 33% of suspected VAP episodes had a PaO2/FIO2 >240. Therefore, the use of this threshold of PaO2/FIO2 in the diagnosis probably would have underestimated the incidence of VAP. This has important implications, either in the development of antimicrobial resistance due to unnecessary treatment, and an increase in mortality due to inappropriate (or none) antibiotic treatment. Using PaO2/FIO2 may be important to stratify patients’ severity in controlled clinical trials, and probably for an earlier or more aggressive treatment, as we report higher hospital mortality for those patients with worse oxygenation. We think, however, that patients with higher PaO2/FIO2 should not be excluded from clinical trials dealing with VAP.
One probable reason to point out oxygenation as a diagnosis marker is due to a wrong interpretation of CPIS as a clinical marker for VAP [6]. The CPIS can be a useful tool for severity identification when the patient has been diagnosed of VAP and can help to address patients’ response to treatment. Luna et al. using PaO2/FIO2 ratio could demonstrate that this was the best parameter for patients’ stratification of treatment response and a marker of clinical cure [34]. Moreover, we have previously reported that lack of improvement of PaO2/FIO2 was among the best predictors for adverse outcomes in ICU-acquired pneumonia [24]. This, however, faces against the use of PaO2/FIO2 as a diagnostic criterion, especially in some subsets of patients such as those with multiple trauma, surgery, or major burns [8]. Indeed, we found in the present study that patients with postoperative VAP episodes presented more hypoxemia compared to patients with multiple trauma. On the other hand, the recent HAP/VAP International guidelines [35] made a weak recommendation for short antibiotic courses in patients with low CPIS (i.e., 6 or less). However, this does not seem to be related to diagnosis purposes.
Diagnosis of VAP including oxygenation has not been universally accepted by many authors. In a European cohort study conducted in 465 patients with VAP, worsening oxygenation was present in 3 out of 4 patients [36], whilst Martin-Loeches et al. found it in two-thirds of their patients with VAP [37]. Similar to severe CAP [38], oxygenation can be and probably should be taken as a severity criteria similar to septic shock development, but it should not substitute current diagnostic parameters widely used. Several studies performed over the last decade have found that prompt antibiotic therapy was the most important determinant for improved outcomes in a general sepsis population [39]. It does not seem reasonable to add a test that based on our results would delay and probably mislead VAP diagnosis.
The current study has important strengths. It includes a long cohort of patients prospectively collected and without significant loss of follow-up due to a careful surveillance with a dedicated research team. In all the patients, blood gases were taken at the moment of diagnosis based on local protocols. Because of inaccuracy to assess the actual FiO2 in non-intubated patients, we did not include cases of non-ventilator ICU-acquired pneumonia [40]. Moreover, all patients had at least one lower respiratory tract sample processed for culture at onset of pneumonia, and the frequency and type of previous antibiotic treatment is reported, without differences related to severity of oxygenation in both variables. An additional value for this study is the acquisition of inflammatory markers at the onset of pneumonia and after three days of antibiotic treatment. However, we have to acknowledge some limitations. The most important would be the single centre analysis, which might limit reproducibility of the results. However, since the same research team worked on the project during the inclusion period, the study appears reliable in terms of diagnosis and homogeneity. Second, we did not collect blood gas data at ICU admission and therefore cannot assess the evolution of oxygenation before patients were suspected of having VAP.
5. Conclusions
In conclusion, we have found that adding PaO2/FIO2 ratio ≤ 240 to the clinical and radiographic criteria does not help in the diagnosis of VAP. PaO2/FIO2 ratio > 240 does not exclude this infection. Using this threshold may underestimate the incidence of VAP. Changing this paradigm might have beneficial effects on the early antibiotic treatment for VAP.
Acknowledgments
The results of this study have not been published elsewhere. We thank the medical and nursing staff at all the ICUs for their co-operation in the data collection. Cillóniz is the recipient of a Postdoctoral Grant (Strategic Plan for Research and Innovation in Health-PERIS 2016-2020).
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/8/8/1217/s1, Supplementary Data, Methods.
Author Contributions
Conception and design: M.F., A.T., T.S.; acquisition, analysis or interpretation of data: M.F., A.T., C.C., C.D., G.L.B. and I.M.-L. Drafting the manuscript for important intellectual content: M.F., A.T., C.C., C.D., G.L.B., I.M.-L.; Statistical analysis: A.T.; administrative, technical or material support: C.C., C.D., A.T.; All authors reviewed, revised, and approved the manuscript for submission; study supervision: M.F., A.T., C.C., C.D., I.M.
Funding
2017-SGR-787: ICREA academia 2013, Juan de la Cierva 2012 (JCI-2012-14801), MEyC, PN I+D (SAF2012-33744), CibeRes (CB06/06/0028)-ISCiii.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- 1.Kalil A.C., Metersky M.L., Klompas M., Muscedere J., Sweeney D.A., Palmer L.B., Napolitano L.M., O’Grady N.P., Bartlett J.G., Carratalà J., et al. Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016;63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalanuria A.A., Ziai W., Zai W., Mirski M. Ventilator-associated pneumonia in the ICU. Crit Care. 2014;18:208. doi: 10.1186/cc13775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Timsit J.-F., Esaied W., Neuville M., Bouadma L., Mourvllier B. Update on ventilator-associated pneumonia. F1000Res. 2017;6:2061. doi: 10.12688/f1000research.12222.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Vught L.A., Klein Klouwenberg P.M.C., Spitoni C., Scicluna B.P., Wiewel M.A., Horn J., Schultz M.J., Nürnberg P., Bonten M.J.M., Cremer O.L., et al. Incidence, Risk Factors, and Attributable Mortality of Secondary Infections in the Intensive Care Unit After Admission for Sepsis. JAMA. 2016;315:1469–1479. doi: 10.1001/jama.2016.2691. [DOI] [PubMed] [Google Scholar]
- 5.Metersky M.L., Wang Y., Klompas M., Eckenrode S., Bakullari A., Eldridge N. Trend in Ventilator-Associated Pneumonia Rates Between 2005 and 2013. JAMA. 2016;316:2427–2429. doi: 10.1001/jama.2016.16226. [DOI] [PubMed] [Google Scholar]
- 6.Pugin J., Auckenthaler R., Mili N., Janssens J.P., Lew P.D., Suter P.M. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am. Rev. Respir. Dis. 1991;143:1121–1129. doi: 10.1164/ajrccm/143.5_Pt_1.1121. [DOI] [PubMed] [Google Scholar]
- 7.Shan J., Chen H.-L., Zhu J.-H. Diagnostic accuracy of clinical pulmonary infection score for ventilator-associated pneumonia: A meta-analysis. Respir. Care. 2011;56:1087–1094. doi: 10.4187/respcare.01097. [DOI] [PubMed] [Google Scholar]
- 8.Zilberberg M.D., Shorr A.F. Ventilator-associated pneumonia: The clinical pulmonary infection score as a surrogate for diagnostics and outcome. Clin. Infect. Dis. 2010;51(Suppl. 1):S131–S135. doi: 10.1086/653062. [DOI] [PubMed] [Google Scholar]
- 9.FAQs: Pneumonia (PNEU) Events | NHSN | CDC. [(accessed on 8 January 2019)]; Available online: https://www.cdc.gov/nhsn/faqs/faq-pneu.html.
- 10.Chastre J., Fagon J.-Y. Ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 11.Hilbert G., Gruson D., Vargas F., Valentino R., Gbikpi-Benissan G., Dupon M., Reiffers J., Cardinaud J.P. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N. Engl. J. Med. 2001;344:481–487. doi: 10.1056/NEJM200102153440703. [DOI] [PubMed] [Google Scholar]
- 12.Fàbregas N., Ewig S., Torres A., El-Ebiary M., Ramirez J., de La Bellacasa J.P., Bauer T., Cabello H. Clinical diagnosis of ventilator associated pneumonia revisited: Comparative validation using immediate post-mortem lung biopsies. Thorax. 1999;54:867–873. doi: 10.1136/thx.54.10.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodhead M., Torres A. Definition and Classification of Community-Acquired and Nosocomial Pneumonias. European Respiratory Society Journals Ltd.; Lausanne, Switzerland: 1997. [Google Scholar]
- 14.Meduri G.U., Chastre J. The standardization of bronchoscopic techniques for ventilator-associated pneumonia. Chest. 1992;102:557S–564S. doi: 10.1378/chest.102.5_Supplement_1.557S. [DOI] [PubMed] [Google Scholar]
- 15.Kollef M.H., Bock K.R., Richards R.D., Hearns M.L. The safety and diagnostic accuracy of minibronchoalveolar lavage in patients with suspected ventilator-associated pneumonia. Ann. Intern. Med. 1995;122:743–748. doi: 10.7326/0003-4819-122-10-199505150-00002. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz M., Torres A., Ewig S., Marcos M.A., Alcón A., Lledó R., Asenjo M.A., Maldonaldo A. Noninvasive versus invasive microbial investigation in ventilator-associated pneumonia: Evaluation of outcome. Am. J. Respir. Crit. Care Med. 2000;162:119–125. doi: 10.1164/ajrccm.162.1.9907090. [DOI] [PubMed] [Google Scholar]
- 17.Van Saene H.K.F., Petros A.J., Ramsay G., Baxby D. All great truths are iconoclastic: Selective decontamination of the digestive tract moves from heresy to level 1 truth. Intensive Care Med. 2003;29:677–690. doi: 10.1007/s00134-003-1722-2. [DOI] [PubMed] [Google Scholar]
- 18.Ioanas M., Cavalcanti M., Ferrer M., Valencia M., Agusti C., Puig de la Bellacasa J., Torres A. Hospital-acquired pneumonia: Coverage and treatment adequacy of current guidelines. Eur. Respir. J. 2003;22:876–882. doi: 10.1183/09031936.03.00045903. [DOI] [PubMed] [Google Scholar]
- 19.Valencia Arango M., Torres Martí A., Insausti Ordeñana J., Alvarez Lerma F., Carrasco Joaquinet N., Herranz Casado M., Tirapu León J.P., Grupo de Estudio de la Neumonía Relacionada con Ventilación Mecánica. Grupo de Trabajo de Enfermedades Infecciosas de la SEMICYUC Diagnostic value of quantitative cultures of endotracheal aspirate in ventilator-associated pneumonia: A multicenter study. Arch. Bronconeumol. 2003;39:394–399. doi: 10.1016/s0300-2896(03)75414-3. [DOI] [PubMed] [Google Scholar]
- 20.Murray P.R., Baron E.J., Joergensen J.H., Pfaller M.A., Yolken R.H. Manual of Clinical Microbiology. 8th ed. American Society for Microbiology; Washington, DC, USA: 2003. [Google Scholar]
- 21.American Thoracic Society; Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 22.Heyland D.K., Dodek P., Muscedere J., Day A., Cook D., Canadian Critical Care Trials Group Randomized trial of combination versus monotherapy for the empiric treatment of suspected ventilator-associated pneumonia. Crit. Care Med. 2008;36:737–744. doi: 10.1097/01.CCM.0B013E31816203D6. [DOI] [PubMed] [Google Scholar]
- 23.Ioanas M., Ferrer M., Cavalcanti M., Ferrer R., Ewig S., Filella X., de la Bellacasa J.P., Torres A. Causes and predictors of nonresponse to treatment of intensive care unit-acquired pneumonia. Crit. Care Med. 2004;32:938–945. doi: 10.1097/01.CCM.0000114580.98396.91. [DOI] [PubMed] [Google Scholar]
- 24.Esperatti M., Ferrer M., Giunta V., Ranzani O.T., Saucedo L.M., Li Bassi G., Blasi F., Rello J., Niederman M.S., Torres A. Validation of predictors of adverse outcomes in hospital-acquired pneumonia in the ICU. Crit. Care Med. 2013;41:2151–2161. doi: 10.1097/CCM.0b013e31828a674a. [DOI] [PubMed] [Google Scholar]
- 25.Ramírez P., Ferrer M., Martí V., Reyes S., Martínez R., Menéndez R., Ewig S., Torres A. Inflammatory biomarkers and prediction for intensive care unit admission in severe community-acquired pneumonia. Crit. Care Med. 2011;39:2211–2217. doi: 10.1097/CCM.0b013e3182257445. [DOI] [PubMed] [Google Scholar]
- 26.Bello S., Lasierra A.B., Mincholé E., Fandos S., Ruiz M.A., Vera E., de Pablo F., Ferrer M., Menendez R., Torres A. Prognostic power of proadrenomedullin in community-acquired pneumonia is independent of aetiology. Eur. Respir. J. 2012;39:1144–1155. doi: 10.1183/09031936.00080411. [DOI] [PubMed] [Google Scholar]
- 27.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.-D., Coopersmith C.M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ARDS Definition Task Force. Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 29.FAQs: Ventilator-Associated (VAE) Events | NHSN | CDC. [(accessed on 8 January 2019)]; Available online: https://www.cdc.gov/nhsn/faqs/faq-vae.html.
- 30.Magill S.S., Klompas M., Balk R., Burns S.M., Deutschman C.S., Diekema D., Fridkin S., Greene L., Guh A., Gutterman D., et al. Developing a new, national approach to surveillance for ventilator-associated events*. Crit. Care Med. 2013;41:2467–2475. doi: 10.1097/CCM.0b013e3182a262db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ewig S., Torres A., Angeles Marcos M., Angrill J., Rañó A., de Roux A., Mensa J., Martínez J.A., de la Bellacasa J.P., Bauer T. Factors associated with unknown aetiology in patients with community-acquired pneumonia. Eur. Respir. J. 2002;20:1254–1262. doi: 10.1183/09031936.02.01942001. [DOI] [PubMed] [Google Scholar]
- 32.Giunta V., Ferrer M., Esperatti M., Ranzani O.T., Saucedo L.M., Li Bassi G., Blasi F., Torres A. ICU-acquired pneumonia with or without etiologic diagnosis: A comparison of outcomes. Crit. Care Med. 2013;41:2133–2143. doi: 10.1097/CCM.0b013e31828a453b. [DOI] [PubMed] [Google Scholar]
- 33.Bellani G., Laffey J.G., Pham T., Fan E., Brochard L., Esteban A., Gattinoni L., van Haren F., Larsson A., McAuley D.F., et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 34.Luna C.M., Blanzaco D., Niederman M.S., Matarucco W., Baredes N.C., Desmery P., Palizas F., Menga G., Rios F., Apezteguia C. Resolution of ventilator-associated pneumonia: Prospective evaluation of the clinical pulmonary infection score as an early clinical predictor of outcome. Crit. Care Med. 2003;31:676–682. doi: 10.1097/01.CCM.0000055380.86458.1E. [DOI] [PubMed] [Google Scholar]
- 35.Torres A., Niederman M.S., Chastre J., Ewig S., Fernandez-Vandellos P., Hanberger H., Kollef M., Bassi G.L., Luna C.M., Martin-Loeches I., et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT) Eur. Respir. J. 2017;50:1700582. doi: 10.1183/13993003.00582-2017. [DOI] [PubMed] [Google Scholar]
- 36.Martin-Loeches I., Deja M., Koulenti D., Dimopoulos G., Marsh B., Torres A., Niederman M.S., Rello J. EU-VAP Study Investigators Potentially resistant microorganisms in intubated patients with hospital-acquired pneumonia: The interaction of ecology, shock and risk factors. Intensive Care Med. 2013;39:672–681. doi: 10.1007/s00134-012-2808-5. [DOI] [PubMed] [Google Scholar]
- 37.Martin-Loeches I., Povoa P., Rodríguez A., Curcio D., Suarez D., Mira J.-P., Cordero M.L., Lepecq R., Girault C., Candeias C., et al. Incidence and prognosis of ventilator-associated tracheobronchitis (TAVeM): A multicentre, prospective, observational study. Lancet Respir. Med. 2015;3:859–868. doi: 10.1016/S2213-2600(15)00326-4. [DOI] [PubMed] [Google Scholar]
- 38.Ferrer M., Travierso C., Cilloniz C., Gabarrus A., Ranzani O.T., Polverino E., Liapikou A., Blasi F., Torres A. Severe community-acquired pneumonia: Characteristics and prognostic factors in ventilated and non-ventilated patients. PLoS ONE. 2018;13:e0191721. doi: 10.1371/journal.pone.0191721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin-Loeches I., Levy M.M., Artigas A. Management of severe sepsis: Advances, challenges, and current status. Drug Des. Devel. Ther. 2015;9:2079–2088. doi: 10.2147/DDDT.S78757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esperatti M., Ferrer M., Theessen A., Liapikou A., Valencia M., Saucedo L.M., Zavala E., Welte T., Torres A. Nosocomial pneumonia in the intensive care unit acquired by mechanically ventilated versus nonventilated patients. Am. J. Respir. Crit. Care Med. 2010;182:1533–1539. doi: 10.1164/rccm.201001-0094OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.