Abstract
Aging and frailty are associated with a high risk of lean mass (LM) loss, which leads to physical disability and can be effectively alleviated by protein supplementation (PS) and muscle strengthening exercise (MSE). In this study, the associations between LM gain and PS + MSE efficacy (measured using physical outcomes) in elderly patients with a high risk of sarcopenia or frailty were identified. A comprehensive search of online databases was performed to identify randomized controlled trials (RCTs) reporting the efficacy of PS + MSE in elderly patients with sarcopenia or frailty. The included RCTs were analyzed using meta-analysis and risk of bias assessment. We finally included 19 RCTs in this meta-analysis with a median (range/total) Physiotherapy Evidence Database score of 7/10 (5–9/10). The PS + MSE group exhibited significant improvements in the whole-body LM (standard mean difference (SMD) = 0.66; p < 0.00001), appendicular LM (SMD = 0.35; p < 0.00001), leg strength (SMD = 0.65; p < 0.00001), and walking capability (SMD = 0.33; p = 0.0006). Meta-regression analyses showed that changes in appendicular LM were significantly associated with the effect sizes of leg strength (β = 0.08; p = 0.003) and walking capability (β = 0.17; p = 0.04), respectively. Our findings suggest that LM gain after PS + MSE significantly contributes to the efficacy of the intervention in terms of muscle strength and physical mobility in elderly patients with a high risk of sarcopenia or frailty.
Keywords: sarcopenia, protein supplement, exercise training, lean body mass, physical function
1. Introduction
Aging is associated with muscle attenuation, which may contribute to common characteristics of muscle weakness and impaired physical mobility observed in elderly individuals at high risks of sarcopenia and frailty [1,2,3]. In addition, the indices for classifying older adults as clinically having sarcopenia [4] or high frailty risk [5] have been established among which low muscle strength and poor physical performance, such as slow walking speed, are common risk factors. Therefore, the maintenance of muscle strength and the prevention of sarcopenia are extremely crucial to enable prefrail and frail elderly adults to successfully perform physical tasks because low levels of lean mass or appendicular skeletal mass are closely associated with physical difficulty and poor health status among elderly patients [6,7].
Various nutrient interventions, exercise therapies or a combination of both are advised to prevent sarcopenia or frailty in elderly individuals [8,9,10,11,12], among which protein supplement (PS) combined with muscle strengthening exercise (MSE) has been known to benefit lean mass gain and function enhancement in elderly individuals regardless of protein type and exercise protocol [11,13,14,15]. However, whether intervention-induced changes in muscle mass contribute to strength gain and physical mobility improvement after PS + MSE remains unclear. An individual with lean mass (LM) gain exhibits improved physical performance, and several previous meta-analysis studies have reported that an increase in LM is accompanied by significant strength gain [14,16,17,18,19] or improvements in physical functioning [14,18] after PS + MSE; however, other authors have reported conflicting results of such synergetic improvements in LM and strength [13,20] or physical function [17,19]. Given that low muscle mass is a well-established factor associated with strength loss and mobility limitations in elderly populations [7,21] and that sarcopenia is associated with suppressed muscle protein turnover and homeostasis [22,23], identifying the effects of muscle mass changes in response to PS + MSE on strength gains and physical improvements can help clinical practitioners to efficiently make clinical decisions and set appropriate intervention strategies for older populations with sarcopenia or frailty.
Previous systematic reviews and meta-analyses have investigated the effects of PS + MSE on either sarcopenic or frail elderly populations; however, the combined meta-analysis approach in elderly adults with sarcopenia and frailty has not yet been confirmed. This study examined the combined effects of PS + MSE in elderly adults who have high risks of sarcopenia and frailty. In addition, meta-regression was used to determine whether LM gain in response to PS + MSE exerted any effect on the intervention outcomes of strength and physical mobility.
2. Method
2.1. Design
The present study was conducted by following the guidelines recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis [24]. The protocol for this study was registered at PROSPERO (registration number: CRD42018109176). The study was carried out based on a comprehensive electronic search from online sources. The articles were obtained from online database, including PubMed, EMBASE, the Cochrane Library Database, the Physiotherapy Evidence Database (PEDro), China knowledge resource integrated database, and Google Scholar databases. Secondary sources included papers cited by articles retrieved from the abovementioned sources. No limitation was imposed on the publication year and language to minimize publication and language bias. Two authors (CDL and HCC) independently searched for relevant articles, screened them, and extracted data. Any disagreement between the authors were resolved through a consensus in which the other team members (THL and SWH) acted as arbitrators.
2.2. Search Strategy
Keywords used for participant conditions were: “older/elderly” OR “frailty/frail” OR “sarcopenia”. Keywords used for intervention were: “exercise training” AND “protein/amino-acid/nutrient supplement”. The detailed search formulas for each database were presented in online Table S1.
2.3. Selection Criteria of Studies
Trials were included if they met the following criteria: (1) the study design was a randomized control trial (RCT); (2) experimental groups received PS (including adequate protein-based diet) plus MSE; (3) control groups received a placebo supplement, PS alone, MSE alone, or none of above; (4) exercise types included resistance training or a multicomponent exercise regime that consisted of MSE, aerobic exercise, balance training, and physical activity training; (5) the supplement intervention used protein sources including whey protein, leucine, casein, and soy, for consumption in isolation or combined with other nutrients (creatine, amino acids); (6) the study enrolled participants with mean age ≥ 60 years; the participants were hospitalized, institutionalized, or community-dwelling elderly individuals and with a high risk of sarcopenia or frailty and physical limitations. (7) the study reported the primary outcome measures of muscle mass or sarcopenia indices, including lean body mass (LBM), fat-free mass, appendicular lean mass (ALM), lean mass index, appendicular mass index, and skeletal mass index; and (8) the study reported the secondary outcomes, such as leg strength or physical function, including mobility and walking capability. Walking capability was measured using walk speed or walk endurance and was defined as 10-m walk time or 6-min walk distance.
Studies were eliminated if (1) the trial was conducted in vitro or in vivo in an animal model or if (2) the trial had a non-RCT design such as a case report, case series, or a prospectively designed trial without a comparison group.
2.4. Data Extraction
Data was extracted from each included trial and presented in an evidence table (Table 1) regarding: (1) characteristics of study design and sample (group design, gender, age); (2) characteristics of exercise training and PS; (3) measured time points; and (4) main outcome results. One author (C.-D.L.) has extracted the relevant data from included trials and the second author (S.-W.H.) checked the extracted data. Any disagreement between two authors was resolved by a consensus procedure. A third author (T.-H.L.) was further consulted if the disagreement persisted.
Table 1.
Summary of included study characteristics.
| Study (Author, Year, Ref) |
Groups 1 | Age (y) 2 | Sex (F/M) | N | Design | Patient Type | Body Composition Assessment Method | Exercise Intervention | Protein supplement | Measured Time Point | Outcome Results | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type, Compliance (%, EG/CG) | Frequency × Duration | Type, Compliance (%, EG/CG) 10 | Intake Amount (g/d or g/session) | ||||||||||
| Bjorkman, 2011 [37] |
EG: MSE + PS | 69.9 ± 7.4 | 20/3 | 23 | RCT, DB | Sarcopenia | DXA | RET | 2 d/wk × 8 wk | WP, | 14.0 g/d | Baseline | ↑ ALM/FM ratio 6,7 |
| CG: MSE | 69.1 ± 6.9 | 22/2 | 24 | Crossover | (PMR) | NA/NA | (16 sessions) | 85.9/89.3 | Posttest: 8 wk | ↑ CRT 6,7; ↑ HG 6,7 |
|||
| Bonnefoy, 2012 [38] |
EG: MSE + PS | 86.3 ± 14.1 4 | 49/4 | 53 | RCT | Pre-frail elder | NA | MET | 7 d/wk × 16 wk | Milk, soy, BCAA | 20.0 g/d | Baseline | FFM 8; PASE 8; ADL 8; GS 8 |
| CG: Control 3 | 86.0 ± 14.8 4 | 39/10 | 49 | adults | 44/NA | (116 sessions) | 44/NA | Posttest: 16 wk |
↓ IADL 5,7; TUG 8 |
||||
| Bonnefoy, 2003 [39] |
EG: MSE + PS | 83.5 ± 1.2 9 | 50/7 9 | 57 9 | RCT, SB | Frail elder | DLW | MET | 3 d/wk × 36 wk | Proteins | 30.0 g/d | Baseline | FFM 8; GS 8; SC 8 |
| CG 1: MSE + PLA-S | individuals | method | 63–70 9 | (108 sessions) | 61/54 | Midtest: 12 wk |
↑ Leg strength 7 | ||||||
| CG 2: CT + PS | Posttest: 36 wk |
||||||||||||
| CG 3: CT + PLA-S | |||||||||||||
| Carlsson, 2011 [40] |
EG: MSE + PS | 84.4 ± 6.3 | 33/9 | 42 | RCT, DB | Frail | BIA | MET | 2–3 d/wk × 13 wk | Milk protein | 7.4 g/session | Baseline | ICW 8; BBS 8 |
| CG 1: MSE + PLA-S | 85.3 ± 5.5 | 28/13 | 41 | elderly | 79/72 | (29 sessions) | 84/79 | Midtest: 12 wk |
|||||
| CG 2: PS | 82.7 ± 6.4 | 34/13 | 47 | Posttest: 24 wk |
|||||||||
| CG 3: PLA-S | 85.4 ± 7.2 | 36/11 | 47 | ||||||||||
| Dirks, 2017 [41] |
EG: MSE + PS | 76.0 ± 8.2 | 11/6 | 17 | RCT, DB | Frail elderly | DXA | RET | 2 d/wk × 24 wk | Milk protein | 30.0 g/d | Baseline | ↑ LBM 6,7; ↑ ALM 6,7 |
| CG: MSE + PLA-S | 77.0 ± 8.2 | 11/6 | 17 | 84 9 | (48 sessions) | NR | Midtest: 12 wk |
↓ CRT 5,6; ↑ SPPB 5,6,8 |
|||||
| Posttest: 24 wk |
↑ LP 1-RM 5,6 | ||||||||||||
| Englund, 2017 [42] |
EG: MSE + PS | 78.1 ± 5.8 | 34/40 | 74 | RCT, DB | Mobility- | DXA | MET | 3 d/wk × 24 wk | Whey proteins | 20.0 g/session | Baseline | ↑ LBM 5,6; ↑ ALM 5,6 |
| CG: MSE + PLA-S | 76.9 ± 4.9 | 35/40 | 75 | limited elderly | >70 | (72 sessions) | >85 | Posttest: 24 wk |
↑ Leg strength 5,6 | ||||
| Fiatarone, 1994 [43] |
EG: MSE + PS | 87.2 ± 6.0 | 16/9 | 25 | RCT, DB | Nursing- | WBP | RET | 3 d/wk × 10 wk | Soy protein | 40.8 g/d | Baseline | WBP 8; ↑ GS 6,7; ↑ SC 6,7 |
| CG 1: MSE + PLA-S | 86.2 ± 5.0 | 16/9 | 25 | home residents | method | 97/100 | (30 sessions) | 99/100 | Posttest: 10 wk |
↑ LP 1-RM 6,7 | |||
| CG 2: PS | 85.7 ± 5.8 | 17/7 | 24 | ||||||||||
| CG 3: PLA-S | 89.2 ± 4.1 | 14/12 | 26 | ||||||||||
| Fielding, 2017 [44] |
EG: MSE + PS | 78.1 ± 5.8 | 34/40 | 74 | RCT, DB | Mobility- | DXA | MET | 3 d/wk × 24 wk | Whey proteins | 20.0 g/session | Baseline | ALM |
| CG: MSE + PLA-S | 76.9 ± 4.9 | 35/40 | 75 | limited elderly | 75/72 | (72 sessions) | 88/86 | Posttest: 24 wk |
↑ GS 5,6,8; ↑ SPPB 5,6,8 |
||||
| Hegerova, 2015 [45] |
EG: MSE + PS | 83.6 ± 3.8 | NR | 100 | RCT | Hospitalized | BIA | MET | 6 d/wk × 3 wk | Protein | 20.0 g/d | Baseline | SMM 8; HG 8 |
| CG: Control 3 | 83.2 ± 3.8 | 100 | elderly adults | NR | (18 sessions) | 83/71.3 | Posttest: 3, 6, 12 mo |
↓ Barthel index 5,6,7 | |||||
| Imaoka, 2016 [46] |
EG: MSE + PS | 87.6 ± 6.5 | 18/5 | 23 | RCT | Institutionalized | BIA | MET | 2 d/wk × 12 wk | Proteins | 4.1 g/d | Baseline | SMI 8; FIM 8 |
| CG 1: MSE | 82.6 ± 9.1 | 16/6 | 22 | frail elderly | NR | (24 sessions) | NR | Posttest: 12 w |
↓ Incidence of falls 7 | ||||
| CG 2: PS | 84.6 ± 7.7 | 20/3 | 23 | Follow up: 26 wk |
|||||||||
| CG 3: Control 3 | 82.5 ± 10.9 | 15/8 | 23 | ||||||||||
| Kim, 2016 [31] |
EG: MSE + PS | 80.9 ± 2.9 | 36/0 | 36 | RCT | Sarcopenic | BIA | MET | 2 d/wk × 12 wk | Leucine, EAA | 3.0 g/d | Baseline | ↑ LLM 7; ↑ GS 7; ↑ HG 7 |
| CG 1: MSE | 81.4 ± 4.3 | 35/0 | 35 | elderly | NR | (24 sessions) | NR | Posttest: 12 wk |
↑ Leg strength 7 | ||||
| CG 2: PS | 81.2 ± 4.9 | 34/0 | 34 | women | |||||||||
| CG 3: Control 3 | 81.1 ± 5.1 | 34/0 | 34 | ||||||||||
| Kim, 2012 [47] |
EG: MSE + PS | 79.5 ± 2.9 | 38/0 | 38 | RCT, DB | Sarcopenic | BIA | MET | 2 d/w × 12 w | Leucine, EAA | 6.0 g/d | Baseline | ↑ LBM 6; ↑ ALM 6 |
| CG 1: MSE | 79.0 ± 2.9 | 39/0 | 39 | elderly | 70.3/71.8–80.5 | (24 sessions) | NR | Posttest: 12 wk |
↑ LLM 6,7; ↑ GS 6,7 |
||||
| CG 2: PS | 79.2 ± 2.8 | 39/0 | 39 | women | ↑ Leg strength 6,7 | ||||||||
| CG 3: Control 3 | 78.7 ± 2.8 | 39/0 | 39 | ||||||||||
| Maltais, 2016 [48] |
EG 1: MSE + Milk PS | 68.0 ± 5.6 | 0/8 | 8 | RCT, DB | Sarcopenic | DXA | RET | 3 d/w × 16 w | Milk protein, | 19–20.5 g/d | Baseline | ↑ LBM 5,6,7; ↓ TUG 6,7 |
| EG 2: MSE + EAA PS | 64.0 ± 4.8 | 0/8 | 8 | elderly men | >90 9 | (48 sessions) | EAA | (3.5 g leucine) | Posttest: 16 wk |
||||
| CG: MSE + PLA-S | 64.0 ± 4.9 | 0/10 | 10 | >90 9 | |||||||||
| Molnar, 2016 [49] |
EG: MSE + PS | 66.6 ± 1.6 | 10/7 | 17 | RCT | Institutionalized | BIA | MET | 2 d/w × 12 w | WP, Leucine | 33.0 g/d | Baseline | ↑ LBM 7 |
| CG: MSE | 66.4 ± 1.8 | 12/5 | 17 | elderly | NR | (24 sessions) | NR | Posttest: 12 wk |
↑ Leg strength 7 | ||||
| Rondanelli, 2016 [50] |
EG: MSE + PS | 80.8 ± 6.3 | 40/29 | 69 | RCT, DB | Sarcopenic | DXA | MET | 5 d/wk × 12 wk | WP | 22.0 g/d | Baseline | ↑ LBM 6,7; ↑ ADL 5,6,7 |
| CG: MSE + PLA-S | 80.2 ± 8.5 | 37/24 | 61 | elderly | NR | (60 sessions) | 100 9 | Posttest: 12 wk |
↑ HG 6,7; ↑ SF-36 PF 7 |
||||
| Tieland, 2012 [51] |
EG: MSE + PS | 78.0 ± 9.0 | 20/11 | 31 | RCT, DB | Frail | DXA | RET | 2 d/wk × 24 wk | Milk protein | 30.0 g/d | Baseline | ↑ LBM 6,7; ↑ ALM 6,7 |
| CG: MSE + PLA-S | 79.0 ± 6.0 | 21/10 | 31 | elderly | ≥98 9 | (48 sessions) | ≥98 9 | Midtest: 12 wk |
↑ LP 1-RM 5,6; ↓ CRT 5,6 |
||||
| Yamada, 2019 [52] |
EG: MSE + PS | 84.9 ± 5.6 | 20/8 | 28 | RCT, SB | Sarcopenic | DXA | RET | 2 d/wk × 12 wk | WP | 10.0 g/d | Baseline | ↑ ALM 6,7; ↑ GS 6,7 |
| CG 1: MSE | 84.7 ± 5.1 | 18/10 | 28 | elderly | 88.1/81 | (24 sessions) | 97.6/98.8–100 | Posttest: 12 wk |
↑ Leg power 5,6,7 | ||||
| CG 2: PS | 83.2 ± 5.7 | 20/8 | 28 | ↓ CRT 5,6; ↑ HG 6,7; SLS 8 |
|||||||||
| CG 3: Control3 | 83.9 ± 5.7 | 15/13 | 28 | ||||||||||
| Yamada, 2015 [53] 11 |
EG: MSE + PS | 78.1 ± 5.7 | 19/12 | 31 | RCT | Frail | BIA | Weighted | 7 d/wk × 24 wk | Protein (BCAA) | 10.0 g/d | Baseline | ↑ SMI 7 |
| CG 1: MSE | 75.7 ± 5.8 | 8/7 | 15 | elderly | walking | 80 (67–92) | Posttest: 24 wk |
||||||
| CG 2: Control 3 | 76.4 ± 6.2 | 15/10 | 25 | NR | |||||||||
| Zdzieblik, 2015 [54] |
EG: MSE + PS | 72.3 ± 3.7 | 0/26 | 26 | RCT, DB | Sarcopenic | DXA | RET | 3 d/wk × 12 wk | EAA | 15.0 g/d | Baseline | ↑ FFM 6,7,8 |
| CG: MSE + PLA-S | 72.1 ± 5.5 | 0/27 | 27 | Elderly men | 86.7/90 | (36 sessions) | NR | Posttest: 12 wk |
|||||
Note: 1 All parallels PS + MSE and control groups are presented for each trial. 2 Values are presented as mean and SD (or range). 3 Non supplement, non-exercise training, standardized care. 4 Data was estimated. 5 Significant within-group difference for control compared with baseline. 6 Significant within-group difference for PS + MSE compared with baseline. 7 Significant between-group difference for PS + MSE compared with control. 8 Non-significant between-group difference for PS + MSE compared with control. 9 Values of all samples. 10 Values denote the compliance of protein and placebo supplement (%) in EG and CG, respectively. 11 Only frail participants’ data were extracted. 6MWD, 6-min walk-for-distance; ADL, activities of daily living; ALM, appendicular lean mass; BBS, Berg’s balance scale; BCAA, branched chain amino acids; BIA, bioelectrical impedance analysis; BMI, body mass index; CG, control group; CRT, chair rise time; CSA, cross-sectional area; CT, cognition training; DB, double blind; DLW, doubly labeled water; DXA, dual-energy X-ray absorptiometry; EAA, essential amino acids; EG, experimental group; FIM, functional independence measure; FFM, fat-free mass; FFMI, fat-free mass index; GS, gait speed; HG, handgrip strength; ICW, intra cellular water; LBM, lean body mass; LLM, leg lean mass; LP 1-RM, leg press one repetition maximum; MET, multicomponent exercise training; MSE, muscle strengthening exercise; NA, not applicable; NR, not reported; PASE, Physical Activity Scale for older people; PLA-S, placebo supplement; PMR, polymyalgia rheumatica; PS, protein supplementation; RCT, randomized controlled trial; Ref = reference number; RET, resistance exercise training; RT, reaction time; SB, single blind; SC, stair climbing; SE, standard exercise; SF-36 PF, Short-Form 36-Item Health Survey (physical function subscore); SMI, skeletal muscle mass index; SMM, skeletal muscle mass; SLS, single leg stance; SPPB, short physical performance battery; TUG, timed up-and-go test; WBP, whole body potassium; WP, whey protein; ↑, significant increase; ↓, significant decrease. d: day; wk: week.
The trial parallels with PS plus MSE group were extracted as experimental groups and those with placebo supplement, PS alone, or MSE alone was extracted as control groups. If the trial had more than one experimental group or control intervention, each of the comparisons was served as an independent one for meta-analyses [25].
2.5. Assessment of Bias Risks and Methodological Quality of Included Studies
Quality assessment was performed using the PEDro quality score to assess the risk of bias. Methodological quality of all the included studies was independently assessed by two researchers in accordance with the PEDro classification scale, which is a valid measure of the methodological quality of clinical trials [26]. The PEDro scale scores 10 items including random allocation, concealed allocation, similarity at baseline, subject blinding, therapist blinding, assessor blinding, >85% follow up for at least one key outcome, intention-to-treat analysis, between-group statistical comparison for at least one key outcome, and point and variability measures for at least one key outcome. Each item is scored as either 1 for present or 0 for absent, and a total sum score ranging from 0 to 10 is obtained by summation of all the 10 items. On the basis of the PEDro score, the methodological quality of the included RCTs was rated as high (≥7/10), medium (4–6/10), and low (≤3/10) [27].
2.6. Data Synthesis and Analysis
We computed effect sizes for each study separately for primary and secondary outcome measures. The primary outcome measure as well as the secondary one was defined as a pooled estimate of the mean difference in change between the mean of the treatment (PS and resistance training) and the placebo (other-type supplement and resistance training) groups. If the exact variance of paired difference was not derivable, it was imputed by assuming a within-participant correlation coefficient of 0.98, 0.92, and 0.80 for lean body mass [28], muscle strength [29,30], and mobility [30,31], respectively, between the baseline and posttest measured data. If data were reported as median (range), they were re-calculated algebraically from the trial data to impute the sample mean and SD [25,32]. All the extracted outcome data were calculated as standard mean difference (SMD) versus placebo or active control, as well as the secondary outcomes including functional mobility. We used SMD for meta-analysis when different scales were used to measure the same concept (e.g., pain, function score).
Fixed effect or random effect models were used, depending on the existence of heterogeneity. Statistical heterogeneity was assessed using the I2 statistic and was estimated for significance (p < 0.05) and χ2 and F values greater than 50% [33]. A fixed effect model was used unless statistical heterogeneity was significant (p < 0.05), after which a random effects model was used.
The duration of follow up (FU) was assessed and defined as immediate (<3 months), short term (≥3 months, <6 months), medium term (≥6 months, <12 months), and long term (≥12 months).
Subgroup analysis was conducted by using methodological quality level, duration of intervention, participant types (i.e., community-dwelling patient or institutionalized resident), and conditions (i.e., sarcopenia, frailty, or others), exercise types (i.e., resistance training or multicomponent exercise regime), PS dose (i.e., <20 g/day or ≥20 g/day [34]), and types of control group (i.e., placebo, PS alone, or exercise training alone) in the included trials. All subgroup differences were tested for significance and an I2 statistics statistic was also computed in order to estimate the degree of subgroup variability. Potential publication bias was investigated using visual inspection of a funnel plot to explore possible reporting bias [35] and was assessed by the Egger’s regression asymmetry test [36] using the SPSS, Version 20.0, statistical software (IBM, Armonk, NY, USA). A value of P less than 0.05 was considered to be statistically significant. All analyses were conducted using RevMan 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark).
To assess the association between muscle mass gain and clinical outcomes (strength and mobility), an inverse-variance weighted meta-regression model was established with percent muscle mass gain as the independent variable and SMD for strength and mobility as dependent variables; the analysis was controlled for age, methodological design, and follow-up duration. If the trial had more than one experimental or control intervention, each comparison was performed independently for meta-regression analysis.
3. Results
3.1. Trial Flow
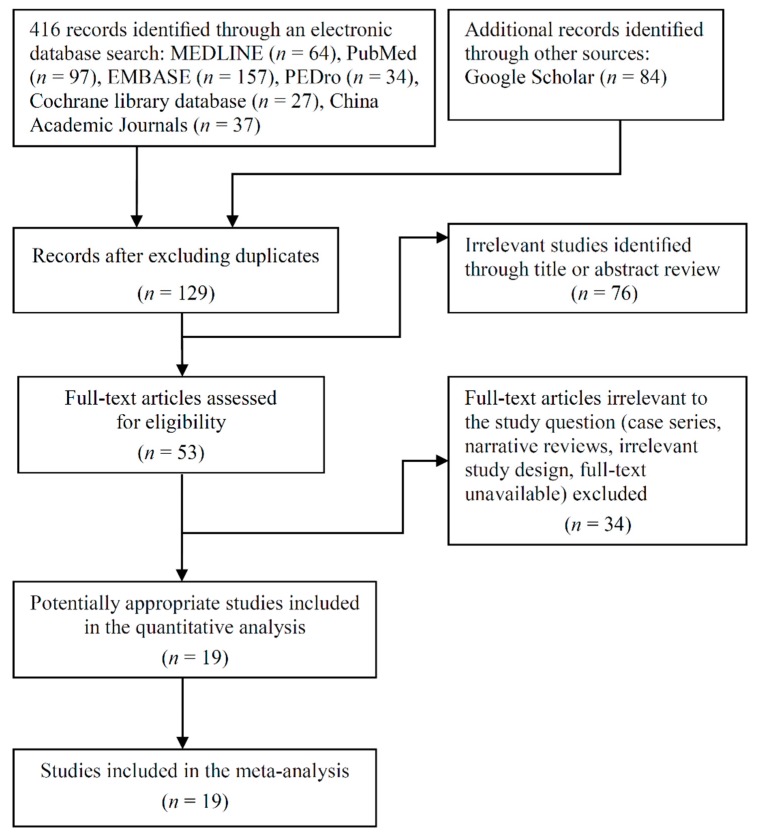
Figure 1 shows a flowchart of the selection processes. The final sample consisted of 19 RCTs [31,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54], which were published between 1994 and 2019. A sample consisting of a total of 1888 participants with a mean (range) age of 78.7 (64.0–89.2) years was enrolled. From the total sample, 738 patients received a protein-type supplement in combination with MSE, 556 received exercise training with or without placebo supplement, 209 received PS alone, and 385 received placebo supplement alone or no intervention.
Figure 1.
Flow chart of enrolled studies.
3.2. Study Characteristics
Table 1 shows a summary of demographic data and study characteristics of the included RCTs. Fifteen RCTs enrolled community-dwelling elderly individuals with frailty, sarcopenia, or mobility limitation [31,37,38,39,40,41,42,44,47,48,50,51,52,53,54,55], whereas the remaining four enrolled institutionalized residents [43,45,46,49]. Mostly all the included RCTs employed an intervention period of 3–6 months [31,38,40,41,42,44,46,47,48,49,50,51,52,53,54]; however, three RCTs had short intervention periods of <3 months [37,43,45], and one had a long period of approximately 9 months [39]. With respect to the follow-up duration, all the 19 included RCTs reported a short-term or medium duration of <9 months; only one RCT had a long-term follow-up period of >12 months [45].
3.3. Protein Supplementation Characteristics
Protocols for PS are summarized in Table 1. The protocol for protein supplementation varied widely across included trials. Regarding the amount of protein, the majority of the included RCTs employed daily PS with amounts of extra protein ranging from 3.0 to 40.8 g/day. Three RCTs used PS of <10.0 g/day [31,46,47], two used PS of >30.0 g/day [43,49], and three RCTs provided supplements immediately after exercise on training days with amounts of extra protein ranging from 7.4 to 20.0 g/session [40,42,44].
3.4. Protocol of Exercise Training
A summary of protocols for MSE is presented in Table 1. Regarding the mode of exercise, seven RCTs used resistance exercise training [37,41,43,48,51,52,54], 11 RCTs used multicomponent exercise regime, and one used aerobic training with weighted walking [53]. One RCTs used a long-term exercise duration of 36 weeks (108 sessions) [39], whereas a medium-period treatment duration of 12–24 weeks (24–116 sessions) was used by 15 RCTs [31,38,40,41,42,44,46,47,48,49,50,51,52,53,54], and the other three RCTs used a short-period intervention of <12 weeks (16–30 sessions) [37,43,45].
3.5. Risk of Bias in Included Studies
The individual PEDro scores are listed in Table 2. Among the 19 RCTs, the methodological quality of 12 was high [37,38,39,40,41,43,45,47,48,50,51,54] and that of the other seven was medium [31,42,44,46,49,52,53], with a median PEDro score of 7/10 (range 5/10 to 9/10). The interrater reliability associated with the cumulative PEDro score was acceptable with an intraclass correlation coefficient of 0.93 (95% CI: 0.82–0.97). Of the 19 included RCTs, all employed random allocation, similarity at the baseline, between-group comparisons, and point estimates and variability; in addition, four employed concealed allocation, nine incorporated subject blinding, four incorporated therapist blinding, 10 incorporated assessor blinding, 17 had adequate follow-up, and 12 employed intention-to-treat analysis.
Table 2.
Summary of methodological quality based on the PEDro classification scale a.
| Study Author (year) (Reference Number) | Overall b | Eligibility Criteria c | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bjorkman 2011 [37] | 8/10 | X | X | X | X | X | X | X | X | X | ||
| Bonnefoy 2012 [38] | 7/10 | X | X | X | X | X | X | X | X | |||
| Bonnefoy 2003 [39] | 7/10 | X | X | X | X | X | X | X | X | |||
| Carlsson 2011 [40] | 9/10 | X | X | X | X | X | X | X | X | X | X | |
| Dirks 2017 [41] | 7/10 | X | X | X | X | X | X | X | X | |||
| Englund 2017 [42] | 6/10 | X | X | X | X | X | X | X | ||||
| Fiatarone 1994 [43] | 8/10 | X | X | X | X | X | X | X | X | X | ||
| Fielding 2017 [44] | 6/10 | X | X | X | X | X | X | X | ||||
| Hegerova 2015 [45] | 7/10 | X | X | X | X | X | X | X | X | |||
| Imaoka 2016 [46] | 6/10 | X | X | X | X | X | X | X | ||||
| Kim 2016 [31] | 5/10 | X | X | X | X | X | X | |||||
| Kim 2012 [47] | 7/10 | X | X | X | X | X | X | X | X | |||
| Maltais 2016 [48] | 8/10 | X | X | X | X | X | X | X | X | X | ||
| Molnar 2016 [49] | 6/10 | X | X | X | X | X | X | X | ||||
| Rondanelli 2016 [50] | 9/10 | X | X | X | X | X | X | X | X | X | X | |
| Tieland 2012 [51] | 7/10 | X | X | X | X | X | X | X | X | |||
| Yamada 2019 [52] | 6/10 | X | X | X | X | X | X | X | ||||
| Yamada 2015 [53] | 6/10 | X | X | X | X | X | X | X | ||||
| Zdzieblik 2015 [54] | 8/10 | X | X | X | X | X | X | X | X | X | ||
| Summary # | 19 | 19 | 4 | 19 | 9 | 4 | 10 | 17 | 12 | 19 | 19 |
a PEDro, Physiotherapy Evidence Database. Guidline of PEDro scale is available from PEDro database (https://www.pedro.org.au/english/downloads/pedro-scale/). b Points of methodological quality are denoted as “X” for fulfilled criteria. c Not used to calculate the total score. Score was determined by a third assessor. # This was calculated as the number of studies satisfied. PEDro classification scale: 1 = random allocation, 2 = concealed allocation, 3 = similarity at the baseline, 4 = subject blinding, 5 = therapist blinding, 6 = assessor blinding, 7 = more than 85% follow-up for at least one key outcome, 8 = intention-to-treat analysis, 9 = between-group statistical comparison for at least one key outcome, 10 = point and variability measures for at least one key outcome. Methodological quality: high, ≥7 points; medium, 4–6 points; low, ≤3 points.
3.6. Effectiveness on Muscle Mass
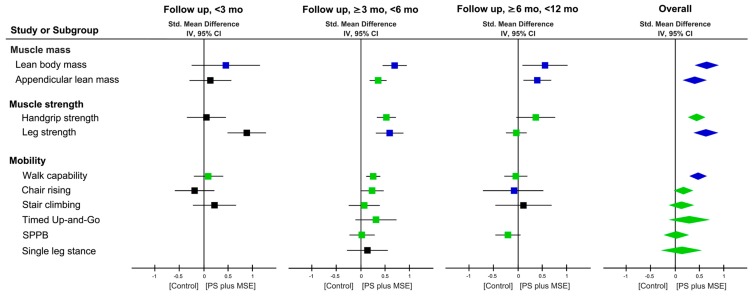
Changes in LBM or fat-free mass after PS + MSE were reported by 18 RCTs (29 comparisons) [31,37,38,39,40,41,42,43,45,46,47,48,49,50,51,52,53,54], and changes in ALM were reported by 10 RCTs (19 comparisons) [31,37,41,42,47,48,50,51,52,53] (Table 1). Results of meta-analyses showed significant short-term (SMD = 0.71, p < 0.00001) and medium-term (SMD = 0.56, p = 0.02) effects on LBM as well as on ALM in favor of PS + MSE (Figure 2 and Figures S1 and S2). The evidence showed an overall effect on LBM with a significant SMD of 0.66 (95%CI: 0.41–0.91, p < 0.00001; I2 = 79%) favoring PS + MSE; similar results was observed in ALM (SMD = 0.40, 95%CI: 0.15–0.66, P = 0.002; I2 = 59%) (Figure 2 and Figures S1 and S2).
Figure 2.
Forest plot summarizing effects of protein supplement (PS) plus muscle strengthening exercise (MSE) on changes of muscle mass, body composition, and physical function at each follow up duration. Each point estimate at each follow up duration (square) and during an overall duration (diamond) presents the combined effect (standard mean difference) of the outcome measure where indicated, with 95% CI (horizontal line). Results plotted on the right-hand side indicate effects in favor of PS plus Ex. The combined effects analyzed by a fixed- or random-effect model are denoted by green and blue colors, respectively; and a black colored square denotes that the combined effect is derived from a single study. 95% CI = 95% confidence interval; Std = standard; IV = inverse variance; SPPB = short physical performance battery.
The results of subgroup analyses for LBM (Table 3) showed significant subgroup differences between participant types (I2 = 84.4%, p = 0.01), among participant conditions (I2 = 88.4%, P = 0.0002), and intervention periods (I2 = 70.8%, p = 0.03). The institutionalized elderly participants appeared to have significant effects on LBM with a greater SMD of 1.34 (p < 0.0001) than their community-dwelling peers (SMD = 0.44, p < 0.00001). The frail elderly participants were more likely to exhibit greater effects on LBM (SMD = 0.90, p < 0.00001) than their peers with sarcopenia (SMD = 0.44, p < 0.00001); similar results were observed for ALM.
Table 3.
Summary of overall effects and subgroup analyses results for muscle mass.
| Subgroups | Lean Body Mass | Appendicular Skeletal Muscle Mass | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Comparison, n | SMD | (95%CI) | p-value | I2 (%) | Comparison, n | SMD | (95%CI) | p-value | I2 (%) | |
| Overall | 29 | 0.66 | (0.41, 0.91) ‡ | <0.00001 | 79 | 19 | 0.35 | (0.20, 0.50) ‡ | <0.00001 | 59 |
| MQ level | ||||||||||
| PEDro score ≥7/10 | 19 | 0.52 | (0.29, 0.75) ‡ | <0.00001 | 64 | 9 | 0.63 | (0.31, 0.95) ‡ | 0.0001 | 56 |
| PEDro score <7/10 | 10 | 1.09 | (0.41, 1.76) ‡ | <0.00001 | 83 | 10 | 0.06 | (−0.17, 0.29) † | n.s. | 39 |
| Subgroup difference | n.s. | 58.8 | 0.03 | 79.2 | ||||||
| Participant type | ||||||||||
| Community-dwelled | 21 | 0.44 | (0.24, 0.64) ‡ | <0.0001 | 57 | 19 | 0.35 | (0.20, 0.50) ‡ | <0.00001 | 59 |
| Institutionalized resident | 8 | 1.34 | (0.67, 2.01) ‡ | <0.0001 | 84 | 0 | ||||
| Subgroup difference | 0.01 | 84.4 | NA | NA | ||||||
| Participant condition | ||||||||||
| Sarcopenia | 11 | 0.44 | (0.27, 0.62) † | <0.00001 | 17 | 13 | 0.49 | (0.14, 0.85) ‡ | 0.007 | 66 |
| Frailty | 17 | 0.90 | (0.52, 1.27) ‡ | <0.00001 | 82 | 2 | 0.75 | (0.32, 1.18) † | 0.0006 | 0 |
| Other conditions | 1 | −0.20 | (−0.56, 0.16) | n.s. | NA | 4 | 0.03 | (−0.26, 0.33) † | n.s. | 0 |
| Subgroup difference | 0.0002 | 88.4 | 0.02 | 76.0 | ||||||
| Gender | ||||||||||
| Men | 3 | 0.57 | (0.12, 1.03) † | 0.01 | 0 | 2 a | −0.03 | (−0.82, 0.76) † | n.s. | 0 |
| Women | 8 | 0.34 | (0.12, 0.56) † | 0.002 | 22 | 7 | 0.41 | (−0.09 0.91) ‡ | n.s. | 77 |
| Mixed | 18 | 0.82 | (0.46, 1.19) ‡ | <0.0001 | 85 | 10 | 0.45 | (0.13, 0.78) ‡ | 0.006 | 49 |
| Subgroup difference | n.s. | 53.6 | n.s. | 0 | ||||||
| Control group type | ||||||||||
| PLA-S or non-exercise | 10 | 0.89 | (0.42, 1.36) ‡ | 0.0002 | 90 | 5 | 0.80 | (0.04, 1.56) ‡ | 0.04 | 89 |
| Exercise | 14 | 0.53 | (0.21, 0.86) ‡ | 0.001 | 77 | 10 | 0.25 | (−0.02, 0.52) ‡ | n.s. | 50 |
| PS | 5 | 0.65 | (0.03, 1.27) ‡ | 0.04 | 85 | 4 | 0.53 | (−0.16, 1.22) ‡ | n.s. | 55 |
| Subgroup difference | n.s. | 0 | n.s. | 5.4 | ||||||
| Exercise type | ||||||||||
| RET | 11 | 0.57 | (0.25, 0.88) ‡ | 0.0005 | 54 | 12 | 0.37 | (0.13, 0.62) † | 0.002 | 42 |
| MET | 18 | 0.73 | (0.38, 1.07) ‡ | <0.0001 | 85 | 7 | 0.40 | (0.01, 0.79) ‡ | 0.04 | 74 |
| Subgroup difference | n.s. | 0 | n.s. | 0 | ||||||
| PS dose (g/day) b | ||||||||||
| <20 | 10 | 1.06 | (0.43, 1.69) ‡ | 0.0009 | 87 | 7 | 0.24 | (−0.08, 0.55) † | n.s. | 50 |
| ≥20 | 13 | 0.57 | (0.24, 0.90) ‡ | 0.0008 | 78 | 6 | 0.37 | (0.16, 0.58) † | 0.0005 | 28 |
| Subgroup difference | n.s. | 46.1 | n.s. | 0 | ||||||
| Intervention duration (week) | ||||||||||
| <12 | 4 | 0.14 | (−0.18, 0.45) † | n.s. | 0 | 1 | 0.14 | (−0.30, 0.58) | n.s. | NA |
| 12–23 | 21 | 0.69 | (0.42, 0.96) ‡ | <0.00001 | 70 | 17 | 0.38 | (0.09, 0.68) ‡ | 0.01 | 58 |
| ≥24 | 9 | 0.44 | (0.05, 0.83) ‡ | 0.03 | 73 | 3 | 0.49 | (−0.02, 1.00) † | n.s. | 65 |
| Subgroup difference | 0.03 | 70.8 | n.s. | 0 | ||||||
† Fixed-model effect. ‡ Random-model effect. a Comparisons were derived from single trial. b Trials with protein supplement were included and those with amino-acid supplement were excluded. SMD, standard mean difference; I2, heterogeneity; MQ, methodological quality; PEDro, Physiotherapy Evidence Database; n.s., nonsignificant (p > 0.05); PLA-S, placebo supplement; PS, protein supplementation; RET, resistance exercise training; MET, multicomponent exercise training; NA, not applicable.
3.7. Effectiveness on Muscle Strength and Physical Mobility Outcome
Changes in the handgrip and leg strength were reported by 6 RCTs (13 comparisons) [31,37,41,50,51,52] and 11 RCTs (23 comparisons) [31,39,41,42,43,47,48,49,51,52,54], respectively. Results of the meta-analysis showed significant combined effects on handgrip and leg strength with SMDs of 0.44 (95% CI: 0.26–0.62; p < 0.00001; I2 = 43%) and 0.65 (95% CI: 0.39–0.90; p < 0.00001; I2 = 62%) during an overall follow-up duration, respectively (Figure 2 and Figures S3 and S4).
The treatment effect of PS + MSE on physical function was assessed using several mobility tests, including walking capability by 18 RCTs (23 comparisons) [31,37,38,39,41,43,44,47,48,51,52], chair-rise test by seven RCTs (13 comparisons) [37,38,39,41,48,51,52], timed up-and-go by two RCTs (three comparisons) [38,48], stair-climb test by three RCTs (five comparisons) [38,39,43], short physical performance battery by three RCTs (three comparisons) [41,44,51], and single leg stance by one RCT (six comparisons) [52]. Significant effects favoring PS + MSE were observed on walking capability (SMD = 0.33, 95% CI: 0.14–0.52; p = 0.0006; I2 = 39%; Figure 2 and Figure S5). No significant effects were identified in other mobility (Figure 2 and Figures S6–S10).
The results of subgroup analyses showed that the control group types exhibited effects on leg strength (I2 = 92.5%, p < 0.00001) and chair-rise scores (I2 = 70.1%, p = 0.04; Table 4). In addition, subgroup analyses for leg strength showed significant subgroup differences between participant types (I2 = 85.2%, p = 0.009) as well as among participant conditions (I2 = 88.7%, p = 0.0001) and intervention periods (I2 = 90.5%, P < 0.0001; Table 4). The institutionalized elderly participants exhibited a greater change in leg strength in response to PS + MSE with a greater SMD on leg strength by 1.02 (p < 0.00001) than the community-dwelled peers (SMD = 0.56, p = 0.0001). No other factor was found to affect subgroup heterogeneity for leg strength, walking capability, and chair-rise test (all p > 0.05) (Table 4).
Table 4.
Summary of overall effects and subgroup analyses results for physical function.
| Subgroups | Leg Muscle Strength | Walk Capability | Chair Rise | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comparison, n | SMD | (95%CI) | p-value | I2 (%) | Comparison, n | SMD | (95%CI) | p-value | I2 (%) | Comparison, n | SMD | (95%CI) | p-value | I2 (%) | |
| Overall | 23 | 0.65 | (0.39, 0.90) ‡ | <0.00001 | 62 | 23 | 0.33 | (0.14, 0.52) ‡ | 0.0006 | 39 | 13 | 0.17 | (−0.02, 0.37) † | n.s. | 0 |
| MQ level | |||||||||||||||
| PEDro score ≥ 7/10 | 12 | 0.79 | (0.42, 1.16) ‡ | <0.0001 | 66 | 13 | 0.24 | (0.07, 0.41) † | 0.006 | 41 | 7 | 0.10 | (−0.12, 0.32) † | n.s. | 9 |
| PEDro score < 7/10 | 11 | 0.31 | (0.09, 0.52) † | 0.005 | 40 | 10 | 0.25 | (0.03, 0.48) † | 0.02 | 43 | 6a | 0.47 | (0.04, 0.91) † | 0.03 | 0 |
| Subgroup difference | n.s. | 50.5 | n.s. | 0 | n.s. | 55.7 | |||||||||
| Participant type | |||||||||||||||
| Community-dwelled | 19 | 0.56 | (0.28, 0.85) ‡ | 0.0001 | 64 | 20 | 0.32 | (0.11, 0.53) ‡ | 0.002 | 44 | 13 | 0.17 | (−0.02, 0.37) † | n.s. | 0 |
| Institutionalized resident | 4 | 1.02 | (0.62, 1.42) † | <0.00001 | 0 | 3a | 0.46 | (0.00, 0.92) † | 0.05 | 0 | 0 | ||||
| Subgroup difference | 0.009 | 85.2 | n.s. | 0 | NA | NA | |||||||||
| Participant condition | |||||||||||||||
| Sarcopenia | 15 | 0.73 | (0.44, 1.03) ‡ | <0.00001 | 43 | 15 | 0.46 | (0.15, 0.77) ‡ | 0.003 | 51 | 9 | 0.12 | (−0.16, 0.40) † | n.s. | 0 |
| Frailty | 7 | 0.58 | (0.32, 0.84) † | <0.0001 | 51 | 7 | 0.27 | (0.05, 0.49) † | 0.02 | 0 | 4 | 0.23 | (−0.05, 0.50) † | n.s. | 24 |
| Other conditions | 1 | −0.21 | (−0.57, 0.15) ‡ | n.s. | 89 | 1 | 0.00 | (−0.34, 0.34) | n.s. | NA | 0 | ||||
| Subgroup difference | 0.0001 | 88.7 | n.s. | 49.3 | n.s. | 0 | |||||||||
| Gender | |||||||||||||||
| Men | 3 | 0.71 | (−0.25, 1.67) ‡ | n.s. | 68 | 2a | 1.15 | (0.27, 2.03) † | 0.01 | 0 | 2 a | 0.15 | (−0.65, 0.95) † | n.s. | 0 |
| Women | 7 | 0.79 | (0.39, 1.20) ‡ | 0.0001 | 59 | 8 | 0.24 | (0.03, 0.46) † | 0.02 | 45 | 2 | 0.03 | (−0.31, 0.37) † | n.s. | 74 |
| Mixed | 13 | 0.51 | (0.18, 0.83) ‡ | 0.002 | 54 | 13 | 0.21 | (0.03, 0.39) † | 0.02 | 36 | 9 | 0.25 | (−0.00, 0.51) † | n.s. | 0 |
| Subgroup difference | n.s. | 0 | n.s. | 41.7 | n.s. | 0 | |||||||||
| Control group type | |||||||||||||||
| PLA-S or non-exercise | 6 | 1.12 | (0.67, 1.57) ‡ | <0.00001 | 67 | 5 | 0.50 | (0.29, 0.72) † | <0.00001 | 56 | 4 | 0.52 | (0.21, 0.83) † | 0.001 | 0 |
| Exercise | 12 | 0.43 | (0.14, 0.73) ‡ | 0.004 | 63 | 15 | 0.25 | (0.00, 0.50) ‡ | 0.05 | 48 | 7 | 0.00 | (−0.24, 0.24) † | n.s. | 0 |
| PS | 5 | 0.69 | (0.43, 0.96) ‡ | <0.00001 | 56 | 3 | 0.33 | (0.04, 0.61) † | 0.03 | 0 | 2a | 0.31 | (−0.22, 0.84) † | n.s. | 0 |
| Subgroup difference | <0.00001 | 92.5 | n.s. | 0 | 0.04 | 70.1 | |||||||||
| Exercise type | |||||||||||||||
| RET | 14 | 0.61 | (0.38, 0.83) † | <0.00001 | 41 | 14 | 0.44 | (0.11, 0.76) ‡ | 0.008 | 48 | 11 | 0.09 | (−0.14, 0.32) † | n.s. | 0 |
| MET | 9 | 0.68 | (0.25, 1.12) ‡ | 0.002 | 77 | 9 | 0.23 | (0.06, 0.41) † | 0.01 | 27 | 2 | 0.41 | (0.02, 0.79) † | 0.04 | 0 |
| Subgroup difference | n.s. | 0 | n.s. | 0 | n.s. | 48.5 | |||||||||
| PS dose (g/day) b | |||||||||||||||
| <20 | 7 | 0.86 | (0.51, 1.22) † | <0.00001 | 21 | 7 | 0.59 | (−0.05, 1.23) ‡ | n.s. | 66 | 7 | 0.12 | (−0.18, 0.41) † | n.s. | 13 |
| ≥20 | 10 | 0.48 | (0.12, 0.84) ‡ | 0.01 | 63 | 10 | 0.28 | (0.05, 0.50) † | 0.02 | 24 | 6 | 0.22 | (−0.05, 0.48) † | n.s. | 0 |
| Subgroup difference | n.s. | 26.6 | n.s. | 0 | n.s. | 0 | |||||||||
| Intervention duration (week) | |||||||||||||||
| <12 | 3a | 1.06 | (0.58, 1.54) † | <0.0001 | 0 | 4 | 0.10 | (−0.20, 0.41) † | n.s. | 44 | 1 | −0.20 | (−0.61, 0.21) | n.s. | NA |
| 12–23 | 19 | 0.60 | (0.32, 0.89) ‡ | <0.0001 | 59 | 19 | 0.26 | (0.11, 0.42) † | 0.0006 | 36 | 11 | 0.23 | (−0.01, 0.48) † | n.s. | 0 |
| ≥24 | 4 | −0.04 | (−0.28, 0.21) † | n.s. | 0 | 4 | −0.05 | (−0.29, 0.19) † | n.s. | 57 | 3 | −0.09 | (−0.71, 0.52) ‡ | n.s. | 69 |
| Subgroup difference | <0.0001 | 90.5 | n.s. | 2.7 | n.s. | 44 | |||||||||
† Fixed-model effect. ‡ Random-model effect. a Comparisons were derived from single trial. b Trials with protein supplement were included and those with amino-acid supplement were excluded. SMD, standard mean difference; I2, heterogeneity; MQ, methodological quality; PEDro, Physiotherapy Evidence Database; n.s., nonsignificant (p > 0.05); PLA-S, placebo supplement; PS, protein supplementation; RET, resistance exercise training; MET, multicomponent exercise training; NA, not applicable.
3.8. Associations of Muscle Mass Change with Muscle Strength and Physical Function
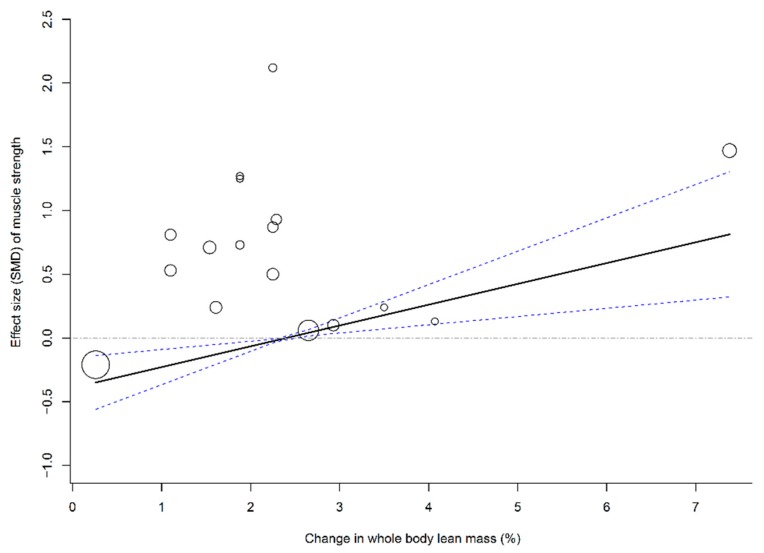
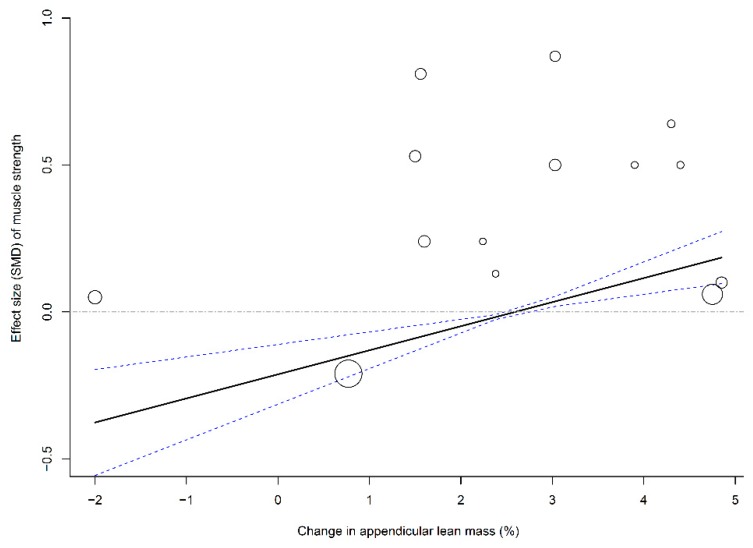
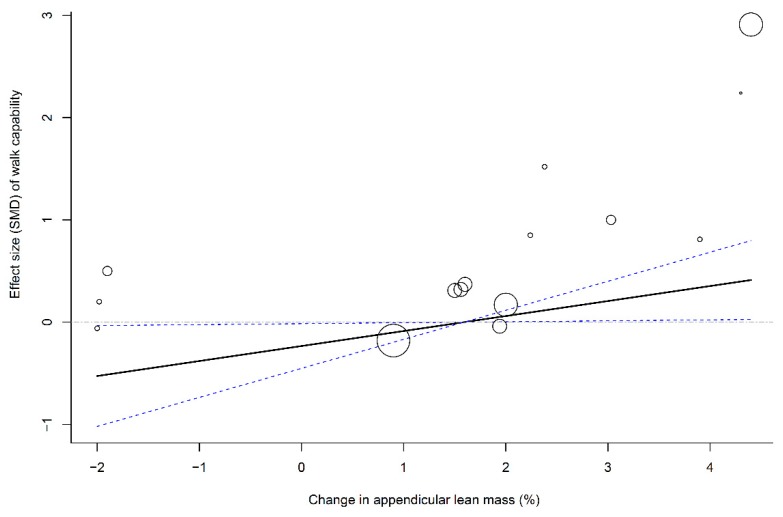
To evaluate the association between muscle mass (i.e., LBM and ALM) and effect sizes of physical outcomes (i.e., leg strength and walking capability), four multivariate meta-regression models that pooled all time frames were established using age, methodological quality, and follow-up duration as covariates. The results of the meta-regression analyses showed that changes in LBM (β = 0.16, 95% CI: 0.04–0.29; p = 0.01; Figure 3) and ALM (β = 0.08, 95% CI: 0.04–0.13; p = 0.003; Figure 4) were significantly associated with SMDs of leg strength; the results further indicated that elderly individuals who responded to PS + MSE by an increase in LBM or ALM of >2.5% may have achieved a positive effect size of leg strength. In addition, a greater change in ALM significantly predicted a greater effect size of walking capability (β = 0.17, 95% CI: 0.01–0.33; p = 0.04; Figure 5); however, no significant association was observed between LBM gain and SMD of walking capability.
Figure 3.
Multivariate meta-regression between percentage change in lean body mass and effects of PS plus MSE on leg strength. Each circle represents an independent comparison. The size of each circle is proportional to that study’s weight (inverse variance weighted). The regression prediction is represented by the solid line for effect size (SMD) of leg strength. Dotted lines represent the 95% CI. The metaregression model was adjusted for age, methodological quality, and follow-up time of each comparison. PS, protein supplementation; MSE, muscle strengthening exercise; SMD, standard mean difference.
Figure 4.
Multivariate meta-regression between percentage change in appendicular lean mass and effects of PS plus MSE on leg strength. Each circle represents an independent comparison. The size of each circle is proportional to that study’s weight (inverse variance weighted). The regression prediction is represented by the solid line for effect size (SMD) of leg strength. Dotted lines represent the 95% CI. The metaregression model was adjusted for age, methodological quality, and follow-up time of each comparison. PS, protein supplementation; MSE, muscle strengthening exercise; SMD, standard mean difference.
Figure 5.
Multivariate meta-regression between percentage change in appendicular lean mass and effects of PS plus MSE on walk capability. Each circle represents an independent comparison. The size of each circle is proportional to that study’s weight (inverse variance weighted). The regression prediction is represented by the solid line for effect size (SMD) of walk capability. Dotted lines represent the 95% CI. The metaregression model was adjusted for age, methodological quality, and follow-up time of each comparison. PS, protein supplementation; MSE, muscle strengthening exercise; SMD, standard mean difference.
3.9. Side Effects and Compliance
No clinically relevant adverse events, side effects, or serious complications were reported after exercise training or protein supplementation in the RCTs. The compliance of resistance-based and multicomponent-based MSE was reported as 84%–100% by six RCTs [41,43,48,51,52,54] and 44%–81% by six RCTs [38,39,40,42,44,47], respectively (Table 1). The compliance of PS was reported as 44%–100% by 13 RCTs [37,38,39,40,42,43,44,45,48,50,51,52,53] (Table 1).
3.10. Publication Bias
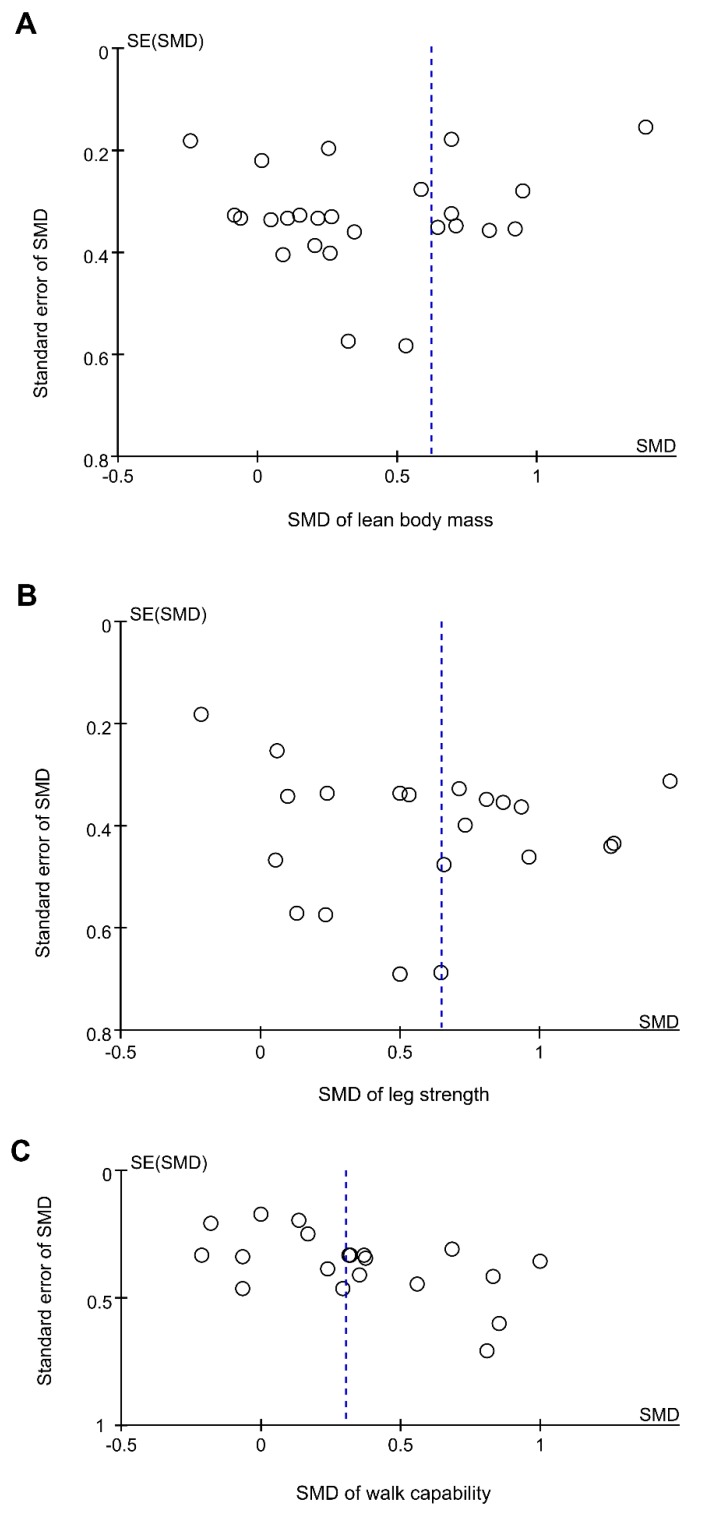
Visual inspection of a funnel plot of increase in LBM, leg strength, and walking capability did not identify substantial asymmetry (Figure 6). The Egger’s linear regression test for LBM also did not indicate any evidence of obvious reporting bias among the comparisons (t = 1.28, p = 0.21) as well as leg strength (t = −0.71, p = 0.48) and walking capability (t = −1.17, p = 0.26).
Figure 6.
Funnel plots of the intervention effects for (A) lean body mass, (B) leg strength, and (C) walk capability. Each circle represents an independent comparison, with the x-axis representing standard mean difference (SMD) the over control comparisons and the y-axis showing the standard error (SE) of SMD. The vertical dotted line indicates the mean value of the SMDs.
4. Discussion
This study demonstrated that PS + MSE exerted overall significant effects on muscle mass (LBM, ALM), muscle strength, and physical mobility in elderly people with high risks of sarcopenia and frailty, regardless of follow-up duration, participant type, exercise type, and type of control group. The results of this study also indicated that muscle mass gains (i.e., increases in LBM or ALM) are significantly associated with improvements in physical outcomes, particularly leg strength and walking capability.
In this meta-analysis, results of subgroup analyses based on control types showed that PS + MSE had greater effects on LBM, leg strength, and walking capability than did MSE-alone control. These results are consistent with the findings of our previous studies, which have indicated that additional PS augments LBM gain and strength gain during resistance training in elderly adults [19,56]. Consistent with previous reviews [57,58] and following the recommendations from the European Society for Clinical Nutrition and Metabolism Expert Group [59], the results of current meta-analysis supported the urgent need for elderly patients with a risk of sarcopenia or frailty to incorporate protein-based nutrition intervention and MSE to prevent the functional decline, particularly institutionalized residents who are at high risk of insufficient protein intake and physical inactivity [60,61,62,63].
PS in combination with resistance-type MSE has been identified as an efficient intervention for LM and strength gain in elderly individuals [11,13,15,19,59,64]. However, an intensity as high as 80%–95% one repetition maximum has been recommended for resistance-type MSE to induce maximal muscle hypertrophy or muscle fiber adaptation [65,66]; this intensity is not permissible for most frail elderly individuals, particularly those with cardiopulmonary dysfunction or physical limitations. Therefore, multicomponent exercise, which incorporates MSE with balance training, aerobic training, and functional activity (i.e., walking) are recommended for elderly patients to improve physical function and prevent fall [58,67,68]. In this study, the results of subgroup analysis based on exercise types showed that PS and multicomponent exercise had significant effects on LBM and ALM as well as PS and resistance exercise, which indicated that elder patients with sarcopenia or frailty responded favorably to a combination of PS and multicomponent exercise in reversing or preventing muscle mass loss.
Previous systemic reviews have shown nonsignificant effects on changes in muscle mass [20,69], muscle strength [20], and physical mobility [19] in response to PS + MSE for elderly adults who mostly were healthy or not frail. In this meta-analysis, we obtained conflicting results showing that PS + MSE is beneficial for LM and strength gain in an elderly population with high risks of sarcopenia and frailty; furthermore, we identified that institutionalized residents appeared to achieve greater effects on LBM and leg strength in response to PS + MSE than their community-dwelling peers. Different populations may explain the inconsistency between the results of previous reviews and the findings in the present meta-analysis, which further confirm the conclusion of previous authors indicating that individuals with sarcopenia or frailty may experience greater benefits in muscle mass gain and physical performance in response to PS + MSE than their healthy peers [15,70]. Therefore, targeting the sarcopenia or frailty indices in response to PS in combination with MSE may hold greater promise in the preservation of independence as well as the prevention of progress to frailty in the prefrail or frail elderly population.
Previous meta-analyses have observed that an increase in LM is accompanied by significant strength gain or function recovery after PS + MSE [14,16,17,18]. The results of meta-regression analyses in this study further confirmed previous results, which indicated that an increase in LM significantly predicts relatively greater strength gain or walking capability after PS + MSE. Furthermore, we identified that an increase of >2.0% to 3.0% in muscle mass predicts a positive effect of PS + MSE on leg strength and walking capability, which may explain the inconsistencies with other authors who reported conflicting results of such synergetic improvements in muscle mass and function [13,17,18,19,20].
Several limitations to our findings should be elucidated. First, based on the variation among protein supplement regimes (protein source, supplied amounts, timing of ingestion) and exercise regimes (training duration, training volume), endorsing a definite conclusion for the effect of specific type of PS or MSE on muscle mass or strength gains was difficult. Second, some of our included trials had small sample sizes [41,48]; the results of these studies that reflected no significant intervention effect on primary or secondary outcomes may have contributed negatively to the overall effect size. Finally, inadequate statistical power for subgroup analyses was noted. Several subgroups (such as intervention durations for ALM) included a small number of RCTs (less than six), which may not have adequate power for detecting differences among subgroups [71,72]; the results of such subgroup analyses should be cautiously interpreted.
5. Conclusions
This systematic review evidenced that PS incorporated with MSE is effective in promoting gain in muscle mass and strength and enhancing performance in physical mobility in elderly adults with a high risk of sarcopenia or frailty, compared with the placebo, PS-alone, or MSE-alone controls. In addition, muscle mass gains have effects on strength gain and function recovery, particularly the walking capability. Therefore, we concluded that PS in addition to resistance-type or multicomponent exercise may have extra effects to prevent or offset muscle loss and functional decline, particularly among elderly individuals who are frail community dwellers or institutionalized residents. The results of this study add knowledge about effective nutrients and exercise intervention strategies and an interdisciplinary practical approach to counteract muscle loss and functional decline in the elderly population. This is relevant for those working in geriatric care and rehabilitation settings such as clinical, hospitalized, institutionalized, and community settings. Based on limitations in our current study, additional studies with relatively large samples, as well as identification of specific supplementation protocols.
Acknowledgments
This study was funded by the Ministry of Science and Technology, Taiwan (grant number: MOST 107-2314-B-038-28) and Taipei Medical University-Shuang Ho Hospital, Ministry of Health and Welfare, Taiwan (grant number: W107HCP-04); and the APC was funded by Taipei Medical University (grant number: IIT-1072-3). All of the funding sources played no role in the design, implementation, data analysis, interpretation, or reporting of the study. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official view of the funding sources.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/11/8/1713/s1, Figure S1: Forest plot summarizing effects of protein supplement (PS) plus muscle strength exercise training (MSE) on lean body mass at an overall duration and each follow-up time point. Figure S2: Forest plot summarizing effects of protein supplement (PS) plus muscle strength exercise training (MSE) on appendicular lean mass at an overall duration and each follow-up time point. Figure S3: Forest plot summarizing effects of protein supplement (PS) plus muscle strength exercise training (MSE) on handgrip strength at an overall duration and each follow-up time point. Figure S4. Forest plot summarizing effects of protein supplement (PS) plus muscle strength exercise training (MSE) on leg strength at an overall duration and each follow-up time point. Figure S5. Forest plot summarizing effects of protein supplement (PS) plus muscle strength exercise training (MSE) on walk capability at an overall duration and each follow-up time point. Figure S6. Forest plot summarizing effects of protein supplement (PS) plus muscle strength exercise training (MSE) on chair rise at an overall duration and each follow-up time point. Figure S7. Forest plot summarizing effects of protein supplement (PS) plus muscle strength exercise training (MSE) on stair climb at an overall duration and each follow-up time point. Figure S8. Forest plot summarizing effects of protein supplement (PS) plus muscle strength exercise training (MSE) on timed up-and-go (TUG) at an overall duration and each follow-up time point. Figure S9. Forest plot summarizing effects of protein supplement (PS) plus muscle strength exercise training (MSE) on SPPB at an overall duration and each follow-up time point. Figure S10. Forest plot summarizing effects of protein supplement (PS) plus muscle strength exercise training (MSE) on single leg stance at an overall duration and each follow-up time point. Table S1: Database search formulas.
Author Contributions
The authors’ responsibilities are outlined as follows—C.-D.L., T.-H.L., and H.-C.C.: conceived of and designed the experiments; C.-D.L., H.-C.C., T.-H.L.: searched for and selected relevant studies; C.-D.L., H.-C.C. and T.-H.L.: extracted the data; C.-D.L. and H.-C.C.: analyzed the data; C.-D.L. and T.-H.L.: wrote the paper; D.-J.H., H.-C.C., S.-W.H., H.-C.C., J.-Y.T., and T.-H.L.: reviewed and verified the paper; C.-D.L., S.-W.H., H.-C.C., and T.-H.L.: take the primary responsibility for the final content of the paper.
Funding
This study was funded by the Ministry of Science and Technology, Taiwan (grant number: MOST 107-2314-B-038-28) and Taipei Medical University-Shuang Ho Hospital, Ministry of Health and Welfare, Taiwan (grant number: W107HCP-04); and the APC was funded by Taipei Medical University (grant number: IIT-1072-3).
Conflicts of Interest
The authors declare that they have no conflict of interest to the publication of this article.
References
- 1.Bernabei R., Martone A.M., Vetrano D.L., Calvani R., Landi F., Marzetti E. Frailty, Physical Frailty, Sarcopenia: A New Conceptual Model. Stud. Health Technol. Inform. 2014;203:78–84. [PubMed] [Google Scholar]
- 2.Dodds R., Sayer A.A. Sarcopenia and frailty: New challenges for clinical practice. Clin. Med. 2016;16:455–458. doi: 10.7861/clinmedicine.16-5-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buch A., Carmeli E., Boker L.K., Marcus Y., Shefer G., Kis O., Berner Y., Stern N. Muscle function and fat content in relation to sarcopenia, obesity and frailty of old age—An overview. Exp. Gerontol. 2016;76:25–32. doi: 10.1016/j.exger.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., Cooper C., Landi F., Rolland Y., Sayer A.A., et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fried L.P., Tangen C.M., Walston J., Newman A.B., Hirsch C., Gottdiener J., Seeman T., Tracy R., Kop W.J., Burke G., et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2001;56:M146–M157. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 6.Lee J.S., Auyeung T.-W., Kwok T., Lau E.M., Leung P.-C., Woo J. Associated Factors and Health Impact of Sarcopenia in Older Chinese Men and Women: A Cross-Sectional Study. Gerontology. 2007;53:404–410. doi: 10.1159/000107355. [DOI] [PubMed] [Google Scholar]
- 7.Janssen I., Heymsfield S.B., Ross R. Low Relative Skeletal Muscle Mass (Sarcopenia) in Older Persons Is Associated with Functional Impairment and Physical Disability. J. Am. Geriatr. Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 8.Trethewey S.P., Brown N., Gao F., Turner A.M. Interventions for the management and prevention of sarcopenia in the critically ill: A systematic review. J. Crit. Care. 2019;50:287–295. doi: 10.1016/j.jcrc.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Jadczak A.D., Makwana N., Luscombe-Marsh N., Visvanathan R., Schultz T.J. Effectiveness of exercise interventions on physical function in community-dwelling frail older people: An umbrella review of systematic reviews. JBI Database Syst. Rev. Implement. Rep. 2018;16:752–775. doi: 10.11124/JBISRIR-2017-003551. [DOI] [PubMed] [Google Scholar]
- 10.Tessier A.-J., Chevalier S. An Update on Protein, Leucine, Omega-3 Fatty Acids, and Vitamin D in the Prevention and Treatment of Sarcopenia and Functional Decline. Nutrients. 2018;10:1099. doi: 10.3390/nu10081099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips S.M. Nutritional Supplements in Support of Resistance Exercise to Counter Age-Related Sarcopenia12. Adv. Nutr. 2015;6:452–460. doi: 10.3945/an.115.008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denison H.J., Cooper C., Sayer A.A., Robinson S.M. Prevention and optimal management of sarcopenia: A review of combined exercise and nutrition interventions to improve muscle outcomes in older people. Clin. Interv. Aging. 2015;10:859–869. doi: 10.2147/CIA.S55842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morton R.W., Murphy K.T., McKellar S.R., Schoenfeld B.J., Henselmans M., Helms E., Aragon A.A., Devries M.C., Banfield L., Krieger J.W., et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports. Med. 2018;52:376–384. doi: 10.1136/bjsports-2017-097608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao C.-D., Lee P.-H., Hsiao D.-J., Huang S.-W., Tsauo J.-Y., Chen H.-C., Liou T.-H. Effects of Protein Supplementation Combined with Exercise Intervention on Frailty Indices, Body Composition, and Physical Function in Frail Older Adults. Nutrients. 2018;10:1916. doi: 10.3390/nu10121916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hidayat K., Chen G.C., Wang Y., Zhang Z., Dai X., Szeto I.M.Y., Qin L.Q. Effects of milk proteins supplementation in older adults undergoing resistance training: A meta-analysis of randomized control trials. J. Nutr. Health Aging. 2018;22:237–245. doi: 10.1007/s12603-017-0899-y. [DOI] [PubMed] [Google Scholar]
- 16.Naclerio F., Larumbe-Zabala E. Effects of Whey Protein Alone or as Part of a Multi-ingredient Formulation on Strength, Fat-Free Mass, or Lean Body Mass in Resistance-Trained Individuals: A Meta-analysis. Sports Med. 2016;46:125–137. doi: 10.1007/s40279-015-0403-y. [DOI] [PubMed] [Google Scholar]
- 17.Luo D., Lin Z., Li S., Liu S.-J. Effect of nutritional supplement combined with exercise intervention on sarcopenia in the elderly: A meta-analysis. Int. J. Nurs. Sci. 2017;4:389–401. doi: 10.1016/j.ijnss.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng H., Kong J., Underwood C., Petocz P., Hirani V., Dawson B., O’Leary F. Systematic review and meta-analysis of the effect of protein and amino acid supplements in older adults with acute or chronic conditions. Br. J. Nutr. 2018;119:527–542. doi: 10.1017/S0007114517003816. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y.-T., Cheng C.-P., Chen H.-C., Liao C.-D., Tsauo J.-Y., Huang Y.-C., Liou T.-H. Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2017;106:1078–1091. doi: 10.3945/ajcn.116.143594. [DOI] [PubMed] [Google Scholar]
- 20.Finger D., Goltz F.R., Umpierre D., Meyer E., Rosa L.H., Schneider C.D. Effects of protein supplementation in older adults undergoing resistance training: A systematic review and meta-analysis. Sports Med. 2015;45:245–255. doi: 10.1007/s40279-014-0269-4. [DOI] [PubMed] [Google Scholar]
- 21.Liu L.-K., Lee W.-J., Liu C.-L., Chen L.-Y., Lin M.-H., Peng L.-N., Chen L.-K. Age-related skeletal muscle mass loss and physical performance in Taiwan: Implications to diagnostic strategy of sarcopenia in Asia. Geriatr. Gerontol. Int. 2013;13:964–971. doi: 10.1111/ggi.12040. [DOI] [PubMed] [Google Scholar]
- 22.Santos M., Gomes W., Pereira D., Oliveira D., Dias J., Ferrioli E., Pereira L. Muscle strength, muscle balance, physical function and plasma interleukin-6 (IL-6) levels in elderly women with knee osteoarthritis (OA) Arch. Gerontol. Geriatr. 2011;52:322–326. doi: 10.1016/j.archger.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Wilson D., Jackson T., Sapey E., Lord J.M. Frailty and sarcopenia: The potential role of an aged immune system. Ageing Res. Rev. 2017;36:1–10. doi: 10.1016/j.arr.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Shamseer L., Moher D., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A. The PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 25.Higgins J.P.T., Deeks J.J., Altman D.G. Chapter 16: Special topics in statistics. In: Higgins J.P.T., Green S., editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; London, UK: 2011. Updated March 2011. [Google Scholar]
- 26.Wu C.-H., Chen K.-T., Hou M.-T., Chang Y.-F., Chang C.-S., Liu P.-Y., Wu S.-J., Chiu C.-J., Jou I.-M., Chen C.-Y. Prevalence and associated factors of sarcopenia and severe sarcopenia in older Taiwanese living in rural community: The Tianliao Old People study 04. Geriatr. Gerontol. Int. 2014;14:69–75. doi: 10.1111/ggi.12233. [DOI] [PubMed] [Google Scholar]
- 27.Briani R.V., Ferreira A.S., Pazzinatto M.F., Pappas E., Silva D.D.O., De Azevedo F.M. What interventions can improve quality of life or psychosocial factors of individuals with knee osteoarthritis? A systematic review with meta-analysis of primary outcomes from randomised controlled trials. Br. J. Sports Med. 2018;52:1031–1038. doi: 10.1136/bjsports-2017-098099. [DOI] [PubMed] [Google Scholar]
- 28.Cermak N.M., Res P.T., De Groot L.C., Saris W.H., Van Loon L.J. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: A meta-analysis. Am. J. Clin. Nutr. 2012;96:1454–1464. doi: 10.3945/ajcn.112.037556. [DOI] [PubMed] [Google Scholar]
- 29.Haaf D.S., Eijsvogels T.M., Bongers C.C., Horstman A.M., Timmers S., Groot L.C., Hopman M.T., Haaf D.S.T., De Groot L.C. Protein supplementation improves lean body mass in physically active older adults: A randomized placebo-controlled trial. J. Cachex Sarcopenia Muscle. 2019;10:298–310. doi: 10.1002/jcsm.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oesen S., Halper B., Hofmann M., Jandrasits W., Franzke B., Strasser E.-M., Graf A., Tschan H., Bachl N., Quittan M., et al. Effects of elastic band resistance training and nutritional supplementation on physical performance of institutionalised elderly—A randomized controlled trial. Exp. Gerontol. 2015;72:99–108. doi: 10.1016/j.exger.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Kim H., Kim M., Kojima N., Fujino K., Hosoi E., Kobayashi H., Somekawa S., Niki Y., Yamashiro Y., Yoshida H. Exercise and Nutritional Supplementation on Community-Dwelling Elderly Japanese Women with Sarcopenic Obesity: A Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2016;17:1011–1019. doi: 10.1016/j.jamda.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 32.Luo D., Wan X., Liu J., Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018;27:1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 33.Bowden J., Tierney J.F., Copas A.J., Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med. Res. Methodol. 2011;11:41. doi: 10.1186/1471-2288-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Churchward-Venne T.A., Holwerda A.M., Phillips S.M., Van Loon L.J. What is the Optimal Amount of Protein to Support Post-Exercise Skeletal Muscle Reconditioning in the Older Adult? Sports Med. 2016;46:1205–1212. doi: 10.1007/s40279-016-0504-2. [DOI] [PubMed] [Google Scholar]
- 35.Beaudreuil J., Coudreuse J.M., Guyen C.N., Deat P., Chabaud A., Pereira B., Lorenzo A., Sailhan F., Rannou F., Coudeyre E. An algorithm to improve knee orthosis prescription for osteoarthritis patients. Ann. Phys. Rehabil. Med. 2016;59:e156. doi: 10.1016/j.rehab.2016.07.347. [DOI] [PubMed] [Google Scholar]
- 36.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Björkman M.P., Pilvi T.K., Kekkonen R.A., Korpela R., Tilvis R.S. Similar effects of leucine rich and regular dairy products on muscle mass and functions of older polymyalgia rheumatica patients: A randomized crossover trial. J. Nutr. Health Aging. 2011;15:462–467. doi: 10.1007/s12603-010-0276-6. [DOI] [PubMed] [Google Scholar]
- 38.Bonnefoy M., Boutitie F., Mercier C., Gueyffier F., Carre C., Guetemme G., Ravis B., Laville M., Cornu C. Efficacy of a home-based intervention programme on the physical activity level and functional ability of older people using domestic services: A randomised study. J. Nutr. Health Aging. 2012;16:370–377. doi: 10.1007/s12603-011-0352-6. [DOI] [PubMed] [Google Scholar]
- 39.Bonnefoy M., Cornu C., Normand S., Boutitie F., Bugnard F., Rahmani A., Lacour J.R., Laville M. The effects of exercise and protein–energy supplements on body composition and muscle function in frail elderly individuals: A long-term controlled randomised study. Br. J. Nutr. 2003;89:731–738. doi: 10.1079/BJN2003836. [DOI] [PubMed] [Google Scholar]
- 40.Carlsson M., Littbrand H., Gustafson Y., Lundin-Olsson L., Lindelöf N., Rosendahl E., Håglin L. Effects of high-intensity exercise and protein supplement on muscle mass in ADL dependent older people with and without malnutrition: A randomized controlled trial. J. Nutr. Health Aging. 2011;15:554–560. doi: 10.1007/s12603-011-0017-5. [DOI] [PubMed] [Google Scholar]
- 41.Dirks M.L., Tieland M., Verdijk L.B., Losen M., Nilwik R., Mensink M., De Groot L.C., Van Loon L.J. Protein Supplementation Augments Muscle Fiber Hypertrophy but Does Not Modulate Satellite Cell Content During Prolonged Resistance-Type Exercise Training in Frail Elderly. J. Am. Med Dir. Assoc. 2017;18:608–615. doi: 10.1016/j.jamda.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Englund D.A., Kirn D.R., Koochek A., Zhu H., Travison T.G., Reid K.F., Von Berens Å., Melin M., Cederholm T., Gustafsson T., et al. Nutritional Supplementation With Physical Activity Improves Muscle Composition in Mobility-Limited Older Adults, The VIVE2 Study: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2017;73:95–101. doi: 10.1093/gerona/glx141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fiatarone M.A., O’Neill E.F., Ryan N.D., Clements K.M., Solares G.R., Nelson M.E., Kehayias J.J., Lipsitz L.A., Roberts S.B., Evans W.J. Exercise Training and Nutritional Supplementation for Physical Frailty in Very Elderly People. N. Engl. J. Med. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 44.Fielding R.A., Travison T.G., Kirn D.R., Koochek A., Reid K.F., Von Berens Å., Zhu H., Folta S.C., Sacheck J.M., Nelson M.E., et al. Effect of structured physical activity and nutritional supplementation on physical function in mobility-limited older adults: Results from the VIVE2 randomized trial. J. Nutr. Heal. Aging. 2017;21:936–942. doi: 10.1007/s12603-017-0936-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hegerová P., Dědková Z., Sobotka L. Early nutritional support and physiotherapy improved long-term self-sufficiency in acutely ill older patients. Nutrition. 2015;31:166–170. doi: 10.1016/j.nut.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 46.Imaoka M., Higuchi Y., Todo E., Kitagwa T., Ueda T. Low-frequency Exercise and Vitamin D Supplementation Reduce Falls among Institutionalized Frail Elderly. Int. J. Gerontol. 2016;10:202–206. doi: 10.1016/j.ijge.2016.02.005. [DOI] [Google Scholar]
- 47.Kim H.K., Suzuki T., Saito K., Yoshida H., Kobayashi H., Kato H., Katayama M. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: A randomized controlled trial. J. Am. Geriatr. Soc. 2012;60:16–23. doi: 10.1111/j.1532-5415.2011.03776.x. [DOI] [PubMed] [Google Scholar]
- 48.Maltais M.L., Ladouceur J.P., Dionne I.J. The Effect of Resistance Training and Different Sources of Postexercise Protein Supplementation on Muscle Mass and Physical Capacity in Sarcopenic Elderly Men. J. Strength Cond. Res. 2016;30:1680–1687. doi: 10.1519/JSC.0000000000001255. [DOI] [PubMed] [Google Scholar]
- 49.Molnar A., Jonasne Sztruhar I., Csontos A.A., Ferencz C., Varbiro S., Szekacs B. Special nutrition intervention is required for muscle protective efficacy of physical exercise in elderly people at highest risk of sarcopenia. Physiol. Int. 2016;103:368–376. doi: 10.1556/2060.103.2016.3.12. [DOI] [PubMed] [Google Scholar]
- 50.Rondanelli M., Klersy C., Terracol G., Talluri J., Maugeri R., Guido D., Faliva M.A., Solerte B.S., Fioravanti M., Lukaski H., et al. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am. J. Clin. Nutr. 2016;103:830–840. doi: 10.3945/ajcn.115.113357. [DOI] [PubMed] [Google Scholar]
- 51.Tieland M., Dirks M.L., Van Der Zwaluw N., Verdijk L.B., Van De Rest O., De Groot L.C., Van Loon L.J. Protein Supplementation Increases Muscle Mass Gain During Prolonged Resistance-Type Exercise Training in Frail Elderly People: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Med Dir. Assoc. 2012;13:713–719. doi: 10.1016/j.jamda.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 52.Yamada M., Kimura Y., Ishiyama D., Nishio N., Otobe Y., Tanaka T., Ohji S., Koyama S., Sato A., Suzuki M., et al. Synergistic effect of bodyweight resistance exercise and protein supplementation on skeletal muscle in sarcopenic or dynapenic older adults. Geriatr. Gerontol. Int. 2019;19:429–437. doi: 10.1111/ggi.13643. [DOI] [PubMed] [Google Scholar]
- 53.Yamada M., Nishiguchi S., Fukutani N., Aoyama T., Arai H. Mail-Based Intervention for Sarcopenia Prevention Increased Anabolic Hormone and Skeletal Muscle Mass in Community-Dwelling Japanese Older Adults: The INE (Intervention by Nutrition and Exercise) Study. J. Am. Med. Dir. Assoc. 2015;16:654–660. doi: 10.1016/j.jamda.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 54.Zdzieblik D., Oesser S., Baumstark M.W., Gollhofer A., König D. Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: A randomised controlled trial. Br. J. Nutr. 2015;114:1237–1245. doi: 10.1017/S0007114515002810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karelis A.D., Messier V., Suppere C., Briand P., Rabasa-Lhoret R. Effect of cysteine-rich whey protein (immunocal(R)) supplementation in combination with resistance training on muscle strength and lean body mass in non-frail elderly subjects: A randomized, double-blind controlled study. J. Nutr. Health Aging. 2015;19:531–536. doi: 10.1007/s12603-015-0442-y. [DOI] [PubMed] [Google Scholar]
- 56.Liao C.-D., Tsauo J.-Y., Chen H.-C., Liou T.-H. Reply to RW Morton and SM Phillips. Am. J. Clin. Nutr. 2018;107:1056–1057. doi: 10.1093/ajcn/nqy069. [DOI] [PubMed] [Google Scholar]
- 57.Coelho-Júnior H.J., Rodrigues B., Uchida M., Marzetti E. Low Protein Intake Is Associated with Frailty in Older Adults: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients. 2018;10:1334. doi: 10.3390/nu10091334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arrieta H., Rezola-Pardo C., Gil S.M., Irazusta J., Rodriguez-Larrad A. Physical training maintains or improves gait ability in long-term nursing home residents: A systematic review of randomized controlled trials. Maturitas. 2018;109:45–52. doi: 10.1016/j.maturitas.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 59.Deutz N.E., Bauer J.M., Barazzoni R., Biolo G., Boirie Y., Bosy-Westphal A., Cederholm T., Cruz-Jentoft A., Krznariç Z., Nair K.S., et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014;33:929–936. doi: 10.1016/j.clnu.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iuliano S., Poon S., Wang X., Bui M., Seeman E. Dairy food supplementation may reduce malnutrition risk in institutionalised elderly. Br. J. Nutr. 2017;117:142–147. doi: 10.1017/S000711451600461X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tieland M., Beelen J., Laan A.C., Poon S., De Groot L.C., Seeman E., Wang X., Iuliano S. An Even Distribution of Protein Intake Daily Promotes Protein Adequacy but Does Not Influence Nutritional Status in Institutionalized Elderly. J. Am. Med Dir. Assoc. 2018;19:33–39. doi: 10.1016/j.jamda.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 62.Douma J.G., Volkers K.M., Engels G., Sonneveld M.H., Goossens R.H.M., Scherder E.J.A. Setting-related influences on physical inactivity of older adults in residential care settings: A review. BMC Geriatr. 2017;17:97. doi: 10.1186/s12877-017-0487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tieland M., Borgonjen-Van den Berg K.J., van Loon L.J., De Groot L.C. Dietary protein intake in community-dwelling, frail, and institutionalized elderly people: Scope for improvement. Eur. J. Nutr. 2012;51:173–179. doi: 10.1007/s00394-011-0203-6. [DOI] [PubMed] [Google Scholar]
- 64.Guimarães-Ferreira L., Cholewa J.M., Naimo M.A., Zhi X., Magagnin D., De Sá R.B.D.P., Streck E.L., Teixeira T.D.S., Zanchi N.E. Synergistic effects of resistance training and protein intake: Practical aspects. Nutrition. 2014;30:1097–1103. doi: 10.1016/j.nut.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 65.Fry A.C. The role of resistance exercise intensity on muscle fibre adaptations. Sports Med. 2004;34:663–679. doi: 10.2165/00007256-200434100-00004. [DOI] [PubMed] [Google Scholar]
- 66.Helms E.R., Cronin J., Storey A., Zourdos M.C. Application of the Repetitions in Reserve-Based Rating of Perceived Exertion Scale for Resistance Training. Strength Cond. J. 2016;38:42–49. doi: 10.1519/SSC.0000000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Molina R.G., Ruíz-Grao M.C., García A.N., Reig M.M., Víctor M.E., Izquierdo M., Abizanda P., Grao M.C.R., Redín M.I., Soler P.A. Benefits of a multicomponent Falls Unit-based exercise program in older adults with falls in real life. Exp. Gerontol. 2018;110:79–85. doi: 10.1016/j.exger.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 68.Gwyther H., Bobrowicz-Campos E., Apostolo J.L.A., Marcucci M., Cano A., Holland C. A realist review to understand the efficacy and outcomes of interventions designed to minimise, reverse or prevent the progression of frailty. Health Psychol. Rev. 2018;12:382–404. doi: 10.1080/17437199.2018.1488601. [DOI] [PubMed] [Google Scholar]
- 69.Miller P.E., Alexander D.D., Perez V. Effects of Whey Protein and Resistance Exercise on Body Composition: A Meta-Analysis of Randomized Controlled Trials. J. Am. Coll. Nutr. 2014;33:163–175. doi: 10.1080/07315724.2013.875365. [DOI] [PubMed] [Google Scholar]
- 70.Gade J., Pedersen R.J., Beck A.M. Effect of Protein or Essential Amino Acid Supplementation During Prolonged Resistance Exercise Training in Older Adults on Body Composition, Muscle Strength, and Physical Performance Parameters: A Systematic Review. Rehabil. Process Outcome. 2018;7:1179572718765760. doi: 10.1177/1179572718765760. [DOI] [Google Scholar]
- 71.Borenstein M., Hedges L.V., Higgins J.P.T., Rothstein H.R. Notes on subgroup analyses and Meta-regression. In: Borenstein M., editor. Introduction to Meta-Analysis. John Wiley & Sons; Hoboken, NJ, USA: 2009. pp. 205–212. [Google Scholar]
- 72.Fu R., Gartlehner G., Grant M., Shamliyan T., Sedrakyan A., Wilt T.J., Griffith L., Oremus M., Raina P., Ismaila A., et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J. Clin. Epidemiol. 2011;64:1187–1197. doi: 10.1016/j.jclinepi.2010.08.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.