Abstract
Cryptomeria fortunei, also known as the Chinese cedar, is an important timber species in southern China. The primary component of its woody tissues is lignin, mainly present in secondary cell walls. Therefore, continuous lignin synthesis is crucial for wood formation. In this study, we aimed to discover key genes involved in lignin synthesis expressed in the vascular cambium of C. fortunei. Through transcriptome sequencing, we detected expression of two genes, 4CL and CCoAOMT, known to be homologous to enzymes involved in the lignin synthesis pathway. We studied the function of these genes through bioinformatics analysis, cloning, vascular cambium expression analysis, and transgenic cross-species functional validation studies. Our results show that Cf4CL and CfCCoAOMT do indeed function in the pathway of lignin synthesis and likely perform this function in C. fortunei. They are prime candidates for future (gene-editing) studies aimed at optimizing C. fortunei wood production.
Keywords: Cryptomeria fortunei, lignin synthetase, gene cloning, expression analysis, functional verification
1. Introduction
Cryptomeria fortunei is a widely cultivated, strongly adaptable woody species that has a high economic value due to its rapid growth. Therefore we aim to improve our understanding of C. fortunei wood production by studying its lignin synthesis pathways. Wood is the secondary xylem of perennial woody plants that is formed as a result of the proliferation and differentiation of vascular cambium cells. It is mainly composed of cellulose, hemicellulose, and lignin, among which lignin is the main component of the secondary cell wall. It occupies approximately 25–35% [1] of the wood dry weight. Lignin can enhance the mechanical support of stems and provide resistance to bacterial infections.
Lignin is a phenylpropane complex composed of three kinds of alcohol monomers: coumarinol, coniferyl alcohol and sinapyl alcohol. Lignin can be classified into three separate categories, each composed of a different monomer: syringyl lignin (S-type lignin), guaiacyl lignin (G-type lignin), and para-hydroxy-phenyl lignin (H-type lignin). Previous studies have shown that in conifers lignin is mainly composed of G-type monomers [2], while primitive conifer species such as Podocarpus macrophyllus may contain S-type lignin monomers [3].
The biosynthesis of lignin is a complex process involving multiple enzymes. The synthetic route can be roughly divided into three steps. The first step is the synthesis of aromatic amino phenylalanine from photosynthetic assimilation products, called the shikimic acid pathway. In the second step, the phenylpropanoid pathway, different compounds are synthesized from phenylalanine, involving enzymes such as phenylalanine ammonia-lyase (PAL), cinnamate 4-hydroxylase (C4H), p-coumarate 3-hydroxylase (C3H), caffeic acid/5-hydroxyferulic acid O-methyltransferase (COMT), caffeoyl CoA O-methyltransferase (CCoAOMT), 4-(hydroxy) cinnamoyl CoA ligase (4CL), etc. The initial reaction of the phenylpropanoid pathway is catalyzed by PAL, which catalyzes the conversion of phenylalanine into cinnamic acid [4]. During the third step, a specific metabolic pathway involving the enzymes cinnamoyl CoA reductase (CCR) and cinnamyl alcohol dehydrogenase (CAD) leads to the synthesis of lignin monomers. Finally, various lignin monomers polymerized to construct lignin, catalyzed by peroxidase and laccase.
Kentaro et al. screened expressed sequence tags in C. japonica (Japanses cedar) cambium tissue using transcriptome sequencing [5]. C. japonica has a growing season from March to October, during which it shows lignification activity in its xylem tissues; activity sharply increases from March to June, then declines again until dormancy in October [5]. Kentaro et al. found the expression of most key enzymes involved in lignin and cell wall synthesis sharply increase from March to April and then slowly decline until dormancy. Interestingly, two lignification enzyme homologues, PAL4 and 4CL3, showed an opposite expression pattern, which increase during dormancy period [5]. This finding indicates that maybe not all such enzymes are involved in the main lignin synthesis needed for growth and some might act as a response to various stresses, such as infection, wounding, or drought.
In order to study the genetic basis of lignin synthesis in C. fortunei further, we selected 4CL and CCoAOMT, homologues of two key lignin synthesis enzymes. The enzymes express strongly correlates to growth activity in both C. japonica and C. fortunei. The enzymes act in close succession of the lignin biosynthesis pathway. 4CL catalyzes the conversion of compounds such as hydroxycinnamic acid and p-Coumaric acid into their corresponding CoA esters, allowing them to be the monomers during the biosynthesis of lignin [4]. Previous studies have shown that 4CL is a member of a multi-gene family: Pinus taeda and Petroselinum hill possess two 4CL gene family members [6], while Nicotiana tabacum and Glycine max contain more gene family members [7]. Not all 4CL genes are involved in lignin synthesis: in Populus trichocarpa, the Ptc4CL1 gene does contribute to lignin biosynthesis in xylem, whereas the Ptc4CL2 gene participates in the biosynthesis of phenolic compounds, such as flavonoids, in epidermal cells [8,9,10,11].
CCoAOMT, another key enzyme involved in lignin synthesis, acts as a methyltransferase, catalyzing the conversion of Caffeoyl CoA into Feruloyl CoA [12]. Ye et al. first confirmed that CCoAOMT participates in lignin biosynthesis in the woody plant Zinnia elegans [13]. In pine, it plays a key role in G-type lignin synthesis [14]. Similar to 4CL, CCoAOMT is part of a multi-gene family: For example, there are 1 to 2 CCoAOMT genes in Medicago Sativa Linn and Populus spp. [15,16] and 5 to 10 genes in Zinnia elegans Jacq. [13].
We demonstrated a functional analysis of C. fortunei 4CL and CCoAOMT, key enzymes involved in the lignin synthesis pathway. We performed a transcriptomic analysis of the vascular cambium [17] and found a large number of differentially expressed genes between growth stages and detected 4CL and CCoAOMT among them. The overexpression of Cf4CL in Arabidopsis leaded to increased lignin deposition, strongly suggesting that it performs a similar function in C. fortunei, accelerating the formation of cedar wood and enhancing wood resistance. CfCCoAOMT is involved in the synthesis of G and S-type lignin; whether it is also involved in the biosynthesis of lignin and interacts with other key enzymes in the phenylpropanoid pathway remains to be further studied. Our results may be of significance towards the improvement of C. fortunei wood cultivation and properties.
2. Materials and Methods
2.1. Plant Material
For our experiments, we used C. fortunei growing in the arboretum of Nanjing Forestry University, aged around 50 years. Nanjing lies in the east of China, locates between 31°14′′ to 32°37′′ north latitude and 118°22′′ to 119°14′′ east longitude.The exact dates of sampling were 15 March, 15 May, 15 July, 15 September, and 15 November in 2017. Samples were marked with the date of collection, as we treated them as 5 successive growth stages of C. fortunei in one year. We collected vascular cambium zone composed of cambium, partial phloem and developing xylem by scratching the stem tissue with a carpet knife [18], collecting the tissue in a sterile enzyme-free 2 mL centrifuge tube and submerging it in liquid nitrogen, after which it was quickly stored at −80˚C. For genetic transformation and functional verification experiments, we used Nicotiana tabacum, a wild type tobacco strain with red flower.
2.2. Extraction of Total RNA and Synthesis of First Strand cDNA
Total C. fortunei vascular cambium RNA was extracted using an RNAprep® Pure Plant Kit (Polysaccharides & Polyphenolics-rich, Tiangen Biotech Co., LTD, Beijing, China). We tested total RNA integrity and concentration using an EasyPure® Quick Gel Extraction Kit (Beijing TransGen Biotech Co., Ltd, Beijing, China) and a Thermo Scientific NanoDrop 2000 (Thermo Fisher Scientific Co., Ltd, Waltham, MA, USA). We achieved cDNA through reverse transcription from total RNA using the PrimeScript™ RT Master Mix (Takara Biomedical Technology (Beijing) Co., Ltd, Beijing, China).
2.3. Molecular Cloning and Genetic Transformation of Tobacco
To determine the Cf4CL and CfCCoAOMT cDNA sequences, 3′ and 5′ race primers were designed based on the analysis of high-throughput sequencing data of the C. fortunei vascular cambium transcriptome (Table 1). The RACE reaction was carried out according to the manufacturer’s instruction using a SMARTer® RACE 5′/3′Kit (Takara Biomedical Technology (Beijing) Co., Ltd, Beijing, China). Target segments were cloned into an Easy®-Blunt Cloning Vector (Beijing TransGen Biotech CO., Ltd, Beijing, China) and transformed into Trans1®-T1 Phage Resistant Chemically Competent Cells (Beijing TransGen Biotech CO., Ltd, Beijing, China). After selecting positive clones, they were sent to Nanjing GenScript Biotech Corp. (Nanjing, China) for sequencing. In order to verify the accuracy of the full-length sequence of the gene we designed primers outside the ORF and then performed PCR amplification verification. We designed primers to amplify the coding region of 4CL and CCoAOMT using CE Design (Vazyme Biotech Co., Ltd, Nanjing, China) and tested the amplification products by 1% agarose gel electrophoresis. We constructed 4CL and CCoAOMT overexpression vectors by linearizing and ligating the pBI121 vector with each insert using a ClonExpresss® II One Step Cloning Kit C112 (Vazyme Biotech Co., Ltd, Nanjing, China). After sequence verification, the recombinant plasmid was transferred into agrobacterium EHA105 (Shanghai Weidi Biotechnology Co.,Ltd, Shanghai, China) using the freeze-thaw method. Transgenic plants were obtained using the tobacco leaf disc transformation referred to the method optimized by Curtis et al. [19], transgenic plants were identified by extracting transgenic tobacco DNA followed by PCR detection.
Table 1.
Primers used for partial cDNA cloning, RACE reaction, qRT-PCR.
| Primer Name | Primer Sequence (5′-3′) | Application |
|---|---|---|
| Longer | CTAATACGACTCACTATAGGGCAAGCAGTGGTATCAACGCAGAGT | Universal primer |
| Shorter | CTAATACGACTCACTATAGGGC | |
| 4CL-5′outer | GGCATTCGCTCTCATCCGCCTCAGTCAG |
4CL RACE reaction |
| 4CL-5′inner | GCTCCACCGGACATGATCAAGC | |
| 4CL-3′outer | ATAGCCCACCTGAAGGTTGCCTCCCGATT | |
| 4CL-3′inner | CTATTCCCTCAATTCGGTGCTC | |
| CCoAOMT-5′outer | GTATTGTCATAGCCGATCACTCCCCCAA | CCoAOMT RACE reaction |
| CCoAOMT-5′inner | CAATTTTGTGGGCAACACCTG | |
| CCoAOMT-3′outer | GCCCTGCCTGATGATGGAAAGATCCTAG | |
| CCoAOMT-3′inner | GATCGGCTATGACAATACTCTG | |
| 4CL-ALL-F | GGGTAAAGATCAATTACTGCTTC | Amplification of the conserved region |
| 4CL-ALL-R | CAAATTTATGTGTGCTGCGAGT | |
| CCoAOMT-ALL-F | GTTTCATTCCTCCAATCCAGT | |
| CCoAOMT-ALL-R | GGGCTGTTCTTAAATCACTCC | |
| RT-4CL-F | CTCCTTTGTGCGCTCCGAGT | RT-PCR |
| RT-4CL-R | GCTCCACCGGACATGATCAAGC | |
| RT-CCoAOMT-F | GCTCATCAATGCCAAGAACACCA | |
| RT-CCoAOMT-R | TCAATTTTGTGGGCAACACCT | |
| CE-4CL-F | ATGGCTTGTGTCGCACCTCT | Enzyme cutting |
| CE-4CL-R | TTAGGCTGCTGCAAGTCTGGC | |
| CE-CCoAOMT-F | ATGGCAACTGCAGAGGCTTC | |
| CE-CCoAOMT-R | AATAACTCTTCTGCAGAGAGTGATGC |
2.4. Bioinformatics Analysis
We used ProtParam [20] to analyze the physical and chemical properties of proteins encoded by Cf4CL and CfCCoAOMT. The number of amino acids, relative molecular weight, theoretical isoelectric point (PI value), protein formula, fat coefficient, hydrophilicity, and stability coefficient were taken as indices. After that, cDNA sequences of Cf4CL and CfCCoAOMT were blasted with the NCBI blastn web tool to find the most closely related known gene sequences from other plant species. We constructed multiple sequence alignments using DNAMAN 6 software (Lynnon Biosoft, San Ramon, CA, USA). The gene phylogenetic trees were constructed using MEGA 6.0 [21]. In addition, we used ProtParam online software to analyze the physical and chemical parameters of protein sequences, including relative molecular weight and theoretical isoelectric point. The NCBI CDS online tool was used to analyze gene-encoded protein domains and SOPMA software [22] used for secondary structure analysis and prediction. We used Swiss-Model Software [23] for analysis and construction of a protein tertiary structure model, and TMHMM v.2.0 software (http://www.cbs.dtu.dk/services/TMHMM/) to analyze the protein transmembrane region. Finally, we made use of ProtScale software [20] for protein hydrophobicity analysis and Signal IP4.0 [24] for the prediction of signal peptide sites.
2.5. Expression Analysis
Oligo7 [25] was used to design primers for qRT-PCR of Cf4CL, CfCCoAOMT, and the reference gene β-Actin (Table 1). The qRT-PCR was conducted using SYBR Premix Ex Taq™ II (Tli RnaseH Plus, TaKaRa Biomedical Technology (Beijing) Co., Ltd, Beijing, China), and ABI 7500 Step One Plus Real-time PCR platform [26]. Reactions were performed in biological triplicate, and six technical repetitions per sample and average Ct values were obtained for each gene at each growth stage. qPCR amplification data were processed with ΔΔCt. The relative quantitative data of the expression changes of three genes at five growth and development stages of C. fortunei vascular cambium formation were analyzed through SPSS v24 (IBM Co., Ltd, New York, NY, USA).
2.6. Transgenic Plant Growth and Phenotyping
Agrobacterium-infected tobacco leaf discs were incubated on MS selection medium (1.0 mg/L 6-BA, 0.1 mg/L NAA, 50 mg/L Kan, 200 mg/L TMT) and fostered for morphological observation at the stage of callus induction, adventitious bud growth and seedlings strengthened and root growth. Seedlings with strong roots were planted in the soil 5 days after adventitious roots appeared. The phenotype of 2-months-old mature tobacco plants, such as plant height, stem diameter, and leaf type were observed and compared. We chose 3 random seedlings of WT and both kinds of transgenic tobaccos for measuring. At the same stage, we observed the anatomical structure of stem segment cross sections with a Quanta 200 environmental scanning electron microscope (SEM) [27].
2.7. Determination of Lignin Content
The lignin content of mature tobacco was determined according to the acetyl bromide method improved by J. Rodrigues et al. [28], and data was collected using a GeneQuant pro ultraviolet (UV) spectrophotometer (Biochrom Ltd, Cambridge, UK).
3. Results
From previous work, we acquired transcriptome data from C. fortunei stem vascular cambium at different growth stages [17], and we identified genes expressed in this tissue. These genes can support the study involved with cambium development. Here, we chose 4CL and CCoAOMT as strong candidate genes in lignin synthesis and performed bioinformatics and functional analysis to confirm their biological function.
3.1. C. fortunei Contains Cf4CL and CfCCoAOMT Genes
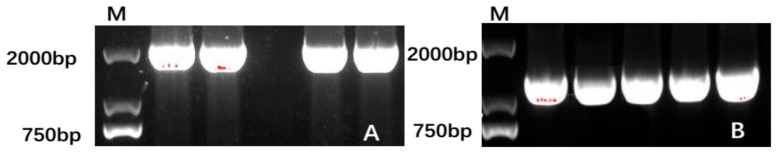
We obtained the 3′ and 5′ terminal sequence and intermediate fragment sequences of Cf4CL and CfCCoAOMT by sequencing. The Cf4CL intermediate fragment was 843 bp in length, and its full-length spliced cDNA was 2065 bp long, of which 1665 bp encoded 554 amino acids (Figure 1A). The CfCCoAOMT intermediate fragment was 685 bp in length, and its full-length spliced cDNA was 1219 bp long, of which 750 bp encoded 249 amino acids (Figure 1B).
Figure 1.
Amplification of the full length Cf4CL and CfCCoAOMT gene cDNA from stem vascular cambium total RNA. Primers used are listed in Table 1. Each lane represents a technical replicate. (A) Cf4CL; (B) CfCCoAOMT.
3.2. Cf4CL and CfCCoAOMT Sequences Are Conserved
3.2.1. Protein Sequence Multiple Alignment and Phylogenetic Tree Construction
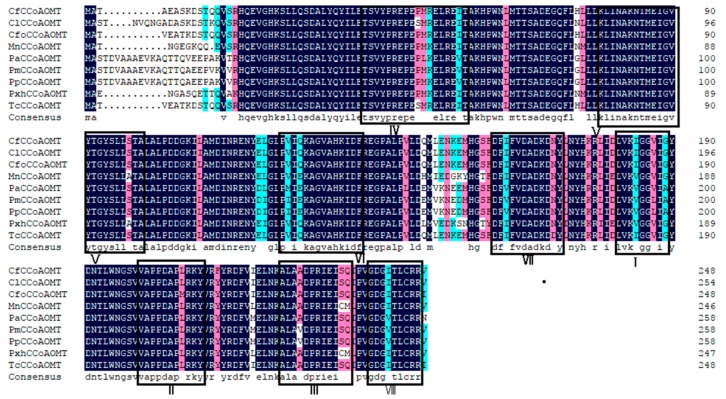
Through comparing the degree of sequence conservation of 4CL and CCoAOMT sequences from multiple plant species, we could identify the nature of our cloned genes [29]. We found that the 4CL amino acid sequence was highly conserved and could detect in total six (Box I-VI) highly conserved regions across the 4CL sequence of all 10 chosen plant species (Figure 2) [30]. Box I (SSGTTGLPKGV) coded for an AMP binding domain required for the reaction catalyzed by 4CL. 4CL substrates, such as p-Coumaric acid, were converted into their corresponding CoA ester together with AMP. The reaction progressesed from the C- to N-terminus of the peptide chain, consumed ATP and used Mg2+ as cofactor [31]. Previous studies have shown that the C-residue found in Box III (GEICIRG), fully conserved across all species, was involved in the catalytic process, and its removal causes the 4CL gene to be inactive [32]. Gly, Glu, and Cys were the most conserved amino acids. 6 highly conserved regions, SSGTTGLPKGV, QGYGMTE, GEICIRG, GWLHTGD, VDRLKELIK, and PKSPSGKILR, were discovered among all known 4CL protein-coding sequences [30]. The amino acid sequence encoded by 4CL of C. fortunei had the highest similarity (94%) to that of C. japonica. This high conservation confirmed that the gene cloned during the experiment to be a 4CL gene and it was named as Cf4CL.
Figure 2.
Multiple sequence alignment of the 4CL protein using 10 plant species. The species represented by the abbreviations on the left side of the figure and their registration numbers in NCBI are as follows: Cf4CL (C. fortunei, MK236392), Cj4CL (C. japonica, AFJ73433.1), Mg4CL (Metasequoia glyptostroboides, AFJ73430.1), Pc4CL (Pseudotaxus chienii, AFJ73459.1), Gb4CL (Ginkgo biloba, AMN10098.1), Pm4CL (Pinus massoniana, ACO40513.1), Pt4CL (P. taeda, AAA92669.1), Pr4CL (P. radiate, ACF35279.1), Cl4CL (Cunninghamia lanceolate, AFX98059.1), Co4CL (Cinnamomum osmophloeum, AFG26323.1), Cs4CL (Camellia sinensis, ASU87411.1). The marked boxes are the six strongly conserved regions.
We then turned to the CCoAOMT protein sequence and found that it was equally highly conserved among 9 different plant species (Figure 3). Altogether, we found 8 conserved amino acid regions among all the known CCoAOMT protein sequences (Box I–VIII). Boxes I, II, and III represented plant methylase specific components, while the other 5 regions, Boxes IV–VIII, were unique to the CCoAOMT gene [30]. We found that all clones of the C. fortunei CCoAOMT gene contained these conserved areas, while mutations only emerged at a few sites. We found that the amino acid sequence encoded by the C. fortunei CfCCoAOMT gene was up to 98% similar to the Chamaecyparis formosensis CCoAOMT gene (Figure 3). The Chamaecyparis formosensis CCoAOMT gene has been annotated to catalyze the methylation of caffeoyl CoA in lignin biosynthesis based on its similarity to CCoAOMT genes discovered in Oryza sativa (ABB89956.1) [12]; it is possible that the C. fortunei CCoAOMT gene had a similar function. We therefore concluded that the cloned gene should be the homologue of CCoAOMT and named it CfCCoAOMT.
Figure 3.
Multiple sequence alignment of the CfCCoAOMT protein using 9 plant species. The species represented by the abbreviations on the left side of the figure and their registration numbers in NCBI are as follows: CfCCoAOMT (C. fortunei, MK236394), CfoCCoAOMT (Chamaecyparis formosensis, ABB89956.1), TcCCoAOMT (Taiwania cryptomerioides, ABB87184.1), ClCCoAOMT (Cunninghamialanceolata, AFX98065.1), CofCCoAOMT (Chamaecyparis obtusa var. formosana, ABB87185.1), PaCCoAOMT (Picea abies, CAK18782.1), PpCCoAOMT (P. pinaster, AFL65039.1), PmCCoAOMT (P. massoniana, AHL67654.1), AtCCoAOMT (Amborella trichopoda, XP_006856484.1), MnCCoAOMT (Morus notabilis, XP_010107916.1), PxhCCoAOMT (Petunia x hybrida, ALP75648.1).
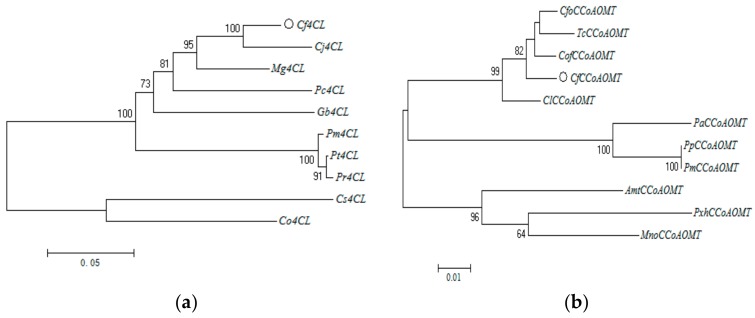
The phylogenetic tree of 4CL was divided into 2 phylogenetic groups: one represented gymnosperms including the 4CL gene of C. fortunei, C. japonica, M. glyptostroboides, P. chienii, G. biloba, P. massoniana, P. taeda and P.radiate, the other represented angiosperms including the 4CL gene of C. osmophloeum and C. sinensis (Figure 4a). The 4CL gene of C. fortunei had the highest similarity with that of C.japonica. The phylogenetic tree of CCoAOMT is divided into 3 phylogenetic groups: one included the CCoAOMT gene of C. fortunei, Chamaecyparis formosensis, Chamaecyparis obtusa var. formosana, T. cryptomerioides, and Cunninghamia lanceolata, the second included the CCoAOMT gene of P. abies, P. pinaster and P. massoniana, while the final group included the CCoAOMT gene of A. trichopoda, M. notabilis and P. x hybrida (Figure 4b). The CCoAOMT gene of C. fortunei was more similar to that of Chamaecyparis formosensis, Chamaecyparis obtusa var. formosana and T. cryptomerioides.
Figure 4.
Phylogenetic trees of 4CL (a) and CCoAOMT (b) proteins using the most closely related protein sequences from 9 (a) and 10 (b) plant species. Displayed are tree diagrams of the interrelationships between the branches of each biological lineage, constructed according to their phylogeny to indicate the genetic relationship between species. Trees were generated using the unrooted Maximum Likelihood algorithm trees with MEGA 6.0 software. Bars represent genetic distance.
3.2.2. Cf4CL and CfCCoAOMT Protein Structure and Function Prediction
We aimed to predict Cf4CL and CfCCoAOMT protein function. First, we analyzed the physicochemical properties of both Cf4CL and CfCCoAOMT using ProtParam online tools (Table 2).
Table 2.
Analysis of protein physical and chemical properties.
| Amino Acids/aa | Molecular Weight/kD | PI Value | Molecular Formula | Fat Coefficient | Hydrophilicity | Unstable Coefficient | |
|---|---|---|---|---|---|---|---|
| Cf4CL | 554 | 59.86 | 5.41 | C2700H4309N699O793S19 | 103.84 | 0.124 | 36.15 |
| CfCCoAOMT | 249 | 28.098 | 5.52 | C1261H1994N338O372S8 | 99.88 | −0.228 | 39.40 |
The Cf4CL protein was composed of 554 amino acids, among which Ala took the largest part with a 9.9% occurrence, followed by Val (9.6%) and Leu (9.0%). The total average hydrophilicity is 0.124, and the instability coefficient was 36.15, indicating Cf4CL protein likely to be a stable, hydrophobic protein. The CfCCoAOMT protein was significantly smaller, been encoded by only 249 amino acids, with Leu taking the largest percentage of 11.6%, followed by Ala (7.6%) and Asp (7.2%).The same physicochemical parameters for CfCCoAOMT (Table 2) leaded us to conclude that it should also be a stable, yet hydrophilic, protein.
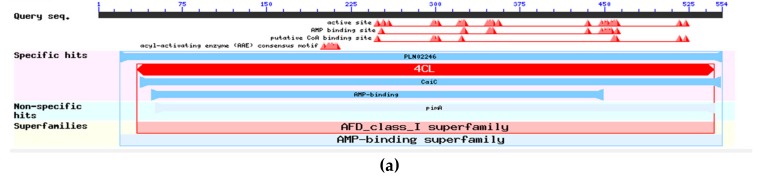
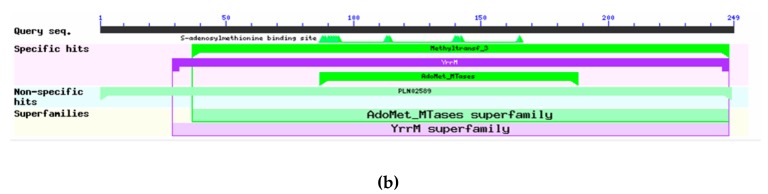
We then used NCBI conserved domains to analyze predicted protein domains in Cf4CL and CfCCoAOMT. Figure 5 indicates the individual Cf4CL and CfCCoAOMT protein domains. The Cf4CL protein contained an AMP binding site and was classified as a unique conserved “4CL” structural domain, belonging to the AFD-class-I family (Figure 5a). Within the CfCCoAOMT protein, we detected a methyltransferase domain (aa28-aa247), which belonged to the AdoMet-Mtases family (Figure 5b).
Figure 5.
Protein domain analysis of Cf4CL (a) and CfCCoAOMT (b) proteins in C. fortunei. The figure was constructed with NCBI conserved domains. Specific hits are the top-ranking RPS-BLAST hits (compared to other hits in overlapping intervals) that meet or exceed a domain-specific E-value threshold (details and illustration). Non-specific hits meet or exceed the RPS-BLAST threshold for statistical significance. Superfamily is the domain cluster to which the specific and/or non-specific hits belong.
We used the online software package SOPMA to predict the secondary structures within the Cf4CL and CfCCoAOMT proteins (Table S1). We found that both proteins have a similar distribution of secondary structure types, with alpha helices and random coils being most frequent (Table S1). For the protein encoded by Cf4CL, the proportions occupied by alpha helix, random coil, extended strand, and beta turn were 30.87%, 35.56%, 24.37%, and 9.20%, respectively. For the protein encoded by CfCCoAOMT, the proportions occupied by alpha helix, random coil, extended strand, and beta turn were 37.35%, 32.53%, 20.88%, and 9.24%, in turn.
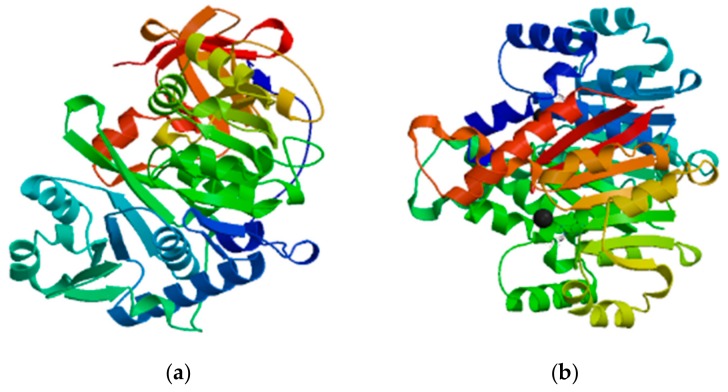
We then predicted the tertiary structure of the Cf4CL and CfCCoAOMT proteins using the online software Swiss-Model (Figure 6). It can be concluded from Table S1 that both proteins mainly consisted of alpha helices and random coils, corresponding well with the prediction of the secondary structures in Table S1. The results of protein hydrophobicity analysis through ProtScale showed that Cf4CL was a hydrophobic protein while CfCCoAOMT was hydrophilic (Figure S1). We predicted the transmembrane domain using TMHMM and found that Cf4CL and CfCCoAOMT both contained no transmembrane structure (Figure S2). In accordance with this result, using Signal IP, we found that both proteins did not contain any predicted signal peptide sequence (Figure S3).
Figure 6.
Tertiary structure model of the C. fortunei Cf4CL (a) and CfCCoAOMT (b) proteins. Color shows the order of peptide chain, starting as dark blue from the N-terminus, going to red at the C-terminus.
3.3. Quantitative Real-time PCR Analysis of Cf4CL and CfCCoAOMT
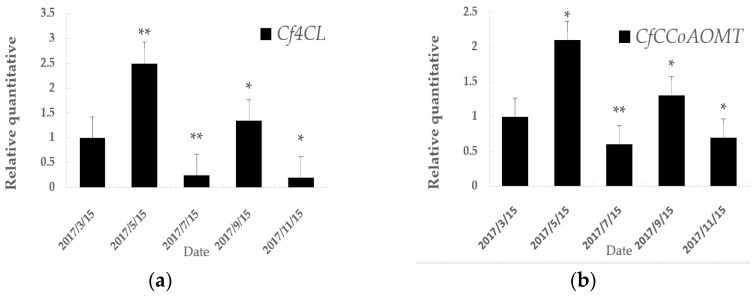
From our bioinformatics analysis we concluded that Cf4CL and CfCCoAOMT were the C. fortunei homologues of two enzymes involved in lignin synthesis, which were highly conserved across plant families. As lignin synthesis in trees was dependent on their seasonal growing activity [33], we aimed to analyze the relationship between the expression level of both genes and seasonal growing activity. Using qRT-PCR analysis of Cf4CL and CfCCoAOMT during different stages of growth and development, we found that expression of both genes could be detected during all five growth stages of the C. fortunei vascular cambium, yet with varying relative expression levels (Figure 7).
Figure 7.
Expression of Cf4CL (a) and CfCCoAOMT (b) in C. fortunei vascular cambium during the different growth stages throughout the year. Plotted are means +/− standard deviation (n = 6 replications for each growing stage). p values were calculated with t-test, * p < 0.05, ** p < 0.01.
The expression level of Cf4CL increased significantly from March to May and peaked at mid-May, reaching its highest expression level (Figure 7a). This was followed by a rapid decline around mid-July, while a second, more gradual increase happened at mid-September. Finally, in mid-November, the expression dropped to its lowest level (Figure 7a). The expression level of CfCCoAOMT showed a very similar trend across the different growth stages, however the decline in expression in mid-July and mid-November was less sharp (Figure 7b).
3.4. Functional Analysis of Cf4CL and CfCCoAOMT
3.4.1. Cf4CL and CfCCoAOMT May Increase Lignin Synthesis
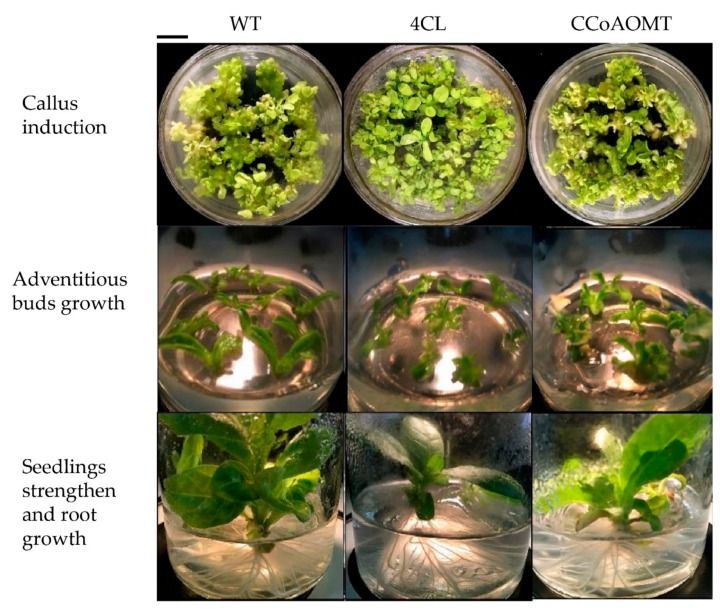
We next tested whether the Cf4CL and CfCCoAOMT genes can indeed affect lignin synthesis and plant growth in vivo, using a transgenic assay. We infected tobacco leaves using agrobacterium transformed with 35S::Cf4CL or 35S::CfCCoAOMT vector to induce high expression of either gene. Next, we placed transfected alongside control leaves on callus induction medium to study the resulting growth of regenerated plantlets. We found that wild type tobacco leaves grew faster than Cf4CL or CfCCoAOMT transfected leaves at every growth stage: callus induction, adventitious bud growth, seedling strengthening, and root growth (Figure 8, top to bottom). Moreover, the transgenic tobacco seedlings showed different degrees of yellowing, vitrification, and plant weakness.
Figure 8.
Overexpression of Cf4CL and CfCCoAOMT initially impedes tobacco growth. The first to third columns show representative images of wild type, Cf4CL transfected, and CfCCoAOMT transfected leaves, respectively. The first to third rows successively show the callus induction, adventitious bud growth, and seedling strengthening/root growth phases. A total of 3 seedlings were randomly chosen for measurements of each genotype. Bar equals 1 cm.
It can be seen from Table S2 that the height difference of mature plants (2 months old) did not reach significance as the average height of wild type tobacco was 37.2 cm while Cf4CL and CfCCoAOMT transfected tobacco plants were 39.4 cm and 40.5 cm high, respectively. However, both transfected plants did develop a higher stem diameter, with the wild type of 4.92 mm, while Cf4CL and CfCCoAOMT transfected plants reached 5.68 mm (1.15× WT) and 6.35 mm (1.29× WT), respectively. Though growing slower than wild type at early growth stages, transgenic seedlings caught up and grew taller than the wild type, which might indicate a delayed function of Cf4CL and CfCCoAOMT in stem growth.
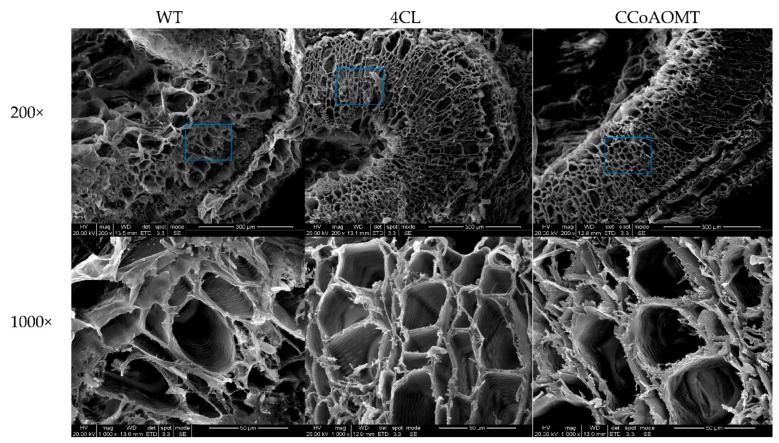
Since we found that the stem diameter of Cf4CL and CfCCoAOMT transfected plants was increased, we wondered whether such plants might have an altered vascular cambium due to heightened lignin synthesis activity. We obtained cross sections of wild type and transfected tobacco stems as samples and soaked them in FAA for fixation. After dehydration with a series of alcohol solutions of increasing strength, samples were sliced and gilded. For further details, check the review of Brenda L. et al and article of Ma J. et al. [27,34]. We found that overexpression of either Cf4CL or CfCCoAOMT causes an increased number of xylem cells, a thickened xylem cell wall, and an increased number of cell layers in vascular tissues, compared to the wild type (Figure 9; Table S3). The average thickness of the xylem cell wall in three Cf4CL transgenic tobacco lines (Cf4CL-1, 2, 3) was 2.458 μm, 2.464 μm, and 2.488 μm, almost twice (~1.8×, p < 0.01) as thick as in the wild type (Table S3). We found similar values for three CfCCoAOMT transgenic lines (CfCCoAOMT-1, 2, 3), also being almost twice (~1.8×, p < 0.01) as thick as the wild type. These results showed that increased expression of Cf4CL and CfCCoAOMT might lead to a thickened xylem wall, most likely by increased lignin deposits.
Figure 9.
Representative SEM micrographs of tobacco stem cross sections, taken at 200× and 1000×. The first to third columns show wild type, Cf4CL and CfCCoAOMT transfected tobacco respectively. The pictures at 1000× show part of the pictures taken at 200×, indicated by a blue square.
3.4.2. Cf4CL and CfCCoAOMT Expression Increases Lignin Content
To determine whether the thickened xylem walls we observed in our transgenic tobacco with higher levels of Cf4CL and CfCCoAOMT expression were caused by increased lignin content, we turned to directly measuring lignin levels of mature tobacco transferred into soil for 2 months using UV spectroscopy. Indeed, we found that in all transgenic lines analyzed, lignin content was significantly higher than in the wild type (Table 3). These results showed that overexpression of Cf4CL and CfCCoAOMT in tobacco leaded to an increase in lignin content, resulting in a thickened xylem cell wall. We therefore concluded that the Cf4CL and CfCCoAOMT genes we identified were indeed functional.
Table 3.
Determination of the content of lignin in tobacco.
| Plant | WT | 4CL | CCoAOMT | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | ||
| Average lignin content % | 4.86 | 8.31 | 10.51 | 7.14 | 9.13 | 7.54 | 8.34 |
| SD | 0.983 | 1.457 | 2.819 | 0.404 | 2.318 | 0.507 | 1.365 |
| Sig | <0.05 | <0.01 | <0.05 | <0.01 | <0.05 | <0.01 | |
For every tobacco genotype 3 plants were measured (n = 3), 3 technical repetitions were done per plant sample. SD, standard deviation from selected lines. Sig, the level of significance, data was compared through t-test.
4. Conclusions and Discussion
In this study, we aimed to understand which genes might be involved in lignin synthesis in C. fortunei in order to understand lignin synthesis in this species and aim to improve its wood characteristics. We chose to study 4CL and CCoAOMT as they are key enzymes in the phenylalanine pathway and have an important role in G-type or S-type lignin monomer biosynthesis. In most plant species, 4CL genes exist as a gene family. It has been confirmed that the isoenzymes of 4CL have different preferences for different substrates. Three At4CL genes were isolated from A. thaliana, and it was found that isoenzymes encoded by At4CL1 and At4CL2 are involved in the biosynthesis of lignin monomers [35]. Research has shown that the overexpression of 4CL may accelerate the formation of wood and enhance the disease resistance and lodging resistance of wood [36,37]. CCoAOMT genes similarly exist as gene families in most plant species, showing high sequence conservation. They may work together in the biosynthesis of lignin with COMT, also a methylase, during which it may affect synthesis of both S-type lignin and G-type lignin [38].
We found that the 4CL and CCoAOMT genes cloned from C. fortunei have high similarity with that of other species and confirmed with the inspiration of method performed by Wang et al. [39] Cf4CL and CfCCoAOMT may increase lignin synthesis and xylem wall thickness when overexpressed in tobacco, confirming their biological functionality. Even though tobacco overexpressing either 4CL or CCoAOMT eventually grew taller than the wild type, initially their growth was delayed. Further study should show focus on whether this has to do with the presence of agrobacterium used for the transformation protocol. Further research needs to be carried out on whether the CfCCoAOMT gene contributes to lignin biosynthesis together with other key enzymes. We sampled C. fortunei vascular cambium at 5 successive growth stages to analyze the relative expression levels of Cf4CL and CfCCoAOMT. Our findings were consistent with previous studies on Populus tomentosa 4CL gene expression dynamics carried out by Zhao et al. [40] and on the Populus tremuloides CCoAOMT gene carried out by Meng et al. [41]. These and our study all showed a ‘double-peak’ pattern during the growing season, with the second expression peak being lower than the first. However, there appeard to be a difference between C. fortunei and the other two species in terms of the timing of the highest expression levels of Cf4CL and CfCCoAOMT. In C. fortunei, this occurred at mid-May and mid-September for both genes. In P. tomentosa the 4CL gene peaked at late June and early August, while in P. tremuloides the CCoAOMT gene peaked around mid-June and late July. We speculate that this difference was due to variations between species and climatic conditions. Soile et al. analyzed the transcriptome data of Picea abies wood formation related genes and found that the seasonal expression of all lignin monomers peaked in summer and did not decrease until the end of August; the second peak was delayed to winter with the lowest temperature [18]. The expression levels of 4CL and CCoAOMT also showed a ‘double-peak’ pattern, which is possible due to diverse climate zones and growth rhythms. Studies have confirmed that temperature change plays a key role in the formation of vascular cambium and the formation of late wood in conifer species [42]. Changes in photoperiod also affect the seasonal expression of genes involved in lignin synthesis in conifer species: Cronn R. et al. used RNA-seq to monitor transcriptional activity in Pseudotsuga menziesii (Douglas-fir) needles at daily and annual cycles and identified 12,042 transcripts that showed significant cyclic variation with changes in photoperiod, including transcripts involved in wood formation [28,43,44].
We found that the expression level of two C. fortunei genes involved in lignin synthesis decreased suddenly in mid-July below the average level. The average temperature in Nanjing at July is 27 °C to 34℃, peaks around the year, is harmful for most plants. We speculate that the reason for this decrease might be that C. fortunei experienced a higher temperature around July, longer illumination time and stronger transpiration, leading to inhibition of vascular formation by inhibiting cell differentiation division. Alternatively, certain internal regulatory factors may be affected during transcription or post-transcriptional regulation due to changes in the external environment, resulting in a change in RNA stability, slowing down the rate of lignin synthesis and resulting in the lower growth speed around mid-July [45]. Future experiments could include more sampling time points for a more accurate and detailed record of expression changes. At the same time, different tissues of C. fortunei can be sampled to study whether lignin synthesis might be differentially regulated in other tissues.
C. fortunei wood is of high value, having a rapid growth rate with a lower density of wood. We demonstrated here that Cf4CL and CfCCoAOMT are involved in lignin synthesis and subject to dynamic regulation of their expression, likely as the lignin build-up within C. fortunei changes throughout the season. Future studies are needed to confirm whether both genes indeed perform the same function in C. fortunei as well, for example, by constructing mutants or performing gene knockdown. Understanding how lignin synthesis operates in C. fortunei will help us to understand how its wood is formed, possibly allowing us to enhance its wood quality.
Acknowledgments
We would like to give thanks to Remco A. Mentink of Plant Research International, Wageningen University and Research Centre for his careful scientific revision on the manuscript. We also thank reviewers for insightful comments on this article.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/10/8/619/s1. Table S1: Analysis of secondary structure of encoded protein; Table S2: Comparison of plant height and stem diameter after Tobacco Maturity; Table S3: Transverse section cell wall thickness of tobacco stem segments; Figure S1: Hydrophobicity curves of the Cf4CL and CfCCoAOMT gene encoding protein of C. fortunei; Figure S2: Transmembrane pattern map of Cf4CLC and fCCoAOMT gene encoding protein of C. fortunei; Figure S3: Pattern diagram of protein signal peptide encoded by C. fortunei Cf4CL and CfCCoAOMT gene.
Author Contributions
Conceptualization, J.X.; formal analysis, Z.G., H.H., H.Z., and J.Y.; funding acquisition, J.X.; investigation, Z.G., H.H., H.Z., and J.Y.; project administration, J.X.; writing—original draft, H.H, Z.G.; writing—review and editing, Z.G., J.M.
Funding
This work was supported by National Forestry and Grassland Administration of China, forestry public welfare industry research (201304104), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Xu Y., Zou M., Tang W., Su F., Hu X., Ban L., Liu W. Difference analyses of tracheid morphological characteristics among provenances of exotic loblolly pine in southern districts of Henan province in China. J. Nanjing. Univ. 2002;26:15–20. [Google Scholar]
- 2.Li L., Cheng X.F., Leshkevich J., Umezawa T., Harding S.A., Chiang V.L. The last step of syringyl monolignol biosynthesis in angiosperms is regulated by a novel gene encoding sinapyl alcohol dehydrogenase. Plant Cell. 2001;13:1567–1585. doi: 10.1105/TPC.010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weng J.K., Chapple C. The origin and evolution of lignin biosynthesis. New Phytol. 2010;187:273–285. doi: 10.1111/j.1469-8137.2010.03327.x. [DOI] [PubMed] [Google Scholar]
- 4.Humphreys J., Chapple C. Rewriting the lignin roadmap. Curr. Opin. Plant Biol. 2002;5:224–229. doi: 10.1016/S1369-5266(02)00257-1. [DOI] [PubMed] [Google Scholar]
- 5.Mishima K., Fujiwara T., Iki T., Kuroda K., Yamashita K., Tamura M., Fujisawa Y., Watanabe A. Transcriptome sequencing and profiling of expressed genes in cambial zone and differentiating xylem of Japanese cedar (Cryptomeria japonica) Bmc Genom. 2014;15:219. doi: 10.1186/1471-2164-15-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X.H., Vincent L.C. Molecular cloning of 4-coumarate: Coenzyme a ligase in loblolly pine and the role of this enzyme in the biosynthesis of lignin in compression wood. Plant Physiol. 1997;113:65–74. doi: 10.1104/pp.113.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee D., Douglas C.J. Two divergent members of a tobacco 4-coumarate-coenzyme a ligase (4CL) gene family, cDNA structure, gene inheritance and expression, and peoperties if recombinant protein. Plant Physiol. 1996;112:193–205. doi: 10.1104/pp.112.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan X. Ph.D. Thesis. Beijing Forestry University; Beijing, China: 2013. Study on the Structure and Function of the 4CL Gene Promoters and APX in Populus tomentosa. (In Chinese) [Google Scholar]
- 9.Lee D., Meyer K., Chapple C., Douglas C.J. Down-regulation of 4-coumarate: CoA ligase (4CL) in Arabidopsis: effect on lignin composition and implications for the control of monolignol biosynthesis. Plant Cell. 1997;9:1985–1998. doi: 10.1105/tpc.9.11.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu H., Zhao Y., Jiang X. Stable and specific expression of 4-coumarate: coenzyme A ligase gene (4CL1) driven by the xylem-specific Pto4CL1 promoter in the transgenic tobacco. Biotechnol. Lett. 2004;26:1147–1152. doi: 10.1023/B:BILE.0000035487.91628.9e. [DOI] [PubMed] [Google Scholar]
- 11.Kajita S., Hishiyama S., Tomimura Y. Structural characterization of modified lignin in transgenic tobacco plants in which the activity of 4-coumarate: Coenzyme A ligase is depressed. Plant Physiol. 1997;114:871–879. doi: 10.1104/pp.114.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hua Z., Qing S., Shi L., Tai W., Yan S. Expression analysis of Oryza sativa CCoAOMT gene. Chin. Sci. Bull. 2004;14:1390–1394. [Google Scholar]
- 13.Ye Z.H., Kneusel R.E., Matern U. An alternative methylation pathway in lignin biosynthesis in Zinnia. Plant Cell. 1994;6:1427–1439. doi: 10.1105/tpc.6.10.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L.G., Osakabe Y.K. Secondary Xylem-specific expressin of caffeoyl-coenzyme A 3-O-methyltransferase plays an important role in the methylation pathway associated with lignin biosynthetic in loblloly pine. Plant Mol. Biol. 1999;40:555–565. doi: 10.1023/A:1006244325250. [DOI] [PubMed] [Google Scholar]
- 15.Inoue K., Sewalt V.J.H., Murray B.G. Developmental expression and substrate specificities of Alfalfa caffeic acid 3-O-mehtyltransferase and coenzyme A-3-O-methyltransferase in relation to lignification. Plant Physiol. 1998;117:761–770. doi: 10.1104/pp.117.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen C., Meyermans H., Burggraeve B., Rycke R.M., Lnoue K., Vleesschauwer V., Steenackers M., Montagu M.C., Engler G.J., Boerjan W.A. Cell-specific and conditional expression of caffeoyl-coenzyme A-3-O-methylytransferase in poplar. Plant Physiol. 2000;123:853–867. doi: 10.1104/pp.123.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao Y. Master’s Thesis. Nanjing Forestry University; Nanjing, China: 2016. Transcriptome Sequencing and Profiling of Expression in Vascular Cambium of Cryptomeria Fortune. [Google Scholar]
- 18.Jokipii-Lukkari S., Delhomme N., Schiffthaler B., Mannapperuma C., Prestele J., Nilsson O., Street N., Tuominen H. Transcriptional roadmap to seasonal variation in wood formation of Norway spruce. Plant Physiol. 2018;176:2851–2870. doi: 10.1104/pp.17.01590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curtis I., Davey M., Power J. Leaf disk transformation. Meth. Mol. Biol. 1995;44:59–70. doi: 10.1385/0-89603-302-3:59. [DOI] [PubMed] [Google Scholar]
- 20.Gasteiger E., Hoogland C., Gattiker A., Duvaud S., Wilkins M.R., Appel R.D., Bairoch A. Protein Identification and Analysis Tools on the ExPASy Server. In: Walker J.M., editor. The Proteomics Protocols Handbook. Humana Press; Totowa, NJ, USA: 2005. pp. 571–607. [Google Scholar]
- 21.Tamura K., Stecher G., Peterson D.S., Filipski A., Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geourjon C., Deleage G. SOPMA: significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Bioinformatics. 1995;11:681–684. doi: 10.1093/bioinformatics/11.6.681. [DOI] [PubMed] [Google Scholar]
- 23.Waterhouse A., Bertoni M., Bienert S., Studer G., Tauriello G., Gumienny M., Heer F.T., de Beer T.A.P., Rempfer C., Bordoli L., et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296–W303. doi: 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen T., Brunak S., Heijne G., Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 25.Rychlik W. OLIGO 7 Primer Analysis Software. In: Yuryev A., editor. Methods in Molecular Biology Vol. 402: PCR Primer Design. Humana Press; Totowa, NJ, USA: 2007. pp. 35–59. [DOI] [PubMed] [Google Scholar]
- 26.Liu S., Li A., Chen C., Xu M., Cai G., Zhang L., Guo C. De novo transcriptome sequencing in Passiflora edulis sims to identify genes and signaling pathways involved in cold tolerance. Forests. 2017;8:435. doi: 10.3390/f8110435. [DOI] [Google Scholar]
- 27.Little B., Wagner P., Ray R., Pope R., Scheetz R. Biofilms—An ESEM evaluation of artifacts introduced during SEM preparation. J. Ind. Microbiol. Biotechnol. 1991;8:213–221. doi: 10.1007/BF01576058. [DOI] [Google Scholar]
- 28.Rodrigues J., Faix O., Pereira H. Improvement of the acetylbromide method for lignin determination within large scale screening programmes. Eur. J. Wood Wood Prod. 1999;57:341–345. doi: 10.1007/s001070050355. [DOI] [Google Scholar]
- 29.Li J., Fan F., Wang L., Zhan Q., Wu P., Du J., Yang X., Liu Y. Cloning and expression analysis of cinnamoyl-CoA reductase (CCR) genes in sorghum. PeerJ. 2016;4:e2005. doi: 10.7717/peerj.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma J., Wei L., Li H., Li J. The analysis of genes and phytohormone metabolic pathways associated with leaf shape development in Liriodendron chinense via de novo transcriptome sequencing. Genes. 2018;9:577. doi: 10.3390/genes9120577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H., Yu Y., Fu C., Zhou G., Gao H. Progress of a key enzyme-caffeoyl-co a 3-o-methyltransferase in lignin biosynthesis. Genom. Appl. Biol. 2014;33:458–466. [Google Scholar]
- 32.Challis G.L., Ravel J., Townsend C.A. Predictive, structure-based model of amino acid recognition by nonribosomal peptide synthetase adenylation domains. Chem. Biol. 2000;7:211–224. doi: 10.1016/S1074-5521(00)00091-0. [DOI] [PubMed] [Google Scholar]
- 33.Stuible H.P., Buttner D., Ehlting J., Hahlbrok K., Kombrink E. Mutation alanalysis of 4-coumarate: CoA ligase identifies functionally important amino acids andverifies its close relationship to othe radenylate-form ingenzymes. Febs Lett. 2000;467:117–122. doi: 10.1016/S0014-5793(00)01133-9. [DOI] [PubMed] [Google Scholar]
- 34.National Center for Biotechnology Information. [(accessed on 1 December 2006)]; Available online: https://www.ncbi.nlm.nih.gov/protein/
- 35.Cronn R., Dolan P.C., Jogdeo S., Wegrzyn J., Neale D., Bradley J., Denver D. Transcription through the eye of a needle: Daily and annual cyclic gene expression variation in Douglas-fir needles. Bmc Genom. 2017;18:558. doi: 10.1186/s12864-017-3916-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cukovic D., Ehlting J., Vanziffle J.A., Douglas C.J. Structureand evolution of 4-coumarate: Coenzyme A ligase (4CL) gene families. Biol. Chem. 2001;382:645–654. doi: 10.1515/BC.2001.076. [DOI] [PubMed] [Google Scholar]
- 37.Huang J., Li W., Qu C., Liu L., Xu X., Wang R., Li J. Expression characteristics of key genes in Lignin pathway among different lodging resistance lines of Brassica napus L. Acta Agron. Sin. 2013;39:1339–1344. doi: 10.3724/SP.J.1006.2013.01339. [DOI] [Google Scholar]
- 38.Pagadala N.S., Arha M., Reddy P., Kumar R., Sirisha V.L., Prashant S., Reddy K., Khan B., Rawal S.K., Kishor P.B. Phylogenetic analysis, homology modelling, molecular dynamics and docking studies of caffeoyl-coa-o-methyl transferase (CCoAOMT1 and 2) isoforms isolated from subabul (Leucaena leucocephala) J. Mol. Model. 2009;15:203–221. doi: 10.1007/s00894-008-0395-8. [DOI] [PubMed] [Google Scholar]
- 39.Wang P., Cheng T., Chen J., Lu M., Liu G., Li M., Shi J., Lu Y., Laux T. Expansion and functional divergence of AP2 group genes in Spermatophytes determined by molecular evolution and Arabidopsis mutant analysis. Front. Plant Sci. 2016;7:1383. doi: 10.3389/fpls.2016.01383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao H., Wei J., Lu J., Song Y., Wang T., Zhu Z. cDNA cloning and functional analysis of 4-coumarate: CoA ligase (4CL) gene in Chinese white aspen. Prog. Nat. Sci. 2003;12:895–900. doi: 10.1080/10020070312331344610. [DOI] [Google Scholar]
- 41.Meng H., Campbell W. Substrate profiles and expression of caffeoyl coenzyme A and caffeic acid O-methyltransferases in secondary xylem of aspen during seasonal development. Plant Mol. Biol. 1998;38:513–520. doi: 10.1023/A:1006071708728. [DOI] [PubMed] [Google Scholar]
- 42.Oribe Y., Funada R., Shibagaki M., Kubo T. Cambial reactivation in locally heated stems of the evergreen conifer Abies sachalinensis (Schmidt) masters. Planta. 2001;212:684–691. doi: 10.1007/s004250000430. [DOI] [PubMed] [Google Scholar]
- 43.O’Connell A., Holt K., Piquemal J., Grima J., Boudet A., Pollet B., Lapierre C., Petit M., Schuch W., Halpin C. Improved paper pulp from plants with suppressed cinnamoyl-CoA reductase or cinnamyl alcohol dehydrogenase. Transgenic Res. 2002;11:495–503. doi: 10.1023/A:1020362705497. [DOI] [PubMed] [Google Scholar]
- 44.Lapierre C., Pollet B., Petit-Conil M. Structural alteration of lignin in transgenic poplars with depressed cinnamyl alcohol dehydrogenase or caffeic acid O-methyl-transferase activity have an opposite impact on the efficiency of industrial kraft pulping. Plant Physiol. 1999;119:153–163. doi: 10.1104/pp.119.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song F. Ph.D. Thesis. Northeast Forestry University; Harbin, China: 2009. Expression and Functional Analysis of Correlative Enzyme Genes of Lignin Phenylpropanoid Biosynthetic Pathway in Betula platphylla. (In Chinese) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.