Abstract
5-Fluorouracil (5-FU) regimen remains the backbone of the first-line agent to treat colon cancer, but often these patients develop resistance. Cancer stem cells (CSC’s) are considered as one of the key contributors in the development of drug resistance and tumor recurrence. We aimed to provide preclinical evidence for Antrodia cinnamomea (AC), as a potential in suppressing colon cancer CSC’s to overcome 5-FU drug-resistant. In-vitro assays including cell viability, colony formation, AC + 5-FU drug combination index and tumor sphere generation were applied to determine the inhibitory effect of AC. Mouse xenograft models also incorporated to evaluate in vivo effect of AC. AC treatment significantly inhibited the proliferation, colony formation and tumor sphere generation. AC also inhibited the expression of oncogenic markers (NF-κB, and C-myc), EMT/metastasis markers (vimentin and MMP3) and stemness associated markers (β-catenin, SOX-2 and Nanog). Sequential treatment of AC and 5-FU synergized and reduces colon cancer viability both in vivo and in vitro. Mechanistically, AC mediated anti-tumor effect was associated with an increased level of tumor suppressor microRNAs especially, miR142-3p. AC can be a potent synergistic adjuvant, down-regulates cancer stemness genes and enhances the antitumor ability of 5-FU by stimulating apoptosis-associated genes, suppressing inflammation and metastasis genes through miR142-3p in colon cancer.
Keywords: colon cancer, AC, 5-FU, EMT, stemness, miR-142-3p
1. Introduction
The prevalence of colorectal cancer (CRC) is expected to be higher than almost 2.2 million, it is expected that by 2030, the new cases and mortality would be around 1.1 million globally [1]. Surgical resection remains the best option for treating the patients with stage II~III colon cancer, but 30–50% of these patients have tumor relapse even after resection and is found to be related with the higher risk of cancer-associated death [2,3]. Most of these recrudescences arise during the period of the first 2 years after the surgery [4]. Despite taking effective measures of cancer surveillance, and major advancement in treatment modalities including chemotherapy and target therapy. The drug resistance in colon cancer still remains a leading hindrance to successful chemotherapy. Recent studies have been associated with Cancer stem cells (CSC’s), which are a small group of cancer cells having the potential to undergo for self-renewal, differentiation and tumor initiation abilities [5]. CSC’s are also thought to be the key player involved in the failure of cancer therapy due to their substantial chemo-resistance properties that eventually leads to disease recurrence and metastasis [6]. More reports have surfaced to indicate for improving the cancer patient’s outcomes, traditional therapies approaches should be now shifted to targeting the specific CSC’s [2]. Therefore, therapeutic approaches that can also target CSC’s are being pursued to decreases the risk of cancer recurrence and metastasis.
It has been seen the effect of compounds isolated from natural source gives better response to cancer therapy, such as Stictic acid a secondary metabolite from Lichen Lobaria pilmonaria inhibits the growth of malignant cells and shows the anticancer effect in human colon adenocarcinoma [7]. Phytol is a diterpene alcohol from chlorophyll induces the concentration-dependent cell cytotoxicity in human cancer cell lines [8].
Antrodia cinnamomea (AC) is a Taiwan endemic species belonging to the genus Antrodia (Polyporaceae). AC is a photophobic parasitic fungus developing in the inner heartwood wall of Cinnamomum kanehirai Hayat (Lauraceae) [9]. It has been used by Taiwanese as a traditional medicine for the treatment of several different ailments, such as diarrhoea, liver disease, hypertension, inflammation, etc. for long times in the history [10]. Isolated compounds from AC are reported to induce apoptosis on colon cancer cells (HT-29 and SW-480) [11]. Other studies also revealed that AC induces apoptosis in human liver and oral cancer cells via mitochondrial-dependent pathways [12,13]. Recent studies also indicate that AC not only causing the apoptosis to tumor cell, but also protects liver cells from free radical-induced oxidative stress through the Nrf-2 activation and up-regulation of the MAP kinase-mediated antioxidant genes [14,15]. Furthermore, AC inhibits the proliferation of head and neck cancer cells and migration of leukaemia cells [16,17,18,19]. These findings strongly suggest AC can be a potential source of new cancer therapeutics. In our research, we aimed to provide experimental supports for inhibiting colon tumorigenesis using AC. We demonstrated AC treatment was able to reduce the colon tumorigenesis, not only in vitro but also in vivo. Notably, AC treatment suppressed CSC’s properties via the downregulating oncogenic (NF-kB, c-Myc), EMT (vimentin) and stemness (β-catenin, Sox2, Nanog) associated markers.
2. Materials and Methods
2.1. Cell Culture, Chemicals and Reagents
The human colon cancer cell lines (SW480, SW620 and HCT116) were purchased from the American Type Culture Collection (ATCC), and cells were cultured according to ATCC’s recommend culture conditions. Colon sphere formation assay was performed according to a previously described method [20]. SW480, SW620 and HCT116 were cultured in Serum-Free Medium composed of DMEM/Ham’s F12 (1:1), human epidermal growth factors (hEGF, 20ng/mL), basic fibroblast growth factor (bFGF; 10ng/mL (PeproTech, Rocky Hill, NJ, USA), 2 ug/mL of 0.2% heparin (Sigma), and 1% penicillin/streptomycin (P/S, 100 U/mL, Hyclone). Cells were seeded (1000 cells/mL) in 12-well low adhesion plates and incubated at 37 °C and 5% CO2 for 5–7 days. Cells aggregates or spheroids which are compact, spherical, non-adherent massed greater than 50 µM in diameter were counted. 5-FU was purchased from SelleckChem, Taiwan (Cat. No. S1209). The mycelia of AC were kindly provided by Balay Biotechnology Corporation (Taipei, Taiwan, Republic of China). The AC extract was isolated according to previously established methods with some modifications [21]. The mycelia of AC were air-dried and extracted with boiling water (at the ration of 1:20, w/v) for 6 h. The liquid and precipitate were then separated by centrifugation and the suspension (non-soluble matters) was filtered out. The filtrate was then mixed with 4 volumes of ethanol (95%) overnight to precipitate crude extract. The crude extract was then spun at 4000× g for 30 min to remove the supernatant. This crude extract was termed aqueous AC mycelia extract and were dissolved in water and stored at room temperature for further analysis. The quality control was done by measuring the sugar content of the AC extract where the average concentration of polysaccharide is 4.00 ± 0.80 mg/g.
2.2. Cell Viability Test
Sulforhodamine B (SRB) assay [22] was used to determine cellular viability. Briefly, the colon cancer cells or colon spheres were seeded in 96 well plates (3.5 × 105 cells/well) and treated with drugs (AC or 5-FU) alone or in combination at the specified concertation and times. After the respective drug treatments relative cell number was estimated by SRB reagent according to the manufacturer’s protocol (Sigma, Ronkonkoma, NY, USA).
2.3. Apoptosis Assay
Annexin V-PI+ viable cells and Annexin-V+ apoptotic cells were estimated by flow cytometry and the later were considered apoptotic cells. Data were collected in FACS Calibur (Becton-Dickinson, Mountain View, CA) and analyzed by using the Cell Quest software (Becton-Dickinson), these experiments were performed thrice.
2.4. RNA Isolation, RT-PCR and Quantitative RT-PCR
TRIzol-based protocol (Life Technologies) was used to isolate total RNA and purify according to manufacturer instructions. One microgram of total RNA was reverse transcribed using QIAGEN OneStep RT-PCR Kit (QIAGEN, Taiwan), and the PCR reaction was carried out using a Roto-Gene SYBR Green PCR Kit (400, QIAGEN, Taiwan). The primer sequence for genes, SOX2, forward: 5′-AAATGGGAGGGGTGCAAAAGAGGAG-3′ and reverse: 5′-CAGCTGTCATTTGCTGTGGGTGATG-3′. Nanog, forward: 5′-AATACCTCAGCCTCCAGCAGATG-3′ and reverse: 5′-TGCGTCACACCATTGCTATTCTTC-3′. β-catenin, forward: 5′-ACTGGCAGCAACAGTCTTACC-3′ and reverse: 5′-TTTGAAGGCAGTCTGTCGTAAT-3′. The primer sequences internal control RPLP0 were: forward: 5′-TGGTCATCCAGCAGGTGTTCGA-3′ and reverse: 5′-ACAGACACTGGCAACATTGCGG-3′. List of microRNA primer sequences was enumerated in Supplementary Table S1.
2.5. Colony Formation Assays
The colony-forming assay was executed according to the previously explained protocols [23] with some modifications. Briefly, a total of 500 colon cancer cells were seeded in 6 well plates and treated with AC (40 μg/mL equivalent of IC20 values). The cells were allowed to grow for another week and then collected, fixed, and counted.
2.6. SDS-Page and Western Blotting
Total protein lysate of colon cancer cells was extracted after the treatments from different experiments were separated using the SDS-PAGE using Mini-Protean III system (Bio-Rad, Taiwan) and transferred onto the PVDF membranes using Trans-Blot Turbo Transfer System (Bio-Rad, Taiwan). The membranes were incubated for overnight at 4 °C with respective primary antibodies PARP (#9542P), β-catenin (#9562), vimentin (#5741P), NF-κB (#6956S), β-actin (Sc-47778), C-myc (Sc-40), MMP3 (Sc-6839), ABCG2 (10051-1-AP). Secondary antibodies purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and ECL detection kit was used for the detection of the protein of interests. The image was captured and analyzed using UVP BioDoc-It system (Upland, CA, USA).
2.7. ALDEFLUOR Assay and ALDH1+ Population Cell Sorting by FACS
The Aldehyde Dehydrogenase 1 (ALDH1) enzymatic activity showing positive cell populations were isolated according to the instruction of the manufacturer (STEMCELL Technologies, Durham, NC, USA). The human colon cancer cells (SW480, SW620 and HCT116) were suspended in the concentration of 1 × 106 cells/mL in ALDEFLUOR assay buffer containing ALDH substrate (BAAA, 1 µmol/L per 1 × 106 cells) and incubated for 40 min at 37 °C. Cells incubated with ALDEFLUOR substrate 50 mmol/L diethylaminobenzaldehyde (DEAB) as reference control, a specific ALDH inhibitor. TO eradicate contamination of cells of mouse origin from the xeno-transplanted tumors, we used staining with anti-H2Kd antibody (BD biosciences, 1/200, 20 min on ice) followed by staining with a secondary antibody labelled with phycoerythrin (PE) (Jackson labs, 1/250, 20 min on ice). The sorting gates were established using as negative controls the cells stained with PI only, for viability, the ALDEFLUOR stained cells treated with DEAB and the staining with secondary antibody alone.
2.8. In Vivo Studies
All the animal experiments and maintenance conformed to the strict compliance to the animal use protocol from Taipei Medical University (protocol LAC-2014-0170). Female NOD/SCID mice were purchased from BioLASCO Taiwan Co., Ltd. Tumor-initiating ability test was first to assess by using the tumor spheres generated from the naïve DLD-1 cells. DLD-1 spheroids cells (1 × 106 cells/injections) were subcutaneously injected into the right flanks of NOD/SCID mice.
Second, for drug treatment test, subcutaneous tumor models were established using the tumor sphere grown from DLD-1 (1 × 106 cells/20µL/injection) in NOD/SCID mice (4–6 weeks old). The treatment commenced when the tumor became palpable. For assessing the effect of AC on tumor growth and 5-FU toxicity. The mice were divided into different groups with matched weight, first the control group receiving the normal saline (vehicle group), second group treated with 10 mg/kg 5-FU, third group treated with 50mg/kg AC, and fourth group treated with 10 mg/kg 5-FU + 50 mg/kg AC (5-FU + AC group),
Dosing regimens are as the following, 5-FU alone (10 mg/kg, two times/week), AC alone (50 mg/kg, five times/ week), and 5-FU + AC combination (10 mg/kg, two times/week; 50mg/kg five times/week, respectively). All the treatments were given intraperitoneal. The change in tumor burden was expressed in fold change in cubic centimeter as compared to its starting volume.
Mice were sacrificed humanely upon the completion of the experiments, and tumor biopsies were collected for further analysis.
2.9. Statistical Analysis
All experiments were executed in triplicates. Statistical analyses were performed using Student’s t-test by GraphPad Prism software, where a p-value < 0.05 was considered as statistically significant and was indicated with an asterisk.
3. Results
3.1. AC Inhibits the CRC Tumorigenesis and Colon Sphere Formation
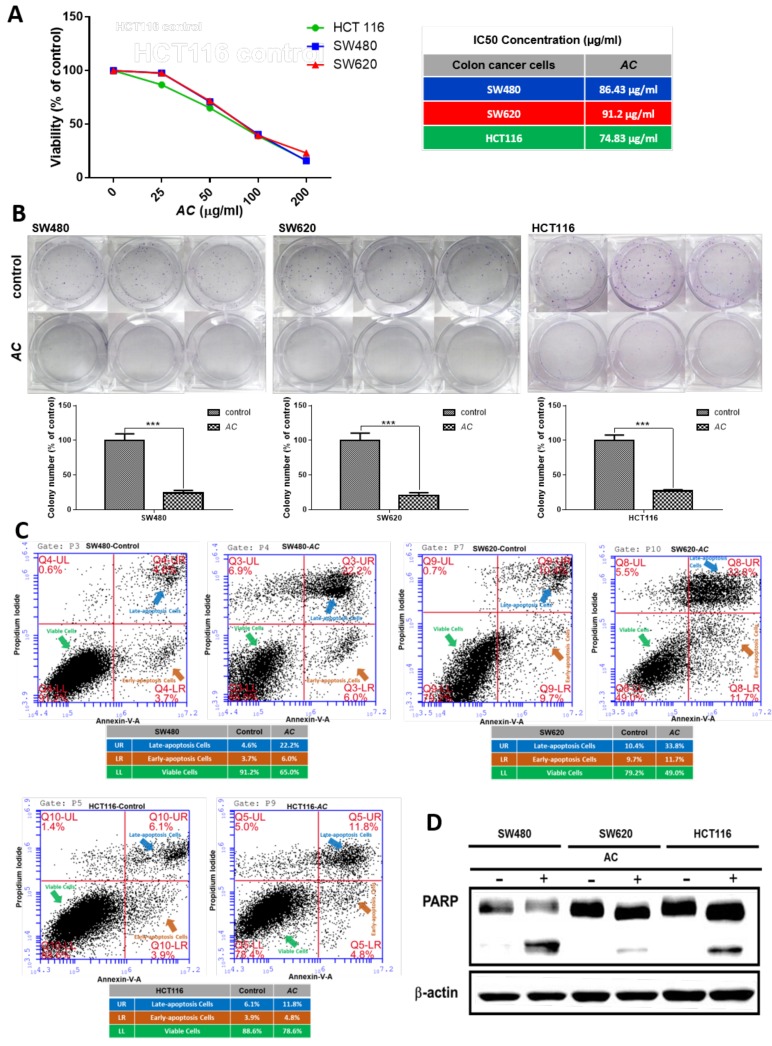
Mechanism of apoptosis was found connected with the advancement and progression of CRC’s [24]. AC treatment was reported to have the ability to suppress tumor growth in various cancers [16,25]. Anti-cancer effect of AC on colon cancer cells were determined by the cellular viability and colony formation assay by SRB test. AC treatment observed to reduces or inhibits the lung cancer cell viability by inducing apoptosis [26]. For further investigation of the effect of AC on CRC cells, apoptosis was examined through Annexin-V-FITC Assay [27]. We first observed AC effectively inhibits the cell viability of SW480, SW620 and HCT116 colon cancer cells as demonstrated in Figure 1A. Furthermore, AC treatment also significantly inhibited colony formation (Figure 1B). We subsequently found that AC treatment significantly induced apoptosis in CRC (SW480, SW620 and HCT116) cells. AC treated samples also exhibited higher percentage of Annexin-V positively stained cells (SW480: 4.6% vs 22.2%; SW620: 10.4% vs 32.8%; HCT116: 6.1% vs 11.8%, control versus AC treatment, respectively) (Figure 1C). The increased apoptosis was associated with increased expression of cleaved PARP (pro-apoptotic marker) (Figure 1D).
Figure 1.
AC treatment decreases the viability of CRC cells by inducing apoptosis. (A) Cell viability assay demonstrates that AC is effectively suppressing the cellular viability in CRC cells. The IC50 values of AC in all three cells (SW480, SW620 and HCT116) are indicated. (B) AC effectively suppresses colony-forming ability. *** p < 0.001. (C) Colon cancer cell lines were treated with AC (100 µg/mL) for 24 h and then analyzed for apoptosis by flow cytometer for Annexin-V+ and PI+ stained cells. AC significantly induces apoptosis in CRC cells (SW480, SW620 and HCT116). Number in the box indicates the percentage of Annexin-V+ cells (control versus AC treatment, left and right respectively). (D) Western blots of the whole-cell lysates from AC treated colon cancer cells showed a significantly increased level of cleaved PARP (pro-apoptotic marker).
3.2. AC Suppresses the Tumorsphere Formation and Reduces the ALDH1+ and Side Population of Stem Likes Cells
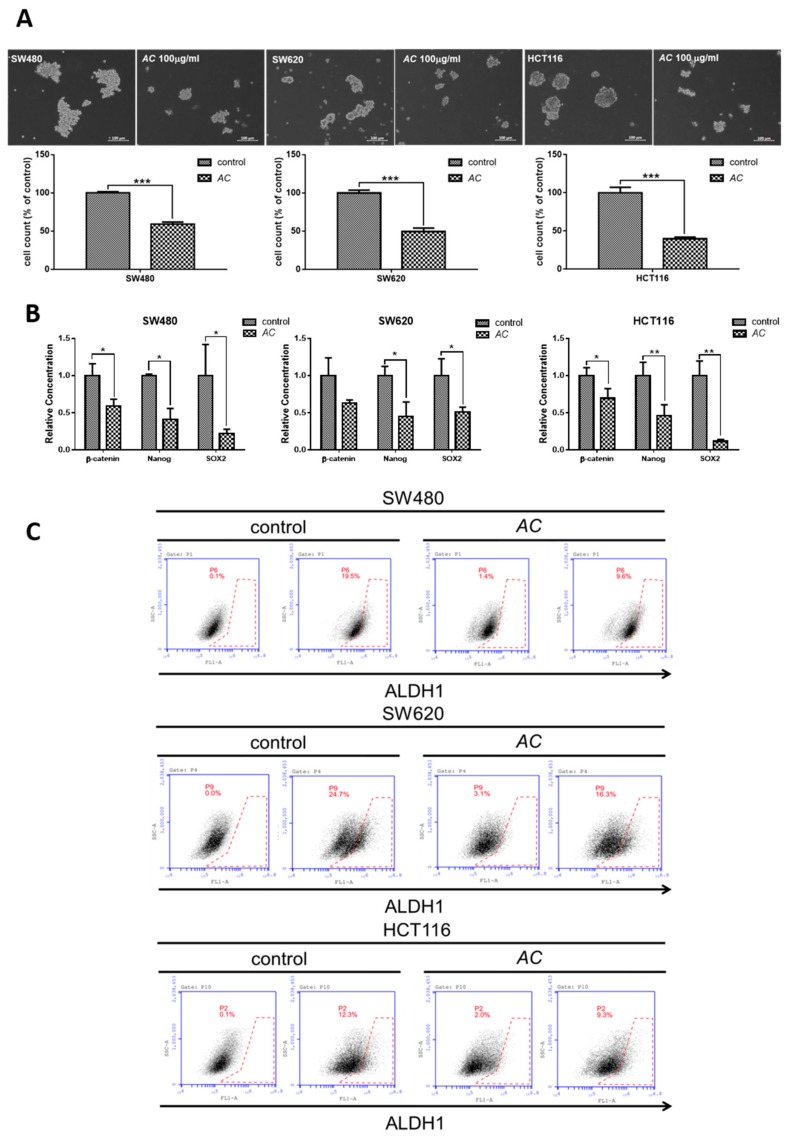
CSC’s are a group of cells within a tumor mass having the sphere-forming and high self-renewal ability, resulting in chemo-resistant properties [28]. ALDH1+ cells expression associated with cancer recurrence and poor prognosis in human cancers stems cells [29]. We first identified that AC suppresses the CRC cells self-renewal and tumor initiation ability when grown under the serum-starved culture condition as exhibited by significantly reduced colon sphere-forming capacity (Figure 2A). Anti-cancer effect of AC acting on CSC’s was related with reduced expression of cancer stem cell markers such as β-catenin, SOX-2, and Nanog (stemness marker) in comparison to control (Figure 2B). We subsequently showed SW480, SW620 and HCT116 colon cancer cells treated with AC (100 µg/mL, 48 h) prominently and dose-dependently reduced the ALDH1 activity (Figure 2C).
Figure 2.
Suppression of cancer stemness by AC treatment. (A) Tumor sphere formation assay. Colon cancer cells treated with AC (100 µg/mL, 48 h) demonstrated a significant reduction in number of tumor spheres generated under serum-deprived culture conditions. *** p < 0.001. (B) q-PCR analysis showed that AC treatment down-regulated cancer stem cell markers β-catenin, SOX-2, Nanog. ** p < 0.01, * p < 0.05. (C) Aldefluor assay showed that AC treatment (100 µg/mL, 48 h) prominently and dose-dependently reduces the ALDH1 enzymatic activity in all three colon cancer cell lines examined.
3.3. AC Treatment Increased 5-FU Sensitivity In Vitro
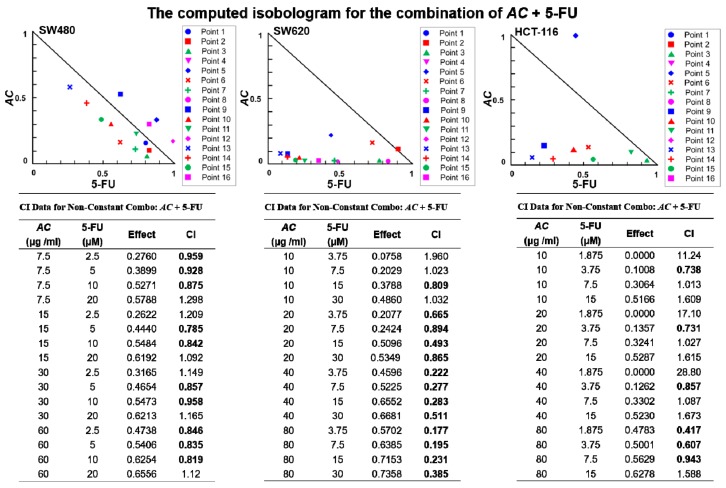
AC has been shown to have adjunctive effects of enhancing radiation therapy in oesophagal cancer patients [30]. Until now, the effect of AC on colon cancer cells has not been yet explored. For a better understanding of the effects of AC on patients with colon cancer, we applied Chou-Talalay’s combination index (CI) theorem for evaluation of combination effects [31] of AC and 5-FU. Furthermore, we examined the chances of AC synergies with 5-FU to suppress the colon cancer cells viability. Isobolograms were generated and assayed by applying different concentrations combination of AC and 5-FU, according to their IC50 values at which drugs were equipotent on respective cell lines. Data analyzed using CompuSyn an open-source software as per their explained methods. Several combinations of AC and 5-FU found to inhibit colon cancer (SW480, SW620 and HCT116) cells viability synergistically (CI index < 1, in bold, Figure 3).
Figure 3.
AC treatment increased 5-FU sensitivity in colon cancer cells. Drug combination assay, different concentrations of AC and 5-FU were used in combination for calculating the combination index (CI). Normalized isobolograms demonstrated a combination of AC and 5-FU synergistically suppressed the cell viability of colon cancer cells. CI values < 1 denotes synergy.
3.4. AC Treatment Effect and Colon Cancer Signaling Pathway
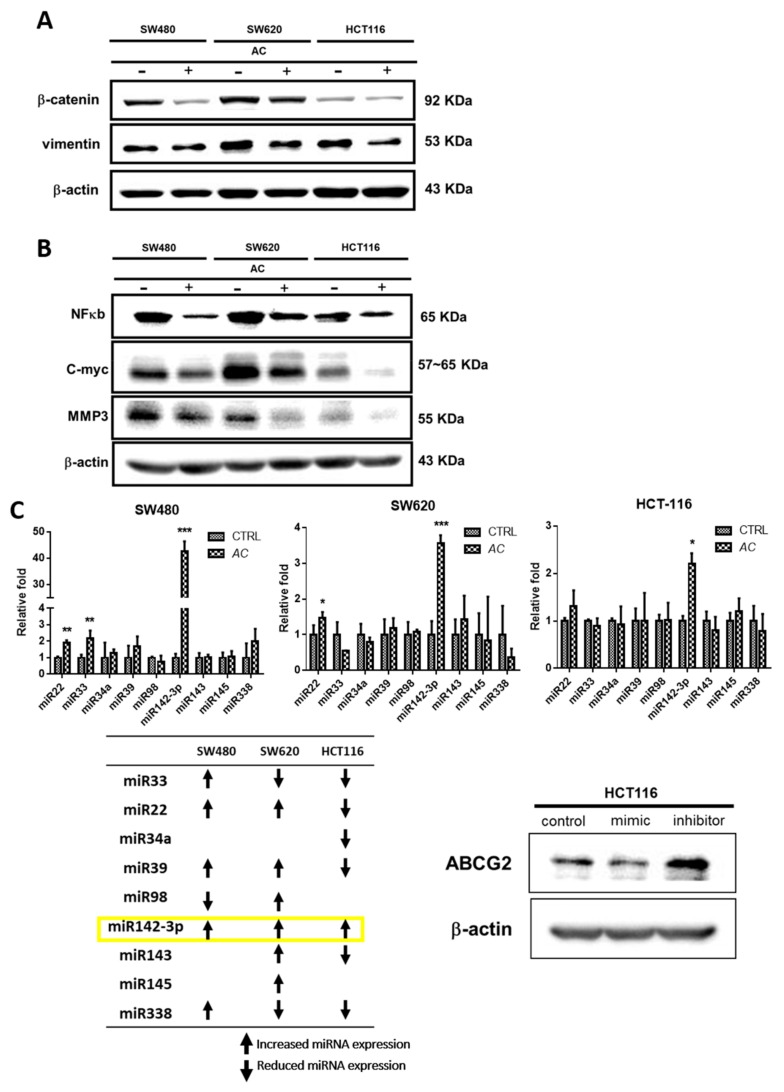
Colorectal cancer connected with a higher recurrence rate and metastasis. Association between inflammatory gene and colorectal cancer has been shown through epidemiological data. A critical feature of these cancer cells has the ability to disintegrate the extracellular matrix (ECM) and basement membranes, largely mediated by matrix metalloproteinases (MMPs) [32]. Previous studies also have shown AC plays an important role in anti-inflammatory and anti-cancer in different cancers and animal models [19,33]. Our results also determine that AC mediated anti-cancer effects in CRC’s were associated with the decreased expression of EMT related β-catenin and vimentin genes (Figure 4A). Additionally, AC treatment also inhibited the expression of NF-κb and C-myc; and MMP3 across all three-colon cancer cell lines (Figure 4B). Furthermore, screening of miRNAs regulating gene expression after AC treatment was validated by q-PCR (Figure 4C). Increased miRNA expression, especially miR142-3p was expressed in colon cancers cells after AC treatment, and ABCG2 genes over-expression is importantly correlated with multidrug resistance mechanism associated with tumorigenic stem cells [34]. ABCG2 expression observed negatively regulated by miR142-3p (Figure 4C). Collectively, AC treatment on CRC cells induces the miR142-3p expression and it negatively regulates ABCG2.
Figure 4.
Impact of AC on the signaling pathway of CRC cell lines. AC (100 μg/mL) treatment were given to CRC cells for 24 h. (A) AC significantly decreases the EMT associated β-catenin and vimentin gene expression, (B) suppression of oncogenic markers NF-κb, C-myc and MMP3, and (C) upregulation of miRNA expression, specially miR142-3p, and miR142-3p expression negatively regulates ABCG2. * p < 0.05, ** p < 0.01 and *** p < 0.001.
3.5. AC treatment Inhibits Tumor-Initiating Capacity
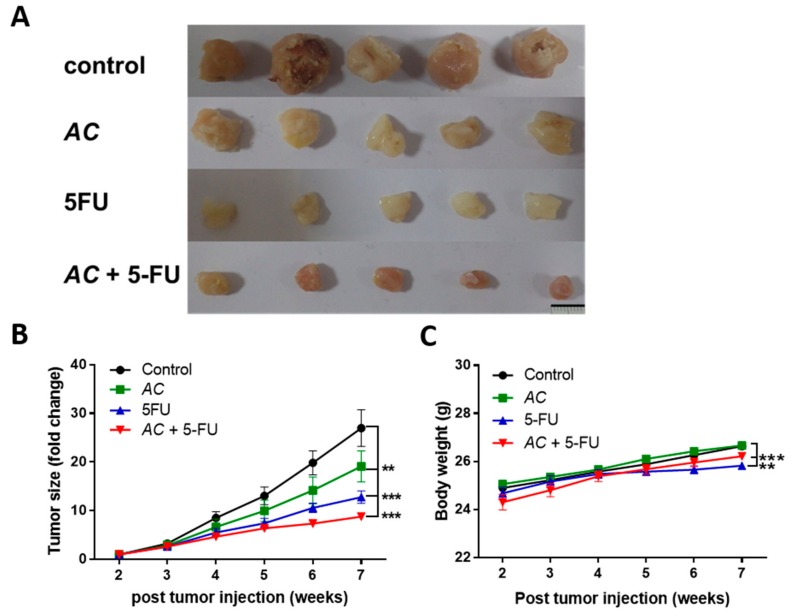
AC enhanced inhibition of tumor-initiating capability tested in vivo. Naïve DLD-1 colon cancer cells (1 × 106 cells/injection) were subcutaneously injected into NOD/SCID mice to establish the xenograft models. After weeks of follow-up, mice were grouped into four different groups when tumor size became noticeable based on their treatment stratification. We found that combine treatment of AC + 5-FU group has got the best effect of tumor growth inhibition in comparison with other treatment arms. The size of tumor was measured for each treatment groups; a significantly lower tumor size was found in combines AC + 5-FU treatment group, reflecting the combination treatment strategy suppressed tumorigenesis (Figure 5A). This was also reflected in the changes in tumor weight across the treatment arms (Figure 5B).
Figure 5.
In-vivo tumor inhibitory effect of AC. (A) Photographs of human colon cancer cells, DLD-1 (1 × 106 cells/injection, subcutaneous) were injected into NDO/SCD mice for establishing tumor xenograft model. When tumor size became palpable, mice were separated into four groups: Vehicle control, AC (50 mg/Kg), 5-FU (10 mg/kg), and combination AC + 5-FU (30 mg/kg 5-FU + 10 mg/kg AC). (B) Evaluation of tumor suppressive effect, synergistic effect of AC + 5-FU significantly decreases the size of tumor in the combination treatment group followed by AC and 5-FU individual treatment. (C) Averaged body weight of the animals, there is no negative impact of treatment on the body weight of animals, except for the 5-FU group. * p < 0.05, ** p < 0.01, and *** p < 0.001.
4. Discussion
To enhance the tumoricidal effect, but at the same time limiting the resistance to chemotherapy has long been approach and the primary task in cancer therapy. Numerous findings and studies have given the clue that some of the natural products holds the potential to achieve these objectives [35,36]. AC has been associated to inhibit liver cancer cells proliferation when used in combination with chemo-agent through suppression of Multi-Drug Resistance (MDR) genes expression and inhibition of COX-2 dependent pathway of phospho-AKT (p-AKT) [37]. AC treatment described to possess anti-cancer activities, but specific focus on its inhibitory effect on colon cancer stemness and drug resistance, and its modelling with a potential clinical application with 5-FU had seldom been reported.
Escape of apoptosis is one of the key hallmarks of cancers that contribute to the cancer progression, as well as drug resistance in cancer [38]. Today’s researchers are more focused on finding natural products with apoptosis-facilitating potentials because that might have the likelihood in the development of medications for cancer and inflammatory diseases [39]. Preventing the creation of oxidative stress and cell death fund to be associated with high levels of AC containing flavonoids, terpenoids and polyphenolics [40]. Besides, AC regulation on the anti-apoptotic pathway was reported to include PI3K/Akt and activator of transcription (STAT), through the over-expressed EGFR in colorectal tumors [41].
Our study results show, AC significantly inhibits the growth and development of colon cancer cells in vitro (Figure 1A). Treatment with AC subsequently induces the apoptosis of colon cancer cells (SW480, SW620, and HCT 116) as demonstrated with Annexin-V assay (Figure 1C). The increased apoptosis was directly associated with increased expression of cleaved PARP (pro-apoptotic marker) (Figure 1D). Traditional cancer treatment is applying chemotherapy to inhibit and induce apoptosis of the hastily proliferating cancer cells [42]. However, cancers grown from a clone of heterogeneous malignant cell subpopulations with diverse characteristics and functions do escape our immunological surveillance, which resultants leads to cancer recurrence and metastasis after the primary response to chemotherapy [43,44]. CSC’s theory provides an alternative elucidation for the above treatment refractoriness of several cancers [45]. CSC’s are the populations of cancer cells with stem cell-like features are tumorigenic, self-renewing and more resistant to chemotherapeutic agents than other cancer cells [46,47]. Considering the emergent role of colon cancer stemness in chemotherapeutic resistance, these markers have become new therapeutic targets for colon cancer patients. The stemness of colon cancer stem cells were found to be associated with higher expression of CD133, ALDH, NANOG, OCT4 and SOX-2, and these markers are found connected to the drug resistance of cancer cells [48,49,50,51,52].
In this study, decreased the number and inhibited self-renewal capacity of colon tumor spheres were seen for AC treated group under serum-deprived condition (Figure 2A). These AC mediated anti-cancer effects were related to the decreased expression of cancer-associated stemness marker β-catenin, SOX-2, and Nanog (Figure 2B). Furthermore, AC also down-regulates the EMT-related gene such as vimentin and MMP3 (Figure 4A) with the upregulation of miRNA expression of miR142-3p across all the three colon cancer cells (Figure 4C), implicating the key regulatory function of miR142-3p in colon cancer stemness and metastasis. The combination therapy strategy where natural agents and chemotherapy agents are combined is a cornerstone of current cancer therapy has gained wide acceptance and popularity these days. For example, combination of Curcumin had shown to enhance the growth inhibition effect of FOLFOX in colon cancer cells [53]. Combination of Nigella sativa seed oil extract with novel octahydropyrazino[2,1-a:5,4-a′]diisoquinoline derivative (OM-90) represents stronger efficacy and enhances the anticancer effect in gastric cancer patients [54]. Resveratrol, a natural cell proliferation inhibitor, works in synergy with 5-FU thus decreasing cancer cell viability and inducing apoptosis [55]. Combination therapy allows targeting to the multiple pathways involved in cancer and applying many different mechanisms to reduce the development of drug resistance [56]. By combining two therapeutic agents into a cancer therapy may results in a synergistic effect, reduced (antagonistic), or identical (additive) effect in comparison to their individual treatment effects. How does the researcher’s make a prediction? It is very important for researchers to assess the interaction mathematically between compounds before actual in vivo or clinical use. Chou-Talay combination index (CI) was applied based on the equation such as CI = a/A + b/B [57]. It is interpreted as an additive interaction between two drugs when CI is equal to 1, synergism when CI < 1, and antagonism when CI > 1 [58]. Here, we found that the combined use of AC increased the 5-FU sensitivity in colon cancer cells. Normalized isobolograms demonstrated that the combination of AC and 5-FU synergistically suppress the colon cancer cell viability (Figure 3). Similarly, the result of in-vivo experiments in the mouse model also reflected the same outcome, that when a combination of AC with 5-FU used it produced the significant tumor inhibitory effect (Figure 5A–C).
5. Conclusions
The results show that AC is a powerful synergistic adjuvant, it down-regulates numerous cancer stemness associated genes and augments antitumor efficacy of 5-FU by triggering the expression of apoptosis-related genes in colon cancer cells, as well as suppressing inflammation and metastasis-related genes through miR142-3p, thus maximizing the therapeutic potential for patients with colon cancer.
Acknowledgments
We are very grateful to the valuable and excellent technical supports from Hui-Hsien Chiang, Jhang Cian-Ling and Po-Yang Huang.
Supplementary Materials
The following are available online at https://www.mdpi.com/2218-273X/9/8/306/s1, Table S1: Primer sequences of microRNA.
Author Contributions
Conceptualization, Y.H. and T.H.; data curation, Y.H.; formal analysis, Y.H., V.K.Y. and T.H.; funding acquisition, Y.H. and T.H.; investigation, Y.H. and V.K.Y.; methodology, Y.H. and V.K.Y.; project administration, A.T.W. and T.H.; resources, Y.H., A.T.W., P.W., C.F.H. and T.H.; supervision, A.T.W., T.H., C.-Y.F.H. and T.H.; validation, A.T.W.; visualization, P.S., A.T.W., T.H. and P.W.; writing—original draft, Y.H., V.K.Y., P.S. and A.T.W.; writing—review & editing, V.K.Y. and A.T.W.
Funding
The study was supported by the following grants to Tse-Hung Huang CMRPG2G0331, CMRPG2G0332, CMRPG2F0141, CMRPG2F0142, CMRPG2H0121 and MOST 107-2320-B-182A-00; Yan-Jiun Huang is supported by TMU106-AE1-B47 and MOST 108-2314-B-038-013.
Conflicts of Interest
The authors declare no conflicts of interest associated with this publication.
References
- 1.Arnold M., Sierra M.S., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 2.Kanwar S.S., Poolla A., Majumdar A.P. Regulation of colon cancer recurrence and development of therapeutic strategies. World J. Gastrointest. Pathophysiol. 2012;3:1–9. doi: 10.4291/wjgp.v3.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abulafi A.M., Williams N.S. Local recurrence of colorectal cancer: The problem, mechanisms, management and adjuvant therapy. BJS. 1994;81:7–19. doi: 10.1002/bjs.1800810106. [DOI] [PubMed] [Google Scholar]
- 4.Ryuk J.P., Choi G.-S., Park J.S., Kim H.J., Park S.Y., Yoon G.S., Jun S.H., Kwon Y.C. Predictive factors and the prognosis of recurrence of colorectal cancer within 2 years after curative resection. Ann. Surg. Treat. Res. 2014;86:143–151. doi: 10.4174/astr.2014.86.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moharil R.B., Dive A., Khandekar S., Bodhade A. Cancer Stem Cells: An Insight. J. Oral Maxillofac. Pathol. JOMFP. 2017;21:463. doi: 10.4103/jomfp.JOMFP_132_16. (In English) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prieto-Vila M., Takahashi R.-U., Usuba W., Kohama I., Ochiya T. Drug Resistance Driven by Cancer Stem Cells and Their Niche. Int. J. Mol. Sci. 2017;18:2574. doi: 10.3390/ijms18122574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pejin B., Iodice C., Bogdanović G., Kojić V., Tešević V. Stictic acid inhibits cell growth of human colon adenocarcinoma HT-29 cells. Arab. J. Chem. 2017;10:S1240–S1242. doi: 10.1016/j.arabjc.2013.03.003. [DOI] [Google Scholar]
- 8.Pejin B., Kojic V., Bogdanovic G. An insight into the cytotoxic activity of phytol at in vitro conditions. Nat. Prod. Res. 2014;28:2053–2056. doi: 10.1080/14786419.2014.921686. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y.-J., Thang M.W.C., Chan Y.-T., Huang Y.-F., Ma N., Yu A.L., Wu C.-Y., Hu M.-L., Chiu K.P. Global assessment of Antrodia cinnamomea-induced microRNA alterations in hepatocarcinoma cells. PLoS ONE. 2013;8:e82751. doi: 10.1371/journal.pone.0082751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yue P.Y., Wong Y.Y., Chan T.Y., Law C.K., Tsoi Y.K., Leung K.S. Review of biological and pharmacological activities of the endemic Taiwanese bitter medicinal mushroom, Antrodia camphorata (M. Zang et C. H. Su) Sh. H. Wu et al. (higher Basidiomycetes) Int. J. Med. Mushrooms. 2012;14:241–256. doi: 10.1615/IntJMedMushr.v14.i3.20. [DOI] [PubMed] [Google Scholar]
- 11.Yeh C.-T., Rao Y.K., Yao C.-J., Yeh C.-F., Li C.-H., Chuang S.-E., Luong J.H.T., Lai G.-M., Tzeng Y.-M. Cytotoxic triterpenes from Antrodia camphorata and their mode of action in HT-29 human colon cancer cells. Cancer Lett. 2009;285:73–79. doi: 10.1016/j.canlet.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh Y.C., Rao Y.K., Wu C.C., Huang C.Y., Geethangili M., Hsu S.L., Tzeng Y.M. Methyl antcinate A from Antrodia camphorata induces apoptosis in human liver cancer cells through oxidant-mediated cofilin- and Bax-triggered mitochondrial pathway. Chem. Res. Toxicol. 2010;23:1256–1267. doi: 10.1021/tx100116a. [DOI] [PubMed] [Google Scholar]
- 13.Tsai W.C., Rao Y.K., Lin S.S., Chou M.Y., Shen Y.T., Wu C.H., Geethangili M., Yang C.C., Tzeng Y.M. Methylantcinate A induces tumor specific growth inhibition in oral cancer cells via Bax-mediated mitochondrial apoptotic pathway. Bioorg. Med. Chem. Lett. 2010;20:6145–6148. doi: 10.1016/j.bmcl.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Gokila Vani M., Kumar K.J., Liao J.W., Chien S.C., Mau J.L., Chiang S.S., Lin C.C., Kuo Y.H., Wang S.Y. Antcin C from Antrodia cinnamomea Protects Liver Cells against Free Radical-Induced Oxidative Stress and Apoptosis In Vitro and In Vivo through Nrf2-Dependent Mechanism. Evid.-Based Complement. Altern. Med. eCAM. 2013:296082. doi: 10.1155/2013/296082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar K.J., Chu F.H., Hsieh H.W., Liao J.W., Li W.H., Lin J.C., Shaw J.F., Wang S.Y. Antroquinonol from ethanolic extract of mycelium of Antrodia cinnamomea protects hepatic cells from ethanol-induced oxidative stress through Nrf-2 activation. J. Ethnopharmacol. 2011;136:168–177. doi: 10.1016/j.jep.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 16.Chang C.W., Chen C.C., Wu M.J., Chen Y.S., Chen C.C., Sheu S.J., Lin T.W., Chou S.H., Lin S.C., Liu C.J., et al. Active Component of Antrodia cinnamomea Mycelia Targeting Head and Neck Cancer Initiating Cells through Exaggerated Autophagic Cell Death. Evid.-Based Complement. Altern. Med. eCAM. 2013:946451. doi: 10.1155/2013/946451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y.Y., Chou P.Y., Chien Y.C., Wu C.H., Wu T.S., Sheu M.J. Ethanol extracts of fruiting bodies of Antrodia cinnamomea exhibit anti-migration action in human adenocarcinoma CL1-0 cells through the MAPK and PI3K/AKT signaling pathways. Phytomed. Int. J. Phytother. Phytopharmacol. 2012;19:768–778. doi: 10.1016/j.phymed.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Liu F.C., Lai M.T., Chen Y.Y., Lin W.H., Chang S.J., Sheu M.J., Wu C.H. Elucidating the inhibitory mechanisms of the ethanolic extract of the fruiting body of the mushroom Antrodia cinnamomea on the proliferation and migration of murine leukemia WEHI-3 cells and their tumorigenicity in a BALB/c allograft tumor model. Phytomed. Int. J. Phytother. Phytopharm. 2013;20:874–882. doi: 10.1016/j.phymed.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y.M., Liu Y.K., Huang P.I., Tsai T.H., Chen Y.J. Antrodia cinnamomea mycelial fermentation broth inhibits the epithelial-mesenchymal transition of human esophageal adenocarcinoma cancer cells. Food Chem. Toxicol. 2018;119:380–386. doi: 10.1016/j.fct.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 20.Dotse E., Bian Y. Isolation of colorectal cancer stem-like cells. Cytotechnology. 2016;68:609–619. doi: 10.1007/s10616-014-9806-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang C.M., Zhou Y.J., Wang R.J., Hu M.L. Anti-angiogenic effects and mechanisms of polysaccharides from Antrodia cinnamomea with different molecular weights. J. Ethnopharmacol. 2009;123:407–412. doi: 10.1016/j.jep.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 22.Vichai V., Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006;1:1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 23.Franken N.A., Rodermond H.M., Stap J., Haveman J., van Bree C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 24.Lee Y.P., Tsai W.C., Ko C.J., Rao Y.K., Yang C.R., Chen D.R., Yang M.H., Yang C.C., Tzeng Y.M. Anticancer effects of eleven triterpenoids derived from Antrodia camphorata. Anticancer Res. 2012;32:2727–2734. [PubMed] [Google Scholar]
- 25.Lin Y.-S., Lin Y.-Y., Yang Y.-H., Lin C.-L., Kuan F.-C., Lu C.-N., Chang G.-H., Tsai M.-S., Hsu C.-M., Yeh R.-A., et al. Antrodia cinnamomea extract inhibits the proliferation of tamoxifen-resistant breast cancer cells through apoptosis and skp2/microRNAs pathway. BMC Complement. Altern. Med. 2018;18:152. doi: 10.1186/s12906-018-2204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang T.-T., Lan Y.-W., Chen C.-M., Ko Y.-F., Ojcius D.M., Martel J., Young J.D., Chong K.-Y. Antrodia cinnamomea induces anti-tumor activity by inhibiting the STAT3 signaling pathway in lung cancer cells. Sci. Rep. 2019;9:5145. doi: 10.1038/s41598-019-41653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang T.-T., Liu F.-G., Wei C.-F., Lu C.-C., Chen C.-C., Lin H.-C., Ojcius D.M., Lai H.-C. Activation of Multiple Apoptotic Pathways in Human Nasopharyngeal Carcinoma Cells by the Prenylated Isoflavone, Osajin. PLoS ONE. 2011;6:e18308. doi: 10.1371/journal.pone.0018308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butler S.J., Richardson L., Farias N., Morrison J., Coomber B.L. Characterization of cancer stem cell drug resistance in the human colorectal cancer cell lines HCT116 and SW480. Biochem. Biophys. Res. Commun. 2017;490:29–35. doi: 10.1016/j.bbrc.2017.05.176. [DOI] [PubMed] [Google Scholar]
- 29.Yasgar A., Titus S.A., Wang Y., Danchik C., Yang S.-M., Vasiliou V., Jadhav A., Maloney D.J., Simeonov A., Martinez N.J. A High-Content Assay Enables the Automated Screening and Identification of Small Molecules with Specific ALDH1A1-Inhibitory Activity. PLoS ONE. 2017;12:e0170937. doi: 10.1371/journal.pone.0170937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y.-M., Liu Y.-K., Wang L.-W., Huang Y.-C., Huang P.-I., Tsai T.-H., Chen Y.-J. The medicinal fungus Antrodia cinnamomea regulates DNA repair and enhances the radiosensitivity of human esophageal cancer cells. OncoTargets Ther. 2016;9:6651–6661. doi: 10.2147/OTT.S96355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chou T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 32.Man S., Gao W., Zhang Y., Liu Z., Yan L., Huang L., Liu C. Formosanin C-inhibited pulmonary metastasis through repression of matrix metalloproteinases on mouse lung adenocarcinoma. Cancer Biol. Ther. 2011;11:592–598. doi: 10.4161/cbt.11.6.14668. [DOI] [PubMed] [Google Scholar]
- 33.Lee M.T., Lin W.C., Wang S.Y., Lin L.J., Yu B., Lee T.T. Evaluation of potential antioxidant and anti-inflammatory effects of Antrodia cinnamomea powder and the underlying molecular mechanisms via Nrf2- and NF-kappaB-dominated pathways in broiler chickens. Poult. Sci. 2018;97:2419–2434. doi: 10.3382/ps/pey076. [DOI] [PubMed] [Google Scholar]
- 34.Dean M., Fojo T., Bates S. Tumour stem cells and drug resistance. Nat. Rev. Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 35.Wu C.P., Ohnuma S., Ambudkar S.V. Discovering natural product modulators to overcome multidrug resistance in cancer chemotherapy. Curr. Pharm. Biotechnol. 2011;12:609–620. doi: 10.2174/138920111795163887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang P., Yang H.L., Yang Y.J., Wang L., Lee S.C. Overcome Cancer Cell Drug Resistance Using Natural Products. Evid.-Based Complement. Altern. Med. eCAM. 2015:767136. doi: 10.1155/2015/767136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang C.Y., Huang Z.N., Yu H.H., Chang L.H., Li S.L., Chen Y.P., Lee K.Y., Chuu J.J. The adjuvant effects of Antrodia Camphorata extracts combined with anti-tumor agents on multidrug resistant human hepatoma cells. J. Ethnopharmacol. 2008;118:387–395. doi: 10.1016/j.jep.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Fernald K., Kurokawa M. Evading apoptosis in cancer. Trends Cell Biol. 2013;23:620–633. doi: 10.1016/j.tcb.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Favaloro B., Allocati N., Graziano V., Di Ilio C., De Laurenzi V. Role of apoptosis in disease. Aging. 2012;4:330–349. doi: 10.18632/aging.100459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiang P.C., Lin S.C., Pan S.L., Kuo C.H., Tsai I.L., Kuo M.T., Wen W.C., Chen P., Guh J.H. Antroquinonol displays anticancer potential against human hepatocellular carcinoma cells: A crucial role of AMPK and mTOR pathways. Biochem. Pharmacol. 2010;79:162–171. doi: 10.1016/j.bcp.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 41.Herbst R.S. Review of epidermal growth factor receptor biology. Int. J. Radiat. Oncol. Biol. Phys. 2004;59(Suppl. 2):21–26. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 42.Gerl R., Vaux D.L. Apoptosis in the development and treatment of cancer. Carcinogenesis. 2005;26:263–270. doi: 10.1093/carcin/bgh283. [DOI] [PubMed] [Google Scholar]
- 43.Mimeault M., Hauke R., Mehta P.P., Batra S.K. Recent advances in cancer stem/progenitor cell research: Therapeutic implications for overcoming resistance to the most aggressive cancers. J. Cell. Mol. Med. 2007;11:981–1011. doi: 10.1111/j.1582-4934.2007.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goranova T.E., Ohue M., Shimoharu Y., Kato K. Dynamics of cancer cell subpopulations in primary and metastatic colorectal tumors. Clin. Exp. Metastasis. 2011;28:427–435. doi: 10.1007/s10585-011-9381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donnenberg V.S., Donnenberg A.D. Multiple drug resistance in cancer revisited: The cancer stem cell hypothesis. J. Clin. Pharmacol. 2005;45:872–877. doi: 10.1177/0091270005276905. [DOI] [PubMed] [Google Scholar]
- 46.Neuzil J., Stantic M., Zobalova R., Chladova J., Wang X., Prochazka L., Dong L., Andera L., Ralph S.J. Tumour-initiating cells vs. cancer ‘stem’ cells and CD133: What’s in the name? Biochem. Biophys. Res. Commun. 2007;355:855–859. doi: 10.1016/j.bbrc.2007.01.159. [DOI] [PubMed] [Google Scholar]
- 47.Scopelliti A., Cammareri P., Catalano V., Saladino V., Todaro M., Stassi G. Therapeutic implications of Cancer Initiating Cells. Expert Opin. Biol. Ther. 2009;9:1005–1016. doi: 10.1517/14712590903066687. [DOI] [PubMed] [Google Scholar]
- 48.Ricci-Vitiani L., Lombardi D.G., Pilozzi E., Biffoni M., Todaro M., Peschle C., De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 49.Lugli A., Iezzi G., Hostettler I., Muraro M.G., Mele V., Tornillo L., Carafa V., Spagnoli G., Terracciano L., Zlobec I. Prognostic impact of the expression of putative cancer stem cell markers CD133, CD166, CD44s, EpCAM, and ALDH1 in colorectal cancer. Br. J. Cancer. 2010;103:382–390. doi: 10.1038/sj.bjc.6605762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meng H.M., Zheng P., Wang X.Y., Liu C., Sui H.M., Wu S.J., Zhou J., Ding Y.Q., Li J. Over-expression of Nanog predicts tumor progression and poor prognosis in colorectal cancer. Cancer Biol. Ther. 2010;9:295–302. doi: 10.4161/cbt.9.4.10666. [DOI] [PubMed] [Google Scholar]
- 51.Saigusa S., Tanaka K., Toiyama Y., Yokoe T., Okugawa Y., Ioue Y., Miki C., Kusunoki M. Correlation of CD133, OCT4, and SOX2 in rectal cancer and their association with distant recurrence after chemoradiotherapy. Ann. Surg. Oncol. 2009;16:3488–3498. doi: 10.1245/s10434-009-0617-z. [DOI] [PubMed] [Google Scholar]
- 52.Jeter C.R., Liu B., Liu X., Chen X., Liu C., Calhoun-Davis T., Repass J., Zaehres H., Shen J.J., Tang D.G. NANOG promotes cancer stem cell characteristics and prostate cancer resistance to androgen deprivation. Oncogene. 2011;30:3833–3845. doi: 10.1038/onc.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patel B.B., Sengupta R., Qazi S., Vachhani H., Yu Y., Rishi A.K., Majumdar A.P.N. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int. J. Cancer. 2008;122:267–273. doi: 10.1002/ijc.23097. [DOI] [PubMed] [Google Scholar]
- 54.Czajkowska A., Gornowicz A., Pawłowska N., Czarnomysy R., Nazaruk J., Szymanowski W., Bielawski K. Anticancer Effect of a Novel Octahydropyrazino[2,1-a:5,4-a’]diisoquinoline Derivative and Its Synergistic Action with Nigella sativa in Human Gastric Cancer Cells. BioMed Res. Int. 2017:9153403. doi: 10.1155/2017/9153403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buhrmann C., Shayan P., Kraehe P., Popper B., Goel A., Shakibaei M. Resveratrol induces chemosensitization to 5-fluorouracil through up-regulation of intercellular junctions, Epithelial-to-mesenchymal transition and apoptosis in colorectal cancer. Biochem. Pharmacol. 2015;98:51–68. doi: 10.1016/j.bcp.2015.08.105. [DOI] [PubMed] [Google Scholar]
- 56.Housman G., Byler S., Heerboth S., Lapinska K., Longacre M., Snyder N., Sarkar S. Drug resistance in cancer: An overview. Cancers. 2014;6:1769–1792. doi: 10.3390/cancers6031769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chou T.C., Talalay P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 58.Tallarida R.J. The interaction index: A measure of drug synergism. Pain. 2002;98:163–168. doi: 10.1016/S0304-3959(02)00041-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.