Abstract
A dose of proanthocyanidins with satiating properties proved to be able to limit body weight increase several weeks after administration under exposure to a cafeteria diet. Here we describe some of the molecular targets and the duration of the effects. We treated rats with 500 mg grape seed proanthocyanidin extract (GSPE)/kg BW for ten days. Seven or seventeen weeks after the last GSPE dose, while animals were on a cafeteria diet, we used reverse transcriptase-polymerase chain reaction (RT-PCR) to measure the mRNA of the key energy metabolism enzymes from the liver, adipose depots and muscle. We found that a reduction in the expression of adipose Lpl might explain the lower amount of adipose tissue in rats seven weeks after the last GSPE dose. The liver showed increased expression of Cpt1a and Hmgs2 together with a reduction in Fasn and Dgat2. In addition, muscle showed a higher fatty oxidation (Oxct1 and Cpt1b mRNA). However, after seventeen weeks, there was a completely different gene expression pattern. At the conclusion of the study, seven weeks after the last GSPE administration there was a limitation in adipose accrual that might be mediated by an inhibition of the gene expression of the adipose tissue Lpl. Concomitantly there was an increase in fatty acid oxidation in liver and muscle.
Keywords: Long-lasting effect, proanthocyanidin, rat, adiposity, Lpl
1. Introduction
Excessive adipose tissue significantly increases the risk and prognosis of metabolic syndrome (diabetes mellitus type 2, cardiovascular disease, hyperlipidemia, nonalcoholic fatty liver disease) and several types of cancer [1]. The causes of excessive body weight are diverse, one of the most prevalent in developing and developed countries being excessive or bad quality food intake [2].
Proanthocyanidins (PACs) are a group of polyphenols that are widespread in nature (in fruits, vegetables and their beverage products). They have been described as bioactive agents against several unhealthy situations. More specifically, they have the well-documented effect of limiting lipid accumulation and favouring lipid oxidation in the organism [3], and as specific inhibitors of fat digestion [4] and absorption [5]. Furthermore, PACs favour a lower respiratory quotient (RQ) [6,7], due to higher fat oxidation in liver and muscle [6]. As they are a group of different compounds, some of the effects could be explained by their interaction with molecules or structures located in the gastrointestinal lumen [6,8]. They protect against cafeteria diet-induced damage to the intestinal barrier and are anti-inflammatory agents [9]. However, the absorbable low-molecular weight flavanols reach intracellular targets inside the body, where they act on different molecular targets to induce increased energy expenditure [3], and prevent cholesterol increase in the organism [10], acting as antihipertensives [10] and antioxidants [11], and maintaining glucose homeostasis [12].
The diversity of structures in proanthocyanidin-rich extracts and their interactions are the reason why some of these effects are highly dependent on the dose used for the studies and the physiological state of the animal [7]. Most studies prove that PACs correct cafeteria diet-induced damage [13,14]. Some studies focus on their possible preventive role in obesity-related pathologies (that is, when they are administered from the beginning of obesogenic diets) [15]. However, only very few studies have analysed their long-term effects after sub-chronic treatment [16,17]. We have recently attempted to determine the best moment to administer grape seed proanthocyanidin extract (GSPE) (500 mg/kg of body weight-BW-) so that it acts most effectively against the damaging effects of an obesogenic diet such as the cafeteria diet [17]. The results showed that PACs had a surprisingly long-lasting effect on body weight, which needed a more in-depth analysis. In the present study, we have further analysed the long-lasting effects of sub-chronic GSPE treatment. We compared the duration of their effects, mainly on the energy metabolism and adipose depots. As we had evident results on adiposity, seven weeks after the last GSPE dose, we analysed it thoroughly. We checked some remaining core effects on the liver, 17 weeks after the last dose, since the effects on adiposity were already lost.
2. Materials and Methods
2.1. Proanthocyanidin Extract
The grape seed proanthocyanidin extract (GSPE) was kindly provided by Les Dérivés Résiniques et Terpéniques (Dax, France). According to the manufacturer, the GSPE used in this study (Batch number: 124029) contains monomers of flavan-3-ols (21.3%), and dimers (17.4%), trimers (16.3%), tetramers (13.3%) and a 31.7% of structures between 5–13 units of proanthocyanidins. A detailed analysis of the monomeric to trimeric structures can be found in the work by Margalef et al. [18].
2.2. Animal Treatments
Female rats (Harlan Rcc:Han), each weighing 240–270 g, were purchased from Charles River Laboratories (Barcelona, Spain). After one week of adaptation, they were individually caged in the animal quarters at 22 °C with a 12-h light/12-h dark cycle and fed ad libitum with a standard chow diet (Panlab 04, Barcelona, Spain) and tap water. Experiments were performed after a period of acclimation.
2.2.1. Short Cafeteria (SC) Experiment
The animals were randomly distributed into two experimental groups (n = 7) and fed a standard chow diet ad libitum (Figure S1, Supplementary Materials). One group of animals received 500 mg GSPE/Kg BW together with a simplified high-fat-high-sucrose diet for 10 days. The diet consisted of a palatable hypercaloric emulsion presented in an independent bottle, containing (by weight) 10% powdered skimmed milk, 40% sucrose, 4% lard and 0.35% xanthan gum as a stabilizer [19]. The GSPE, dissolved in tap water, was orally gavaged to the animals at 18:00 in a volume of 500 µL, one hour after all the available food had been removed. The animals not supplemented with GSPE received water as a vehicle. After 10 days of treatment, all of the animals were kept for 18 days on a standard chow diet. Afterwards, the rats started with the cafeteria diet challenge for 35 days (SC). The cafeteria diet consisted of standard chow, bacon, carrots, and sugared milk, which induces voluntary hyperphagia [20]. This diet was offered ad libitum every day.
2.2.2. Long Cafeteria (LC) Experiment
A second group of thirty female Wistar rats was challenged with a long-term cafeteria treatment (LC), which was initially similar to the treatment described above. They were organized into three experimental groups (n = 10), (Figure S1, Supplementary Materials). One group was given the same amount of GSPE every day for 10 days at the same time, and the control group received the same amount of tap water. During the GSPE treatment period, all the rats were fed standard chow diet. On day eleven, a standard group (STD) stayed on the chow diet, and the two remaining groups started a cafeteria challenge, which in this case was maintained for 17 weeks.
In both experiments, the GSPE treatments were intragastrically (i.g.) administered 1 h before the onset of the dark cycle. Food intake was measured 20 h after the daily chow replacement with an accuracy of 0.01 g.
2.3. Blood and Tissue Collection
At the end of the study, the rats were anesthetized with sodium pentobarbital (70 mg/kg body weight; Fagron Iberica, Barcelona, Spain) and exsanguinated from the abdominal aorta after 1–4 h since light opened, and food was removed. The blood was collected using heparin (Deltalab, Barcelona, Spain) as an anticoagulant. Plasma was obtained by centrifugation (1500 g, 15 min, 4 °C) and stored at −80 °C until analysis. The different white adipose tissue depots (retroperitoneal (rWAT), mesenteric (mWAT) and periovaric (oWAT)), brown adipose tissue (BAT), liver and pancreas were rapidly removed weighed and stored at −80 °C until analysis
All of the procedures were approved by the Experimental Animal Ethics Committee of the Universitat Rovira i Virgili (code: 0152S/4655/2015).
2.4. Plasma Metabolites and Hormones
Plasma β-hydroxybutyrate was analysed by colorimetry (BEN, Mainz, Alemania). Total ghrelin from plasma samples was analysed with an extraction-free total ghrelin enzyme immunoassay (Phoenix Pharmaceuticals, Burlingame, CA, USA) [21]. Plasma insulin and glucagon levels were analysed with rat ELISA kits (Mercodia, Uppsala, Sweeden). Plasma leptin levels were analysed with an ELISA kit (Millipore, Billerica, MA, USA).
2.5. Tissue Triglycerides and mRNA Quantification
Pancreatic triglycerides were assayed according to Castell et al. [22]. Total RNA was extracted using Trizol (Ambion, Austin, TX, USA) and trichloromethane-ethanol (Panreac, Barcelona, Spain) and purified using an RNA extraction kit (Qiagen, Hilden, Germany). Complementary DNA was obtained using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Madrid, Spain), and the quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) amplification was performed using TaqMan Universal polymerase chain reaction (PCR) Master Mix and the respective specific TaqMan probes (Applied Biosystems, Madrid, Spain). The relative expression of each mRNA was calculated against the control group using the 2−ΔΔCt method, with cyclophilin A as reference.
2.6. Statistical Analysis
The data are shown as the mean ± standard error of the mean (S.E.M.). Statistical comparisons between groups were assessed using the Students’ t-test. Analyses were performed with XLStat 2017.01 (Addinsoft, Barcelona, Spain). p-values < 0.05 were considered statistically significant.
3. Results
3.1. Sub-Chronic Treatment with GSPE Reduces Food Intake in Rats on a Palatable Diet
In previous studies we used a GSPE dose that had satiating properties in animals on a chow diet [7,23]. Here, we reproduced this effect in animals with an enhanced appetite because they were offered a tasty diet. Figure 1a shows a 10% reduction in the total food intake of the healthy females while they were treated with GSPE. Table 1 shows that this reduction was on the amount of hypercaloric emulsion ingested, not on the amount of chow ingested.
Figure 1.
Food intake in the short-cafeteria study in different diet periods. Food intake was measured 20 h after the daily food had been replaced for each diet administered. The black column indicates animals not treated with grape seed proanthocyanidin extract (GSPE). The white column indicates animals treated with 0.5 g/Kg BW of GSPE for the first 10 days of treatment. The results showed the mean data obtained from each measurement throughout the period. (a) Mean food intake from the daily measurement for the first ten days of treatment with a tasty diet. (b) Mean food intake from measurements taken during the eighteen days of treatment with a chow diet. (c) Mean food intake from measurements during the thirty-five days with a cafeteria diet. Statistical differences identified by t-test are defined by * when p < 0.05 between treatments.
Table 1.
Characteristics of food intake during the short cafeteria study.
| Cafeteria | GSPE Pre-Treated Rats | |
|---|---|---|
| Initial treatment: 10 days, tasty diet | ||
| Chow ingested (g) | 8.34 ± 0.4 | 9.10 ± 0.5 |
| Hypercaloric emulsion ingested (g) | 14.01 ± 0.8 | 10.54 ±1.1 * |
| Carbohydrates (Kcal) | 43.04 ± 0.8 | 39.15 ± 0.6 * |
| Lipids (Kcal) | 6.84 ± 0.2 | 5.90 ± 0.2 * |
| Protein (Kcal) | 7.07 ± 0.2 | 7.12 ± 0.2 |
| Final treatment: 35-day cafeteria diet | ||
| Carbohydrates (Kcal) | 43 ± 4.0 | 38 ± 2.0 |
| Lipids (Kcal) | 28 ± 1.0 | 28 ± 0.6 |
| Protein (Kcal) | 8.4 ± 0.3 | 8.0 ± 0.2 |
GSPE was administered for 10 days together with a tasty diet. After the GSPE treatment stopped, the rats were put on an 18-day chow diet and then a 35-day cafeteria diet. *: (p < 0.05 vs. C; t-test).
The effects on food intake disappeared when GSPE administration finished, as previously shown [7]. Figure 1b shows that there was no difference between groups in the total kilocalories (Kcal) ingested over the eighteen days after GSPE treatment, when animals received a standard chow diet. During this period, the rats obtained 68% of energy from CH, 12% from lipids and 20% from protein. When the animals were subsequently subjected to a short (35 days) cafeteria diet, the amount of Kcal ingested was not different between the groups, either (Figure 1c). During this last period of the study, animals obtained 54% ± 2.2 of energy from carbohydrates (CH) (mainly from the sucrose included in the milk), 36% ± 1.9 from lipids and 11% ± 0.4 from protein. As mentioned, these percentages were not statistically different for GSPE treated animals (51% ± 1.0; 38% ± 0.9; 11% ± 0.1, from CH, lipids and protein, respectively).
3.2. A Reduction in the Expression of Adipose Lpl might Explain the Lower Amount of Adipose Tissue in GSPE Pre-Treated Rats
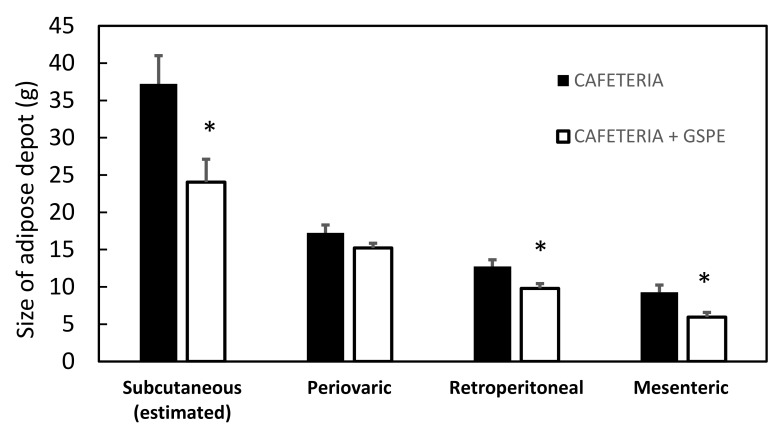
We have shown that a 10-day pre-treatment with GSPE followed by a cafeteria diet led to a reduction in adiposity and RQ, fifty-three days after the last dose of GSPE [17]. Here we analyse each adipose depot size and the expression level of the key enzymes of fatty acid metabolism on most of each. Figure 2 shows that in the GSPE pre-treated group there is a statistically significant reduction of around 35% in the size of subcutaneous depots (estimated by the difference between total adipose contents measured by NMR [17] and the weighed intraabdominal depots). Although the mRNA levels of lipid metabolism genes did not show a clear change, there was a statistically significant effect on the ratio between Cpt1b (carnitine palmitoyltransferase 1b) and Fasn (fatty acid synthase), suggesting a trend towards a higher oxidative profile in the subcutaneous depot (Table 2). The next most abundant WAT depot is the periovaric WAT, which did not show any differences between the control and GSPE groups in either weight or gene expression. Instead, the retroperitoneal and mesenteric depots were of statistically different sizes due to the GSPE treatment (reductions of 23 and 35%, respectively). However, there were no significant changes in the gene expression profile, only a tendency to present lower Fasn mRNA levels in mesenteric WAT (Table 2).
Figure 2.
GSPE effects on white adipose depots in the short cafeteria study. Adipose depots were obtained at the end of the treatment and each depot was weighed. Rats were treated with 0.5 g/Kg BW for the first 10 days, and then they were put on a chow diet for 18 days and a cafeteria diet for 35 days. The black column indicates animals not treated with GSPE. The white column indicates animals treated with 0.5 g/Kg BW of GSPE for the first 10 days of treatment. The data are the mean ± standard error of the mean (S.E.M.) (n = 7). Statistical differences identified by t-test are defined by * when p < 0.05 between treatments.
Table 2.
GSPE effects on mRNA levels of white adipose depots in the short cafeteria study.
| Cafeteria | GSPE Pre-Treated Rats | |
|---|---|---|
| Subcutaneous WAT | ||
| Cpt1b | 1.03 ± 0.09 | 1.09 ± 0.12 |
| Lipe | 0.94 ± 0.09 | 0.70 ± 0.05 # |
| Fasn | 1.03 ± 0.09 | 0.74 ± 0.18 |
| Dgat2 | 0.98 ± 0.08 | 0.90 ± 0.08 |
| Cpt1b/Fasn (ratio between them) | 0.93 ± 0.06 | 1.40 ± 0.15 * |
| Periovaric WAT | ||
| Cpt1b | 1.01 ± 0.07 | 1.17 ± 0.05 |
| Retroperitoneal WAT | ||
| Cpt1b | 1.15 ± 0.20 | 1.27 ± 0.29 |
| Fasn | 1.00 ± 0.08 | 0.95 ± 0.12 |
| Dgat2 | 1.00 ± 0.05 | 0.99 ± 0.09 |
| Cpt1b/Fasn (ratio between them) | 1.26 ± 0.24 | 1.56 ± 0.41 |
| Mesenteric WAT | ||
| Cpt1b | 1.05 ± 0.08 | 0.97 ± 0.1 |
| Fasn | 0.95 ± 0.13 | 0.62 ± 0.1 # |
| Dgat2 | 1.01 ± 0.09 | 0.78 ± 0.16 |
| Cpt1b/Fasn (ratio between them) | 1.22 ± 0.27 | 1.50 ± 0.18 |
Reverse transcriptase-polymerase chain reaction (RT-PCR) was used for each gene (results are presented as arbitrary units versus cafeteria group, except detailed). The T test was used to determine statistical differences highlighted as * p < 0.05 vs. cafeteria treatment; # p < 0.1 vs. cafeteria group. Lipe (lipase E, hormone sensitive type).
Brown adipose tissue was also analysed but there were no changes due to GSPE either in weight (0.91 ± 0.07 for the cafeteria group; 0.77 ± 0.04 for the GSPE-pre-treated group) or in Cpt1b gene expression (1.02 ± 0.0 for the cafeteria group; 1.15 ± 0.2 for the GSPE-pre-treated group) suggesting a lack of effect on oxidative activity in this tissue.
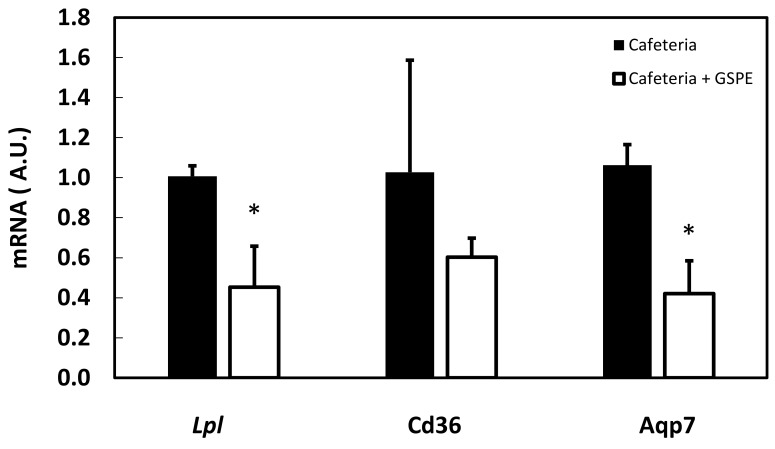
As mesenteric depot was the highest reduced by GSPE treatment, to identify the effects of GSPE on triglyceride entry into adipose tissue, we measured the gene expression of the genes related to this process. We assayed Lpl (lipoprotein lipase), the enzyme that hydrolyses triglycerides into fatty acids and glycerol, before their uptake into the cell; Cd36 (CD36 molecule), involved in free fatty acid uptake into the cell; and Aqp7 (aquaporin 7), which facilitates the efflux of glycerol from the cell. Figure 3 shows that the amount of Lpl in the mesenteric depot was highly reduced, as was the amount of Aqp7. There were no statistically significant differences for the fatty acid transporter (Cd36).
Figure 3.
Effect of GSPE pre-treatment on mesenteric adipose gene expression in the short-cafeteria study at the end of the study. Rats were treated with 0.5 g/Kg BW for the first 10 days, and then they were put on a chow diet for 18 days and a cafeteria diet for 35 days. The black column indicates animals not treated with GSPE. The white column indicates animals treated with 0.5 g/Kg BW of GSPE for the first 10 days of treatment. The mRNA extracted from mesenteric adipose was quantified by RT-PCR and the relative gene expression of Lpl, Cd36 and AqpP7 was obtained by the DDCt method in each gene. The data are the mean ± S.E.M. (n = 7). Statistical differences identified by t-test are defined by * when p < 0.05 between treatments.
3.3. Lipid Oxidation in Liver and Muscle Is Higher
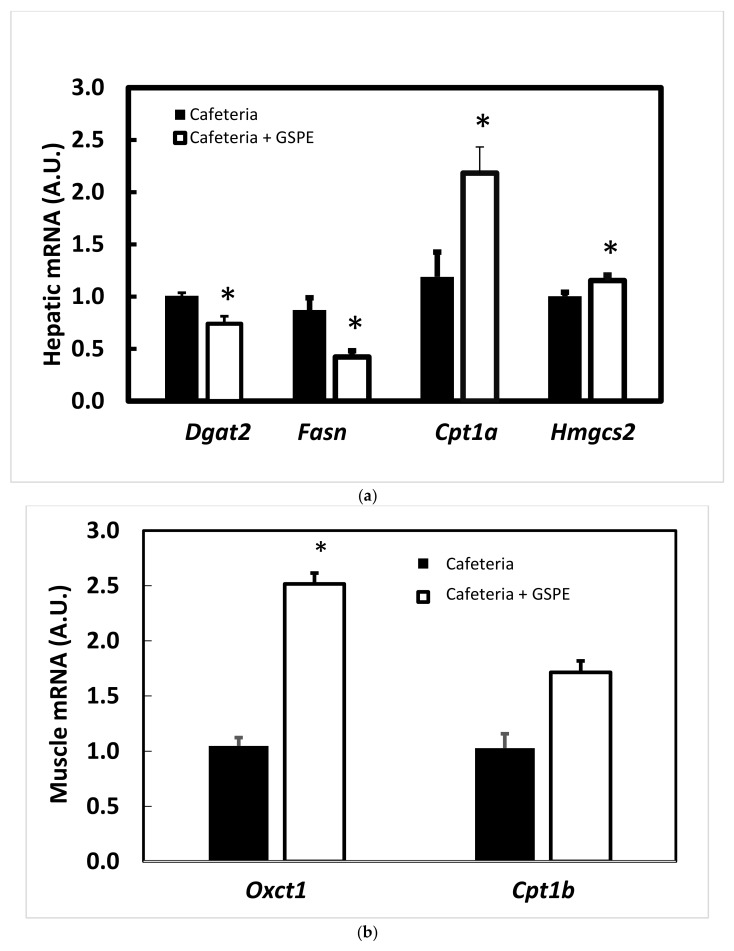
In our search for an explanation for the lower RQ observed in GSPE pre-treated rats [17], we analysed liver and muscle gene expressions. Figure 4a shows a significant increase in Cpt1a (carnitine palmitoyltransferase 1a) and Hmgcs2 (3-hydroxy-3-methylglutaryl-CoA synthase 2), suggesting the increased oxidation of fatty acids and the active synthesis of ketone bodies in the liver of GSPE-pre-treated animals. In addition, decreased Fasn and Dgat 2 (diacylglycerol O-acyltransferase 2) expression suggested decreased synthesis and esterification of fatty acids.
Figure 4.
Effect of GSPE pre-treatment on gene expression in the short cafeteria study at the end of the study. Rats were treated with 0.5 g/Kg BW for the first 10 days, and then they were put on a chow diet for 18 days and a cafeteria diet for 35 days. The black column indicates animals not treated with GSPE. The white column indicates animals treated with 0.5 g/Kg BW of GSPE for the first 10 days of treatment. The mRNA extracted from liver was quantified by RT-PCR and the relative gene expression detailed gens was obtained by the DDCt method in each gene. Figure 4a resumes liver results. Figure 4b summarizes muscle gene expression. The data are the mean ± S.E.M. (n = 7). Statistical differences identified by t-test are defined by * when p < 0.05 between treatments.
Plasma ketone bodies were not significantly modified (control: 4.14 ± 0.35; GSPE: 3.89 ± 0.56; mM), so we measured the extent to which they could be oxidized by muscle. Figure 4b shows a strong significant increase in Oxct1 (3-oxoacid CoA transferase 1) due to GSPE treatment, concomitantly with a tendency to increased Cpt1b, which suggests that ketone bodies and fatty acids are the energy source in the muscle.
3.4. Hormonal Status of GSPE-Treated Rats after the Short-Cafeteria Study
Next, we analysed the effects of GSPE on key hormones for the regulation of energetic homeostasis, seven weeks after finishing the GSPE treatment.
There were no statistical differences in insulin plasma levels [17], but we checked the effects on mRNA in pancreas. GSPE pre-treatment did not change insulin mRNA levels (1.16 ± 0.25 in controls; 0.66 ± 0.11 in the GSPE pre-treated group; A.U.) but showed a tendency towards a lower glucagon mRNA (1.16 ± 0.23; 0.60 ± 0.14; A. U.; p = 0.06). The amount of triglycerides (TG) in pancreas was not modified in GSPE pre-treated rats (29.66 ± 2.21 in the control group and 27.10 ± 4.21 in the GSPE pre-treated group- μg TG/ mg tissue).
GSPE pre-treatment statistically increased the plasma levels of total ghrelin (ng/mL; control: 4.09 ± 0.15; GSPE: 6.10 ± 0.42; p < 0.05), although stomach mRNA levels of this hormone were not modified by GSPE pre-treatment (control: 1.04 ± 0.16; GSPE: 1.05 ± 0.12). In addition, GSPE pre-treatment led to a trend towards lower leptinemia (ng/mL; control: 28.0 ± 4.04; GSPE: 18.02 ± 2.15; p = 0.07).
3.5. Duration of GSPE Effects
Finally, to estimate the duration of some of the effects described above, we analysed several parameters after a longer (17 weeks) cafeteria challenge.
The effects on total adiposity and visceral adiposity were lost [17], and the gene expression profile in the liver differed considerably from that found in the short-cafeteria study (Table 3). GSPE pre-treatment led to an increase in Fasn and Dgat2 and a decrease in Cpt1a compared to the cafeteria treatment. This profile resembled that of the standard group of animals.
Table 3.
Effects of GSPE on liver seventeen weeks after treatment (long cafeteria study).
| Chow Diet | Cafeteria Diet | GSPE Pre-Treated Rats | |
|---|---|---|---|
| Cpt1a (A.U.) | 0.34 ± 0.12 * | 1.01 ± 0.08 | 0.35 ± 0.09 * |
| Fasn (A.U.) | 4.24 ± 0.80 * | 0.92 ± 0.18 | 2.69 ± 0.87 # |
| Dgat2 (A.U.) | 1.23 ± 0.10 | 1.04 ± 0.12 | 1.36 ± 0.07 * |
Liver samples were obtained at the end of the treatment. RT-PCR was used for each gene. The t-test was used to determine statistical differences highlighted as * p < 0.05 vs. Cafeteria treatment; #: p < 0.1 vs. cafeteria group.
The insulin/glucagon ratio of plasma levels in the GSPE pre-treated animals differed significantly from that of the cafeteria-fed animals and produced a relationship closer to that of the standard-fed group (Chow: 0.86 ± 0.14 *; cafeteria: 0.39 ± 0.10; GSPE: 0.95 ± 0.19 *; *: p < 0.05 vs. cafeteria group).
4. Discussion
Grape-seed derived proanthocyanidins have been extensively studied, but few studies use doses that are higher than can be provided by standard food ingestion but which may be interesting for a potential functional food. We showed that a dose of 0.5 g GSPE/kg BW has satiating properties in healthy rats [7] and limits adipose accumulation induced by a cafeteria diet [17]. We have also shown that GSPE maintains some of its antiobesogenic effects for a long period after GSPE administration finishes. In the present study, we analysed the mechanisms that explain this. Here, we show that GSPE limits adipose fat pad accumulation until seven weeks after the last GSPE administration due to reduced expression in the adipose tissue Lpl. An increase in fatty acid oxidation in liver and muscle compensates for the inability of fatty acids to accumulate in WAT. All of these effects had been lost at seventeen weeks after the last GSPE dose.
First, we showed that GSPE also inhibits food intake if the diet is a tasty one (energy dense). During the ten-day GSPE treatment, these animals reduced energy intake by 10% in comparison to the control group. Furthermore, these rats gained 30% less weight than the control (cafeteria) group [17]. These results confirm that GSPE effects on food intake are additive to slimming effects, because the lipid oxidation of GSPE is activated [7]. In our short-cafeteria experiment, after GSPE intake, animals changed to a chow diet, and showed no changes in body weight accrual. Unexpectedly, when the rats were again fed a cafeteria diet (still without GSPE), the differences in body weight reappeared. These differences were around 40% between GSPE pre-treated animals and the control group, which correlated with the lower adiposity (79%) [17], and there were no differences in either the quantity or quality of food intake. Our results, therefore, show that GSPE only has long-lasting anti-obesity effects under exposure to a high fat and/or a high sucrose diet.
A key element in triglyceride food-derived storage is the adipose lipoprotein lipase (Lpl). GSPE pre-treatment limited the amount of Lpl mRNA in mesenteric adipose tissue, which suggested an impairment of triglyceride storage in this tissue. Lpl has been shown to be a target for GSPE by Del Bas et al. [24]. They showed that five hours after an acute dose of 250 mg GSPE/kg BW there was a reduction in adipose Lpl mRNA. Similarly, Yoon et al. showed that Allomyrina dichotoma larvae treatment limits adipose tissue growth in mice fed a high-fat diet concomitantly to a reduction on Lpl mRNA [25]. However, adipose Lpl limitation by itself is not sufficient to explain all GSPE effects. Weinstock et al., working with Lpl knockout mice that maintain Lpl expression only in muscle, found no changes in the various adipose depots or in total lipid content [26]. This suggests that in our study GSPE might also act on other targets in the body.
If triglycerides cannot be stored in WAT after GSPE treatment, they might be derived to other organs. One of these organs is the liver, where GSPE pre-treatment directed fatty acids towards β-oxidation, as we found increased expression of Cpt1a concomitantly with lower esterification (Dgat2). Similar effects were obtained with lower doses of GSPE but measured during treatment. Cpt1a was also found to be up-regulated after two acute doses of 250 mg GSPE/kg BW [27] in chow-fed rats but not after a subchronic treatment for 10 days with 25 mg/kg BW [5] in 13-week cafeteria-fed rats. Baselga et al. [28] found a similar trend to ours in Fasn and Cpt1a mRNA levels after three weeks with a dose of 25 mg/kg BW in rats that had previously been on a cafeteria diet for 10 weeks. The main difference between our results and Baselga et al.’s is that that they found that GSPE decreased liver triglycerides but we did not. On the contrary, our results show that triglycerides increased in the liver of the GSPE pre-treated rats [29]. However, when we analysed the long cafeteria challenge (17 weeks) the triglyceride content in the GSPE pre-treated group did not differ from content in the vehicle-treated group, which suggests a limited trend toward their accumulation in the liver. Yang-Xue et al. [30], working with partially KO mice for Lpl, showed a strong Lpl mRNA inhibition in the youngest animals that was partially reduced with aging. These animals also showed an increased deposition of triglycerides in the liver in adulthood due to the KO gene that reverted the aging period. We worked with different treatments, different species and different durations, but we noticed a changing relationship between GSPE pre-treated rats and the amount of liver triglycerides, which suggests that time affects the accrual of triglycerides in the liver.
In the short-cafeteria study, GSPE pre-treated rats sent more triglycerides to the liver, oxidized fatty acids and produced ketone bodies, which were then removed by muscle. In fact, del Bas et al. also showed an increase in the mRNA of muscle Lpl [24], which suggests a derivation of fatty acids from adipose tissue to muscle that we cannot ignore. Similarly, fatty-acid uptake and oxidation were also found to be activated in muscle (higher mRNA Cpt1b, Lpl and Cd36) in males on a 10-week cafeteria diet that subsequently received 21 days of 25 mg GSPE/kg BW [6]. Besides, the dose of GSPE does not seem to have a critical effect on muscle, as Crescenti et al. found an overexpression of genes related to fatty acid uptake (Fatp1 and CD36) and b-oxidation in the skeletal muscle of STD-GSPE offspring [16]. So the higher oxidation of fatty acids in the liver and muscle explains the lower RQ found in the GSPE pre-treated rats, a common trait of several GSPE treatments [3].
It is important to point out that the metabolic changes remain for some considerable time after GSPE treatment. There may be several explanations for this. On the one hand, GSPE is an extract composed of absorbable compounds and non-absorbable structures. Absorbable compounds can reach the various tissues assayed [31,32] and non-absorbable structures can interact with intestinal sensors [33]. Through their interaction with microbiota, they can produce new compounds that can reach different targets in the body [34,35]. Thus, we cannot discount that there might be flavonoids remaining in the tissues. However, previous studies with a lower dose (100 mg GSPE/kg BW) administered for a longer time (12 weeks) suggested that flavonoids do not accumulate in liver or mesenteric adipose tissue [36]. Another explanation might be that some epigenetic activity is taking place in the target tissues. GSPE modified liver miR-33a and miR-122 [37] at doses as low as 5 mg/kg BW for 3 weeks after a 15-week cafeteria diet. Similarly, Milenkovinc et al. [38] found changes in hepatic miRNA working with doses of proanthocyanidins closer to 5 mg/kg BW for two weeks. GSPE activity on histone deacetylases was proved by Downing et al. [39], who showed that GSPE regulates liver HDAC and Pparα activities to modulate lipid catabolism and reduce serum triglycerides in vivo. Similarly, Bladé et al. proved that PACs modulate hepatic class III HDACs, which are often called sirtuins (SIRT1-7), in a dose-dependent manner. This was associated with significant protection against hepatic triglyceride and cholesterol accumulation in healthy rats [10]. All of this evidence, in conjunction with our findings on the regulation of gene expression in liver 7 weeks after GSPE treatment, suggests an epigenetic modelling of hepatic functioning by GSPE. The duration of these effects after GSPE administration is not clear. Crescenti et al. showed effects in offspring 24 weeks after GSPE had been administered to their mothers during pregnancy [40]. We observe that seventeen weeks after GSPE treatment, Ctp1a, Fasn and Dgat2 expression in the liver changed profile compared to 7 weeks after GSPE, which suggests that hepatic epigenetic regulation had come to an end. Instead, at this time point, the changes in liver clearly agree with the insulin/glucagon ratio modulation by GSPE. In fact, we showed that GSPE (45 days with 25 mg GSPE per kg of body weight) modulates pancreatic miRNAs [41]. miR-483, which we showed is down-regulated by GSPE treatment in rat pancreatic islets, has been related to the optimum equilibrium between β-cells and α-cells (that is, its upregulation leads to increased insulin production and decreased glucagon synthesis) [42]. Therefore, our results also point to epigenetic changes in pancreas, which will need to be addressed in future work.
In conclusion, a short-term pre-treatment with GSPE repressed adipose Lpl and activated fatty oxidation in the liver for a period of at least seven weeks after the last dose. In conjunction with a greater utilization of ketone bodies in muscle, this would help to prevent an increase in body weight caused by a long-term high-fat diet after the end of treatment.
Acknowledgments
We would like to thank Niurka Llopiz and the master student Marieke Schorstein for their technical support.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/10/8/598/s1, Figure S1: Schematic diagram of the experimental design. (1) CAF-Short Cafeteria: rats receiving a GSPE preventive treatment for 10 days together with a high fat/high sucrose diet followed by an 18-day chow diet (standard diet) and then the 35-day cafeteria diet; (2) CAF-Long Cafeteria: rats receiving a GSPE preventive treatment 10 days before the 17-week cafeteria intervention. GSPE: grape seed proanthocyanidin extract; SC: Short Cafeteria; LC: Long Cafeteria.
Author Contributions
Conceptualization, A.A., J.S. and À.C.-M.; Data curation, X.T.; Formal analysis, M.L.; Funding acquisition, X.T. and M.B.; Investigation, I.G., J.S., À.C.-M. and M.P.; Methodology, K.G.-C.; Resources, X.T.; Validation, M.B.; Writing—original draft, A.A. and I.G.
Funding
Ministerio de Ciencia e Innovación: AGL2014-55347-R, AGL2017-83477-R, Universitat Rovira i Virgili: Martí Franqués, Departament d’Innovació, Universitats i Empresa, Generalitat de Catalunya: Serra Húnter, Departament d’Innovació, Universitats i Empresa, Generalitat de Catalunya: FI.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.World Health Organization WHO Obesity and overweight. [(accessed on 14 August 2019)];2015 World Health Organ. Media Cent. Fact Sheet No. 311. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/
- 2.Williams E.P., Mesidor M., Winters K., Dubbert P.M., Wyatt S.B. Overweight and Obesity: Prevalence, Consequences, and Causes of a Growing Public Health Problem. Curr. Obes. Rep. 2015;4:363–370. doi: 10.1007/s13679-015-0169-4. [DOI] [PubMed] [Google Scholar]
- 3.Salvadó M.J., Casanova E., Fernández-Iglesias A., Arola L., Bladé C. Roles of proanthocyanidin rich extracts in obesity. Food Funct. 2015;6:1053–1071. doi: 10.1039/C4FO01035C. [DOI] [PubMed] [Google Scholar]
- 4.Lamothe S., Azimy N., Bazinet L., Couillard C., Britten M. Interaction of green tea polyphenols with dairy matrices in a simulated gastrointestinal environment. Food Funct. 2014;5:2621–2631. doi: 10.1039/C4FO00203B. [DOI] [PubMed] [Google Scholar]
- 5.Quesada H., Del Bas J.M., Pajuelo D., Díaz S., Fernandez-Larrea J., Pinent M., Arola L., Salvadó M.J., Bladé C. Grape seed proanthocyanidins correct dyslipidemia associated with a high-fat diet in rats and repress genes controlling lipogenesis and VLDL assembling in liver. Int. J. Obes. 2009;33:1007–1012. doi: 10.1038/ijo.2009.136. [DOI] [PubMed] [Google Scholar]
- 6.Casanova E., Baselga-Escudero L., Ribas-Latre A., Cedó L., Arola-Arnal A., Pinent M., Bladé C., Arola L., Salvadó M.J. Chronic intake of proanthocyanidins and docosahexaenoic acid improves skeletal muscle oxidative capacity in diet-obese rats. J. Nutr. Biochem. 2014;25:1003–1010. doi: 10.1016/j.jnutbio.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Serrano J., Casanova-Martí À., Gual A., Pérez-Vendrell A.M., Blay M.T., Terra X., Ardévol A., Pinent M., Casanova-Martí À., Gual A., et al. A specific dose of grape seed-derived proanthocyanidins to inhibit body weight gain limits food intake and increases energy expenditure in rats. Eur. J. Nutr. 2016;56:1629–1636. doi: 10.1007/s00394-016-1209-x. [DOI] [PubMed] [Google Scholar]
- 8.Serrano J., Casanova-Martí À., Depoortere I., Blay M.T., Terra X., Pinent M., Ardévol A. Subchronic treatment with grape-seed phenolics inhibits ghrelin production despite a short-term stimulation of ghrelin secretion produced by bitter-sensing flavanols. Mol. Nutr. Food Res. 2016;60:2554–2564. doi: 10.1002/mnfr.201600242. [DOI] [PubMed] [Google Scholar]
- 9.Gil-Cardoso K., Ginés I., Pinent M., Ardévol A., Arola L., Blay M., Terra X. Chronic supplementation with dietary proanthocyanidins protects from diet-induced intestinal alterations in obese rats. Mol. Nutr. Food Res. 2017;61:1601039. doi: 10.1002/mnfr.201601039. [DOI] [PubMed] [Google Scholar]
- 10.Bladé C., Aragonès G., Arola-Arnal A., Muguerza B.B., Bravo F.I., Salvadó M.J., Arola L., Suárez M., Blade C., Aragones G., et al. Proanthocyanidins in health and disease. Biofactors. 2016;42:5–12. doi: 10.1002/biof.1249. [DOI] [PubMed] [Google Scholar]
- 11.Puiggros F., Llópiz N., Ardévol A., Bladé C., Arola L., Salvadó M.J.J., Puiggròs F., Llópiz N., Ardévol A., Bladé C., et al. Grape Seed Procyanidins Prevent Oxidative Injury by Modulating the Expression of Antioxidant Enzyme Systems. J. Agric. Food Chem. 2005;53:6080–6086. doi: 10.1021/jf050343m. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Abuin N., Pinent M., Casanova-Marti A., Arola L., Blay M., Ardevol A. Procyanidins and their healthy protective effects against type 2 diabetes. Curr. Med. Chem. 2015;22:39–50. doi: 10.2174/0929867321666140916115519. [DOI] [PubMed] [Google Scholar]
- 13.Terra X., Montagut G., Bustos M., Llopiz N., Ardèvol A., Bladé C., Fernández-Larrea J., Pujadas G., Salvadó J., Arola L., et al. Grape-seed procyanidins prevent low-grade inflammation by modulating cytokine expression in rats fed a high-fat diet. J. Nutr. Biochem. 2009;20:210–218. doi: 10.1016/j.jnutbio.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Castell-Auvi A., Cedo L., Pallares V., Blay M., Pinent M., Ardevol A., Castell-Auví A., Cedó L., Pallarès V., Blay M., et al. Grape seed procyanidins improve β-cell functionality under lipotoxic conditions due to their lipid-lowering effect. J. Nutr. Biochem. 2013;24:948–953. doi: 10.1016/j.jnutbio.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Abuin N., Martinez-Micaelo N., Blay M., Ardevol A., Pinent M. Grape-seed procyanidins prevent the cafeteria diet-induced decrease of glucagon-like peptide-1 production. J. Agric. Food Chem. 2014;62:1066–1072. doi: 10.1021/jf405239p. [DOI] [PubMed] [Google Scholar]
- 16.Crescenti A., Del Bas J.M., Arola-Arnal A., Oms-Oliu G., Arola L.L., Caimari A. Grape seed procyanidins administered at physiological doses to rats during pregnancy and lactation promote lipid oxidation and up-regulate AMPK in the muscle of male offspring in adulthood. J. Nutr. Biochem. 2015;26:912–920. doi: 10.1016/j.jnutbio.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Ginés I., Gil-Cardoso K., Serrano J., Casanova-Martí À., Blay M., Pinent M., Ardévol A., Terra X., Gin I., Gil-Cardoso K., et al. Effects of an Intermittent Grape-Seed Proanthocyanidin (GSPE) Treatment on a Cafeteria Diet Obesogenic Challenge in Rats. Nutrients. 2018;10:15. doi: 10.3390/nu10030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margalef M., Pons Z., Iglesias-Carres L., Arola L., Muguerza B., Arola-Arnal A. Gender-related similarities and differences in the body distribution of grape seed flavanols in rats. Mol. Nutr. Food Res. 2016;60:760–772. doi: 10.1002/mnfr.201500717. [DOI] [PubMed] [Google Scholar]
- 19.Sánchez J., Priego T., Palou M., Tobaruela A., Palou A., Picó C., Sa J., Priego T., Palou M., Tobaruela A., et al. Oral Supplementation with Physiological Doses of Leptin During Lactation in Rats Improves Insulin Sensitivity and Affects Food Preferences Later in Life. Endocrinology. 2008;149:733–740. doi: 10.1210/en.2007-0630. [DOI] [PubMed] [Google Scholar]
- 20.Sampey B.P., Vanhoose A.M., Winfield H.M., Freemerman A.J., Muehlbauer M.J., Fueger P.T., Newgard C.B., Makowski L. Cafeteria Diet Is a Robust Model of Human Metabolic Syndrome With Liver and Adipose Inflammation: Comparison to High-Fat Diet. Obesity. 2011;19:1109–1117. doi: 10.1038/oby.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serrano J., Casanova-Martí À., Gil-Cardoso K., Blay M.T., Terra X., Pinent M., Ardévol A. Acutely administered grape-seed proanthocyanidin extract acts as a satiating agent. Food Funct. 2016;7:483–490. doi: 10.1039/C5FO00892A. [DOI] [PubMed] [Google Scholar]
- 22.Castell-Auvi A., Cedo L., Pallares V., Blay M., Ardevol A., Pinent M. The effects of a cafeteria diet on insulin production and clearance in rats. Br. J. Nutr. 2012;108:1155–1162. doi: 10.1017/S0007114511006623. [DOI] [PubMed] [Google Scholar]
- 23.Casanova-Martí À., Serrano J., Portune K.J., Sanz Y., Blay M.T., Terra X., Ardévol A., Pinent M. Grape seed proanthocyanidins influence gut microbiota and enteroendocrine secretions in female rat. Food Funct. 2018;9:1672–1682. doi: 10.1039/c7fo02028g. [DOI] [PubMed] [Google Scholar]
- 24.Del Bas J.M.M., Fernández-larrea J., Blay M., Ardèvol A., Salvadó M.J., Arola L., Bladé C., Fernandez-Larrea J., Blay M., Ardevol A., et al. Grape seed procyanidins improve atherosclerotic risk index and induce liver CYP7A1 and SHP expression in healthy rats. FASEB J. 2005;19:479–481. doi: 10.1096/fj.04-3095fje. [DOI] [PubMed] [Google Scholar]
- 25.Fried S.K., Russell C.D., Grauso N.L., Brolin R.E. Omental Adipose. Insulin. 1993;92:2191–2198. doi: 10.1172/JCI116821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinstock P.H., Bisgaier C.L., Aalto-Set„l„ K., Radner H., Ramakrishnan R., Levak-Frank S., Essenburg A.D., Zechner R., Breslow J.L. Severe hypertriglyceridemia, reduced high density lipoprotein, and neonatal death in lipoprotein lipase knockout mice. J. Clin. Investig. 1995;96:2555–2568. doi: 10.1172/JCI118319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Del Bas J.M.J.M., Ricketts M.L.M.L., Baiges I., Quesada H., Ardevol A., Salvado M.J., Pujadas G., Blay M., Arola L., Blade C., et al. Dietary procyanidins lower triglyceride levels signaling through the nuclear receptor small heterodimer partner. Mol. Nutr. Food Res. 2008;52:1172–1181. doi: 10.1002/mnfr.200800054. [DOI] [PubMed] [Google Scholar]
- 28.Baselga-Escudero L., Arola-Arnal A., Pascual-Serrano A., Ribas-Latre A., Casanova E., Salvadó M.-J.J., Arola L., Blade C. Chronic Administration of Proanthocyanidins or Docosahexaenoic Acid Reversess the Increase of miR-33a and miR-122 in Dyslipidemic Obese Rats. PLoS ONE. 2013;8:e69817. doi: 10.1371/journal.pone.0069817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon Y.I., Chung M., Hwang J.S., Han M., Goo T.W., Yun E.Y. Allomyrina dichotoma (Arthropoda: Insecta) Larvae Confer Resistance to Obesity in Mice Fed a High-Fat Diet. Nutrients. 2015;7:1978–1991. doi: 10.3390/nu7031978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y.X., Han T.T., Liu Y., Zheng S., Zhang Y., Liu W., Hu Y.M. Insulin resistance caused by lipotoxicity is related to oxidative stress and endoplasmic reticulum stress in LPL gene knockout heterozygous mice. Atherosclerosis. 2015;239:276–282. doi: 10.1016/j.atherosclerosis.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 31.Ardévol A., Motilva M.J.J., Serra A., Blay M., Pinent M., Ardevol A., Motilva M.J.J., Serra A., Blay M., Pinent M., et al. Procyanidins target mesenteric adipose tissue in Wistar lean rats and subcutaneous adipose tissue in Zucker obese rat. Food Chem. 2013;141:160–166. doi: 10.1016/j.foodchem.2013.02.104. [DOI] [PubMed] [Google Scholar]
- 32.Margalef M., Pons Z., Bravo F.I., Muguerza B., Arola-Arnal A. Tissue distribution of rat flavanol metabolites at different doses. J. Nutr. Biochem. 2015;26:987–995. doi: 10.1016/j.jnutbio.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Roland W.S.U., Gouka R.J., Gruppen H., Driesse M., Van Buren L., Smit G., Vincken J.P. 6-methoxyflavanones as bitter taste receptor blockers for hTAS2R39. PLoS ONE. 2014;9:e94451. doi: 10.1371/journal.pone.0094451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casanova-Marti A. Grape seed proanthocyanidins influence gut microbiota and enteroendocrine secretions in rats. Food Funct. 2018;9:1672–1682. doi: 10.1039/C7FO02028G. [DOI] [PubMed] [Google Scholar]
- 35.Margalef M., Pons Z., Bravo F.I., Muguerza B., Arola-Arnal A. Plasma kinetics and microbial biotransformation of grape seed flavanols in rats. J. Funct. Foods. 2015;12:478–488. doi: 10.1016/j.jff.2014.12.007. [DOI] [Google Scholar]
- 36.Margalef M., Pons Z., Iglesias-Carres L., Bravo F.I., Muguerza B., Arola-Arnal A. Lack of Tissue Accumulation of Grape Seed Flavanols after Daily Long-Term Administration in Healthy and Cafeteria-Diet Obese Rats. J. Agric. Food Chem. 2015;63:9996–10003. doi: 10.1021/acs.jafc.5b03856. [DOI] [PubMed] [Google Scholar]
- 37.Baselga-Escudero L., Pascual-Serrano A., Ribas-Latre A., Casanova E., Salvadó M.J., Arola L., Arola-Arnal A., Bladé C. Long-term supplementation with a low dose of proanthocyanidins normalized liver miR-33a and miR-122 levels in high-fat diet-induced obese rats. Nutr. Res. 2015;35:337–345. doi: 10.1016/j.nutres.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Milenkovic D., Deval C., Gouranton E., Landrier J.F., Scalbert A., Morand C., Mazur A. Modulation of miRNA expression by dietary polyphenols in apoE deficient mice: A new mechanism of the action of polyphenols. PLoS ONE. 2012;7:e29837. doi: 10.1371/journal.pone.0029837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Downing L.E., Ferguson B.S., Rodriguez K., Ricketts M.L. A grape seed procyanidin extract inhibits HDAC activity leading to increased Pparα phosphorylation and target-gene expression. Mol. Nutr. Food Res. 2017;61:1600347. doi: 10.1002/mnfr.201600347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Del Bas J.M., Crescenti A., Arola-Arnal A., Oms-Oliu G., Arola L., Caimari A. Grape seed procyanidin supplementation to rats fed a high-fat diet during pregnancy and lactation increases the body fat content and modulates the inflammatory response and the adipose tissue metabolism of the male offspring in youth. Int. J. Obes. 2015;39:7–15. doi: 10.1038/ijo.2014.159. [DOI] [PubMed] [Google Scholar]
- 41.Castell-Auvi A., Cedo L., Movassat J., Portha B., Sanchez-Cabo F., Pallares V., Blay M., Pinent M., Ardevol A., Castell-Auví A., et al. Procyanidins Modulate MicroRNA Expression in Pancreatic Islets. J. Agric. Food Chem. 2013;61:355–363. doi: 10.1021/jf303972f. [DOI] [PubMed] [Google Scholar]
- 42.Mohan R., Mao Y., Zhang S., Zhang Y.W., Xu C.R., Gradwohl G., Tang X. Differentially expressed microRNA-483 confers distinct functions in pancreatic β -and α-Cells. J. Biol. Chem. 2015;290:19955–19966. doi: 10.1074/jbc.M115.650705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.