Abstract
Tacrine was the first drug to be approved for Alzheimer’s disease (AD) treatment, acting as a cholinesterase inhibitor. The neuropathological hallmarks of AD are amyloid-rich senile plaques, neurofibrillary tangles, and neuronal degeneration. The portfolio of currently approved drugs for AD includes acetylcholinesterase inhibitors (AChEIs) and N-methyl-d-aspartate (NMDA) receptor antagonist. Squaric acid is a versatile structural scaffold capable to be easily transformed into amide-bearing compounds that feature both hydrogen bond donor and acceptor groups with the possibility to create multiple interactions with complementary sites. Considering the relatively simple synthesis approach and other interesting properties (rigidity, aromatic character, H-bond formation) of squaramide motif, we combined this scaffold with different tacrine-based derivatives. In this study, we developed 21 novel dimers amalgamating squaric acid with either tacrine, 6-chlorotacrine or 7-methoxytacrine representing various AChEIs. All new derivatives were evaluated for their anti-cholinesterase activities, cytotoxicity using HepG2 cell line and screened to predict their ability to cross the blood-brain barrier. In this contribution, we also report in silico studies of the most potent AChE and BChE inhibitors in the active site of these enzymes.
Keywords: tacrine, bis(7)-tacrine, 6-chlorotacrine, 7-methoxytacrine, squaramides, Alzheimer’s disease, cholinesterases, in vitro, in silico
1. Introduction
Alzheimer’s disease (AD), the most common form of dementia [1], is symptomatically treated with inhibitors of acetylcholinesterase (AChE, 3.1.1.7). This type of serine protease catalyzes the hydrolysis of the neurotransmitter acetylcholine (ACh) to choline and acetic acid [2]. Low levels of ACh lead to cognitive impairment and dementia [3], thus the inactivation of AChE increases the pool of ACh and ameliorates the cognitive dysfunction associated with AD [4]. Three AChE inhibitors (AChEIs), namely, donepezil, galantamine, and rivastigmine, are the representatives of so-called “cholinergic hypothesis” used currently as the main drugs for AD treatment [5]. The etiology of AD is complex, and several factors are considered as causative. Indeed, AD has been recognized by deposition of two types of proteins, extracellularly accumulated β-amyloid (Aβ) protein due to abnormal processing of amyloid precursor protein (APP), and intracellular neurofibrillary tangles composed from hyperphosphorylated tau protein [6]. Furthermore, various other neurochemical abnormalities contribute to the progression of AD, such as oxidative stress [7], excitotoxicity [8], imbalance of biometals [9], or neuro-inflammation [10]. Memantine, an antagonist of N-methyl-d-aspartate (NMDA) receptor, is also approved for AD treatment [11].
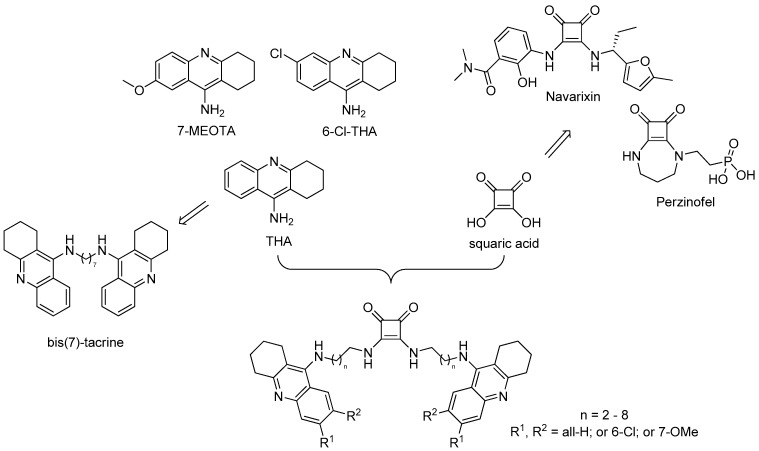
Tacrine (THA, 9-amino-1,2,3,4-tetrahydroacridine, Figure 1) is the centrally acting reversible AChEI that launched the market in 1993 as the first drug to alleviate symptoms of AD [12]. THA is a non-selective cholinesterase agent, inhibiting also butyrylcholinesterase (BChE, E.C. 3.1.1.8). Moreover, it affects several other biological systems, which contributes to its complex action against AD [13]. THA has been reported to also modulate muscarinic and nicotinic receptors [14] and the amyloidogenic pathway [15,16]. The clinical benefits of THA were limited by its poor oral bioavailability and considerable side effects mainly defined as hepatotoxicity. This fact caused THA withdrawal from the market [17,18]. On the other hand, 1,2,3,4-tetrahydroaminoacridine scaffold still stands at the forefront of the scientific research given by its relatively simple structure that can be easily modified to find novel, more potent, and less toxic analogues. Indeed, these endeavors yielded, for instance, 7-methoxytacrine (7-MEOTA) [14], 6-chlorotacrine (6-Cl-THA) [19], or homodimer bis(7)-tacrine [20] (Figure 1). 7-MEOTA possesses suppressed toxicity profile compared to THA due to distinct metabolic pathway preserving anti-ChE potency [14]. The chlorine atom at position 6- of the THA core strongly enhances the inhibitory activity against AChE. 6-Cl-THA has two orders of magnitude higher AChE inhibition potency than THA [21]. Bis-tacrine homodimers were developed as multifunctional analogues capable to simultaneously contact both the catalytic site (CAS) and the peripheral anionic site (PAS) of AChE [20,22]. The most active derivative is connected via seven methylene units (bis(7)-tacrine), being almost 400-fold more potent than THA in inhibiting human AChE [23].
Figure 1.
Schematic drawing for the design of tacrine-squaramide homodimers.
Squaramides (SQ) are derivatives of squaric acid (3,4-dihydroxycyclobut-3-ene-1,2-dione) that are widely used in a variety fields of expertise (Figure 1). They mostly dominate asymmetric synthesis as chiral ligands and H-bonding catalysts [24,25,26,27], but are also valuable building blocks in medicinal chemistry. Examples of small molecules with incorporated squaramide scaffold, that reached the various stage of clinical trials, are Perzinfotel (NMDA receptor antagonist) [28], and Navarixin (chemokine receptors 1 and 2 antagonist) [29] (Figure 1). Squaramide can be considered as a motif of interest enabling formation of H-bonds bioisosteric to functionalities like carboxylic and amino acids, urea, guanidine, cyanoguanidine, and different phosphate groups [30]. More recently, mono- and bis-squaramide-based derivatives were investigated as treatment for insect-transmitted parasite diseases such as leishmaniasis [31], malaria [32], and Chagas disease [33].
Considering the simple synthesis and other properties (aromaticity, rigidity, H-bond formation) suitable for binding of squaramide motif, we envisaged to combine this scaffold with different tacrine-based derivatives following the successful story of bis(7)-tacrine. Bis(7)-tacrine has a broad and complex pharmacological profile. Besides highly selective inhibition potency towards AChE, it emerged as a potent inhibitor of other enzymes like β-secretase 1 (BACE-1), nitric-oxide synthase and receptors including NMDA, serotonin 5-HT3, gamma-aminobutyric acid GABAA, nicotinic and others, all of them being involved in the pathophysiology of neurodegenerative disorders [23,34,35,36]. Bis(7)-tacrine is able to inhibit self-aggregation and also AChE-induced aggregation of Aβ protein [37]. Moreover, bis(7)-tacrine has been shown to provide multiple neuroprotective effects in vitro and in vivo [38,39,40,41,42,43,44]. Bis(7)-tacrine has spurred the development of so-called multi-target directed ligands (MTDLs) targeting several pathological mechanisms pathways in AD. In this study, we developed 21 novel dimers amalgamating squaric acid scaffold with THA, 6-Cl-THA, and 7-MEOTA into so-called tacrine-squaramide homodimers (Figure 1). All new derivatives were evaluated for their anti-ChEs properties, cytotoxicity using the HepG2 cell line, and selected compounds were screened to predict their ability to cross the blood-brain barrier (BBB). In this article, we also report in silico studies of the most potent AChE and BChE inhibitors in the active site of respective enzyme.
2. Results and Discussion
2.1. Chemistry
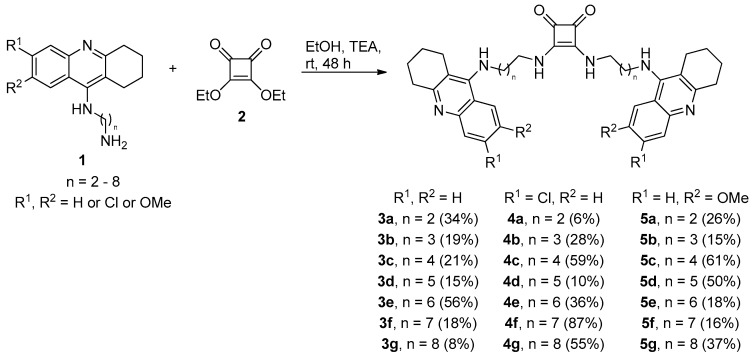
Symmetrical squaramides are generally prepared by the reaction of dialkyloxysquarate derivatives with an excess of primary or secondary amines [30]. Aliphatic amine condensation of corresponding tacrine derivatives 1 with diethoxysquarate (2) in ethanol in the presence of TEA afforded desired products with 6–87% yields (THA family—3a–3g; 6-Cl-THA family—4a–4g and 7-MEOTA family—5a–5g; Scheme 1). Intermediates of general structure 1 varying in the length of alkyl chains were prepared according to previously published methods [45,46]. During the synthesis, we also observed the formation of monomeric analogues of squaramides (not shown in the Scheme 1), but these were chemically unstable when isolated and thus were excluded from further biological studies. All final compounds were characterized by 1H, 13C-NMR spectra and HRMS analysis. LC analysis confirmed their purity >95%.
Scheme 1.
Synthetic procedure for tacrine-squaramide derivatives 3a–3g, 4a–4g and 5a–5g.
2.2. Evaluation of Cholinesterase Inhibitory Activity
Three series of tacrine-squaramide homodimers were tested for their inhibitory potential against human AChE (hAChE) and human BChE (hBChE) enzymes using modified spectrophotometric method of Ellman et al. [47,48,49,50]. THA, 6-Cl-THA and 7-MEOTA were used as reference compounds. The IC50 values of all tested compounds and their selectivity index (SI) for hAChE are summarized in Table 1. All tested derivatives showed good to excellent inhibitory potencies for both cholinesterases with IC50 values ranging from micromolar to the single digit nanomolar scale.
Table 1.
hAChE and hBChE inhibitory activities of tacrine-squaramide homodimers and reference compounds THA, 6-Cl-THA and 7-MEOTA.
| Compound | R1 | R2 | n | IC50 (nM) a | SI b | |
|---|---|---|---|---|---|---|
| hAChE | hBChE | |||||
| 3a | H | H | 2 | 3.8 | 62 | 16 |
| 3b | H | H | 3 | 8.4 | 55 | 6.5 |
| 3c | H | H | 4 | 5.5 | 75 | 14 |
| 3d | H | H | 5 | 8.1 | 69 | 8.5 |
| 3e | H | H | 6 | 13 | 21 | 1.6 |
| 3f | H | H | 7 | 32 | 32 | 1 |
| 3g | H | H | 8 | 72 | 50 | 0.7 |
| 4a | Cl | H | 2 | 4.2 | 150 | 36 |
| 4b | Cl | H | 3 | 2.0 | 110 | 55 |
| 4c | Cl | H | 4 | 4.6 | 170 | 37 |
| 4d | Cl | H | 5 | 8.2 | 310 | 38 |
| 4e | Cl | H | 6 | 3.2 | 540 | 170 |
| 4f | Cl | H | 7 | 16 | 450 | 28 |
| 4g | Cl | H | 8 | 10 | ˃100,000 | ˃10,000 |
| 5a | H | OMe | 2 | 170 | 130 | 0.7 |
| 5b | H | OMe | 3 | 1100 | 8900 | 8.1 |
| 5c | H | OMe | 4 | 4500 | 4700 | 0.9 |
| 5d | H | OMe | 5 | 120 | 680 | 5.7 |
| 5e | H | OMe | 6 | 150 | 1100 | 7.3 |
| 5f | H | OMe | 7 | 170 | 1300 | 7.6 |
| 5g | H | OMe | 8 | 490 | 2900 | 5.9 |
| THA c | H | H | - | 320 | 80 | 0.3 |
| 6-Cl-THA c | Cl | H | - | 20 | 1800 | 90 |
| 7-MEOTA c | H | OMe | - | 10,000 | 17,000 | 1.7 |
All homodimers bearing THA (3a–g) and 6-Cl-THA (4a–g) units displayed IC50 values in the nanomolar range for hAChE (2–72 nM). As expected, derivatives of 7-MEOTA (5a–g) were less potent, displaying IC50 values in the sub-micromolar to micromolar range (0.1–4.5 µM). According to hAChE inhibition potency, the top ranked derivative from all the derivatives under the study was 4b (IC50 = 2.0 nM) being only 2.5-times less active compared to bis(7)-tacrine (IC50 = 0.8 nM) [51], but still one order of magnitude more potent hAChEI than the parent 6-Cl-THA. From THA and 7-MEOTA families, the most pronounced derivatives in terms of hAChE inhibition were 3a and 5d with IC50 values 3.8 and 120 nM, being 84- and 83-times more potent than references THA and 7-MEOTA, respectively. The length of the spacer between two tacrine sub-units significantly affected AChE inhibition potency. THA- and 6-Cl-THA-based dimers (compounds 3f, 3g, and 4f, 4g) with the longest chains were the least active AChEIs. Surprisingly, for the 7-MEOTA-dimers, negligible inhibitory potency was associated with derivatives bearing shorter linkers, especially to 5b and 5c.
Almost all homodimers were also good inhibitors of hBChE with IC50 values in the micromolar to nanomolar range. The most active hBChEIs were THA-based derivatives (IC50 = 21–75 nM) followed by 6-Cl-THA and 7-MEOTA subsets. THA-derivative 3e exerted the highest hBChE inhibition potency (IC50 = 21 nM).
The selective/non-selective inhibition profile for ChEs in drugs potentially useful in AD treatment is largely discussed [52]. Currently used ChEIs are AChE-selective (donepezil) or possess more or less non-selective pattern of inhibition (rivastigmine, galantamine). It has been proved that levels of BChE in the brain of AD patients are elevated with age while levels of AChE are decreased. This finding underlies the importance of selective-BChEIs in the treatment of moderate-to-severe stages of AD [53]. Given this fact, only 3g revealed small preference for hBChE inhibition (SI = 0.7), while most of the tacrine-squaramides were AChE-selective, highlighting 4g from the 6-Cl-THA family with remarkable SI ˃ 10,000. In the THA-based subset, SI strongly correlated with the length of alkyl chain where the four-methylene tethered analogue (3c) displayed the most pronounced AChE selectivity.
2.3. Kinetic Study of AChE and BChE Inhibition
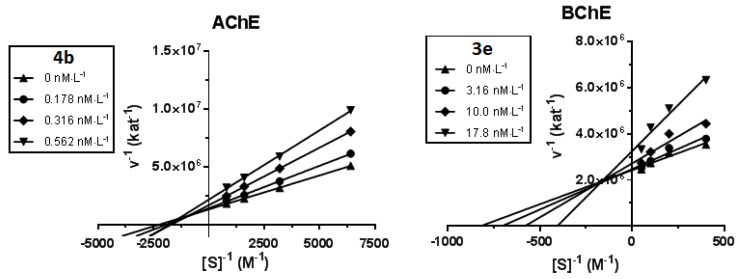
Kinetic study was performed in order to describe the interactions of the compounds 4b and 3e with hAChE and hBChE, respectively. Inhibition kinetics were elucidated from velocity curves that were measured at several concentrations of the corresponding substrate (AChE—acetylthiocholine, BChE—butyrylthiocholine) and tested compounds. The type of enzyme inhibition and corresponding kinetic parameters (Ki and Ki’) were determined by nonlinear regression analysis. Results for each type model of inhibition (competitive, non-competitive, uncompetitive and mixed) were compared by sum-of-squares F-test. Statistical analysis showed mixed type of inhibition (p ˂ 0.05), which is in line with the Lineweaver–Burk plot, used for visualization of obtained data (Figure 2).
Figure 2.
Steady state inhibition of cholinesterase substrate hydrolysis by compound 4b (hAChE) and 3e (hBChE) at different concentrations. Lineweaver−Burk plots of initial velocity at increasing substrate concentrations (AChE: 0.1563–1.250 mM; BChE: 2.5–20.0 mM) are presented. Lines were derived from a linear regression of the data points.
The intersection of lines is located above the x-axis for both measured inhibitors, which means reversible binding mode to both free enzyme and enzyme-substrate complex, with higher affinity to the free enzyme (Ki < Ki’), interacting with its allosteric peripheral anionic site (PAS). This interaction causes conformational changes of the cholinesterase, resulting also in changes of its active site. Km was slightly increased and Vmax was reduced at higher concentration of both inhibitors. A Ki value of 0.4 ± 0.2 nM and Ki’ of 0.5 ± 0.2 nM were measured for 4b on AChE and a Ki value of 8.1 ± 1.9 nM and Ki’ of 100 ± 28 nM for 3e on BChE, respectively.
2.4. Cytotoxicity
The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) colorimetric assay performed on the human hepatocyte carcinoma (HepG2) cell line was applied to investigate the preliminary toxicity profile of all the developed derivatives [56]. The results are summarized in Table 2. The lowest cytotoxic effect displayed analogues with the shortest alkyl chains from all three series (3a, 4a, and 5a). These compounds were nontoxic at the highest tested concentrations (256 µM for 3a and 5a, and 128 µM for 4a). The linker elongation negatively affected cytotoxicity in THA- and 7-MEOTA-squaramides. In other words, the most toxic hybrids were those having the longest spacers, i.e., compounds 3g and 5g, with IC50 values 6.6 and 3.5 µM, respectively. Derivatives of 6-Cl-THA were nontoxic even at the highest tested concentration of 64 µM and, intriguingly, were also less toxic than reference 6-Cl-THA (IC50 = 43 µM). These data have to be considered carefully since MTT cytotoxicity screening is performed on isolated cell lines, thus these results provide only preliminary toxicity insight and do not reflect the toxicity profile under in vivo conditions. For comparative purposes, we also included cytotoxicity data for other AChEIs like donepezil, galantamine and rivastigmine. Interestingly, donepezil toxicity lies in the same range as some of the least toxic tacrine-squaramides (3c, 4a and 5c), whereas rivastigmine and galantamine exerted a one to three orders lower toxic profile.
Table 2.
Cytotoxicity of tacrine-squaramides evaluated using HepG2 cells measured by colorimetric MTT assay.
| Compound | R1 | R2 | n | IC50 (µM) a |
|---|---|---|---|---|
| 3a | H | H | 2 | >256 |
| 3b | H | H | 3 | >32 |
| 3c | H | H | 4 | 150 |
| 3d | H | H | 5 | 69 |
| 3e | H | H | 6 | 76 |
| 3f | H | H | 7 | 23 |
| 3g | H | H | 8 | 6.6 |
| 4a | Cl | H | 2 | >128 |
| 4b | Cl | H | 3 | >64 |
| 4c | Cl | H | 4 | >64 |
| 4d | Cl | H | 5 | >64 |
| 4e | Cl | H | 6 | >64 |
| 4f | Cl | H | 7 | >64 |
| 4g | Cl | H | 8 | >64 |
| 5a | H | OMe | 2 | >256 |
| 5b | H | OMe | 3 | >64 |
| 5c | H | OMe | 4 | 190 |
| 5d | H | OMe | 5 | 98 |
| 5e | H | OMe | 6 | 26 |
| 5f | H | OMe | 7 | 9 |
| 5g | H | OMe | 8 | 3.5 |
| THA | H | H | - | 169 |
| 6-Cl-THA | Cl | H | - | 43 |
| 7-MEOTA | H | OMe | - | 44 |
| Donepezil | - | - | - | 150 |
| Rivastigmine | - | - | - | 3400 |
| Galantamine | - | - | - | 4200 |
a The IC50 values are expressed as the mean of at least three independent experiments.
2.5. In Vitro BBB Permeation
An important feature for drugs targeting the brain is their ability to cross the BBB. The parallel artificial membrane permeability assay (PAMPA) is a high-throughput screening tool applicable for prediction of passive transport across BBB [57]. The results of selected homodimers and positive (THA, donepezil, ibuprofen) and negative (furosemide, chlorothiazide, ranitidine) controls are outlined in Table 3. The studied compound 4e is likely endowed with a high probability to cross the BBB. Derivatives 3a and 5d with lower values of Pe cannot presumably cross the BBB; other tested derivatives have uncertain BBB permeation (3e, 4b, and 5a). It also has to be bear in mind, that in its current form using lipid porcine, the PAMPA-BBB assay possesses several limitations. These include mainly (i) the different composition of the human phospholipid bilayer may cause deviation in predicted permeability, (ii) omitting the absorption of compounds that are actively transported by influx transporters and pumped-out by various efflux mechanisms with the glycoprotein P at the forefront or (iii) transport through the paracellular route which is mostly exploited by small hydrophobic molecules [58]. Even considering these drawbacks, PAMPA-BBB is conceived as very simple but powerful tool for BBB prediction.
Table 3.
Prediction of BBB penetration of the studied compounds expressed as Pe values.
| Compound | R1 | R2 | n | Pe (× 10−6 cm.s−1) a | CNS (+/−) b |
|---|---|---|---|---|---|
| 3a | H | H | 3 | 1.1 | CNS − |
| 3e | H | H | 3 | 2.6 | CNS +/− |
| 4b | Cl | H | 3 | 2.5 | CNS +/− |
| 4e | Cl | H | 6 | 17.0 | CNS + |
| 5a | H | OMe | 2 | 2.3 | CNS +/− |
| 5d | H | OMe | 5 | 1.3 | CNS − |
| THA | H | H | - | 6.0 | CNS + |
| donepezil | 21.0 | CNS + | |||
| ibuprofen | 12.0 | CNS + | |||
| furosemide | 0.0 | CNS − | |||
| chlorothiazide | 0.3 | CNS − | |||
| ranitidine | 0.0 | CNS − |
a Results are expressed as the mean of at least three experiments; b CNS +: high BBB permeation predicted with Pe (10−6 cm·s−1) > 4.0, CNS −: low BBB permeation predicted with Pe (10−6 cm·s−1) < 2.0, CNS +/−: BBB permeation uncertain with Pe (10−6 cm·s−1) from 4.0 to 2.0.
2.6. In Silico Studies
Molecular docking studies were carried out with the hAChE and hBChE enzymes to analyze the binding data of the developed compounds. We employed the crystal structures of hAChE (PDB entry: 4EY7) bound to dual anionic site ligand donepezil and hBChE (PDB entry: 4BDS) complexed with THA [59,60]. Both of them possess satisfactory resolution at 2.35 Å and 2.10 Å, respectively, ensuring reliable outputs. In line with the results from in vitro and following enzyme kinetic analysis, we selected 4b and 3e, two most pronounced hAChE and hBChE inhibitors from 6-Cl-THA and THA families, respectively. Docking analyses were carried out using Autodock Vina software (v. 1.1.2) [61].
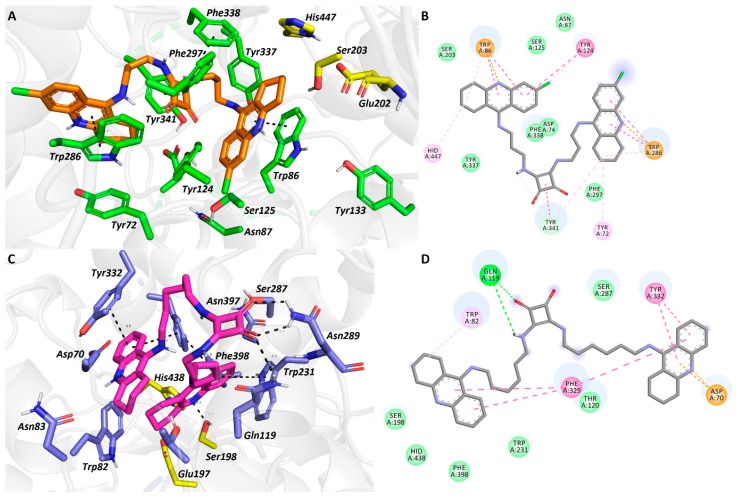
Tacrine-squaramide derivative 4b spans the cavity gorge of hAChE from the bottom to the entrance (Figure 3A,B). Proximal 6-Cl-THA unit occupies CAS region by showing typical parallel π-π/cation-π interaction with Trp86 (3.9 Å; distance measured from center-to-center of aromatic rings). Chlorine atom is directed towards hydrophobic groove of the enzyme contacting Tyr124, Ser125 and Asn87. This topology orientation of 6-Cl-THA moiety has been observed more valuable in improving the inhibition potency compared to 7-MEOTA derivatives. The latter generally displays unfavorable 180° rotation of core tacrine scaffold yielding lower enzyme affinity [14,62]. Catalytic triad residues (Glu202, Ser203 and His447) are also involved in ligand stabilization revealing alkyl-π (His447) or van der Waals contacts (Glu202 and Ser203). Connecting linker is anchored via a complex web of hydrophobic interactions in the mid-gorge enzyme region. These also involve distorted π-π contacts between central squaramide scaffold with Tyr341 (4.5 Å), Tyr124 (4.5 Å), Phe297 (4.2 Å) and Phe338 (5.1 Å). The most strikingly aromatic character of squaramide and its interaction with aromatic residues in the mid-gorge region can be denoted as culprits for extraordinary inhibition potency of 4b. At the rim of the gorge, the distal 6-Cl-THA subunit faces towards key amino acid residue from PAS region, i.e., Trp286 (parallel π-π stacking, 3.7 Å). In general, 4b-hAChE complex mimics structural features in crystallographically refined bis(7)-tacrine in Torpedo californica AChE [63].
Figure 3.
Docking results for the tacrine-squaramide hybrids 4b and 3e within hAChE (A,B) and hBChE (C,D) active sites, respectively (PDB IDs: 4EY7, 4BDS). A and C—Superimposed analogues 4b and 3e (orange and purple carbon atoms, respectively) as 3D figures; B and D—2D figures 4b and 3e, respectively. Generally to A and C—important amino acid residues involved in the ligand-enzyme interactions are displayed as green and blue carbon atoms, respectively; catalytic triad residues are shown in yellow, the rest of the enzyme is represented as a light-grey cartoon. Figures A and C were generated with PyMol 1.5.0.4 (The PyMOL Molecular Graphics System, Version 1.5.0.4 Schrödinger, LLC, Mannheim, Germany), Figures B and D were created with Discovery Studio 2016 Client software.
In the 3e-hBChE complex, the ligand revealed atypical U-shaped conformation (Figure 3C,D). This can be nicely explained by the structural differences between AChE/BChE enzymes in the PAS region, where most of the aromatic amino acid residues in BChE are substituted by aliphatic ones enabling entrance of more bulky substrates [64]. From this point of view, the structural properties of BChE enabled lodging of both THA subunits of 3e deep into cavity of hBChE. In this case, catalytic triad residues form either hydrophobic (His438 and Glu197) or hydrogen-bond contacts (2.5 Å). The ligand orientation is mainly orchestrated by Phe329 stabilizing both tacrine-subunits and allow ligand U-shaped conformation. Interestingly, the squaramide moiety exerted a complex web of hydrogen bonds with Asn289 (2.3 and 2.3 Å) and Gln119 (2.7 Å) at the mouth of the enzyme.
3. Conclusions
The never-ending quest to find effective therapy for AD is on one hand a challenging task and on the other hand, a daunting process. Enormous efforts are made to find disease-modifying agents that would improve quality of life for the AD sufferers. A wealth of scientific studies currently still indicate that ChEIs can serve as a useful tool in drug development of potential therapeutics to tackle symptomatology of AD as well their beneficial effects on pathophysiological pathways associated with the progression of the disease. Building on them, so-called MTDLs flourished as an emerging direction [65,66]. These findings, given by the drug evolution, can renew the interest in ChEIs but only if rationally designed [67]. In this study, we described the design, synthesis, and in vitro evaluation of squaramide-containing tacrine homodimers represented by THA, 6-Cl-THA, and 7-MEOTA scaffolds. All newly developed compounds were good to excellent inhibitors of both ChEs, with IC50 in the micromolar to single-digit nanomolar range. Compounds 4b and 3e were found to demonstrate the highest inhibition potencies against hAChE and hBChE, respectively. For 4b and 3e, the kinetic analysis revealed their mixed-type inhibition which is indicative of concurrent interaction with both anionic sites of the enzymes. The lowest cytotoxic effect on HepG2 cell line displayed analogues with the shortest alkyl chains from all three series (3a, 4a, and 5a). Increasing the number of methylene units in the linker increases the cytotoxicity. Interestingly, this finding does not apply to the 6-Cl-THA family. However, from the six selected squaramide-tacrine homodimers, only 4e is predicted to be BBB permeable. In silico studies predicted orientation of 4b in hAChE active site correlating well with previously published crystallographic structure of bis(7)-tacrine in AChE. Compound 3e displayed rather atypical U-shaped conformation in the hBChE active site, given presumably by the topological properties of cholinesterase. With this in mind, we believe that squaramides can be further recognized as interesting building blocks in the drug discovery with particular emphasis on AD.
4. Experimental Section
4.1. General Chemistry Methods
All chemical solvents and reagents were used in the highest available purity without further purification and they were purchased from Sigma-Aldrich (Prague, Czech Republic). The reactions were monitored by thin layer chromatography (TLC) on silica gel plates (60 F254, Merck, Prague, Czech Republic) and the spots were visualized by ultraviolet light (254 nm). Purification of crude products was carried out using columns of silica gel (silica gel 100, 0.063–0.200 mm, 70–230 mesh ASTM, Fluka, Prague, Czech Republic). NMR spectra were recorded in deuterated chloroform (CDCl3), deuterated methanol (CD3OD) and deuterated dimethyl sulfoxide (DMSO-d6) on a Varian S500 spectrometer. Chemical shifts (δ) are reported in parts per millions (ppm) and spin multiplicities are given as bold singlet (bs), doublet (d), doublet of doublet (dd), triplet (t), quartet (q), pentet (p), or multiplet (m). Coupling constants (J) are reported in Hz. Recorded NMR data are available at Supplementary Information. The synthesized compounds were analyzed by an LC-MS system consisting of UHLPC Dionex Ultimate 3000 couplet with Q Exaxtive Plus mass spectrometer to obtain high resolution mass spectra (Thermo Fisher Scientific, Bremen, Germany). Melting points were measured using an automated melting point recorder M-565 (Büchi, Switzerland). The final compounds were analyzed by LC-MS consisting of UHPLC Dionex Ultimate 3000 RS coupled with Q Exactive Plus orbitrap mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) to obtain high-resolution mass spectra. Gradient LC analysis confirmed > 95% purity.
General Procedure for the Preparation of Tacrine-Squaramides (3a–3g; 4a–4g and 5a–5g)
To the solution of diethoxysquarate 2 (1.0 eq) in EtOH (1 M) were added corresponding tacrine derivative (2.0 eq) and TEA (2.0 eq). The reaction mixture was stirred at room temperature for 48 h. After completion of the reaction, the solvent was evaporated and crude product was purified by column chromatography using a mixture of eluents (DCM:MeOH:NH3 = 20:1:0.1).
Bis({2-[(1,2,3,4-tetrahydroacridin-9-yl)amino]ethyl}amino)cyclobut-3-ene-1,2-dione (3a). Yield 34%; yellow solid; Mp = 159–160 °C; 1H-NMR (500 MHz, CD3OD): δ 8.06–7.99 (m, 2H), 7.74–7.69 (m, 2H), 7.54–7.47 (m, 2H), 7.36–7.28 (m, 2H), 3.79–3.60 (m, 8H), 2.95–2.88 (m, 4H), 2.72–2.61 (m, 4H), 1.88–1.75 (m, 8H); 13C-NMR (126 MHz, CD3OD): δ 183.6 (CO), 158.7, 153.2, 146.8, 137.8, 130.3, 127.2, 125.3, 124.2, 121.1, 117.6, 46.1, 33.6, 26.1, 23.8, 23.4; HRMS [M + H]+: 561.2972; (calculated for [C34H36N6O2]+: 561.2973).
Bis({3-[(1,2,3,4-tetrahydroacridin-9-yl)amino]propyl}amino)cyclobut-3-ene-1,2-dione (3b). Yield 19%; yellow solid; Mp = 196–198 °C; 1H-NMR (500 MHz, DMSO-d6): δ 8.20–8.10 (m, 2H), 7.74–7.66 (m, 2H), 7.60–7.50 (m, 2H), 7.39–7.30 (m, 2H), 3.65–3.44 (m, 8H), 2.92–2.85 (m, 4H), 2.72–2.62 (m, 4H), 1.90–1.56 (m, 12H); 13C-NMR (126 MHz, DMSO-d6): δ 182.5, 159.2, 151.6, 147.3, 132.8, 126.5, 125.5, 123.7, 118.5, 116.1, 48.8, 44.9, 41.1, 33.5, 32.3, 25.2, 22.7, 22.3; HRMS [M + H]+: 589.3218 (calculated for [C36H40N6O2]+: 589.3286).
Bis({4-[(1,2,3,4-tetrahydroacridin-9-yl)amino]butyl}amino)cyclobut-3-ene-1,2-dione (3c). Yield 21%; yellow solid; Mp = 47–49 °C; 1H-NMR (500 MHz, CD3OD): δ 8.11–8.06 (m, 2H), 7.76–7.71 (m, 2H), 7.57–7.51 (m, 2H), 7.38–7.33 (m, 2H), 3.61–3.55 (m, 4H), 2.98–2.92 (m, 4H), 2.74–2.67 (m, 4H), 1.87 (dd, J = 7.3, 4.4 Hz, 8H), 1.69–1.63 (m, 8H), 1.31–1.24 (m, 4H); 13C-NMR (126 MHz, CD3OD): δ 183.5, 169.3, 158.5, 153.4, 147.0, 130.2, 127.2, 125.0, 124.5, 120.9, 116.7, 79.5, 60.2, 44.8, 33.7, 29.7, 28.9, 26.1, 23.9, 23.5, 8.5; HRMS [M + H]+: 617.3603 (calculated for [C38H44N6O2]+: 617.3599).
Bis({5-[(1,2,3,4-tetrahydroacridin-9-yl)amino]pentyl}amino)cyclobut-3-ene-1,2-dione (3d). Yield 15%; brown solid; Mp = 76–77 °C; 1H-NMR (500 MHz, CDCl3): δ 7.98–7.92 (m, 2H), 7.86–7.81 (m, 2H), 7.54–7.47 (m, 2H), 7.34–7.28 (m, 2H), 4.44–4.37 (m, 2H), 3.81 (bs, 5H), 3.63 (t, J = 7.0 Hz, 4H), 3.45 (q, J = 6.8 Hz, 4H), 3.03–2.96 (m, 4H), 2.66–2.60 (m, 4H), 1.89–1.83 (m, 8H), 1.67–1.60 (m, 8H), 1.47–1.36 (m, 4H); 13C-NMR (126 MHz, CDCl3): δ 182.2, 168.1, 157.3, 151.3, 146.1, 128.7, 126.9, 123.6, 123.0, 119.5, 115.2, 50.0, 48.8, 43.9, 32.9, 30.8, 30.6, 24.5, 23.5, 22.7, 22.3; HRMS [M + H]+: 645.3903 (calculated for [C40H48N6O2]+: 645.3912).
Bis({6-[(1,2,3,4-tetrahydroacridin-9-yl)amino]hexyl}amino)cyclobut-3-ene-1,2-dione (3e). Yield 56%; yellow solid; Mp = 93–95 °C; 1H-NMR (500 MHz, CDCl3): δ 7.90 (dd, J = 8.6, 1.4 Hz, 2H), 7.81 (dd, J = 8.5, 1.2 Hz, 2H), 7.66 (bs, 2H), 7.51–7.44 (m, 2H), 7.32–7.25 (m, 2H), 4.14 (t, J = 6.0 Hz, 2H), 3.62–3.53 (m, 4H), 3.39 (q, J = 6.7 Hz, 4H), 3.10 (bs, 2H), 3.02–2.91 (m, 4H), 2.67–2.55 (m, 4H), 1.91–1.78 (m, 8H), 1.60–1.46 (m, 8H), 1.34–1.19 (m, 8H); 13C-NMR (126 MHz, CDCl3): δ 182.4, 168.1, 157.9, 151.1, 146.9, 128.5, 127.8, 123.6, 122.9, 119.8, 115.5, 50.4, 49.0, 44.1, 33.6, 31.4, 30.9, 26.3, 25.9, 24.7, 22.9, 22.6; HRMS [M + H]+: 673.4218 (calculated for [C42H52N6O2]+: 673.4225).
Bis({7-[(1,2,3,4-tetrahydroacridin-9-yl)amino]heptyl}amino)cyclobut-3-ene-1,2-dione (3f). Yield 18%; yellow solid; Mp = 64–66 °C; 1H-NMR (500 MHz, CD3OD): δ 8.11 (d, J = 8.6 Hz, 2H), 7.75–7.70 (m, 2H), 7.61–7.55 (m, 2H), 7.40–7.34 (m, 2H), 3.61–3.53 (m, 8H), 3.00–2.92 (m, 4H), 2.72–2.66 (m, 4H), 1.94–1.84 (m, 8H), 1.68–1.61 (m, 4H), 1.58–1.51 (m, 4H), 1.38–1.29 (m, 12H); 13C-NMR (126 MHz, CD3OD): δ 183.5, 169.4, 157.4, 154.2, 146.1, 130.7, 126.2, 125.0, 124.9, 120.3, 115.8, 45.1, 33.1, 32.1, 32.0, 29.8, 27.7, 27.2, 25.9, 23.8, 23.3; HRMS [M + H]+: 701.4534 (calculated for [C44H56N6O2]+: 701.4538).
Bis({8-[(1,2,3,4-tetrahydroacridin-9-yl)amino]octyl}amino)cyclobut-3-ene-1,2-dione (3g). Yield 8%; yellow solid; Mp = 64–65 °C; 1H-NMR (500 MHz, CDCl3): δ 7.97 (d, J = 8.5 Hz, 2H), 7.91–7.78 (m, 4H), 7.51 (t, J = 7.6 Hz, 2H), 7.32 (t, J = 7.6 Hz, 2H), 4.34 (t, J = 6.0 Hz, 2H), 3.73–3.35 (m, 12H), 3.03–2.95 (m, 4H), 2.72–2.61 (m, 4H), 1.96–1.78 (m, 8H), 1.63–1.47 (m, 8H), 1.35–1.09 (m, 12H); 13C-NMR (126 MHz, CDCl3): δ 182.4, 168.2, 157.5, 151.6, 146.5, 128.8, 127.3, 123.7, 123.2, 119.6, 115.2, 50.4, 48.9, 44.3, 33.3, 31.5, 30.9, 28.8, 26.4, 26.1, 24.6, 22.8, 22.5; HRMS [M + H]+: 729.4848 (calculated for [C46H60N6O2]+: 729.4851).
Bis({2-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]ethyl}amino)cyclobut-3-ene-1,2-dione (4a). Yield 6%; yellow solid; Mp = 113–114 °C; 1H-NMR (500 MHz, DMSO-d6): δ 8.10 (d, J = 9.1 Hz, 2H), 7.72–7.66 (m, 2H), 7.35–7.24 (m, 2H), 3.73–3.43 (m, 8H), 2.91–2.75 (m, 4H), 2.70–2.59 (m, 4H), 1.80–1.67 (m, 8H); 13C-NMR (126 MHz, DMSO-d6): δ 182.6, 159.4, 150.5, 147.4, 132.7, 126.6, 125.5, 123.8, 118.8, 116.8, 69.9, 48.8, 44.3, 33.5, 25.1, 22.7, 22.3; HRMS [M + H]+: 629.2179 (calculated for [C34H34Cl2N6O2]+: 629.2194).
Bis({3-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]propyl}amino)cyclobut-3-ene-1,2-dione (4b). Yield 28%; Mp = 86–88 °C; 1H-NMR (500 MHz, DMSO-d6): δ 8.11 (d, J = 9.1 Hz, 2H), 7.70–7.65 (m, 2H), 7.42 (bs, 2H), 7.32–7.25 (m, 2H), 3.52 (bs, 4H), 3.49–3.41 (m, 8H), 2.90–2.81 (m, 4H), 2.70–2.60 (m, 4H), 1.86–1.69 (m, 12H); 13C-NMR (126 MHz, DMSO-d6): δ 182.5, 159.2, 150.6, 147.3, 132.8, 126.5, 125.5, 123.67, 118.5, 116.1, 48.8, 44.9, 41.1, 33.5, 32.3, 25.2, 22.7, 22.3; HRMS [M + H]+: 657.2513 (calculated for [C36H38Cl2N6O2]+: 657.2507).
Bis({4-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]butyl}amino)cyclobut-3-ene-1,2-dione (4c). Yield 59%; yellow solid; Mp = 99–100 °C; 1H-NMR (500 MHz, DMSO-d6): δ 8.13 (d, J = 9.1 Hz, 2H), 7.69 (d, J = 2.2 Hz, 2H), 7.42 (bs, 2H), 7.32 (dd, J = 9.0, 2.3 Hz, 2H), 3.64–3.27 (m, 8H), 2.90–2.82 (m, 4H), 2.68–2.60 (m, 4H), 1.84–1.66 (m, 8H), 1.60–1.46 (m, 8H); 13C-NMR (126 MHz, DMSO-d6): δ 182.4, 167.9, 158.8, 150.9, 146.9, 133.0, 126.1, 125.7, 123.8, 118.4, 115.9, 47.6, 43.1, 33.2, 28.3, 27.6, 25.1, 22.6, 22.2; HRMS [M + H]+: 685.2819 (calculated for [C38H42Cl2N6O2]+: 685.2820).
Bis({5-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]pentyl}amino)cyclobut-3-ene-1,2-dione (4d). Yield 10%; yellow solid; Mp = 85–86 °C; 1H-NMR (500 MHz, DMSO-d6): δ 8.11 (d, J = 9.1 Hz, 2H), 7.69 (d, J = 2.3 Hz, 2H), 7.31 (dd, J = 9.1, 2.3 Hz, 2H), 3.42–3.24 (m, 8H), 2.92–2.81 (m, 4H), 2.71–2.60 (m, 4H), 1.84–1.69 (m, 8H), 1.62–1.52 (m, 4H), 1.51–1.40 (m, 4H), 1.35–1.23 (m, 4H); 13C-NMR (126 MHz, DMSO-d6): δ 182.4, 167.9, 159.4, 150.7, 147.6, 132.6, 126.7, 125.6, 123.6, 118.7, 116.1, 47.9, 43.3, 33.6, 30.6, 30.3, 25.2, 23.4, 22.7, 22.4; HRMS [M + H]+: 713.3129 (calculated for [C40H46Cl2N6O2]+: 713.3133).
Bis({6-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]hexyl}amino)cyclobut-3-ene-1,2-dione (4e). Yield 36%; yellow solid; Mp = 87–88 °C; 1H-NMR (500 MHz, DMSO-d6): δ 8.11 (d, J = 9.1 Hz, 2H), 7.69 (d, J = 2.3 Hz, 2H), 7.31 (dd, J = 9.1, 2.3 Hz, 2H), 5.55 (t, J = 6.3 Hz, 2H), 4.10 (bs, 2H), 3.44–3.35 (m, 8H), 2.86 (t, J = 6.2 Hz, 4H), 2.65 (t, J = 6.0 Hz, 4H), 1.83–1.71 (m, 8H), 1.57–1.49 (m, 4H), 1.48–1.40 (m, 4H), 1.31–1.20 (m, 8H); 13C-NMR (126 MHz, DMSO-d6): δ 182.4, 167.9, 159.3, 150.7, 147.5, 132.7, 126.7, 125.6, 123.6, 118.7, 115.9, 48.0, 43.3, 33.6, 30.9, 30.6, 26.1, 25.7, 25.2, 22.7, 22.4; HRMS [M + H]+: 741.3434 (calculated for [C42H50Cl2N6O2]+: 741.3446).
Bis({7-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]heptyl}amino)cyclobut-3-ene-1,2-dione (4f). Yield 87%; yellow solid; Mp = 85–86 °C; 1H-NMR (500 MHz, CDCl3): δ 7.85 (d, J = 9.1 Hz, 2H), 7.79 (d, J = 2.2 Hz, 2H), 7.54 (bs, 2H), 7.21 (dd, J = 9.0, 2.2 Hz, 2H), 4.12 (t, J = 6.0 Hz, 2H), 3.59 (q, J = 6.7 Hz, 4H), 3.43 (q, J = 6.7 Hz, 4H), 3.01–2.90 (m, 4H), 2.66–2.55 (m, 4H), 1.92–1.81 (m, 8H), 1.61–1.43 (m, 8H), 1.31–1.16 (m, 12H); 13C-NMR (126 MHz, CDCl3): δ 182.4, 168.1, 159.3, 151.1, 147.7, 134.1, 126.9, 124.8, 124.1, 118.2, 115.5, 50.6, 49.0, 44.4, 33.8, 31.4, 30.9, 28.6, 26.4, 26.2, 24.5, 22.8, 22.5; HRMS [M + H]+: 769.3745 (calculated for [C44H54Cl2N6O2]+: 769.8446).
Bis({8-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]octyl}amino)cyclobut-3-ene-1,2-dione (4g). Yield 55%; brown solid; Mp = 58–60 °C; 1H-NMR (500 MHz, CDCl3): δ 7.87 (d, J = 9.0 Hz, 2H), 7.80 (d, J = 2.1 Hz, 2H), 7.60 (bs, 2H), 7.23 (dd, J = 9.0, 2.2 Hz, 2H), 4.13 (t, J = 6.0 Hz, 2H), 3.62 (q, J = 6.7 Hz, 4H), 3.47 (q, J = 6.7 Hz, 4H), 3.01–2.92 (m, 4H), 2.67–2.59 (m, 4H), 1.93–1.83 (m, 8H), 1.59 (p, J = 7.1 Hz, 4H), 1.51 (p, J = 7.0 Hz, 4H), 1.29–1.09 (m, 16H); 13C-NMR (126 MHz, CDCl3): δ 190.0, 189.8, 184.6, 184.4, 178.0, 177.4, 174.8, 174.7, 160.2, 160.2, 153.3, 148.5, 135.5, 126.6, 126.6, 126.5, 124.9, 119.4, 116.7, 70.6, 45.5, 45.3, 34.2, 32.2, 32.2, 31.8, 31.5, 30.2, 30.1, 30.1, 29.9, 27.8, 27.7, 27.3, 27.2, 25.9, 25.9, 23.9, 23.6, 16.2, 16.1; HRMS [M + H]+: 797.4061 (calculated for [C44H54Cl2N6O2]+: 797.4072).
Bis({2-[(7-methoxy-1,2,3,4-tetrahydroacridin-9-yl)amino]ethyl}amino)cyclobut-3-ene-1,2-dione (5a). Yield 26%; yellow solid; Mp = 106–107 °C; 1H-NMR (500 MHz, DMSO-d6): δ 7.63 (d, J = 9.1 Hz, 2H), 7.51 (bs, 2H), 7.41–7.35 (m, 2H), 7.18 (dd, J = 9.1, 2.7 Hz, 2H), 5.36 (bs, 2H), 3.84 (s, 6H), 3.74–3.57 (m, 4H), 3.54–3.40 (m, 4H), 2.90–2.80 (m, 4H), 2.77–2.66 (m, 4H), 1.86–1.64 (m, 8H); 13C-NMR (126 MHz, DMSO-d6): δ 182.6, 168.1, 155.8, 155.6, 149.3, 142.4, 129.7, 121.5, 120.3, 117.9, 101.6, 55.5, 48.8, 48.4, 44.2, 33.2, 25.4, 22.9, 22.6; HRMS [M + H]+: 621.3178 (calculated for [C36H40N6O4]+: 621.3184).
Bis({3-[(7-methoxy-1,2,3,4-tetrahydroacridin-9-yl)amino]propyl}amino)cyclobut-3-ene-1,2-dione (5b). Yield 15%; yellow solid; Mp = 64–65 °C; 1H-NMR (500 MHz, DMSO-d6): δ 7.63 (d, J = 9.1 Hz, 2H), 7.42 (d, J = 2.6 Hz, 2H), 7.19 (dd, J = 9.1, 2.6 Hz, 2H), 5.48 (bs, 2H), 3.85 (s, 6H), 3.55 (bs, 4H), 3.42–3.31 (m, 8H), 2.86 (t, J = 6.5 Hz, 4H), 2.70 (t, J = 6.3 Hz, 4H), 1.85–1.69 (m, 12H); 13C-NMR (126 MHz, DMSO-d6): δ 182.5, 167.9, 155.8, 155.5, 149.6, 142.3, 130.2, 129.6, 123.6, 121.3, 120.3, 118.8, 117.2, 101.6, 55.6, 44.5, 41.2, 33.1, 32.4, 25.6, 22.9, 22.6; HRMS [M + H]+: 649.3488 (calculated for [C38H44N6O4]+: 649.3497).
Bis({4-[(7-methoxy-1,2,3,4-tetrahydroacridin-9-yl)amino]butyl}amino)cyclobut-3-ene-1,2-dione (5c). Yield 61%; yellow solid; Mp = 91–92 °C; 1H-NMR (500 MHz, CDCl3): δ 7.84 (bs, 2H), 7.71 (d, J = 9.1 Hz, 2H), 7.18 (d, J = 2.7 Hz, 2H), 7.15 (d, J = 2.6 Hz, 1H), 7.14 (d, J = 2.6 Hz, 1H), 4.23–4.12 (m, 2H), 3.81 (s, 6H), 3.59–3.54 (m, 4H), 3.40–3.23 (m, 4H), 2.96–2.88 (m, 4H), 2.65–2.55 (m, 4H), 1.86–1.73 (m, 8H), 1.69–1.55 (m, 8H); 13C-NMR (126 MHz, CD3OD): δ 183.5, 169.3, 157.7, 156.4, 152.4, 142.8, 128.9, 122.2, 118.0, 102.7, 56.1, 44.9, 33.6, 29.8, 28.9, 26.3, 24.0, 23.6; HRMS [M + H]+: 677.3799 (calculated for [C40H48N6O4]+: 677.3810).
Bis({5-[(7-methoxy-1,2,3,4-tetrahydroacridin-9-yl)amino]pentyl}amino)cyclobut-3-ene-1,2-dione (5d). Yield 50%; yellow solid; Mp = 70–72 °C; 1H-NMR (500 MHz, CDCl3): δ 7.85 (s, 2H), 7.73 (d, J = 9.1 Hz, 2H), 7.21 (d, J = 2.7 Hz, 2H), 7.17 (dd, J = 9.1, 2.6 Hz, 2H), 4.29–4.13 (m, 2H), 3.84 (s, 6H), 3.64–3.59 (m, 4H), 3.35 (q, J = 6.8 Hz, 4H), 2.94 (m, J = 6.8 Hz, 4H), 2.66–2.61 (m, 4H), 1.85–1.81 (m, 8H), 1.62–1.56 (m, 8H), 1.44–1.35 (m, 4H); 13C-NMR (126 MHz, CDCl3): δ 182.4, 168.2, 156.0, 155.3, 150.6, 141.8, 128.5, 120.8, 120.8, 116.6, 101.9, 55.6, 48.4, 44.0, 32.9, 31.0, 30.7, 24.7, 23.7, 22.8, 22.5; HRMS [M + H]+: 705.4119 (calculated for [C42H52N6O4]+: 705.4123).
Bis({6-[(7-methoxy-1,2,3,4-tetrahydroacridin-9-yl)amino]hexyl}amino)cyclobut-3-ene-1,2-dione (5e). Yield 18%; yellow solid; Mp = 157–159 °C; 1H-NMR (500 MHz, CDCl3): δ 8.20 (bs, 2H), 7.75 (d, J = 9.2 Hz, 2H), 7.32 (d, J = 2.7 Hz, 2H), 7.18 (dd, J = 9.2, 2.6 Hz, 2H), 4.83 (bs, 2H), 4.20–3.93 (m, 4H), 3.87 (s, 6H), 3.67–3.60 (m, 4H), 2.99–2.94 (m, 4H), 2.70–2.65 (m, 4H), 1.88–1.81 (m, 8H), 1.69–1.55 (m, 10H), 1.47–1.32 (m, 10H); 13C-NMR (126 MHz, CDCl3): δ 182.2, 168.3, 156.2, 154.1, 151.5, 140.2, 127.1, 121.4, 120.1, 115.4, 102.1, 55.7, 50.5, 48.1, 44.0, 32.0, 31.1, 30.7, 26.1, 25.8, 24.8, 22.7, 22.1; HRMS [M + H]+: 733.4434 (calculated for [C44H56N6O4]+: 733.4436).
Bis({7-[(7-methoxy-1,2,3,4-tetrahydroacridin-9-yl)amino]heptyl}amino)cyclobut-3-ene-1,2-dione (5f). Yield 16%; yellow solid; Mp = 132–134 °C; 1H-NMR (500 MHz, CDCl3): δ 8.32 (bs, 2H), 7.81 (dd, J = 9.5, 4.7 Hz, 2H), 7.36 (d, J = 2.6 Hz, 2H), 7.26–7.17 (m, 2H), 3.88 (s, 4H), 3.68–3.58 (m, 4H), 3.58–3.48 (m, 4H), 3.05–2.95 (m, 4H), 2.75–2.62 (m, 4H), 1.92–1.80 (m, 8H), 1.72–1.50 (m, 8H), 1.38–1.29 (m, 12H); 13C-NMR (126 MHz, CDCl3): δ 182.2, 168.3, 156.2, 153.5, 152.0, 139.5, 126.5, 121.7, 119.8, 114.9, 102.4, 55.7, 53.4, 48.2, 44.2, 31.6, 31.1, 30.7, 28.5, 26.4, 25.9, 24.7, 22.7, 21.9; HRMS [M + H]+: 761.4736 (calculated for [C46H60N6O4]+: 761.4749).
Bis({8-[(7-methoxy-1,2,3,4-tetrahydroacridin-9-yl)amino]octyl}amino)cyclobut-3-ene-1,2-dione (5g). Yield 37%; yellow solid; Mp = 87–89 °C; 1H-NMR (50 MHz, CDCl3): δ 7.78–7.72 (m, 2H), 7.65 (bs, 2H), 7.25–7.19 (m, 4H), 3.95 (t, J = 6.4 Hz, 2H), 3.88 (s, 6H), 3.61 (q, J = 6.5 Hz, 4H), 3.43 (q, J = 6.7 Hz, 4H), 3.03–2.91 (m, 4H), 2.73–2.67 (m, 4H), 2.61 (bs, 2H), 1.93–1.83 (m, 8H), 1.58 (p, J = 7.0 Hz, 4H), 1.47 (m, 4H), 1.27–1.06 (m, 16H); 13C-NMR (126 MHz, CDCl3): δ 182.6, 168.2, 155.9, 155.9, 150.6, 142.9, 129.3, 120.9, 120.6, 116.8, 101.9, 55.5, 50.5, 48.4, 44.3, 33.5, 31.8, 30.7, 28.8, 28.8, 26.4, 25.9, 24.7, 22.9, 22.7; HRMS [M + H]+: 789.5064 (calculated for [C48H64N6O4]+: 789.5062).
4.2. In Vitro Anti-ChE Assay
The AChE/BChE inhibitory activity of the tested drugs was determined using Ellman’s method [47,48,49,50] and is expressed as IC50, the i.e., concentration that reduces the cholinesterase activity by 50%. Human plasmatic butyrylcholinesterase (BChE; EC 3.1.1.8) and recombinant acetylcholinesterase (AChE; 3.1.1.7) were prepared at the Department of Toxicology and Military Pharmacy. 5,5′-dithiobis(2-nitrobenzoic acid) (Ellman’s reagent, DTNB), phosphate buffer (PB, pH 7.4), acetylthiocholine (ATC) and butyrylthiocholine (BTC), were purchased from Sigma-Aldrich, Prague, Czech Republic. For measuring purposes – polystyrene Nunc 96-well microplates with flat bottom shape (ThermoFisher Scientific, USA) were utilized.
All the assays were carried out in 0.1 M KH2PO4/K2HPO4 buffer, pH 7.4. Enzyme solutions were prepared at 2.0 units/mL in 2 mL aliquots. The assay medium (100 µL) consisted of 40 µL of 0.1 M phosphate buffer (pH 7.4), 20 µL of 0.01 M DTNB, 10 µL of the enzyme, and 20 µL of 0.01 M substrate (ATC/BTC iodide solution).
Inhibitor solutions in concentration range 10−3–10−11 M were prepared and IC50 values were calculated. Tested compounds were preincubated for 5 min. The reaction was started by immediate addition of 20 µL of the substrate. The activity was determined by measuring the increase in absorbance at 412 for AChE/BChE at 37 °C at 2 min intervals - using a Multi-mode microplate reader Synergy 2 (Winooski, VT, USA). Each concentration was assayed in triplicate. Software GraphPad Prizm 5 (San Diego, CA, USA) was used for the statistical data evaluation.
4.3. Kinetic Study of AChE and BChE Inhibition
The kinetic study of AChE and BChE was performed by using the above-mentioned modified Ellman’s method. The values of Vmax and Km of the Michaelis-Menten kinetics as well as the values of Ki and Ki’ were calculated by nonlinear regression from the substrate velocity curves. Linear regression was used for calculation of Lineweaver-Burk plots. All calculations were performed using GraphPad Prism software version 6.07 for Windows (San Diego, CA, USA).
4.4. Evaluation of Cytotoxicity by MTT Assay
The human origin cell line HepG2 (ATCC, Mannassas, VA, USA) isolated from liver hepatocellular carcinoma was used to evaluate cytotoxicity of tested compounds. The cells were cultivated in Dublecco’s modified Eagle’s medium (DMEM; Biosera, Nuaille, France) supplemented with 10% fetal bovine serum (Biosera) and 1% penicillin-streptomycin antibiotic solution (Sigma-Aldrich, St. Louis, MO, USA), incubated at 37 °C in a CO2 incubator (Binder CO2 incubator BC 160, Tuttlingen, Germany) and routinely passaged by trypsinization at 75–85% confluence.
The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetraziolium bromide (Sigma–Aldrich) reduction assay was used for measurement of compounds’ cytotoxicity according to [68]. MTT is a water soluble tetrazolium salt and it is converted to purple formazan by succinate dehydrogenase in mitochondria of viable cells [69,70]. Cell viability was detected after 24-h incubation with the tested substances. For the assay, HepG2 cells were seeded into 96-well plates in 100 µL volume and density of 15 × 103 per well. Cells were allowed to attach overnight before the treatment. The stock solutions of tested compounds were prepared in dimethyl sulfoxide (DMSO; Sigma-Aldrich), which were further serially diluted in DMEM and added to the cells in 96-well culture plates. The final concentration of DMSO was less than 0.25% per well.
After 24-h incubation, the medium containing serially diluted substances was aspirated from each well and replaced by 100 µL of fresh medium containing MTT (0.5 mg/mL). Plates were subsequently incubated at 37 °C in CO2 incubator for 45 min. The medium containing MTT was then aspirated and formazan dissolved in 100 μL of DMSO. The optical density of each well was measured using Synergy 2 Multi-Mode Microplate Reader (BioTek Instruments, Inc., Winooski, VT, USA) at 570 nm. The cell viability was expressed as the percentage of untreated control. Each experiment was performed in triplicate and repeated three independent times.
The IC50 values were calculated using four parametric nonlinear regression by statistic GraphPad Prism software (version 5.04; GraphPad Software Inc., San Diego, CA) from the logarithmic dose–response curve. The IC50 values were expressed as a mean ± standard error of the mean (SEM).
4.5. Determination of in Vitro BBB Permeation
PAMPA (the parallel artificial membrane permeability assay) is a high-throughput screening tool applicable for prediction of the passive transport of potential drugs across the blood-brain barrier (BBB) [57]. In this study, it has been used as a non-cell-based in vitro assay carried out in a coated 96-well membrane filter. The filter membrane of the donor plate was coated with PBL (Polar Brain Lipid, Avanti, USA) in dodecane (4 µl of 20 mg/mL PBL in dodecane) and the acceptor well was filled with 300 µl of phosphate buffer saline, (PBS pH 7.4; VA). The tested compounds were dissolved first in DMSO and then diluted with PBS pH 7.4 to reach the final concentrations 50–500 µM in the donor well. The final concentration of DMSO did not exceed 0.5% (v/v) in the donor solution; 300 µl of the donor solution (VD) was added to the donor wells and the donor filter plate was carefully put on the acceptor plate so that the coated membrane was “in touch” with both donor solution and acceptor buffer. In principle, test compound diffuse from the donor well through the polar brain lipid membrane (area = 0.28 cm2) to the acceptor well. The concentration of the tested compound in both donor and the acceptor wells were assessed after 3, 4, 5 and 6 h of incubation in quadruplicate using the UV plate reader Synergy HT (Biotek, USA) at the maximum absorption wavelength of each compound (n = 3). Besides that, the solution of theoretical compound concentration, simulating the equilibrium state, established if the membrane was ideally permeable was prepared and assessed as well. The concentration of the compounds in the donor and acceptor well and equilibrium concentration were calculated from the standard curve and expressed as the permeability (Pe) according the equation [57]
| (1) |
where .
4.6. Molecular Modeling Studies
From the online PDB database (www.pdb.org) models of hAChE (PDB ID: 4EY7, resolution: 2.35 Å) and hBChE (PDB ID: 4BDS, resolution: 2.10 Å) were downloaded and prepared for flexible molecular docking by MGL Tools utilities [59,60]. The preparation of these receptors involved removal of the surplus copies of the enzyme chains, non-bonded inhibitors, addition of polar hydrogens and merging of non-polar ones. Default Gasteiger charges were assigned to all atoms. Flexible parts of the enzymes were determined by a spherical selection of residues (R = 11 Å) approximately around the center of the active site. In the same points, the centers of the grid box of 33 × 33 × 33 Å were positioned. The rotatable bonds in the flexible residues were detected automatically by AutoDock Tools 1.5.4 program. Given the limitation of the program used for flexible molecular docking, water molecules had to be removed from the system. The flexible receptor parts contained 40 residues for hAChE and 39 residues for hBChE. The following xyz coordinates of the grid box centers were applied: hAChE (10.698, −58.115, −23.192); hBChE (140.117, 122.247, 38.986). The studied ligands were firstly drawn in HyperChem 8.0, then manually protonated as suggested by MarvinSketch 6.2.0. software (http://www.chemaxon.com), geometrically optimized by semi-empirical quantum-chemistry PM3 method and stored as pdb files. The structures of the ligands were processed for docking in a similar way as abovementioned flexible parts of the receptor by AutoDock Tools 1.5.4 program. Molecular docking was carried out in AutoDock Vina 1.1.2 program utilizing computer resources of the Czech National Grid Infrastructure MetaCentrum. The search algorithm of AutoDock Vina efficiently combines a Markov chain Monte Carlo like method for the global search and a Broyden-Fletcher-Goldfarb-Shano gradient approach for the local search [61]. It is a type of memetic algorithm based on interleaving stochastic and deterministic calculations [70]. Each docking task was repeated 20 times with the exhaustiveness parameter set to 16, employing 16 CPUs in parallel multithreading. From the obtained results, the solutions reaching the minimum predicted Gibbs binding energy were taken as the top-scoring modes. The graphic representations of the docked poses were shown in PyMOL (The PyMOL Molecular Graphics System, Version 1.5.0.4 Schrödinger, LLC.). 2D diagrams were generated using Discovery Studio Visualizer v16.1.0.15350 (Dassault Systèmes Biovia Corp., 2016, San Diego, CA, USA).
Acknowledgments
The authors express their appreciation to L. Krbalek for her excellent technical assistance.
Supplementary Materials
The data containing 1H and 13C NMR spectra are available online at https://www.mdpi.com/2218-273X/9/8/379/s1.
Author Contributions
Organic synthesis, writing original draft, NMR spectra interpretation, B.S. and E.M.; in vitro measurement of cholinesterase activity, V.H. and M.H.; cytotoxicity screening, L.M.; BBB permeation prediction, T.K. and O.S.; enzyme kinetic analysis, D.J.; NMR measurement, M.L.J.; in silico data interpretation, J.K.; supervision, J.K. and J.M.-C.
Funding
This project was conceived by JMC (IQOG, CSIC, Madrid, Spain), initially carried out experimentally by EM, in Madrid, thanks to a Short-Term Scientific Mission (from 30 January until the 28 April 2017) granted by EU COST Action (CA15135: “Multi-target paradigm for innovative ligand identification in the drug discovery process (MuTaLig)”), and continued in Hradec Kralove (Czech Republic) by EM in collaboration with BS, under the supervision of JK, who organized and planned all the biological analysis and wrote the manuscript. JMC is very thankful to EM, BS, and JK for their collaboration, and to the COST Action CA15135 for supporting this project. The study was supported by a grant of Ministry of Defence “Long Term Development Plan” Medical Aspects of Weapons of Mass Destruction of the Faculty of Military Health Sciences, University of Defence, by the Ministry of Education, Youth and Sports of Czech Republic (project ERDF no. CZ.02.1.01/0.0/0.0/18_069/0010054) and by MH CZ - DRO (University Hospital Hradec Kralove, No. 00179906). JMC thanks MINECO (Government of Spain) (SAF2015-65586-R) for support.
Conflicts of Interest
The authors have declared no conflict of interest.
References
- 1.Ferri C.P., Prince M., Brayne C., Brodaty H., Fratiglioni L., Ganguli M., Hall K., Hasegawa K., Hendrie H., Huang Y., et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long J.Z., Cravatt B.F. The Metabolic Serine Hydrolases and Their Functions in Mammalian Physiology and Disease. Chem. Rev. 2011;111:6022–6063. doi: 10.1021/cr200075y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartus R.T., Dean R.L., Beer B., Lippa A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 4.Zemek F., Drtinova L., Nepovimova E., Sepsova V., Korabecny J., Klimes J., Kuca K. Outcomes of Alzheimer’s disease therapy with acetylcholinesterase inhibitors and memantine. Expert Opin. Drug Saf. 2014;13:759–774. doi: 10.1517/14740338.2014.914168. [DOI] [PubMed] [Google Scholar]
- 5.Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. 2006:CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vickers J.C., Mitew S., Woodhouse A., Fernandez-Martos C.M., Kirkcaldie M.T., Canty A.J., McCormack G.H., King A.E. Defining the earliest pathological changes of Alzheimer’s disease. Curr. Alzheimer Res. 2016;13:281–287. doi: 10.2174/1567205013666151218150322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim G.H., Kim J.E., Rhie S.J., Yoon S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015;24:325–340. doi: 10.5607/en.2015.24.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang R., Reddy P.H. Role of Glutamate and NMDA Receptors in Alzheimer’s Disease. J. Alzheimers Dis. JAD. 2017;57:1041–1048. doi: 10.3233/JAD-160763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Budimir A. Metal ions, Alzheimer’s disease and chelation therapy. Acta Pharm. Zagreb Croat. 2011;61:1–14. doi: 10.2478/v10007-011-0006-6. [DOI] [PubMed] [Google Scholar]
- 10.Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M., et al. Neuroinflammation in Alzheimer’s Disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonkusare S.K., Kaul C.L., Ramarao P. Dementia of Alzheimer’s disease and other neurodegenerative disorders--memantine, a new hope. Pharmacol. Res. 2005;51:1–17. doi: 10.1016/j.phrs.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Crismon M.L. Tacrine: first drug approved for Alzheimer’s disease. Ann. Pharmacother. 1994;28:744–751. doi: 10.1177/106002809402800612. [DOI] [PubMed] [Google Scholar]
- 13.Horak M., Holubova K., Nepovimova E., Krusek J., Kaniakova M., Korabecny J., Vyklicky L., Kuca K., Stuchlik A., Ricny J., et al. The pharmacology of tacrine at N-methyl-d-aspartate receptors. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2017;75:54–62. doi: 10.1016/j.pnpbp.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Soukup O., Jun D., Zdarova-Karasova J., Patocka J., Musilek K., Korabecny J., Krusek J., Kaniakova M., Sepsova V., Mandikova J., et al. A resurrection of 7-MEOTA: a comparison with tacrine. Curr. Alzheimer Res. 2013;10:893–906. doi: 10.2174/1567205011310080011. [DOI] [PubMed] [Google Scholar]
- 15.Lahiri D.K., Lewis S., Farlow M.R. Tacrine alters the secretion of the beta-amyloid precursor protein in cell lines. J. Neurosci. Res. 1994;37:777–787. doi: 10.1002/jnr.490370612. [DOI] [PubMed] [Google Scholar]
- 16.Lahiri D.K., Farlow M.R., Sambamurti K. The secretion of amyloid beta-peptides is inhibited in the tacrine-treated human neuroblastoma cells. Brain Res. Mol. Brain Res. 1998;62:131–140. doi: 10.1016/S0169-328X(98)00236-8. [DOI] [PubMed] [Google Scholar]
- 17.Watkins P.B., Zimmerman H.J., Knapp M.J., Gracon S.I., Lewis K.W. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. JAMA. 1994;271:992–998. doi: 10.1001/jama.1994.03510370044030. [DOI] [PubMed] [Google Scholar]
- 18.Zeiger E., Erexson G., Mortelmans K., Thilagar A. Genetic toxicity studies of 1,2,3,4-tetrahydro-9-acridinamine (tacrine) Mutat. Res. 1997;393:189–197. doi: 10.1016/S1383-5718(97)00096-X. [DOI] [PubMed] [Google Scholar]
- 19.Misik J., Nepovimova E., Pejchal J., Kassa J., Korabecny J., Soukup O. Cholinesterase Inhibitor 6-Chlorotacrine—In Vivo Toxicological Profile and Behavioural Effects. [(accessed on 2 August 2019)]; doi: 10.2174/1567205015666171212105412. Available online: http://www.eurekaselect.com/158225/article. [DOI] [PubMed]
- 20.Pang Y.P., Quiram P., Jelacic T., Hong F., Brimijoin S. Highly potent, selective, and low cost bis-tetrahydroaminacrine inhibitors of acetylcholinesterase. Steps toward novel drugs for treating Alzheimer’s disease. J. Biol. Chem. 1996;271:23646–23649. doi: 10.1074/jbc.271.39.23646. [DOI] [PubMed] [Google Scholar]
- 21.Recanatini M., Cavalli A., Belluti F., Piazzi L., Rampa A., Bisi A., Gobbi S., Valenti P., Andrisano V., Bartolini M., et al. SAR of 9-amino-1,2,3,4-tetrahydroacridine-based acetylcholinesterase inhibitors: synthesis, enzyme inhibitory activity, QSAR, and structure-based CoMFA of tacrine analogues. J. Med. Chem. 2000;43:2007–2018. doi: 10.1021/jm990971t. [DOI] [PubMed] [Google Scholar]
- 22.Carlier P.R., Han Y.F., Chow E.S., Li C.P., Wang H., Lieu T.X., Wong H.S., Pang Y.P. Evaluation of short-tether bis-THA AChE inhibitors. A further test of the dual binding site hypothesis. Bioorg. Med. Chem. 1999;7:351–357. doi: 10.1016/S0968-0896(98)00213-2. [DOI] [PubMed] [Google Scholar]
- 23.Korábečný J. Prokognitivní Potenciál Bis(7)-takrinu Jako Zvažovaného Terapeutika Neurodegenerativních Onemocnění. MMSL. 2018;87:34–44. doi: 10.31482/mmsl.2018.006. [DOI] [Google Scholar]
- 24.Chauhan P., Mahajan S., Kaya U., Hack D., Enders D. Bifunctional Amine-Squaramides: Powerful Hydrogen-Bonding Organocatalysts for Asymmetric Domino/Cascade Reactions. Adv. Synth. Catal. 2015;357:253–281. doi: 10.1002/adsc.201401003. [DOI] [Google Scholar]
- 25.Zhao B.-L., Li J.-H., Du D.-M. Squaramide-Catalyzed Asymmetric Reactions. Chem. Rec. 2017;17:994–1018. doi: 10.1002/tcr.201600140. [DOI] [PubMed] [Google Scholar]
- 26.Karahan S., Tanyeli C. Squaramide catalyzed α-chiral amine synthesis. Tetrahedron Lett. 2018;59:3725–3737. doi: 10.1016/j.tetlet.2018.08.034. [DOI] [Google Scholar]
- 27.Brandão P., Burke A.J. Recent advances in the asymmetric catalytic synthesis of chiral 3-hydroxy and 3-aminooxindoles and derivatives: Medicinally relevant compounds. Tetrahedron. 2018;74:4927–4957. doi: 10.1016/j.tet.2018.06.015. [DOI] [Google Scholar]
- 28.Kinney W.A., Abou-Gharbia M., Garrison D.T., Schmid J., Kowal D.M., Bramlett D.R., Miller T.L., Tasse R.P., Zaleska M.M., Moyer J.A. Design and Synthesis of [2-(8,9-Dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)- ethyl]phosphonic Acid (EAA-090), a Potent N-Methyl-d-aspartate Antagonist, via the Use of 3-Cyclobutene-1,2-dione as an Achiral α-Amino Acid Bioisostere. J. Med. Chem. 1998;41:236–246. doi: 10.1021/jm970504g. [DOI] [PubMed] [Google Scholar]
- 29.Dwyer M.P., Yu Y., Chao J., Aki C., Chao J., Biju P., Girijavallabhan V., Rindgen D., Bond R., Mayer-Ezel R., et al. Discovery of 2-Hydroxy-N,N-dimethyl-3-{2-[[(R)-1-(5- methylfuran-2-yl)propyl]amino]-3,4-dioxocyclobut-1-enylamino}benzamide (SCH 527123): A Potent, Orally Bioavailable CXCR2/CXCR1 Receptor Antagonist. J. Med. Chem. 2006;49:7603–7606. doi: 10.1021/jm0609622. [DOI] [PubMed] [Google Scholar]
- 30.Storer R.I., Aciro C., Jones L.H. Squaramides: physical properties, synthesis and applications. Chem. Soc. Rev. 2011;40:2330–2346. doi: 10.1039/c0cs00200c. [DOI] [PubMed] [Google Scholar]
- 31.Marín C., Ximenis M., Ramirez-Macías I., Rotger C., Urbanova K., Olmo F., Martín-Escolano R., Rosales M.J., Cañas R., Gutierrez-Sánchez R., et al. Effective anti-leishmanial activity of minimalist squaramide-based compounds. Exp. Parasitol. 2016;170:36–49. doi: 10.1016/j.exppara.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 32.Ribeiro C.J.A., Espadinha M., Machado M., Gut J., Gonçalves L.M., Rosenthal P.J., Prudêncio M., Moreira R., Santos M.M.M. Novel squaramides with in vitro liver stage antiplasmodial activity. Bioorg. Med. Chem. 2016;24:1786–1792. doi: 10.1016/j.bmc.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Martín-Escolano R., Marín C., Vega M., Martin-Montes Á., Medina-Carmona E., López C., Rotger C., Costa A., Sánchez-Moreno M. Synthesis and biological evaluation of new long-chain squaramides as anti-chagasic agents in the BALB/c mouse model. Bioorg. Med. Chem. 2019;27:865–879. doi: 10.1016/j.bmc.2019.01.033. [DOI] [PubMed] [Google Scholar]
- 34.Fu H., Li W., Luo J., Lee N.T.K., Li M., Tsim K.W.K., Pang Y., Youdim M.B.H., Han Y. Promising anti-Alzheimer’s dimer bis(7)-tacrine reduces β-amyloid generation by directly inhibiting BACE-1 activity. Biochem. Biophys. Res. Commun. 2008;366:631–636. doi: 10.1016/j.bbrc.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 35.Li C., Carlier P.R., Ren H., Kan K.K.W., Hui K., Wang H., Li W., Li Z., Xiong K., Clement E.C., et al. Alkylene tether-length dependent γ-aminobutyric acid type A receptor competitive antagonism by tacrine dimers. Neuropharmacology. 2007;52:436–443. doi: 10.1016/j.neuropharm.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 36.Ros E., Aleu J., Gomez De Aranda I., Cantí C., Pang Y.-P., Marsal J., Solsona C. Effects of Bis(7)-Tacrine on Spontaneous Synaptic Activity and on the Nicotinic ACh Receptor of Torpedo Electric Organ. J. Neurophysiol. 2001;86:183–189. doi: 10.1152/jn.2001.86.1.183. [DOI] [PubMed] [Google Scholar]
- 37.Minarini A., Milelli A., Tumiatti V., Rosini M., Simoni E., Bolognesi M.L., Andrisano V., Bartolini M., Motori E., Angeloni C., et al. Cystamine-tacrine dimer: A new multi-target-directed ligand as potential therapeutic agent for Alzheimer’s disease treatment. Neuropharmacology. 2012;62:997–1003. doi: 10.1016/j.neuropharm.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Han Y.-F., Wu D.-C., Xiao X.-Q., Chen P.M.Y., Chung W., Lee N.T.K., Pang Y.-P., Carlier P.R. Protection against ischemic injury in primary cultured astrocytes of mouse cerebral cortex by bis(7)-tacrine, a novel anti-Alzheimer’s agent. Neurosci. Lett. 2000;288:95–98. doi: 10.1016/S0304-3940(00)01198-8. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Y., Li W., Chow P.C.Y., Lau D.T.K., Lee N.T.K., Pang Y., Zhang X., Wang X., Han Y. Bis(7)-tacrine, a promising anti-Alzheimer’s dimer, affords dose- and time-dependent neuroprotection against transient focal cerebral ischemia. Neurosci. Lett. 2008;439:160–164. doi: 10.1016/j.neulet.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Li J., Lu Z., Xu L., Wang Q., Zhang Z., Fang J. Neuroprotective effects of bis(7)-tacrine in a rat model of pressure-induced retinal ischemia. Cell Biochem. Biophys. 2014;68:275–282. doi: 10.1007/s12013-013-9707-4. [DOI] [PubMed] [Google Scholar]
- 41.Xiao X.Q., Lee N.T., Carlier P.R., Pang Y., Han Y.F. Bis(7)-tacrine, a promising anti-Alzheimer’s agent, reduces hydrogen peroxide-induced injury in rat pheochromocytoma cells: comparison with tacrine. Neurosci. Lett. 2000;290:197–200. doi: 10.1016/S0304-3940(00)01357-4. [DOI] [PubMed] [Google Scholar]
- 42.Li W., Xue J., Niu C., Fu H., Lam C.S.C., Luo J., Chan H.H.N., Xue H., Kan K.K.W., Lee N.T.K., et al. Synergistic Neuroprotection by Bis(7)-tacrine via Concurrent Blockade of N-Methyl-d-aspartate Receptors and Neuronal Nitric-Oxide Synthase. Mol. Pharmacol. 2007;71:1258–1267. doi: 10.1124/mol.106.029108. [DOI] [PubMed] [Google Scholar]
- 43.Li W., Lee N.T.K., Fu H., Kan K.K.W., Pang Y.-P., Li M., Tsim K.W.K., Li X., Han Y. Neuroprotection via inhibition of nitric oxide synthase by bis(7)-tacrine. NeuroReport. 2006;17:471–474. doi: 10.1097/01.wnr.0000209014.09094.72. [DOI] [PubMed] [Google Scholar]
- 44.Li W., Pi R., Chan H.H.N., Fu H., Lee N.T.K., Tsang H.W., Pu Y., Chang D.C., Li C., Luo J., et al. Novel dimeric acetylcholinesterase inhibitor bis7-tacrine, but not donepezil, prevents glutamate-induced neuronal apoptosis by blocking N-methyl-D-aspartate receptors. J. Biol. Chem. 2005;280:18179–18188. doi: 10.1074/jbc.M411085200. [DOI] [PubMed] [Google Scholar]
- 45.Nepovimova E., Korabecny J., Dolezal R., Babkova K., Ondrejicek A., Jun D., Sepsova V., Horova A., Hrabinova M., Soukup O., et al. Tacrine–Trolox Hybrids: A Novel Class of Centrally Active, Nonhepatotoxic Multi-Target-Directed Ligands Exerting Anticholinesterase and Antioxidant Activities with Low In Vivo Toxicity. J. Med. Chem. 2015;58:8985–9003. doi: 10.1021/acs.jmedchem.5b01325. [DOI] [PubMed] [Google Scholar]
- 46.Spilovska K., Korabecny J., Kral J., Horova A., Musilek K., Soukup O., Drtinova L., Gazova Z., Siposova K., Kuca K. 7-Methoxytacrine-adamantylamine heterodimers as cholinesterase inhibitors in Alzheimer’s disease treatment--synthesis, biological evaluation and molecular modeling studies. Molecules. 2013;18:2397–2418. doi: 10.3390/molecules18022397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ellman G.L., Courtney K.D., Andres V., Feather-Stone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 48.Pohanka M., Jun D., Kuca K. Improvement of acetylcholinesterase-based assay for organophosphates in way of identification by reactivators. Talanta. 2008;77:451–454. doi: 10.1016/j.talanta.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 49.Sepsova V., Karasova J.Z., Korabecny J., Dolezal R., Zemek F., Bennion B.J., Kuca K. Oximes: inhibitors of human recombinant acetylcholinesterase. A structure-activity relationship (SAR) study. Int. J. Mol. Sci. 2013;14:16882–16900. doi: 10.3390/ijms140816882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pohanka M., Karasova J.Z., Kuca K., Pikula J., Holas O., Korabecny J., Cabal J. Colorimetric dipstick for assay of organophosphate pesticides and nerve agents represented by paraoxon, sarin and VX. Talanta. 2010;81:621–624. doi: 10.1016/j.talanta.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 51.Bolognesi M.L., Cavalli A., Valgimigli L., Bartolini M., Rosini M., Andrisano V., Recanatini M., Melchiorre C. Multi-Target-Directed Drug Design Strategy: From a Dual Binding Site Acetylcholinesterase Inhibitor to a Trifunctional Compound against Alzheimer’s Disease. J. Med. Chem. 2007;50:6446–6449. doi: 10.1021/jm701225u. [DOI] [PubMed] [Google Scholar]
- 52.Nordberg A., Ballard C., Bullock R., Darreh-Shori T., Somogyi M. A review of butyrylcholinesterase as a therapeutic target in the treatment of Alzheimer’s disease. Prim. Care Companion CNS Disord. 2013;15 doi: 10.4088/PCC.12r01412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greig N.H., Utsuki T., Ingram D.K., Wang Y., Pepeu G., Scali C., Yu Q.-S., Mamczarz J., Holloway H.W., Giordano T., et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA. 2005;102:17213–17218. doi: 10.1073/pnas.0508575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nepovimova E., Uliassi E., Korabecny J., Peña-Altamira L.E., Samez S., Pesaresi A., Garcia G.E., Bartolini M., Andrisano V., Bergamini C., et al. Multitarget drug design strategy: quinone-tacrine hybrids designed to block amyloid-β aggregation and to exert anticholinesterase and antioxidant effects. J. Med. Chem. 2014;57:8576–8589. doi: 10.1021/jm5010804. [DOI] [PubMed] [Google Scholar]
- 55.Spilovska K., Korabecny J., Horova A., Musilek K., Nepovimova E., Drtinova L., Gazova Z., Siposova K., Dolezal R., Jun D., et al. Design, synthesis and in vitro testing of 7-methoxytacrine-amantadine analogues: a novel cholinesterase inhibitors for the treatment of Alzheimer’s disease. Med. Chem. Res. 2015;24:2645–2655. doi: 10.1007/s00044-015-1316-x. [DOI] [Google Scholar]
- 56.Muckova L., Pejchal J., Jost P., Vanova N., Herman D., Jun D. Cytotoxicity of acetylcholinesterase reactivators evaluated in vitro and its relation to their structure. Drug Chem. Toxicol. 2019;42:252–256. doi: 10.1080/01480545.2018.1432641. [DOI] [PubMed] [Google Scholar]
- 57.Di L., Kerns E.H., Fan K., McConnell O.J., Carter G.T. High throughput artificial membrane permeability assay for blood-brain barrier. Eur. J. Med. Chem. 2003;38:223–232. doi: 10.1016/S0223-5234(03)00012-6. [DOI] [PubMed] [Google Scholar]
- 58.Li C., Wainhaus S., Uss A.S., Cheng K.-C. High-Throughput Screening Using Caco-2 Cell and PAMPA Systems. In: Ehrhardt C., Kim K.-J., editors. Drug Absorption Studies: In Situ, In Vitro and In Silico Models. Springer US; Boston, MA, USA: 2008. pp. 418–429. Biotechnology: Pharmaceutical Aspects. [Google Scholar]
- 59.Cheung J., Rudolph M.J., Burshteyn F., Cassidy M.S., Gary E.N., Love J., Franklin M.C., Height J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012;55:10282–10286. doi: 10.1021/jm300871x. [DOI] [PubMed] [Google Scholar]
- 60.Nachon F., Carletti E., Ronco C., Trovaslet M., Nicolet Y., Jean L., Renard P.-Y. Crystal structures of human cholinesterases in complex with huprine W and tacrine: elements of specificity for anti-Alzheimer’s drugs targeting acetyl- and butyryl-cholinesterase. Biochem. J. 2013;453:393–399. doi: 10.1042/BJ20130013. [DOI] [PubMed] [Google Scholar]
- 61.Trott O., Olson A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hepnarova V., Korabecny J., Matouskova L., Jost P., Muckova L., Hrabinova M., Vykoukalova N., Kerhartova M., Kucera T., Dolezal R., et al. The concept of hybrid molecules of tacrine and benzyl quinolone carboxylic acid (BQCA) as multifunctional agents for Alzheimer’s disease. Eur. J. Med. Chem. 2018;150:292–306. doi: 10.1016/j.ejmech.2018.02.083. [DOI] [PubMed] [Google Scholar]
- 63.Rydberg E.H., Brumshtein B., Greenblatt H.M., Wong D.M., Shaya D., Williams L.D., Carlier P.R., Pang Y.-P., Silman I., Sussman J.L. Complexes of alkylene-linked tacrine dimers with Torpedo californica acetylcholinesterase: Binding of Bis5-tacrine produces a dramatic rearrangement in the active-site gorge. J. Med. Chem. 2006;49:5491–5500. doi: 10.1021/jm060164b. [DOI] [PubMed] [Google Scholar]
- 64.Bajda M., Więckowska A., Hebda M., Guzior N., Sotriffer C.A., Malawska B. Structure-Based Search for New Inhibitors of Cholinesterases. Int. J. Mol. Sci. 2013;14:5608–5632. doi: 10.3390/ijms14035608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cavalli A., Bolognesi M.L., Minarini A., Rosini M., Tumiatti V., Recanatini M., Melchiorre C. Multi-target-directed ligands to combat neurodegenerative diseases. J. Med. Chem. 2008;51:347–372. doi: 10.1021/jm7009364. [DOI] [PubMed] [Google Scholar]
- 66.León R., Garcia A.G., Marco-Contelles J. Recent advances in the multitarget-directed ligands approach for the treatment of Alzheimer’s disease. Med. Res. Rev. 2013;33:139–189. doi: 10.1002/med.20248. [DOI] [PubMed] [Google Scholar]
- 67.Proschak E., Stark H., Merk D. Polypharmacology by Design: A Medicinal Chemist’s Perspective on Multitargeting Compounds. J. Med. Chem. 2019;62:420–444. doi: 10.1021/acs.jmedchem.8b00760. [DOI] [PubMed] [Google Scholar]
- 68.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 69.Riss T.L., Moravec R.A., Niles A.L., Duellman S., Benink H.A., Worzella T.J., Minor L. Cell Viability Assays. In: Sittampalam G.S., Coussens N.P., Nelson H., Arkin M., Auld D., Austin C., Bejcek B., Glicksman M., Inglese J., Iversen P.W., et al., editors. Assay Guidance Manual. Eli Lilly & Company and the National Center for Advancing Translational Sciences; Bethesda, MD, USA: 2004. [Google Scholar]
- 70.Liu B., Wang L., Jin Y.-H. An effective PSO-based memetic algorithm for flow shop scheduling. IEEE Trans. Syst. Man Cybern. Part B Cybern. Publ. IEEE Syst. Man Cybern. Soc. 2007;37:18–27. doi: 10.1109/TSMCB.2006.883272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.