Abstract
Meningitis, encephalitis, and myelitis are various forms of acute central nervous system (CNS) inflammation, which can coexist and lead to serious sequelae. Known aetiologies include infections and immune-mediated processes. Despite advances in clinical microbiology over the past decades, the cause of acute CNS inflammation remains unknown in approximately 50% of cases. High-throughput sequencing was performed to search for viral sequences in cerebrospinal fluid (CSF) samples collected from 26 patients considered to have acute CNS inflammation of unknown origin, and 10 patients with defined causes of CNS diseases. In order to better grasp the clinical significance of viral sequence data obtained in CSF, 30 patients without CNS disease who had a lumbar puncture performed during elective spinal anaesthesia were also analysed. One case of human astrovirus (HAstV)-MLB2-related meningitis and disseminated infection was identified. No other viral sequences that can easily be linked to CNS inflammation were detected. Viral sequences obtained in all patient groups are discussed. While some of them reflect harmless viral infections, others result from reagent or sample contamination, as well as index hopping. Altogether, this study highlights the potential of high-throughput sequencing in identifying previously unknown viral neuropathogens, as well as the interpretation issues related to its application in clinical microbiology.
Keywords: acute central nervous system inflammation, meningitis, encephalitis, myelitis, high throughput sequencing, viruses, cerebrospinal fluid, viral sequences
1. Introduction
Acute central nervous system (CNS) inflammation, encompassing meningitis, encephalitis, myelitis, or any combination of these entities, results either from infection or dysimmunity [1,2]. Among infectious causes, viruses are the main culprits clinicians must search for, once pyogenic bacterial meningitis and nonpyogenic agents, such as Mycobacterium tuberculosis or Listeria monocytogenes (Lm), have been excluded [2,3,4]. Among neurotropic viruses, herpes simplex virus types 1 and 2 (HSV-1 and -2), varicella zoster virus (VZV), enteroviruses (EV), parechovirus, human immunodeficiency virus (HIV), as well as flaviviruses (whose types and prevalence vary according to the local epidemiology) are the predominant causes [5,6].
Despite significant improvements in microbiological investigations in the past decades through implementation of sensitive PCR-based assays performed on cerebrospinal fluid (CSF) or on cerebral biopsy samples, the cause of acute meningo-encephalitis remains unknown in approximately 50% of cases [2,3]. As the initial clinical features of this syndrome are often unspecific and cannot assert an aetiology, a large panel of molecular assays that are by essence formatted to screen predefined targets is often used. High-throughput sequencing (HTS) represents a tool enabling an unbiased microbial detection [7,8].
In the present investigation, HTS was used to screen for RNA and DNA viral signatures in CSF withdrawn from both patients admitted and investigated for acute CNS inflammation and in a control group of individuals undergoing spinal anaesthesia for an elective surgical procedure. This strategy intended not only to detect unexpected or divergent viral neuropathogens, but also to explore the human CNS virome.
2. Materials and Methods
The protocol of this study was approved by the Geneva Cantonal Ethics Commission (project numbers #13-074 and #2016-00549). Patients or their next of kin provided written informed consent before enrolment.
This study is a monocentric prospective unmatched case-control study performed at the Geneva University Hospitals, Switzerland. Inclusion criteria were: paediatric and adult patients hospitalized at Geneva University Hospitals from May 2013 through June 2017, presenting with suspected CNS inflammation, and for which no diagnosis was made in the first 48 h. Exclusion criteria were: the absence of a signed informed consent for the present study.
CNS inflammations were classified as three entities: encephalitis/meningoencephalitis, meningitis, and myelitis/meningomyelitis (see Table 1 for definitions) [9,10,11].
Table 1.
Acute central nervous system (CNS) inflammation definitions.
| Encephalitis and Meningo-Encephalitis | Meningitis | Myelitis and Meningomyelitis |
|---|---|---|
|
Major criteria (required) Altered mental status (defined as decreased or altered level of consciousness, lethargy or personality change) lasting ≥24 hours with no alternative cause identified Minor criteria (2 required for possible encephalitis, ≥3 required for probable, or confirmed encephalitis)
|
≥2 following criteria
AND CSF leukocyte count ≥5 M/L |
Major criteria (≥1 required)
Minor criteria (optional)
|
Concerning the control group, inclusion criteria were: adult patients without any known CNS disease, hospitalized from January 2016 through December 2017 either for elective surgery (orthopaedic, urologic, or visceral) under spinal anaesthesia; or women who underwent elective caesarean section under spinal anaesthesia. Exclusion criteria were: the absence of a signed informed consent for the present study.
CSF samples were collected through lumbar puncture performed for diagnostic purposes in the case group and during spinal anaesthesia, before injecting the anaesthetic mixture, in the control group. In the event that several CSF samples were collected from the same patient, only the first was selected for analysis. No patient received antiviral treatment before lumbar puncture except one immunocompromised patient treated with valaciclovir for secondary prevention of a herpes zoster infection, and an HIV-infected patient treated with atazanavir and ritonavir. All samples were stored at −80 °C before HTS analysis.
For all patients with CNS disease, a workup according to the standard of care was performed for diagnostic purposes. All CSFs were screened for EV, HSV-1 and -2, and VZV by in-house real-time (reverse transcription)-PCR rRT-PCR assays until June 2017, when the FilmArray Meningitis/Encephalitis (ME) Panel [12] was introduced as a routine tool to screen CSF for pathogens. Serological screening for HIV (n = 32/36), syphilis (n = 29/36), and Lyme disease (n = 33/36) was also performed in most patients. A cerebral CT scan was performed for all patients having encephalitis or myelitis. Additional microbiologic investigations, as well as radiological evaluation by CT-scan and/or MRI, electroencephalogram, immunological and oncologic investigations, were performed on a case-by-case basis.
In the control group, no analysis other than HTS on CSF samples was performed.
Medical records of the patients with CNS disease (n = 36) and controls (n = 30) were collected using a standardized CRF that included the main patients’ characteristics, and for patients with CNS disease, clinical, laboratory, and radiological data.
The viral enrichment process, the viral nucleic acid extraction, and the library preparations were done using two specific protocols for RNA and DNA genome viruses as previously described [13]. Briefly, two hundred microliters (μL) of each sample were treated with 40 U of Turbo DNAse (Ambion, Rotkreuz, Switzerland). Then, half the volume (120 μL) was used to perform RNA extraction using the TRIzol protocol (Invitrogen, Carlsbad, CA, USA), while the second half was used to perform a DNA extraction with a NucliSens easyMAG system (bioMérieux, Geneva, Switzerland), followed by a double-stranded DNA synthesis step (Klenow, New England BioLabs, Ipswich, MA, USA). Concerning the RNA sequencing library preparation, ribosomal RNA was removed prior to the preparation of the RNA libraries with the TruSeq total RNA preparation protocol (Illumina, San Diego, CA, US). DNA libraries were prepared using the Illumina Nextera XT protocol from Illumina. Then, concentrations of both RNA and DNA libraries were measure with a Q-bit (Life Technologies, Carlsbad, CA, USA) and the size distribution of fragments was checked with a 2200 TapeStation (Agilent, Santa Clara, CA, USA). For samples drawn from patients with CNS disease, RNA and DNA libraries were both run on a HiSeq 2500 (Illumina, San Diego, CA, USA; paired-end sequencing, 100-bp protocol). For the 30 control samples, RNA and DNA libraries were run on a HiSeq 2500 and 4000, respectively (Illumina, paired-end sequencing, 100-bp protocol). Raw data were analysed using an updated version of the ezVIR pipeline [13]. A ratio of 0.3% was used for cross-talk check [14]. In parallel, a de novo analysis was done on nonhuman reads using the assembly IDBA-UD software (v.1.1.3) [15]. Contigs > 1000 bp were blasted (blastx BLAST 2.3.0+) against a clustered version of the RVDB-prot 12.2 (http://rvdb-prot.pasteur.fr/) protein database.
In order to confirm HTS findings or confirm suspected sequence cross-lane, a set of specific rRT-PCR assays were applied to CSF with a sufficient initial specimen leftover to extract the viral genomes using the NucliSENS easyMAG (bioMérieux, Geneva, Switzerland). Such assays were performed when needed and available for adenovirus (ADV), Torque teno virus (TTV), Epstein-Barr virus (EBV), Merkel cell polyomavirus (MCPyV), human pegivirus-1 (HPgV-1), and Human herpesvirus 7 (HHV-7) (Table S1) [16,17,18,19,20]. The rRT-PCR assays were performed using the one-step QuantiTect Probe RT-PCR Kit (Qiagen, Hombrechtikon, Switzerland) in a StepOne Plus instrument (Applied Biosystems, Rotkreuz, Switzerland) for the HPgV-1 assays or using the TaqMan™ Universal PCR Master Mix (ThermoFisher, Reinach, Switzerland) on a QuantStudio 5 instrument (Applied Biosystems) for the others assays.
Data from atients and controls were described as frequency and percentage for categorical parameters and as mean ± standard deviation (SD) for continuous parameters. Comparisons between groups were performed using the Mann–Whitney test for continuous variables. A two-sided p-value of <0.05 was considered significant. Statistics were performed using Stata (StataCorp. 2015, College Station, TX, USA).
3. Results
3.1. Clinical and Paraclinical Data
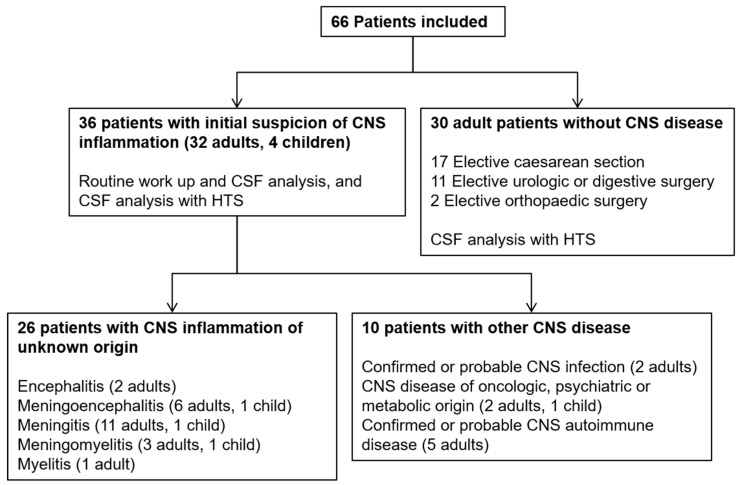
A total of 36 patients with suspected clinical acute CNS inflammation were included, and 30 patients without any CNS symptoms were included in the control group. Among the 36 enrolled cases with suspected CNS disease, a definitive diagnosis using routine investigations was made in 10 of them (nine adults and one child), who were collectively called the “diagnosed CNS disease” group. One patient had Lm meningo-encephalitis, one had an HIV-associated encephalitis, and eight had a documented noninfectious cause of CNS disease (Figure 1). The remaining 26 cases (23 adults and three children) composed a group with “acute CNS inflammation of unknown origin” and presented clinical signs compatible with acute encephalitis (n = 2), acute meningitis (n = 12), meningo-encephalitis (n = 7), myelitis (n = 1), and meningo-myelitis (n = 4) (Figure 1).
Figure 1.
Study flowchart showing patient groups included in the study.
Demographic characteristics for patients and controls are shown in Table 2 and Table 3, respectively.
Table 2.
Demographic characteristics of patients with central nervous system (CNS) disease.
| All | CNS Inflammation of Unknown Origin | Diagnosed CNS Disease | |
|---|---|---|---|
| Number (%) | 36 (100) | 26 (72.2) | 10 (27.8) |
| Male | 22 (61.1) | 16 (61.5) | 6 (60) |
| Mean age, years (SD) | 41.6 (18.5) | 38.7 (18.5) | 49.2 (17.1) |
| <18 years old (%) | 4 (11.1) | 3 (11.5) | 1 (10.0) |
Table 3.
Demographic characteristics of the control patients.
| All | Urologic or Digestive Surgery | Orthopaedic Surgery | Caesarean Section | |
|---|---|---|---|---|
| Number (%) | 30 (100) | 11 (36.6) | 2 (6.7) | 17 (56.7) |
| Male | 9 (30) | 9 (81.8) | 0 | 0 |
| Mean age, years (SD) | 53.4 (22.5) | 77.4 (7.5) | 81 (1.4) | 34.7 (4.1) |
Three patients with CNS disease (8.3%) were under immunosuppressive treatment (one liver and one kidney transplant recipients, and one patient with systemic lupus erythematosus).
The 26 cases with meningitis, encephalitis, and/or myelitis of undetermined cause were classified into three groups according to the definitions proposed in Table 1. Clinical and laboratory data are described in Table 4. Regarding CSF analysis, the patients’ median white blood cell count at the time of the CSF collection was 76 M/L (range 6–3706 M/L), most of the patients had proteinorachia above 0.45 g/L (range 0.24–2.32 g/L), and median glycorachia was 3.1 mmol/L (range 2.5–5.4 mmol/L) (Table 4). The median CRP level was 4.3 mg/L (range 0–266 mg/L) and median peripheral blood leucocyte count was 10 G/L (range 3.9–25.1 G/L) (Table 4).
Table 4.
Clinical and laboratory data of the 26 patients with CNS inflammation of unknown origin.
| All (N = 26) | Encephalitis and Meningo-encephalitis (n = 9) | Meningitis (n = 12) | Myelitis and Meningo-myelitis (n = 5) | |
|---|---|---|---|---|
| Clinical features, n (%) | ||||
| Headache | 19 (73.1) | 5 (55.6) | 11 (91.7) | 3 (60.0) |
| Neck stiffness | 3 (11.5) | 2 (22.2) | 1 (8.3) | 0 |
| Fever (≥38,2 °C) *1 | 13 (50.0) | 3 (33.3) | 7 (58.3) | 3 (60.0) |
| Nausea/vomiting | 2(7.7) | 0 | 2 (16.7) | 0 |
| Photophobia | 8 (30.8) | 2 (22.2) | 5 (41.6) | 1 (20.0) |
| Phonophobia | 4 (15.4) | 0 | 3 (25.0) | 1 (20.0) |
| Altered mental status *2 | 8 (30.8) | 8 (88.9) | 0 | 0 |
| Seizure | 2 (7.7) | 2 (22.2) | 0 | 0 |
| Suggestive abnormality on neuroimaging | 13 (50.0) | 5 (55.5) | 4 (33.3) | 4 (80.0) |
| Suggestive abnormality on EEG *3 | 8 (30.8) | 7 (77.8) | 1 (8.3) | 0 |
| Sensory or motor focal neurologic deficit | 9 (34.6) | 0 | 4 (33.3) | 5 (100.0) |
| Requiring intensive care | 4 (15.4) | 2 (22.2) | 0 | 2 (40.0) |
| Requiring immunosuppressive therapy | 6 (23.1) | 1 (11.1) | 1 (8.3) | 4 (80.0) |
| Clinical outcome *4 | ||||
| Complete resolution of signs and symptoms | 16 (61.5) | 3 (33.3) | 11 (91.6) | 2 (40.0) |
| Death | 0 | 0 | 0 | 0 |
| Laboratory features | ||||
| Cerebrospinal fluid | ||||
| WBC *5 count ≥ 5 M/L (%) | 26 (100.0) | 9 (100.0) | 12 (100.0) | 5 (100) |
| Median WBC count, M/L (range) | 76 (6–3706) | 53 (18–72) | 75(6–3706) | 161 (50–275) |
| Median glycorachia, mmol/L (range) (2.8–4.0 mmol/L) | 3.1 (2.5–5.4) | 3.2 (2.7–5.4) | 3.2 (2.7–4.5) | 2.8 (2.5–4.4) |
| Median proteinorachia (g/L) (range) (0.15–0.45 g/L) | 0.61 (0.24–2.32) | 0.7 (0.37–2.32) | 0.61 (0.24–2.27) | 0.53 (0.40–1.58) |
| Proteinorachia > 0.45 (%) | 21 (80.8) | 8 (88.9) | 9 (75.0) | 3 (60.0) |
| Proteinorachia < 0.15 (%) | 0 | 0 | 0 | 0 |
| Blood | ||||
| Median C-reactive protein mg/L, (range) (0–10 mg/L) *6 | 4.3 (0.0–266.1) | 1.7 (0.0–80.8) | 4.8 (1.6–266.1) | 18.1 (7.7–33.7) |
| Median Leucocytes G/L, (range) (4–11 G/L) *6 | 10.0 (3.9–25.1) | 7.4 (3.9–13.9) | 10.9 (4.9–25.1) | 14.4 (6.9–15.1) |
*1 Documented fever within 72 h before or after presentation to medical attention, *2 cf. Table 3, *3 electroencephalogram, *4 within the three months after the first day of hospitalization, *5 white blood cells, *6 Laboratory reference ranges.
Among the 26 patients with CNS inflammation of unknown cause using routine investigations, the time between onset of neurologic symptoms and lumbar puncture was available for 23 patients, and the median value was 3 days (range 0–8 days).
3.2. HTS Analysis and r(RT-)PCR Results
Due to the use of different HTS platforms over time for the DNA libraries and multiplexing protocols between cases and controls (see methods), the total million paired reads were lower in the samples from patients without CNS disease (control group) compared to those of patients with CNS inflammation and other CNS diseases (Figure S1). In order to confirm the sensitivity of the HTS method despite this variability, screening TTV with quantitative real-time PCR of all samples from patients without CNS disease was performed and revealed to be positive in one CSF (CSF31, viral load < 250 copies/mL), leading to concordant results with HTS and PCR assay in 29/30 (96.7%) samples.
3.2.1. Detection of Nonsignificant Viral Sequences and Reagent Contamination
Patients undergoing CSF puncture for anaesthesia purposes were considered as asymptomatic controls (n = 30) and a negative control for the whole HTS procedure was used. This led us to identify viral sequences reflecting HTS reagents contaminants (Figure S2).
Among the 12 MCPyV sequences identified in this group, none were confirmed by rPCR (Figure S2, panel A).
Papillomavirus and Polyomavirus sequences were obtained in 26/30 and 9/30 samples, respectively, as well as in the negative control. Although no specific rPCR assay was available for these two virus families, these findings most likely indicate environment or reagent contaminations.
Reagent contaminations with vector sequences were detected in three samples. Two were positive for adenovirus (CSF36 and CSF37) and one for parvovirus (CSF12) sequences.
3.2.2. Sequence Cross-Contamination
In 1/9 samples positive for anelloviruses by HTS (P36), reads were falsely assigned because of sequence cross-contamination (Figure S2, panel B).
3.2.3. Viral Sequences Assigned to Pathogenic or Commensal Viruses
Control Patients
Anelloviruses were not detected in the CSF samples collected from the 30 controls.
HPgV-1 sequences detected by HTS in two CSF samples were confirmed by rRT-PCR in samples CSF33 and CSF37 (Figure S2, panel A).
Two samples tested positive for HHV-7 sequences by HTS. One of these was considered to result from index hopping. None of these two samples was confirmed by rPCR (Figure S2, panel A).
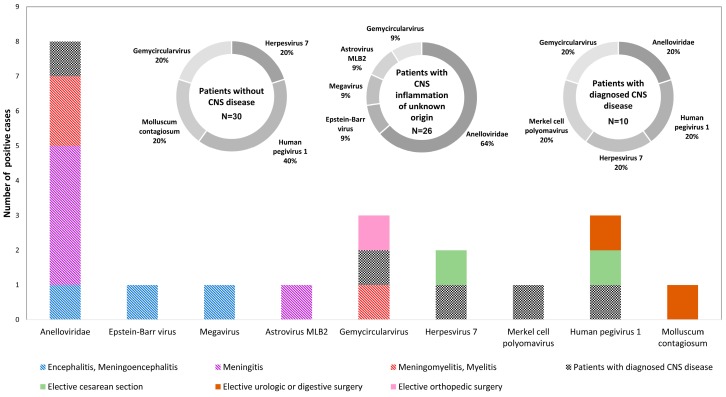
Gemycircularvirus (GemyCV) sequences were detected by HTS in one sample, and Molluscum contagiosum virus sequences in another sample in this group. These findings could not be confirmed due to the lack of specific rPCR assays for these viruses in our laboratory (Figure 2, and Figure S2, panel A).
Figure 2.
Schematic representation of the different RNA and DNA viruses detected by high throughput sequencing (HTS) in patients with and without CNS disease.
Patients with CNS Inflammation of Unknown Origin
In this group, HTS analysis allowed the identification of a potential viral causal agent in one case only. Indeed, the presence of novel human astrovirus (HAstV)-MLB2 sequences (155 reads, 35% genome coverage) were detected in the CSF of an immunocompetent patient, as well as in the plasma, anal swab, and urine samples retrospectively tested. This virus, which is not screened for in a routine work-up, was considered to be associated with the patient’s clinical presentation and a final diagnosis of HAstV-MLB2-associated meningitis was retained [21].
Anelloviruses’ reads were detected in eight of the 26 patients with acute CNS inflammation of unknown origin (26.9%). A specific rPCR assay confirmed the detection of TTV DNA in 7/8 samples (87.5%) (Figure S2, panel B).
Of note, the mean lymphocyte count of the seven TTV positive CSF samples (524.4 G/L, SD 1068.3 G/L) was higher compared to the 19 TTV negative CSF samples (mean 65.7 G/L, SD 54.7 G/L), although statistical significance was not achieved (p = 0.201).
An EBV sequence detected by HTS was not confirmed by an EBV rPCR assays (Figure 2, and Figure S2, panel B).
Megavirus sequences were detected by HTS in one sample, and GemyCV sequences in another one. These findings could not be confirmed due to the lack of specific rPCR assays for these viruses in our laboratory (Figure 2, and Figure S2, panel B).
Patients with Diagnosed CNS Disease
In this group, HPgV-1 sequences were detected by HTS in one patient, and were confirmed by rRT-PCR (Figure S2, panel C).
In one of the four samples in which MCPyV sequences were detected by HTS, MCPyV DNA detection was confirmed by rPCR (Figure S2, panel C).
Anelloviruses’ reads were detected in one CSF sample. This finding could not be confirmed by rPCR due to insufficient leftover sample volume.
HHV-7 sequences were detected by HTS but not by rPCR in one sample (Figure 2, and Figure S2, panel C).
GemyCV sequences were detected by HTS in one sample. This finding could not be confirmed due to the lack of a specific rPCR assay in our laboratory (Figure 2, and Figure S2, panel C).
Of note, in a case of HIV escape in the CNS diagnosed by rRT-PCR (110 RNA copies/mL), HIV sequences were not detected by HTS (Figure S2, panel C, P04), possibly due to the low viral load and limited amount of CSF analysed.
3.2.4. De Novo Analysis of the HTS Data
Finally, a de novo analysis of the HTS data did not reveal the presence of other relevant viral sequences.
4. Discussion
An original approach of the present investigation was the inclusion of a control group of patients without CNS disease, providing the opportunity to explore the CSF virome of asymptomatic humans. This study reports the HTS analysis results of CSF samples collected from 26 patients presenting with CNS inflammation of unknown origin, 10 patients with various identified causes of CNS disease, and 30 control adult patients.
Among the 26 patients with CNS inflammation, an unexpected viral pathogen was identified by HTS and considered to be responsible for the CNS disease in only one case, reported by Cordey et al. [21]. HTS led to the identification of other sequences assigned to viruses, some of them known to be associated with human infection and diseases but their causal role in CNS inflammation in our cases cannot be ascertained. HPgV-1 was detected in the CSF of two patients without CNS disease and one adult patient with CNS HIV escape. HPgV-1 has for the first time been detected in CNS using HTS on a brain sample of a patient with multiple sclerosis [22] and with RT-PCR in CSF samples from HIV infected patients, and its detection could possibly be linked to concomitant viremia [23,24]. Thus far, HPgV-1 detection in CNS samples has not been associated with any overt disease. TTV detection has been reported in CSF samples of patients with infectious and noninfectious CNS diseases, including patients with concomitant viremia [25,26,27]. Since TTV is known to be lymphocytotropic [28,29], the correlation between high CSF lymphocyte counts and increased TTV DNA detection [28,29] suggests that TTV enters the CSF in situations where the blood–brain barrier is altered, either associated to lymphocytes or as free circulating virus [30]. Furthermore, TTV was not detected in any CSF from patients without CNS disease by HTS or by rPCR.
GemyCV detection in CSF samples in both cases (two samples) and controls (one sample) reflects the uncertainty about the origin of GemyCV in CSF samples and its potential role in CNS disease [27,31,32]. Although no GemyCV sequences were detected in the negative control, these viruses, which are known to infect fungi, are not expected to cause human diseases and their detection could be associated with punctual environment or reagent contamination [33].
Papillomavirus, MCPyV, and other polyomavirus sequences were detected with HTS in CSF samples of both patients with and without CNS disease, as well as in the HTS whole process negative control (Figure 2), suggesting HTS contaminations. As mentioned earlier, real-time PCR results for MCPyV were all negative except in one case, a liver transplant recipient. Data are conflicting regarding the detection of MCPyV DNA in different types of brain tumours and its detection in cases with CNS infections has not been reported [34,35,36].
Sequence cross-contaminations were considered for two samples positive for HHV-7 (CSF23 and P33). Indeed, the detection of HHV-7 specific reads by HTS but not confirmed by real-time PCR likely results from experimental cross-contaminations during the library preparation with another clinical specimen prepared in the same series but that is not part of the present study. Although we cannot rule out that in these latter cases HTS was more sensitive that the specific real-time PCR, these results highlight that this issue must systematically be considered. Furthermore, in three healthy individuals, reagents contaminations with known vector sequences were also observed for two adenovirus and one parvovirus positive samples.
The paucity of relevant viral sequences detection by HTS in CSF collected in controls but also from patients who have CNS inflammation of undetermined aetiology may be explained by the following hypotheses. First, some viruses are known to be undetectable in CSF by the time of CNS disease. This is the case of tick-borne encephalitis virus (TBEV), whose associated CNS disease is probably rather related to the host immune and inflammatory response, rather than to tissue damage resulting from uncontrolled viral multiplication. The same phenomenon might apply for other neurotropic viruses. Second, the time between onset of neurologic symptoms and lumbar puncture might be too long in some cases, although this time interval was relatively short in our study (median 3 days, range 0–8 days). In such instances, serology represents an essential complementary diagnostic tool. Third, for some viral CNS infections, the microbiologic diagnostic yield might be higher in samples other than CSF. This is particularly true for enteric viruses, for which nucleic acid detection can be more important in terms of quantity and more prolonged in stool samples or anal swabs than in CSF. Fourth, a large, potentially under-recognised proportion of encephalitis and myelitis might result from autoimmunity, either related to post infectious, paraneoplastic, or idiopathic processes. Fifth, drug-related aseptic meningitis might also account for a portion of negative CSF HTS results. Finally, while being the ideal sample for investigating meningitis, CSF is only a suboptimal surrogate in cases of brain parenchymal infection.
The enrolment of patients with an initial suspicion of CNS inflammation started in 2013 and the respective CSF samples were consecutively analysed by HTS, generating a large number of runs. Therefore, given costs constraints (although since HTS-related costs have gradually decreased), a shortcoming of the study is the absence of positive (positive sample or spiked internal RNA/DNA controls) and negative controls run in parallel to evaluate the analysis efficiency (such as sensitivity loss, potential detection of reagent, or environmental contaminations). A HTS whole-process negative control was however included for CSF samples collected from patients without CNS disease. Finally, although our pipeline has been challenged on different specimens positive for a wide range of RNA and DNA viruses with an overall success rate suggesting a sensitivity threshold close to that of rRT-PCR assays [13,21,37,38], and benchmarked to the VirCapSeq-VERT pipeline, which is based on a positive selection of the viral template prior to sequencing using a probe set [39], with similar results obtained, we cannot exclude that some viral sequences were not detected by our unbiased method.
Concerning the control group, the results of HTS and confirmatory rRT-PCR assays revealed the absence of relevant confirmed viral sequences, except HPgV-1 sequences in two CSF samples. This information will be useful for future interpretations of HTS results in samples from patients presenting with CNS inflammation.
Regarding the case group, the low diagnostic yield could probably be interpreted according to the multiple factors described above. Nonetheless, the added value of HTS in the etiologic identification of a viral cause of CNS infections has been demonstrated [7,8]. HTS will undoubtedly be implemented in routine clinical virology laboratories as a second line technique or in parallel to an extensive work up driven by guidelines and local epidemiology. Special caution should be taken when interpreting HTS results in the context of CNS disease, due to possible environmental or reagent contaminations, sequence cross-contaminations, detection of viral latency, and identification of nonpathogenic viral bystanders, as illustrated in this study and recently reported data [33].
Acknowledgments
We thank Lara Turin, Gaël Vieille, and Brice Petit for technical assistance. We are also grateful to the patients and their respective family members who have accepted to be enrolled in this study.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/10/8/625/s1. Table S1. Real-time (reverse transcription-) PCR assays used in this study; Figure S1. Boxplot showing the million paired reads according to the nucleic acid preparation method and to the patient groups; Figure S2. Grids showing viral sequences results obtained with HTS and by specific rRT-PCR in CSF of patients with CNS inflammation of unknown origin (panel A), in CSF of patients with other CNS disease (panel B), and in CSF of patients without CNS disease (panel C). Colour codes are used to display sequencing depth and types of CNS inflammation.
Author Contributions
Conceptualization, L.K., S.C., and S.M.; methodology, L.K., S.C., M.S, M.-C.Z., F.B., and F.L.; formal analysis, S.C., F.B., F.L., M.-C.Z., S.M., and L.L.; investigation, S.M., J.A., A.G.L., N.W., K.M., P.B, E.S., G.L.S., R.F., F.B., F.L., M.D., C.S, and M.-C.Z.; resources, L.K.; data curation, S.C., F.B., F.L., M.-C.Z., L.L., S.M., and L.K.; writing—original draft preparation, S.M., M.-C.Z., and S.C.; writing—review and editing, L.K., E.S., G.L.S., B.F., F.L., J.A., A.G.L., N.W., K.M.P.-B., S.M., M.-C.Z., and S.C.; visualization, F.L., S.C., M.-C.Z., and S.M.; supervision, S.C., E.M.Z., and L.K.; funding acquisition, L.K. and S.M.
Funding
This work was supported by the Swiss National Science Foundation (grant No. 32003B_146993 to L.K.) and partly supported by the research Fund of the Department of Internal Medicine of the University Hospital and the Faculty of Medicine of Geneva (M.S.), this Fund receives an unrestricted grant from AstraZeneca Switzerland.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Dalmau J., Graus F. Antibody-Mediated Encephalitis. N. Engl. J. Med. 2018;378:840–851. doi: 10.1056/NEJMra1708712. [DOI] [PubMed] [Google Scholar]
- 2.Granerod J., Ambrose H.E., Davies N.W., Clewley J.P., Walsh A.L., Morgan D., Cunningham R., Zuckerman M., Mutton K.J., Solomon T., et al. Causes of encephalitis and differences in their clinical presentations in England: A multicentre, population-based prospective study. Lancet Infect. Dis. 2010;10:835–844. doi: 10.1016/S1473-3099(10)70222-X. [DOI] [PubMed] [Google Scholar]
- 3.Mailles A., Stahl J.P., Steering C., Investigators G. Infectious encephalitis in france in 2007: A national prospective study. Clin. Infect. Dis. 2009;49:1838–1847. doi: 10.1086/648419. [DOI] [PubMed] [Google Scholar]
- 4.Swanson P.A., II, McGavern D.B. Viral diseases of the central nervous system. Curr. Opin. Virol. 2015;11:44–54. doi: 10.1016/j.coviro.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boucher A., Herrmann J.L., Morand P., Buzele R., Crabol Y., Stahl J.P., Mailles A. Epidemiology of infectious encephalitis causes in 2016. Med. Mal. Infect. 2017;47:221–235. doi: 10.1016/j.medmal.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Mailles A., Stahl J.P., Bloch K.C. Update and new insights in encephalitis. Clin. Microbiol. Infect. 2017;23:607–613. doi: 10.1016/j.cmi.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Wilson M.R., Sample H.A., Zorn K.C., Arevalo S., Yu G., Neuhaus J., Federman S., Stryke D., Briggs B., Langelier C., et al. Clinical Metagenomic Sequencing for Diagnosis of Meningitis and Encephalitis. N. Engl. J. Med. 2019;380:2327–2340. doi: 10.1056/NEJMoa1803396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zanella M.C., Lenggenhager L., Schrenzel J., Cordey S., Kaiser L. High-throughput sequencing for the aetiologic identification of viral encephalitis, meningoencephalitis, and meningitis. A narrative review and clinical appraisal. Clin. Microbiol. Infect. 2019;25:422–430. doi: 10.1016/j.cmi.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C.Y., Chang Y.C., Huang C.C., Lui C.C., Lee K.W., Huang S.C. Acute flaccid paralysis in infants and young children with enterovirus 71 infection: MR imaging findings and clinical correlates. Am. J. Neuroradiol. 2001;22:200–205. [PMC free article] [PubMed] [Google Scholar]
- 10.Kraushaar G., Patel R., Stoneham G.W. West Nile Virus: A case report with flaccid paralysis and cervical spinal cord: MR imaging findings. Am. J. Neuroradiol. 2005;26:26–29. [PMC free article] [PubMed] [Google Scholar]
- 11.Venkatesan A., Tunkel A.R., Bloch K.C., Lauring A.S., Sejvar J., Bitnun A., Stahl J.P., Mailles A., Drebot M., Rupprecht C.E., et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: Consensus statement of the international encephalitis consortium. Clin. Infect. Dis. 2013;57:1114–1128. doi: 10.1093/cid/cit458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leber A.L., Everhart K., Balada-Llasat J.M., Cullison J., Daly J., Holt S., Lephart P., Salimnia H., Schreckenberger P.C., DesJarlais S., et al. Multicenter Evaluation of BioFire FilmArray Meningitis/Encephalitis Panel for Detection of Bacteria, Viruses, and Yeast in Cerebrospinal Fluid Specimens. J. Clin. Microbiol. 2016;54:2251–2261. doi: 10.1128/JCM.00730-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petty T.J., Cordey S., Padioleau I., Docquier M., Turin L., Preynat-Seauve O., Zdobnov E.M., Kaiser L. Comprehensive human virus screening using high-throughput sequencing with a user-friendly representation of bioinformatics analysis: A pilot study. J. Clin. Microbiol. 2014;52:3351–3361. doi: 10.1128/JCM.01389-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright E.S., Vetsigian K.H. Quality filtering of Illumina index reads mitigates sample cross-talk. BMC Genom. 2016;17:876. doi: 10.1186/s12864-016-3217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng Y., Leung H.C., Yiu S.M., Chin F.Y. IDBA-UD: A de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics. 2012;28:1420–1428. doi: 10.1093/bioinformatics/bts174. [DOI] [PubMed] [Google Scholar]
- 16.Arvia R., Sollai M., Pierucci F., Urso C., Massi D., Zakrzewska K. Droplet digital PCR (ddPCR) vs quantitative real-time PCR (qPCR) approach for detection and quantification of Merkel cell polyomavirus (MCPyV) DNA in formalin fixed paraffin embedded (FFPE) cutaneous biopsies. J. Virol. Methods. 2017;246:15–20. doi: 10.1016/j.jviromet.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Chivero E.T., Bhattarai N., Rydze R.T., Winters M.A., Holodniy M., Stapleton J.T. Human pegivirus RNA is found in multiple blood mononuclear cells in vivo and serum-derived viral RNA-containing particles are infectious in vitro. J. Gen. Virol. 2014;95:1307–1319. doi: 10.1099/vir.0.063016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimura H., Morita M., Yabuta Y., Kuzushima K., Kato K., Kojima S., Matsuyama T., Morishima T. Quantitative analysis of Epstein-Barr virus load by using a real-time PCR assay. J. Clin. Microbiol. 1999;37:132–136. doi: 10.1128/jcm.37.1.132-136.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masouridi-Levrat S., Pradier A., Simonetta F., Kaiser L., Chalandon Y., Roosnek E. Torque teno virus in patients undergoing allogeneic hematopoietic stem cell transplantation for hematological malignancies. Bone Marrow Transplant. 2016;51:440–442. doi: 10.1038/bmt.2015.262. [DOI] [PubMed] [Google Scholar]
- 20.Verheyen J., Timmen-Wego M., Laudien R., Boussaad I., Sen S., Koc A., Uesbeck A., Mazou F., Pfister H. Detection of adenoviruses and rotaviruses in drinking water sources used in rural areas of Benin, West Africa. Appl. Environ. Microbiol. 2009;75:2798–2801. doi: 10.1128/AEM.01807-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cordey S., Vu D.L., Schibler M., L’Huillier A.G., Brito F., Docquier M., Posfay-Barbe K.M., Petty T.J., Turin L., Zdobnov E.M., et al. Astrovirus MLB2, a New Gastroenteric Virus Associated with Meningitis and Disseminated Infection. Emerg. Infect. Dis. 2016;22:846–853. doi: 10.3201/eid2205.151807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kriesel J.D., Hobbs M.R., Jones B.B., Milash B., Nagra R.M., Fischer K.F. Deep sequencing for the detection of virus-like sequences in the brains of patients with multiple sclerosis: Detection of GBV-C in human brain. PLoS ONE. 2012;7:e31886. doi: 10.1371/journal.pone.0031886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z., Zhang Y., Wei F., Xu M., Mou D., Zhang T., Li W., Chen D., Wu H. Detection of GB virus C genomic sequence in the cerebrospinal fluid of a HIV-infected patient in China: A case report and literature review. Epidemiol. Infect. 2016;144:106–112. doi: 10.1017/S0950268815001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardie D., Smuts H. Human pegivirus-1 in the CSF of patients with HIV-associated neurocognitive disorder (HAND) may be derived from blood in highly viraemic patients. J. Clin. Virol. 2017;91:58–61. doi: 10.1016/j.jcv.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Maggi F., Fornai C., Vatteroni M.L., Siciliano G., Menichetti F., Tascini C., Specter S., Pistello M., Bendinelli M. Low prevalence of TT virus in the cerebrospinal fluid of viremic patients with central nervous system disorders. J. Med. Virol. 2001;65:418–422. doi: 10.1002/jmv.2051. [DOI] [PubMed] [Google Scholar]
- 26.Pollicino T., Raffa G., Squadrito G., Costantino L., Cacciola I., Brancatelli S., Alafaci C., Florio M.G., Raimondo G. TT virus has a ubiquitous diffusion in human body tissues: Analyses of paired serum and tissue samples. J. Viral Hepat. 2003;10:95–102. doi: 10.1046/j.1365-2893.2003.00417.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhou C., Zhang S., Gong Q., Hao A. A novel gemycircularvirus in an unexplained case of child encephalitis. Virol. J. 2015;12:197. doi: 10.1186/s12985-015-0431-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maggi F., Fornai C., Zaccaro L., Morrica A., Vatteroni M.L., Isola P., Marchi S., Ricchiuti A., Pistello M., Bendinelli M. TT virus (TTV) loads associated with different peripheral blood cell types and evidence for TTV replication in activated mononuclear cells. J. Med. Virol. 2001;64:190–194. doi: 10.1002/jmv.1035. [DOI] [PubMed] [Google Scholar]
- 29.Desai M., Pal R., Deshmukh R., Banker D. Replication of TT virus in hepatocyte and leucocyte cell lines. J. Med. Virol. 2005;77:136–143. doi: 10.1002/jmv.20426. [DOI] [PubMed] [Google Scholar]
- 30.Maggi F., Bendinelli M. Human anelloviruses and the central nervous system. Rev. Med. Virol. 2010;20:392–407. doi: 10.1002/rmv.668. [DOI] [PubMed] [Google Scholar]
- 31.Phan T.G., Mori D., Deng X., Rajindrajith S., Ranawaka U., Fan Ng T.F., Bucardo-Rivera F., Orlandi P., Ahmed K., Delwart E. Small circular single stranded DNA viral genomes in unexplained cases of human encephalitis, diarrhea, and in untreated sewage. Virology. 2015;482:98–104. doi: 10.1016/j.virol.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamberto I., Gunst K., Muller H., Zur Hausen H., de Villiers E.M. Mycovirus-like DNA virus sequences from cattle serum and human brain and serum samples from multiple sclerosis patients. Genome Announc. 2014;2 doi: 10.1128/genomeA.00848-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asplund M., Kjartansdottir K.R., Mollerup S., Vinner L., Fridholm H., Herrera J.A.R., Friis-Nielsen J., Hansen T.A., Jensen R.H., Nielsen I.B., et al. Contaminating viral sequences in high-throughput sequencing viromics: A linkage study of 700 sequencing libraries. Clin. Microbiol. Infect. 2019 doi: 10.1016/j.cmi.2019.04.028. [DOI] [PubMed] [Google Scholar]
- 34.Sadeghi F., Salehi-Vaziri M., Alizadeh A., Ghodsi S.M., Bokharaei-Salim F., Fateh A., Monavari S.H., Keyvani H. Detection of Merkel cell polyomavirus large T-antigen sequences in human central nervous system tumors. J. Med. Virol. 2015;87:1241–1247. doi: 10.1002/jmv.24178. [DOI] [PubMed] [Google Scholar]
- 35.Lam W.Y., Leung B.W., Chu I.M., Chan A.C., Ng H.K., Chan P.K. Survey for the presence of BK, JC, KI, WU and Merkel cell polyomaviruses in human brain tissues. J. Clin. Virol. 2010;48:11–14. doi: 10.1016/j.jcv.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 36.Giraud G., Ramqvist T., Pastrana D.V., Pavot V., Lindau C., Kogner P., Orrego A., Buck C.B., Allander T., Holm S., et al. DNA from KI, WU and Merkel cell polyomaviruses is not detected in childhood central nervous system tumours or neuroblastomas. PLoS ONE. 2009;4:e8239. doi: 10.1371/journal.pone.0008239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cordey S., Bel M., Petty T.J., Docquier M., Sacco L., Turin L., Cherpillod P., Emonet S., Louis-Simonet M., Zdobnov E.M., et al. Toscana virus meningitis case in Switzerland: An example of the ezVIR bioinformatics pipeline utility for the identification of emerging viruses. Clin. Microbiol. Infect. 2015;21:387.e1–387.e4. doi: 10.1016/j.cmi.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 38.Cordey S., Hartley M.A., Keitel K., Laubscher F., Brito F., Junier T., Kagoro F., Samaka J., Masimba J., Said Z., et al. Detection of novel astroviruses MLB1 and MLB2 in the sera of febrile Tanzanian children. Emerg. Microbes Infect. 2018;7:1–3. doi: 10.1038/s41426-018-0025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams S.H., Cordey S., Bhuva N., Laubscher F., Hartley M.A., Boillat-Blanco N., Mbarack Z., Samaka J., Mlaganile T., Jain K., et al. Investigation of the Plasma Virome from Cases of Unexplained Febrile Illness in Tanzania from 2013 to 2014: A Comparative Analysis between Unbiased and VirCapSeq-VERT High-Throughput Sequencing Approaches. mSphere. 2018;3 doi: 10.1128/mSphere.00311-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.