Abstract
Autosomal dominantly inherited genetic disorders such as corneal dystrophies are amenable to allele-specific gene silencing with small interfering RNA (siRNA). siRNA delivered to the cornea by injection, although effective, is not suitable for a frequent long-term treatment regimen, whereas topical delivery of siRNA to the cornea is hampered by the eye surface’s protective mechanisms. Herein we describe an attractive and innovative alternative for topical application using cell-penetrating peptide derivatives capable of complexing siRNA non-covalently and delivering them into the cornea. Through a rational design approach, we modified derivatives of a cell-penetrating peptide, peptide for ocular delivery (POD), already proved to diffuse into the corneal layers. These POD derivatives were able to form siRNA-peptide complexes (polyplexes) of size and ζ-potential similar to those reported able to undergo cellular internalization. Successful cytoplasmic release and gene silencing in vitro was obtained when an endosomal disruptor, chloroquine, was added. A palmitoylated-POD, displaying the best delivery properties, was covalently functionalized with trifluoromethylquinoline, an analog of chloroquine. This modified POD, named trifluoromethylquinoline-palmitoyl-POD (QN-Palm-POD), when complexed with siRNA and topically applied to the eye in vivo, resulted in up to 30% knockdown of luciferase reporter gene expression in the corneal epithelium. The methods developed within represent a valid standardized approach that is ideal for screening of a range of delivery formulations.
Keywords: siRNA, cornea, peptide, CPP, delivery
Introduction
The eye, and in particular the ocular surface, is one of the most accessible sites for local drug treatment, allowing direct application without the need for systemic administration. This, taken together with the fact that the area to be treated is small and the success of any treatment is easily monitored, makes topical drug delivery an attractive option for ophthalmology. In addition, an immune-privileged status has been proposed that minimizes risk for unwanted side effects.1, 2
However, despite its unique attributes, drug delivery through and to the cornea, which represents one of the main components of the ocular surface, has proved to be challenging.3, 4 The main obstacles to achieving a therapeutic dose, by the diffusion of a drug through the eye surface, are the protective mechanisms and underlying ocular anatomy.1, 5 The cornea is a tear film-covered, 500-μm-deep tissue composed of three layers, from anterior to posterior, the epithelium, stroma, and endothelium, separated by Bowman’s layer and Descemet’s membrane, respectively. A drug may be eliminated from the ocular surface by various mechanisms including lacrimation and tear turnover, drug metabolism, and preferential conjunctival absorption.4, 6 The corneal epithelium is a non-keratinized, stratified squamous epithelium, approximately five to six cell layers thick, joined together by tight junctions. This, together with the tear film, make the cornea a difficult barrier to overcome, and when the desired target is other parts of the eye, such as the retina, bypass of the cornea by direct injection of the drug (e.g., intravitreal injection) is often the preferred pathway to delivery in the clinic.6, 7
Although methods such as intrastromal injection1 and iontophoresis8, 9 have been shown to be successful for delivery to the cornea, the development of a user-friendly, non-invasive method such as self-administrated eye drops has proved challenging.10, 11, 12 Drugs and delivery agents need to be able to overcome the tear barrier and remain associated with the corneal epithelium for the time necessary to allow cellular internalization. Subsequently, once internalized into the target cell, the drug cargo must be released to function within the cell with optimal bioavailability.
Among the different drugs for which delivery to the front of the eye is sought, large hydrophilic oligonucleotides represent a unique and effective approach for selective gene therapy treatment of a wide spectrum of corneal diseases.13, 14, 15 In particular, small interfering RNA (siRNA)-induced gene silencing has been shown to hold great potential for the treatment of different ocular pathologies, reaching phase II and III clinical trials for glaucoma and dry eye,2, 16 and more recently for corneal pathologies1, 11 such as corneal dystrophies (CDs). CDs represent a spectrum of eye diseases associated with one or more different layers of the cornea, affecting its shape and transparency, and in some cases, leading to a partial or complete loss of vision.17 Corneal transplantation is the only intervention that can be used currently in the case of a damaged cornea. CDs are frequently caused by missense mutations or small in-frame insertions or deletions,18 and therefore stable gene editing and transient gene silencing are promising tools for a gene therapy approach.
To investigate this further, our group previously developed promising siRNA molecules for a personalized therapeutic approach for CDs.19, 20, 21, 22, 23 siRNAs that can be used for a transient, reversible, and dosage-variable treatment24, 25 were developed that were highly specific with single-nucleotide discrimination at the mutation site.26, 27
Due to the short half-life of siRNA molecules, a daily reversible and dosage-variable treatment regimen by non-invasive topical delivery is necessary. However, effective delivery remains a challenge, and presently no published research reports significant siRNA delivery to an intact corneal epithelium.11, 28
In addition to overcoming pre-corneal tear film turnover and the other protective mechanisms described, it is necessary to promote cellular uptake of siRNA through the cellular membranes of the epithelial cells and increase corneal bioavailability. Shielding the negatively charged siRNA with positively charged delivery molecules represents a promising option.29 A positively charged formulation can, in the first instance, interact with the negatively charged ocular components, such as the epithelial cell membranes and the external mucus, in order to increase the persistence of the drug on the eye surface,30 and then mediate cellular uptake.
Cationic polymers have been extensively used for drug delivery, in particular of nucleic acids. Examples of these polymers include polyethyleneimine (PEI), polyamidoamine dendrimers (pAMAMs), and chitosan, which are routinely used for cell transfection in vitro and in vivo.31 Different cationic polymers may be used to deliver oligonucleotides to the eye surface,32 and among these, cationic cell-penetrating peptides (CPPs) are very versatile and promising.33 Their positive charges can be exploited both to generate an ionic interaction with the negatively charged siRNA and to drive ocular penetration, and when compared with other nanoparticles, CPPs have the advantage of forming nanoparticles by simply mixing the peptide with siRNA in aqueous solution. CPPs have been used extensively to deliver various macromolecules,34 including siRNA,35, 36 to cells both in vitro and in vivo. They can be easily modified by the addition of chemical blocks to address different delivery hurdles; for example, lipid moieties (such as palmitoyl- and cholesteryl-) may be added in order to increase the hydrophobicity of CPPs, and thus favor the destabilization of the endosomal membrane. CPPs can also be used together with other molecules involved in delivery, offering a wide range of potential combinations. Moreover, in contrast with other cationic polymers, CPPs are well-defined chemical entities, allowing better control of the CPPs/siRNA molar ratio.
CPPs, such as POD37, 38 and PEP-1,39 have demonstrated the ability to penetrate the corneal tissues when applied topically to the eye. Herein, we present the development of a modified POD for corneal delivery of siRNA that overcomes poor endosomal escape (when siRNA remains trapped in endosomes and is trafficked into lysosomes, where it is degraded).34, 35, 40 In this study, chloroquine (Chlq)41 was first applied together with the polyplexes (i.e., nucleotide-peptide complexes42) to elicit siRNA release from the endosomes, in order to confirm the endosomal entrapment and to determine whether a lysogenic compound was able to enhance siRNA release in vitro and in vivo. Subsequently, the combination that showed the best delivery properties when tested in vitro and in vivo was selected and covalently modified with a Chlq analog. A corneal epithelium cell line was used as an in vitro model, whereas in vivo experiments were performed on a novel murine model expressing, under the regulation of the corneal-specific Krt12 promoter, the luciferase gene and Meesmann epithelial CD mutations.
This covalently modified peptide, once topically applied on the eye surface, proved capable of delivering bioavailable siRNA into corneal epithelial cells, allowed effective release of the siRNA from the endosomes, and achieved significant knockdown of gene expression.
Results
Evaluation of In Vitro siRNA Delivery Using Modified Versions of Peptide for Ocular Delivery (POD)
To improve the delivery and bioavailability of siRNA by the POD peptide, novel chemical modifications were introduced (Table 2). To minimize the number of possible candidates to be tested in vivo with the mouse corneal reporter model, the different modified versions of POD were first tested to determine their delivery activity in vitro in a corneal epithelial cell model.43
Table 2.
POD Structures and Molecular Weights
| Name | Structure | MW (g/mol) |
|---|---|---|
| POD | CGGG[ARKKAAKA]4 | 3,592.4 |
| Palm-POD | CH3-(CH2)14-CO-POD | 3,829.9 |
| Chol-POD | C28H45O-CO-POD | 4,004.1 |
| QN-Palm-POD | C66H100F6N12O6-CO-POD | 4,873.0 |
| PLGA-PEG-POD | [C3H4O2]x[C2H2O2]y[C2H4O]zC9H11N2O3-POD | 29,500–43,500 |
Initially, POD was modified with either a palmitoyl group, a cholesteryl group, or poly(lactic-co-glycolic acid) (PLGA)-PEG and tested for its ability to deliver siRNA in corneal epithelial cells. POD was functionalized with a palmitoyl- or a cholesteryl- group because these modifications, increasing the ability of a peptide to fuse with the plasma membrane, were previously shown to enhance the performance of other siRNA-delivering peptides.44, 45 PLGA-PEG-POD was selected for this study because PEG-POD was reported to efficiently deliver nucleotides in vivo, whereas the addition of PLGA was shown to enhance the capability of PEG-POD to penetrate corneal tissues.37, 46
Evaluation of POD-siRNA Complexes
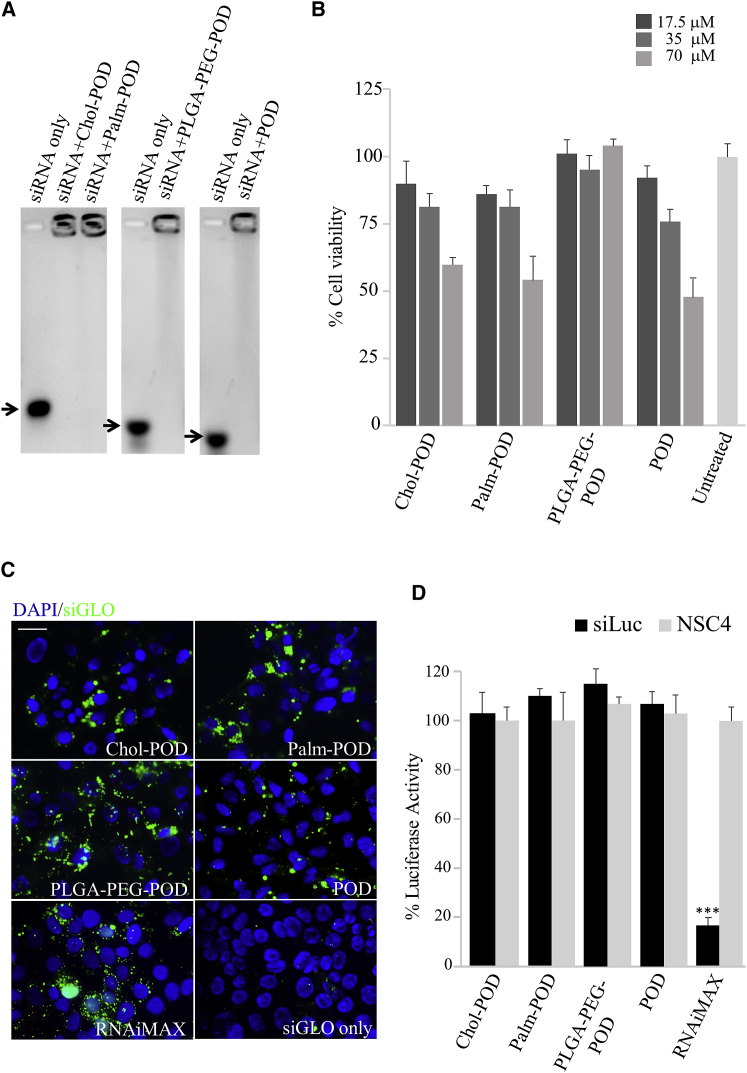
A molar ratio of 35:1 PODs/siRNA was used to determine the POD-siRNA complex formation in a gel retardation assay. This molar ratio was chosen because molar ratios between 30:1 and 50:1 have been demonstrated previously to result in the maximum incorporation of siRNA into complexes for other CPPs.47 Moreover, a molar ratio of 25:1 was shown to achieve efficient knockdown of GFP expression in transiently transfected cells,38 whereas Vasconcelos et al.37 determined the cell viability in cells treated with the PLGA-PEG-POD to be higher than 60% at a concentration of 2.5 mg/mL (herein we used 1.4 mg/mL PLGA-PEG-POD).37, 38 At 35:1 molar ratio, cholesteryl-POD (Chol-POD), Palm-POD, PLGA-PEG-POD, and POD all showed complete complexation of siRNA (Figure 1A). Uncomplexed siRNA (siRNA only) migrates into the agarose gel, whereas siRNA complexed with PODs does not escape from the wells; this POD/siRNA ratio was used for all further experiments.
Figure 1.
Palm-POD and Chol-POD Encapsulate siRNA and Penetrate into HCE-S Cells
(A) Gel retardation assay showing uncomplexed siRNA (arrow, siRNA only) migrated through the agarose gel, whereas the siRNA complexed with the PODs remained immobilized within the wells. 1 μM siRNA was complexed with: Chol-POD, Palm-POD, PLGA-PEG-POD, and POD (35 μM) to a final molar ratio of 35:1 POD:siRNA. (B) MTT cell viability assay was performed on HCE-S at 24 h after treatment with different concentrations of PODs-siRNA. Chol-, Palm-, PLGA-PEG-POD, and POD were used at 17.5, 35, and 70 μM (dark gray bars) and complexed with siRNA at 35:1 molar ratio. Untreated control (light gray bar) is represented on the right. (C) Fluorescence images of HCE-S cells collected 24 h after transfection with 1 μM green siGLO and Chol-POD, Palm-POD, PLGA-PEG-POD, and POD at 35:1 molar ratio. Transfection with RNAiMAX and 1 μM green siGLO was used as positive control, whereas 1 μM green siGLO only was used as negative control. The nuclei are stained with DAPI (blue), whereas the green dots represent green siGLO. (D) Dual-luciferase reporter gene expression assay was performed in vitro: luminescence was measured 72 h after treating HCE-S cells with POD-siRNA (35:1 molar ratio, 1 μM siRNA). Black bars represent the luciferase activity in the siLuc-transfected wells, whereas the gray bars represent the luciferase in the NSC4-transfected wells. The mean values of the NSC4 were normalized to 100%, and the siLuc mean values were expressed as a percentage of the negative control. A positive control with RNAiMAX and 1 μM siLuc was also assessed (***p < 0.001). Error bars represent SEM; n = 4 biological replicates.
PODs ζ-Potential and Size
CPP-siRNA complexes that have the highest rate of endocytic uptake and tissue diffusion are generally smaller than 200 nm47 and have a positive ζ-potential (generally lower than +40 mV) in aqueous solution.47, 48, 49 The size and ζ-potential of each POD-siRNA complex was therefore determined. The analysis showed that the particles from each formulation in PBS had mean diameters and mean charges (Table 1) that fall within the described parameters and are thus suitable for cell delivery. The analysis was also performed in water to assess whether a buffered pH may have an effect on the properties of the complexes (data not shown). However, only a minimal reduction of the charge and dimension was observed when prepared in PBS, which was used from this point to prepare the other formulations described in this study.
Table 1.
POD Dimensions and ζ-potentials
| POD with siRNA | Dimension (nm) | ζ-Potential (mV) |
|---|---|---|
| Chol-POD in PBS | 150.8 ± 0.28 | +15.6 ± 0.8 |
| Palm-POD in PBS | 142.5 ± 3.1 | +14.5 ± 1.8 |
| PLGA-PEG-POD in PBS | 127 ± 10.9 | +11.9 ± 3.5 |
| QN-Palm-POD in PBS | 107 ± 3.2 | +14.9 ± 4.2 |
POD/siRNA molar ratio was 140:1 for QN-Palm-POD and 35:1 for the other PODs.
Evaluation of siRNA Cellular Delivery
A human epithelial corneal epithelial cell line (HCE-S)50 was used for initial in vitro screening in order to evaluate cellular transfection and toxicity properties of the POD-siRNA complexes and to reduce the number of animals needed for the subsequent in vivo analysis. Although corneal epithelial cell lines have molecular features that differ from the original epithelium51 and might respond differently to the treatment if grown in different culture conditions,52 they represent a valid cellular model of the cornea to initially investigate cellular transfection and toxicity.53 Delivery of a green fluorescently labeled non-targeting siRNA (siGLO) into HCE-S cells by each of the different PODs was tested. The majority of HCE-S cells, transfected with all three different POD-siRNA formulations, showed punctuate cytoplasmic fluorescence (Figure 1B) with a perinuclear concentration (nuclei stained blue, DAPI), in agreement with previous observations in corneal epithelial cells of a rabbit cornea treated with fluorescently labeled POD.37 No intracellular fluorescence was detectable in cells treated with non-complexed siGLO (Figure 1B). The perinuclear distribution pattern of the POD-delivered siGLO is characterized by fluorescent dots, larger than those observed using the commercially available cationic lipid transfection agent RNAiMAX (Figure 1B). The perinuclear distribution of fluorescence suggests that the nanoparticles are internalized along an endocytic pathway.47, 54, 55
Evaluation of POD Cellular Toxicity
siRNAs used herein were previously reported not to elicit any toxicity or immunological response in HCE-S cells.43 The cellular toxicity of POD-siRNA complexes was assessed by measuring cell viability using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay in HCE-S cells treated for 24 h with PODs at different concentrations (Figure 1C). Under these conditions, cells treated with Chol- and Palm-POD showed, when compared with the untreated control, an ∼87% and 75% cell viability with 17.5 and 35 μM POD that fell to ∼55% at 70 μM. PLGA-PEG-POD was instead showing ∼100% viability. Similar results were obtained for the POD-siRNA comparison, showing that the presence of Chol-, Palm-, and PLGA-PEG groups did not elicit cellular toxicity.
Evaluation of Gene Knockdown
The 35:1 PODs/siRNA ratio, determined by a gel retardation assay to be sufficient for complexing all of the siRNA, was then used to assess knockdown of luciferase reporter gene expression in HCE-S cells using a dual-luciferase assay, as previously described.22, 26 No significant knockdown was observed when cells were transfected with luciferase targeting siRNA (siLuc) complexed with any of the four different PODs (Figure 1D, black bars) compared with PODs complexed with non-targeting siRNA (NSC4) (Figure 1D, gray bars), whereas knockdown was achieved in the positive control using RNAiMAX (p < 0.001). The results obtained are consistent with those previously observed in studies with other CPPs where, even in the case of strong cell association, no significant gene expression knockdown was measured.47
Evaluation of Endosomal Release
Because all of the PODs tested were able to deliver siRNA in vitro but failed to knock down luciferase expression, we hypothesized that the siRNA-peptide complexes were entrapped in endosomes, a well-known cellular barrier that prevents the cytosolic release of siRNAs.56, 57, 58 To test this hypothesis, we repeated assessment of delivery and knockdown, treating the cells with the POD-siRNA formulations together with Chlq,41 reported to increase endosomal escape. This should result in a release of siRNA to the cytoplasm, where it can target mRNA and be observed as reduction in luciferase reporter expression.
In combination with Chlq, POD-siRNA complexes were observed in a more diffuse cytoplasmic pattern (Figure 2A) when compared with the same complexes without Chlq (Figure 1B), and achieved a significant knockdown of luciferase expression (Figure 2B). Based on these in vitro analyses, Chol- and Palm-POD were selected for the subsequent in vivo experiments. In further experiments, these two PODs were tested to identify which demonstrated the best in vivo delivery and might thus be covalently modified with an endosomal disruptor. Direct derivatization of a POD is sought to maximize endosomal release while minimizing corneal toxicity. PLGA-PEG-POD was excluded from this in vivo comparison because its chemical and structural features made it unsuitable for any further chemical derivatization.
Figure 2.
Chlq Addition to PODs Disrupts Endosomes and Promotes siRNA Release and Luciferase Knockdown
(A) Fluorescence images of HCE-S cells 24 h after transfection with 1 μM green siGLO and Chol-POD, Palm-POD, PLGA-PEG-POD, and POD with Chlq. The nuclei are stained with DAPI (blue), whereas the green dots represent green siGLO. Scale bar, 25 μM. (B) Dual-luciferase reporter gene expression assays were performed as described above. HCE-S cells were treated with a 35:1 molar ratio (POD/siRNA) and Chlq, and luminescence was measured 72 h later. Black bars represent the luciferase activity in the siLuc-transfected wells, and the gray bars the NSC4 siRNA-transfected wells. *p < 0.05, **p < 0.01, ***p < 0.001). Error bars represent SEM; n = 4 biological replicates. A positive control with RNAiMAX and 1 μM siLuc was also assessed.
Corneal Delivery and Knockdown of Luciferase Expression by Intrastromal Injection of Accell-siRNA
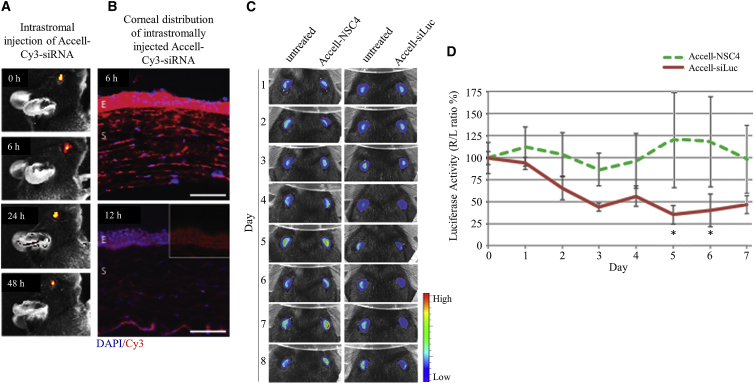
Previous in vivo studies have demonstrated that direct intradermal injection of siRNA can: (1) specifically silence co-injected target alleles in murine epidermis;27, 59, 60 (2) silence expression of epidermal reporter transgene;61 and (3) show efficacy in a phase 1b clinical trial.62 “Pressure-fection” intrastromal injection of plasmid into the corneal stroma has been shown to result in GFP expression in all layers of the cornea.63, 64 To determine whether the siLuc siRNA was able to knock down luciferase expression in the cornea of the Krt12+/luc2 mice, we first sought to demonstrate that intrastromal injection can deliver siRNA to all layers of the murine cornea. Live animal imaging was performed following intrastromal injection of Cy3-labeled Accell siRNA, a nuclease-resistant siRNA with “self-delivery” properties.27 Strong fluorescent signals were observed in the mouse eye for up to 72 h following injection; however, the signal was most intense at 6 h post-injection (Figure 3A). Fluorescence microscopy showed that Cy3-labeled Accell siRNA localized to the corneal epithelium and stroma after the initial injection, with pronounced distribution within the corneal epithelium visible 6 h after injection (Figure 3B). The fluorescence in the stroma declined within 12 h. These findings confirmed that intrastromal injection results in siRNA delivery to the corneal epithelium and suggest that the retention times should be sufficient to study siRNA gene silencing in living Krt12+/luc2 mice.
Figure 3.
In Vivo Intrastromal Injection of Accell Cy3-Labeled siRNA Efficiently Knocked Down Luciferase Expression
(A) Fluorescent images of a live mouse at time points following intrastromal injection of Cy3-labeled Accell siRNA showing strongest fluorescent signal at 0 and 6 h post-injection, with a readily detectable signal still evident at 48 h. (B) Fluorescence microscopy of transverse mice corneal sections shows that the siRNA (red) is well distributed and strongly accumulates in the anterior epithelium by 6 h post-injection. By 12 h post-injection, the signal has diminished; however, there is still a prominent Cy3 signal in the epithelium, which is more clearly seen within the inset portion of the image where the blue DAPI channel has been masked. Scale bar, 150 μm. (C) In vivo gene inhibition by intrastromal injection of luc2 siRNA. Representative images of animals (n = 3 per group) imaged over 7 days. Left eyes were untreated. Right eyes were treated with a single intrastromal injection of Accell-modified siRNA after imaging on day 0. (D) Quantification of luciferase activity for each treatment group expressed as a percentage of control (right eye/left eye [R/L] ratio %). Inhibition of luciferase with siLuc was sustained through days 2–7. NSC4 had no effect. E, epithelium; NSC4, nonspecific control siRNA; S, stroma; siLuc, luciferase-specific siRNA.
In order to assess the ability of the described siRNAs to knock down the expression of the luciferase gene in vivo, intrastromal injections of Accell-modified siRNA in mice expressing luciferase in the cornea were performed.
Before in vivo treatment experiments began, corneal luciferase activity in Krt12+/luc2 mice was quantified every 24 h for 3 days to confirm a consistent right-to-left ratio. Accell control (Accell-NSC4) or luc2 siRNAs (Accell-siLuc) were delivered by intrastromal injection (n = 3 mice/group), and corneal epithelial luciferase expression was evaluated daily by live animal imaging over 7 days (Figure 3C). Accell-siLuc inhibited luciferase expression in vivo, with >50% repression achieved 72 h post-injection. Maximal inhibition (64%) was observed at day 5, and silencing persisted at day 6 (Figure 3D); data were statistically significant (p < 0.05) for both time points. Importantly, intrasomal injection of non-targeting Accell-NSC4 had no significant effect (Figure 3D).
In parallel experiments, no significant knockdown of expression was observed when luciferase expression was measured following topical application of Accell siRNA (data not shown). Therefore, to investigate whether siRNA-POD polyplexes can mediate knockdown of corneal gene expression following topical application, we chose to use the siLuc siRNA that we had proved to knock down luciferase expression in vivo by intrastromal injection. However, we combined PODs with native and not Accell-modified siRNA because it is not known whether this modification interferes in the peptide-siRNA interaction and CPP-mediated delivery.
In Vivo Evaluation of Topical Delivery of POD-siRNA Complexes to the Cornea Using a Fluorescent siRNA
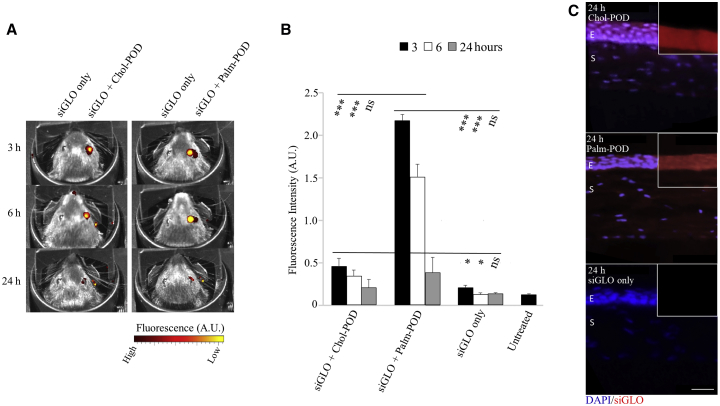
Following the demonstration that successful delivery and gene knockdown in vitro were facilitated by the addition of Chlq, the two modified versions of the POD (Palm-POD and Chol-POD) were assessed for in vivo delivery of siRNA. PODs were first complexed with red fluorescent siGLO at the same molar ratios used for in vitro delivery and applied topically to the eye surface of wild-type mice, and fluorescence was monitored for up to 24 h. All of the eyes treated with siGLO in combination with a POD showed fluorescence up to 24 h, whereas the siGLO-only-treated eyes did not show any visible fluorescence (Figure 4A). At 3 and 6 h after application, fluorescence signals from siGLO-Palm-POD and of siGLO-Chol-POD were significantly higher than the siGLO-only control. siGLO-Palm-POD fluorescence was between three and four times more intense than the one measured for Chol-POD (Figure 4B).
Figure 4.
In Vivo Topical Application of Chol- and Palm-POD Showed Corneal Delivery of siGLO
(A) Comparison between wild-type mice treated with Chol- and Palm-POD combined with red siGLO (right eye) and siGLO alone (left eye, negative control). The images were collected at 3, 6, and 24 h after treatment with IVIS Xenogen. The red and yellow colors represent the intensity of the fluorescence and not the color of the siGlo signal. (B) Fluorescence measurements of the treated eye at the different conditions reported in (A). Palm-POD demonstrated a significant increase in the fluorescent signal either at 3 and 6 h after treatment when compared with Chol-POD and siGlo only. The values represent the mean (and the SEs) of two treated eyes in n = 2 mice. (C) Corneal sections of the eyes collected from the treated mice at 24 h with either Chol- or Palm-POD and siGLO. The nuclei are stained blue (DAPI), whereas the siGLO is red in all images. Fluorescence is present in eyes treated with both Chol- and Palm-POD, whereas no red fluorescence distinguishable from the background is visible in the negative control (siGLO only). E, corneal epithelium; S, stroma.
Twenty-four hours after the application of POD-siRNA, the eyes were collected and distribution of the siRNA throughout the cornea observed. Red fluorescence was detected in all of the treated sections throughout the corneal layers, particularly the corneal epithelium, whereas no fluorescence above background was observed in the siGLO-only control (Figure 4C).
Because PODs alone are not sufficient to deliver siRNA into the cytoplasm, with the siRNA-POD complexes probably retained into the endosomes, and Chlq has been proved to elicit endosomal escape in vitro, but is known to result in in vivo toxicity,65 we decided to covalently modify POD with an analog of Chlq that shows low toxicity. We also chose to use Palm-POD rather than Chol-POD because it showed better delivery of siGLO in vivo (Figure 4B).
Evaluation of In Vitro and In Vivo siRNA Delivery Using a Palmitoylated Version of POD Functionalized with Chlq
An analog of Chlq, trifluoromethylquinoline (QN), was selected to covalently functionalize Palm-POD because a peptide previously developed for siRNA delivery was similarly derivatized and showed successful in vivo delivery with low toxicity66 (Figure 5A).
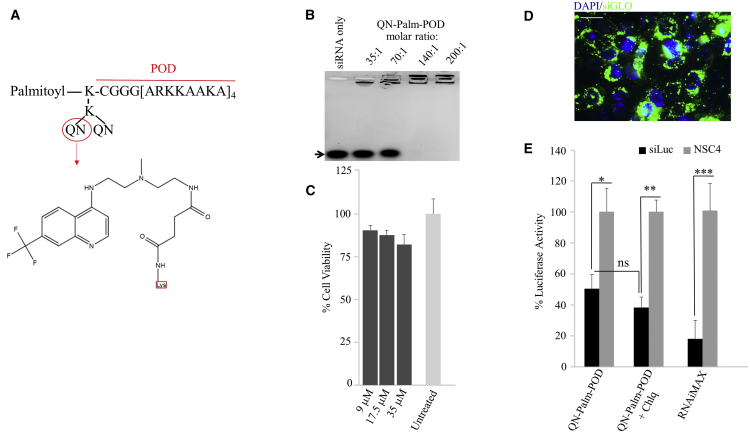
Figure 5.
In Vitro Characterization of QN-Palm-POD-siRNA
(A) QN-Palm-POD with the amino acidic chain, the palmitoyl group, and the QN group, the chemical structure of which is shown in detail. (B) Gel retardation assay of QN-Palm-POD complexed with 1 μM siRNA at 35:1, 70:1, 140:1, and 200:1 molar ratios. The siRNA alone (siRNA only) migrated through the agarose gel, whereas the siRNA complexed with the PODs remained immobilized within the wells, either partially (35:1 and 70:1) or completely (140:1 and 200:1). (C) MTT assay performed on HCE-S treated with siRNA and QN-Palm-PODs 24 h after the treatment. POD was used at 8.75, 17.5, and 35 μM (dark gray bars) at a 140:1 molar ratio. Light gray bar represents untreated control. (D) Fluorescence image of HCE-S cells 24 h after transfection with 1 μM green siGLO (green) and QN-Palm-POD. The nuclei are stained with DAPI (blue). Scale bar, 25 μM. (E) In dual-luciferase reporter gene expression assays, HCE-S cells were treated with a 35:1 molar ratio (QN-Palm-POD:siRNA), and luminescence was measured 72 h later. Black bars represent the luciferase activity in the siLuc-transfected wells, and the gray bars the NSC4 siRNA-transfected wells. *p < 0.05, **p < 0.01, ***p < 0.001. Error bars represent SEM. n = 4 biological replicates. A positive control with RNAiMAX and 1 μM siLuc was also assessed.
Evaluation of PODs-siRNA Complexes
To assess whether the functionalization of Palm-POD with QN altered the ability of the POD to bind siRNA and to determine the optimum ratio of QN-Palm-POD/siRNA for POD-siRNA complex formation, QN-Palm-POD was titrated at different molar ratios with siRNA. Although partial complexation of siRNA was observed at 35:1 and 70:1 (with slightly more siRNA bound to the POD at a 70:1 molar ratio), a 140:1 molar ratio achieved complete siRNA complexation (Figure 5B). This result suggests that the presence of the covalently attached QN molecule reduces the ability of the peptide to interact with siRNA by approximately 75% because a 35:1 molar ratio of Palm-POD/siRNA was sufficient to achieve complete complexation (Figure 1A).
QN-Palm-POD ζ-Potential and Size
Biophysical analysis of the QN-Palm-POD-siRNA complexes (140:1 ratio) showed that they had a mean diameter (±SD) of 107.1 ± 3.2 nm and a mean charge (±SD) of +14.9 ± 4.2 mV in PBS (Table 2). Thus, the presence of the QN groups does not have an effect on the charge of complexes formed in PBS (14 mV), and although the dimensions of the QN-Palm-POD complexes are reduced compared with Palm-POD in PBS (142 to 107 nm), these values remain in the range suitable for cellular delivery (as described earlier in PODs ζ-Potential and Size47, 48, 49).
Evaluation of QN-Palm-POD Cellular Toxicity
Cellular toxicity of QN-Palm-POD-siRNA in HCE-S cells treated with POD concentrations of 9, 17.5, and 35 μM (140:1 molar ratio) was measured with an MTT assay (Figure 5C). Cell viability was reduced to ∼80% in cells treated with 9, 17.5, and 35 μM QN-Palm-POD when compared with untreated cells. Thus, to achieve complete complexation of siRNA (140:1 molar ratio) while minimizing cellular toxicity, the concentrations used for the transfection experiments of QN-Palm-POD and siGLO/siLuc were reduced to 17.5 μM and 125 nM, respectively.
Evaluation of siRNA Cellular Delivery
To investigate the effect of Palm-POD functionalization with QN, we used 17.5 μM QN-Palm-POD complexed with 125 nM siGLO (molar ratio of 140:1) to transfect HCE-S cells. Efficient transfection (>90%, counting nuclei surrounded by fluorescent green dots) was achieved (Figure 5D). siGLO is distributed in the cells with a punctuate pattern both around the nuclei and throughout the cytoplasm. When compared with the non-functionalized Palm-POD, the fluorescent dots have a reduced dimension and are less defined, more similar in appearance to that observed in RNAiMAX-transfected cells. This suggests an endocytic uptake, but also an improved endosomal release.
Evaluation of Knockdown of Reporter Gene Expression
Knockdown of luciferase reporter gene expression was assessed in HCE-S using QN-Palm-POD:siLuc complexes. Seventy-two hours after transfection, a 50% knockdown of luciferase expression (p < 0.05) was observed. Although addition of 30 μM Chlq to the QN-Palm-POD:siLuc complex-transfected cells increased knockdown of luciferase gene expression to 62% (p < 0.01), this was not significantly higher than with the QN-Palm-POD:siLuc complex alone (Figure 5E).
In Vivo Evaluation of QN-Palm-POD-siRNA Complex Delivery to the Cornea
To assess the ability of the QN-functionalized POD to deliver siRNA in vivo, we measured fluorescence as described above using a 140:1 molar ratio (700 μM QN-Palm-POD and 5 μM siGLO), following topical application of siGLO-QN-Palm-POD complexes. Although the fluorescence intensity was lower than that observed for the non-functionalized Palm-POD, fluorescence in the cornea treated with QN-Palm-POD-siRNA persisted for up to 24 h (Figure 6A) and is significantly higher than in corneas treated with the siGLO alone at all the time points (p < 0.001 at 3 h, p < 0.05 at 6 and 24 h) (Figure 6B). Sections of the treated corneas show uptake of siGLO in all of the corneal layers (epithelium, stroma, and endothelium) (Figure 6C), whereas siGLO was not observed in the posterior segment (data not shown).
Figure 6.
In Vivo Topical Application of siRNA with QN-PALM-POD Demonstrated Efficient Delivery and Knockdown in Cornea
(A) In vivo comparison of wild-type mice treated with QN-Palm-POD and red siGLO with and without amphotericin B (AmB). The formulation was applied to the right eye, whereas siGLO alone was applied to the left eye (negative control). The images were collected with IVIS Xenogen at 3, 6, and 24 h after treatment. The red and yellow colors represent the intensity of the fluorescence and not the color of the fluorescent signal. (B) Fluorescence measurements of the treated eye at the different conditions reported in (A). The values represent the mean (and the SEs) of two treated eyes in n = 2 mice. (C) Corneal sections of the eyes collected from the treated mice at 24 h reported in (A). The nuclei are stained with DAPI (blue), whereas the red fluorescence represents siGLO. Fluorescence is present in eyes treated with QN-Palm-POD in all of the layers of the cornea. (D) Representative daily images of one of the four mice during the treatment (10 days) with siLuc and QN-Palm-POD in the right eye and with NSC4 and QN-Palm-POD in the left eye. (E) Quantification of luciferase activity for a treatment group (n = 4) expressed as a percentage of the right eye/left eye ratio (R/L ratio %). Significant knockdown of luciferase with siLuc was persistent from day 7 to day 9. The black arrows indicate the days of treatment. The mice were observed for 3 days before the treatment and 4 days after the treatment. *p < 0.05, ***p < 0.001. (F) Representative section of the corneas treated with QN-Palm-POD-siRNA and stained with H&E. No abnormality or signs of inflammation were observed in any treated cornea.
In Vivo Evaluation of QN-Palm-POD-siRNA Nanoparticle Knockdown of Luciferase Expression
The ability of QN-Palm-POD to deliver siRNA to the cornea and achieve knockdown of corneal epithelial gene expression in vivo was assessed using siLuc knockdown of luciferase expression in a Krt12+/luc2 mouse model. In a split body control experiment, mice (n = 5) were treated with QN-Palm-POD complexed with siLuc (right eye) and NSC4 (left eye) (140:1 molar ratio with 700 μM QN-Palm-POD and 5 μM siRNA) in parallel daily for 4 days. NSC4 was shown earlier not to decrease luciferase signal when injected in the stroma, suggesting that any observed effect on luciferase gene expression is not due to non-specific or toxic effects.
Although no significant knockdown of expression was observed during the 4 days of treatment, a significant knockdown was detected in the 3 days following the termination of the treatment with QN-Palm-POD-siRNA, reaching a maximum of 30% (p < 0.001) at day 9 (day 3 after withdrawal of treatment) (Figures 6D and 6E), whereas luciferase gene expression returned to pretreatment level 4 days after treatment.
In Vivo Evaluation of Cellular Toxicity
To assess whether topical application of QN-Palm-POD-siRNA complexes caused toxicity and damage to the cornea, corneal sections of the treated eyes collected after the termination of the experiment were examined but did not show any alteration of the corneal layers, nor signs of inflammation or cellular infiltration (Figure 6F). Mice eyes were examined by an ophthalmic surgeon at various time points during treatment and up to 15 days post-treatment, and all eyes presented as quiet eyes. At no time point were any signs of swelling, edema, or inflammation noted.
Discussion
The results presented here are the first example of successful topical delivery of a mixed siRNA-delivery agent to the cornea. siRNAs have been successfully and extensively used to treat different diseases,67 but their application for the treatment of corneal pathologies has proved to be difficult, despite the external accessibility of this organ.1, 11, 28 To date, administration of oligonucleotides by intrastromal injection is the preferred route,6, 64, 65 although it is not suitable for a prolonged and repeated treatment regimen.68 Commercially available transfection agents, including Lipofectamine 2000, Entranster-in vivo, PEI, and PEO-PPO-PEO polymers, are unable to deliver Cy3-siRNA to mouse cornea in vivo.11
For successful polynucleotide delivery, a vehicle should fulfill three requirements: (1) delivery to the desired tissue, (2) release of cargo into the cytoplasm, and (3) low toxicity. Cell-penetrating peptides have been proved to satisfy all of these requirements, delivering siRNA and peptides to cells,56, 69 with an increasing number of peptides used for this purpose.34, 47, 70, 71, 72, 73 Some CPPs have been used in vivo to deliver siRNA, targeting tumors, the brain-blood barrier, and other tissues,74, 75 but none was topically applied, either to the skin or to the ocular surface.34 A CPP with proven ability to overcome the corneal barrier,37, 38 POD with a PEG moiety, showed improved functionalizing76 able to deliver a luciferase expression vector to retinal cells in vivo46, 76, 77 and more efficiently than other CPPs such as HIV-Tat and CK30.76 Further modification of PEG-POD by the addition of one moiety of PLGA, previously proved to be biomedically compatible and with recognized delivery features,36, 37 resulting in PLGA-PEG-POD, improved the in vivo bioavailability of POD.37
In the present study, we compared PLGA-PEG-POD with the native POD and two other PODs modified with either a palmitoyl- or a cholesteryl- group (Palm-POD and Chol-POD), in an attempt to improve the performance of siRNA delivery.44, 45 Cholesterol-functionalized siRNAs have been extensively used for this purpose, and their therapeutic use has progressed to clinical trial;78 palmitoylation has proved to enhance peptide absorption by the lipid bilayer of the cell membrane.79, 80
In vitro, all of the PODs tested were able to achieve cellular delivery of siRNA with a low toxicity, which did not exceed that previously reported for the cationic lipid transfection agent, Lipofectamine,81 and CPPs at high concentrations.47 However, bioavailability was not achieved, and none of the formulations achieved gene silencing of a luciferase reporter gene in in vitro-transfected HCE-S cells, consistent with other CPPs, where cellular delivery of siRNA was not matched with gene silencing.47
We attributed the lack of bioavailability and gene silencing in cells treated with POD-siRNA complexes to endosomal entrapment, assuming whichever internalization pathway is utilized by CPP,72 it is fundamental to develop a method that permits the siRNA to escape from the endosomes.35 Herein, we demonstrated Chlq, a known endosomal disruptor (coupled to and derivatized with the POD peptide), to elicit siRNA endosomal release and gene silencing, in agreement with previous reports.41, 54, 82 However, the structural and chemical features of PLGA-PEG-POD make it unsuitable for further covalent modification with endosomal disruptors and because the latter are essential to improve the release and decrease corneal toxicity, PLGA-PEG-POD was excluded from the in vivo study. Both Palm- and Chol-POD achieved a significantly higher knockdown than the POD, and they were thus selected for the subsequent in vivo analysis.
Topical drug delivery to the eye surface has proved difficult because of several anatomical barriers. The tear film in particular reduces the contact time of an applied drug to the eye surface. Absorption through the conjunctival pathway is responsible for the removal of more than 75% of any administrated drug on the ocular surface.83 Topical delivery of POD to the cornea is promising: a fluorescently tagged POD was visible in mouse corneas 45 min after application and persisted, with a decreased intensity, for 24 h afterward, penetrating into the different corneal layers.38 Similarly, a fluorescently labeled PLGA-PEG-POD was visible in rabbit corneas 2 h after topical application.37 siRNA alone cannot penetrate in vivo into the murine cornea unless injected under pressure into the stroma. In comparison, topical delivery of siRNA combined with Chol- and Palm-POD effectively penetrated into all corneal layers and demonstrated some gene silencing of the target gene. The persistence of the fluorescent siRNA for up to 48 h after topical application suggests that these PODs have the capacity to interact with the ocular surface, thus increasing the effective time of exposure and the amount of complex that can be internalized, which is in contrast with results previously described for modified, single-filament fluorescent siRNA, which completely cleared from the cornea in about 3 h.84
We demonstrate here that to achieve knockdown of gene expression, an endosomal disruptor, like Chlq, is necessary to release the siRNA into the cytoplasm. However, because Chlq has been reported to display strong systemic and corneal toxicity,65 we sought a way to use it in vivo that would minimize these harmful side effects. An analog of Chlq, QN, linked to the peptide PepFect6, was previously shown to deliver siRNA and miRNA and achieve knockdown, both in vivo and in vitro66, 85 without significantly enhancing cytokine levels in serum and cellular toxicity in kidney, lung, liver, and spleen.66, 86 We modified Palm-POD by the covalent addition of two moieties of QN. The ability of the Palm-POD to complex siRNA was reduced by QN-functionalization, probably because of the presence of the bulky QN moiety; to compensate for this, subsequent in vitro experiments were all conducted with a 140:1 molar ratio and 125 nM siRNA. Enhanced endosomal release was confirmed through bioavailability and significant knockdown of luciferase expression 72 h after transfection, which was not significantly increased when the cells were pre-treated with Chlq.
The functionalized formulation was also able to deliver siRNA in vivo, performing as well as unmodified Palm-POD, with the siGLO fluorescent signal persistent in the cornea for up to 24 h. This is despite the fact that, as a consequence of the reduced complexation capacity of QN-Palm-POD, the amount of siGLO applied was reduced. Furthermore, the luciferase reporter gene expression knockdown observed (up to 30%) in the corneal epithelium was greater than previously achieved despite a reduced amount of siRNA, and the effect was prolonged for 3 days after treatment and had no observable toxic or inflammatory effect on the cornea in vivo. Enhancing gene silencing within the cornea, using non-invasive eye drop delivery, to a therapeutic level remains a challenge, and we have not matched the 60% knockdown previously reported in mice skin using Accell-siRNA.61 We can match this level of corneal gene silencing using intrastromal injection of Accell siRNA (64%) as described within, but this is not suitable for repeated and long-term siRNA therapeutic application in the ophthalmology clinic. To our knowledge, the modest 30% gene silencing result we achieved is the first report of a decreased protein expression in corneal epithelium after siRNA-mediated knockdown persisting up to 72 h after eye drop treatment. Taketani et al.84 observed knockdown in mouse cornea only at the mRNA level and only for 24 h after the treatment, whereas mRNA expression returned to the untreated level by 48 h.
Moreover, in agreement with reports that PepFect6-siRNA does not elicit an inflammatory response in vivo66, 86 and does not alter the lipid bilayer,87 no toxic effect of QN-Palm-POD-siRNA was observed in mouse corneas in vivo, suggesting that the reduced amount of this Chlq analog is not toxic to the corneal epithelium.
In summary, we designed a novel version of a cell-penetrating POD capable of complexing the siRNA, delivering it into the corneal layers, and releasing functional siRNA into the cytoplasm, which ultimately results in targeted gene expression reduction.
The Palm-POD-siRNA complex gave the best knockdown of in vivo gene expression and, when chemically modified by covalent attachment of the Chlq analog, QN, was able to deliver siRNA to the cytoplasm and to knock down gene expression up to 40% in vitro and 30% in vivo. We acknowledge that this knockdown is relatively modest and will require further improvement to reach levels of knockdown sufficient for therapeutic application. This study confirmed that functionalization of a cell-penetrating peptide for siRNA delivery with an endosomal disruptor is an effective approach to target the cornea in vivo. It also represents a valid proof-of-principle that can applied to safer and more effective endosomal disruptors.88 Different treatment regimens and adjuvants that might increase the persistence of the drug on the eye surface may be tested as well, together with modified siRNAs having an increased resistance to nuclease degradation. The rational design presented in this study, combining an in vitro pre-screening with the in vivo assessment of therapeutic siRNA delivery and function in a corneal bioluminescence reporter mouse, represents a methodology to evaluate the efficacy and topical delivery in the corneal epithelium.
Materials and Methods
Synthesis of Peptides and Preparation of Nanoparticles
POD (CGGG[ARKKAAKA]4)30 was modified in order to obtain: a Palm-POD (molecular weight [MW] 3,829.9), a Chol-POD (MW 4,004.1), and a QN-Palm-POD (MW 4,873.0) as described in Figure S1. The lipophilic derivatization was carried out in solid-phase at the N terminus of the POD sequence. A fraction of the peptidyl-resin was treated with 3-fold molar excesses of palmitic acid, N’, N’ diisopropylcarbodiimide and 1-hydroxybenzotriazole (HOBt) (all of the reagents were from Fluka-Sigma-Aldrich, St. Louis, MO, USA) in dimethylformamide (DMF) (Scharlau, Barcelona, Spain) at room temperature overnight.
Cholesterol was also conjugated at the N terminus of another fraction of peptidyl resin. Modifications were introduced at the N terminus of the cell-penetrating peptide (POD) in order to not alter or modify its secondary structure. The coupling took place by reaction of cholesteryl chloroformate (Fluka-Sigma-Aldrich, St. Louis, MO, USA) (10 equiv) dissolved in dichloromethane (DCM) (Merck, Darmstad, Germany), together with triethylamine (Fluka-Sigma-Aldrich, St. Louis, CO, USA) (3 equiv) at room temperature overnight.
Both peptidyl-resins were treated with a mixture of 95% (v/v) trifluoroacetic acid (TFA) (Scharlau, Barcelona, Spain), 2% (v/v) MilliQ water, 1% (v/v) triisopropylsilane, and 2% (v/v) β-mercaptoethanol (Fluka-Sigma-Aldrich, St. Louis, MO, USA) for 3 h at room temperature. The TFA was removed under N2 flow, and the crude peptides were precipitated with diethyl ether (Merck, Darmstad, Germany). The solids were dissolved in 30% (v/v) acetic acid (Panreac; AppliChem, Darmstad, Germany) in MilliQ water and lyophilized.
To obtain the QN-Palm-POD, an N-α-9-fluorenylmethyloxycarbonyl-N-ε-4-methyltrityl-L-lysine [Fmoc-Lys(Mtt)-OH, 3 equiv] (Novabiochem; Merck Millipore, Darmstad, Germany) amino acid derivative was coupled on solid phase to the N terminus of the POD throughout activation with 2-(1H-7-azabenzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate methanaminium (HATU) (3 equiv) (Genscript, Piscataway, NJ, USA) and diisopropylethylamine (DIPEA) (6 equiv) (Fluka-Sigma-Aldrich, St. Louis, MO, USA) in DMF. After removing the Fmoc-protecting group by reaction with piperidine (Fluka-Sigma-Aldrich, St. Louis, MO, USA) in DMF (20% v/v), the palmitic acid was coupled to the free N-α-amino group as described above. Subsequently, the methyltrityl protecting group of the N-Ɛ-amine of the lysine was selectively removed after repeated treatments with 1% TFA in DCM. A Fmoc-Lys(Fmoc)-OH (Novabiochem; Merck Millipore, Darmstad, Germany) derivative was then incorporated through activation with HATU and DIPEA in DMF. Three-fold molar excess of reagents was used. The deprotection of the Fmoc group by repeated treatment with piperidine in DMF (20% v/v) rendered two free amino groups that were afterward treated with succinic anhydride (Fluka-Sigma-Aldrich, St. Louis, MO, USA) (1.5 equiv) and DIPEA (3 equiv) in DMF. The efficiency of the reactions was evaluated by the ninhydrin colorimetric test. The synthetic scheme of QN and QN-Palm-POD are described in Figures S2A and S2B.
In order to obtain the final QN-Palm-POD derivative, the QN derivative was first synthesized through reaction of 4-chloro-7-(trifluoromethyl)quinoline (Fluka-Sigma-Aldrich, St. Louis, MO, USA) (16.4 mmol) and 2,2′-diamino-N-methyldiethylamine (TCI, Tokyo, Japan) (194.1 mmol).24 The product, N-(2-aminoethyl)-N-methyl-N’-[7-(trifluoromethyl)-quinolin-4-yl]ethane-1,2-diamine (QN), was characterized by MALDI-TOF (Figure S2C) and proton NMR (NMR-H+) (Figures S2 and 2D). QN (2.5 equiv) was coupled overnight to the succinic acid-modified peptidyl resin previously activated with 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethylaminium tetrafluoroborate (TBTU) (Fluka-Sigma-Aldrich, St. Louis, MO, USA) (3 equiv), HOBt (3 equiv), and DIPEA (6 equiv). Crude peptide was obtained after cleavage and final deprotection of the peptidyl-resin with TFA/water/β-mercaptoethanol/TIS (95:2:2:1).
The crude peptides were purified by semi-preparative high-pressure liquid chromatography (HPLC; 1260 Infinity; Agilent Technologies, Santa Clara, CA, USA) in an XBridge Prep BEH 130 C18 column (Waters; 5 μm, 10 × 250 mm) at a flow rate of 3 mL/min. The peptides were purified with a linear gradient of 5%–100% B (0.05% [v/v] TFA in acetonitrile) into A (0.05% [v/v] TFA in water) for 20 min. Their identity was confirmed by electrospray ionization mass spectrometry (ES-MS). Thus, purified peptides were characterized by an analytical ultra-performance liquid chromatograph (UPLC; Waters, Milford, MA, USA) coupled to a time-of-flight (LC-TOF) detector, LCT Premier XE (Micromass; Waters, Milford, MA, USA). Samples were analyzed in the UPLC at a flow rate of 0.3 mL/min. The mass spectra were recorded in positive ion mode in the m/z 500–2,500 range. UPLC was performed in an Acquity UPLC BEH C18 reverse-phase column (2.1 × 100 mm, 1.7-μm particle size). Solvent A was 20 mM formic acid in acetonitrile, and solvent B was 20 mM formic acid in water. Elution was performed with linear gradients of 5%–100% A into B over 10 min. Figures S3 and S4 show the characterization of the pure peptides by ES-MS and MALDI-TOF.
PLGA-PEG-POD-nanoparticles (NPs) were prepared by covalently binding POD to the pegylated polymer PLGA as described in Figure S5. With this aim, PLGA was preactivated before polyethylene glycosylation (PEGylation) with maleimide-PEG-amine. The obtained PLGA-PEG copolymer was dried under vacuum and stored at 4°C. To conjugate the peptide with the PLGA-PEG-maleimide, POD was dissolved in acetonitrile/DMF and added to the polymer dissolved in chloroform. The mixture was covered tightly and stirred overnight. The product was precipitated with 3 mL of an ice-cold 80/20 mixture of diethyl ether/methanol, centrifuged at 2,600 × g for 10 min, the supernatant discarded, and the product re-dissolved in 1 mL of chloroform. This cycle was repeated twice more and the purified PLGA-PEG-POD dried under vacuum.
1H-NMR was used to assess the grafting of PEG to PLGA and the conjugation with POD. The PLGA-PEG was dissolved in deuterated chloroform and the PLGA-PEG-POD in DMSO-d6. The spectra were recorded at 298K on a Varian Inova 500 MHz spectrometer (Agilent Technologies, Santa Clara, CA, USA). PLGA-PEG-POD NPs were prepared following the solvent displacement technique.29 In brief, an organic solution of the polymer containing the POD (PLGA-PEG-POD) in acetone was poured, with moderate stirring, into an RNase-free aqueous solution containing Poloxamer 188 (Lutrol F68). The resulting colloidal suspension was stirred for 5 min, and the acetone was then evaporated and the NP dispersion was concentrated under reduced pressure. The mean particle size, polydispersity index (PI), and ζ-potential were determined by dynamic light scattering (DLS) measurement using a Zetasizer nano ZS (Malvern Instruments, Malvern, UK) at 25°C.
Cell Culture
HCE-S, a spontaneously immortalized human corneal epithelial cell line (a gift from J.T. Daniels, Institute of Ophthalmology, University College London, London, UK),50 was grown in DMEM (GlutaMAX; Invitrogen, UK) supplemented with 10% fetal bovine serum (FBS) (Thermo Fisher, UK). Cells were incubated under 5% CO2 at 37°C and passaged following standard laboratory procedures.
Gel Retardation Assay
To assess the formation of polyplexes between the siRNA and the PODs,89 Chol-POD, Palm-POD, PEG-PLGA-POD, and POD (Table 2) were mixed with siRNA in a PBS solution to give a final concentration of 35 μM for the PODs and 1 μM for the siRNA in a final volume of 10 μL and incubated for 30 min at room temperature. These formulations were then analyzed by electrophoresis on a 1% agarose, 0.5 × TBE (Tris-Borate-EDTA; UltraPure Agarose, Thermo Fisher, UK) gel for 40 min at 100 V and the gel visualized using the Gel Logic 100 Imaging System (Kodak). To determine the optimal ratio for formation of polyplexes, the same procedure was repeated for the QN-Palm-POD at four different molar ratios (35:1, 70:1, 140:1, and 200:1 POD:siRNA).
Measurement of Dimension and ζ-Potential
To measure the dimensions and the ζ-potentials of the polyplexes, POD-siRNA formulations were prepared in a final volume of 50 μL by mixing PODs and siRNA in PBS, and incubated for 40 min at 25°C before analysis. For the measurement of particle size, a molar ratio of 140:1 for the QN-Palm-POD and 35:1 for all the other PODs was used. The samples were then diluted to 1 mL in distilled water before the measurement of ζ-potential (the charge of the POD-siRNA polyplexes) using a Nano ZS Zetasizer and DTS software (Malvern Instruments, Malvern, UK). Three measurements were collected for each sample, and the values expressed as mean ± SD.
Measurement of Effect of Formulation upon Cell Viability (MTT Assay)
HCE-S cells were plated at a density of 1.5 × 104 cells/well in 96-well plates and transfected 24 h later with POD-siRNA polyplexes at 17.5, 35, and 70 μM POD at 35:1 molar ratio in PBS for Chol-, Palm-, and POD and at 9, 17.5, and 35 μM POD at 140:1 molar ratio for the QN-Palm-POD. For each condition, n = 5 replicates were tested. Twenty-four hours post-transfection, 0.5 mg/mL MTT reagent (Sigma-Aldrich, UK) was added to the media, and the cells were incubated for 2 h at 37°C, under 5% CO2. Absorbance was then measured at 570 and 650 nm in a plate reader (LUMIstar OPTIMA; BMG LABTECH, UK). The absorbance at 650 nm, subtracted from that at 570 nm, indicates cell viability; the results obtained were compared with an internal untreated control (maximum cell viability).
In Vitro Fluorescence siRNA Analysis with PODs-siRNA Formulations
Green fluorescent siRNA (siGLO; GE Dharmacon) was complexed with PODs and used to transfect HCE-S cells. Chol-, Palm-, and POD were used at 35:1 molar ratio in PBS with 1 μM green fluorescent siRNA, whereas QN-Palm-POD was used at 17.5 μM with 0.125 μM siGLO, for a final molar ratio of 140:1. HCE-S were seeded on coverslips at 105 cells/well in a 24-well plate, 24 h before POD-siRNA transfection, and 24 h after transfection, the coverslips were collected, fixed for 10 min in 4% paraformaldehyde in PBS (Thermo Scientific, USA), and mounted in Ultracruz Mounting media (Santa Cruz Biotechnology). Fluorescence was then assessed with an AxioScope A1 microscope equipped with a 20×/40× N Archoplan lens on an AxioCam MRc camera (Carl Zeiss, Germany).
To study the effect of endosomal disruptors on siRNA release, the experiment was repeated using the formulation at 35:1 molar ratio alone or in combination with 30 μM Chlq (Sigma-Aldrich, UK) added 1 h before transfection.41
In Vitro Luciferase Assay with POD-siLuc Formulations
A modified in vitro dual-luciferase assay was performed, as previously reported,22, 26 in which expression of Firefly luciferase, the siRNA target, is normalized to Renilla luciferase expression as an internal control of cell transfection: HCE-S cells were plated at 6.5 × 103 cells/well in a 96-well plate, transfected after 24 h with the luciferase reporter plasmids, and then treated 24 h later with the different POD-siRNA formulations (1 μM siRNA 35:1 POD/siRNA in a final volume of 100 μL), using luciferase-specific siRNA (siLuc, 5′- CGACAAGCCUGGCGCAGUAUU-3′, with dTdT overhang at 3′ in both strands; Eurogentech, Belgium) and non-specific control siRNA (NSC4, 5′-UAGCGACUAAACACAUCAAUU-3′, inverted β-galactosidase sequence, with dTdT overhang at 3′ in both strands; Eurogentech, Belgium).22, 43 Luciferase expression was measured 72 h after POD-siRNA transfection, and the values obtained were expressed as a percentage of the luciferase activity measured with NSC4 (100%). The effect of Chlq upon knockdown of gene expression was investigated by transfecting cells with the POD-siRNA formulation, as above, along with 30 μM Chlq, added 1 h before transfection. As a positive control, cells were transfected with 1 μM siLuc/NSC4 siRNA complexed with RNAiMAX (Thermo Fisher, Invitrogen, UK) as previously described.90 The experiment was further repeated with QN-Palm-POD at a 140:1 ratio and with QN-Palm-POD in combination with free Chlq.
Live Animal Imaging
Animals were used for the following experiments in accordance with the UK Animal Welfare Act; the experiments were approved by the Home Office (Scotland) and the Department of Health, Social Services, and Public Safety (DHSSPS; Northern Ireland). The experiments to assess delivery of fluorescent siRNA (siGLO) to the cornea were performed on wild-type C57BL/6 mice. To assess functionality of the delivered siRNA, we used a transgenic mouse line expressing luciferase in the cornea epithelium. This animal model was developed by inserting a synthetic multi-target cassette composed of Meesmann epithelial CD-causing mutations (L132P and R135T in keratin 12, and E509K, R503P, and E498V in keratin 391, 92) with 40-bp flanking regions into the 3′ UTR of the firefly luciferase reporter gene luc2 (codon-optimized for mammalian expression) under the control of the endogenous Krt12 promoter on a C57BL/6 background. Mice were genotyped by extracting genomic DNA (gDNA) from ear biopsies by standard protocols. A common reverse primer was used (K12KI.R): 5′-TGAACGGAACTGTACTTCTGTG-3′ with primers K12KI.2F: 5′-ACGTCCAGACACAGCATAGG-3′ and K12KI.1F: 5′-GCTGTGGAGGCCTCTTTTC-3′) in equimolar concentrations, in order to detect either the luciferase knock-in allele with a 299-bp product or the WT allele with a 553-bp product (Figure S6).
For live imaging, mice between 12 and 25 weeks old were anesthetized using 1.5%–2% isoflurane in oxygen (Abbott Laboratories, Berkshire, UK) at a flow rate of ∼1.5 L/min. To measure luciferase reporter gene expression, luciferin substrate (30 mg/mL D-luciferin potassium salt; Gold Biotechnology, St. Louis, MO, USA) mixed 1:1 w/v with Viscotears gel (Novartis, Camberley, UK) was applied to the eye of heterozygous luc2 transgenic mice 1 min prior to imaging. A Xenogen IVIS Lumina (Perkin Elmer, Cambridge, UK) was used to quantify luminescence and fluorescence. In each mouse eye, a region of interest (ROI) was selected for quantification. ROIs parameters (size and shape) were kept constant throughout, using protocols as previously described.61
Intrastromal Injection
Intrastromal injection of Accell siRNA was performed by a trained ophthalmic surgeon as previously described.64 Two microliters of 150 pmol/μL Cy3-labeled Accell-modified siRNA was injected intrastromally into the right eyes of WT C57BL/6J mice. To assess the persistence of Cy3-labeled siRNA, animals were imaged on the Xenogen IVIS Lumina system at 0, 6, 24, 48, and 72 h post-injection (n = 3). Mice were sacrificed at 0, 6, and 12 h after injection (n = 3); eyes were enucleated and frozen at −80°C. Tissue was fixed in OCT and cryosectioned for fluorescence microscopy. To assess luciferase knockdown, mice were treated in a split body control (untreated versus Accell-siRNA and untreated versus Acell-NSC4) for 7 days after the treatment (n = 3). Luciferase signal was quantified as described earlier in Live Animal Imaging. Baseline luciferase reporter gene expression (day 0) was a mean value obtained by measurement of ocular luminescence daily for 3 days before treatment.
In Vivo POD-siRNA Studies
Mice between 12 and 25 weeks old were anesthetized using 1.5%–2% isoflurane in air (Abbott Laboratories, Berkshire, UK) at a flow rate of ∼1.5 L/min. Formulations containing 35:1 molar ratio POD/siRNA (Chol- and Palm-), with 18 μM siRNA and 625 μM POD or QN-Palm-POD:siRNA at a 140:1 molar ratio with 5 μM siRNA, in a total volume of 2.5 μL of PBS per eye were prepared, incubated at room temperature for 30 min, and then applied as a drop to the cornea of anesthetized mice, which were maintained with the eye in a horizontal position. After application, the mouse was kept anesthetized for a further 15 min, over which period the droplet was observed to remain on the eye, to allow absorption and maximize uptake. Following treatment, fluorescence and luciferase experiments were performed as described below.
Assessment of siRNA Uptake by In Vivo Fluorescence Assay
To assess the uptake of siRNA by the corneal epithelium, in vivo fluorescence assays were performed by treating wild-type mice (n = 2 for each condition). 100 μM red siGLO (#D-001630-02; GE Dharmacon) was used in combination with Chol- or Palm-POD. The siRNA-POD polyplexes were applied to the right eye, whereas siGLO alone was applied to the left eye of each mouse as control. Measurements were obtained from two untreated mice to determine background fluorescence. Fluorescence was detected with a Xenogen IVIS with LivingImage 3.2 software (both Perkin Elmer, Cambridge, UK) using DS Red filters (excitation [Ex.] 570 nm, emission [Em.] 620 nm) at 3, 6, and 24 h following application. Fluorescence was quantified after selecting a ROI tightly cropped to the fluorescent regions in the eyes, and the ROI was kept constant in all subsequent measurements. After the final measurement, mice were sacrificed, and the eyes were enucleated, fixed in 4% paraformaldehyde (prepared in PBS, pH 7.4) for 30 min at room temperature, submerged in Poly-Freeze (P0091 SIGMA; Sigma-Aldrich), and immediately frozen at −80°C. Five-micrometer sections were cut with a cryostat (CM 1850; Leica), mounted on 3-aminopropyltriethoxysilane (APES) (Sigma Aldrich, UK)-coated slides, and treated with a mounting medium containing DAPI (DAPI I; Vysis, USA), to stain the nuclei, and fluorescence was visualized with a fluorescence microscope (as described above).
Assessment of siRNA-Mediated Gene Expression Knockdown by In Vivo Luciferase Expression Analysis
In vivo luciferase experiments were performed using a split body control by comparing the treatment under a test, in one eye, with a negative control in the other eye of the same animal: the right eye was treated with QN-Palm-POD and NSC4, whereas the left one was treated with QN-Palm-POD and siLuc. QN-Palm-POD:siRNA were at a 140:1 molar ratio with 5 μM siRNA. Experiments and treatment were performed in n = 4 mice. Baseline luciferase reporter gene expression was determined by measurement of ocular luminescence daily for 3 days before treatment. Ocular luminescence was measured before POD-siRNA complexes were applied daily, as described above, for 4 days, and ocular luminescence was then measured daily for a further 4 days after cessation of treatment.
H&E Staining of the Mouse Cornea
After the final measurement of luminescence, mice were sacrificed and eyes enucleated, paraformaldehyde fixed, dehydrated through graduated ethanol solutions, and paraffin embedded. Five-micrometer sections were obtained using a microtome (Leica RM 2135), mounted on APES (Sigma Aldrich, UK)-coated slides, dewaxed and rehydrated, and stained with H&E solution (both from Sigma-Aldrich, UK). Sections were visualized using an AxioScope A1 microscope as described previously.
Chemical Compounds
The chemical compounds studied in this article were D-Luciferin potassium salt (compound identifier [CID], PubMed: 23703111), propylene glycol (PubMed: 1030), Chlq (PubMed: 2719), and amphotericin B (PubMed: 5386092).
Statistical Analysis
Statistical analysis was performed using Microsoft Excel 2010 and GraphPad Prism 5 software. Data were presented as mean ± SEM. The different treatment groups were compared using two-tailed Student’s t test and ANOVA. For in vitro assays, a Student’s t test was performed on treatment groups composed of n = 5 replicates. Significance was set at p < 0.05. For the in vivo POD luciferase experiments, the statistical comparison was done by comparing the average right/left ratio for five mice in the first 3 days before the beginning of the treatment with the ratios measured on each of the single days after the beginning of the treatment.
Author Contributions
D.S., T.M., M.A.N., and I.H. conceived of and designed the experiments. D.S., M.J.G., E.M., S.D.A., L.M., K.A.C., D.F.C., and C.M.M. performed the experiments. D.S., E.M., S.D.A., T.M., and I.H. analyzed the data. D.S., E.M., M.A.N., and T.M. wrote the paper.
Acknowledgments
This work was supported by the United Kingdom Fight for Sight grant (to C.B.T.M.), The Belfast Association for the Blind (S.D.A., M.A.N., and T.M.), and Northern Ireland Clinical Research Network Vision Research Translation Research Group (M.A.N. and T.M.). Partial financial support from the Spanish Ministry of Economy, Industry and Competitiveness (MINECO) and the European Regional Development Fund (grant CTQ2015-63919-R to I.H.) is also gratefully acknowledged.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtn.2019.07.017.
Supplemental Information
References
- 1.Guzman-Aranguez A., Loma P., Pintor J. Small-interfering RNAs (siRNAs) as a promising tool for ocular therapy. Br. J. Pharmacol. 2013;170:730–747. doi: 10.1111/bph.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bobbin M.L., Rossi J.J. RNA Interference (RNAi)-Based Therapeutics: Delivering on the Promise? Annu. Rev. Pharmacol. Toxicol. 2016;56:103–122. doi: 10.1146/annurev-pharmtox-010715-103633. [DOI] [PubMed] [Google Scholar]
- 3.Yavuz B., Kompella U.B. Pharmacologic Therapy of Ocular Disease. In: Whitcup S., Azar D., editors. vol. 242. Springer; 2016. pp. 57–93. (Handbook of Experimental Pharmacology). [Google Scholar]
- 4.Järvinen K., Järvinen T., Urtti A. Ocular absorption following topical delivery. Adv. Drug Deliv. Rev. 1995;16:3–19. [Google Scholar]
- 5.Subrizi A., Del Amo E.M., Korzhikov-Vlakh V., Tennikova T., Ruponen M., Urtti A. Design principles of ocular drug delivery systems: importance of drug payload, release rate, and material properties. Drug Discov. Today. 2019 doi: 10.1016/j.drudis.2019.02.001. Published online February 7, 2019. [DOI] [PubMed] [Google Scholar]
- 6.Kim Y.C., Chiang B., Wu X., Prausnitz M.R. Ocular delivery of macromolecules. J. Control. Release. 2014;190:172–181. doi: 10.1016/j.jconrel.2014.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Deliv. Rev. 2006;58:1131–1135. doi: 10.1016/j.addr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 8.Berdugo M., Valamanesh F., Andrieu C., Klein C., Benezra D., Courtois Y., Behar-Cohen F. Delivery of antisense oligonucleotide to the cornea by iontophoresis. Antisense Nucleic Acid Drug Dev. 2003;13:107–114. doi: 10.1089/108729003321629647. [DOI] [PubMed] [Google Scholar]
- 9.Eljarrat-Binstock E., Domb A.J. Iontophoresis: a non-invasive ocular drug delivery. J. Control. Release. 2006;110:479–489. doi: 10.1016/j.jconrel.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 10.Hao J., Li S.K., Liu C.-Y., Kao W.W. Electrically assisted delivery of macromolecules into the corneal epithelium. Exp. Eye Res. 2009;89:934–941. doi: 10.1016/j.exer.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z., Duan F., Lin L., Huang Q., Wu K. A new approach of delivering siRNA to the cornea and its application for inhibiting herpes simplex keratitis. Curr. Mol. Med. 2014;14:1215–1225. doi: 10.2174/1566524014666141021145909. [DOI] [PubMed] [Google Scholar]
- 12.Souza J.G., Dias K., Pereira T.A., Bernardi D.S., Lopez R.F. Topical delivery of ocular therapeutics: carrier systems and physical methods. J. Pharm. Pharmacol. 2014;66:507–530. doi: 10.1111/jphp.12132. [DOI] [PubMed] [Google Scholar]
- 13.Solinís M.Á., del Pozo-Rodríguez A., Apaolaza P.S., Rodríguez-Gascón A. Treatment of ocular disorders by gene therapy. Eur. J. Pharm. Biopharm. 2015;95(Pt B):331–342. doi: 10.1016/j.ejpb.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Williams K.A., Irani Y.D. Gene Therapy and Gene Editing for the Corneal Dystrophies. Asia Pac. J. Ophthalmol. (Phila.) 2016;5:312–316. doi: 10.1097/APO.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 15.Mohan R.R., Rodier J.T., Sharma A. Corneal gene therapy: basic science and translational perspective. Ocul. Surf. 2013;11:150–164. doi: 10.1016/j.jtos.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozcan G., Ozpolat B., Coleman R.L., Sood A.K., Lopez-Berestein G. Preclinical and clinical development of siRNA-based therapeutics. Adv. Drug Deliv. Rev. 2015;87:108–119. doi: 10.1016/j.addr.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klintworth G.K. Corneal dystrophies. Orphanet J. Rare Dis. 2009;4:7. doi: 10.1186/1750-1172-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss J.S., Møller H.U., Aldave A.J., Seitz B., Bredrup C., Kivelä T., Munier F.L., Rapuano C.J., Nischal K.K., Kim E.K. IC3D classification of corneal dystrophies—edition 2. Cornea. 2015;34:117–159. doi: 10.1097/ICO.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 19.Allen E.H., Courtney D.G., Atkinson S.D., Moore J.E., Mairs L., Poulsen E.T., Schiroli D., Maurizi E., Cole C., Hickerson R.P. Keratin 12 missense mutation induces the unfolded protein response and apoptosis in Meesmann epithelial corneal dystrophy. Hum. Mol. Genet. 2016;25:1176–1191. doi: 10.1093/hmg/ddw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Courtney D.G., Atkinson S.D., Allen E.H., Moore J.E., Walsh C.P., Pedrioli D.M., MacEwen C.J., Pellegrini G., Maurizi E., Serafini C. siRNA silencing of the mutant keratin 12 allele in corneal limbal epithelial cells grown from patients with Meesmann’s epithelial corneal dystrophy. Invest. Ophthalmol. Vis. Sci. 2014;55:3352–3360. doi: 10.1167/iovs.13-12957. [DOI] [PubMed] [Google Scholar]
- 21.Courtney D.G., Atkinson S.D., Moore J.E., Maurizi E., Serafini C., Pellegrini G., Black G.C., Manson F.D., Yam G.H., Macewen C.J. Development of allele-specific gene-silencing siRNAs for TGFBI Arg124Cys in lattice corneal dystrophy type I. Invest. Ophthalmol. Vis. Sci. 2014;55:977–985. doi: 10.1167/iovs.13-13279. [DOI] [PubMed] [Google Scholar]
- 22.Liao H., Irvine A.D., Macewen C.J., Weed K.H., Porter L., Corden L.D., Gibson A.B., Moore J.E., Smith F.J., McLean W.H., Moore C.B. Development of allele-specific therapeutic siRNA in Meesmann epithelial corneal dystrophy. PLoS ONE. 2011;6:e28582. doi: 10.1371/journal.pone.0028582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLean W.H., Moore C.B. Keratin disorders: from gene to therapy. Hum. Mol. Genet. 2011;20(R2):R189–R197. doi: 10.1093/hmg/ddr379. [DOI] [PubMed] [Google Scholar]
- 24.Unniyampurath U., Pilankatta R., Krishnan M.N. RNA Interference in the Age of CRISPR: Will CRISPR Interfere with RNAi? Int. J. Mol. Sci. 2016;17:291. doi: 10.3390/ijms17030291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boettcher M., McManus M.T. Choosing the right tool for the job: RNAi, TALEN, or CRISPR. Mol. Cell. 2015;58:575–585. doi: 10.1016/j.molcel.2015.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atkinson S.D., McGilligan V.E., Liao H., Szeverenyi I., Smith F.J., Moore C.B., McLean W.H. Development of allele-specific therapeutic siRNA for keratin 5 mutations in epidermolysis bullosa simplex. J. Invest. Dermatol. 2011;131:2079–2086. doi: 10.1038/jid.2011.169. [DOI] [PubMed] [Google Scholar]
- 27.Hickerson R.P., Smith F.J., Reeves R.E., Contag C.H., Leake D., Leachman S.A., Milstone L.M., McLean W.H., Kaspar R.L. Single-nucleotide-specific siRNA targeting in a dominant-negative skin model. J. Invest. Dermatol. 2008;128:594–605. doi: 10.1038/sj.jid.5701060. [DOI] [PubMed] [Google Scholar]
- 28.Wilkes R.P., Ward D.A., Newkirk K.M., Adams J.K., Kania S.A. Evaluation of delivery agents used for introduction of small interfering RNAs into feline corneal cells. Am. J. Vet. Res. 2013;74:243–247. doi: 10.2460/ajvr.74.2.243. [DOI] [PubMed] [Google Scholar]
- 29.Rabinovich-Guilatt L., Couvreur P., Lambert G., Dubernet C. Cationic vectors in ocular drug delivery. J. Drug Target. 2004;12:623–633. doi: 10.1080/10611860400015910. [DOI] [PubMed] [Google Scholar]
- 30.Patel A., Cholkar K., Agrahari V., Mitra A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013;2:47–64. doi: 10.5497/wjp.v2.i2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang S., Zhao B., Jiang H., Wang B., Ma B. Cationic lipids and polymers mediated vectors for delivery of siRNA. J. Control. Release. 2007;123:1–10. doi: 10.1016/j.jconrel.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Calvo P., Vila-Jato J.L., Alonso M.J. Evaluation of cationic polymer-coated nanocapsules as ocular drug carriers. Int. J. Pharm. 1997;153:41–50. [Google Scholar]
- 33.Lindgren M., Langel Ü. Classes and prediction of cell-penetrating peptides. Methods Mol. Biol. 2011;683:3–19. doi: 10.1007/978-1-60761-919-2_1. [DOI] [PubMed] [Google Scholar]
- 34.Kurrikoff K., Gestin M., Langel Ü. Recent in vivo advances in cell-penetrating peptide-assisted drug delivery. Expert Opin. Drug Deliv. 2016;13:373–387. doi: 10.1517/17425247.2016.1125879. [DOI] [PubMed] [Google Scholar]
- 35.Endoh T., Ohtsuki T. Cellular siRNA delivery using cell-penetrating peptides modified for endosomal escape. Adv. Drug Deliv. Rev. 2009;61:704–709. doi: 10.1016/j.addr.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Juliano R.L. The delivery of therapeutic oligonucleotides. Nucleic Acids Res. 2016;44:6518–6548. doi: 10.1093/nar/gkw236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vasconcelos A.C., Vega E., Pérez Y., Gómara M.J., García M.L., Haro I. Conjugation of cell-penetrating peptides with poly(lactic-co-glycolic acid)-polyethylene glycol nanoparticles improves ocular drug delivery. Int. J. Nanomedicine. 2015;10:609–631. doi: 10.2147/IJN.S71198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson L.N., Cashman S.M., Kumar-Singh R. Cell-penetrating peptide for enhanced delivery of nucleic acids and drugs to ocular tissues including retina and cornea. Mol. Ther. 2008;16:107–114. doi: 10.1038/sj.mt.6300324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pescina S., Sala M., Padula C., Scala M.C., Spensiero A., Belletti S., Gatti R., Novellino E., Campiglia P., Santi P. Design and synthesis of new cell penetrating peptides: diffusion and distribution inside the cornea. Mol. Pharm. 2016;13:3876–3883. doi: 10.1021/acs.molpharmaceut.6b00658. [DOI] [PubMed] [Google Scholar]
- 40.Bouheraoua N., Jouve L., Borderie V., Laroche L. Three Different Protocols of Corneal Collagen Crosslinking in Keratoconus: Conventional, Accelerated and Iontophoresis. J. Vis. Exp. 2015;105:e53119. doi: 10.3791/53119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhattarai S.R., Muthuswamy E., Wani A., Brichacek M., Castañeda A.L., Brock S.L., Oupicky D. Enhanced gene and siRNA delivery by polycation-modified mesoporous silica nanoparticles loaded with chloroquine. Pharm. Res. 2010;27:2556–2568. doi: 10.1007/s11095-010-0245-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gebhart C.L., Kabanov A.V. Evaluation of polyplexes as gene transfer agents. J. Control. Release. 2001;73:401–416. doi: 10.1016/s0168-3659(01)00357-1. [DOI] [PubMed] [Google Scholar]
- 43.Allen E.H.A., Atkinson S.D., Liao H., Moore J.E., Pedrioli D.M.L., Smith F.J.D., McLean W.H.I., Moore C.B.T. Allele-specific siRNA silencing for the common keratin 12 founder mutation in Meesmann epithelial corneal dystrophy. Invest. Ophthalmol. Vis. Sci. 2013;54:494–502. doi: 10.1167/iovs.12-10528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jing X., Foged C., Martin-Bertelsen B., Yaghmur A., Knapp K.M., Malmsten M., Franzyk H., Nielsen H.M. Delivery of siRNA Complexed with Palmitoylated α-Peptide/β-Peptoid Cell-Penetrating Peptidomimetics: Membrane Interaction and Structural Characterization of a Lipid-Based Nanocarrier System. Mol. Pharm. 2016;13:1739–1749. doi: 10.1021/acs.molpharmaceut.5b00309. [DOI] [PubMed] [Google Scholar]
- 45.Qin B., Chen Z., Jin W., Cheng K. Development of cholesteryl peptide micelles for siRNA delivery. J. Control. Release. 2013;172:159–168. doi: 10.1016/j.jconrel.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Binder C., Cashman S.M., Kumar-Singh R. Extended duration of transgene expression from pegylated POD nanoparticles enables attenuation of photoreceptor degeneration. PLoS ONE. 2013;8:e82295. doi: 10.1371/journal.pone.0082295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Asbeck A.H., Beyerle A., McNeill H., Bovee-Geurts P.H., Lindberg S., Verdurmen W.P., Hällbrink M., Langel U., Heidenreich O., Brock R. Molecular parameters of siRNA—cell penetrating peptide nanocomplexes for efficient cellular delivery. ACS Nano. 2013;7:3797–3807. doi: 10.1021/nn305754c. [DOI] [PubMed] [Google Scholar]
- 48.Kim S.W., Kim N.Y., Choi Y.B., Park S.H., Yang J.M., Shin S. RNA interference in vitro and in vivo using an arginine peptide/siRNA complex system. J. Control. Release. 2010;143:335–343. doi: 10.1016/j.jconrel.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 49.Hatakeyama H., Ito E., Akita H., Oishi M., Nagasaki Y., Futaki S., Harashima H. A pH-sensitive fusogenic peptide facilitates endosomal escape and greatly enhances the gene silencing of siRNA-containing nanoparticles in vitro and in vivo. J. Control. Release. 2009;139:127–132. doi: 10.1016/j.jconrel.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 50.Notara M., Daniels J.T. Characterisation and functional features of a spontaneously immortalised human corneal epithelial cell line with progenitor-like characteristics. Brain Res. Bull. 2010;81:279–286. doi: 10.1016/j.brainresbull.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 51.Greco D., Vellonen K.S., Turner H.C., Häkli M., Tervo T., Auvinen P., Wolosin J.M., Urtti A. Gene expression analysis in SV-40 immortalized human corneal epithelial cells cultured with an air-liquid interface. Mol. Vis. 2010;16:2109–2120. [PMC free article] [PubMed] [Google Scholar]
- 52.Toropainen E., Hornof M., Kaarniranta K., Johansson P., Urtti A. Corneal epithelium as a platform for secretion of transgene products after transfection with liposomal gene eyedrops. J. Gene Med. 2007;9:208–216. doi: 10.1002/jgm.1011. [DOI] [PubMed] [Google Scholar]
- 53.Rönkkö S., Vellonen K.-S., Järvinen K., Toropainen E., Urtti A. Human corneal cell culture models for drug toxicity studies. Drug Deliv. Transl. Res. 2016;6:660–675. doi: 10.1007/s13346-016-0330-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El-Andaloussi S., Johansson H.J., Lundberg P., Langel U. Induction of splice correction by cell-penetrating peptide nucleic acids. J. Gene Med. 2006;8:1262–1273. doi: 10.1002/jgm.950. [DOI] [PubMed] [Google Scholar]
- 55.Chiu Y.-L., Ali A., Chu C.Y., Cao H., Rana T.M. Visualizing a correlation between siRNA localization, cellular uptake, and RNAi in living cells. Chem. Biol. 2004;11:1165–1175. doi: 10.1016/j.chembiol.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 56.Erazo-Oliveras A., Muthukrishnan N., Baker R., Wang T.-Y., Pellois J.-P. Improving the endosomal escape of cell-penetrating peptides and their cargos: strategies and challenges. Pharmaceuticals (Basel) 2012;5:1177–1209. doi: 10.3390/ph5111177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma D. Enhancing endosomal escape for nanoparticle mediated siRNA delivery. Nanoscale. 2014;6:6415–6425. doi: 10.1039/c4nr00018h. [DOI] [PubMed] [Google Scholar]
- 58.Varkouhi A.K., Scholte M., Storm G., Haisma H.J. Endosomal escape pathways for delivery of biologicals. J. Control. Release. 2011;151:220–228. doi: 10.1016/j.jconrel.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 59.Leslie Pedrioli D.M., Fu D.J., Gonzalez-Gonzalez E., Contag C.H., Kaspar R.L., Smith F.J., McLean W.H. Generic and personalized RNAi-based therapeutics for a dominant-negative epidermal fragility disorder. J. Invest. Dermatol. 2012;132:1627–1635. doi: 10.1038/jid.2012.28. [DOI] [PubMed] [Google Scholar]
- 60.Smith F.J., Hickerson R.P., Sayers J.M., Reeves R.E., Contag C.H., Leake D., Kaspar R.L., McLean W.H. Development of therapeutic siRNAs for pachyonychia congenita. J. Invest. Dermatol. 2008;128:50–58. doi: 10.1038/sj.jid.5701040. [DOI] [PubMed] [Google Scholar]
- 61.Hegde V., Hickerson R.P., Nainamalai S., Campbell P.A., Smith F.J., McLean W.H., Pedrioli D.M. In vivo gene silencing following non-invasive siRNA delivery into the skin using a novel topical formulation. J. Control. Release. 2014;196:355–362. doi: 10.1016/j.jconrel.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leachman S.A., Hickerson R.P., Hull P.R., Smith F.J., Milstone L.M., Lane E.B., Bale S.J., Roop D.R., McLean W.H., Kaspar R.L. Therapeutic siRNAs for dominant genetic skin disorders including pachyonychia congenita. J. Dermatol. Sci. 2008;51:151–157. doi: 10.1016/j.jdermsci.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moore J.E., McMullen T.C., Campbell I.L., Rohan R., Kaji Y., Afshari N.A., Usui T., Archer D.B., Adamis A.P. The inflammatory milieu associated with conjunctivalized cornea and its alteration with IL-1 RA gene therapy. Invest. Ophthalmol. Vis. Sci. 2002;43:2905–2915. [PubMed] [Google Scholar]
- 64.Courtney D.G., Moore J.E., Atkinson S.D., Maurizi E., Allen E.H., Pedrioli D.M., McLean W.H., Nesbit M.A., Moore C.B. CRISPR/Cas9 DNA cleavage at SNP-derived PAM enables both in vitro and in vivo KRT12 mutation-specific targeting. Gene Ther. 2016;23:108–112. doi: 10.1038/gt.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bernstein H.N. Chloroquine ocular toxicity. Surv. Ophthalmol. 1967;12:415–447. [PubMed] [Google Scholar]
- 66.Andaloussi S.E., Lehto T., Mäger I., Rosenthal-Aizman K., Oprea I.I., Simonson O.E., Sork H., Ezzat K., Copolovici D.M., Kurrikoff K. Design of a peptide-based vector, PepFect6, for efficient delivery of siRNA in cell culture and systemically in vivo. Nucleic Acids Res. 2011;39:3972–3987. doi: 10.1093/nar/gkq1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wittrup A., Lieberman J. Knocking down disease: a progress report on siRNA therapeutics. Nat. Rev. Genet. 2015;16:543–552. doi: 10.1038/nrg3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liang S.Y.-W., Lee G.A. Intrastromal injection of antibiotic agent in the management of recalcitrant bacterial keratitis. J. Cataract Refract. Surg. 2011;37:960–962. doi: 10.1016/j.jcrs.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 69.Liu C., Jiang K., Tai L., Liu Y., Wei G., Lu W., Pan W. Facile noninvasive retinal gene delivery enabled by penetratin. ACS Appl. Mater. Interfaces. 2016;8:19256–19267. doi: 10.1021/acsami.6b04551. [DOI] [PubMed] [Google Scholar]
- 70.Arukuusk P., Pärnaste L., Hällbrink M., Langel Ü. PepFects and NickFects for the intracellular delivery of nucleic acids. Methods Mol. Biol. 2015;1324:303–315. doi: 10.1007/978-1-4939-2806-4_19. [DOI] [PubMed] [Google Scholar]
- 71.Hou K.K., Pan H., Schlesinger P.H., Wickline S.A. A role for peptides in overcoming endosomal entrapment in siRNA delivery—a focus on melittin. Biotechnol. Adv. 2015;33:931–940. doi: 10.1016/j.biotechadv.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pae J., Pooga M. Peptide-mediated delivery: an overview of pathways for efficient internalization. Ther. Deliv. 2014;5:1203–1222. doi: 10.4155/tde.14.72. [DOI] [PubMed] [Google Scholar]
- 73.Reissmann S. Cell penetration: scope and limitations by the application of cell-penetrating peptides. J. Pept. Sci. 2014;20:760–784. doi: 10.1002/psc.2672. [DOI] [PubMed] [Google Scholar]
- 74.Fonseca S.B., Pereira M.P., Kelley S.O. Recent advances in the use of cell-penetrating peptides for medical and biological applications. Adv. Drug Deliv. Rev. 2009;61:953–964. doi: 10.1016/j.addr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 75.Vivès E., Schmidt J., Pèlegrin A. Cell-penetrating and cell-targeting peptides in drug delivery. Biochim. Biophys. Acta. 2008;1786:126–138. doi: 10.1016/j.bbcan.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 76.Read S.P., Cashman S.M., Kumar-Singh R. A poly(ethylene) glycolylated peptide for ocular delivery compacts DNA into nanoparticles for gene delivery to post-mitotic tissues in vivo. J. Gene Med. 2010;12:86–96. doi: 10.1002/jgm.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Read S.P., Cashman S.M., Kumar-Singh R. POD nanoparticles expressing GDNF provide structural and functional rescue of light-induced retinal degeneration in an adult mouse. Mol. Ther. 2010;18:1917–1926. doi: 10.1038/mt.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tiemann K., Rossi J.J. RNAi-based therapeutics-current status, challenges and prospects. EMBO Mol. Med. 2009;1:142–151. doi: 10.1002/emmm.200900023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rojo N., Gómara M.J., Alsina M.A., Haro I. Lipophilic derivatization of synthetic peptides belonging to NS3 and E2 proteins of GB virus-C (hepatitis G virus) and its effect on the interaction with model lipid membranes. J. Pept. Res. 2003;61:318–330. doi: 10.1034/j.1399-3011.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- 80.Pérez-López S., Vila-Romeu N., Asunción Alsina Esteller M., Espina M., Haro I., Mestres C. Interaction of GB virus C/hepatitis G virus synthetic peptides with lipid langmuir monolayers and large unilamellar vesicles. J. Phys. Chem. B. 2009;113:319–327. doi: 10.1021/jp806938y. [DOI] [PubMed] [Google Scholar]
- 81.McCarthy H.O., McCaffrey J., McCrudden C.M., Zholobenko A., Ali A.A., McBride J.W., Massey A.S., Pentlavalli S., Chen K.H., Cole G. Development and characterization of self-assembling nanoparticles using a bio-inspired amphipathic peptide for gene delivery. J. Control. Release. 2014;189:141–149. doi: 10.1016/j.jconrel.2014.06.048. [DOI] [PubMed] [Google Scholar]
- 82.El-Sayed A., Futaki S., Harashima H. Delivery of macromolecules using arginine-rich cell-penetrating peptides: ways to overcome endosomal entrapment. AAPS J. 2009;11:13–22. doi: 10.1208/s12248-008-9071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sánchez-López E., Espina M., Doktorovova S., Souto E.B., García M.L. Lipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye—Part I—Barriers and determining factors in ocular delivery. Eur. J. Pharm. Biopharm. 2017;110:70–75. doi: 10.1016/j.ejpb.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 84.Taketani Y., Usui T., Toyono T., Shima N., Yokoo S., Kimakura M., Yamagami S., Ohno S., Onodera R., Tahara K. Topical Use of Angiopoietin-like Protein 2 RNAi-loaded Lipid Nanoparticles Suppresses Corneal Neovascularization. Mol. Ther. Nucleic Acids. 2016;5:e292. doi: 10.1038/mtna.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Urgard E., Lorents A., Klaas M., Padari K., Viil J., Runnel T., Langel K., Kingo K., Tkaczyk E., Langel Ü. Pre-administration of PepFect6-microRNA-146a nanocomplexes inhibits inflammatory responses in keratinocytes and in a mouse model of irritant contact dermatitis. J. Control. Release. 2016;235:195–204. doi: 10.1016/j.jconrel.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 86.Suhorutsenko J., Oskolkov N., Arukuusk P., Kurrikoff K., Eriste E., Copolovici D.M., Langel U. Cell-penetrating peptides, PepFects, show no evidence of toxicity and immunogenicity in vitro and in vivo. Bioconjug. Chem. 2011;22:2255–2262. doi: 10.1021/bc200293d. [DOI] [PubMed] [Google Scholar]
- 87.Anko M., Majhenc J., Kogej K., Sillard R., Langel U., Anderluh G., Zorko M. Influence of stearyl and trifluoromethylquinoline modifications of the cell penetrating peptide TP10 on its interaction with a lipid membrane. Biochim. Biophys. Acta. 2012;1818:915–924. doi: 10.1016/j.bbamem.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 88.Joris F., De Backer L., Van de Vyver T., Bastiancich C., De Smedt S.C., Raemdonck K. Repurposing cationic amphiphilic drugs as adjuvants to induce lysosomal siRNA escape in nanogel transfected cells. J. Control. Release. 2018;269:266–276. doi: 10.1016/j.jconrel.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 89.Gary D.J., Lee H., Sharma R., Lee J.S., Kim Y., Cui Z.Y., Jia D., Bowman V.D., Chipman P.R., Wan L. Influence of nano-carrier architecture on in vitro siRNA delivery performance and in vivo biodistribution: polyplexes vs micelleplexes. ACS Nano. 2011;5:3493–3505. doi: 10.1021/nn102540y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maurizi E., Schiroli D., Atkinson S.D., Mairs L., Courtney D.G., O’Hagan B., McGilligan V.E., Pagnamenta A.T., Taylor J.C., Vasquez J.J.D. A novel role for CRIM1 in the corneal response to UV and pterygium development. Exp. Eye Res. 2019;179:75–92. doi: 10.1016/j.exer.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 91.Kao W.W., Liu C.Y., Converse R.L., Shiraishi A., Kao C.W., Ishizaki M., Doetschman T., Duffy J. Keratin 12-deficient mice have fragile corneal epithelia. Invest. Ophthalmol. Vis. Sci. 1996;37:2572–2584. [PubMed] [Google Scholar]
- 92.Irvine A.D., Corden L.D., Swensson O., Swensson B., Moore J.E., Frazer D.G., Smith F.J., Knowlton R.G., Christophers E., Rochels R. Mutations in cornea-specific keratin K3 or K12 genes cause Meesmann’s corneal dystrophy. Nat. Genet. 1997;16:184–187. doi: 10.1038/ng0697-184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.