Abstract
Short-term administration of Lactobacillus gasseri CP2305 improves stress-associated symptoms and clinical symptoms in healthy young adults and in patients with irritable bowel syndrome, respectively. We evaluated the efficacy and health benefits of the long-term use of a tablet containing heat-inactivated, washed Lactobacillus gasseri CP2305 (CP2305) in healthy young adults. Sixty Japanese medical students (41 men and 19 women) preparing for the national examination for medical practitioners ingested CP2305-containing or placebo tablets once daily for 24 weeks. Intake of the CP2305 tablet significantly reduced anxiety and sleep disturbance relative to placebo, as quantitated by the Spielberger State-Trait Anxiety Inventory and the Pittsburgh Sleep Quality Index. Single-channel sleep electroencephalograms show that CP2305 significantly shortened sleep latency and wake time after sleep onset and increased the delta power ratio in the first sleep cycle. CP2305 also significantly lowered salivary chromogranin A levels compared with placebo. Furthermore, 16S rRNA gene sequencing of participant feces demonstrated that CP2305 administration attenuated the stress-induced decline of Bifidobacterium spp. and the stress-induced elevation of Streptococcus spp. We conclude that the long-term use of CP2305-containing tablets may improve the mental state, sleep quality, and gut microbiota of healthy adults under stressful conditions.
Keywords: heat-inactivated Lactobacillus gasseri CP2305, psychobiotics, healthy young adults, stress, mental health, sleep quality, fecal microbiota
1. Introduction
The bidirectional communication system between the gut and brain, the gut–brain axis, has been shown to have a crucial role in the maintenance of intestinal homeostasis and brain function [1,2]. The microbial community of the gastrointestinal tract affects this communication system through immune, endocrine, and neural pathways [3]. Indeed, several lines of evidence suggest that the gut microbiome has a significant impact on brain function, affecting mood, recognition, and behavior [4]. Thus, the concept of the gut–brain axis has expanded to the “microbiota–gut–brain axis” [4].
Probiotics, defined as live microorganisms which confer a health benefit to the host when administered in adequate amounts [5], have also been shown to interact with the brain. In particular, animal studies have reported that probiotic administration modulates the hippocampus-mediated negative feedback regulation of the hypothalamic–pituitary–adrenal (HPA) axis and mitigates stress-induced visceral pain and behavior [6,7]. Probiotics have also been shown to transduce signals to the brain via the afferent vagal nerve and relieve mood disturbances [8,9]. Accordingly, the term “psychobiotics” refers to live microbes that confer a positive mental health benefit [10]. In human trials, the probiotic Lactobacillus plantarum 299v complemented treatment with selective serotonin reuptake inhibitors, improving the cognitive performance of patients with major depressive disorder [11]. Similarly, administration of Bifidobacterium longum NCC3001 reduced depression, but not anxiety scores, and improved the quality of life in patients with irritable bowel syndrome (IBS). These observations were shown to be associated with reduction of the amygdala and fronto-limbic reactivity [12]. Lactobacillus casei strain Shirota has also been reported to relieve stress-associated symptoms in young adults experiencing stressful situations [9].
In addition to live bacteria, heat-inactivated probiotics have been shown to exert beneficial effects. For example, administration of heat-killed Lactobacillus acidophilus L-92 reduced symptoms of atopic dermatitis in adults [13]. In hamsters, supplementation of a high-fat diet with heat-killed Lactobacillus reuteri GMNL-263 mitigated fatty liver syndrome and fibrosis of the liver and heart through a reduction in the expression of transforming growth factor β [14]. Wei et al. [15] showed that both live and heat-killed Lactobacillus paracasei PS23 reversed chronic corticosterone-induced anxiety- and depression-like behaviors via prevention of the corticosterone-induced decline of brain-derived neurotrophic factor, mineralocorticoid, and glucocorticoid receptor levels in the hippocampus.
Similarly, Lactobacillus gasseri CP2305, which was originally isolated from the stool of a healthy volunteer and colonizes the digestive tract of 40% of recipients [16], has been shown to relieve stress. Indeed, daily intake of live CP2305 for four weeks ameliorated stress-associated symptoms in medical students participating in a cadaver dissection course [16] and improved the clinical symptoms of patients with IBS [17]. Heat-inactivated CP2305 exerted similar stress-relieving effects when tested using the same cadaver dissection stress [18]. Further, the daily intake of a heat-inactivated, washed CP2305-containing beverage for 12 weeks significantly reduced stress-associated mental and physical symptoms, sleep disturbance, and elevation of stress-responsive microRNAs in peripheral blood associated with preparation for a national certification examination [19].
Formulation of heat-inactivated bacteria in tablets has the following advantages: preservation, portability, less sugar, and fewer artificial ingredients. Tablets containing heat-inactivated CP2305 do not include any bioactive metabolites produced by live bacteria. However, heat-inactivated CP2305 itself exhibited bioactivity in our previous studies [16,17,18,19]. Based on these observations, this study was designed to evaluate a tablet containing heat-inactivated, washed, and dried CP2305 as a stress-relieving para-psychobiotic, using the national examination stress model. We used psychological questionnaire scores and biological stress responses based on salivary cortisol and chromogranin A levels as the primary outcomes, and sleep electroencephalogram (EEG) and changes in the gut microbiome as the secondary outcomes.
2. Materials and Methods
2.1. Participants and Study Design
The present clinical trial was approved by the Institutional Review Board of Tokushima University Hospital and was conducted according to the ethical standards established in the 1964 Declaration of Helsinki. This study was registered with the University Hospital Medical Information Network (UMIN) Clinical Trials Registry as “Research on a lactic acid bacterium preparation for stress relief” (UMIN000027303).
The study was designed as a double-blind, placebo-controlled, parallel-group clinical trial and ran from July 2017 to March 2018 (Table 1). Sample size calculation was performed with G*Power 3.1 [20] using F-test between factors with two groups (CP2305 and placebo) and three evaluation sessions (before, 12, and 24 weeks after administration). Assuming an a priori effect size of 0.25 estimated from our previous study [19], an α error probability of 0.05, and a power (1-β error probability) of 0.80, the resulting total sample size is 68. Seventy-four sixth-grade medical students were recruited at Tokushima University, Tokushima, Japan. Written informed consent was obtained from all participants, and they were randomly allocated to either the CP2305 or placebo group with a stratified randomization by gender. Seven of the 74 participants were excluded as they were taking medications that could affect sleep quality; three females were excluded for hormonal contraceptives; and four others were excluded owing to active disease (mental disease, inflammatory disease, bone disease, and hormonal disorder). BMI, alcohol consumption, and smoking were not exclusion criteria. Finally, 29 participants (20 males and nine females) were allocated to the CP2305 group and 31 (21 males and 10 females) to the placebo group. All participants had no history of taking medications within the three months prior to study enrollment or during the study period; none of the participants had a history of psychiatric or other disease. Females were not taking hormonal contraceptives. The participants were instructed to ingest two tablets (placebo or CP2305) once daily for 24 weeks. To assess compliance, participants self-recorded tablet intake. During the trial, the participants were asked not to consume fermented milks, foods containing live lactic acid bacteria, or other probiotic or prebiotic products.
Table 1.
Experimental schedule.
| Measurements/Events | Period of Time (Weeks) | ||||
|---|---|---|---|---|---|
| −2 | 0 | 12 | 24 | 26 | |
| Tablet intake |

|
||||
| Questionnaires | ● | ● | ● | ||
| Weekly diary |

|
||||
| Saliva sampling (1 day) | ● | ● | ● | ||
| EEG waves measurement (3 days) |
|
|
|||
| Fecal sampling (3 days) |
|
|
|||
| Defecation diary (7 days) |

|
|

|
||
| National examination (2 days) |
 * * |
||||
●: Spot event,  : Once a day in the indicated period * All subjects took the national examination for medical practitioners. EEG: electroencephalogram.
: Once a day in the indicated period * All subjects took the national examination for medical practitioners. EEG: electroencephalogram.
2.2. Tablets
Both the CP2305-containing and placebo tablets were prepared using the same procedures and formula except for the presence or absence of heat-inactivated, washed, and dried CP2305 (1 × 1010 bacterial cells per 2 tablets). The active tablet was composed of maltose, dextrin, starch, heat-inactivated lactic acid bacteria powder, and vegetable oil. The placebo tablet was similarly composed except that the lactic acid bacteria powder was replaced with dextrin. The formula was allergen-free.
2.3. Questionnaires to Assess Mental and Physical States
The physical and mental health of the participants was evaluated using the following questionnaires: the Spielberger State-Trait Anxiety Inventory (STAI) [21], the 28-item General Health Questionnaire (GHQ-28) [22], and the Hospital Anxiety and Depression Scale (HADS) [23]. Sleep was assessed using the Pittsburgh Sleep Quality Index (PSQI) [24]. Participants completed the questionnaires three times, two weeks before and 12 or 24 weeks after the intervention. Stress-associated symptoms (mental irritability, abdominal discomfort, feeling tired, and sleep disturbance) were also assessed using a 100 mm visual analog scale (VAS) ranging from none (0 mm) to worst (100 mm). The participants reported their symptoms by marking the VAS every week during the experimental period.
2.4. Measurements of Salivary Cortisol and Chromogranin A (CGA)
Saliva was collected within three days before and 12 or 24 weeks after the intervention start between 16:00 and 17:00, to avoid diurnal fluctuations, using Salivette sampling devices (Sarstadt Inc., Rommelsdorf, Germany) as previously described [25]. Concentrations of salivary CgA (YK070 Human CgA EIA kit; Yanaihara Institute, Shizuoka, Japan), cortisol (Expanded Range High Sensitivity Salivary Cortisol Enzyme Immunoassay kit; Salimetrics Inc., LLC, Carlsbad, CA, USA), and protein (Protein Quantification Kit-Wide Range; Dojindo Inc., Kumamoto, Japan) were measured according the manufacturer’s instructions. Saliva samples were stored at −80 °C until analysis.
2.5. Measurement and Assessment of Single-Channel Sleep Electroencephalogram (EEG)
A single-channel EEG was used to record brain activity overnight (Sleep ScopeTM; SleepWell Co., Osaka, Japan). This instrument is approved as medical equipment (Certification No. 27ADBZX00087000) in Japan and has been widely used in sleep studies [26,27]. After the participants were trained, they wore the portable EEG monitor on three separate occasions before and 24 weeks after starting the supplements. All subjects were required not to consume alcohol on the day the EEG was performed. The data from the first measurement was treated as practice. The data collected from the second measurement were analyzed unless data collection was unsuccessful owing to a failure in data gathering, alcohol intake, or a lack of sufficient sleep-associated data. In those cases, the third record was used for analysis. The sleep stages were scored in accordance with the American Academy of Sleep Medicine Manual [28].
2.6. Assessment of Stool Properties and Bowel Habits
All participants recorded the frequency of their bowel movements and fecal characteristics (form, color tone, and output volume) seven consecutive days before and 12 or 24 weeks after the intervention began. Fecal form and color tone were assessed using the Bristol Stool Scale [29]. The fecal output volume was measured using a circular cylinder with a base diameter of 2.5 cm and a length of 5.0 cm.
2.7. Measurement of Short-Chain Fatty Acid (SCFA) Concentrations in Feces
Fecal samples were collected within three days before the start of tablet intake and at the end of the study. Concentrations of acetic acid, propionic acid, n-butyric acid, isobutyric acid, n-valeric acid, and isovaleric acid were measured according to the method of Ikeda et al. [30] by high-performance liquid chromatography with a pH indicator (LaChrom Elite; Hitachi High-Technologies Corporation, Tokyo, Japan).
2.8. Fecal Microbiota Analysis
To analyze the participants’ intestinal microbiota, fecal samples were collected at two time points, within three days before and 24 weeks after the start of the supplementation. The intestinal microbiota analysis was performed by high-throughput sequencing of the 16S rRNA gene using a MiSeq V2 kit (Illumina, San Diego, CA, USA) as described by Hatanaka et al. [31]. Briefly, the fecal samples were washed in phosphate-buffered saline and centrifuged. Pellets were resuspended in 166 mmol L−1 Tris–HCl buffer (pH 9.0) containing 66 mmol l−1 EDTA, 8.3% sodium dodecyl sulfate, and 66% saturated phenol in Tris–EDTA (TE) buffer (10 mmol l−1 Tris–HCl (pH 8.0), 1 mmol l−1 EDTA (pH 8.0)). Glass beads (0.1 mm diameter) were added to the suspension, and the mixture was vortexed vigorously for 60 s using a Multi Beads ShockerR (Yasui Kikai Corporation, Osaka, Japan). After centrifugation at 18,700 g for 5 min at 4 °C, the supernatant was extracted with phenol–chloroform–isoamyl alcohol (25:24:1), and DNA was precipitated with isopropanol. The precipitates were washed with 70% ethanol and dissolved with TE buffer. For further purification, a High Pure PCR Template Preparation Kit (Roche, Tokyo, Japan) was used according to the manufacturer’s instructions. Gene sequencing of 16S rRNA was performed as described previously [32]. Briefly, the purified DNA was used as the template for amplicon PCR, and the V4 fragment of the 16S rRNA was amplified with a primer set of Tru357F (5’-CGCTCTTCCGATCTCTG TACGGRAGGCAGCAG-3’) and Tru806R (5’-CGCTCTTCCGATCTGAC GGACTACHVGGGTWTCTAAT-3’). After the PCR products were purified by Agencourt AMPure XP (Beckman Coulter, Inc., CA, USA), the products were amplified using the Nextera Index Kit (Illumina, CA, USA). After the second PCR, amplified products were purified using Agencourt AMPure XP. The library was quantified, normalized, and pooled in an equimolar amount. Sequencing was performed with an Illumina MiSeq system and MiSeq Reagent Kit v.2 (300 Cycle). Sequence data were analyzed as described previously [32]. In brief, Quantitative Insights Into Microbial Ecology (QIIME) ver.1.8.0 was used for sequence filtering and analysis. Quality filtering was performed using fastq files, and sequences with a quality score <29 were removed. Chimeric sequences were removed using USEARCH. Assignment to operational taxonomic units (OTUs) was performed using open-reference OTU picking with a 97% threshold for pairwise identity. After removing the OTUs containing <5 sequences, the OTUs were classified taxonomically using the Greengenes reference database (https://greengenes.secondgenome.com/?prefix=downloads/greengenes_database/gg_13_5/). For detecting overall variation of abundant bacteria (relative abundance of genera accounting for >1% of the total of sequences in feces), normalization was not performed to analyze the relative abundance of major genera in feces.
2.9. Statistical Analysis
Statistical analysis was performed using JMP v.13.0 (SAS Japan, Tokyo, Japan). The data are presented as the mean ± standard error of the mean (SEM). The time-dependent changes of the questionnaire scores, salivary stress marker levels, and stool property scores of the participants were analyzed by two-way ANOVA with repeated measures. The VAS scores measured were averaged every six weeks, and their changes were analyzed by two-way ANOVA with repeated measures. The changes in sleep EEG parameters, relative abundances of fecal microbiota, and fecal SCFA levels were analyzed by analysis of covariance (ANCOVA) with each initial value as the covariate. Differences were considered significant at p < 0.05. Effect size was estimated from partial eta squared, calculated as the ratio of variance associated with an effect plus that effect and its associated error variance.
3. Results
3.1. Participant Demographics
Participants were randomly assigned to one of two groups: CP2305 (21 males and 10 females) or placebo (20 males and 9 females). As shown in Table 2, there were no significant differences in age, male/female ratio, body mass index (BMI), or STAI state, STAI trait, HADS anxiety, HADS depression, GHQ 28, and PSQI global scores between the two groups. The mean scores of the questionnaires were within the normal limits. The tablet consumption rates were 95.3 ± 1.0 and 92.6 ± 1.6% in the CP2305 and the placebo groups, respectively. No adverse events were observed throughout the trial.
Table 2.
Comparison of baseline characteristics between CP2305 and placebo groups.
| Parameters | CP2305 | Placebo | p Value |
|---|---|---|---|
| Age (years) | 24.9 ± 0.5 | 25.3 ± 0.6 | 0.54 |
| Sex (male/female) | 21/10 | 20/9 | 0.91 |
| BMI (kg/m2) | 21.1 ± 0.5 | 20.8 ± 0.5 | 0.65 |
| STAI state anxiety | 37.1 ± 1.6 | 35.8 ± 1.6 | 0.58 |
| STAI trait anxiety | 41.8 ± 1.7 | 39.7 ± 1.8 | 0.40 |
| HADS anxiety | 4.8 ± 0.5 | 4.3 ± 0.5 | 0.45 |
| HADS depression | 5.8 ± 0.5 | 5.2 ± 0.5 | 0.43 |
| GHQ-28 total | 4.1 ± 0.6 | 3.7 ± 0.6 | 0.64 |
| PSQI global score | 4.2 ± 0.4 | 3.4 ± 0.4 | 0.16 |
| % of daily test tablet consumption | 95.3 ± 1.0 | 92.6 ± 1.6 | 0.17 |
Data are presented as mean ± standard error of the mean (SEM). Data were analyzed by the Student’s t-test. The χ2 test was used for analysis of the male/female ratio. BMI: body mass index; STAI: Spielberger State-Trait Anxiety Inventory; HADS: Hospital Anxiety and Depression Scale; GHQ-28: 28-item General Health Questionnaire; PSQI: Pittsburgh Sleep Quality Index.
3.2. Effects of CP2305 on Mental and Physical Conditions
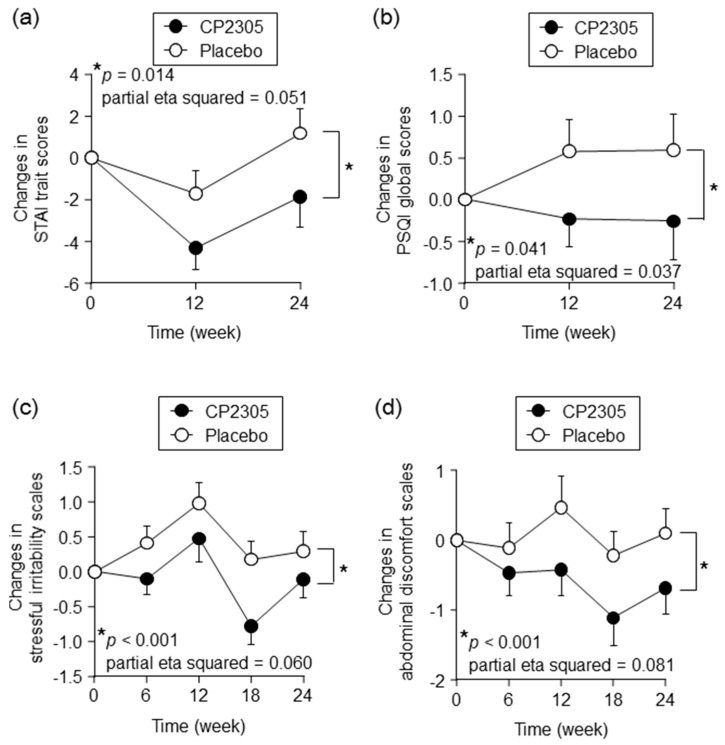
Time-dependent changes in the questionnaire scores are summarized in Table S1. The mean values of the STAI-state scores in the CP2305 and placebo groups were within normal limits before and 12 weeks after starting the interventions, and they were increased above the threshold value of 40 at 24 weeks (two weeks before the examination). However, there was no significant difference in the elevation of STAI-state score between the two groups. Although STAI-trait scores remained within normal limits during the intervention period, CP2305 intake significantly reduced STAI-trait anxiety scores compared to the placebo intake (Figure 1a). The CP2305 supplement also significantly improved sleep quality as assessed by the PSQI questionnaire when compared with the placebo (Figure 1b). There was no significant difference in the global GHQ-28 scores between the two groups, but the CP2305 group documented significantly lower depression scores using the GHQ-28 (Table S1). The HADS questionnaire also show that CP2305 intake ameliorated anxiety and depressive moods relative to placebo (Table S1).
Figure 1.
Effects of CP2305 intake on time-dependent changes in questionnaire and visual analog scores. Time-dependent changes in scores of STAI trait anxiety (a) and PSQI global score (b) in the CP2305 and placebo groups are shown. In addition, the visual analog scales were used to assess stressful irritability (c) and abdominal discomfort (d). The black circles represent CP2305 and the white represent the placebo groups. Data are presented as the mean ± SEM. The p values and partial eta squared values are shown in each panel; these were determined by repeated-measures ANOVA between the groups without multiple comparison correction. Asterisks indicate overall significance between the groups across time points. * p < 0.05.
In addition to the above questionnaires, stress-associated symptoms were also evaluated using the VAS, where daily intake of CP2305 significantly improved subjective feelings of irritability (Figure 1c) and abdominal discomfort (Figure 1d).
3.3. Effects of CP2305 on Sleep
The quality of sleep was assessed using a single-channel sleep EEG, and the results are summarized in Table 3. There was no significant time-dependent change nor group difference in total rapid eye movement (REM) and non-REM sleep times (Table 3). Delta power (high-amplitude slow wave: 0.5–2.0 Hz, 75 μV) is an indicator of deep sleep observed during the N3 stages of non-REM sleep, a period also referred to as slow wave sleep (SWS). Although there were no significant time-dependent changes nor group differences in total delta power, the intake of CP2305-containing tablets significantly increased the ratio of EEG delta power in the first sleep cycle, compared to placebo intake. In addition, the CP2305 intake significantly shortened the sleep latency of the first N3 stage and wake time after sleep onset compared to placebo intake (Table 3). Thus, CP2305 seemed to improve the quality of sleep in participants experiencing chronic stress.
Table 3.
Sleep measurements.
| Parameters | Treatment | Values * | ||
|---|---|---|---|---|
| Week 0 | Week 24 | Changes | ||
| Total N3 stage (min) | CP2305 | 41.6 ± 5.0 | 46.1 ± 5.3 | 4.5 ± 3.4 |
| Placebo | 43.9 ± 4.9 | 46.1 ± 5.2 | 2.2 ± 3.3 | |
| Total REM sleep time (min) | CP2305 | 93.4 ± 5.7 | 84.8 ± 5.7 | −8.5 ± 5.5 |
| Placebo | 79.4 ± 5.6 | 81.8 ± 5.6 | 2.4 ± 5.4 | |
| Delta power in total sleep period time (μV2) | CP2305 | 520,644 ± 61,030 | 483,302 ± 54,273 | −37,342 ± 25,529 |
| Placebo | 482,451 ± 59,889 | 456,930 ± 53,259 | −25,522 ± 25,052 | |
| Delta power ratio (%) †,‡ | CP2305 | 37.2 ± 2.7 | 50.4 ± 2.9 | 13.2 ± 2.9 |
| Placebo | 41.2 ± 2.6 | 44.4 ± 2.9 | 3.2 ± 2.8 | |
| Sleep latency of first N3 stage (min) † | CP2305 | 23.7 ± 3.6 | 17.6 ± 5.6 | −6.0 ± 5.4 |
| Placebo | 22.0 ± 3.5 | 26.3 ± 5.5 | 4.3 ± 5.3 | |
| Wake time after sleep onset (min) † | CP2305 | 22.7 ± 2.1 | 18.4 ± 2.1 | −4.2 ± 2.0 |
| Placebo | 22.7 ± 1.0 | 21.8 ± 2.1 | −0.9 ± 1.9 | |
* Data are presented as the mean ± SEM. † Significant differences between CP2305 and placebo group (p < 0.05) by analysis of covariance (ANCOVA) with each initial value as a covariate. Partial eta squared values of delta power ratio, sleep latency of first N3 stage, and wake time after sleep onset are 0.099, 0.085, and 0.120, respectively. ‡ Delta power ratio is representative of the delta power in the first sleep cycle relative to the delta power in the total sleep period. Abbreviations: REM, rapid eye movement; N3, non-REM sleep stage 3.
3.4. Effects of CP2305 on Salivary Stress Markers
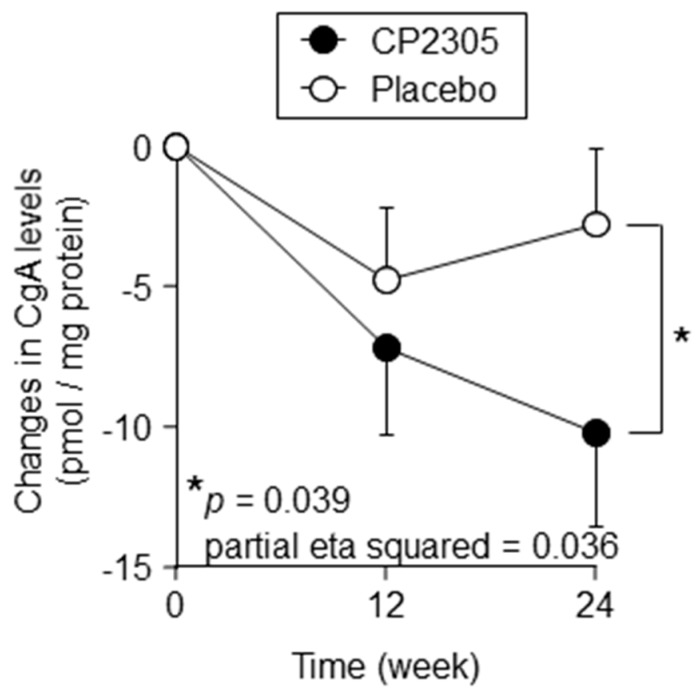
The stress response during the preparative period for the national examination was also evaluated by measuring salivary CgA and cortisol levels. Salivary CgA levels were significantly decreased in the CP2305 group relative to those of the placebo group (Figure 2 and Table S2), whereas there was no significant difference in salivary cortisol levels between the two groups during the intervention period (Table S2).
Figure 2.
Effects of CP2305 on time-dependent changes in salivary chromogranin A (CgA) levels. Saliva was collected at a constant time within 3 days before and 12 or 24 weeks after the intervention start. Data are presented as the mean ± SEM. Black circles are indicative of CP2305 and the white circles of placebo. Data were analyzed by repeated-measures ANOVA between the groups without multiple comparison correction. p and partial eta squared values are shown in the panel. Asterisks indicate overall significance between the groups across time points. * p < 0.05.
3.5. Effect of CP2305 on Bowel Habits
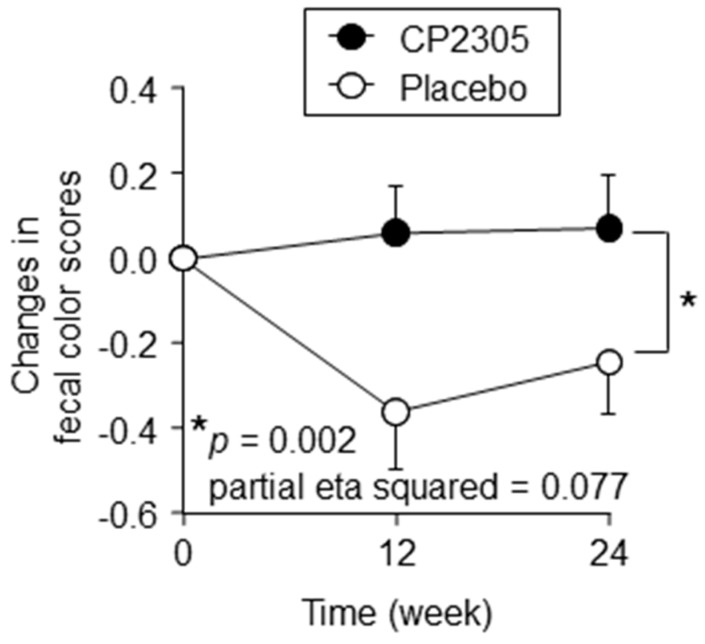
As shown in Table S3, there were no significant changes in the frequency of defecation, stool output, and stool form over time or between groups. However, the daily intake of the CP2305 tablets significantly changed the color tone relative to placebo, wherein the stool was lightened by CP2305 intake (Figure 3).
Figure 3.
Effects of CP2305 on the time-dependent changes in fecal color. Fecal color tone was assessed using the Bristol Stool Scale. Black circles represent data from participants receiving CP2305 and white circles placebo. Data are presented as the mean ± SEM. Data were analyzed by repeated-measures ANOVA between the groups without multiple comparison correction. p and partial eta squared value are shown in the panel. Asterisks indicate overall significance between the groups across time points. * p < 0.05.
3.6. Effects of CP2305 on Fecal Microbiota and SCFAs in Feces
The stress-induced changes in the fecal microbiota and modification of the bacterial communities with the daily CP2305 intake were examined at the genus level using next-generation sequencing. The results are shown in Table 4. Notably, the stress encountered during preparations for the national examination decreased the relative abundance of Bifidobacterium and increased that of Streptococcus in the placebo group. The daily intake of CP2305 significantly mitigated the reduction in Bifidobacterium and prevented the elevation of Streptococcus (Table 4).
Table 4.
Relative abundance of major genera within the fecal microbiota.
| Genus | Treatment | Composition of Bacterial Genus (%) * | ||
|---|---|---|---|---|
| Baseline | 24 Weeks | Change | ||
| Bifidobacterium † | CP2305 | 9.8 ± 1.8 | 6.1 ± 1.3 | −3.6 ± 1.2 |
| Placebo | 14.7 ± 2.6 | 6.8 ± 1.3 | −7.9 ± 2.3 | |
| Faecalibacterium | CP2305 | 10.5 ± 1.8 | 16.5 ± 2.0 | 6.0 ± 1.4 |
| Placebo | 6.8 ± 1.0 | 12.4 ± 1.9 | 5.6 ± 1.7 | |
| Roseburia | CP2305 | 4.5 ± 1.2 | 4.7 ± 1.0 | 0.2 ± 1.2 |
| Placebo | 2.8 ± 0.6 | 6.6 ± 1.5 | 3.7 ± 1.4 | |
| Streptococcus † | CP2305 | 1.8 ± 0.7 | 1.6 ± 0.6 | −0.2 ± 0.3 |
| Placebo | 1.3 ± 0.4 | 1.8 ± 0.5 | 0.5 ± 0.6 | |
| Dorea | CP2305 | 1.5 ± 0.2 | 1.8 ± 0.3 | 0.3 ± 0.3 |
| Placebo | 1.7 ± 0.2 | 1.9 ± 0.4 | 0.2 ± 0.4 | |
| Lachnospiraceae; Other † | CP2305 | 2.1 ± 0.4 | 1.6 ± 0.3 | −0.5 ± 0.2 |
| Placebo | 1.4 ± 0.3 | 1.4 ± 0.2 | 0.0 ± 0.2 | |
| Ruminococcus | CP2305 | 1.7 ± 0.4 | 2.9 ± 0.6 | 1.2 ± 0.7 |
| Placebo | 1.2 ± 0.3 | 2.8 ± 0.8 | 1.6 ± 0.8 | |
* Data are presented as the mean ± SEM. † Significant differences between CP2305 and placebo groups (p < 0.05) by ANCOVA with each initial value as a covariate. Partial eta squared values of Bifidobacterium, Streptococcus, and Lachnospiraceae; Other are 0.096, 0.152, and 0.158, respectively.
The effect of daily CP2305 intake on the concentrations of SCFAs (acetic acid, propionic acid, n-butyric acid, isobutyric acid, n-valeric acid, and isovaleric acid) in feces was also tested (Table 5). Among the SCFAs measured, only n-valeric acid concentrations were significantly increased in the CP2305 group relative to the placebo group.
Table 5.
Concentration of short-chain fatty acids (SCFAs) in feces.
| SCFAs | Treatment | Values * (mg g−1 feces) | ||
|---|---|---|---|---|
| Week 0 | Week 24 | Change | ||
| Acetic acid | CP2305 | 38.3 ± 2.4 | 32.0 ± 1.9 | −6.3 ± 2.7 |
| Placebo | 38.5 ± 2.6 | 30.5 ± 2.0 | −8.0 ± 2.8 | |
| Propionic acid | CP2305 | 13.0 ± 1.1 | 12.9 ± 0.9 | −0.1 ± 1.2 |
| Placebo | 12.6 ± 1.1 | 11.8 ± 0.9 | −0.8 ± 1.3 | |
| n-Butyric acid | CP2305 | 8.5 ± 1.1 | 9.3 ± 1.1 | 0.8 ± 1.3 |
| Placebo | 8.6 ± 1.1 | 7.8 ± 1.1 | −0.9 ± 1.3 | |
| iso-Butyric acid | CP2305 | 0.9 ± 0.1 | 1.4 ± 0.2 | 0.5 ± 0.2 |
| Placebo | 1.1 ± 0.1 | 1.0 ± 0.2 | −0.1 ± 0.2 | |
| n-Valeric acid † | CP2305 | 1.2 ± 0.2 | 1.6 ± 0.2 | 0.4 ± 0.2 |
| Placebo | 1.5 ± 0.2 | 1.0 ± 0.3 | −0.6 ± 0.2 | |
| iso-Valeric acid | CP2305 | 1.3 ± 0.2 | 1.9 ± 0.3 | 0.6 ± 0.3 |
| Placebo | 1.6 ± 0.2 | 1.4 ± 0.3 | −0.2 ± 0.3 | |
* Data are presented as the mean ± SEM. † Significant differences between CP2305 and placebo group (p < 0.05) by ANCOVA with each initial value as a covariate. Partial eta squared value of n-valeric acid is 0.114.
4. Discussion
This study aimed to assess whether long-term use of a tablet containing heat-inactivated, washed, and dried Lactobacillus gasseri CP2305 would have health benefits in young adults preparing for the national examination for medical practitioners. This model has been used in multiple studies of chronic psychological stress and is considered appropriate [25,33]. Of note, our research group used this model to study the effects of daily intake of a beverage containing heat-inactivated, washed Lactobacillus gasseri CP2305 for 12 weeks on stress-associated symptoms [19]. Although the stress-associated symptoms were less remarkable in the study presented here, the experimental period was longer (12 vs. 24 weeks), and the present study confirms that the regular intake of a tablet containing heat-inactivated CP2305 improves stress-associated symptoms. Particularly, the CP2305 tablet reduced the trait anxiety score of STAI, which was not detected in shorter studies [16,17,18,19]. This study did not document CP2305-associated suppression of basal salivary cortisol levels, which was documented with the use of the CP2305 beverage [19]. Salivary cortisol levels may be influenced by the menstrual cycle in women. Further analysis of the results that were different among men and women showed no significant effect on stress-induced salivary cortisol levels between the sexes (Table S4). However, the CP2305 tablet significantly reduced salivary CgA levels.
More importantly, these results confirm the significant improvement of sleep quality with the administration of a CP2305 tablet, which was also demonstrated by the CP2305 beverage [19]. Psychological stressors prolong sleep latency and reduce delta power preferentially in the first sleep cycle [34]. In addition to the improvement of sleep quality subjectively assessed by PSQI, a single-channel sleep EEG demonstrated that the daily intake of the CP2305 tablet significantly shortened the sleep latency to the N3-stage and decreased total wake time after sleep onset. Concerning delta power, the CP2305 tablet intake significantly increased the delta power ratio in the first non-REM sleep period compared with the placebo tablet. SWS in the first sleep cycle is particularly important for physiological functions. For instance, growth hormone (GH) is preferentially released during this stage [35]. Similarly, live Lactobacillus casei strain Shirota ameliorated academic stress-induced sleep disturbance in healthy adults in association with an increase in delta power [36]. These data suggest that distinct Lactobacillus strains may improve the quality of sleep during stressful situations. Some reports suggest a negative correlation between trait anxiety and sleep quality. Indeed, in our subjects, a positive correlation between PSQI and STAI-trait scores (Pearson’s r = 0.3138, p < 0.001) was observed. Although the precise mechanism remains unknown, long-term administration of CP2305 may improve sleep quality and result in alteration of the anxiety trait.
Unfortunately, earlier studies did not assess changes to the gut microbiota while taking heat-inactivated, washed CP2305. Given that stress has been reported to change the fecal microbiota composition in animal [37,38,39] and human studies [40,41], this parameter was included in the present study. Indeed, the daily intake of the CP2305 tablet significantly prevented the stress-induced reduction in the relative abundance of Bifidobacterium in feces, which was observed in the placebo group. Bifidobacterium have been linked to improvements in the intestinal environment by regulating immunological responses, preventing infection, reducing pathogenic bacteria, and producing health-promoting metabolites, thus exerting beneficial effects on allergies, inflammatory bowel disease, IBS, and cancer [42,43]. Of note, Logan and Katzman [44] reported that emotional stress leads to acute and long-term reductions in Bifidobacterium, suggesting the high sensitivity of this genus to emotional stress. Additionally, the administration of Bifidobacterium reduces anxiety and depression-like behaviors while suppressing peripheral proinflammatory cytokines and increasing plasma tryptophan, which have been implicated in depression in animal models [45]. The preservation of a high abundance of Bifidobacterium in the microbiota may improve stress-associated mental and physical symptoms. The CP2305 tablet also prevented the increase in the relative abundance of Streptococcus, which was noted in the placebo. This is supported by work from Suzuki et al. [37], who found that the abundance of Streptococcus was significantly increased in rats exposed to crowding stress. Such an increase in Streptococcus may be considered harmful in humans, as streptococci are associated with increased risk of colorectal cancer [46].
Previous studies have also demonstrated that some probiotics alter the gut microbiota production of SCFAs [47,48,49]. The production of SCFAs can stimulate colonocytes, including endocrine epithelial cells, and gut hormones, subsequently contributing to gut–brain axis activation [50,51]. Thus, the concentrations of major SCFAs in feces were measured; the concentrations of n-valeric acid were significantly increased in participants that received CP2305 during the intervention period compared to placebo. Yuille et al. [52] have shown that n-valeric acid is a potent inhibitor of class-I histone deacetylase (HDAC) using HT-29 human colon cancer cells. The concentration of n-valeric acid in Yuille’s study (approximately 50 mM) was higher than that found in the feces in the current study (approximately 10–15 mM). The physiological significance of n-valeric acid in the feces is not fully understood. Further studies on this point are needed. A significant improvement in fecal color tone was also observed in response to CP2305 tablet intake, which is suggestive of more acidic conditions. Thus, the long-term consumption of CP2305 likely improves the intestinal environment even under stressful situations and ameliorates stress-associated symptoms.
Our study has some important limitations. No dietary data were collected to determine whether dietary habits in both groups influenced changes in the n-valeric acid concentration in the stool. No information regarding the menstrual cycle was obtained from the women participants, which may influence salivary cortisol levels.
The mechanism underlying the stress-relieving effects of Lactobacillus gasseri CP2305 is unknown. However, the strain is known to colonize the intestines, and it has been observed that heat-inactivated cells can stimulate the afferent vagal nerve when administered to rat stomach or intestine (unpublished observations). Thus, these features may effectively stimulate the gut–brain axis directly or indirectly and may modify HPA axis activity, resulting in the improvement of stress-associated symptoms and the intestinal environment. However, this remains to be proven. Regardless, the present study suggests that a tablet containing heat-inactivated, washed, and dried Lactobacillus gasseri CP2305 may be beneficial for young adults experiencing stressful conditions. Moreover, the development of a tablet containing CP2305 widens the commercial applications of this bacterium.
Acknowledgments
We thank Tomonori Sugawara and Hiroto Morita for their technical support.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/11/8/1859/s1, Table S1. Time-dependent changes in questionnaire scores. Table S2. Time-dependent changes in salivary stress markers. Table S3. Time-dependent changes in stool properties and bowel habits, Table S4. Gender-stratified analysis of salivary cortisol.
Author Contributions
Conceptualization, K.N., D.S., Y.K., and K.R.; Data curation, K.N., D.S., and Y.K.; formal analysis, K.N. and D.S.; funding acquisition, K.R.; investigation, K.N., D.S., Y.K., and H.T.; methodology, K.N. and D.S.; project administration, K.N.; resources, D.S. and K.R.; supervision, K.R.; validation, K.N. and D.S.; visualization, D.S.; writing—original draft preparation, K.N., D.S., and K.R.; writing—review and editing, Y.K. and K.R.
Funding
This study was funded by Asahi Group Holdings, Ltd., Japan.
Conflicts of Interest
D.S. is an employee of Asahi Quality & Innovations, Ltd., related to Asahi Group Holdings, Ltd. The other authors declare that the research was conducted in the absence of any financial relationships that could be construed as a potential conflict of interest.
References
- 1.Cryan J.F., O’Mahony S.M. The microbiome-gut-brain axis: From bowel to behavior. Neurogastroenterol. Motil. 2011;23:187–192. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- 2.Mayer E.A. Gut feelings: The emerging biology of gut-brain communication. Nat. Rev. Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhee S.H., Pothoulakis C., Mayer E.A. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cryan J.F., Dinan T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 5.Food and Agriculture Organization of the United Nations. World Health Organization . Guidelines for the Evaluation of Probiotics in Food. Food and Agriculture Organization of the United Nations/World Health; London, ON, Canada: 2002. Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food. [Google Scholar]
- 6.Abildgaard A., Elfving B., Hokland M., Wegener G., Lund S. Probiotic treatment reduces depressive-like behaviour in rats independently of diet. Psychoneuroendocrinology. 2017;79:40–48. doi: 10.1016/j.psyneuen.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Ait-Belgnaoui A., Payard I., Rolland C., Harkat C., Braniste V., Theodorou V., Tompkins T.A. Bifidobacterium longum and Lactobacillus helveticus Synergistically Suppress Stress-related Visceral Hypersensitivity Through Hypothalamic-Pituitary-Adrenal Axis Modulation. J. Neurogastroenterol. Motil. 2018;24:138–146. doi: 10.5056/jnm16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bercik P., Park A.J., Sinclair D., Khoshdel A., Lu J., Huang X., Deng Y., Blennerhassett P.A., Fahnestock M., Moine D., et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011;23:1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takada M., Nishida K., Kataoka-Kato A., Gondo Y., Ishikawa H., Suda K., Kawai M., Hoshi R., Watanabe O., Igarashi T., et al. Probiotic Lactobacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut-brain interaction in human and animal models. Neurogastroenterol. Motil. 2016;28:1027–1036. doi: 10.1111/nmo.12804. [DOI] [PubMed] [Google Scholar]
- 10.Dinan T.G., Stanton C., Cryan J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry. 2013;74:720–726. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Rudzki L., Ostrowska L., Pawlak D., Malus A., Pawlak K., Waszkiewicz N., Szulc A. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology. 2019;100:213–222. doi: 10.1016/j.psyneuen.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Pinto-Sanchez M.I., Hall G.B., Ghajar K., Nardelli A., Bolino C., Lau J.T., Martin F.P., Cominetti O., Welsh C., Rieder A., et al. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients with Irritable Bowel Syndrome. Gastroenterology. 2017;153:448–459.e8. doi: 10.1053/j.gastro.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Inoue Y., Kambara T., Murata N., Komori-Yamaguchi J., Matsukura S., Takahashi Y., Ikezawa Z., Aihara M. Effects of oral administration of Lactobacillus acidophilus L-92 on the symptoms and serum cytokines of atopic dermatitis in Japanese adults: A double-blind, randomized, clinical trial. Int. Arch. Allergy Immunol. 2014;165:247–254. doi: 10.1159/000369806. [DOI] [PubMed] [Google Scholar]
- 14.Ting W.J., Kuo W.W., Hsieh D.J., Yeh Y.L., Day C.H., Chen Y.H., Chen R.J., Padma V.V., Chen Y.H., Huang C.Y. Heat Killed Lactobacillus reuteri GMNL-263 Reduces Fibrosis Effects on the Liver and Heart in High Fat Diet-Hamsters via TGF-beta Suppression. Int. J. Mol. Sci. 2015;16:25881–25896. doi: 10.3390/ijms161025881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei C.L., Wang S., Yen J.T., Cheng Y.F., Liao C.L., Hsu C.C., Wu C.C., Tsai Y.C. Antidepressant-like activities of live and heat-killed Lactobacillus paracasei PS23 in chronic corticosterone-treated mice and possible mechanisms. Brain Res. 2019;1711:202–213. doi: 10.1016/j.brainres.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 16.Sawada D., Kawai T., Nishida K., Kuwano Y., Fujiwara S., Rokutan K. Daily intake of Lactobacillus gasseri CP2305 improves mental, physical, and sleep quality among Japanese medical students enrolled in a cadaver dissection course. J. Funct. Foods. 2017;31:188–197. doi: 10.1016/j.jff.2017.01.042. [DOI] [Google Scholar]
- 17.Nobutani K., Sawada D., Fujiwara S., Kuwano Y., Nishida K., Nakayama J., Kutsumi H., Azuma T., Rokutan K. The effects of administration of the Lactobacillus gasseri strain CP2305 on quality of life, clinical symptoms and changes in gene expression in patients with irritable bowel syndrome. J. Appl. Microbiol. 2017;122:212–224. doi: 10.1111/jam.13329. [DOI] [PubMed] [Google Scholar]
- 18.Nishida K., Sawada D., Kawai T., Kuwano Y., Fujiwara S., Rokutan K. Para-psychobiotic Lactobacillus gasseri CP2305 ameliorates stress-related symptoms and sleep quality. J. Appl. Microbiol. 2017;123:1561–1570. doi: 10.1111/jam.13594. [DOI] [PubMed] [Google Scholar]
- 19.Nishida K., Sawada D., Kuwano Y., Tanaka H., Sugawara T., Aoki Y., Fujiwara S., Rokutan K. Daily administration of paraprobiotic Lactobacillus gasseri CP2305 ameliorates chronic stress-associated symptoms in Japanese medical students. J. Funct. Foods. 2017;36:112–121. doi: 10.1016/j.jff.2017.06.031. [DOI] [Google Scholar]
- 20.Faul F., Erdfelder E., Lang A.G., Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 21.Kvaal K., Ulstein I., Nordhus I.H., Engedal K. The Spielberger State-Trait Anxiety Inventory (STAI): The state scale in detecting mental disorders in geriatric patients. Int. J. Geriatr. Psychiatry. 2005;20:629–634. doi: 10.1002/gps.1330. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg D.P., Hillier V.F. A scaled version of the General Health Questionnaire. Psychol. Med. 1979;9:139–145. doi: 10.1017/S0033291700021644. [DOI] [PubMed] [Google Scholar]
- 23.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 24.Buysse D.J., Reynolds C.F., 3rd, Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 25.Kurokawa K., Tanahashi T., Murata A., Akaike Y., Katsuura S., Nishida K., Masuda K., Kuwano Y., Kawai T., Rokutan K. Effects of chronic academic stress on mental state and expression of glucocorticoid receptor alpha and beta isoforms in healthy Japanese medical students. Stress. 2011;14:431–438. doi: 10.3109/10253890.2011.555930. [DOI] [PubMed] [Google Scholar]
- 26.Goto S., Masaki C., Mukaibo T., Takahashi H., Kondo Y., Nakamoto T., Hosokawa R. The effects of nocturnal electromyographic biofeedback on sleep quality and psychological stress. Int. J. Stomatol. Occlusion Med. 2015;8:63–69. doi: 10.1007/s12548-015-0131-9. [DOI] [Google Scholar]
- 27.Matsuo M., Masuda F., Sumi Y., Takahashi M., Yamada N., Ohira M.H., Fujiwara K., Kanemura T., Kadotani H. Comparisons of Portable Sleep Monitors of Different Modalities: Potential as Naturalistic Sleep Recorders. Front. Neurol. 2016;7:110. doi: 10.3389/fneur.2016.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berry R.B., Budhiraja R., Gottlieb D.J., Gozal D., Iber C., Kapur V.K., Marcus C.L., Mehra R., Parthasarathy S., Quan S.F., et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis S.J., Heaton K.W. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997;32:920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda N., Saito Y., Shimizu J., Ochi A., Mizutani J., Watabe J. Variations in concentrations of bacterial metabolites, enzyme activities, moisture, pH and bacterial composition between and within individuals in faeces of seven healthy adults. J. Appl. Bacteriol. 1994;77:185–194. doi: 10.1111/j.1365-2672.1994.tb03063.x. [DOI] [PubMed] [Google Scholar]
- 31.Hatanaka M., Yamamoto K., Suzuki N., Iio S., Takara T., Morita H., Takimoto T., Nakamura T. Effect of Bacillus subtilis C-3102 on loose stools in healthy volunteers. Benef. Microbes. 2018;9:357–365. doi: 10.3920/BM2017.0103. [DOI] [PubMed] [Google Scholar]
- 32.Imoto N., Morita H., Amanuma F., Maruyama H., Watanabe S., Hashiguchi N. Maternal antimicrobial use at delivery has a stronger impact than mode of delivery on bifidobacterial colonization in infants: A pilot study. J. Perinatol. 2018;38:1174–1181. doi: 10.1038/s41372-018-0172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Honda M., Kuwano Y., Katsuura-Kamano S., Kamezaki Y., Fujita K., Akaike Y., Kano S., Nishida K., Masuda K., Rokutan K. Chronic academic stress increases a group of microRNAs in peripheral blood. PLoS ONE. 2013;8:e75960. doi: 10.1371/journal.pone.0075960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vein A.M., Sudakov K.V., Levin Y.I., Yumatov E.A., Strygin K.N., Kovrov G.V. Stages of sleep after psychoemotional tension: The individual character of changes. Neurosci. Behav. Physiol. 2002;32:513–518. doi: 10.1023/A:1019859606601. [DOI] [PubMed] [Google Scholar]
- 35.Gronfier C., Luthringer R., Follenius M., Schaltenbrand N., Macher J.P., Muzet A., Brandenberger G. A quantitative evaluation of the relationships between growth hormone secretion and delta wave electroencephalographic activity during normal sleep and after enrichment in delta waves. Sleep. 1996;19:817–824. doi: 10.1093/sleep/19.10.817. [DOI] [PubMed] [Google Scholar]
- 36.Takada M., Nishida K., Gondo Y., Kikuchi-Hayakawa H., Ishikawa H., Suda K., Kawai M., Hoshi R., Kuwano Y., Miyazaki K., et al. Beneficial effects of Lactobacillus casei strain Shirota on academic stress-induced sleep disturbance in healthy adults: A double-blind, randomised, placebo-controlled trial. Benef. Microbes. 2017;8:153–162. doi: 10.3920/BM2016.0150. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki K., Harasawa R., Yoshitake Y., Mitsuoka T. Effects of crowding and heat stress on intestinal flora, body weight gain, and feed efficiency of growing rats and chicks. Jpn. J. Vet. Sci. 1983;45:331–338. doi: 10.1292/jvms1939.45.331. [DOI] [PubMed] [Google Scholar]
- 38.Tannock G.W., Savage D.C. Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect. Immun. 1974;9:591–598. doi: 10.1128/iai.9.3.591-598.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bailey M.T., Lubach G.R., Coe C.L. Prenatal stress alters bacterial colonization of the gut in infant monkeys. J. Pediatr. Gastroenterol. Nutr. 2004;38:414–421. doi: 10.1097/00005176-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Holdeman L.V., Good I.J., Moore W.E. Human fecal flora: Variation in bacterial composition within individuals and a possible effect of emotional stress. Appl. Environ. Microbiol. 1976;31:359–375. doi: 10.1128/aem.31.3.359-375.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kato-Kataoka A., Nishida K., Takada M., Kawai M., Kikuchi-Hayakawa H., Suda K., Ishikawa H., Gondo Y., Shimizu K., Matsuki T., et al. Fermented Milk Containing Lactobacillus casei Strain Shirota Preserves the Diversity of the Gut Microbiota and Relieves Abdominal Dysfunction in Healthy Medical Students Exposed to Academic Stress. Appl. Environ. Microbiol. 2016;82:3649–3658. doi: 10.1128/AEM.04134-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tojo R., Suarez A., Clemente M.G., De Los Reyes-Gavilan C.G., Margolles A., Gueimonde M., Ruas-Madiedo P. Intestinal microbiota in health and disease: Role of bifidobacteria in gut homeostasis. World J. Gastroenterol. 2014;20:15163–15176. doi: 10.3748/wjg.v20.i41.15163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arboleya S., Watkins C., Stanton C., Ross R.P. Gut Bifidobacteria Populations in Human Health and Aging. Front. Microbiol. 2016;7:1204. doi: 10.3389/fmicb.2016.01204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Logan A.C., Katzman M. Major depressive disorder: Probiotics may be an adjuvant therapy. Med. Hypotheses. 2005;64:533–538. doi: 10.1016/j.mehy.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 45.Desbonnet L., Garrett L., Clarke G., Bienenstock J., Dinan T.G. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 2008;43:164–174. doi: 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 46.Kasai C., Sugimoto K., Moritani I., Tanaka J., Oya Y., Inoue H., Tameda M., Shiraki K., Ito M., Takei Y., et al. Comparison of human gut microbiota in control subjects and patients with colorectal carcinoma in adenoma: Terminal restriction fragment length polymorphism and next-generation sequencing analyses. Oncol. Rep. 2016;35:325–333. doi: 10.3892/or.2015.4398. [DOI] [PubMed] [Google Scholar]
- 47.Ferrario C., Taverniti V., Milani C., Fiore W., Laureati M., De Noni I., Stuknyte M., Chouaia B., Riso P., Guglielmetti S. Modulation of fecal Clostridiales bacteria and butyrate by probiotic intervention with Lactobacillus paracasei DG varies among healthy adults. J. Nutr. 2014;144:1787–1796. doi: 10.3945/jn.114.197723. [DOI] [PubMed] [Google Scholar]
- 48.Nyangale E.P., Farmer S., Keller D., Chernoff D., Gibson G.R. Effect of prebiotics on the fecal microbiota of elderly volunteers after dietary supplementation of Bacillus coagulans GBI-30, 6086. Anaerobe. 2014;30:75–81. doi: 10.1016/j.anaerobe.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 49.Berni Canani R., De Filippis F., Nocerino R., Laiola M., Paparo L., Calignano A., De Caro C., Coretti L., Chiariotti L., Gilbert J.A., et al. Specific Signatures of the Gut Microbiota and Increased Levels of Butyrate in Children Treated with Fermented Cow’s Milk Containing Heat-Killed Lactobacillus paracasei CBA L74. Appl. Environ. Microbiol. 2017;83 doi: 10.1128/AEM.01206-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bliss E.S., Whiteside E. The Gut-Brain Axis, the Human Gut Microbiota and Their Integration in the Development of Obesity. Front. Physiol. 2018;9:900. doi: 10.3389/fphys.2018.00900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoyles L., Snelling T., Umlai U.K., Nicholson J.K., Carding S.R., Glen R.C., McArthur S. Microbiome-host systems interactions: Protective effects of propionate upon the blood-brain barrier. Microbiome. 2018;6:55. doi: 10.1186/s40168-018-0439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuille S., Reichardt N., Panda S., Dunbar H., Mulder I.E. Human gut bacteria as potent class I histone deacetylase inhibitors in vitro through production of butyric acid and valeric acid. PLoS ONE. 2018;13:e0201073. doi: 10.1371/journal.pone.0201073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.