Abstract
As a source of bioactive compounds, species of the genus Lupinus are interesting legumes from a nutritional point of view. Although wild species are abundant and represent a potential source of nutrients and biologically active compounds, most research has focused on domesticated and semi-domesticated species, such as Lupinus angustifolius, Lupinus albus, Lupinus luteus, and Lupinus mutabilis. Therefore, in this review, we focus on recent research conducted on the wild Lupinus species of Mexico. The nutritional content of these species is characterized (similar to those of the domesticated species), including proteins (isolates), lipids, minerals, dietary fiber, and bioactive compounds, such as oligosaccharides, flavonoids, and alkaloids.
Keywords: legumes, Mexican lupins, protein isolates, dietary fiber, nutrients, bioactives compounds
1. Introduction
At present, epidemiological studies have shown that plant-based diets reduce the risks of developing chronic diseases [1,2]. In this sense, legumes represent one of the most important food categories. They have been widely cultivated and used as basic food sources to cover protein and energy needs throughout human history [3]. Legume seeds are used as a source of proteins, lipids, and dietary fiber in human and animal nutrition, and they are also adapted to marginal soils and climates. In many regions of the world, legumes are the only source of dietary protein and have been used as an effective substitute for animal protein at a lower cost.
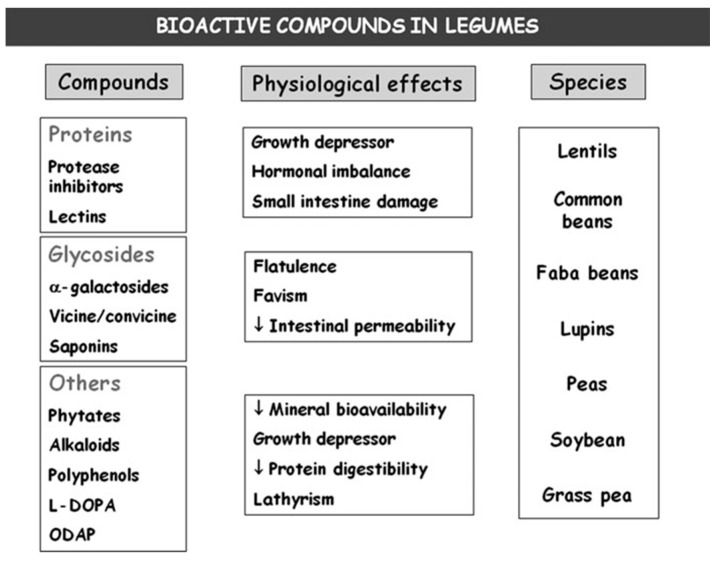
Additionally, it has been proven that legumes contain a large number of bioactive compounds with potential health benefits, such as the prevention of coronary heart disease, cancer, and diabetes. Among these compounds are oligosaccharides, phenols, and alkaloids. The interest in these compounds is due to their possible beneficial applications (Figure 1) as metabolic, hormonal, and digestive system regulators, as well as prebiotics [4], so it is of great interest to increase the knowledge of legumes as functional foods and the importance of their bioactive compounds [2].
Figure 1.
Bioactive compounds in legume seeds and their and physiological effects (adapted from Muzquiz et al., 2012 [4]). L-DOPA (L-Dopaminie), ODAP (oxalyl-L-α,β-diaminopropionic acid).
2. Domesticated Lupins
Despite their high protein and dietary fiber content and potential health benefits, lupins are undervalued legumes. There is a great diversity of species, but few are cultivated. Lupinus angustifolius, Lupinus albus, Lupinus luteus, and Lupinus mutabilis (Figure 2, Figure 3, Figure 4 and Figure 5) are among those that are domesticated. Domesticated species have been improved and have (a) low alkaloid content to increase edibility for humans and animals, (b) soft seeds with high germination, and (c) indehiscent pods that keep their seeds and yield efficient harvests [5].
Figure 2.
Lupinus angusifolius (narrow-leafed lupin), Photo: P. White.
Figure 3.
Lupinus albus (withe lupin) Photo: B.Buirchell.
Figure 4.
Lupinus luteus (Yellow lupin) Photo: P. White.
Figure 5.
Lupinus mutabilis (pearl lupin) Photo: P. White.
In relation to their nutritional properties, the protein content in lupine seeds is similar to that in soy (Table 1), with an acceptable content of essential amino acids. In addition, the presence of prolamins and low glutelin content makes lupine proteins desirable for the preparation of foods low in gluten. A gluten-low diet is one that excludes most grains and is recommended for people who have celiac disease or gluten sensitivity. For other people, however, going gluten-free can be unhealthy. The benefits and risks of a gluten-free diet should be carefully weighed, especially if the person starting the new diet does not truly need to restrict gluten intake.
Table 1.
Chemical composition and alkaloids in the seeds of four lupine species (g/100 g DM).
| Component | L. albus | L. angustifolius | L. luteus | L. mutabilis |
|---|---|---|---|---|
| Dry matter | 90.4 | 90.6 | 91.7 | 62.0 |
| Proteins | 36.3 | 33.0 | 46.5 | 44.7 |
| Ashes | 3.9 | 3.7 | 3.7 | 3.0 |
| Crude fat | 11.5 | 6.8 | 4.6 | 14.07 |
| Crude fiber | 14.4 | 14.0 | 13.9 | 7.04 |
| Alkaloids | 0.04 | 0.06 | 0.1 | 1.27 |
The protein fractions of several species of lupines have been isolated, and the two most important are albumins and globulins, which are present at a ratio of 1:9. Globulins represent about 90% of the total protein content in the seeds, and they can be separated by ultracentrifugation and chromatography into two main components, vicilins and legumins (also called α-conglutin and β-conglutin), and two minor proteins, γ-conglutin and δ-conglutin [8].
Lupinus albus γ-conglutin in vitro models have demonstrated the biological activity that potentiates insulin and metformin activity on cellular glucose consumption, thereby presenting a potential use for γ-conglutin in glycemic control [9]. Furthermore, protein isolates of L. albus have been reported to have a hypolipidemic, anti-atherosclerotic, and hypocholesterolemia effect in rabbits, rats, and chickens, and were shown to increase LDL receptor activity in HepG2 cells [10,11,12].
Several clinical human studies have shown that the incorporation of 25 g of lupine protein into different foods decreases total LDL and HDL cholesterol, as well as triglycerides and uric acid, in hypercholesterolemic subjects [13].
Lupine seeds are an important source of nutritionally important lipids. Table 2 shows the average composition of oils in the seeds of different lupine species.
Table 2.
Fatty acid composition (g/kg DM) in different lupine species.
| Fatty Acid | L. albus | L. luteus | L. angustifolius |
|---|---|---|---|
| 16:0 | 78 | 48 | 110 |
| 18:0 | 16 | 25 | 382 |
| 18:1 | 530 | 210 | 382 |
| 18:2 | 172 | 475 | 371 |
| 18:3 | 95 | 75 | 53 |
| 20:0 | 55 | 45 | 12 |
| 22:0 | 58 | 79 | 19 |
| 22:1 | 58 | 79 | 19 |
| n3/n6 | 0.55 | 0.16 | 0.14 |
Source: Reference [8].
The content of essential fatty acids, such as linoleic acid (18:2), is higher in L. luteus than in the other two species in Table 2. However, L. albus has a higher content of linolenic acid (18:3). Similarly, the fatty acid composition of L. angustifolius seeds is excellent because of its high palmitic and stearic acid content, which makes it an attractive ingredient for food products and nutraceuticals [14].
The lipid content of L. albus is particularly promising since its composition is very close to the dietary recommendations for the prevention of cardiovascular diseases. Among these recommendations is the intake of similar proportions of omega-6 and omega-3 fatty acids, while the diet of Western countries has a large omega-6/omega-3 ratio of 15:1. Excess omega-6 relative to omega-3 represents a risk factor, whereas a 2:1 ratio has a positive effect and reduces the mortality associated with cardiovascular disease [15].
The beneficial effect of dietary fiber consumption on the digestive system is known, especially for the prevention of colon cancer. According to the Nordic Nutrition recommendations, an adequate dietary fiber consumption reduces the risk of constipation, which, in turn, reduces the risk of colorectal cancer and other chronic diseases, such as cardiovascular disease and diabetes. Thus, the consumption of foods rich in fiber is favorable to health. A dietary fiber intake of 25–35 g per day is recommended.
Lupinus angustifolius is a rich source of dietary fiber (41.5%), of which 11% is soluble fiber and 30.5% is insoluble fiber [16]. In L. albus, a content of 50.4% total dietary fiber has been reported, with 2.0% soluble fiber and 48.4% insoluble fiber. Fiber incorporated into food products can benefit intestinal functions and fecal parameters by decreasing the risk of colon cancer and promoting a healthy digestive system [17,18].
Lupine seeds are a good source of minerals, such as calcium, phosphorus, and iron, among others, which are important for various bodily functions and are part of many tissue structures. The most important minerals in human nutrition are calcium, phosphorus, iron, iodine, fluorine, and zinc.
Lupines, similar to other legumes, are generally a good source of minerals (Table 3), the content of which varies among species and differs from that of other legumes [19]. Compared with other legumes, lupines have low levels of calcium (Ca) and phosphorus (P) but similar contents of microelements, such as iron (Fe), zinc (Zn), and copper (Cu) [20]. Lupinus angustifolius var. troll presents a higher Ca content, while L. luteus var. 4492 contains more Mg, P, Cu, Fe, and Zn [21].
Table 3.
Mineral Composition of Lupin Seed Flours (mg/100 g of Dry Matter).
| Lupines | Ca | Mg | P | Cu | Fe | Mn | Zn |
|---|---|---|---|---|---|---|---|
| L. albus var. multolupa | 139.0 ± 2.72 | 145.0 ± 1.65 | 332.1 ± 5.12 | 0.72 ± 0.06 | 3.80 ± 0.05 | 90.1 ± 1.34 | 4.30 ± 0.09 |
| L. albus var. marta | 133.6 ± 0.53 | 193.2 ± 0.86 | 468.1 ± 1.76 | 0.75 ± 0.03 | 6.20 ± 0.06 | 35.0 ± 0.32 | 5.24 ± 0.02 |
| L. angustifolius var. troll | 161.8 ± 1.49 | 191.9 ± 1.00 | 543.3 ± 2.25 | 1.02 ± 0.11 | 4.15 ± 0.03 | 7.6 ± 0.06 | 3.65 ± 0.03 |
| L. angustifolius var. emir | 143.0 ± 1.05 | 219.1 ± 1.25 | 613.4 ± 2.05 | 0.95 ± 0.05 | 4.26 ± 0.03 | 8.4 ± 0.04 | 3.79 ± 0.02 |
| L. luteus var. 4486 | 134.8 ± 0.61 | 294.0 ± 2.39 | 715.5 ± 1.39 | 1.10 ± 0.03 | 5.84 ± 0.03 | 5.6 ± 0.03 | 5.90 ± 0.02 |
| L. luteus var. 4492 | 110.4 ± 0.66 | 308.8 ± 1.84 | 845.7 ± 2.72 | 1.25 ± 0.04 | 7.05 ± 0.05 | 6.8 ± 0.02 | 6.42 ± 0.02 |
Source: [19].
3. Bioactive Compounds
Additionally, lupines contain bioactive compounds, such as oligosaccharides, phenolic compounds, and alkaloids, some of the main biomolecules that can prevent and protect against chronic diseases, such as cancer, diabetes, and neurodegenerative and cardiovascular diseases [22,23].
The beneficial effect of these compounds depends on their chemical structures, concentrations, times of exposure, interactions with other compounds, and, especially, their bioavailabilities [24,25]. These compounds are considered to be antinutrients and/or pronutrients with negative and/or positive effects on health [23,26]. Thus, it is important to know the quantity and type of compounds in food and understand how they affect the organism.
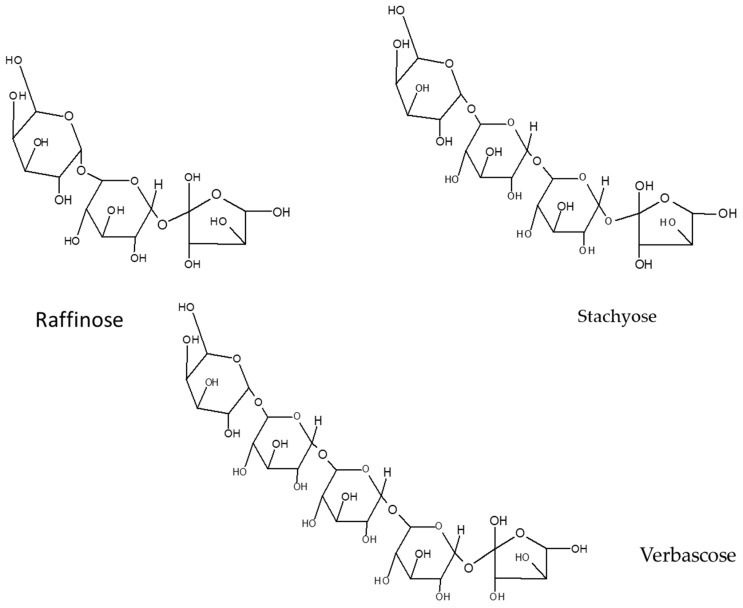
On the other hand, most of the oligosaccharides of plant origin are α-galactosides and belong to the raffinose family [27], which includes raffinose (a trisaccharide), stachyose (a tetrasaccharide), verbascose (a pentasaccharide), and ajugose (a hexasaccharide) (Figure 6).
Figure 6.
Raffinose family oligosaccharides structures.
In domesticated lupine cultivars and species, there is a variation in the content of total oligosaccharides, with a range from 5.46 (in L. albus) to 12.3 g/100 g for dry samples (in L. luteus). Stachyose is the main oligosaccharide, with values that range from 3.62 to 8.61 g/100 g for dry sample (Table 4).
Table 4.
Oligosaccharide content of lupine cultivars (g/100 g DM).
| Lupine cultivar | Sucrose | Raffinose | Stachyose | Verbascose | Total |
|---|---|---|---|---|---|
| Lupinus albus var. marta | 0.73 ± 0.0 | 5.5 ± 0.01 | 1.1 ± 0.0 | ||
| Lupinus albus var. multolupa | 0.63 ± 0.0 | 5.9 ± 0.01 | 1.2 ± 0.03 | ||
| L. albus cv. multolupa | 2.58 ± 0.06 | 0.62 ± 0.03 | 5.7 ± 0.06 | 0.19 ± 0.10 | 7.56 ± 0.10 |
| L. albus cv. marta | 3.09 ± 0.08 | 0.33 ± 0.02 | 7.2 ± 0.11 | 0.94 ± 0.01 | 8.51 ± 0.13 |
| L. albus LO-3844 | 2.16 ± 0.20 | 0.44 ± 0.03 | 7.2 ± 0.31 | ND | 7.71 ± 0.33 |
| L. albus LO-3846 | 3.13 ± 0.06 | 0.54 ± 0.02 | 6.85 ± 0.03 | ND | 7.39 ± 0.03 |
| L. albus LO-3848 | 2.41 ± 0.19 | 0.36 ± 0.02 | 5.71 ± 0.57 | ND | 6.07 ± 0.55 |
| L. albus LO-3855 | 2.84 ± 0.09 | 0.48 ± 0.02 | 4.98 ± 0.22 | ND | 5.46 ± 0.24 |
| L. luteus LO-4486 | 1.38 ± 0.13 | 0.56 ± 0.11 | 7.01 ± 1.22 | 3.54 ± 0.37 | 11.1 ± 1.69 |
| L. luteus LO-4492 | 1.21 ± 0.04 | 0.64 ± 0.04 | 8.61 ± 0.20 | 3.04 ± 0.02 | 12.3 ± 0.20 |
| L. luteus LO-4500 | 1.01 ± 0.09 | 0.54 ± 0.03 | 6.13 ± 0.31 | 2.79 ± 0.14 | 9.46 ± 0.41 |
| L. angustifolius LO-4817 | 5.05 ± 0.22 | 1.24 ± 0.09 | 5.11 ± 0.36 | 2.48 ± 0.07 | 8.82 ± 0.43 |
| L. angustifolius LO-4820 | 2.55 ± 0.24 | 0.89 ± 0.07 | 3.62 ± 0.06 | 0.79 ± 0.03 | 5.30 ± 0.12 |
| L. angustifolius LO-4822 | 2.91 ± 0.19 | 1.15 ± 0.07 | 5.19 ± 0.14 | 1.36 ± 0.01 | 7.70 ± 0.11 |
| L. angustifolius cv. zapaton | 3.16 ± 0.17 | 0.63 ± 0.04 | 4.52 ± 0.18 | 1.39 ± 0.13 | 6.54 ± 0.34 |
Similarly, phenolic compounds are bioactive secondary metabolites that are found mainly in plants. They have anti-allergenic, anti-arteriogenic, anti-inflammatory, antimicrobial, antioxidant, anti-thrombotic, cardioprotective, and vasodilator effects [29]. There is variation in the content of phenolic compounds among lupine species, as well as among cultivars, growing sites, and parts of the plant. The total phenol varies from 212 to 317 mg per 100 g DM in the seeds of cultivars of L. albus, L. luteus, and L. angustifolius [30], but these values are inferior to leaves of traditional medicinal plants used in China, India, and Europa (Pimpinella anisum), with values of 234 to 1178.5 mg GAE/100 g DM [31,32,33].
Lupinus albus was revealed to have the highest total flavonoid content, i.e., 1100 μg catechin/g DM, compared with varieties of L. angustifolius (Table 5) [34].
Table 5.
The content of total phenolic compounds (gallic acid equivalent) and flavonoids (catechin equivalent) from seeds of lupine varieties.
| Sample | Cultivar | Total Phenolic Compounds (mg GAE/100 g DM) | Total Flavonoids (µg Catechin/g DM) |
|---|---|---|---|
| Lupinus albus | Butan | 212.12 ± 2.24 | |
| Boros | 271.25 ± 3.75 | ||
| Multolupa | 1100 ± 17.6 | ||
| Lupinus luteus | Lord | 249.32 ± 4.72 | |
| Parys | 317.88 ± 2.69 | ||
| Lupinus angustifolius | Bojar | 269.72 ± 9.97 | |
| Zeus | 258.42 ± 7.21 | ||
| Troll | 133 ± 12.6 | ||
| Emir | 362 ± 9.00 |
The majority of phenolic compounds in lupine species are flavones, dihydroflavones, phenolic acids, and isoflavones (Table 6).
Table 6.
Content of phenols and phenolic acids in Lupinus species (mg/kg DW).
| Sample | Lupinus albus | Lupinus luteus | Lupinus angustifolius | |||
|---|---|---|---|---|---|---|
| Butan | Boros | Lord | Parys | Bojar | Zeus | |
| Apigenin-6,8-di-C-b-glucopyranoside (mg/100 g DM) | 11.90 ± 0.33 | 14.3 ± 0.33 | 53.63 ± 0.44 | 63.14 ± 0.14 | 30.25 ± 0.22 | 27.78 ± 0.07 |
| Gallic acid (mg/kg DM) | 3.53 ± 0.24 | 3.43 ± 0.1 | 3.50 ± 0.33 | 4.21 ± 0.27 | 0.63 ± 0.01 | 0.62 ± 0.04 |
| Protocatechuic (mg/kg DM) | 12.96 ± 0.14 | 14.69 ± 0.36 | 35.90 ± 0.54 | 73.60 ± 1.71 | 12.50 ± 0.39 | 13.77 ± 0.39 |
| p-Hydroxybenzoic (mg/kg DM) | 22.77 ± 0.30 | 27.82 ± 0.68 | 1.06 ± 0.11 | 2.24 ± 0.21 | 43.73 ± 0.48 | 42.73 ± 0.31 |
| Caffeic acid (mg/kg DM) | 0.58 ± 0.05 | 0.09 ± 0.01 | 1.02 ± 0.06 | 1.22 ± 0.11 | 0.84 ± 0.09 | 0.56 ± 0.07 |
| p-Coumaric acid (mg/kg DM) | 0.11 ± 0.01 | 0.18 ± 0.01 | 0.03 ± 0.01 | 0.68 ± 0.14 | 0.42 ± 0.08 | 0.34 ± 0.06 |
| Total (mg/kg DM) | 39.96 ± 0.15 | 46.23 ± 1.22 | 41.52 ± 0.68 | 82.06 ± 1.52 | 58.14 ± 1.02 | 58.03 ± 0.87 |
Source: [30].
The varieties of L. luteus have the highest content of apigenin-6,8-di-C-b-glucopyranoside and protocatechuic acid compared with the varieties of the other two species, while the main phenolic acid in L. albus is p-hydroxybenzoic.
In L. angustifolius seeds, a higher concentration of p-hydroxybenzoic acid was observed. Additionally, in other studies, flavones (mainly luteolin glycosides, apigenin, and diosmetin), isoflavones (mainly derived from genistein), and dihydroflavones have been reported in this species [35].
Many of these phenolic acids and flavonoids are known for their high antioxidant capacity, which has been the subject of several studies aiming to understand this property, as seen in Table 7.
Table 7.
Antioxidant capacity of lupine seed extracts.
| Sample | Cultivar | DPPH (mM Trolox) Eq/g | TRAP (mMTrolox) Eq/g |
|---|---|---|---|
| Lupinus albus | Butan | 3.51 ± 0.2 | 0.33 ± 0.01 |
| Boros | 6.78 ± 0.28 | 0.71 ± 0.01 | |
| Lupinus luteus | Lord | 9.03 ± 0.33 | 1.44 ± 0.02 |
| Parys | 8.12 ± 0.10 | 0.96 ± 0.07 | |
| Lupinus angustifolius | Bojar | 6.89 ± 0.35 | 1.60 ± 0.05 |
| Zeus | 7.47 ± 0.29 | 1.78 ± 0.04 |
Source: Reference [30]. DPPH, 2,2-diphenyl-1-picrilhydrazil; TRAP, total peroxil radical-trapping potential.
It can be observed that the highest antioxidant capacity has been found in L. luteus varieties using the DPPH technique (2,2-diphenyl-1-picrilhydrazil), while varieties of L. angustifolius have shown the highest antioxidant activity with the TRAP technique (total peroxil radical-trapping potential).
4. Mexican Wild Lupinus
At the moment, diverse investigations focusing on wild and underused legumes are underway. It is known that wild legumes, such as Lupinus spp., have significant quantities of proteins, essential amino acids, polyunsaturated fatty acids (PUFAs), dietary fiber, minerals, and essential vitamins, comparable to edible legumes, in addition to the presence of beneficial bioactive compounds [36].
The study of Mexican lupine species is very recent. Dr. Ruiz-López started to study these species in 2000 by describing their distribution in Mexico, analyzing their chemical-nutritional and antinutrient composition, and obtaining their protein isolates [37,38].
4.1. Distribution in Mexico
Because of the biological, geographic, and climatic diversity in Mexico, nearly 100 wild species have been identified and are distributed throughout the country. The highest concentration of these species is found in the Sierra Madre Occidental and the Transverse Neovolcanic Axis [39], mainly in areas with an altitude between 1500 and 3500 m above sea level (Figure 7, Figure 8, Figure 9 and Figure 10).
Figure 7.
Lupinus exaltatus Zucc. Photo: M. Ruiz.
Figure 8.
Lupinus rotundiflorus M.E. Jones. Photo: M. Ruiz.
Figure 9.
Lupinus aschenbornii S. Schauer. Photo: J. Ruiz.
Figure 10.
Lupinus montanus Kunth ssp. montanus. Photo: J. Ruiz.
4.2. Nutritional Composition
The wild lupines of Mexico present nutritional values similar to those of domesticated species.
The protein content (37.2–45.4 g 100 g−1) (Table 8) is higher than that of other wild legumes, such as Caesalpinia bracteosa (29.3 g 100 g−1 DM), Dimorphandra gardneriana (32.3 g 100 g−1 DM), Pterogyne nitens (28.3 g 100 g−1 DM), Hymenaea courbaril (10.9 g 100 g−1 DM), Senna obtusifolia (22.3 g 100 g−1 DM), and Senna rugosa (22.3 g 100 g−1 DM) [37], and similar to that of domesticated lupine species.
Table 8.
Proximate composition of seeds from species of Mexican wild lupins (g /100 g DM).
| Component | Ash | Lipids | Crude Fiber | Crude Protein (n = 6.25) | Carbohydrates |
|---|---|---|---|---|---|
| L. elegans | 4.2 | 5.8–7.3 | 12.9 | 43.6–45.4 | 31.7 |
| L. exaltatus | 3.4–5.2 | 5.8–8.7 | 14.6–27.0 | 32.1–43.9 | 22.9–32.8 |
| L. reflexus | 3.6–7.2 | 6.6–7.9 | 15.2–16.6 | 37.3–38.8 | 32.1–34.6 |
| L. rotundiflorus | 3.1–4.1 | 5.5–6.4 | 151 | 41.9–42.8 | 32.5 |
| L. simulans | 3.6 | 6.3 | 14.4 | 40.7 | 35.0 |
| L. splendens | 3.3–4.3 | 8.9–13.0 | 12.7–16.4 | 34.1–37.2 | 32.1–38.1 |
| L. mexicanus | 3.8–4.1 | 6.1–8.0 | 16.8 | 34.7–36.7 | 34.3 |
| L. madrensis | 3.5 | 6.8 | 15.4 | 41.4 | 32.8 |
| L. campestris | 4.4 | 7.5 | 40.5 | 39.3 | |
| L. montanus | 3.6–4.3 | 7.1–10.0 | 26.5 | 42.4–45.9 | 28.3 |
| L. hintonni | 6.3 | 7.0 | 32.5 | 24.4 |
4.3. Protein Isolates and Their Functionality
The nutritional value of food proteins is determined by their quality, which depends on their composition and the biological availability of their essential amino acids to humans: phenylalanine, isoleucine, leucine, lysine, methionine, threonine, tryptophan, valine, and histidine. Thus, protein isolates of wild lupins from Mexico have been prepared, as can be observed in Table 9.
Table 9.
Approximate chemical composition of protein isolates of Lupinus species from Mexico (g/100 g on dry base sample).
| Protein | Lipids | Ash | Fiber | Carbohydrates | Alkaloids | |
|---|---|---|---|---|---|---|
| L. campestris | 93.2 | -- | 2.4 | 0.5 | 3.9 | 0.005 |
| L. exaltatus | 95.0 | 0.57 | 2.5 | 0.0 | 1.97 | 0.09 |
| L. elegans | 95 | 0.6 | 1.2 | 0.0 | 1.2 | 0.003 |
In Mexican species, protein isolates of 93–95% protein were obtained by isoelectric precipitation. The meal was suspended in water (10% w/v) and the pH was adjusted to 9.0. It was centrifugated, and the suspension was adjusted to pH 4.5 for protein precipitation. Then, the suspension was left at 4 °C overnight to allow the proteins to precipitate. Then, centrifugation was performed at 10,000× g for 10 min at 4 °C. The protein precipitate was freeze dried in a Freezone Dry System (Labconco).
In addition, protein isolates from wild Mexican species have α, β, and γ conglutin, which may be promising as hypoglycemic, hypolipidemic, anti-atherosclerotic, and hypocholesterolemic agents. Fatty acids are the organic components of fats and are crucial because they supply energy to our body and enable the healthy formation and maintenance of different tissues.
The fatty acid content was determined in Mexican species, which have a high lipid content of up to 13%. Table 10 shows the composition of fatty acids in three lupine species from different localities in Mexico. Fatty acid methyl esters (FAME) were prepared as follows: lupin oil was placed into a glass tube with a screw-cap. The tubes were flushed with nitrogen until dry. After cooling, 2 mL of hexane was added to each tube, re-capped, mixed, and left standing to allow the hexane layer to separate. 1 mL of each hexane layer was diluted 10-fold with hexane, and 1 mL was transferred to autosampler vials.
Table 10.
Saturated and unsaturated fatty acid content (g/kg) of wild lupin, collected at several locations of Jalisco and Zacatecas states in 1996–1997.
| State Location | L. exaltatus, Jalisco | L. montanus, Jalisco | L. stipulatus, Zacatecas | ||||
|---|---|---|---|---|---|---|---|
| CG 1 | CG 2 | Z 3 | T 4 | T 5 | B 6 | ME 7 | |
| C14:0 | 2 | 2 | 2 | 8 | 9 | 7 | 8 |
| C16:0 | 200 | 202 | 206 | 259 | 257 | 273 | 264 |
| C18:0 | 48 | 56 | 66 | 36 | 37 | 69 | 76 |
| C20:0 | - | - | - | - | - | - | - |
| C22:0 | - | - | - | 23 | 22 | 23 | 25 |
| C16:1 | 2 | 2 | 1 | 7 | 6 | 7 | 7 |
| C18:1 | 138 | 124 | 138 | 96 | 99 | 171 | 137 |
| C18:2 | 534 | 544 | 520 | 430 | 423 | 324 | 370 |
| C18:3 | 76 | 71 | 67 | 142 | 146 | 126 | 113 |
| C22:1 | - | - | - | - | - | - | - |
| n6/n3 | 7/1 | 7.5/1 | 7.7/1 | 3/1 | 2.9/1 | 2.6/1 | 3.2/1 |
| Ratio I/S | 3.0 | 2.8 | 2.6 | 2.1 | 2.1 | 1.7 | 1.7 |
1 Ciudad Guzmán (1996), 2 Ciudad Guzmán (1997), 3 Zapopan (1996), 4 El Refugio, Tonila (1996), 5 El Refugio, Tonila (1997), 6 Bolaños (1997), 7 Monte Escobedo (1997). Source: Reference [47].
The analysis of FAME was performed using a Perkin-Elmer Autosystem 1-A gas chromatograph equipped with a flame ionization detector. FAME were identified by comparing their retention times with known concentrations of methyl ester standards, including nonadecanoic methyl esters (Sigma Chemical Co. Ltd, St Louis, MO, USA) that were analyzed under similar conditions.
There have been few reports on the fatty acid content of Mexican lupines, with only one report on three species. In that study, it was observed that the fatty acid profile of the three Mexican species presented a predominance of unsaturated fatty acids (USFAs). Those existing in higher quantities are linoleic acid (C18:2), followed by oleic acid (C18:1), and gamma-linolenic acid (C18:3). The undesirable erucic acid (C22:1) was not found in any of the species studied [47].
Essential fatty acids, such as linoleic acid, are higher in Lupinus exaltatus than in all wild species of lupine; in Lupinus montanus and Lupinus exaltatus, linoleic acid was higher than in L. angustifolius and L. albus, respectively [47]. Similarly, L. montanus and L. stipulatus had higher linolenic acid content than domesticated lupine species, and L. exaltatus had a greater concentration of this fatty acid than L. angustifolius. The n6/n3 ratio was lower in L. exaltatus than in domesticated lupines analyzed for this trait, and this ratio was lower in L. montanus and L. stipulatus than in L. albus. These results are interesting given the recommended intake of similar amounts of omega-6 and omega-3 fatty acids for the prevention of cardiovascular diseases. Moreover, a good ratio of unsaturated vs. saturated fatty acids is observed, which could be beneficial for the development and maintenance of brain functions, as well as immune and inflammatory responses. Mammalian cells cannot convert omega-6 to omega-3 fatty acids because they lack the converting enzyme, omega-3 desaturase [15].
4.4. Dietary Fiber
Dietary fiber consumption is critical. Several studies have shown that fiber reduces blood pressure in patients with hypertension and also suggest a small reduction in normotensive ones [48].
The content of dietary fiber (DF) in the seeds of Mexican species (Table 11) varies considerably, with values ranging from 17.72 (L. exaltatus) to 27.93 g/100 g (L. rotundiflorus) [49]. These values are lower than those of domesticated species, such as L. albus with 50.4 g/100 g and L. angustifolius with 41.5 g/100 g [16,18]. However, the DF values of lupines are higher than those reported in other legumes for human consumption, such as soybean, beans, and mung beans.
Table 11.
Dietary fiber (DF) content in five Mexican species of lupines.
| Species | Dietary Fiber |
|---|---|
| L. exaltatus | 17.72 ± 0.1 |
| L. elegans | 21.07 ± 0.0 |
| L. mexicanus | 20.90 ± 0.5 |
| L. montanus | 24.63 ± 0.1 |
| L. rotundiflorus | 27.93 ± 0.1 |
Source: [49].
4.5. Minerals and Bioavailability
In Mexican lupine seeds, the mineral content has been evaluated, as shown in the following table. Mineral concentration is highly variable, depending on the species (Table 12). Lupinus mexicanus has the highest values of Ca (3252 mg/kg), while L. montanus has the highest P content, with 6500–7690 mg/kg. L. rotundiflorus has the highest Fe content with 82.8 mg/kg, and L. exaltatus has the highest Zn and Cu content recorded, with values of 89.6 and 184 mg/kg, respectively.
Table 12.
Mineral content in wild lupine seeds (mg/kg in dry basis).
| Species | Ca | P | Mg | Fe | Zn | Cu |
|---|---|---|---|---|---|---|
| L. exaltatus | 1600–2052 | 584–5600 | 2300–2330 | 61.8–81.3 | 46.97–89.6 | 74.4–184 |
| L. elegans | 1777 | 6441 | 2656 | 70.9 | 73.6 | 64.8 |
| L. mexicanus | 3252 | 5865 | 2651 | 63.1 | 73.7 | 70.8 |
| L. montanus | 800–2074 | 6500–7690 | 2443–3000 | 77.7–73.7 | 46.87–73.3 | 56.2–89.7 |
| L. rotundiflorus | 1887 | 6166 | 2213 | 82.8 | 79.0 | 64.9 |
| L. campestris | 1200 | 6300 | 2300 | 70.74 | 46.97 | 93.3 |
| L. hintonii | 1000 | 5400 | 2600 | 49.39 | 38.18 | 67.7 |
The values of Ca in L. mexicanus, L. montanus, and L. rotundiflorus are higher than those reported in varieties of domesticated species of lupine, such as L. angustifolius (1430–1618 mg/kg), L. albus (1336–1390 mg/kg), and L. luteus (1104–1348 mg/kg). The values of P in L. montanus are similar to those found in varieties of L. luteus (7155–8457 mg/kg) and higher than those of L. albus and L. angustifolius. Lupinus rotundiflorus and L. montanus have a higher Fe content than domesticated lupines, since they contain values ranging from 38 to 70 mg/kg, which makes them an interesting source of Fe.
Fe is one of the most important nutritional minerals because its deficiency is associated with anemia. Thus, in a study on Lupinus rotundiflorus, the bioavailability of Fe was evaluated using a rat model. It was found that the concentration of Fe was higher in roots than in cooked seeds, and both showed good bioavailability, with 13.8% and 13.7%, respectively, compared to iron sulfate (18.38%). Thus, this species could be considered as a source of bioavailable Fe and incorporated into foods [51].
5. Bioactive Compounds
5.1. Oligosaccharides
The raffinose family of oligosaccharides (RFOs) are indigestible carbohydrates (α-galactosides) in the intestinal tract. They are fermented in the colon and selectively stimulate the establishment and development of beneficial bacteria, such as Bifidobacterium, which have beneficial effects on the health of the host, including improvement in mineral absorption, modulation of lipid levels, reduction of colon cancer risk, influence on glucose levels, reduction and prevention of intestinal infections, and stimulation of the immune system [52].
Table 13 shows the content of oligosaccharides in Mexican wild species, obtained by using 50% ethanol twice. The obtained extract was boiled under reflux for 10 min. The precipitated solids were removed by centrifugation, and then the solids were concentrated under a vacuum to subsequently precipitate the oligosaccharides with 100% ethanol. These oligosaccharides were separated by centrifugation, and the ethanol was removed by vacuum. In the purification process, the sample was resuspended in 20 mL of water and filtered through 300 mL of a 1:1 (w/w) mixture of soil of diatoms and activated carbon. Oligosaccharides recovered with 500 mL of ethanol at 70% (v/v). Oligosaccharideswas finally eluted by a column DOWEX 50WX8 cation exchange [53] and analyzed using HPLC-RID equipment.
Table 13.
Oligosaccharide content in Mexican lupine seeds (mg/g of a dry sample).
| Species | Sucrose | Raffinose | Stachyose | Verbascose | Totals |
|---|---|---|---|---|---|
| L. campestris | 21.5 ± 0.76 | 11.7–13.65 | 57.16 ± 0.95 | 19.45 ± 0.59 | 90.26 ± 0.73 |
| Lupinus elegans | 7.82 ± 0.42 | 25.50 ± 1.21 | 37.20 ± 2.08 | 70.50 ± 3.71 | |
| Lupinus montanus | 14.55 ± 0.92 | 37.32 ± 2.52 | 12.44 ± 0.94 | 64.31 ± 4.38 | |
| Lupinus rotundiflorus | 5.60 ± 0.34 | 19.34 ± 0.82 | 29.57 ± 1.87 | 54.50 ± 3.03 |
Mexican lupine species have a good total oligosaccharide content, especially Lupinus campestris, with 90.26 mg/g per sample, which is greater than the rest of the wild species (Table 13) and similar to L. albus varieties (5.46–8.51 g/100 g), L. angustifolius (5.3–8.82 g/100 g), and L. luteus varieties (9.46–12.3 g/100 g). The sucrose level in L. campestris is higher than that in varieties of L. luteus and some varieties of L. albus but lower than varieties of L. angustifolius (2.55–5.05 g/100 g). Lupinus montanus, with the highest raffinose content of all wild species (14.55 mg/g), also contains more of this oligosaccharide compared with domesticated lupine varieties, except for L. albus varieties. In general, Mexican species show lower stachyose values compared to domesticated lupine varieties, but their verbascose content is higher.
5.2. Phenols
Most phenolic compounds are considered to potentially benefit health, for example, by reducing the risks of cardiovascular and neurodegenerative disease, as well as cancer, diabetes, and osteoporosis [25]. In addition, polyphenols are the most attractive natural antioxidants, as they are associated with preventing degenerative diseases, such as cancer and atherosclerosis, as well as for the nutraceutical, cosmetic, and pharmaceutical industries, because they can replace synthetic antioxidants [56].
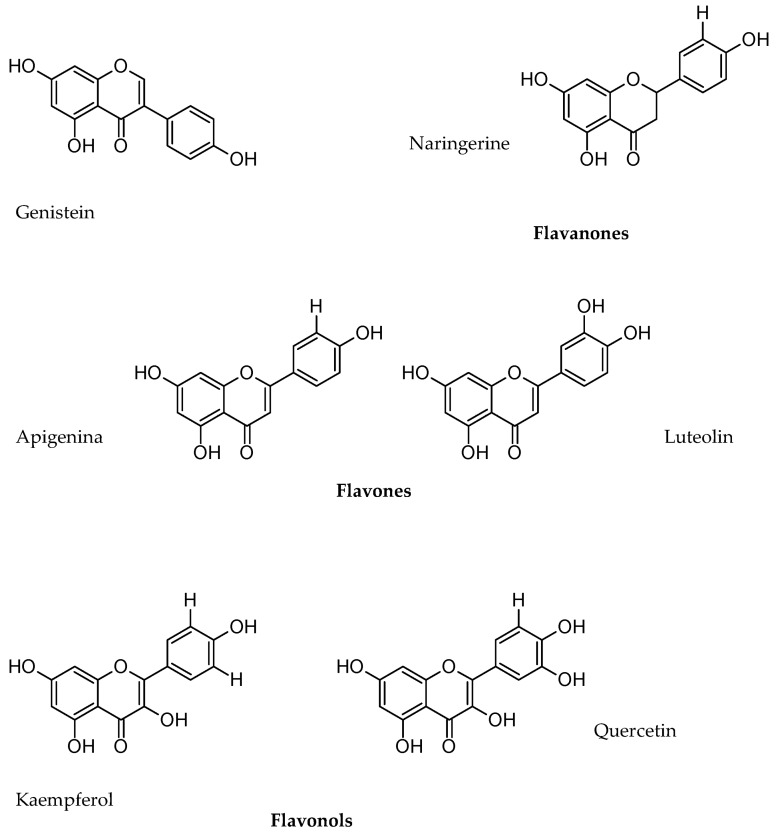
Few studies have been conducted on the phenol content of Mexican lupine species. For the extraction of flavonoids, the samples were homogenized in 80% MeOH and placed in an ultrasonic bath for 30 min. Subsequently, the bath was filtered with a vacuum pump and centrifuged at 14,000 rpm for 5 min. Finally, the bath was decanted and the supernatant was freeze-dried in a lyophilizer (LAB CONCO Corporation, Kansas City, MI, USA). The lyophilized supernatant was kept at −80 °C until its use in analysis by LC-MS techniques. It reported a predominance of glycosylated and acidulated flavonoids of genistein, isoflavone, flavone, and flavonol in the leaves and roots of nine wild lupine species: Lupinus reflexus, Lupinus elegans, L. exaltatus, Lupinus hintonii, L. mexicanus, L. montanus, L. rotundiflorus, L. stipulatus, and Lupinus sp. Among these species, the following phenols have been found: genistein’s glycated and acidulated flavonoids, such as 2′-hydroxygenistein 4′,7-O-diglucoside, 2′-hydroxygenistein 7-O-glucosylglucoside, genistein 4′,7-O-diglucoside, 2′-hydroxygenistein C,O-diglucoside, 2′-hydroxygenistein 4′,7-O-diglucoside malylated, genistein 6,8-C-diglucoside, 2′-hydroxygenistein 4′,7-O-diglucoside malonylated, and luteone 4′,7-O-diglucoside [57]. Wojakowska et al. (2013) [58] reported the isoflavones genistein, 2′hydroxygenistein, luteolin, wighteona; the flavones acacetin, apigenin, chrysoeriol, luteolin; the flavonols isorhamnetin, kaempferol, quercetin; and the flavanones eriodictyol and naringerina.
The flavonoids present in wild lupins are important. These flavonoids include the flavones (acacetin, apigenin, chrysoeriol, luteolin), flavonols (isorhamnetin, kaempferol, quercetin), and flavanols (quercetin, kaempferol) (Figure 11), since they have been shown to reduce coronary heart disease and cancer and trap reactive oxygen and nitrogen species, which is why they are potent antioxidants [56].
Figure 11.
Genistein, flavones, flavonols, flavanones.
No analysis of the content of phenolic acids or anthocyanins in these species has been conducted, so information is lacking regarding the potential for bioactive phenolic compounds with antioxidant properties, among others.
In seeds of L. campestris, a study was conducted to evaluate the effect of germination in their antioxidant capacity, using the DPPH method, and an increase of 51%– 585% was observed only in the second germination day but decreased on the ninth germination day (a decrease of 38%) [55].
The antioxidant activity depends on the type, category, and abundance of these compounds, which have substantial impacts and potential health benefits [33]. It has been reported that phenolic compounds are the main metabolites that have a positive effect on health, largely due to their antioxidant properties, through their reduction power, their ability to eliminate and inhibit free radicals, and their metal chelating activity, which avoids DNA damage and lipid peroxidation [59].
5.3. Alkaloids
In general, most of the alkaloids present in the seeds of edible legumes have been reported in species of the genus Lupinus. However, sweet varieties have been generated by selection and improvement, leading to low alkaloid content in species to be used in human and animal food [60]. Wild lupine species are rich in quinolizidinic alkaloids (more than 150 different quinolizidine alkaloids have been reported), and their content and composition depends on the phenology, organ of the plant (leaves, stems, flowers, roots, and seeds), as well as their environmental conditions and interactions with herbivores and phytopathogenic microorganisms. Seeds contain the highest total alkaloid content, which varies from 1.5% to 4%, depending on the species [40].
The alkaloid extraction was performed as follows: One half gram of seed flour was homogenized in 5% trichloroacetic acid (3 × 5 mL) and centrifuged at 3000 r.p.m. for 5 min. Later, 10 M NaOH was added to the supernatant, and the alkaloids were extracted with dichloromethane (3 × 5 mL). The dichloromethane was evaporated, and the alkaloids were dissolved in methanol [61].
Table 14 shows that sparteine was either not abundant or not present in the species studied, with the exception of L. montanus and L. reflexus, which had 3.97 and 26.63 mg of alkaloids/g, respectively, in the sample. Lupanine was present in all samples analyzed, especially in L. mexicanus, with 5.05–21.2 mg of alkaloids/g (Figure 12). Additionally, gas chromatography/mass spectrometry (GLC/MS) was used to identify the following alkaloids in L. campestris: Aphyllidine, 5,6-dehydrolupanine, Aphylline, Lupanine, a-lsolupanine, and Hydroxyaphyllidine, which was the main alkaloid compound [62]. Similar testing on Lupinus aschenbornii identified angustifoline, esparteine, tetrahydrorombifoline, lupanine, multiflorane, and 17-Oxolupanine [63].
Table 14.
Quinolizidine alkaloid content in seeds from wild Mexican lupines (mg of alkaloids/g DM).
| Lupinus species | Sparteine | Lupanine | 3-hydroxy lupanine | 13-hydroxy lupanine | Multiflorine | Hydroxyaphylline | Hydroxyaphyllidine |
|---|---|---|---|---|---|---|---|
| L. exaltatus | 0.03 | 1.47–5.83 | 1.53 | 0.015 | 0.004 | ||
| L. elegans | nd | 0.03 | 3.73 | nd | |||
| L. splendens | 0.29 | 0.89 | 1.05 | 1.00 | |||
| L. reflexus | 26.63 | 2.91 | 0.16 | 0.08 | |||
| L. rotundiflorus | 0.11 | 11.50 | 4.19 | nd | |||
| L. simulans | 0.40 | 8.87 | 2.76 | 0.09 | |||
| L. madrensis | 0.02 | 10.63 | 2.08 | 0.03 | |||
| L. montanus | 3.97 | 1.65 | 0.10 | 0.07 | |||
| L. stipulatus | 0.04 | 0.1 | 0.12 | 0.2 | |||
| L. mexicanus | 5.05–21.2 | 0.015 | 0.096–1.09 | ||||
| L. campestris | 1.2 | 498.0 | 1475.0 |
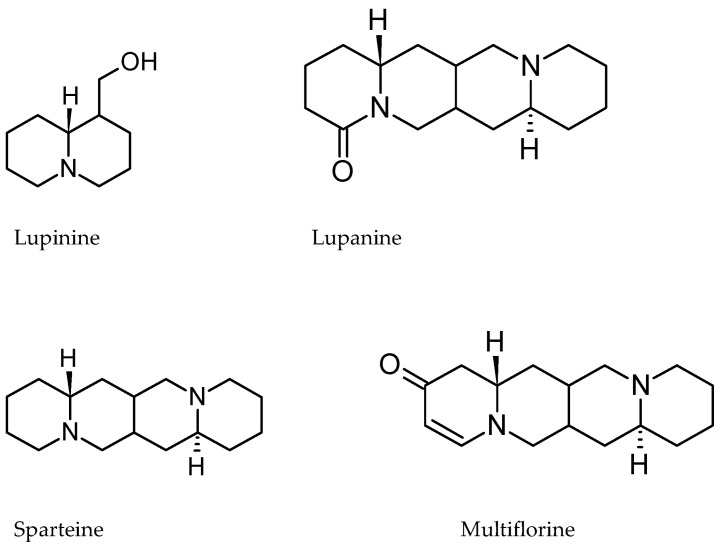
Figure 12.
Main alkaloids of lupin species.
The presence of quinolizidinic alkaloids is an advantage of wild lupines over domesticated lupines since these compounds have pharmacological properties. For example, sparteine is used in the treatment of cardiac arrhythmias and induces uterine contractions. In addition, it has been shown to have depressant effects on the central nervous system and hypotensive, diuretic, and anti-inflammatory activities [65,66]. Similarly, lupanine, 13-hydroxylupanine, and multifluorine have pharmacological activities as anticonvulsant, antipyretic, and hypoglycemic agents [67,68,69,70].
In this sense, L. reflexus represents an excellent source of esparteine, even greater than that of Cytisus scoparius, which is the commercial source of pharmacologically used sparteine. Further, the species L. mexicanus, L. rotundiflorus, and Lupinus madrensis have shown high lupanine concentrations, which could be used in various treatments.
Unlike chemical drugs that have negative physical and sometimes mental side effects, treatments with medicinal plants have shown minimal or no side effects. Consequently, there is currently a tendency to use plants with medicinal properties as food ingredients for the prevention of diseases [59].
In Mexico, there are close to 100 wild species of Lupinus widely distributed. These species show great potential due to their high protein content, which varies from 30 to 43 g/100 g, as well as their oil content, which ranges from 5.8 to 13 g/100 g, depending on the species, variety, and environmental conditions.
The wild lupins under consideration represent a potential protein supply and could be domesticated and used for feed if the alkaloids were eliminated and the protein were supplemented with methionine, or if the lupins were used in a mixture with cereals (as an addition to popular foods, such as breads, cookies, salads, etc.).
The wild Mexican lupin has high potential nutritional value for its high protein, essential fatty acids, essential minerals (such as iron with high bioavailability), and low gluten content. This species is also a future prospective nutraceutical because it contains bioactive compounds, such as a prebiotic of oligosaccharides that improves mineral absorption, the modulation of lipid levels, and reduces colon cancer risk, among other benefits. In Mexican lupins, polyphenols, such as genistein, flavonoids, flavones, and flavolos, show significant potential human health benefits. For example, they can serve as natural antioxidants by acting as scavengers of free radicals, thereby inhibiting lipid peroxidation and acting as agents for chelating metal ions. These benefits have attracted marked attention from the nutraceutical and cosmetic industries, and also from preventive medicine because of their potential application in making food, cosmetic, and pharmaceutical products that can replace synthetic antioxidants associated with health concerns, such as putative carcinogenic effects [13]. Furthermore, alkaloids, such as spartein, lupanin, 13-hydroxylupanine, and multifluorine, which are very abundant in wild species, have pharmacological activities in the central nervous system and a hypoglycemic effect on the pharmaceutic sector. However, it is very important that the molecules do not change when ingested, because their effect could be different, as in the alkaloid Frangufoline, a sedative cyclopeptide alkaloid that does not change in the gastric juices, so it is absorbed unchanged and performs its function [71].
6. Conclusions
Mexican wild lupines have substantial uses because they are an important source of protein with an adequate balance of amino acids, which can be extracted and used as ingredients in the preparation of various foods. They have the advantage of low gluten content and are also important sources of dietary fiber, minerals, and essential lipids. Additionally, they contain bioactive compounds, such as oligosaccharides with prebiotic functions, polyphenols, and alkaloids, which have been shown to have various physiological–metabolic properties. Wild lupine species have not yet been the subject of adequate study. However, these species represent a potentially important future nutritional, nutraceutical, and pharmacological source. Thus, it would be interesting to further expand the present knowledge of these species, including those outside of Mexico, since there is a great diversity of species around the world.
Acknowledgments
In addition to the funding body, the first-named author gratefully acknowledges to Ana Cristina Espinoza Gallardo by Translate this manuscript.
Author Contributions
Conceptualization, M.A.R.-L.; methodology, L.B.-R., and P.M.G.-L.; software, J.B.-P.; validation, M.A.R.-L. and J.J.V.-R.; resources, R.R.-M. and E.S.-P.; writing—review and editing, J.J.V.-R., and E.H.V.-M.; supervision, M.A.R.-L., E.H.V.-M., J.F.Z.-N.; funding acquisition, M.A.R.-L. and R.R.-M.
Funding
This research was funded by University of Guadalajara and National Research of Science and Technology (CONACYT).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Dillard C.J., German J.B. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 2000;80:1744–1756. doi: 10.1002/1097-0010(20000915)80:12<1744::AID-JSFA725>3.0.CO;2-W. [DOI] [Google Scholar]
- 2.Rochfort S., Panozzo J. Phytochemicals for health, the role of pulses. J. Agric. Food Chem. 2007;55:7981–7994. doi: 10.1021/jf071704w. [DOI] [PubMed] [Google Scholar]
- 3.Dilis V., Trichopoulou A. Nutritional and health properties of pulses. Mediterr. J. Nutr. Metab. 2009;1:149–157. doi: 10.3233/s12349-008-0023-2. [DOI] [Google Scholar]
- 4.Muzquiz M., Varela A., Burbano C., Cuadrado C., Guillamón E., Pedrosa M.M. Bioactive compounds in legumes: Pronutritive and antinutritive actions. Implications for nutrition and health. Phytochem. Rev. 2012;11:227–244. doi: 10.1007/s11101-012-9233-9. [DOI] [Google Scholar]
- 5.Villarino C., Jayasena V., Coorey R., Chakrabarti-Bell S., Johnson S. Nutritional, health, and technological functionality of lupin flour addition to bread and other baked products: Benefits and challenges. Crit. Rev. Food Sci. Nutr. 2016;56:835–857. doi: 10.1080/10408398.2013.814044. [DOI] [PubMed] [Google Scholar]
- 6.Petterson D.S. Composition and food uses of Lupinus. In: Gladstones J.S., Atkins C., Hamblin J., editors. Lupin as Crop Plants. Biology, Production and Utilization. CAB International; Wallingford, UK: 1998. pp. 353–384. [Google Scholar]
- 7.Sujak A., Kotlarz A., Strobel W. Compositional and nutritional evaluation of several lupin seeds. Food Chem. 2006;98:711–719. doi: 10.1016/j.foodchem.2005.06.036. [DOI] [Google Scholar]
- 8.Arnoldi A., Greco S. Nutritional and nutraceutical characteristics of lupin protein. Nutrafoods. 2011;10:23–29. doi: 10.1007/BF03223356. [DOI] [Google Scholar]
- 9.Lovati M.A., Manzoni C., Castiglioni S., Parolari A., Magni C., Duranti M. Lupin seed g-conglutin lowers blood glucose in hyperglycemic rats and increases glucose consumption of HepG2 cells. Br. J. Nutr. 2012;107:67–73. doi: 10.1017/S0007114511002601. [DOI] [PubMed] [Google Scholar]
- 10.Marchesi M., Parolini C., Diani E., Rigamonti E., Cornelli L., Arnoldi A., Sirtori C.R., Chiesa G. Hypolipidaemic and anti-atherosclerotic effects of lupin proteins in a rabbit model. Br. J. Nutr. 2008;100:707–710. doi: 10.1017/S000711450894215X. [DOI] [PubMed] [Google Scholar]
- 11.Sirtori C.R., Lovati M.R., Manzoni C., Castiglioni S., Duranti M., Magni C., Morandi S., D’Agostina A., Arnoldi A. Proteins of white lupin seed, a naturally isoflavone-poor legume, reduce cholesterolemia in rats and increase LDL receptor activity in HepG2 cells. J. Nutr. 2004;134:18–23. doi: 10.1093/jn/134.1.18. [DOI] [PubMed] [Google Scholar]
- 12.Viveros A., Centeno C., Arija I., Brenes A. Cholesterol-Lowering Effects of Dietary Lupin (Lupinus albus var Multolupa) in Chicken Diets. Poult. Sci. 2007;86:2631–2638. doi: 10.3382/ps.2007-00128. [DOI] [PubMed] [Google Scholar]
- 13.Pihlanto A., Mattila P., Mäkinen S., Pajari A.M. Bioactivities of alternative protein sources and their potential health benefits. Food Funct. 2017;8:3443–3458. doi: 10.1039/C7FO00302A. [DOI] [PubMed] [Google Scholar]
- 14.Lizarazo C.I., Lampi A., Liu J., Sontag-Strohm T., Piironen V., Stoddard F.L. Nutritive quality and protein production from grain legumes in a boreal climate. J. Sci. Food Agric. 2015;95:2053–2064. doi: 10.1002/jsfa.6920. [DOI] [PubMed] [Google Scholar]
- 15.Simopoulos A.P. Importance of the ratio of omega-6/omega-3 essential fatty acids: Evolutionary aspects. World Rev. Nutr. Diet. 2003;92:1–22. doi: 10.1159/000073788. [DOI] [PubMed] [Google Scholar]
- 16.Hall R.S., Thomas S.J., Johnson S.K. Australian sweet lupin flour addition reduces the glycaemic index of a white bread breakfast without affecting palatability in healthy human volunteers. Asia Pac. J. Clin. Nutr. 2005;14:91–97. [PubMed] [Google Scholar]
- 17.Johnson S.K., Chua V., Hall S.R., Baxter L.A. Lupin kernel fibre foods improve bowel function and beneficially modify some putative faecal risk factors for colon cancer in men. Br. J. Nutr. 2006;95:372–378. doi: 10.1079/BJN20051648. [DOI] [PubMed] [Google Scholar]
- 18.Písaříková B., ZralýA Z. Dietary fibre content in lupine (Lupinus albus L.) and soya (Glycine max L.) seeds. Acta Vet. Brno. 2010;79:211–216. doi: 10.2754/avb201079020211. [DOI] [Google Scholar]
- 19.Porres J.M., Aranda P., Lopez-Jurado M.A., Urbano G. Nitrogen Fractions and Mineral Content in Different Lupin Species (Lupinus albus, Lupinus angustifolius, and Lupinus luteus). Changes induced by the a-Galactoside extraction process. J. Agric. Food Chem. 2007;55:7445−7452. doi: 10.1021/jf070718z. [DOI] [PubMed] [Google Scholar]
- 20.Donangelo C.M., Pedersen B., Eggum O.B. Protein energy and mineral utilization in rats fed rice: Legume diets. Plant Foods Hum. Nutr. 1986;36:119–137. doi: 10.1007/BF01092139. [DOI] [Google Scholar]
- 21.Trugo L.C., Donangelo C.M., Duarte Y.A., Tavares C.L. Phytic acid and selected mineral composition of seed from wild species and cultivated varieties of lupin. Food Chem. 1993;47:391–394. doi: 10.1016/0308-8146(93)90183-G. [DOI] [Google Scholar]
- 22.Jayasena V., James A.P. Total phenolic and phytosterol compounds and the radical scavenging activity of germinated Australian sweet lupin flour. Plant Foods Hum. Nutr. 2013;68:352–357. doi: 10.1007/s11130-013-0377-6. [DOI] [PubMed] [Google Scholar]
- 23.Champ M.M. Non-nutrient bioactive substances of pulses. Br. J. Nutr. 2002;88:307–319. doi: 10.1079/BJN2002721. [DOI] [PubMed] [Google Scholar]
- 24.Muzquiz M., Wood J.A. Antinutritional Factors. In: Yadav S.S., Redden R., Chen W., Sharma B., editors. Chickpea Breeding Management. CABI; Wallingford, UK: 2007. pp. 143–166. [Google Scholar]
- 25.Del Rio D., Rodriguez-Mateos A., Spencer J.P., Tognolini M., Borges G., Crozier A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013;18:1818–1892. doi: 10.1089/ars.2012.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campos-Vega R., Loarca-Piña G.F., Oomah B.D. Minor components of pulses and their potential impact on human health. Food Res. Int. 2010;43:461–482. doi: 10.1016/j.foodres.2009.09.004. [DOI] [Google Scholar]
- 27.Kadlec P., Bjergegaard C., Gulewicz K., Horbowicz M., Jones A., Kintia P., Kratchanov C., Kratchanova M., Lewandowicz G., Soral-Smietana M., et al. Carbohydrate chemistry. In: Hedley C.L., editor. Carbohydrates in Grain Legume Seeds: Improving Nutritional Quality and Agronomic Characteristics. CAB International; Wallingford, UK: 2000. pp. 15–59. [Google Scholar]
- 28.Martínez-Villaluenga C., Frías J., Vidal-Valverde C. Raffinose family oligosaccharides and sucrose contents in 13 Spanish lupin cultivars. Food Chem. 2005;91:645–649. doi: 10.1016/j.foodchem.2004.06.034. [DOI] [Google Scholar]
- 29.Balasundram N., Sundram K., Samman S. Phenolic compounds in plants and agro-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006;99:191–203. doi: 10.1016/j.foodchem.2005.07.042. [DOI] [Google Scholar]
- 30.Siger A.l., Czubinski J., Kachlicki P., Dwiecki K., Lampart-Szczapa E., Nogala-Kalucka M. Antioxidant activity and phenolic content in three Lupin species. J. Food Compos. Anal. 2012;25:190–197. doi: 10.1016/j.jfca.2011.10.002. [DOI] [Google Scholar]
- 31.Song F.L., Gan R.Y., Zhang Y., Xiao Q., Kuang L., Li H.B. Total Phenolic Contents and Antioxidant Capacities of Selected Chinese Medicinal Plants. Int. J. Mol. Sci. 2010;11:2362–2372. doi: 10.3390/ijms11062362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bordoloi M., Bordoloi P.K., Dutta P.P., Singh V., Nath S., Narzary B., Bhuyan P.D., Rao P.G., Barua I.C. Studies on some edible herbs: Antioxidant activity, phenolic content, mineral content and antifungal properties. J. Funct. Foods. 2016;23:220–229. doi: 10.1016/j.jff.2016.02.028. [DOI] [Google Scholar]
- 33.Vahid F., Gominho J., Pereira H., Carvalho I.S. Screening of the Antioxidant and Enzyme Inhibition Potentials of Portuguese Pimpinella anisum L. Seeds by GC-MS. Food Anal. Method. 2018;11:2645–2656. [Google Scholar]
- 34.Martínez-Villaluenga C., Zielinski H., Frias J., Piskula M.K., Kozlowska H., Vidal-Valverde C. Antioxidante capacity and polyphenolic content of high-protein lupin products. Food Chem. 2009;112:84–88. doi: 10.1016/j.foodchem.2008.05.040. [DOI] [Google Scholar]
- 35.Khan M.K., Karnpanit W., Nasar-Abbas S.M., Huma Z., Jayasena V. Phytochemical composition and bioactivities of lupin: A review. Int. J. Food Sci. Technol. 2015;50:2004–2012. doi: 10.1111/ijfs.12796. [DOI] [Google Scholar]
- 36.Rajeev B., Karim A.A. Exploring the Nutritional Potential of Wild and Underutilized Legumes. Compr. Rev. Food. Sci. Saf. 2009;8:305–331. [Google Scholar]
- 37.Ruiz L.M.A., Garcia-López P.M., Castañeda-Vazquez H., Zamora-Natera J.F., Garzón-De la Mora P., Bañuelos-Pineda J., Burbano C., Pedrosa M.M., Cuadrado C., Muzquiz M. Chemical composition and antinutrient content of three Lupinus species from Jalisco, Mexico. J. Food Compos. Anal. 2000;13:193–199. doi: 10.1006/jfca.1999.0887. [DOI] [Google Scholar]
- 38.Medina-Gonzalez E., Martinez-Herrera J., Ruiz-López M.A., Martinez-Ayala A.L. Análisis de alcaloides quinolizidinicos en harina y aislados proteicos de L. exaltatus y L. elegans. Rev. Latinoam. Quím. 2000;28:137–142. [Google Scholar]
- 39.Ruiz-Moreno J.J., Ruiz-Lopez M.A., Zamora-Natera J.F. The genus Lupinus: Taxonomy and distribution in Jalisco, Mexico; Proceedings of the 9th International Lupin Conference; Klink/Muritz, Germany. 20–24 June 1999; pp. 297–300. [Google Scholar]
- 40.Ruiz M.A., Sotelo A. Chemical composition, nutritive value, and toxicology evaluation of Mexican wild lupins. J. Agric. Food Chem. 2001;49:5336–5339. doi: 10.1021/jf010247v. [DOI] [PubMed] [Google Scholar]
- 41.Ruiz-López M.A., Rodríguez-Macías R., Navarro-Pérez S. Evaluación químico-nutricional de Lupinus exaltatus Zucc, del nevado de colima, México, como fuente potencial de forraje. Interciencia. 2006;31:758–761. [Google Scholar]
- 42.Pablo-Pérez M., Lagunes-Espinoza L.C., López-Upton J., Aranda-Ibáñez E.M., Ramos-Juárez J. Composición química de especies silvestres del género Lupinus del estado de Puebla, México. Rev. Fitotec. Mex. 2015;38:49–55. [Google Scholar]
- 43.Juárez-Fuentes B., Lagunes-Espinoza L.C., Bucio-Galindo A., Delgado-Alvarado A., Pérez-Flores J., López-Upton J. Efecto de tratamientos hidrotérmico, remojo y germinación en la composición química de semillas de Lupinus silvestres. Agroproductividad. 2018;11:41–47. [Google Scholar]
- 44.Porras Saavedra J., Guemez-Vera N., Montañez-Soto J.L., Fernández-Martínez M.C., Yañez-Fernández J. Comparative study of functional properties of protein isolates obtained from three Lupinus species. Adv. Bioresour. 2013;4:106–116. [Google Scholar]
- 45.Rodríguez-Ambriz S.L., Martínez-Ayala A.L., Millan F., Davila-Ortíz G. Composition and functional properties of Lupinus campestris protein isolates. Plant Foods Hum. Nutr. 2005;60:99–107. doi: 10.1007/s11130-005-6835-z. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-López P.M., Caro-Ojeda A., Ruiz-López M.A., Bañuelos-Pineda J., Zamora-Natera F., Ruiz-Moreno J. Opportunieties for High Quality, Healthy and Added-Value Crops to Meet European Demands, Proceeding of 3rd European Conference on Grain Legumes, Valladolid, Spain, 14–19 November 1998. European Association for Grain Legume Research; Valladolid, Espana: 1998. Functional properties of protein isolate obtained from Lupinus exaltatus Zucc. [Google Scholar]
- 47.García-Lopez P.M., Muzquiz M., Ruiz-Lopez M.A., Zamora-Natera J.F., Burbano C., Pedrosa M.M., Cuadrado C., Garzon-De la Mora P. Chemical composition and fatty acid profile of several Mexican wild lupins. J. Food Compos. Anal. 2001;14:645–651. [Google Scholar]
- 48.Whelton S.P., Hyre D.A., Pedersen B., Yi Y., Whelton P.K., He J. Effect of dietary fiber intake on blood pressure: A meta-analysis of randomized, controlled clinical trials. J. Hypertens. 2005;23:475–481. doi: 10.1097/01.hjh.0000160199.51158.cf. [DOI] [PubMed] [Google Scholar]
- 49.Valdés-Miramontes E.H., López-Espinoza A., Rodríguez-Macías R., Salcedo-Pérez E., Ruiz-López M.A. Efecto del tratamiento térmico sobre la composición química y minerales en semillas de lupinos silvestres. Rev. Chil. Nutr. 2015;42:186–190. doi: 10.4067/S0717-75182015000200011. [DOI] [Google Scholar]
- 50.Pablo-Pérez M., Lagunes-Espinoza L.D.C., López-Upton J., Ramos-Juárez J., Aranda-Ibáñez E.M. Morfometría, germinación y composición mineral de semillas de Lupinus silvestres. Bioagro. 2013;25:101–108. [Google Scholar]
- 51.Valdes-Miramontes E.H., López-Espinoza A., Martínez M.A.G., Zamora-Natera J.F., Rodriguez-Macias R., Ruiz-López M.A. Iron bioavailability of Lupinus rotundiflorus seeds and roots inlow-iron-diet treated rats. Rev. Nutr. 2017;30:827–834. doi: 10.1590/1678-98652017000600014. [DOI] [Google Scholar]
- 52.Gibson G.R., Roberfroid M.B. Handbook of Prebiotics. CRC Press; Boca Raton, FL, USA: 2008. p. 504. [Google Scholar]
- 53.Maya-Zepeda L., Hernández-Gobora J., Rodríguez-Macías R., García-López P.M., Ruiz-López M.A. Contenido de oligosacáridos en semillas de leguminosas silvestres mexicanas. Chil. J. Agric. Anim. Sci. 2013;29:161–167. [Google Scholar]
- 54.Jiménez M.C., Loarca-Piña G., Dávila O.G. Antimutagenic activity of phenolic compounds, oligosaccharides and quinolizidinic alkaloids from Lupinus campestris seeds. Food Addit. Contam. 2003;20:940–948. doi: 10.1080/02652030310001605998. [DOI] [PubMed] [Google Scholar]
- 55.Jimenez-Martınez C., Cardador-Martınez A., Martinez-Ayala A.L., Muzquiz M., Pedrosa M.M., Davila-Ortiz G. Changes in protein, nonnutritional factors, and antioxidant capacity during germination of L. campestris Seeds. Int. J. Agron. 2012;2012:1–7. doi: 10.1155/2012/387407. [DOI] [Google Scholar]
- 56.Oleszek W. Dietary phytochemicals and human health. Phytochem. Rev. 2002;1:163–166. doi: 10.1023/A:1022572509383. [DOI] [Google Scholar]
- 57.Stobiecki M., Staszkow A., Piasecka A., Garcia-Lopez P.M., Zamora-Natera F., Kachlicki P. LC-MSMS Profiling of Flavonoid Conjugates in Wild Mexican Lupine, Lupinus reflexus. J. Nat. Prod. 2010;73:1254–1260. doi: 10.1021/np100139d. [DOI] [PubMed] [Google Scholar]
- 58.Wojakowska A., Piasecka A., García-López P.M., Zamora-Natera F., Krajewski P., Marczak L., Kachlicki P., Stobiecki M. Structural analysis and profiling of phenolic secondary metabolites of Mexican lupine species using LC–MS techniques. Phytochemistry. 2013;92:71–86. doi: 10.1016/j.phytochem.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 59.Farzaneh V., Carvalho I.S. A review of the health benefit potentials of herbal plant infusions and their mechanism of actions. Ind. Crops Prod. 2015;65:247–258. doi: 10.1016/j.indcrop.2014.10.057. [DOI] [Google Scholar]
- 60.Hymowitz T. Grain legumes. In: Janick J., Simon J.E., editors. Advances in New Crops. Timber Press; Portland, OR, USA: 1990. pp. 54–57. [Google Scholar]
- 61.Ruiz-López M.M., García-López P.M., Rodríguez-Macias R., Zamora Natera J.F. Mexican wild lupines as a source of quinolizidine alkaloids of economic potential. Polibotanica. 2010;29:159–164. [Google Scholar]
- 62.Martínez-Herrera J., Robledo-Quintos N., Mora-Escobedo R., Dávila-Ortiz G. Alkaloid composition of Lupinus campestris from México. J. Food Biochem. 2001;25:117–125. doi: 10.1111/j.1745-4514.2001.tb00728.x. [DOI] [Google Scholar]
- 63.Montes H.E., Corona R.M.L., Encarnación C.A., Cantor A.J.A., Sánchez L.A., Sporer F., Wink M., Bermúdez T.K. Quinolizidine alkaloid composition in different organs of Lupinus aschenbornii. Rev. Bras. Farmacogn. 2011;21:824–828. [Google Scholar]
- 64.Zamora-Natera F., García-López P., Ruiz-López M.A., Salcedo-Pérez E. Composición de alcaloides en semillas de Lupinus mexicanus (fabaceae) y evaluación antifúngica y alelopática del extracto alcaloideo. Agrociencia. 2008;42:185–192. [Google Scholar]
- 65.Schmeller T., Wink M. Utilization of alkaloids in modern medicine. In: Roberts M.F., Wink M., editors. Alkaloids. Biochemistry, Ecology and Medicinal Applications. Plenum Press; New York, NY, USA: 1998. pp. 435–459. [Google Scholar]
- 66.Szczawinska K., Bobkiewicz K.T., Kozaryn M., Peretiatkowicz K., Gulewicz K. Some pharmacological properties of an extract from bitter Lupin (L. angustifolius) seeds. In: Neves-Martin J.M., Beirão da Costa M.L., editors. Advances in Lupin Research, Proceeding of the VII th International Lupin Conference, Evora, Portugal, 18–23 April 1993. Instituto Superior de Agronomia; Lisbon, Portugal: 1994. pp. 297–300. [Google Scholar]
- 67.Hatzold T., Elmadfa I., Gross R., Wink M., Hartmann T., Witte L. Quinolizidine alkaloids in seeds of Lupinus mutabilis. J. Agric. Food Chem. 1983;31:934–938. doi: 10.1021/jf00119a003. [DOI] [Google Scholar]
- 68.Kubo H., Inoue M., Kamei J., Higashiyama K. Hypoglycemic effect of multiflorine derivatives in normal mice. Biol. Pharm. Bull. 2006;29:2046–2050. doi: 10.1248/bpb.29.2046. [DOI] [PubMed] [Google Scholar]
- 69.García-López P.M., Garzon de la Mora P., Wysocka W., Maiztegui B., Alzugaray M.E., Del Zotto H., Borelli M.I. Quinolizidine alkaloids isolated from Lupinus enhance insulin secretion. Eur. J. Pharm. 2004;504:139–142. doi: 10.1016/j.ejphar.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 70.Bobkiewicz K.T., Dworacka M., Kuczynski S., Abramczyk M., Kolanos R., Wysocka W., García-López P.M., Winiarska H. Hypoglycaemic effect of quinolizidine alkaloids-lupanine and 2-tionosparteine on non diabetic and streptozotocin-induced diabetic rats. Eur. J. Pharm. 2007;565:240–244. doi: 10.1016/j.ejphar.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 71.El-Seedi H.R., Maram H.Z., Goransson U., Verpoorte R. Cyclopeptide alkaloids. Phytochem. Rev. 2017;6:143–165. doi: 10.1007/s11101-006-9029-x. [DOI] [Google Scholar]