Abstract
Hispidin, a polyphenol compound isolated from Phellinus linteus, has been reported to possess antioxidant activities. In this study, we aimed to investigate the mechanisms underlying the protective effect of hispidin against hydrogen peroxide (H2O2)-induced oxidative stress on Adult Retinal Pigment Epithelial cell line-19 (ARPE-19) cells. Hispidin was not cytotoxic to ARPE-19 cells at concentrations of less than 50 μM. The levels of intracellular reactive oxygen species (ROS) were analyzed by dichlorofluorescin diacetate (DCFDA) staining. Hispidin significantly restored H2O2-induced cell death and reduced the levels of intracellular ROS. The expression levels of antioxidant enzymes, such as NAD(P)H:Quinine oxidoreductase-1 (NQO-1), heme oxygenase-1 (HO-1), glutamate-cysteine ligase catalytic subunit (GCLC), and glutamate-cysteine ligase modifier subunit (GCLM) were examined using real-time PCR and Western blotting. Our results showed that hispidin markedly enhanced the expression of nuclear factor erythroid 2-related factor 2 (Nrf2), HO-1, NQO-1, GCLM, and GCLC in a dose-dependent manner. Furthermore, knockdown experiments revealed that transfection with Nrf2 siRNA successfully suppresses the hispidin activated Nrf2 signaling in ARPE-19 cells. Moreover, activation of the c-Jun N-terminal kinase (JNK) pathway is involved in mediating the protective effects of hispidin on the ARPE-19 cells. Thus, the present study demonstrated that hispidin provides protection against H2O2-induced damage in ARPE-19 cells via activation of Nrf2 signaling and up-regulation of its downstream targets, including Phase II enzymes, which might be associated with the activation of the JNK pathway.
Keywords: ARPE-19, hispidin, hydrogen peroxide, Nrf2, oxidative stress, age-related macular degeneration
1. Introduction
Age-related macular degeneration (AMD) is the leading cause of blindness among the elderly in the developed world [1]. The most common type of AMD, also known as the dry-type or atrophic AMD, is initiated by changes in the pigmentation of the retinal pigment epithelial (RPE) cells and the sub-retinal deposits between the RPE and Bruch’s membrane. These progressions eventually result in RPE cell death, photoreceptors degeneration, and eventually loss of vision [2,3]. Unfortunately, there is no specific treatment available for dry/atrophic AMD.
Oxidative stress is characterized by the generation of reactive oxygen species (ROS), which plays a key role in the pathogenesis of AMD [4]. RPE cells have a high metabolic rate and exist in an environment which is abundant of endogenous ROS, such as O2−, H2O2 and OH·[5]. Long-term accumulation of oxidative damage leads to dysfunction in RPE cells and increases their susceptibility to oxidative stress. Several dietary supplements of antioxidants, including lutein, β-carotene, and vitamin C, appropriately alleviate the progression of AMD [6,7]. Thus, protecting RPE cells from oxidative stress is deemed to be a sustainable option for preventing the occurrence or decelerating the progression of AMD.
Nuclear factor erythroid 2-related factor 2 (Nrf2) is known for being a major regulator of the anti-oxidative responses which binds to the AREs (antioxidant response elements); thus, it plays a role in the up-regulation of the expression of antioxidant enzymes, including heme oxygenase-1 (HO-1), NAD(P)H:Quinine oxidoreductase-1 (NQO-1), glutamate-cysteine ligase catalytic subunit (GCLC), and glutamate-cysteine ligase modifier subunit (GCLM) [8,9]. Interestingly, a recent report shows that Nrf2-deficient mice present retinal pathology which is analogous to human AMD [10]. Recent studies also indicate that antioxidants work against oxidative stresses in RPE cells by activating Nrf2 signaling [11,12]. These findings suggest the key role of Nrf2 signaling in the protective effects of antioxidant on RPE cells.
Phellinus linteus, a species of fungus belonging to the genus Phellinus and the family Hymenochaetaceae has been extensively used as a medicinal mushroom in Africa and East Asia [13]. It has been reported to be rich in several polysaccharides and polyphenol compounds, including hispidin [14,15]. Hispidin is well-known for its antioxidant [15], anti-inflammatory [16,17], anti-proliferative, and anti-metastatic effects [18]. In addition, hispidin has been reported to function as an antioxidant agent by preventing the ROS associated damage in the pancreatic β-cells [19,20]. However, it still remains uncertain whether hispidin has the ability to protect the RPE cells from oxidative-stress-induced damage. In order to fill this gap in the existing knowledge, H2O2 was used to induce oxidative stress in the human RPE cell line, Adult Retinal Pigment Epithelial cell line-19 (ARPE-19)—which is isolated from human retinal pigmented epithelium—that has the structural and functional properties of RPE cells [21]. The purpose of this study was to determine the cytoprotective effect of hispidin on H2O2-induced oxidative stress in ARPE-19 cells and to investigate the association of Nrf2 signaling with the underlying mechanism.
2. Results
2.1. Effects of Hispidin on the Viability of ARPE-19 Cells
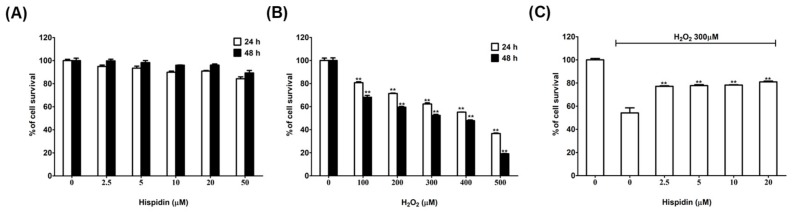
To determine the optimal concentration without toxic effect of hispidin, ARPE-19 cells were treated with hispidin in various concentrations for 24 h or 48 h. The MTT assays revealed that there was no significant change in the viability of ARPE-19 cells after treated with hispidin at concentrations ranging from 2.5–50 μM (Figure 1A). Thus, this data indicates that hispidin is relatively non-toxic for use in ARPE-19 cells up to a concentration of 50 μM.
Figure 1.
Effects of hispidin and H2O2 on the viability of Adult Retinal Pigment Epithelial cell line-19 (ARPE-19) cells. (A) ARPE-19 cells were treated with various concentrations (2.5–50 μM) of hispidin or (B) H2O2 (100–500 μM) for 24 (□) and 48 h (■), respectively. Cell survival was measured by MTT assay (C) ARPE-19 cells were pre-treated with hispidin (0–20 μM) for 24 h, followed by 300 μM H2O2 treatment for 24 h, cell survival was measured by MTT assay. ** p < 0.01 versus vehicle control.
Using H2O2 to explore the protective effect against oxidative stress in RPE cells is a well-known model [22,23]. Thus, H2O2 was selected as the oxidative-stress inducer for our studies, and a working concentration of H2O2 that killed 50% of ARPE-19 cells after a 48 h incubation was determined by performing a dose-response experiment. The results showed that the viability of ARPE-19 cells decreased in a dose-dependent fashion in response to H2O2 treatment (Figure 1B). It was found that treatment with 300 μM H2O2 decreases cell viability by around 50% (52.4%); therefore, this concentration of H2O2 was selected for use in subsequent experiments.
To determine the protective effects of hispidin against H2O2-induced cell death on ARPE-19 cells, MTT assays were performed. The results showed that treatment with 300 μM H2O2 led to a significant reduction in cell viability (by 54.2%) as compared with the control cells; whereas, pre-treatment with hispidin (2.5–20 μM) for 24 h resulted in the prevention of H2O2-induced cell death (Figure 1C). Furthermore, pre-treatment of ARPE-19 cells with 20 μM hispidin restored the cell viability up to 80.9% with respect to the untreated cells. These results suggest that hispidin can help protect ARPE-19 cells from H2O2-induced cell death.
2.2. Hispidin Protects ARPE-19 Cells Against H2O2-Induced Oxidative Stress
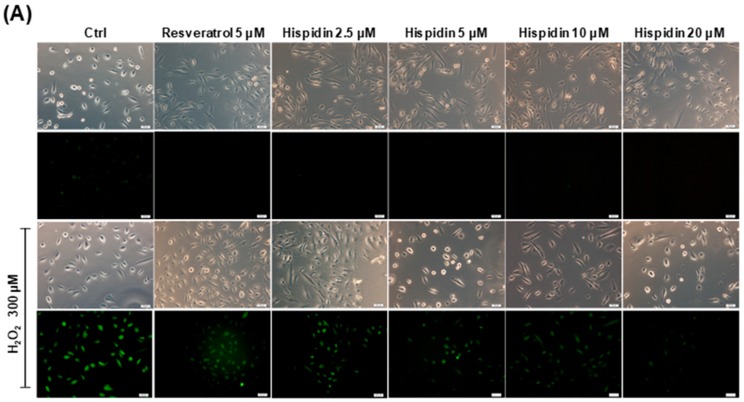
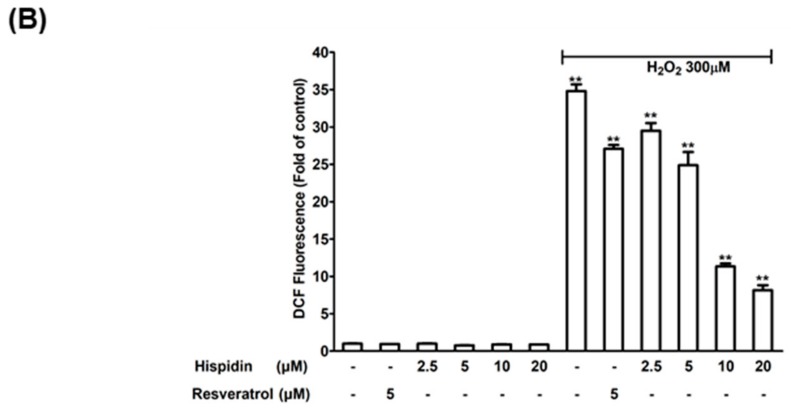
Hispidin has been reported to possess quenching effects against free radicals. To evaluate the ROS scavenging ability of hispidin on ARPE-19 cells, dichlorofluorescin diacetate (DCFDA) assay was performed. The fluorescence microscopy results revealed that the levels of ROS in 300 μM H2O2-treated cells were enhanced as compared to the vehicle group (Figure 2A). However, pre-treatment with hispidin (2.5–20 μM) for 24 h prominently decreased the fluorescence intensity as compared to the H2O2-only group. The fluorescence signal at 535 nm was measured by a fluorescence plate reader (Figure 2B). Cells treated with 300 μM H2O2 showed a 34.8-fold induction of intracellular ROS as compared to the non-treated group. However, pre-treatment with hispidin at concentrations of 2.5 μM, 5 μM, 10 μM, and 20 μM significantly reduced the intracellular ROS to 29.5-, 24.9-, 11.3-, and 8.2-fold, respectively. Cells treated with 5 μM resveratrol as a positive control showed a 27.1-fold induction in the concentration of intracellular ROS. These results indicate that hispidin reduces H2O2-induced intracellular ROS in a dose-dependent manner.
Figure 2.
Protective effect of hispidin against H2O2-induced oxidative stress on ARPE-19 cells. (A) ARPE-19 cells were pre-treated with various concentrations (2.5–20 μM) of hispidin for 24 h and then treated with 300 μM H2O2 for 6 h. The H2O2-induced reactive oxygen species (ROS) generation was measured by dichlorofluorescin diacetate (DCFDA) assay. The green fluorescence represents the cells stained for ROS (magnification 200x); (B) Fluorescence was then measured with a multi-well fluorescence reader at 535 nm (emission). ** p < 0.01 versus vehicle control.
2.3. Hispidin Activates Nrf2 and Its Target Genes Involved in Anti-Oxidative Response in ARPE-19 Cells
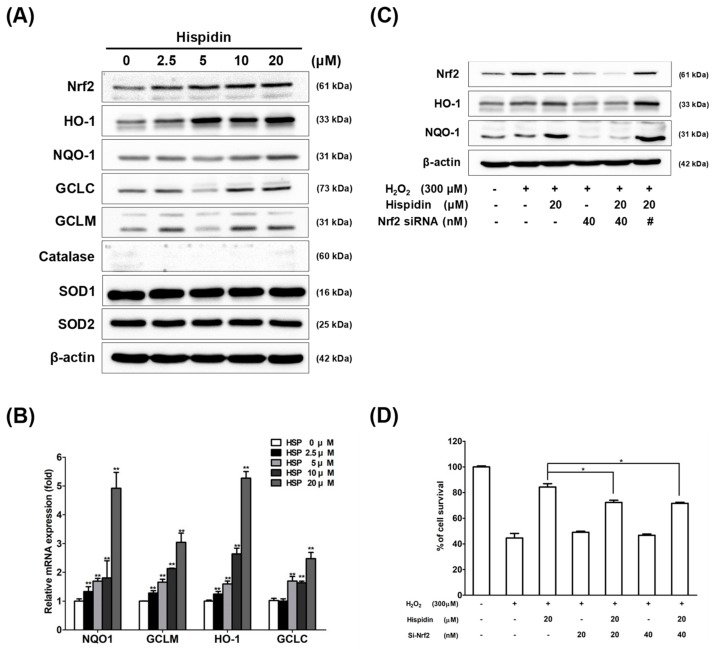
To further elucidate whether the activation of antioxidant enzymes is involved in the ROS scavenging activities of hispidin, ARPE-19 cells were treated with various concentrations of hispidin for 48 h, and the protein-expression levels of antioxidants, including Nrf2, HO-1, NQO-1, GCLC, GCLM, catalase, and superoxide dismutase (SOD, SOD1:Cu/ZnSOD, and SOD2:MnSOD), were examined by Western blot (Figure 3A). The expression of Nrf2, HO-1, NQO-1, GCLC, and GCLM were up-regulated after treatment with hispidin. However, the expression of catalase, SOD1, and SOD2 did not increase on treatment with hispidin. Consistent with the results of the Western blot, all the indicated concentrations of hispidin (2.5–20 μM) treatment increased the expression of NQO-1, GCLM, HO-1, and GCLC, transcripts as assessed by real-time PCR analysis (Figure 3B). These results demonstrated that the expression of Nrf2 and its downstream target genes, such as NQO-1, HO-1, GCLC, and GCLM, were up-regulated after hispidin treatment in ARPE-19 cells.
Figure 3.
Activation of nuclear factor erythroid 2-related factor 2 (Nrf2) signaling is corelated with the protective effect of hispidin against H2O2-induced cell death on ARPE-19 cells. (A) Cells were treated with various concentrations (2.5–20 μM) of hispidin for 24 h, and the expression levels of Nrf2, heme oxygenase-1 (HO-1), NAD(P)H:Quinine oxidoreductase-1 (NQO-1), glutamate-cysteine ligase catalytic subunit (GCLC), glutamate-cysteine ligase modifier subunit (GCLM), catalase, superoxide dismutase 1 (SOD1), and superoxide dismutase 2 (SOD2) were analyzed by Western blot or real-time PCR (B). (C) Cells were transfected with 40 μM Nrf2 siRNA or scramble siRNA (#) for 48 h, and then pre-treated with hispidin for 24 h, followed by 24 h H2O2 treatment. Protein levels of Nrf2, HO-1, and NQO-1 were analyzed by Western blot, and cell viability was assessed by MTT assay (D). * p < 0.05 versus hispidin+H2O2 group.
In order to determine the role of Nrf2 in mediating the protective effects of hispidin on RPE cells, the siRNA approach was employed. Cells were transfected with 40 nM Nrf2 siRNA or control scramble siRNA for 48 h, and then pre-treated with or without 20 μM hispidin for 24 h, followed by treatment with 300 μM H2O2 for 24 h (Figure 3C). Immunoblotting analysis revealed that transfection with Nrf2 siRNA suppressed the hispidin-induced Nrf2 expression in ARPE-19 cells, as compared with the vehicle group (scrambled siRNA transfected). The down-regulated expression of the Nrf2 downstream target genes HO-1 and NQO-1 were also observed. In addition, upon transfection with Nrf2 siRNA, the cell viability after H2O2 treatment was attenuated from 84.3% to 72.3% (20 nM) or 71.5% (40 nM), as measured by MTT assays (Figure 3D). Taken together, these results indicate that hispidin-induced protective effects against H2O2 are due to the up-regulation of Nrf2 and its downstream target genes in ARPE-19 cells.
2.4. Hispidin Induces C-Jun N-Terminal Kinase (JNK) Activation Involved in Anti-Oxidative Response in ARPE-19 Cells
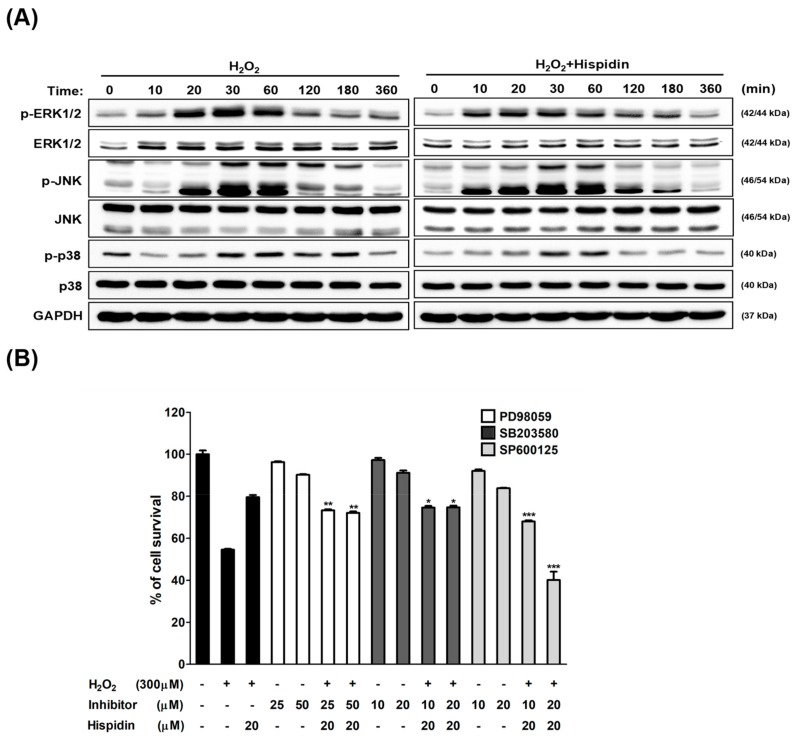
To determine the roles of mitogen-activated protein kinase (MAPK) signaling involved in the protective effect of hispidin on ARPE-19 cells, cells were pre-treated with 20 μM hispidin for 24 h and then treated with 300 μM H2O2 for 10–360 min. After analyzed by Western blot, protein levels of phospho-JNK in cells treated with hispidin were augmented during 10–180 min post H2O2 treatment as compared to the H2O2 alone group (Figure 4A). To further elucidate how the JNK pathway is associated with the protective effects of hispidin against oxidative stress, cells were treated with 20 μM hispidin for 24 h in the presence or absence of the following MAPK signaling inhibitors: MEK1/2 inhibitor PD98059 (25 μM or 50 μM), the p38 inhibitor SB203580 (10 μM or 20 μM), or the JNK inhibitor SP600125 (10 μM or 20 μM). Then cells were subjected to a 300 μM H2O2 treatment for 24 h, followed by MTT assay analysis (Figure 4B). The results showed that only SP600125 treatment can significantly reduce the cell viability to 72.7% (10 μM) and 40.1% (20 μM) as compared to the vehicle control, which suggested that treatment with JNK but not MEK1/2 or p38 inhibitor abolished the protective effects of hispidin in H2O2-treated ARPE-19 cells. These results indicate that the activation of the JNK pathway plays a crucial role in mediating the protective effect of hispidin on H2O2-induced cell death in ARPE-19 cells.
Figure 4.
Hispidin induces c-Jun N-terminal kinase (JNK) activation in ARPE-19 cells exposed to H2O2-induced oxidative stress. (A) ARPE-19 cells were treated with or without hispidin (20 μM) for 24 h and then treated with 300 μM H2O2 for 10–360 min. Proteins were collected and subjected to analyze the expression levels of p-ERK, p-JNK, and p-p38, which were then evaluated by Western blot. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. (B) ARPE-19 cells were treated with hispidin for 24 h in the presence or absence of the mitogen-activated protein kinase (MAPK) pathway inhibitors, followed by a 300 μM H2O2 treatment for 24 h. Cell viability was analyzed by MTT assay. * p < 0.05, ** p < 0.01, *** p < 0.001 versus hispidin+H2O2 group.
2.5. JNK Pathway is Essential for Hispidin-Induced Activation of Nrf2 Signaling in ARPE-19 Cells
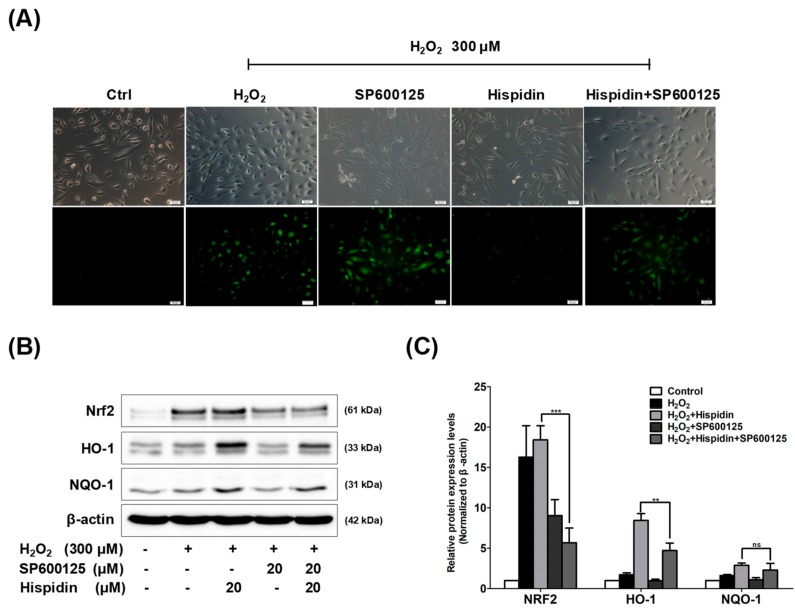
To elucidate the possible role of JNK activation in the hispidin-induced antioxidant effect, ARPE-19 cells were treated with hispidin (20 μM) for 24 h in the presence and absence of the SP600125 (20 μM), then treated with 300 μM H2O2 for 6 h, followed by dichlorofluorescin diacetate (DCFDA) staining (Figure 5A). The data showed that the fluorescence intensity was enhanced in the H2O2- or SP600125-alone groups, but not in the hispidin pre-treatment group. However, treatment with SP600125 significantly enhanced the fluorescence intensity in the hispidin pre-treatment group. The involvement of JNK activation in the regulation of Nrf2 signaling was also investigated. ARPE-19 cells were treated with hispidin (20 μM) for 24 h in the presence and absence of the SP600125 (20 μM), followed by 300 μM H2O2 treatment for 24 h, and then subjected to Western blot analysis (Figure 5B). The protein expression levels of Figure 5B were quantified and normalized to the β-actin (Figure 5C). The results revealed that SP600125 significantly blocks the hispidin-induced expression of genes such as Nrf2 and HO-1, as compared to the indicated group. Thus, the overall data suggests that the activation of the JNK pathway is critical for the hispidin-induced antioxidant effect and the activation of Nrf2 signaling.
Figure 5.
The role of JNK activation in hispidin-induced Nrf2 signaling on ARPE-19 cells. (A) Cells were pre-treated with 20 μM hispidin, 20 μM SP600125, or both for 24 h and then treated with 300 μM H2O2 for 6 h. The ROS generation was measured by dichlorofluorescin diacetate (DCFDA) assay. The green fluorescence represents the cells stained for ROS (magnification 200×). (B) Cells were pre-treated with 20 μM hispidin or 20 μM SP600125 for 24 h followed by 300 μM H2O2 treatment for 24 h. The expression levels of Nrf2, HO-1, and NQO-1 were evaluated by Western blot. (C) Quantitative analysis was performed by measuring the intensity relative to the control. ** p < 0.01, *** p < 0.001, ns: not significant.
3. Discussion
Hispidin has been demonstrated to protect pancreatic β-cells from H2O2-induced damage through ROS scavenging activity [19]. Tu et al. have reported that hispidin inhibits ROS and nitric oxide (NO) production in adipocytes [24]. Park et al. have demonstrated the cytoprotective effect of hispidin on myotubes from oxidative-stress-induced injury by inhibiting oxidative stress and suppressing apoptosis [25]. However, the effects of hispidin on RPE cells to protect against H2O2-induced oxidative stress is not elucidated yet. Thus, the aim of the present study is to analyze the antioxidant effects of hispidin on H2O2-induced oxidative stress in RPE cells and to determine its underlying molecular mechanism of action. In this study, an MTT assay showed that hispidin does not have a toxic effect on ARPE-19 cells at concentrations less than 50 μM. Pre-treatment of hispidin significantly increases the viability of ARPE-19 cells from H2O2-induced oxidative injury. The increased cell viability in response to 2.5 μM was similar to that of 5 μM, 10 μM, and 20 μM. However, as the protective effect of hispidin against oxidative stress was significantly better at 20 μM, this concentration of hispidin was determined to be optimal for subsequent experiments in this study. These results indicate that hispidin exerts a cytoprotective effect on ARPE-19 cells exposed to H2O2-induced oxidative stress by enhancing cell viability. The chemical structure of hispidin is very similar to resveratrol, which is well-known as a strong antioxidant [26]. In the present study, hispidin, as well as resveratrol pre-treatment, reduced ROS production, as observed by DCFDA staining. Several antioxidant enzymes, including SOD, protect against ROS-induced damage in RPE cells [27]. However, hispidin up-regulated the expression of HO-1 and NQO-1, but not that of SODs (SOD1, SOD2, and catalase) in H2O2-treated ARPE-19 cells. These results suggest that the protective effects of hispidin are due to its role as a ROS scavenger, whereby it enhances the expression of antioxidant enzyme, thereby attenuating the oxidative damage.
Upon further investigating the possible pathways involved in mediating the cytoprotective ability of hispidin against oxidative stress in ARPE-19 cells, it was revealed that the downstream target genes of Nrf2-signaling HO-1 and NQO-1, play crucial roles in protecting the cells from oxidative stress [28,29]. Recent reports have indicated the beneficial effects of Nrf2 signaling on RPE cells [30,31]. Various studies have demonstrated that the activation of Nrf2/HO-1 signaling is essential for the reduction of the oxidative damage to RPE cells [32,33,34]. In this study, it was proposed that the anti-oxidative effects of hispidin might incorporate with Nrf2 signaling. Our data demonstrates that hispidin protects the ARPE-19 cells from H2O2-induced oxidative damage by activating the Nrf2-signaling pathway and inducing the expression of Nrf2, HO-1, NQO-1, GCLC, and GCLM. In addition, Nrf2 silencing attenuates the protective effects of hispidin and suppresses the expression of its downstream targets, HO-1 and NQO-1.
In the current study, activation of the JNK pathway appeared to be involved in the protective effect of hispidin against H2O2-induced oxidative stress. Our findings have revealed that hispidin treatment induces significant JNK phosphorylation, whereas treatment with a JNK inhibitor (SP600125) reduces the JNK phosphorylation and attenuates the cytoprotective effect of hispidin against H2O2 in ARPE-19 cells. Moreover, the ROS scavenging activity of hispidin was found to be abolished by SP600125 pre-treatment. Besides, hispidin-induced Nrf2/HO-1 expression was directly regulated by the JNK pathway. In addition, treatment with SP600125 reduced the hispidin-induced protein expression such as Nrf2, HO-1, and NQO-1. Therefore, to our knowledge, our study has for the first time demonstrated that naturally occurring hispidin isolated from P. linteus suppresses cellular damage and oxidative stress induced by H2O2 via JNK-Nrf2 dependent HO-1 expression in ARPE-19 cells.
The blood-retina barrier (BRB) is a physiological barrier that regulating material transport between the retina and circulating blood [35]. The development of the drug delivery system across the BRB is widely expected to improve the treatment of retinal diseases such as AMD and diabetic retinopathy. Since positively charged molecule could penetrate across the BRB [36,37], hispidin could be formulated into either cationic macromolecule or nanoparticles for further in vivo application. The cationic bio-degradable polymers are ideal candidates for hispidin formulation. Naturally derived biocompatible polymers, including chitosan, gelatin, dextran, and cellulose, were not only applied in gene delivery but also for various therapeutic purpose [38]. Besides, various phospholipid-based nano-formulated drugs have been approved in clinical use [39]. The cationic lipid was also applied to encapsulate the p53 gene for delivery and restoring the wild-type p53 protein in cancer treatment. According to the relative research, a cationic formulated particle system exhibits great potential for hispidin application, and the in vivo experiments are worthy of further investigation.
4. Conclusion
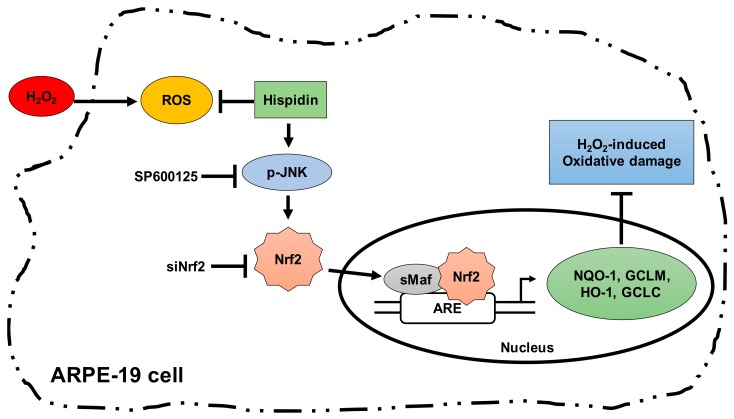
In conclusion, the present study presents the novel functions of hispidin that are responsible for protection of ARPE-19 cells against H2O2-induced oxidative damage by suppressing ROS levels. To our knowledge, our data has demonstrated for the first time that hispidin suppresses H2O2-induced oxidative stress and cell death through induction of Nrf2/HO-1 expression via a JNK-Nrf2-dependent pathway in ARPE-19 cells (Figure 6). These results suggest that hispidin has the potential to serve as a therapeutic candidate for AMD treatment or prevention.
Figure 6.
Schematic representation a proposed pathway for hispidin-induced Nrf2/ARE-mediated cytoprotective proteins. Up-regulation of Nrf2 target genes such as HO-1 explains the protective effects against H2O2-induced oxidative stress in ARPE-19 cells. ARE: Antioxidant response elements; sMaf: Small Maf (musculoaponeurotic fibrosarcoma).
5. Materials and Methods
5.1. ARPE-19 Cells
The ARPE-19 cell line was purchased from BCRC (Bioresource Collection and Research Center, Hsinchu, Taiwan), authenticated by short-tandem repeat analysis, and cultured in its standard medium, as recommended by the BCRC. The culture medium, fetal bovine serum, and supplements were all purchased from Invitrogen, Carlsbad, CA, USA. For cell viability and DCFDA assays, ARPE-19 cells were seeded at a density of 3 × 104 in 24-well plates. For real-time PCR and Western blot analyses, cells were seeded at a density of 3 × 105 in a 6 cm culture petri dish.
5.2. Chemicals and Reagents
The hispidin was first isolated from Polyporus hispidulus, and the production and structure elucidation came from the mycelial broth of Phellinus linteus [15]. It can also be synthesized [40]. In this study, hispidin (C13H10O5, 98%, soluble in DMSO at 20 mg/mL), MTT, and DCFDA were purchased from Sigma Chemical Co. (St. Louis, MO, USA). The Nrf2 (ab52352) antibody was purchased from Abcam (Cambridge, MA, USA), and all other antibodies were purchased from GeneTex Inc. (San Antonio, TX, USA).
5.3. Cell Viability Assay
The viability of the cells was evaluated using MTT assay, as described [41,42].
5.4. DCFDA Assay
ROS were detected using 2′,7′-dichlorofluorescein diacetate (DCFDA) assay. Cells were loaded with 5 μM DCFDA, followed by incubation for 6 h. Fluorescence signal at 535 nm (excitation at 482 nm) was measured using an Infinite 200 Pro TecanTM (Tecan, Mannedorf, Switzerland). The background fluorescence signal was measured immediately after addition of reagent.
5.5. Western Blot Analysis
5.6. Real-Time RT-PCR Analysis
Real-time RT-PCR analysis was performed as described [41,42]. Real-time RT-PCR primer sequences used in this study were GCLC F-5′-AAGCCATTCACTCCAGATTTTACC-3′, GCLM F-5′-ACTGACTTAGGAGCATAACTTACC-3′, GAPDH F-5′-CCATGGAGAAGGCTGGGG -3′, R-5′-CAAAGTTGTCATGGATGACC -3′, R-5′-AAGAATATCTGCCTCAATGACACC-3′, HO-1 F-5′- ATGACACCAAGGACCAGAGC-3′, R-5′- GTAAGGACCCATCGGAGAAGC-3′, R-5′-ACAACAAACTTCAACGCAAAGC-3′, NQO1 F-5′-TATCCTGCCGAGTCTGTTCTG-3′, and R-5′-AACTGGAATATCACAAGGTCTGC-3′.
5.7. Small Interfering RNA (siRNA) Transfection
For siRNA transfection, ARPE-19 cells were transfected with 20 or 40 nM siRNA, using RNAifect Transfection Reagent (QIAGEN) and analyzed 48 h post-transfection. Nrf2 siRNA were purchased from Dhamarcon RNAi Technologies (Lafayette, CO, USA). ON-TARGETplus SMARTpool Nrf2 siRNA sequences: (1) UAAAGUGGCUGCUCAGAAU, (2) GAGUUACAGUGUCUUAAUA, (3) UGGAGUAAGUCGAGAAGUA, and (4) CACCUUAUAUCUCGAAGUU.
5.8. Statistical Analysis
All data were analyzed using the Student’s t test for normally distributed values and by the nonparametric Mann–Whitney U test for values with a non-normal distribution as described [41,42].
Author Contributions
Conceptualization, S.-Y.H., S.-F.C. (Siu-Fung Chau), and S.-C.C.; data curation, S.-F.C. (Shu-Fang Chang) and S.-C.C.; formal analysis, S.-F.C. (Shu-Fang Chang) and S.-C.C.; funding acquisition, S.-Y.H. and S.-C.C.; investigation, S.-Y.H., S.-F.C. (Shu-Fang Chang), and S.-F.C. (Siu-Fung Chau); writing—original draft, S.-Y.H. and S.-C.C.; writing—review and editing, S.-F.C. (Siu-Fung Chau) and S.-C.C.
Funding
This work was supported by grants from the Hsinchu Mackay Memorial Hospital, Hsinchu, Taiwan (MMH-HB-10521), and Taichung Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Taichung, Taiwan (TTCRD105-17, TTCRD106-05).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Klein R., Klein B.E. The prevalence of age-related eye diseases and visual impairment in aging: Current estimates. Invest. Ophthalmol Vis. Sci. 2013 doi: 10.1167/iovs.13-12789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holz F.G., Schmitz-Valckenberg S., Fleckenstein M. Recent developments in the treatment of age-related macular degeneration. J. Clin. Invest. 2014;124:1430–1438. doi: 10.1172/JCI71029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarbin M.A., Casaroli-Marano R.P., Rosenfeld P.J. Age-related macular degeneration: Clinical findings, histopathology and imaging techniques. Dev. Ophthalmol. 2014;53:1–32. doi: 10.1159/000358536. [DOI] [PubMed] [Google Scholar]
- 4.Khandhadia S., Lotery A. Oxidation and age-related macular degeneration: Insights from molecular biology. Expert Rev. Mol. Med. 2010 doi: 10.1017/S146239941000164X. [DOI] [PubMed] [Google Scholar]
- 5.Mettu P.S., Wielgus A.R., Ong S.S., Cousins S.W. Retinal pigment epithelium response to oxidant injury in the pathogenesis of early age-related macular degeneration. Mol. Aspects Med. 2012;33:376–398. doi: 10.1016/j.mam.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Ho L., van Leeuwen R., Witteman J.C., van Duijn C.M., Uitterlinden A.G., Hofman A., de Jong P.T., Vingerling J.R., Klaver C.C. Reducing the genetic risk of age-related macular degeneration with dietary antioxidants, zinc, and omega-3 fatty acids: The Rotterdam study. Arch. Ophthalmol. 2011;129:758–766. doi: 10.1001/archophthalmol.2011.141. [DOI] [PubMed] [Google Scholar]
- 7.Tan J.S., Wang J.J., Flood V., Rochtchina E., Smith W., Mitchell P. Dietary antioxidants and the long-term incidence of age-related macular degeneration: The Blue Mountains Eye Study. Ophthalmology. 2008;115:334–341. doi: 10.1016/j.ophtha.2007.03.083. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen T., Nioi P., Pickett C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang M., An C., Gao Y., Leak R.K., Chen J., Zhang F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog. Neurobiol. 2013;100:30–47. doi: 10.1016/j.pneurobio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Z., Chen Y., Wang J., Sternberg P., Freeman M.L., Grossniklaus H.E., Cai J. Age-related retinopathy in NRF2-deficient mice. PLoS ONE. 2011 doi: 10.1371/journal.pone.0019456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vu K.T., Hulleman J.D. An inducible form of Nrf2 confers enhanced protection against acute oxidative stresses in RPE cells. Exp. Eye Res. 2017;164:31–36. doi: 10.1016/j.exer.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sachdeva M.M., Cano M., Handa J.T. Nrf2 signaling is impaired in the aging RPE given an oxidative insult. Exp. Eye Res. 2014;119:111–114. doi: 10.1016/j.exer.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu T., Kim S.H., Chen C.Y. A medicinal mushroom: Phellinus linteus. Curr. Med. Chem. 2008;15:1330–1335. doi: 10.2174/092986708784534929. [DOI] [PubMed] [Google Scholar]
- 14.Jung J.Y., Lee I.K., Seok S.J., Lee H.J., Kim Y.H., Yun B.S. Antioxidant polyphenols from the mycelial culture of the medicinal fungi Inonotus xeranticus and Phellinus linteus. J. Appl. Microbiol. 2008;104:1824–1832. doi: 10.1111/j.1365-2672.2008.03737.x. [DOI] [PubMed] [Google Scholar]
- 15.Park I.H., Chung S.K., Lee K.B., Yoo Y.C., Kim S.K., Kim G.S., Song K.S. An antioxidant hispidin from the mycelial cultures of Phellinus linteus. Arch. Pharm. Res. 2004;27:615–618. doi: 10.1007/BF02980159. [DOI] [PubMed] [Google Scholar]
- 16.Kim B.C., Jeon W.K., Hong H.Y., Jeon K.B., Hahn J.H., Kim Y.M., Numazawa S., Yosida T., Park E.H., Lim C.J. The anti-inflammatory activity of Phellinus linteus (Berk. & M.A. Curt.) is mediated through the PKCdelta/Nrf2/ARE signaling to up-regulation of heme oxygenase-1. J. Ethnopharmacol. 2007;113:240–247. doi: 10.1016/j.jep.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 17.Kim H.G., Yoon D.H., Lee W.H., Han S.K., Shrestha B., Kim C.H., Lim M.H., Chang W., Lim S., Choi S. Phellinus linteus inhibits inflammatory mediators by suppressing redox-based NF-kappaB and MAPKs activation in lipopolysaccharide-induced RAW 264.7 macrophage. J. Ethnopharmacol. 2007;114:307–315. doi: 10.1016/j.jep.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Chandimali N., Huynh D.L., Jin W.Y., Kwon T. Combination Effects of Hispidin and Gemcitabine via Inhibition of Stemness in Pancreatic Cancer Stem Cells. Anticancer Res. 2018;38:3967–3975. doi: 10.21873/anticanres.12683. [DOI] [PubMed] [Google Scholar]
- 19.Jang J.S., Lee J.S., Lee J.H., Kwon D.S., Lee K.E., Lee S.Y., Hong E.K. Hispidin produced from Phellinus linteus protects pancreatic beta-cells from damage by hydrogen peroxide. Arch. Pharm. Res. 2010;33:853–861. doi: 10.1007/s12272-010-0607-5. [DOI] [PubMed] [Google Scholar]
- 20.Lee J.H., Lee J.S., Kim Y.R., Jung W.C., Lee K.E., Lee S.Y., Hong E.K. Hispidin isolated from Phellinus linteus protects against hydrogen peroxide-induced oxidative stress in pancreatic MIN6N beta-cells. J. Med. Food. 2011;14:1431–1438. doi: 10.1089/jmf.2010.1493. [DOI] [PubMed] [Google Scholar]
- 21.Dunn K.C., Aotaki-Keen A.E., Putkey F.R., Hjelmeland L.M. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp. Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 22.Kaczara P., Sarna T., Burke J.M. Dynamics of H2O2 availability to ARPE-19 cultures in models of oxidative stress. Free Radic. Biol. Med. 2010;48:1064–1070. doi: 10.1016/j.freeradbiomed.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu W.C., Hu D.N., Gao H.X., Chen M., Wang D., Rosen R., McCormick S.A. Subtoxic levels hydrogen peroxide-induced production of interleukin-6 by retinal pigment epithelial cells. Mol. Vis. 2010;16:1864–1873. [PMC free article] [PubMed] [Google Scholar]
- 24.Be Tu P.T., Chompoo J., Tawata S. Hispidin and related herbal compounds from Alpinia zerumbet inhibit both PAK1-dependent melanogenesis in melanocytes and reactive oxygen species (ROS) production in adipocytes. Drug Discov. Ther. 2015;9:197–204. doi: 10.5582/ddt.2015.01038. [DOI] [PubMed] [Google Scholar]
- 25.Park J.M., Lee J.S., Song J.E., Sim Y.C., Ha S.J., Hong E.K. Cytoprotective effect of hispidin against palmitate-induced lipotoxicity in C2C12 myotubes. Molecules. 2015;20:5456–5467. doi: 10.3390/molecules20045456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin A.R., Villegas I., La Casa C., de la Lastra C.A. Resveratrol, a polyphenol found in grapes, suppresses oxidative damage and stimulates apoptosis during early colonic inflammation in rats. Biochem. Pharmacol. 2004;67:1399–1410. doi: 10.1016/j.bcp.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 27.Hu X., Wu X., Zhao B., Wang Y. Scutellarin protects human retinal pigment epithelial cells against hydrogen peroxide (H2O2)-induced oxidative damage. Cell Biosci. 2019 doi: 10.1186/s13578-019-0276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu Y., Duan M., Liang S., Wang Y., Feng Y. Senkyunolide I protects rat brain against focal cerebral ischemia-reperfusion injury by up-regulating p-Erk1/2, Nrf2/HO-1 and inhibiting caspase 3. Brain Res. 2015;1605:39–48. doi: 10.1016/j.brainres.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Vurusaner B., Gamba P., Gargiulo S., Testa G., Staurenghi E., Leonarduzzi G., Poli G., Basaga H. Nrf2 antioxidant defense is involved in survival signaling elicited by 27-hydroxycholesterol in human promonocytic cells. Free Radic. Biol. Med. 2016;91:93–104. doi: 10.1016/j.freeradbiomed.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Hu H., Hao L., Tang C., Zhu Y., Jiang Q., Yao J. Activation of KGFR-Akt-mTOR-Nrf2 signaling protects human retinal pigment epithelium cells from Ultra-violet. Biochem. Biophys. Res. Commun. 2018;495:2171–2177. doi: 10.1016/j.bbrc.2017.12.078. [DOI] [PubMed] [Google Scholar]
- 31.Wang K., Jiang Y., Wang W., Ma J., Chen M. Escin activates AKT-Nrf2 signaling to protect retinal pigment epithelium cells from oxidative stress. Biochem. Biophys. Res. Commun. 2015;468:541–547. doi: 10.1016/j.bbrc.2015.10.117. [DOI] [PubMed] [Google Scholar]
- 32.Arumugam B., Palanisamy U.D., Chua K.H., Kuppusamy U.R. Protective effect of myricetin derivatives from Syzygium malaccense against hydrogen peroxide-induced stress in ARPE-19 cells. Mol. Vis. 2019;25:47–59. [PMC free article] [PubMed] [Google Scholar]
- 33.Hu X., Liang Y., Zhao B., Wang Y. Thymoquinone protects human retinal pigment epithelial cells against hydrogen peroxide induced oxidative stress and apoptosis. J. Cell Biochem. 2019;120:4514–4522. doi: 10.1002/jcb.27739. [DOI] [PubMed] [Google Scholar]
- 34.Zhu C., Dong Y., Liu H., Ren H., Cui Z. Hesperetin protects against H2O2-triggered oxidative damage via upregulation of the Keap1-Nrf2/HO-1 signal pathway in ARPE-19 cells. Biomed. Pharmacother. 2017;88:124–133. doi: 10.1016/j.biopha.2016.11.089. [DOI] [PubMed] [Google Scholar]
- 35.Cunha-Vaz J.G. The blood-retinal barriers system. Basic concepts and clinical evaluation. Exp. Eye Res. 2004;78:715–721. doi: 10.1016/S0014-4835(03)00213-6. [DOI] [PubMed] [Google Scholar]
- 36.Kubo Y., Shimizu Y., Kusagawa Y., Akanuma S., Hosoya K. Propranolol transport across the inner blood-retinal barrier: Potential involvement of a novel organic cation transporter. J. Pharm. Sci. 2013;102:3332–3342. doi: 10.1002/jps.23535. [DOI] [PubMed] [Google Scholar]
- 37.Martini D., Monte M.D., Ristori C., Cupisti E., Mei S., Fiorini P., Filippi L., Bagnoli P. Antiangiogenic effects of beta2 -adrenergic receptor blockade in a mouse model of oxygen-induced retinopathy. J. Neurochem. 2011;119:1317–1329. doi: 10.1111/j.1471-4159.2011.07530.x. [DOI] [PubMed] [Google Scholar]
- 38.Samal S.K., Dash M., Van Vlierberghe S., Kaplan D.L., Chiellini E., van Blitterswijk C., Moroni L., Dubruel P. Cationic polymers and their therapeutic potential. Chem. Soc. Rev. 2012;41:7147–7194. doi: 10.1039/c2cs35094g. [DOI] [PubMed] [Google Scholar]
- 39.Bulbake U., Doppalapudi S., Kommineni N., Khan W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics. 2017;9:12. doi: 10.3390/pharmaceutics9020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonindard C., Bergonzi C., Denier C., Sergheraert C., Klaebe A., Chavant L., Hollande E. Synthetic hispidin, a PKC inhibitor, is more cytotoxic toward cancer cells than normal cells in vitro. Cell Biol. Toxicol. 1997;13:141–153. doi: 10.1023/A:1007321227010. [DOI] [PubMed] [Google Scholar]
- 41.Chiu S.C., Chiu T.L., Huang S.Y., Chang S.F., Chen S.P., Pang C.Y., Hsieh T.F. Potential therapeutic effects of N-butylidenephthalide from Radix Angelica Sinensis (Danggui) in human bladder cancer cells. BMC Complement. Altern. Med. 2017 doi: 10.1186/s12906-017-2034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang S.Y., Chang S.F., Liao K.F., Chiu S.C. Tanshinone IIA Inhibits Epithelial-Mesenchymal Transition in Bladder Cancer Cells via Modulation of STAT3-CCL2 Signaling. Int. J. Mol. Sci. 2017:1616. doi: 10.3390/ijms18081616. [DOI] [PMC free article] [PubMed] [Google Scholar]