Abstract
This systematic review provides a qualitative appraisal of 24 high-quality metabolomics-based studies published over the past decade exploring exercise-induced alterations of the human metabolome. Of these papers, 63% focused on acute metabolite changes following intense and prolonged exercise. The best studies utilized liquid chromatography mass spectrometry (LC-MS/MS) analytical platforms with large chemical standard libraries and strong, multivariate bioinformatics support. These studies reported large-fold changes in diverse lipid-related metabolites, with more than 100 increasing two-fold or greater within a few hours post-exercise. Metabolite shifts, even after strenuous exercise, typically return to near pre-exercise levels after one day of recovery. Few studies investigated metabolite changes following acute exercise bouts of shorter durations (< 60 min) and workload volumes. Plasma metabolite shifts in these types of studies are modest in comparison. More cross-sectional and exercise training studies are needed to improve scientific understanding of the human system’s response to varying, chronic exercise workloads. The findings derived from this review provide direction for future investigations focused on the body’s metabolome response to exercise.
Keywords: exercise, sports, metabolomics, metabolism
1. Introduction
Acute and chronic physical activity causes extensive adaptations in organs and systems, leading to health benefits [1]. Improvements in technology have allowed investigators to quantify these adaptations using a biological systems approach, overlaying gene information with transcriptomics, proteomics, and metabolomics [1,2,3,4,5,6,7,8,9,10]. Combined data from multi-omics approaches will improve scientific understanding regarding the complex modulating effect that physical activity has on the phenotype at the individual level and related molecular mechanisms.
Metabolomics is defined as the simultaneous measurement of numerous low molecular metabolites that participate as substrates, reactants, signaling agents, intermediates, and products of enzyme-mediated reactions [3,4]. Metabolites are the final endpoints of upstream biochemical processes, and closely reflect the expressed phenotype. With the support of advanced analytical platforms and bioinformatics, metabolomics data can provide valuable insights regarding the biological impact of physical activity, pharmacological treatment, nutritional interventions, and other exposures [3].
Global metabolomics procedures were first performed in the 1960s and 1970s when gas chromatography mass spectrometry (GC-MS) was used to measure human metabolites in blood and urine samples [4]. Despite this, metabolomics was considered an emerging field of scientific endeavor as late as 2010, the year when the earliest studies investigating exercise effects in human athletes were published [3]. Since then, a growing number of research groups have used metabolomics in exercise-based studies. This is due, in large part, to the widespread availability of mass spectrometry platforms, freely accessible online databases of metabolites such as the Human Metabolome Database (HMDB), the expansion of chemical standards libraries, and advanced bioinformatics support to analyze and make sense of the large volumes of data. The net effect has been an improved capacity to accurately detect a greater number of metabolites and then interpret the overall effect on the human metabolome in a wide variety of matrixes.
This systematic review provides a qualitative appraisal of metabolomics-based studies published during the past decade exploring exercise-induced alterations on the human metabolome. The conclusions derived from this review will provide an evidence-based framework for future investigations.
2. Results
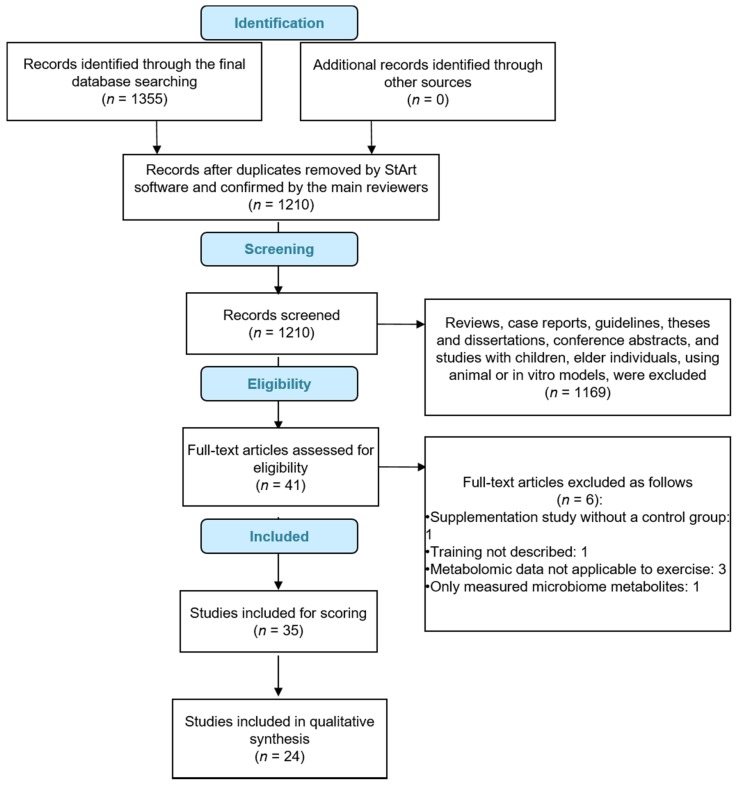
A total of 1355 articles were retrieved for this analysis. Of these, 1314 were excluded for not meeting analysis criteria after review of the abstracts. Of the 41 studies selected for full text examination, six were excluded for not meeting analysis criteria. Of the 35 studies included for scoring, 24 achieved a minimum score of 6, and were included in the final analysis (Table 1 and Figure 1).
Table 1.
Studies classification according to score system.
| Investigators, Year Published |
Research Design | Methodology | Novelty | Final Score | Classification | ||
|---|---|---|---|---|---|---|---|
| Subjects Number | Studies Characteristics | Analysis Methods | Statistical Support | ||||
| Nieman et al., 2015 [5] | 2 | 2 | 3 | 2 | 2 | 11 | Excellent |
| Jacobs et al., 2014 [6] | 2 | 2 | 3 | 2 | 2 | 11 | Excellent |
| Nieman et al., 2014 [7] | 2 | 2 | 3 | 1 | 2 | 10 | Excellent |
| Nieman et al., 2017 [8] | 2 | 1 | 3 | 2 | 2 | 10 | Excellent |
| Davison et al., 2018 [9] | 2 | 2 | 3 | 1 | 1 | 9 | Excellent |
| Hodgson et al., 2012 [10] | 0 | 2 | 3 | 2 | 2 | 9 | Excellent |
| Karl et al., 2017 [11] | 0 | 1 | 3 | 2 | 2 | 8 | Good |
| Lehman et al., 2010 [12] | 0 | 1 | 3 | 2 | 2 | 8 | Good |
| Lewis et al., 2010 [13] | 2 | 1 | 3 | 0 | 2 | 8 | Good |
| Nieman et al., 2013 [14] | 0 | 2 | 3 | 1 | 2 | 8 | Good |
| Al-Khelaifi et al., 2018 [15] | 2 | 0 | 3 | 2 | 1 | 8 | Good |
| Knab at al., 2013 [16] | 0 | 2 | 1 | 2 | 2 | 7 | Good |
| Manaf et al., 2018 [17] | 0 | 1 | 3 | 2 | 1 | 7 | Good |
| Messier et al., 2017 [18] | 2 | 1 | 1 | 2 | 1 | 7 | Good |
| Nieman et al., 2013 [19] | 0 | 1 | 3 | 1 | 2 | 7 | Good |
| Nieman et al., 2014 [20] | 0 | 1 | 3 | 1 | 2 | 7 | Good |
| Ra et al., 2014 [21] | 2 | 1 | 1 | 2 | 1 | 7 | Good |
| Stander et al., 2018 [22] | 2 | 1 | 1 | 2 | 1 | 7 | Good |
| Danaher et al., 2015 [23] | 0 | 1 | 1 | 2 | 2 | 6 | Good |
| Howe et al., 2018 [24] | 0 | 0 | 3 | 2 | 1 | 6 | Good |
| Neal et al., 2013 [25] | 0 | 1 | 1 | 2 | 2 | 6 | Good |
| Peake et al., 2014 [26] | 0 | 2 | 1 | 1 | 2 | 6 | Good |
| Pechlivanis et al., 2013 [27] | 0 | 1 | 1 | 2 | 2 | 6 | Good |
| Zafeiridis et al., 2016 [28] | 0 | 1 | 1 | 2 | 2 | 6 | Good |
| Muhsen Ali et al., 2016 [29] | 0 | 0 | 3 | 2 | 0 | 5 | Fair |
| Castro et al., 2019 [30] | 2 | 1 | 1 | 1 | 0 | 5 | Fair |
| Enea et al., 2010 [31] | 0 | 1 | 1 | 2 | 1 | 5 | Fair |
| Andersson Hall et al., 2015 [32] | 0 | 1 | 1 | 2 | 1 | 5 | Fair |
| Pechlivanis et al., 2010 [33] | 0 | 1 | 1 | 2 | 1 | 5 | Fair |
| Wang et al., 2015 [34] | 0 | 1 | 1 | 2 | 1 | 5 | Fair |
| Yan et al., 2009 [35] | 0 | 1 | 1 | 2 | 1 | 5 | Fair |
| Prado et al., 2017 [36] | 2 | 0 | 2 | 0 | 0 | 4 | Fair |
| Sun et al., 2017 [37] | 0 | 0 | 1 | 2 | 1 | 4 | Fair |
| Berton et al., 2017 [38] | 0 | 1 | 1 | 0 | 1 | 3 | Poor |
| Valério et al., 2018 [39] | 0 | 1 | 1 | 0 | 1 | 3 | Poor |
Figure 1.
Outcomes of review flow diagram.
2.1. Exercise Intensity and Duration Effects on Metabolism
Metabolic responses to exercise depend on the intensity and duration of effort. For the purposes of this review, heavy and moderate-intensity were differentiated using an intensity threshold of 60% of the oxygen uptake and heart rate reserve, and long and short-duration using a duration threshold of 60 min [40].
2.2. High-Intensity and Long-Duration
More than half of the studies included in this analysis (62.5%; n = 15) measured metabolite responses to long-duration, high-intensity running (n = 8) [8,9,12,13,14,19,22,24], cycling (n = 5) [5,7,17,18,20], soccer (n = 1), and swimming (n = 1) [16] (Table 2). Liquid chromatography mass spectrometry (LC-MS) with or without GC-MS was used for metabolite identification in 11 [5,7,8,9,12,13,14,17,19,20,24] of these studies, with GC-MS as the primary method in two studies [16,22], capillary electrophoresis time-of-flight mass spectrometry (CE-TOFMS) in one study [21] and nuclear magnetic resonance (NMR) for one study [18]. Large-fold changes in metabolites from the lipid super pathway were reported by most investigators, including increases in plasma medium- and long-chain fatty acids, fatty acid oxidation products (dicarboxylate and monohydroxy fatty acids, acylcarnitines), and ketone bodies, with corresponding decreases in triacylglycerol esters (Table 2). Other metabolite changes included shifts in amino acids and increases in energy tricarboxylic acid (TCA) cycle components.
Table 2.
High-intensity and long-duration studies.
| Investigators Year Published | Study Population | Analytical Platform/Matrix | Research Design | Key Findings Exercise Effect Separate from Other Interventions | Intensity |
|---|---|---|---|---|---|
| Nieman et al., 2015 [5] | 20 male cyclists (aged 39.2 ± 1.9 years) | UPLC-MS/MS; Plasma |
Randomized, cross-over design; three trials of a 75-km cycling protocol ingesting: water only, bananas and water, pears and water (2-week washout); blood samples timepoints: pre- and post-exercise (0-h, 1.5-h, 21-h) | 509 metabolites were chemically identified; ↑ ratio > 2-fold: 107 metabolites increased in the water only trial (exercise effects); ↑ ratio > 5-fold: 35 metabolites increased, all from the lipid super pathway, all significantly elevated 1.5-h post exercise, 8 only remained after 21-h post-exercise. | High-intensity, long-duration |
| Nieman et al., 2014* [7] | 19 male cyclists (aged 38.06 ± 1.6 years) | GC-MS and UHPLC-MS/MS; Plasma |
Randomized, cross-over design; two trials of a 75-km cycling protocol with pistachio or no pistachio supplementation (2-week washout); blood samples timepoints: pre- and post-exercise (0-h, 1.5-h, 21-h) | 423 metabolites were chemically identified; Exercise increased 167 metabolites; All but 26 of these metabolites were related to ↑ lipid and carnitine metabolism, with the largest fold changes seen for ketones, dicarboxylate fatty acids, and long chain fatty acids. | High-intensity, long-duration |
| Nieman et al., 2017 [8] | 24 trained male runners (aged 36.5 ± 1.8 years) | GC-MS and UHPLC-MS/MS; Plasma |
Repeated measures, ANOVA analysis, one group design; blood samples collected pre- and post-exercise (0-h), one bout run to exhaustion at 70%VO2max | 209 chemically identified metabolites changed with exercise, especially long and medium-chain ↑ fatty acids, ↑ fatty acids oxidation products (dicarboxylate and monohydroxy fatty acids and acylcarnitines), and ↑ ketone bodies. Minor relationship with ↑ IL-6. | High-intensity, long-duration |
| Davison et al., 2018 [9] | 24 healthy males (aged 28 ± 5 years) | LC-MS; Serum |
Double-blind, randomized, cross-over design; 60-min run 75% VO2max in hypoxia (FiO2 = 0.16%) (hypoxia chamber) and normoxia (FiO2 = 0.21%) (1-week washout); blood samples timepoints: pre- (after 30-min rest in hypoxia, normoxia), post-exercise (0 h, 3-h) | 27 metabolites, identified using internet databases, changed with exercise; Most related to ↑ lipid metabolism (several acylcarnitines molecules identified) and purine metabolism [↑adenine, ↑adenosine and ↓ (3 h after recovery) hypoxanthine]; ↑ 4.3-fold increase in 18 acylcarnitines post-exercise, above pre-exercise at 3-h recovery. | High-intensity, long-duration |
| Lehman et al., 2010 [12] | Healthy subjects; 1st study: n = 13 (32.6 ± 6.1 years) 2nd study: n = 8 (30.9 ± 5.8 years) | UPLC-qTOF-MS; Plasma |
Parallel group design; 1st study: treadmill run 60min at 75% VO2, blood samples timepoints: pre- and post-exercise (0-h, 3-h, 24-h); 2nd study: treadmill run > 120 min at 70%VIAT, blood samples timepoints: pre- (1h 45 min after breakfast) and post-exercise (0-h, 3-h, 24-h) | 10 metabolites, chemically identified, characterized the separation between the timepoints; Most part non-esterified free fatty acids; ↑ 9-fold increases in acylcarnitines. | High-intensity, long-duration |
| Lewis et al., 2010 [13] | 25 amateur runners (aged 42 ± 9 years) | LC-MS; Plasma |
Repeated measures, one group; Boston Marathon; blood samples time points: pre- and post-marathon | Metabolites chemically identified; ↑ in glycolysis, lipolysis, adenine nucleotide catabolism, and amino acid catabolism; ↑ indicators of glycogenolysis (glucose-6-phosphate and 3-phosphoglycerate), and small molecules that reflect oxidative stress (allantoin), and that modulate insulin sensitivity (niacinamide) | High-intensity, long-duration |
| Nieman et al., 2013 [14] | 35 long-distance male runners (supplemented group: aged 33.7 ± 6.8 years; placebo: aged 35.2 ± 8.7 years) | GC-MS and UHPLC-MS/MS; Serum |
Double-blind, parallel group design; 2-week supplementation (polyphenol-enriched protein) followed by a 3-day intensified exercise (2.5-h at 70%VO2max bouts); blood samples timepoints: pre- and post- 14-day supplementation, and immediately and 14-h after the 3rd day of running | 324 chemically identified metabolites that changed with 3-day period of exercise; ↑ metabolites related to fatty acid oxidation and ketogenesis including free fatty acids, acylcarnitines, 3-hydroxy-fatty acids, and dicarboxylic acids, amino acid and carbohydrate metabolism, energy production, nucleotides, and cofactors and vitamins. | High-intensity, long-duration |
| Knab et al., 2013 [16] | 9 elite male sprint and middle-distance swim athletes; 7 control subjects (healthy and exercised less than 150 min/week) (aged 24.6 ± 0.7 years) | GC-MS; Serum |
Randomized, crossover design, 10-day supplementation with juice (8 fl oz pre- and post-training) or non-juice, 10-d practice of 2-h swimming, approximately 5500-m swim interval training (3-week washout). Blood samples timepoints: pre- and post- each 10-days supplementation period and post-exercise (0-h) | 325 metabolites were chemically identified; No effects of juice on exercise-induced measures; ↑ Oxidative stress and ↓ antioxidant capacity in swimmers group compared to nonathletic control group; Metabolites that differed mostly related to substrate utilization and supplements used by the swimmers. Pre and post-exercise small but significant shift in metabolites related to substrate utilization: pyruvic acid, propanoic acid, d-fructose, mannose, n-acetylglutamine, norleucine, alloisoleucine, and d-glucuronic acid. | High-intensity, long-duration |
| Manaf et al., 2018 [17] | 18 healthy and recreationally active males (aged 24.7 ± 4.8 years) | LC-MS; Plasma |
Repeated measures, ANOVA analysis, one group design; time-to-exhaustion (81-min) cycling test at a workload 3 mM/l lactate; blood samples timepoints: pre-exercise, during exercise (10-min, before fatigue), point of exhaustion (immediately after fatigue), post-exercise (20-min after fatigue) | 80 metabolites identified using internet databases; 68 metabolites changed during exercise; ↑ Free-fatty acids and ↓ tryptophan contributed to differences in plasma metabolome at fatigue. | High-intensity, long-duration |
| Messier et al., 2017 [18] | 20 healthy male (aged 39 ± 4.3 years) | 1H NMR; Plasma |
Cross-over design; cycling 60-min at ventilatory threshold 1 at 70 rpm, at sea level and above 2150 m of the sea level (2-week washout); blood samples timepoints: pre- and post-exercise (0-h) | 18 metabolites identified using internet databases; ↓ glucose and free amino acid levels; No differences in lipid metabolism between altitudes; Fuel shift from lipid oxidation to carbohydrate oxidation at 2150 above sea level. | High-intensity, long-duration |
| Nieman et al., 2013 [19] | 15 runners (7 males, 8 females) (aged 35.2 ± 8.7 years) | GC-MS and UHPLC-MS/MS; Serum |
Cross-sectional design, 3-day period exercise (2.5 h per day running bouts at approximately 70% VO2max); blood samples timepoints: pre- and post-exercise (0-h, 14-h) | Metabolites chemically identified; ↑ ≥ 2-fold increases in 75 metabolites immediately post 3-day exercise period, 22 related to lipid/carnitine metabolism, 13 related to amino acid/peptide metabolism, 4 to hemoglobin/porphyrin metabolism, and 3 to Krebs cycle intermediates. After 14-h recovery: 50 of 75 metabolites still elevated. ↓ 22 metabolites post-exercise related to lysolipid and bile acid metabolism. | High-intensity, long-duration |
| Nieman et al., 2014* [20] | 19 male cyclists (aged 38.06 ± 1.6 years) | GC-MS and UHPLC-MS/MS; Plasma |
Repeated measures, ANOVA analysis, one group design; blood samples timepoints: pre- and post-exercise (0-h, 1.5-h, 21-h); 75-km cycling protocol | 221 chemically identified metabolites changed with exercise; all but 26 related to ↑ lipid and carnitine metabolism; largest fold changes seen for ↑ ketones, dicarboxylate fatty acids, and long chain fatty acids. | High-intensity, long-duration |
| Ra et al., 2014 [21] | 37 male soccer players (aged 20.6 ± 0.04 years) | CE-TOFMS; Saliva |
Repeated measures, ANOVA analysis, one group design; 3-day game program (90-min per day); saliva samples timepoints: pre-exercise (1-month before) and post-exercise (24-h after) | 144 metabolites chemically identified; ↑12 metabolites (e.g., 3-methylhistidine, glucose 1- and 6-phosphate, taurine, amino acids) related to muscle catabolism, glucose metabolism, lipid metabolism, amino acid metabolism and energy metabolism. | High-intensity, long-duration |
| Stander et al., 2018 [22] | 31 recreational marathon athletes (19 males and 12 females) (aged 41 ± 12 years) | GC-TOFMS; Serum |
Repeated measures, ANOVA analysis, one group design; 42-km marathon; blood samples timepoints: pre- and post-marathon (0-h) | 70 metabolites chemically identified; ↑ carbohydrates, fatty acids, tricarboxylic acid cycle intermediates, ketones, and ↓ amino acids; ↑odd-chain fatty acids and α-hydroxy acids. | High-intensity, long-duration |
| Howe et al., 2018 [24] | 9 male ultramarathon runners (aged 34 ± 7 years) | HILIC-MS; Plasma |
Repeated measures, ANOVA analysis, one group design; 80.5-km treadmill simulated ultramarathon run; blood samples timepoints: pre- and post-exercise (0-h) | 446 metabolites chemically identified; ↓ amino acids metabolism post-80.5 km; ↑ in the formation of medium-chain unsaturated, partially oxidized fatty acids and conjugates of fatty acids with carnitines. | High-intensity, long-duration |
UPLC-MS: ultra-performance liquid chromatography mass spectrometry; UHPLC-MS: ultra-high-performance liquid chromatography mass spectrometry; GC-MS: gas chromatography mass spectrometry; LC-MS: liquid chromatography mass spectrometry; UHPLC/Q-TOF MS: ultra-high-performance liquid chromatography quadrupole time-of-flight mass spectrometry; 1H NMR: proton nuclear magnetic resonance; CE-TOFMS: capillary electrophoresis time-of-flight mass spectrometry; HILIC-MS: hydrophilic interaction chromatography mass spectrometry; VO2max = maximal oxygen uptake; FiO2 = fraction of inspired oxygen; VIAT = velocity at individual anaerobic threshold. * References [7,20] were from the same study but the data sets provided additive information.
2.3. High-Intensity and Short-Duration, Moderate-Intensity and Short/Long-Duration, Cross-Sectional and Training Studies
(A) High-Intensity, Short-Duration
Two studies measured metabolite responses to high-intensity, short-duration (18 to 30 min) exercise in recreationally active males and soccer athletes [23,28] (Table 3). Metabolite data from these studies were derived from GC-MS and NMR analytical platforms. Relatively small post-exercise changes were reported for metabolites related to the TCA cycle and related bioenergetics pathways.
Table 3.
Summaries of study characteristics and findings from nine [6,10,11,15,23,25,26,27,28] studies using other types of exercise designs.
| Investigators Year Published | Study Population | Analytical Platform/Matrix | Research Design | Key Findings Exercise Effect Separate from Other Interventions | Intensity |
|---|---|---|---|---|---|
| Danaher et al., 2015 [23] | 7 active males (aged 22.9 ± 5.0 years) | GC-MS; Plasma |
Randomized, cross-over design; two supramaximal low volume high-intensity exercise protocols (1-week washout) (HIE); (1) HIE150%: 30 × 20 s cycling at 150% VO2peak, 40 s rest (348 ± 27W); (2) HIE300%: 30x 10s cycling at 300% VO2peak, 50 s rest (697 ± 54 W); blood samples timepoints: pre- and post-exercise (0-h, 1-h) | 55 chemically identified metabolites detected; HIE300% produced greater metabolic perturbations compared to HIE150%; Changes more pronounced during recovery than exercise, with ↑ glycolytic pathway and fatty acids and lipid metabolism. | High-intensity, short-duration |
| Zafeiridis et al., 2016 [28] | 9 healthy young men (aged 20.5 ± 0.7 years). Soccer training 4−5 times per week. | 1H NMR; Plasma |
Randomized, cross-over design; three running protocols (2-week washout): intense continuous (18-min, 80% of maximum aerobic velocity (MAV)), long-interval (29-min, 3 min at 95% of MAV, 3 min recovery at 35% of MAV) and short-interval (18-min, 30 s at 110% of MAV, 30 s recovery at 50% of MAV); blood sample timepoints: pre- and post-exercise (5-min). | 17 metabolites identified using internet databases; No detectable difference in metabolites; ↑ carbohydrate/lipid metabolism and activation of the TCA cycle in all three protocols. |
High-intensity, short-duration |
| Jacobs et al., 2014 [6] | 19 healthy physically active males (aged 21 ± 2 years) | GC-MS and LC-MS/MS; Plasma |
Double-blind, randomized, cross-over design; 6-day supplementation with decaffeinated green tea or placebo ingestion (28-day washout) 2-h before a 30 min cycle exercise at 55%VO2max | 152 chemically identified metabolites changed with exercise; ↑ metabolites related to adrenergic and energy metabolism (e.g., lactate, pyruvate, malate, succinate, glycerol, cortisol); ↓ 2-hydrxobutyrate. | Moderate-intensity, short-duration |
| Hodgson et al., 2012 [10] | 27 healthy physically active males (aged 22 ± 5 years) | GC-MS and LC-MS/MS; Plasma |
Double-blind, randomized, parallel design; 7-day supplementation with caffeinated green tea or placebo ingestion 2-h before 60-min cycle exercise at 50%VO2max | 238 metabolites chemically detected changed with exercise; ↑ ratio > 2: lactate, pyruvate, succinate, noradrenaline and glycerol; ↓ 2-hydroxybutyrate, trans-4-hydroxyproline, mannose, certain triacylglycerides (TAGs) and nicotinamide. | Moderate-intensity, long-duration |
| Karl et al., 2017 [11] | 25 male highly trained soldiers (aged 19.0 ± 1.0 years) | UPLC-MS/MS; Plasma |
Double-blind, randomized, parallel design; 4-day, 51-km cross-country ski march carrying 45 kg pack; blood sample timepoints: pre- and post-exercise (early completers: 8 to 10-h or late completers: 2 to 3-h). | 478 chemically identified metabolites changed pre- and post-exercise ↑ 88% of the free fatty acids; ↑ 91% of the acylcarnitines; ↓ 88% of the mono- and diacylglycerols detected within lipid metabolism pathways; Smaller ↑ 75% of the tricarboxylic acid cycle intermediates; ↑ 50% of the branched chain amino acid metabolites | Moderate-intensity, long-duration |
| Peake et al., 2014 [26] | 10 well-trained male cyclists and triathletes (aged 33.2 ± 6.7 years) | GC-MS; Plasma |
Randomized, cross-over design; HIIT (60-min, ≈ 82% VO2max,) and a moderate-intensity continuous exercise (MOD) (61-min, ≈ 67% VO2max); blood samples timepoints: pre- and post-exercise (0-h, 1-h, 2-h). | 49 metabolites chemically identified; 29 changed with exercise (11 changed with both HIIT and MOD; 13 changed with HIIT only; 5 changed with MOD only); ↑ in carbohydrate oxidation and ↓ in fat oxidation in HIIT exercise compared to MOD; Glucose and lactate higher at 0-h in HIIT compared to MOD. | High and moderate-intensity, long-duration |
| Al-Khelaifi et al., 2018 [15] | 191 elite athletes (171 males, 20 females) | UPLC-MS/MS; Serum |
Cross-sectional design using elite athletes from various sport disciplines being monitored for doping; blood samples collected IN or OUT competition (1 timepoint) | Metabolites chemically identified; ↑ Oxidative stress common to both high-power and high-endurance sports alike; ↑ steroids and polyamine pathways more prominent in endurance; ↑ sterols, adenine-containing purines, and energy metabolites most evident with power. | Cross-section elite athletes |
| Neal et al., 2013 [25] | 12 male cyclists (aged 36 ± 6 years) | 1H NMR; Urine |
Randomized, cross-over design; 6-week training of polarized training-intensity (80% low intensity, 0% moderate-intensity, 20% high-intensity) and a training-intensity distribution (57% low intensity, 43% moderate-intensity, 0% high-intensity) (4-week washout); urine samples timepoints: pre- and post- each training period. | Method used to identify metabolites not reported; metabolites identified as ↓ hippuric acid, ↑ creatinine, ↑ dimethylamine, ↑ 3-methylxanthine, ↓ hypoxanthine. | Chronic training, Low, moderate and high-intensity |
| Pechlivanis et al., 2013 [27] | 14 young moderately trained healthy males (aged 21 ± 2 years) | 1H NMR; Serum |
Randomized, parallel group design; two 8-week programs (3 sessions/week), two and three sets of two 80-m maximal runs (interval between runs: group A = 10 s; group B = 1 min), 20 min interval between sets; blood timepoints: pre- and post-training. | 18 chemically identified metabolites changed after training period; separation after training mainly due to ↓ lactate, ↓ pyruvate, ↑ methylguanidine, ↑ citrate, ↑ glucose, ↑ valine, ↑ taurine, ↑ trimethylamine N-oxide, ↑ choline-containing compounds, ↑ histidines, ↑ acetoacetate/acetone, ↓ glycoprotein acetyls, and ↓ lipids; no significant difference between training intervals. | Chronic training, high-intensity |
GC-MS: gas chromatography mass spectrometry; LC-MS: liquid chromatography mass spectrometry; UPLC-MS: ultra-performance liquid chromatography mass spectrometry; 1H NMR: proton nuclear magnetic ressonance; UPLC-MS: ultra-performance liquid chromatography mass spectrometry; HIE = high-intensity exercise; HIE150% = high-intensity exercise at 150% of VO2 peak; HIE300% = high-intensity exercise at 300% of VO2 peak; W = watts; MAV = maximum aerobic velocity; TCA = tricarboxylic acid cycle; HIIT: high-intensity interval training; MOD = moderate-intensity continous exercise.
(B) Moderate-Intensity, Short-Duration
One study reported metabolite responses following moderate-intensity, short-duration (30 min) cycling [6] (Table 3). Metabolites were identified using GC-MS and LC-MS/MS analytical platforms. Small-fold post-exercise changes were reported for metabolites linked to energy metabolism (lipolysis, glycolysis, TCA cycle intermediates, and catecholamines).
(C) Moderate-Intensity, Long-Duration
Two studies investigated metabolite responses to moderate-intensity, long-duration cycling and cross-country skiing [10,11]. Small to moderate post-exercise changes were reported for metabolites related to glycolytic and lipid pathways including free fatty acids, branched chain amino acids, acylcarnitines, mono- and diacylglycerols, and TCA intermediates.
(D) High- and Moderate-Intensity, Long-Duration
One study compared metabolite responses of high-intensity interval training (HIIT) and 60 min of moderate-intensity cycling (MOD) using GC-MS [26]. Small- to moderate-fold changes were reported for metabolites related to energy metabolism, and glycolytic and lipid pathways. HIIT compared to MOD induced higher post-exercise levels for glycolytic-related metabolites, with lower levels of lipid related metabolites.
(E) Cross-Section Elite Athletes
One study compared plasma metabolite levels in athletes from high- (n = 121) and moderate- (n = 70) endurance sports [15] (Table 3). Metabolomics was performed by ultra-performance liquid chromatography mass spectrometry (UPLC-MS/MS). The cross-sectional analysis showed some group differences, including higher levels for metabolites related to oxidative stress, fatty acid metabolism, steroid biosynthesis, and energy metabolism in high power and high endurance athletes. Plasma levels of metabolites related to steroid and polyamine pathways were more prominent in endurance athletes, with sterols, adenine-containing purines, and energy metabolites more evident in power athletes.
(F) Chronic Training, Low-, Moderate-, and High-Intensity
One study compared the chronic effects of cycling training at different intensities [25]. Plasma metabolites were measured with NMR, and only small group differences were reported in a few selected metabolites (hippuric acid, hypoxanthine, creatinine, dimethylamine, 3-methylxanthine).
(G) Chronic Training, High-Intensity
One study investigated the effects of chronic, high-intensity, short-duration running on plasma metabolite levels using NMR [27]. Small changes in selected metabolites were reported including lactate, pyruvate, TCA intermediates, and phospholipids.
3. Discussion
Advances in mass spectrometry since 2010 have led to an increasing number of metabolomics-based studies targeted on whole-body metabolite responses to varying acute and chronic exercise workloads. This systematic review of 24 high-quality papers published during the past decade revealed that the primary focus (63% of studies) has been on acute metabolite perturbations to long-duration, high-intensity aerobic exercise. Little information is available regarding metabolite changes coupled to acute bouts of exercise with lower workload volumes or those linked to long-term exercise training. The best studies utilized LC-MS/MS analytical platforms with large chemical standards libraries to identify and detect exercise-induced shifts in hundreds of metabolites. Strong bioinformatics support has improved predictive and descriptive modelling, discriminative variable selection, and the overall understanding of the body’s metabolome response to exercise.
This review indicates that a bout of prolonged and intensive exercise causes large-fold changes in numerous and diverse lipid-related metabolites [5,7,8,9,12,14,17,19,20,21,22,24]. In a typical study with human athletes exercising intensely for more than two hours, significant increases in at least 300 identified metabolites can be measured by LC-MS/MS analytical platforms, with more than 100 increasing twofold or greater [5,7,14,20,24]. This response includes post-exercise increases in plasma medium- and long-chain fatty acids, ketone bodies, fatty acid oxidation products, and sulfated bile acids. At the same time, related decreases occur in plasma triacylglycerol esters, primary and secondary bile acids, and minor phospholipids such as lysophosphatidylcholines and lysophosphatidylethanolamines [12,19,20,41]. Untargeted metabolomics has revealed post-exercise increases in both common (e.g., oleate/vaccinate, palmitate, linoleate, stearate, palmitoleate, myristate), and atypical fatty acids (adrenate, docosapentaenoate, dihomo-linolenate, dihomolinoleate, docosadienoate, and eicosenoate). The corresponding fatty acid oxidation signature includes acylcarnitines, 3-hydroxybutyrate (BHBA), and dicarboxylate and monohydroxy fatty acids. Other important shifts have been measured for plasma concentrations of tryptophan- and other amino acid-related metabolites, and energy tricarboxylic acid (TCA) cycle components including malate, aconitate, citrate, fumarate, succinate, and alpha-ketoglutarate [13,16,17,18,19,22,41].
Most of the changes in plasma metabolites after prolonged and intensive exercise reach their nadir within a few hours. Plasma deviations in many of these metabolites are still apparent, but largely abated, after one day of recovery [5,7,12,19,20,21]. The large and varied metabolite response to heavy exercise workloads reflects the physiological stress and diminished glycogen stores experienced by the participant [12,21,24,41].
An increasing number of studies are utilizing metabolomics to measure the influence of various nutritional interventions on metabolite perturbations during recovery from prolonged and intensive exercise [5,7,14,16,41,42]. Metabolomics is ideally suited to measuring the impact of nutritional interventions during acute exercise by simultaneously measuring and identifying shifts in hundreds of metabolites from diverse pathways. Emerging data indicate that carbohydrates from both sugar beverages and fruits such as bananas, and flavonoids from food and beverage sources such as blueberries and green tea, have a large effects on the human metabolome response to intense exercise workloads [3,5,14,16,42,43].
Relatively few studies have investigated exercise-induced metabolite changes following acute bouts with lower durations (< 60 min) and workload volumes [23,26]. Half of these studies performed metabolomics using GC-MS or NMR analytical platforms, limiting the number of identified metabolites and the usefulness of these data. As expected, post-exercise shifts in plasma metabolite levels are modest in comparison to high exercise volume workloads due to a moderated reliance on underlying carbohydrate and lipid substrate pathways.
More cross-sectional studies are needed to compare plasma and urine metabolite levels between sedentary and physically active individuals, and athletes from different sports. These studies could provide important information for future randomized, exercise training trials. Using a cross-sectional design, one study showed some metabolite differences between power and endurance athletic groups [15]. The athletes were not tested at the same time or in similar resting states, however, making it difficult to draw definitive conclusions.
Few randomized, exercise training studies have been conducted to investigate potential adaptations in the human metabolome [25,27]. These two studies employed different training protocols and study designs, and performed metabolomics using NMR, limiting the usefulness of these data. Future metabolomics-based randomized exercise training studies, especially when combined with genomics and proteomics outcomes, will improve scientific understanding of the human system’s response to varying exercise workloads [44].
4. Materials and Methods
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [45] and was preregistered in the International Prospective Register of Systematic Review (PROSPERO). To systematize the search and data extraction, a free standardized electronic tool called State of the Art through Systematic Review (StArt) [46] was used. The software StArt tracked duplicated studies during extraction, and this was confirmed with manual examination by the two main reviewers. The studies were selected, extracted and included independently by two researchers (CAS and EFS), and a third independent researcher (RMA) verified the inclusion process in order to solve any disagreement between the two main researchers.
4.1. Search Strategy
The electronic search was performed from inception to November 26th, 2018 and updated on April 10th, 2019. The articles were retrieved from the following electronic databases: PubMed (via National Library of Medicine), Science Direct, SCOPUS (Elsevier) and Web of Science. The MeSH terms were selected and combined according to analysis method (metabolomics) and mandatory activity (sports OR exercise). Moreover, the search strategy was limited to humans (population of interest), English language and clinical trial studies.
4.2. Eligibility Criteria for Inclusion
The abstracts were first examined and evaluated for the listed criteria. Studies were selected if metabolomics were utilized to measure exercise-induced changes in metabolites in healthy study participants using serum, plasma, saliva, or urine samples. Exercise-based studies with nutrition interventions were included, but this review only included data collected from the control groups. Reviews, case reports, guidelines, theses and dissertations, conference abstracts, and studies using animal or in vitro models were not included.
4.3. Data Extraction and Study Inclusion
The following data from the selected studies were extracted: name of the first author, year of publication, characteristics of participants and groups (population, sample size, groups, gender, age, physical activity level), research design elements (type of research, exercise mode, duration, and intensity), metabolomics procedures (analytical platform, metabolite data), and summary comments.
4.4. Studies Quality Assessment
The quality of the studies was assessed by two researchers (CAS and EFS) using a scoring system created for this analysis (see Table 4 and Figure 2).
Table 4.
Score setting for metabolomic studies quality assessment.
| Score Setting | |||
|---|---|---|---|
| Section | Maximum Score | Aspects | Score Attribution |
| Research Design | 2 | Number of Participants | Parallel Studies 0 – N < 20 2 – N > 20 Crossover Studies 0 – N < 13 2 – N > 13 |
| 2 | Study Characteristics | Randomized control group Proper matrix > 2 timepoints data collection Duration ≥ 3 week (chronic studies only) 0—None of the previous items 1—At least 2 of the first 3 criteria listed 3—All 3 of the first 3 criteria listed |
|
| Methodology | 3 | Analysis Methods | 3—LC-MS/MS with extensive standards 1—NMR 1H, limited standards 1—GC-MS, limited standards |
| 2 | Statistical Support | 0—simple univariate statistics 1—Univariate statistics + additional analyses to sort and group the data, and to control for confounding factors 2—Univariate statistics + PCA, OPLS-DA, PLS-DA, or similar advanced bioinformatics procedures |
|
| Novelty | 2 | New information in the literature | |
Figure 2.
Classification of studies based on the total score.
5. Conclusions and Future Directions
The first decade of metabolomics-based exercise studies, especially those utilizing sensitive LC-MS/MS analytical platforms with large chemical standards libraries and rigorous bioinformatics support, provided useful systems biology information on the biochemical mechanisms underlying exercise-induced effects on metabolism [13,47]. This area of scientific endeavor is still emerging, and much remains to be discovered, especially in the areas of the metabolite response to acute and chronic moderate exercise workloads. The sensitivity of the analytical platforms will continue to improve, expanding the number of small molecule metabolites that can be detected. These improvements in technology, coupled with improved quality control, bioinformatics support, the expansion of biochemical standards, and an emphasis on larger study groups of both genders, will improve the identification and quantitation of currently known and unknown metabolites in a variety of human matrixes. More emphasis is needed on the influence of activity reduction and physical inactivity on metabolite shifts. These improvements in study design and methodology will broaden our understanding of the influence of acute and chronic exercise on the human metabolome. An increasing number of studies, including the National Institutes of Health project, ’Molecular Transducers of Physical Activity in Humans’, will combine metabolomics with genetics, epigenetics, lipidomics, and proteomics to examine all aspects of the physiological, biochemical, and molecular response to both aerobic- and resistance-based exercise training interventions [44].
Acknowledgments
The authors would like to acknowledge the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil (CAPES, Postgraduate Program in Physiotherapy, grant: 001).
Author Contributions
C.A.S., D.C.N. and A.M.C. organized the study and created the scoring and classification system; C.A.S., D.C.N., E.F.S., R.M.A. searched, extracted the studies from the databases and selected for inclusion; D.C.N., C.A.S., E.F.S. wrote; D.C.N., C.A.S., E.F.S., R.M.A., A.M.C. edited and reviewed the study.
Funding
São Paulo Research Fundation—FAPESP (grant #2016/222157).
Conflicts of Interest
Authors declare no conflict of interest.
References
- 1.Grazioli E., Dimauro I., Mercatelli N., Wang G., Pitsiladis Y., Di Luigi L., Caporossi D. Physical activity in the prevention of human diseases: Role of epigenetic modifications. BMC Genom. 2017;18:802. doi: 10.1186/s12864-017-4193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wackerhage H., Smith J., Wisniewski D. Molecular Exercise Physiology. 1st ed. Oxford University Press; Oxford, UK: 2014. p. 1. [Google Scholar]
- 3.Nieman D.C., Mitmesser S.H. Potential impact of nutrition on immune system recovery from heavy exertion: A metabolomics perspective. Nutrients. 2017;9:513. doi: 10.3390/nu9050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunn W.B., Broadhurst D.I., Atherton H.J., Goodacre R., Griffin J.L. Systems level studies of mammalian metabolomes: The roles of mass spectrometry and nuclear magnetic resonance spectroscopy. Chem. Soc. Rev. 2011;40:387–426. doi: 10.1039/B906712B. [DOI] [PubMed] [Google Scholar]
- 5.Nieman D.C., Gillitt N.D., Sha W., Meaney M.P., John C., Pappan K.L., Kinchen J.M. Metabolomics-based analysis of banana and pear ingestion on exercise performance and recovery. J. Proteome Res. 2015;14:5367–5377. doi: 10.1021/acs.jproteome.5b00909. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs D.M., Hodgson A.B., Randell R.K., Mahabir-Jagessar-T K., Garczarek U., Jeukendrup A.E., Mela D.J., Lotito S. Metabolic response to decaffeinated green tea extract during rest and moderate-intensity exercise. J. Agric. Food Chem. 2014;62:9936–9943. doi: 10.1021/jf502764r. [DOI] [PubMed] [Google Scholar]
- 7.Nieman D.C., Scherr J., Luo B., Meaney M.P., Dréau D., Sha W., Dew D.A., Henson D.A., Pappan K.L. Influence of pistachios on performance and exercise-induced inflammation, oxidative stress, immune dysfunction, and metabolite shifts in cyclists: A randomized, crossover trial. PLoS ONE. 2014;9:e113725. doi: 10.1371/journal.pone.0113725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nieman D.C., Sha W., Pappan K.L. IL-6 linkage to exercise-induced shifts in lipid-related metabolites: A metabolomics-based analysis. J. Proteome Res. 2017;16:970–977. doi: 10.1021/acs.jproteome.6b00892. [DOI] [PubMed] [Google Scholar]
- 9.Davison G., Vinaixa M., McGovern R., Beltran A., Novials A., Correig X., McClean C. Metabolomic response to acute hypoxic exercise and recovery in adult males. Front. Physiol. 2018;9:1682. doi: 10.3389/fphys.2018.01682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodgson A.B., Randell R.K., Boon N., Garczarek U., Mela D.J., Jeukendrup A.E., Jacobs D.M. Metabolic response to green tea extract during rest and moderate-intensity exercise. J. Nutr. Biochem. 2013:325–334. doi: 10.1016/j.jnutbio.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Karl J.P., Margolis L.M., Murphy N.E., Carrigan C.T., Castellani J.W., Madslien E.H., Teien H.-K., Martini S., Montain S.J., Pasiakos S.M. Military training elicits marked increases in plasma metabolomic signatures of energy metabolism, lipolysis, fatty acid oxidation, and ketogenesis. Physiol. Rep. 2017;5:e13407. doi: 10.14814/phy2.13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehmann R., Zhao X., Weigert C., Simon P., Fehrenbach E., Fritsche J., Machann J., Schick F., Wang J., Hoene M., et al. Medium chain acylcarnitines dominate the metabolite pattern in humans under moderate intensity exercise and support lipid oxidation. PLoS ONE. 2010;5:e11519. doi: 10.1371/journal.pone.0011519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis G.D., Farrell L., Wood M.J., Martinovic M., Arany Z., Rowe G.C., Souza A., Cheng S., McCabe E.L., Yang E., et al. Metabolic signatures of exercise in human plasma. Sci. Transl. Med. 2010;2:33–37. doi: 10.1126/scitranslmed.3001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieman D.C., Gillitt N.D., Knab A.M., Shanely R.A., Pappan K.L., Jin F., Lila M.A. Influence of a polyphenol-enriched protein powder on exercise-induced inflammation and oxidative stress in athletes: A randomized trial using a metabolomics approach. PLoS ONE. 2013;8:e72215. doi: 10.1371/journal.pone.0072215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Khelaifi F., Diboun I., Donati F., Botrè F., Alsayrafi M., Georgakopoulos C., Suhre K., Yousri N.A., Elrayess M.A. A pilot study comparing the metabolic profiles of elite-level athletes from different sporting disciplines. Sports Med. Open. 2018;4:2. doi: 10.1186/s40798-017-0114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knab A.M., Nieman D.C., Gillitt N.D., Shanely R.A., Cialdella-Kam L., Henson D.A., Sha W. Effects of a flavonoid-rich juice on inflammation, oxidative stress, and immunity in elite swimmers: A metabolomics-based approach. Int. J. Sport Nutr. Exerc. Metab. 2013;23:150–160. doi: 10.1123/ijsnem.23.2.150. [DOI] [PubMed] [Google Scholar]
- 17.Manaf F.A., Lawler N., Peiffer J.J., Maker G.L., Boyce M.C., Fairchild T.J., Broadhurst D. Characterizing the plasma metabolome during and following a maximal exercise cycling test. J. Appl. Physiol. 2018;125:1193–1203. doi: 10.1152/japplphysiol.00499.2018. [DOI] [PubMed] [Google Scholar]
- 18.Messier F.M., Le Moyec L., Santi C., Gaston A.-F., Triba M.N., Roca E., Durand F. The impact of moderate altitude on exercise metabolism in recreational sportsmen: A nuclear magnetic resonance metabolomic approach. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2017;42:1135–1141. doi: 10.1139/apnm-2016-0717. [DOI] [PubMed] [Google Scholar]
- 19.Nieman D.C., Shanely R.A., Gillitt N.D., Pappan K.L., Lila M.A. Serum metabolic signatures induced by a three-day intensified exercise period persist after 14 h of recovery in runners. J. Proteome Res. 2013;12:4577–4584. doi: 10.1021/pr400717j. [DOI] [PubMed] [Google Scholar]
- 20.Nieman D.C., Shanely R.A., Luo B., Meaney M.P., Dew D.A., Pappan K.L. Metabolomics approach to assessing plasma 13- and 9-hydroxy-octadecadienoic acid and linoleic acid metabolite responses to 75-km cycling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;307:68–74. doi: 10.1152/ajpregu.00092.2014. [DOI] [PubMed] [Google Scholar]
- 21.Ra S.-G., Maeda S., Higashino R., Imai T., Miyakawa S. Metabolomics of salivary fatigue markers in soccer players after consecutive games. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2014;39:1120–1126. doi: 10.1139/apnm-2013-0546. [DOI] [PubMed] [Google Scholar]
- 22.Stander Z., Luies L., Mienie L.J., Keane K.M., Howatson G., Clifford T., Stevenson E.J., Loots D.T. The altered human serum metabolome induced by a marathon. Metabolomics Off. J. Metabolomic Soc. 2018;14:150. doi: 10.1007/s11306-018-1447-4. [DOI] [PubMed] [Google Scholar]
- 23.Danaher J., Gerber T., Wellard R.M., Stathis C.G., Cooke M.B. The use of metabolomics to monitor simultaneous changes in metabolic variables following supramaximal low volume high intensity exercise. Metabolomics. 2015;12:7. doi: 10.1007/s11306-015-0883-7. [DOI] [Google Scholar]
- 24.Howe C.C.F., Alshehri A., Muggeridge D., Mullen A.B., Boyd M., Spendiff O., Moir H.J., Watson D.G. Untargeted metabolomics profiling of an 80.5 km simulated treadmill ultramarathon. Metabolites. 2018;8:14. doi: 10.3390/metabo8010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neal C.M., Hunter A.M., Brennan L., O’Sullivan A., Hamilton D.L., De Vito G., Galloway S.D.R. Six weeks of a polarized training-intensity distribution leads to greater physiological and performance adaptations than a threshold model in trained cyclists. J. Appl. Physiol. 2013;114:461–471. doi: 10.1152/japplphysiol.00652.2012. [DOI] [PubMed] [Google Scholar]
- 26.Peake J.M., Tan S.J., Markworth J.F., Broadbent J.A., Skinner T.L., Cameron-Smith D. Metabolic and hormonal responses to isoenergetic high-intensity interval exercise and continuous moderate-intensity exercise. Am. J. Physiol. Endocrinol. Metab. 2014;307:539–552. doi: 10.1152/ajpendo.00276.2014. [DOI] [PubMed] [Google Scholar]
- 27.Pechlivanis A., Kostidis S., Saraslanidis P., Petridou A., Tsalis G., Veselkov K., Mikros E., Mougios V., Theodoridis G.A. 1H NMR study on the short- and long-term impact of two training programs of sprint running on the metabolic fingerprint of human serum. J. Proteome Res. 2013;12:470–480. doi: 10.1021/pr300846x. [DOI] [PubMed] [Google Scholar]
- 28.Zafeiridis A., Chatziioannou A.C., Sarivasiliou H., Kyparos A., Nikolaidis M.G., Vrabas I.S., Pechlivanis A., Zoumpoulakis P., Baskakis C., Dipla K., et al. Global metabolic stress of isoeffort continuous and high intensity interval aerobic exercise: A comparative 1H NMR metabonomic study. J. Proteome Res. 2016;15:4452–4463. doi: 10.1021/acs.jproteome.6b00545. [DOI] [PubMed] [Google Scholar]
- 29.Muhsen Ali A., Burleigh M., Daskalaki E., Zhang T., Easton C., Watson D.G. Metabolomic profiling of submaximal exercise at a standardized relative intensity in healthy adults. Metabolites. 2016;6:9. doi: 10.3390/metabo6010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castro A., Duft R.G., Ferreira M.L.V., de Andrade A.L.L., Gáspari A.F., de Marchi Silva L., de Oliveira-Nunes S.G., Cavaglieri C.R., Ghosh S., Bouchard C., et al. Association of skeletal muscle and serum metabolites with maximum power output gains in response to continuous endurance or high-intensity interval training programs: The TIMES study—A randomized controlled trial. PLoS ONE. 2019;14:e0212115. doi: 10.1371/journal.pone.0212115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Enea C., Seguin F., Petitpas-Mulliez J., Boildieu N., Boisseau N., Delpech N., Diaz V., Eugène M., Dugué B. 1H NMR-based metabolomics approach for exploring urinary metabolome modifications after acute and chronic physical exercise. Anal. Bioanal. Chem. 2010;396:1167–1176. doi: 10.1007/s00216-009-3289-4. [DOI] [PubMed] [Google Scholar]
- 32.Andersson Hall U., Edin F., Pedersen A., Madsen K. Whole-body fat oxidation increases more by prior exercise than overnight fasting in elite endurance athletes. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2016;41:430–437. doi: 10.1139/apnm-2015-0452. [DOI] [PubMed] [Google Scholar]
- 33.Pechlivanis A., Kostidis S., Saraslanidis P., Petridou A., Tsalis G., Mougios V., Gika H.G., Mikros E., Theodoridis G.A. 1H NMR-based metabonomic investigation of the effect of two different exercise sessions on the metabolic fingerprint of human urine. J. Proteome Res. 2010;9:6405–6416. doi: 10.1021/pr100684t. [DOI] [PubMed] [Google Scholar]
- 34.Wang F., Han J., He Q., Geng Z., Deng Z., Qiao D. Applying (1)H NMR spectroscopy to detect changes in the urinary metabolite levels of Chinese half-pipe snowboarders after different exercises. J. Anal. Methods Chem. 2015;2015:315217. doi: 10.1155/2015/315217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan B., A J., Wang G., Lu H., Huang X., Liu Y., Zha W., Hao H., Zhang Y., Liu L., et al. Metabolomic investigation into variation of endogenous metabolites in professional athletes subject to strength-endurance training. J. Appl. Physiol. Bethesda Md 1985. 2009;106:531–538. doi: 10.1152/japplphysiol.90816.2008. [DOI] [PubMed] [Google Scholar]
- 36.Prado E., Souza G.H.M.F., Pegurier M., Vieira C., Lima-Neto A.B.M., Assis M., Guedes M.I.F., Koblitz M.G.B., Ferreira M.S.L., Macedo A.F., et al. Non-targeted sportomics analyses by mass spectrometry to understand exercise-induced metabolic stress in soccer players. Int. J. Mass Spectrom. 2017;418:1–5. doi: 10.1016/j.ijms.2017.02.002. [DOI] [Google Scholar]
- 37.Sun T., Wu Y., Wu X., Ma H. Metabolomic profiles investigation on athletes’ urine 35 minutes after an 800-meter race. J. Sports Med. Phys. Fitness. 2017;57:839–849. doi: 10.23736/S0022-4707.17.06254-5. [DOI] [PubMed] [Google Scholar]
- 38.Berton R., Conceição M.S., Libardi C.A., Canevarolo R.R., Gáspari A.F., Chacon-Mikahil M.P.T., Zeri A.C., Cavaglieri C.R. Metabolic time-course response after resistance exercise: A metabolomics approach. J. Sports Sci. 2017;35:1211–1218. doi: 10.1080/02640414.2016.1218035. [DOI] [PubMed] [Google Scholar]
- 39.Valério D.F., Berton R., Conceição M.S., Canevarolo R.R., Chacon-Mikahil M.P.T., Cavaglieri C.R., Meirelles G.V., Zeri A.C., Libardi C.A. Early metabolic response after resistance exercise with blood flow restriction in well-trained men: A metabolomics approach. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2018;43:240–246. doi: 10.1139/apnm-2017-0471. [DOI] [PubMed] [Google Scholar]
- 40.Nieman D.C., Wentz L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019;8:201–217. doi: 10.1016/j.jshs.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nieman D.C., Gillitt N.D., Sha W. Identification of a select metabolite panel for measuring metabolic perturbation in response to heavy exertion. Metabolomics. 2018;14:147. doi: 10.1007/s11306-018-1444-7. [DOI] [PubMed] [Google Scholar]
- 42.Nieman D.C., Gillitt N.D., Sha W., Esposito D., Ramamoorthy S. Metabolic recovery from heavy exertion following banana compared to sugar beverage or water only ingestion: A randomized, crossover trial. PLoS ONE. 2018;13:e0194843. doi: 10.1371/journal.pone.0194843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nieman D.C., Gillitt N.D., Henson D.A., Sha W., Shanely R.A., Knab A.M., Cialdella-Kam L., Jin F. Bananas as an energy source during exercise: A metabolomics approach. PLoS ONE. 2012;7:e37479. doi: 10.1371/journal.pone.0037479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sparks L.M. Exercise training response heterogeneity: Physiological and molecular insights. Diabetologia. 2017;60:2329–2336. doi: 10.1007/s00125-017-4461-6. [DOI] [PubMed] [Google Scholar]
- 45.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fabbri S., Silva C., Hernandes E., Octaviano F., Di Thommazo A., Belgamo A. Improvements in the StArt tool to better support the systematic review process; Proceedings of the 20th International Conference on Evaluation and Assessment in Software Engineering; Limerick, Ireland. 1–3 June 2016; New York, NY, USA: ACM; 2016. [Google Scholar]
- 47.Heaney L.M., Deighton K., Suzuki T. Non-targeted metabolomics in sport and exercise science. J. Sports Sci. 2017;37:959–967. doi: 10.1080/02640414.2017.1305122. [DOI] [PubMed] [Google Scholar]