Abstract
Since vegetable oils (usually triacylglycerol [TAG]) are extensively used as food and raw materials, an increase in storage oil content and production of valuable polyunsaturated fatty acids (PUFAs) in transgenic plants is desirable. In this study, a gene encoding glycerol-3-phosphate acyltransferase 9 (GPAT9), which catalyzes the synthesis of lysophosphatidic acid (LPA) from a glycerol-3-phosphate and acyl-CoA, was isolated from Physcomitrella patens, which produces high levels of very-long-chain PUFAs in protonema and gametophores. P. patens GPAT9 shares approximately 50%, 60%, and 70% amino acid similarity with GPAT9 from Chlamydomonas reinhardtii, Klebsormidium nitens, and Arabidopsis thaliana, respectively. PpGPAT9 transcripts were detected in both the protonema and gametophores. Fluorescent signals from the eYFP:PpGPAT9 construct were observed in the ER of Nicotiana benthamiana leaf epidermal cells. Ectopic expression of PpGPAT9 increased the seed oil content by approximately 10% in Arabidopsis. The levels of PUFAs (18:2, 18:3, and 20:2) and saturated FAs (16:0, 18:0, and 20:0) increased by 60% and 43%, respectively, in the storage oil of the transgenic seeds when compared with the wild type. The transgenic embryos with increased oil content contained larger embryonic cells than the wild type. Thus, PpGPAT9 may be a novel genetic resource to enhance storage oil yields from oilseed crops.
Keywords: arabidopsis, glycerol-3-phosphate acyltransferase, GPAT9, polyunsaturated fatty acids, Physcomitrella patens, storage oil, triacylglycerol
1. Introduction
In plants, triacylglycerol (TAG) is an energy-rich storage compound in which three fatty acid molecules are esterified to glycerol [1,2]. TAGs obtained mainly from seeds and fruits are used as edible oils, such as frying oils, and salad dressing or as raw materials to produce detergents, lubricants, paints, linoleum, coatings, and biodiesel [2,3]. In addition, global warming and depletion of fossil fuels have led to increased production of vegetable oils as alternative and sustainable fuels and biomaterial resources [4,5,6]. In particular, the production of vegetable oils with enhanced polyunsaturated fatty acids (PUFAs), which are essential for human health and nutrition, has been a major area of focus in the field of plant biotechnology [7,8].
De novo fatty acid biosynthesis occurs exclusively in plastids, and glycerolipids are synthesized via prokaryotic pathway in the plastids and eukaryotic pathway in the endoplasmic reticulum (ER) [9]. The synthesized fatty acyl-acyl carrier protein (ACP) or acyl-CoA is esterified to glycerol-3-phosphate by glycerol-3-phosphate acyltransferase (GPAT) [10,11,12]. The produced lysophosphatidic acid is subsequently converted to phosphatidic acid (PA) via acylation by lysophosphatidic acid acyltransferase [13]. In plastids, PA is used for the synthesis of phosphatidyl glycerol (PG) or dephosphorylated to diacylglycerol (DAG), which is the precursor of galactolipid and sulfolipid syntheses, by PA phosphatase (PAP) [14]. The 16:0 and 18:1 fatty acids on PG, monogalactosyl diacylglycerol (MGDG), and digalactosyl diacylglycerol (DGDG) are desaturated by fatty acid desaturase (FAD)4, FAD5, FAD6, FAD7, or FAD8 [15,16,17,18,19]. In the ER, PA, which is the common precursor for the synthesis of phospholipids, is converted to DAG by PP for the synthesis of phosphatidylcholine (PC), phosphatidylethanolamine (PE), and phosphatidylserine (PS) or cytidine diphosphate (CDP)-DAG by CDP-diacylglycerol synthetase for the synthesis of phosphatidylinositol (PI) [14,20,21]. DAG is acylated by acyl-CoA to TAG by diacylglycerol acyltransferase (DGAT) [22]. Phospholipid:diacylglycerol acyltransferase (PDAT) also contributes TAG biosynthesis by transferring the acyl-CoA from PC to DAG [23]. The C18:1 fatty acid on PC is further desaturated to C18:2 and C18:3 by FAD2 and FAD3 [24,25], respectively, or C16:0- and C18:0-CoAs are further elongated to very long chain FAs by the fatty acid elongase complex, which is composed of β-ketoacyl-CoA synthase, β-ketoacyl-CoA reductase, β-hydroxy acyl-CoA dehydratase, and enoyl-CoA reductase [26].
Since the activity of GPAT, which catalyzes the committed step in the synthesis of glycerolipids, has been identified in liver cell extracts of the guinea pig (Cavia porcellus) [27] and microsomal fraction of avocado (Persea americana) mesocarps [28], the presence of GPATs has been reported in various organisms, including bacteria, fungi, diatoms, and plants [29,30,31,32]. Recently, in vivo functions of GPAT9, which is the homolog of a microsomal GPAT involved in the production of storage oil in mammalian cells, were characterized in Arabidopsis [11]. Loss-of-function mutant atgpat9 displayed a lethal embryo phenotype, suggesting the essential role of GPAT9 in membrane lipid synthesis [11]. Total seed oil content was reduced by 26 to 44% in seed-specific GPAT9 knockdown Arabidopsis plants generated by the expression of artificial microRNA of GPAT9, indicating that GPAT9 is involved in TAG synthesis in Arabidopsis seeds [11]. In addition, AtGPAT9 exhibits sn-1 acyltransferase activity with preferential specificity for acyl-CoAs in the microsomal fractions prepared from yeast cells expressing AtGPAT9 [33]. Recently, GPAT9-type GPAT overexpression greatly enhances TAG accumulation in red alga (Cyanidioschyzon merolae) [34]. Overexpression of AtGPAT9 increased polar lipid and TAG levels in the leaves and enhanced lipid droplet accumulation in the pollen grains of Arabidopsis [33], suggesting that GPAT9 can be a novel genetic resource for the enhancement of storage oil yield from seeds or vegetative tissues.
The moss Physcomitrella patens is one of the earliest land plants, and it is a lower plant model. In the life cycle of P. patens, the haploid spore forms filamentous protonema with two distinct chloronema and caulonema cell types. The protonema forms buds, which are able to generate gametophores, shoot-like stems bearing phylloids, and rhizoids [35,36]. Female and male sex organs are formed at the tip of the gametophore, motile spermatozoids are fertilized with the egg cell, and then the diploid sporophyte develops from the zygote. The haploid spores are generated by meiosis in an apical spore capsule of the sporophyte. In particular, P. patens protonema and gametophore possess approximately 65% long-chain (LC)-PUFAs and very-long-chain (VLC)-PUFAs, indicating that characterization of genes encoding enzymes involved in LC- and VLC-PUFA biosyntheses may be important in the production of PUFAs in transgenic plants [36,37,38].
In this study, a gene encoding GPAT9, which catalyzes the first step in the synthesis of membrane lipids and TAG, was isolated from P. patens by using homology searches with the deduced amino acid sequences of Arabidopsis GPAT9. The deduced amino acid sequences of GPAT9 from P. patens and other plant species were compared to examine the genetic diversity of GPAT in plants. To investigate the role of the isolated PpGPAT9, its mRNA expression pattern in the gametophore and protonema and subcellular localization of eYFP:PpGPAT9 were examined. An increase in the size of the seeds and embryonic cells and in seed mass was observed in transgenic Arabidopsis lines ectopically expressing PpGPAT9 driven by the CaMV 35S promoter. Finally, ectopic expression of PpGPAT9 increased the total seed oil content, with significantly higher levels of PUFAs in the transgenic Arabidopsis. These results indicate that PpGPAT9 may be used for the enhanced production of storage oils in transgenic oilseed crops.
2. Results
2.1. Isolation of GPAT9 From P. patens
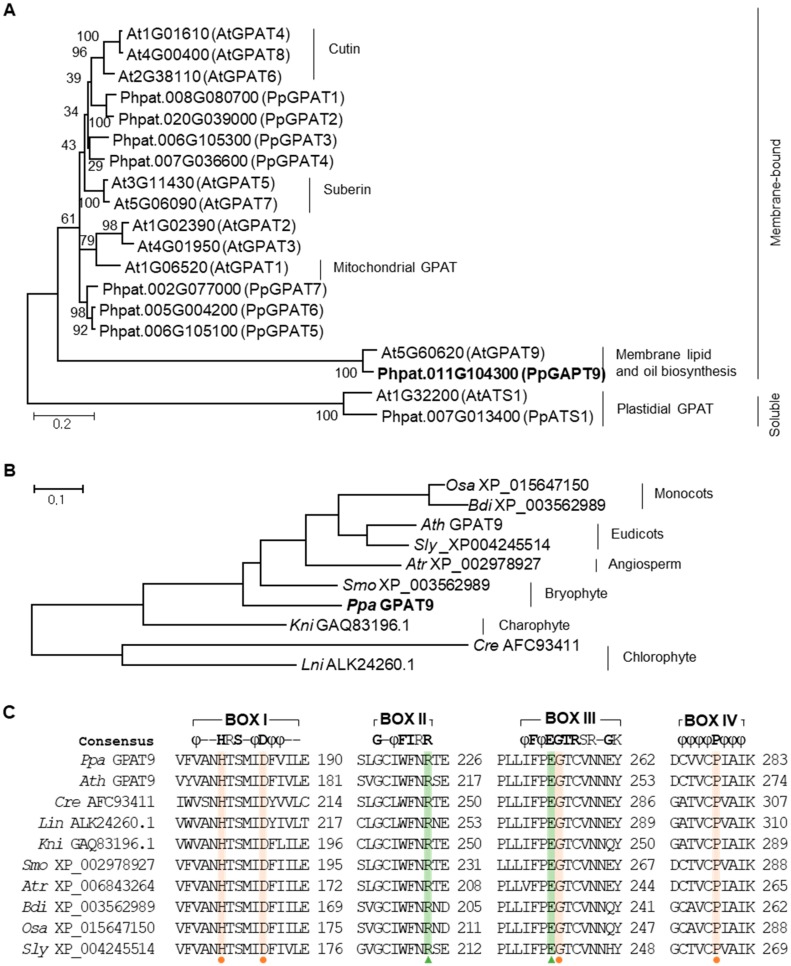
BLAST was used to isolate GPAT9 from P. patens with the deduced amino acid sequence of Arabidopsis GPAT9, which is functionally characterized [11,33] at the Phytozome v12.1 public database (https://www.phytozome.net; e-value ≤ 1 × 10−5). Ten genes encoding GPAT were present in the genome of P. patens. When a phylogenetic tree of Arabidopsis and P. patens GPATs was constructed (Figure 1A), plastidial soluble GPAT/ATS1 was initially classified with the other membrane-bound GPAT groups. GPAT9, involved in membrane lipid and TAG biosyntheses, was further classified with other GPATs, including GPATs required for the synthesis of cutin (AtGPAT4, AtGPAT8, and AtGPAT6), suberin (AtGPAT5 and AtGPAT7), and functions in the mitochondria (AtGPAT1) [11,33,39,40,41,42,43].
Figure 1.
Phylogenetic trees for PpGPATs and AtGPATs (A) and PpGPAT9 and GPAT9s from other organisms (B), and conserved acyltransferase motifs (C) of GPAT9s. (A) The phylogenetic trees were constructed using the MEGA6 program and amino acid sequences of A. thaliana and P. patens GPATs obtained from the Phytozome v11.0 database (https://phytozome.jgi.doe.gov/) by using BLAST with a cutoff e-value of 1 × 10−5. (B) Homologs of PpGPAT9 were isolated from the genome database by using BLAST with a cutoff e-value of 1e-5 in Phytozome v12.1 (https://www.phytozome.net), NCBI (https://ncbi.nlm.nih.gov/), and UniProt (http://www.uniprot.org). Phylogenetic analysis was conducted using the Maximum Likelihood method in MEGA6 with multiple amino acid sequences. PpGPAT9 indicated in bold was used in this study. (C) Amino acid sequence alignment of GPAT9 isoforms from P. patens and other organisms by using CLUSTALW. Four conserved acyltransferase motifs (BOX I–IV) are shown in colored boxes, according to Lewin et al. [44]. The amino acid residues indicated using orange circles in BOX I, BOX III, and BOX IV are required for GPAT catalysis. Hydrophobic amino acids are represented by “φ.” The following amino acids are considered hydrophobic: V, I, L, F, W, Y, and M. The amino acid residues indicated using green triangles in BOX II and BOX III are required for binding glycerol-3-phosphate. Ppa GPAT9 (Physcomitrella patens XP_024389305), Ath GPAT9 (Arabidopsis thaliana NP_568925), Cre AFC93411 (Chlamydomonas reinhardtii), Lin ALK24260 (Lobosphaera incisa), Kni GAQ83196.1 (Klebsormidium nitens), Smo XP_002978927 (Selaginella moellendorffii), Atr XP_006843264 (Amborella trichopoda), Bdi XP_003562989 (Brachypodium distachyon), Osa XP_015647150 (Oryza sativa), and Sly XP_004245514 (Solanum lycopersicum) are GenBank accession numbers.
In addition, P. patens GPAT9 homologues were isolated from algae (Lobosphaera incisa, Chlamydomonas reinhardtii, and Klebsormidium nitens) and lower (Selaginella moellendorffii) and higher (Amborella trichopoda, Solanum lycopersicum, Arabidopsis thaliana, Brachypodium distachyon, and Oryza sativa) plants to examine the genetic diversity of GPAT9. In a phylogenetic analysis of PpGPAT and other GPAT homologues (Figure 1B), GPAT9 is evolutionarily conserved and a single-copy gene in the examined plant species. The four conserved motifs (BOX I to IV) in GPAT9 are shown in Figure 1C, and the amino acid residues, His and Asp in BOX I, Gly in BOX III, and Pro in BOX IV, which are essential for the E. coli GPAT enzyme activity assay [44], are highly conserved in plant GPAT9s. Although four amino acid residues, Phe and Arg in BOX II and Glu and Ser in BOX III, are involved in the binding of the glycerol-3-phosphate substrate in E. coli [44], only two, Arg and Glu, residues were conserved in plant GPAT9s (Figure 1C).
2.2. Expression of PpGPAT9 in the Protonema and Gametophores
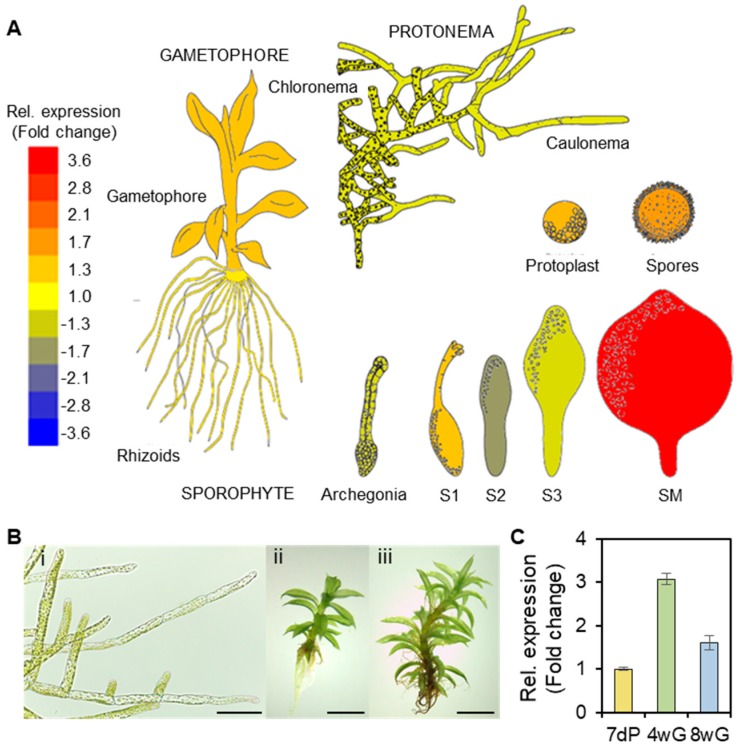
To investigate the expression patterns of PpGPAT9 during the life cycle of P. patens, the expression of PpGPAT9 was analyzed in the Physcomitrella eFP browser (http://bar.utoronto.ca/efp_physcomitrella/cgi-bin/efpWeb.cgi). When the relative expression of PpGPAT9 (Pp1s138_27V6.1, Phpat.011G104300) vs. PpActin2 (PpACT2, Pp1s198_157V6.1, Phpat.003G133100) was calculated, the highest expression of PpGPAT9 was detected in the mature sporophyte (SM), followed by the spores. The expression of PpGPAT9 relative to PpActin2 was approximately 1.2-, 1.3-, 1.4-, and 1.4-fold higher in the rhizoids, protoplasts, gametophores, and S1 sporophyte stage, but approximately 0.6- to 0.8-fold lower in the protonema, archegonia, and S2 and S3 sporophyte stages (Figure 2A); this indicated that PpGPAT9 was ubiquitously expressed in all the tested P. patens organs. To examine PpGPAT9 transcript levels, quantitative RT-PCR was performed using 7-day-old protonema and 4- and 8-week-old gametophores (Figure 2B). The PpGPAT9 transcript levels were approximately 3-fold and 1.6-fold higher in 4-week-old and 8-week-old gametophores than in the protonema (Figure 2C).
Figure 2.
Expression pattern of PpGPAT9 in P. patens. (A) The expression of PpGPAT9 (Pp1s138_27V6.1, Phpat.011G104300) relative to PpActin2 (Pp1s198_157V6.1, Phpat.003G133100). Images and values were obtained from P. patens eFP-Browser (http://bar.utoronto.ca/efp_physcomitrella/cgi-bin/efpWeb.cgi). Three- to 4-week-old gametophore; sporophyte 1 (S1), comprising sporophytes collected 5–6 days after fertilization (AF); sporophyte 2 (S2), 9–11 days AF; sporophyte 3 (S3), 18–20 days AF; and sporophyte M (SM), 28–33 days AF. (B) Morphology of P. patens. (i), Seven-day-old filamentous protonema, Bar = 0.4 mm. (ii), Four-week-old gametophore, Bar = 2 mm. (iii), Eight-week-old gametophore, Bar = 2 mm. (C) Quantitative RT-PCR analysis of PpGPAT9. Total RNA was isolated from 7-day-old protonema (7dP), 4-week-old gametophores (4wG), and 8-week-old gametophores (8wG). PpActin2 was used as the reference gene to assess RNA quality and quantity. Each value is the mean ± SD of five independent measurements.
2.3. Subcellular Localization of PpGPAT9
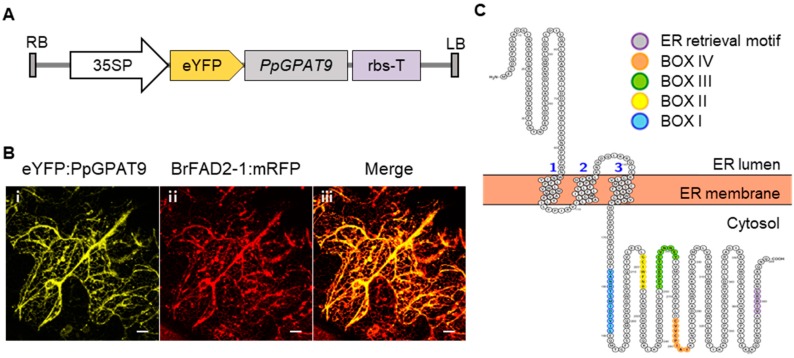
To examine the subcellular localization of PpGPAT9, we constructed a binary vector, peYFP:PpGPAT9, in which the enhanced yellow fluorescent protein (eYFP) was fused to the 5′-end of PpGPAT9 under the control of the CaMV 35S promoter (Figure 3A). The generated construct was co-transformed into Nicotiana benthamiana leaves with the BrFAD2-1:mRFP construct, which is localized in the ER [45], via Agrobacterium-mediated infiltration. The yellow fluorescent signals from peYFP:PpGPAT9 were merged with the red fluorescent signals from BrFAD2-1:mRFP (Figure 3B). In the topology analysis of PpGPAT9 by using an open-source tool for visualization of proteoforms (http://topcons.net/; http://wlab.ethz.ch/protter/start/), PpGPAT9 was predicted to possess an N-terminal region oriented to the ER lumen and one ER lumen loop, three transmembrane domains, and a long C-terminal cytoplasmic region (Figure 3C). Four acyltransferase motifs (BOX I to BOX IV) and an ER retrieval motif (“VIRRL” residues) [46] were observed to be present in the long C-terminal cytoplasmic regions. Therefore, subcellular localization and topology analysis of PpGPAT9 indicate that PpGPAT9 is localized in the ER and may be involved in eukaryotic pathway of glycerolipid synthesis in the ER.
Figure 3.
Subcellular localization of PpGPAT9. (A and B) Subcellular localization of eYFP:PpGPAT9 in N. benthamiana leaf epidermal cells. (A) Binary vector construct harboring eYFP:PpGPAT9. 35SP, cauliflower mosaic virus 35S promoter. eYFP, enhanced yellow fluorescent protein; LB, left border; RB, right border; rbs-T, terminator of ribulose-1,5-bisphosphate carboxylase and oxygenase small subunit from pea (Pisum sativum). (B) Two fluorescent signals in N. benthamiana leaf. Agrobacterium tumefaciens cells harboring eYFP:PpGPAT9 and BrFAD2-1:mRFP were co-infiltrated into N. benthamiana leaf epidermal cells. The fluorescent signals were visualized under a confocal laser-scanning microscope at 48 h after infiltration. (i) Yellow fluorescent signals from eYFP:PpGPAT9. (ii) Red fluorescent signals from BrFAD2-1:mRFP. (iii) Merged image of eYFP:PpGPAT9 with BrFAD2-1:mRFP. Bars = 10 μm. (C) Topology of PpGPAT9. The topology of PpGPAT9 was analyzed using the open-source tool for visualization of proteoforms (http://topcons.net/; http://wlab.ethz.ch/protter/start/) [47]. Computational analysis showed that PpGPAT9 harbors an N-terminal region and two non-cytoplasmic loops, three transmembrane regions, and one cytoplasmic loop and long C-terminal cytoplasmic regions. Acyltransferase motifs (BOX I–IV) and ER retrieval motif of long C-terminal cytoplasmic regions are shown in blue, yellow, green, orange, and purple, respectively. The numbers indicate the counts of each transmembrane domain.
2.4. Generation of Arabidopsis Transgenic Plants Ectopically Expressing PpGPAT9
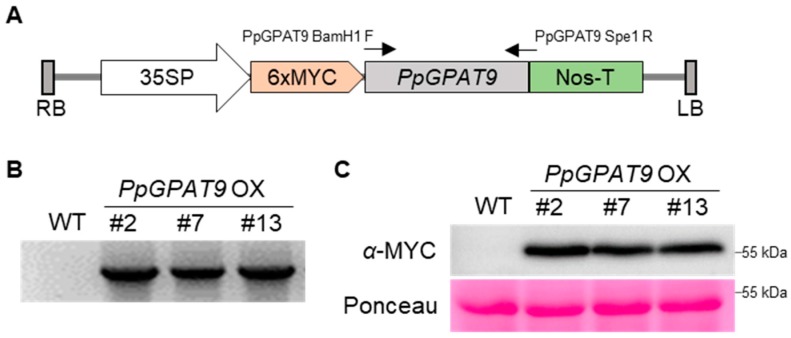
To examine the in vivo activity of PpGPAT9, a pBA002 binary vector construct, in which PpGPAT9 was translationally fused to the 3′-end of 6x MYC under the control of the CaMV 35S promoter was generated, and then introduced to Arabidopsis plants via Agrobacterium-mediated dip floral method (Figure 4A). Transgenic Arabidopsis seeds were selected from half-strength MS medium supplemented with 10 μg/mL phosphinothricin (PPT). Chromosomal DNA was extracted from the leaves of PPT-resistant plants, and PCR was performed with a gene-specific primer set (PpGPAT9 BamH1 F1 and PpgPAT9 Spe1 R1; Table S1) to ensure the presence of the transferred DNA (T-DNA) region in the Arabidopsis chromosome. Figure 4B shows that the PCR bands were detected in three independent transgenic lines (#2, #7, and #13) but not in the wild type, indicating that the T-DNA region harboring PpGPAT9 is integrated into the Arabidopsis chromosome. To examine the expression of PpGPAT9 in the transgenic Arabidopsis lines, total proteins were extracted from #2, #7, and #13 seedlings (T2 generation); separated using 10% SDS-polyacrylamide gel electrophoresis (PAGE); and then visualized using anti-c-Myc antibody and an ECL chemiluminescence detection system. Bands corresponding to approximately 57 kDa were clearly detected in three transgenic lines, but not in the wild type (Figure 4C), indicating that the PpGPAT9 proteins were expressed in the transgenic Arabidopsis plants.
Figure 4.
Generation of transgenic A. thaliana plants ectopically expressing PpGPAT9 under the control of the CaMV 35S promoter. (A) Schematic diagram of the 35SP:6xMYC-PpGPAT9 construct. 35SP, Cauliflower mosaic virus 35S promoter; LB, left border; Nos-T, nopaline synthase terminator; RB, right border. The arrows indicate forward and reverse primer sites. (B) Genomic DNA (gDNA) PCR. The gDNA was isolated from the wild type (WT) and 3-week-old transgenic leaves ectopically expressing PpGPAT9 (#2, #7, and #13) of the T1 generation. (C) Immunoblot analysis. Total proteins were extracted from 10-day-old seedlings of WT and transgenic lines ectopically expressing PpGPAT9 of the T2 generation. Anti-myc antibodies were used to detect chemiluminescence (upper image) after 1 min of exposure. Protein quality and quantity were confirmed using Ponceau S (bottom image).
2.5. Morphological and Microscopic Analyses of Transgenic Arabidopsis Seeds Ectopically Expressing PpGPAT9
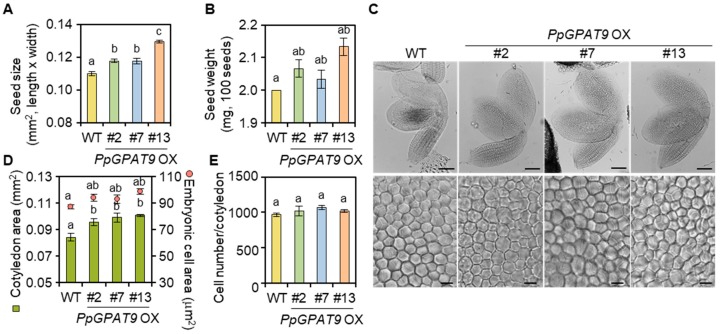
During the growth and development of transgenic Arabidopsis plants ectopically expressing PpGPAT9 (PpGPAT9 OX), no noticeable differences were observed relative to the wild type. We next examined whether the ectopic expression of PpGPAT9 affects morphological changes in the PpGPAT9 OX seeds. When the size and weight of seeds from the wild type and three PpGPAT9 OX lines were measured, the size of seeds from the #2, #7, and #13 transgenic plants increased by approximately 6%, 6%, and 16%, respectively, compared with the wild type (Figure 5A). We also observed that the weight of seeds from the #2, #7, and #13 transgenic plants was approximately 3%, 2%, and 6% higher, respectively, than that from the wild type (Figure 5B).
Figure 5.
Microscopic analysis of seeds of the WT and transgenic Arabidopsis ectopically expressing PpGPAT9 (#2, #7, and #13). (A) Length and width of seeds of the WT and transgenic lines (#2, #7, and #13). The data were statistically examined using ANOVA with Tukey’s test at 95% confidence interval. (B) Seed weight per 100 mature dried seeds of the WT and transgenic lines (#2, #7, and #13). The data were statistically examined using ANOVA with Tukey’s test at 95% confidence interval. (C) Images of mature embryos (upper image) and embryonic cells in the central region of the cotyledon (bottom image) obtained from dried seeds of the WT and transgenic lines (#2, #7, and #13). Bars = 0.1 mm (upper image). Bars = 0.01 mm (bottom image). (D) Cotyledon size and epidermal cell size of embryonic cells of the WT and transgenic lines (#2, #7, and #13). Each value is the mean ± SE of three independent measurements. The data were statistically analyzed using ANOVA with Tukey’s test at 95% confidence interval. (E) Epidermal cell number of adaxial cotyledons in the WT and transgenic lines (#2, #7, and #13). Each value is the mean ± SE of three independent measurements. The data were statistically analyzed using ANOVA with Tukey’s test at 95% confidence interval.
We subsequently analyzed if the increase in PpGPAT9 OX seed size is associated with cell division or expansion. After the wild type and PpGPAT9 OX seeds were cleared using Visikol, the size of the cotyledons and embryonic cells was measured under a microscope ICC50 HD (Leica, Wetzlar, Germany). The size of #2, #7, and #13 PpGPAT9 OX cotyledons was approximately 13, 18, and 15% larger than that of the wild type (Figure 5C,D). The size of #2, #7, and #13 PpGPAT9 OX embryonic cells also increased by approximately 10%, 7%, and 8%, respectively, when compared with the wild type (Figure 5C,D). When the number of embryonic cells in the wild type and transgenic cotyledons was calculated, significant differences were not observed (Figure 5E). These results suggest that the increased seed size of PpGPAT9 OX lines may be attributable to cell expansion, but not cell division.
2.6. Fatty Acid Analysis of Arabidopsis Seeds and Leaves Ectopically Expressing PpGPAT9
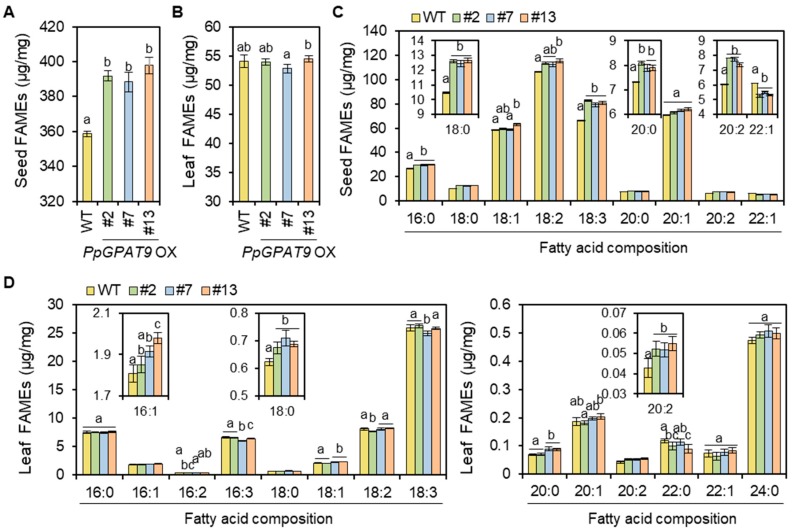
To examine the fatty acid composition and levels in the Arabidopsis seeds and leaves ectopically expressing PpGPAT9, total fatty acids were extracted from dry seeds and leaves of 4 week-old-plants and subsequently analyzed using GC-FID. The levels of total fatty acid methyl esters (FAMEs), which are mainly from TAGs [48,49,50], increased by approximately 10% in the seeds of three transgenic lines (#2, #7, and #13) relative to the wild type (Figure 6A). The levels of both saturated (16:0, 18:0, and 20:0) and polyunsaturated (18:2, 18:3, and 20:2) fatty acids increased by approximately 44% and 60%, whereas the levels of monounsaturated FAs (C18:1 and C20:1) were not significantly altered or C22:1 levels decreased in all the transgenic seeds when compared with the wild type (Figure 6C). The largest increase (approximately 53%) was detected in the levels of C18:3 plus C20:2 fatty acids in the transgenic seeds.
Figure 6.
Fatty acid content and composition in the seeds (A and C) and leaves (B and D) of the WT and transgenic lines ectopically expressing PpGPAT9 (#2, #7, and #13). Hundred dry seeds and 4-week-old leaves were used to extract the fatty acids. FAMEs were determined using GC-FID. Error bars indicate mean ± SE of three independent measurements. The data were statistically examined using ANOVA with Tukey’s test at 95% confidence interval. The percentage values indicate the portion of increased fatty acids when compared with the wild type.
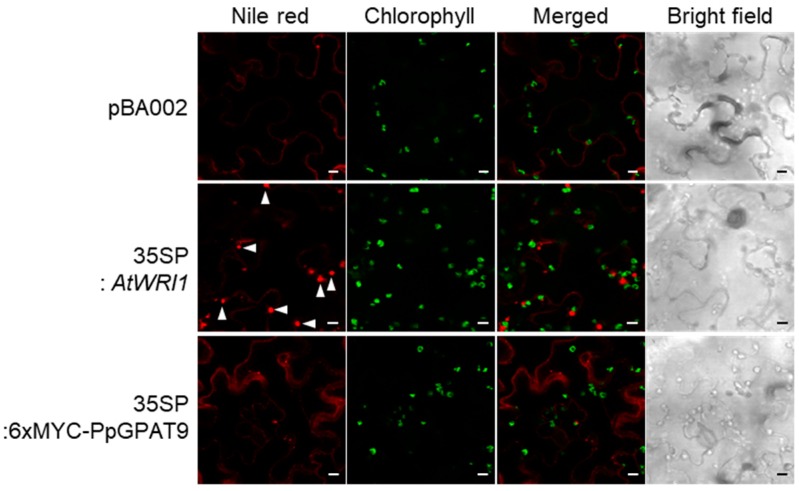
In PpGPAT9 OX leaves relative to the wild type, the levels of C16:1, C18:0, and C20:2 increased by approximately 2 to 9%, 11%, and 24%, respectively, but no significant changes were observed in the levels of total fatty acids, which are mainly from membrane lipids [48,49,50] (Figure 6B,D). No noticeable accumulation of oil bodies was observed during the transient expression of PpGPAT9 in N. benthamiana leaves (Figure 7); under similar conditions, oil bodies were clearly detected by the transient expression of Arabidopsis WRINKLED1 [51]. These results indicate that PpGPAT9 functions in glycerolipid synthesis and may perform preferential acylation of LC- and VLC-PUFAs to glycerol-3-phosphate.
Figure 7.
Transient expression of PpGPAT9 in N. benthamiana leaves. Agrobacterium harboring pBA002 (for the control); AtWRI1 or PpGPAT9 was infiltrated into N. benthamiana leaves, and the leaf disks were stained with Nile red solution. The fluorescent signals were visualized using confocal laser scanning microscopy. The arrowheads indicate oil bodies. Bars = 20 μm.
3. Discussion
The lower plant P. patens harbors relatively higher amounts of LC-PUFAs and VLC-PUFAs such as C18:2, C18:3, and C20:4 in cellular membranes [36,37,52]. However, there is limited information on the activity and substrate specificity of acyltransferases required for the synthesis of glycerolipids in P. patens. Here, we have reported that (1) the expression of PpGPAT9 in the protonema and gametophores was detected using qRT-PCR. (2) PpGPAT9 is localized in the ER. (3) Ectopic expression of PpGPAT9 increased the size of the seeds and embryonic cells, which is related to the increased cell expansion. (4) A significant increase in the levels of total FAMEs was observed in PpGPAT9 OX seeds relative to the wild type, but not in PpGPAT9 OX leaves. (5) An increase in LC-FA and VLC-FA levels relative to saturated FA levels was prominent in the PpGPAT9 OX seeds and leaves. Our current study shows that PpGPAT9 is a novel genetic material that can be used for the increased production of storage oils with more PUFAs in transgenic oilseed plants.
Genome-wide analysis has shown that multi-copy GPAT genes are present in land plants. Arabidopsis harbors 10 GPATs, which function in the ER (GPAT4-9, ER membrane-bound form), mitochondria (GPAT1-3, membrane-bound form), and chloroplast (ATS1, soluble form) [11,42,43,46,53]. All GPATs, including PpGPAT9, contain highly conserved acyltransferase domains, which include conserved amino acid residues, His and Asp in BOX I, Arg in BOX II, Glu and Gly in BOX III, and Pro in BOX IV. GPAT4, GPAT6, and GPAT8 have additional conserved domains (the first Asp in motif I, the first Lys in motif III, and the first Asp in motif IV), which are essential for the phosphatase activity that enables the production of 2-monoacylglycerol, a precursor for cutin synthesis [42,54]. In contrast, other GPATs, including GPAT5 and GPAT7 grouped in the suberin-biosynthesis clade (Figure 1A), have lost some amino acid residues at the active site required for phosphatase activity [37,41], indicating the functional divergence of the GPAT family.
GPAT9 is present as a single-copy gene in most organisms, including P. patens (Figure 1) [46]. The Arabidopsis gpat9 mutant displayed the lethal-embryo phenotype [11], indicating that GPAT9 is essential in Arabidopsis. In this study, we observed the ubiquitous expression of PpGPAT9 during the life cycle of P. patens life (Figure 2). Interestingly, we tried to generate P. patens gpat9 mutants by homologous recombination [35]; however, it was not successful under similar conditions in which knockout mutants of PpGPAT2 and PpGPAT4 involved in cutin synthesis were generated [55]. When PpGPAT9 is overexpressed in Arabidopsis, the total FAME levels increased in PpGPAT9 OX seeds, and alterations in the fatty acid composition of PpGPAT9 OX seeds and leaves were observed when compared with the wild type (Figure 6). Therefore, these results indicate that PpGPAT9 is involved in glycerolipid synthesis and PpGPAT9, like AtGPAT9, may have an essential role in P. patens.
Gidda et al. [46] reported that Arabidopsis GPAT9 is localized to the ER in tobacco BY-2 suspension cells. A hydrophobic pentapeptide motif (-φ-X-X-K/R/D/E-φ-, φ; large hydrophobic amino acid residues) is essential for the ER retrieval of AtGPAT9 and AtFAD2 [46,56]. We also observed a similar result; the yellow fluorescent signals from the eYFP:PpGPAT9 construct were merged with the red fluorescent signals from the BrFAD2-1:mRFP construct in the ER of N. benthamiana leaves (Figure 3). Therefore, the “VIRRL” motif in the C-terminal region of PpGPAT9, which corresponds to the hydrophobic pentapeptide motif of AtGPAT9 and BrFAD2-1 [45,46], may be the ER retention signal of PpGPAT9. Alignment of the deduced amino acid sequences of lower and higher plant GPAT9s showed that the hydrophobic pentapeptide motif has been conserved from charophyte to monocot, except chlorophyte, during plant evolution. In the topology prediction analysis of PpGPAT9, we observed that PpGPAT9 harbors three membrane-spanning domains and its N-terminal and C-terminal regions were directed to the ER lumen and cytoplasm, respectively (Figure 3A), although both N- and C- terminal residues of AtGPAT9 were predicted to be oriented to the cytosol [46]. However, the catalytic domains of both AtGPAT9 and PpGPAT9 were observed to be present in the cytoplasm.
There have been attempts to increase storage oil yield by metabolic flux control during TAG biosynthesis. Vanhercke et al. [57] reported three critical steps: (1) The “PUSH” step to increase the levels of fatty acid precursors required for TAG synthesis, (2) “PULL” step to enhance TAG assembly, and (3) “PROTECT” step to prevent the degradation of synthesized TAG. In the “PUSH” step, overexpression of transcription factors such as WRI1 and LEC led to an increase in seed oils by approximately more than 40% [48,58,59,60,61]. In the “PULL” step, the oil content was increased by up to 28% in the transgenic Arabidopsis seeds expressing DGAT1 or PDAT1 [62,63]. In the “PROTECT” step, the inhibition of SDP1 lipase, which degrades TAG, resulted in increased oil content by up to 8% in the seeds or vegetative tissues of the transgenic Arabidopsis lines [64,65]. In particular, previous studies have reported that GPAT9 genes of Arabidopsis and the marine diatom Phaeodactylum tricornutum also contribute to elevated TAG content in the transgenic Arabidopsis plants and P. tricornutum, respectively [29,33]. Ectopic expression of PpGPAT9, which shares approximately 70% amino acid sequence similarity with AtGPAT9, led to increased TAG production in the transgenic Arabidopsis seeds, confirming that GPAT9 as well as DGAT and PDAT can be genetic resources involved in the “PULL” mechanism to increase the oil yield in a sustainable manner.
According to previous studies [48,57,60,66,67], seed mass and oil content increased in the transgenic lines expressing Arabidopsis or Camelina (Camelina sativa) DGAT1 or Arabidopsis or rapeseed (Brassica napus) WRINKLED1 (WRI1). In C. sativa cotyledons overexpressing AtWRI1, the increase in the size of embryonic cells was closely related to an increase in embryonic cell expansion rather than cell division [48]. However, both cell expansion and division increased in transgenic C. sativa cotyledons overexpressing CsDGAT1B [66]. In the current study, increased TAG levels in PpGPAT9 OX seeds were observed to be proportional to the increased cotyledon size, which is closely related to the increased embryonic cell expansion rather than embryonic cell division (Figure 5). Weber et al. [68] reported that a high ratio of hexoses to sucrose activates embryonic cell growth via cell division, whereas a low ratio of hexoses to sucrose promotes differentiation and storage product synthesis. Therefore, the altered ratio of carbohydrate metabolites may be related with the cotyledon size in PpGPAT9 OX seeds. However, the molecular networks between embryonic cell expansion and seed oil yield during seed development need to be studied further.
In the in vitro enzyme activity assay for sunflower (Helianthus annuus) GPAT9-1 by using yeast microsomal fractions, the substrate activity of HaGPAT9-1 was the strongest for C16:0-CoA, followed by C18:2-CoA and C18:1-CoA [69]. The result was consistent with the increased TAG species with C16:0 in yeast cells expressing HaGPAT9 [69]. AtGPAT9 showed the highest substrate preference for C18:1-CoA relative to C16:0-CoA, C18:0-CoA, and C18:3-CoA, which is correlated with elevated C18:1 levels in the storage oils of transgenic seeds ectopically expressing AtGPAT9 [35]. When PtGPAT9 was expressed in P. tricornutum, a remarkable increase in PUFA levels and concomitant decrease in saturated FA and monounsaturated FA levels were observed. In particular, a significant increase (~40%) in the levels of C20:5, which are abundant (~18% per dry weight) fatty acids in P. tricornutum, and decrease (~45%) in C16:0 levels were detected in PtGPAT9 overexpressing P. tricornutum when compared with the wild type [33]. Resemann et al. [38] reported that approximately 70% and 67% PUFAs are present in P. patens protonema and gametophores, respectively. The major PUFAs were C16:2, C16:3, C18:3, C20:4, and C20:5 in the protonema and C18:2, C18:3, and C20:4 in the gametophore [38]. In this study, the levels of C18:3 and C20:2 FAs were higher than that of saturated FAs in the transgenic seeds ectopically expressing PpGPAT9 (Figure 6). It is interesting that ectopic expression of PpGPAT9 caused a significant increase in C20:2 content, which is not the major component in Arabidopsis seeds. Overall, these results suggest that GPAT9 may exhibit a substrate preference for certain FAs with specific carbon chain length and/or specific degree of unsaturation and can be used for the improvement of oil quality in oilseed crops. In conclusion, this study shows that ER-localized PpGPAT9 is involved in glycerolipid synthesis and is a genetic resource that can be used to increase the production of storage oils with more PUFAs in oilseed crops.
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
The wild type P. patens (Gransden 2007 strain) was obtained from Jeong Sheop Shin at Korea University and grown on BCD medium supplemented with 5 mM diammonium tartrate and 1 mM CaCl2, according to previous studies [70,71]. A. thaliana (ecotype Columbia-0, Col-0) and transgenic Arabidopsis plants were grown under moss growth conditions. Seeds were sterilized with 75% ethanol containing 0.05% Triton X-100 and then washed with 100% ethanol. Germinated 7-day-old seedlings grown on half-strength MS media containing 1% sucrose and 0.7% phytoagar were transplanted to an autoclaved soil mixture containing soil, vermiculite, and pearlite (3:2:1 v/v/v). The photoperiod was 16 h day and 8 h dark, and light intensity was 100–120 µmol/m/s at 23 °C.
4.2. Phylogenetic Tree Construction
Amino acid sequences of GPAT9 homolog genes from P. patens, A. thaliana, C. reinhardtii, L. incisa, K. nitens, S. moellendorffii, A. trichopoda, B. distachyon, O. sativa, and S. lycopersicum were obtained using BLAST with cutting threshold 1 × 10−5 from Phytozome v12.1 (www.phytozome.net), NCBI (www.ncbi.nlm.nih.gov), and UniProt (www.uniprot.org). The amino acid sequences were used for domain and similarity analyses with CLUSTALW software. The phylogenetic tree was created using the MEGA6 program with the Maximum Likelihood method [72].
4.3. RNA Isolation and Gene Expression Analysis
To isolate the total RNA from 7-day-old protonema and 4- and 8-week-old gametophores, the tissues were ground in liquid nitrogen and the total RNA was extracted using TRIzol reagent (Ambion, CA, USA), according to the manufacturer’s instructions. Two micrograms of the isolated total RNA was converted to cDNA by using the GoScript™ Reverse Transcription System (Promega, WI, USA), according to the manufacturer’s instructions. Using the gene-specific primers listed in Table S1, qRT-PCR and RT-PCR were performed as follows: First hold at 94 °C for 5 min, followed by cycles at 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s and second hold at 72 °C for 5 min. The KAPA SYBR FAST qRT-PCR kit (KAPA Biosystem, Cape Town, South Africa) and CFX 96 thermal cycler (Bio-Rad, CA, USA) were used for qRT-PCR. To normalize gene expression and compare RNA levels, PpACT2 (P.patens.003G133100) was used [73].
4.4. Subcellular Localization Using Fluorescent Reporter Proteins
To generate the eYFP-fused PpGPAT9 in the pPZP212 binary vector [74] harboring the CaMV 35S promoter, eYFP and rbs S3′ terminator, amplified cDNA fragment of PpGPAT9 (primer sets are listed in Table S1) was inserted using the restriction enzymes BamHI and SalI (referred to as peYFP:PpGPAT9). The recombinant binary vector was transformed into Agrobacterium strain GV3101 by using the freeze-thaw method. Agrobacterium cells harboring the peYFP:PpGPAT9 construct was incubated up to OD600 = 0.8. The composition of the infiltration medium was as follows: 10 mM MES (pH 5.7), 10 mM MgCl2, and 0.2 mM acetosyringone. Agrobacterium cells in the infiltration media were inoculated using a 1 mL syringe. After 2 days, the fluorescent signal was observed using a TCS SP5 AOBS/tandem laser confocal scanning microscope (Leica, Wetzlar, Germany). Agrobacterium cells harboring pBrFAD2-1:mRFP construct were co-infiltrated to confirm ER localization [45].
4.5. Ectopic Expression and Transformation
To generate transgenic Arabidopsis ectopically expressing PpGPAT9, the amplified cDNA fragment of PpGPAT9 (primer sets are listed in Table S1) was inserted into the pBA002 binary vector [75] harboring the CaMV 35S promoter, 6x MYC, and NOS terminator. The recombinant binary vector was transformed into Agrobacterium strain GV3101 by using the freeze-thaw method. The Agrobacterium cells were incubated up to OD600 = 0.8 on YEP media containing rifampicin (50 µg/mL) and spectinomycin (50 µg/mL), and then they were collected and resuspended in transformation media (5% sucrose and 0.05% Silwet L-77). Arabidopsis transformation was performed using the floral-drop method [76].
4.6. Protein Extraction and Western Blotting
Crude proteins from 10-day-old seedlings of the wild type and transgenic Arabidopsis (T2) ectopically expressing PpGPAT9 were extracted using extraction buffer (125 mM Tris-HCl buffer [pH 6.8], 0.2 M DTT, 4% SDS, 10% glycerol, 2 mM PMSF, 5 µg/mL aprotinin, 3 µg/mL pepstatin A, and 3 µg/mL leupeptin). Approximately 50 µg proteins were used for SDS-PAGE (10% gel) analysis (Mini-Protein Tetra System, Bio-Rad) and immunoblot assay. The proteins were transferred onto PVDF membrane and fixed with 5% formaldehyde and washed with pure water. The membrane was treated with blocking solution (5% skim milk in 1× TBST) on the rocker at room temperature for 1 h and then incubated with MYC antibody (clone 9E10, Millipore) on the rocker at 4 °C overnight. The membrane was incubated with anti-mouse IgG (GE Healthcare) at room temperature for 1 h. To detect the proteins, enhanced chemiluminescence reaction (ECL, Thermo Scientific, MA, USA) was performed using Amersham Imager 600 (GE Healthcare Life Sciences, MA, USA).
4.7. Microscopic Analysis of Seeds
Dry seeds (approximately 100 seeds) were treated with 8 M NaOH solution for 5 min to obtain soft tissues. The seeds were washed with distilled water and then cleared in Visikol (https://visikol.com/) for more than 10 h until they were submerged and transparent. The seeds were spread on a microscope slide with a few drops of Visikol solution. Then, the coverslip was placed on the microscope slide and gently pushed using forceps to separate the embryo from the seed coat. The microscopic image analyses were performed using LEICA ICC50 HD with Leica Application Suite (LAS) software (Leica, Wetzlar, Germany).
4.8. Fatty Acid Analysis
The dry seeds (100 seeds) and plant tissue powder (about 10 mg) were incubated in methylation solution (500 µL toluene, 1 mL of 5% sulfuric acid in methanol, and 500 µg glyceryl triheptadecanate as the standard) at 90 to 95 °C for 1.5 h. The FAMEs were extracted by adding 1.5 mL of 0.9% NaCl and sequential hexane. The supernatant was evaporated with nitrogen gas, and the FAMEs were concentrated with 100 µL hexane. The FAMEs were analyzed with GC-2010 (Shimadzu, Japan) and DB-23 column (30 mm × 0.25 mm, 0.25 µm film thickness; J&W Scientific, Folsom. CA, USA) as follows: temperature hold at 190 °C for 10 min, followed by 190 °C to 230 °C with 5 °C increase per min, and hold at 230 °C for 10 min.
4.9. Transient Expression in N. benthamiana Leaves
Agrobacterium harboring the pBA002 construct, 35S pro:AtWRI1, and 35SP:6xMYC-PpGPAT9 construct or P19 construct was incubated up to OD600 = 0.8 and diluted to OD600 = 0.2 by using infiltration media in the following combinations: pBA002 and P19 or AtWRI1 and P19 or 35SP:6xMYC-PpGPAT9 and P19. To visualize the oil bodies in N. benthamiana leaves, the leaf disks were stained with Nile red solution (10 mg/mL in 0.1 M Tris-HCl buffer [pH 8]; Sigma-N3013) at room temperature for 30 min after 5 days of infiltration. The fluorescence was observed using the TCS SP5 AOBS/Tandem confocal laser scanning microscope (Leica Microsystems, Wetzlar, Germany). The emission wavelength was 615 nm, and the excitation wavelength was 560–572 nm.
Acknowledgments
We thank Jeong Sheop Shin (Korea University) for providing P. patens (Gransden 2007 strain) and Ryeo Jin Kim for technical assistance.
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/8/8/284/s1, Table S1: List of DNA primers used in this study.
Author Contributions
M.C.S. designed the research. S.U.Y., J.K., and H.K. performed the experiments and analyzed the data with M.C.S. J.K., H.K., and M.C.S. wrote the paper.
Funding
This work was supported by grants from the Next-Generation BioGreen 21 Program (no. PJ013422) of the Rural Development Administration, Republic of Korea and the National Research Foundation (NRF-2019R1A2B5B02070204) of Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Xu C., Shanklin J. Triacylglycerol metabolism, function, and accumulation in plant vegetative tissues. Annu. Rev. Plant Biol. 2016;67:179–206. doi: 10.1146/annurev-arplant-043015-111641. [DOI] [PubMed] [Google Scholar]
- 2.Xu Y., Caldo K.M.P., Pal-Nath D., Ozga J., Lemieux M., Weselake R.J., Chen G. Properties and biotechnological applications of Acyl-CoA:diacylglycerol acyltransferase and phospholipid:diacylglycerol acyltransferase from terrestrial plants and microalgae. Lipids. 2018;5:663–688. doi: 10.1002/lipd.12081. [DOI] [PubMed] [Google Scholar]
- 3.Biermann U., Bornscheuer U., Meier M.A., Metzger J.O., Schafer H.J. Oils and fats as renewable raw materials in chemistry. Angew. Chem. Int. Ed. Engl. 2011;50:3854–3871. doi: 10.1002/anie.201002767. [DOI] [PubMed] [Google Scholar]
- 4.Foley P.M., Beach E.S., Zimmerman J.B. Algae as a source of renewable chemicals: Opportunities and challenges. Green Chem. 2011;13:1399–1405. doi: 10.1039/c1gc00015b. [DOI] [Google Scholar]
- 5.Ho D.P., Ngo H.H., Guo W. A mini review on renewable sources for biofuel. Bioresour. Technol. 2014;169:742–749. doi: 10.1016/j.biortech.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Goncalves E.C., Wilkie A.C., Kirst M., Rathinasabapathi B. Metabolic regulation of triacylglycerol accumulation in the green algae: Identification of potential targets for engineering to improve oil yield. Plant Biotechnol. J. 2016;14:1649–1660. doi: 10.1111/pbi.12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrie J.R., Shrestha P., Belide S., Kennedy Y., Lester G., Liu Q., Divi U.K., Mulder R.J., Mansour M.P., Nichols P.D., et al. Metabolic engineering Camelina sativa with fish oil-like levels of DHA. PLoS ONE. 2014;9:e85061. doi: 10.1371/journal.pone.0085061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruiz-Lopez N., Sayanova O., Napier J.A., Haslam R.P. Metabolic engineering of the omega-3 long chain polyunsaturated fatty acid biosynthetic pathway into transgenic plants. J. Exp. Bot. 2012;63:2397–2410. doi: 10.1093/jxb/err454. [DOI] [PubMed] [Google Scholar]
- 9.Li-Beisson Y., Shorrosh B., Beisson F., Andersson M.X., Arondel V., Bates P.D., Baud S., Bird D., Debono A., Durrett T.P., et al. Acyl-lipid metabolism. Arab. Book. 2013;11:e0161. doi: 10.1199/tab.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gimeno R.E., Cao J. Thematic review series: Glycerolipids. Mammalian glycerol-3-phosphate acyltransferases: New genes for an old activity. J. Lipid Res. 2008;49:2079–2088. doi: 10.1194/jlr.R800013-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Shockey J., Regmi A., Cotton K., Adhikari N., Browse J., Bates P.D. Identification of Arabidopsis GPAT9 (At5g60620) as an essential gene involved in triacylglycerol biosynthesis. Plant Physiol. 2016;170:163–179. doi: 10.1104/pp.15.01563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou J., Wei Y., Jako C., Kumar A., Selvaraj G., Taylor D.C. The Arabidopsis thaliana TAG1 mutant has a mutation in a diacylglycerol acyltransferase gene. Plant J. 1999;19:645–653. doi: 10.1046/j.1365-313x.1999.00555.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim H.U., Li Y.B., Huang A.H.C. Ubiquitous and endoplasmic reticulum-located lysophosphatidyl acyltransferase, LPAT2, is essential for female but not male gametophyte development in Arabidopsis. Plant Cell. 2005;17:1073–1089. doi: 10.1105/tpc.104.030403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura Y., Tsuchiya M., Ohta H. Plastidic phosphatidic acid phosphatases identified in a distinct subfamily of lipid phosphate phosphatases with prokaryotic origin. J. Biol. Chem. 2007;282:29013–29021. doi: 10.1074/jbc.M704385200. [DOI] [PubMed] [Google Scholar]
- 15.Falcone D.L., Gibson S., Lemieux B., Somerville C. Identification of a gene that complements an Arabidopsis mutant deficient in chloroplast omega 6 desaturase activity. Plant Physiol. 1994;106:1453–1459. doi: 10.1104/pp.106.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao J., Ajjawi I., Manoli A., Sawin A., Xu C., Froehlich J.E., Last R.L., Benning C. FATTY ACID DESATURASE4 of Arabidopsis encodes a protein distinct from characterized fatty acid desaturases. Plant J. 2009;60:832–839. doi: 10.1111/j.1365-313X.2009.04001.x. [DOI] [PubMed] [Google Scholar]
- 17.Heilmann I., Mekhedov S., King B., Shanklin J. Identification of the Arabidopsis palmitoyl-monogalactosyldiacylglycerol delta7-desaturase gene FAD5, and effects of plastidial retargeting of Arabidopsis desaturases on the fad5 mutant phenotype. Plant Physiol. 2004;136:4237–4245. doi: 10.1104/pp.104.052951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iba K., Gibson S., Nishiuchi T., Fuse T., Nishimura M., Arondel V., Hugly S., Somerville C. A gene encoding a chloroplast omega-3 fatty acid desaturase complements alterations in fatty acid desaturation and chloroplast copy number of the fad7 mutant of Arabidopsis thaliana. J. Biol. Chem. 1993;268:24099–24105. [PubMed] [Google Scholar]
- 19.McConn M., Hugly S., Somerville C. A mutation at the fad8 locus of Arabidopsis identifies a second chloroplast [omega]-3 desaturase. Plant Physiol. 1994;106:1609–1614. doi: 10.1104/pp.106.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eastmond P.J., Quettier A.L., Kroon J.T.M., Craddock C., Adams N., Slabas A.R. Phosphatidic acid phosphohydrolase 1 and 2 regulate phospholipid synthesis at the endoplasmic reticulum in Arabidopsis. Plant Cell. 2010;22:2796–2811. doi: 10.1105/tpc.109.071423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Y.H., Peisker H., Weth A., Baumgartner W., Dormann P., Frentzen M. Extraplastidial cytidinediphosphate diacylglycerol synthase activity is required for vegetative development in Arabidopsis thaliana. Plant J. 2013;75:867–879. doi: 10.1111/tpj.12248. [DOI] [PubMed] [Google Scholar]
- 22.Browse J., McConn M., James D., Jr., Miquel M. Mutants of Arabidopsis deficient in the synthesis of alpha-linolenate: Biochemical and genetic characterization of the endoplasmic reticulum linoleoyl desaturase. J. Biol. Chem. 1993;268:16345–16351. [PubMed] [Google Scholar]
- 23.Stahl U., Carlsson A.S., Lenman M., Dahlqvist A., Huang B.Q., Banas W., Banas A., Stymne S. Cloning and functional characterization of a Phospholipid:Diacylglycerol acyltransferase from Arabidopsis. Plant Physiol. 2004;135:1324–1335. doi: 10.1104/pp.104.044354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okuley J., Lightner J., Feldmann K., Yadav N., Lark E., Browse J. Arabidopsis FAD2 gene encodes the enzyme that is essential for polyunsaturated lipid synthesis. Plant Cell. 1994;6:147–158. doi: 10.1105/tpc.6.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Millar A.A., Kunst L. Very-long-chain fatty acid biosynthesis is controlled through the expression and specificity of the condensing enzyme. Plant J. 1997;12:121–131. doi: 10.1046/j.1365-313X.1997.12010121.x. [DOI] [PubMed] [Google Scholar]
- 26.Kornberg A., Pricer W.E., Jr. Enzymatic esterification of alpha-glycerophosphate by long chain fatty acids. J. Biol. Chem. 1953;204:345–357. [PubMed] [Google Scholar]
- 27.Barron E.J., Stumpf P.K. Fat metabolism in higher plants. XIX. The biosynthesis of triglycerides by avocado-mesocarp enzymes. Biochim. Biophys. Acta. 1962;60:329–337. doi: 10.1016/0006-3002(62)90408-0. [DOI] [PubMed] [Google Scholar]
- 28.Gao Q., Shang Y., Huang W., Wang C. Glycerol-3-phosphate acyltransferase contributes to triacylglycerol biosynthesis, lipid droplet formation, and host invasion in Metarhizium robertsii. Appl. Environ. Microbiol. 2013;79:7646–7653. doi: 10.1128/AEM.02905-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niu Y.F., Wang X., Hu D.X., Balamurugan S., Li D.W., Yang W.D., Liu J.S., Li H.Y. Molecular characterization of a glycerol-3-phosphate acyltransferase reveals key features essential for triacylglycerol production in Phaeodactylum tricornutum. Biotechnol. Biofuels. 2016;9:60. doi: 10.1186/s13068-016-0478-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Röttig A., Steinbüchel A. Acyltransferases in bacteria. Microbiol. Mol. Biol. Rev. 2013;77:277–321. doi: 10.1128/MMBR.00010-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waschburger E., Kulcheski F.R., Veto N.M., Margis R., Margis-Pinheiro M., Turchetto-Zolet A.C. Genome-wide analysis of the glycerol-3-phosphate acyltransferase (GPAT) gene family reveals the evolution and diversification of plant GPATs. Genet. Mol. Biol. 2018;41:355–370. doi: 10.1590/1678-4685-gmb-2017-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cove D.J., Knight C.D. The Moss Physcomitrella patens, a model system with potential for the study of plant reproduction. Plant Cell. 1993;5:1483–1488. doi: 10.2307/3869798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singer S.D., Chen G., Mietkiewska E., Tomasi P., Jayawardhane K., Dyer J.M., Weselake R.J. Arabidopsis GPAT9 contributes to synthesis of intracellular glycerolipids but not surface lipids. J. Exp. Bot. 2016;67:4627–4638. doi: 10.1093/jxb/erw242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukuda S., Hirasawa E., Takemura T., Takahashi S., Chokshi K., Pancha I., Tanaka K., Imamura S. Accelerated triacylglycerol production without growth inhibition by overexpression of a glycerol-3-phosphate acyltransferase in the unicellular red alga Cyanidioschyzon merolae. Sci. Rep. 2018;8:12410. doi: 10.1038/s41598-018-30809-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strotbek C., Krinninger S., Frank W. The moss Physcomitrella patens: Methods and tools from cultivation to targeted analysis of gene function. Int. J. Dev. Biol. 2013;57:553–564. doi: 10.1387/ijdb.130189wf. [DOI] [PubMed] [Google Scholar]
- 36.Beike A.K., Jaeger C., Zink F., Decker E.L., Reski R. High contents of very long-chain polyunsaturated fatty acids in different moss species. Plant Cell Rep. 2014;33:245–254. doi: 10.1007/s00299-013-1525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grimsley N.H., Grimsley J.M., Hartmann E. Fatty acid composition of mutants of the moss Physcomitrella patens. Phytochemistry. 1981;20:1519–1524. doi: 10.1016/S0031-9422(00)98523-6. [DOI] [Google Scholar]
- 38.Resemann H.C., Lewandowska M., Gomann J., Feussner I. Membrane lipids, waxes and oxylipins in the moss model organism Physcomitrella patens. Plant Cell Physiol. 2019;60:1166–1175. doi: 10.1093/pcp/pcz006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beisson F., Li Y., Bonaventure G., Pollard M., Ohlrogge J.B. The acyltransferase GPAT5 is required for the synthesis of suberin in seed coat and root of Arabidopsis. Plant Cell. 2007;19:351–368. doi: 10.1105/tpc.106.048033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li-Beisson Y., Pollard M., Sauveplane V., Pinot F., Ohlrogge J., Beisson F. Nanoridges that characterize the surface morphology of flowers require the synthesis of cutin polyester. Proc. Natl. Acad. Sci. USA. 2009;106:22008–22013. doi: 10.1073/pnas.0909090106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y., Beisson F., Koo A.J.K., Molina I., Pollard M., Ohlrogge J. Identification of acyltransferases required for cutin biosynthesis and production of cutin with suberin-like monomers. Proc. Natl. Acad. Sci. USA. 2007;104:18339–18344. doi: 10.1073/pnas.0706984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang W., Simpson J.P., Li-Beisson Y., Beisson F., Pollard M., Ohlrogge J.B. A land-plant-specific glycerol-3-phosphate acyltransferase family in Arabidopsis: Substrate specificity, sn-2 preference, and evolution. Plant Physiol. 2012;160:638–652. doi: 10.1104/pp.112.201996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng Z., Xia Q., Dauk M., Shen W., Selvaraj G., Zou J. Arabidopsis AtGPAT1, a member of the membrane-bound glycerol-3-phosphate acyltransferase gene family, is essential for tapetum differentiation and male fertility. Plant Cell. 2003;15:1872–1887. doi: 10.1105/tpc.012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewin T.M., Wang P., Coleman R.A. Analysis of amino acid motifs diagnostic for the sn-glycerol-3-phosphate acyltransferase reaction. Biochemistry. 1999;38:5764–5771. doi: 10.1021/bi982805d. [DOI] [PubMed] [Google Scholar]
- 45.Jung J.H., Kim H., Go Y.S., Lee S.B., Hur C.G., Kim H.U., Suh M.C. Identification of functional BrFAD2-1 gene encoding microsomal delta-12 fatty acid desaturase from Brassica rapa and development of Brassica napus containing high oleic acid contents. Plant Cell Rep. 2011;30:1881–1892. doi: 10.1007/s00299-011-1095-x. [DOI] [PubMed] [Google Scholar]
- 46.Gidda S.K., Shockey J.M., Rothstein S.J., Dyer J.M., Mullen R.T. Arabidopsis thaliana GPAT8 and GPAT9 are localized to the ER and possess distinct ER retrieval signals: Functional divergence of the dilysine ER retrieval motif in plant cells. Plant Physiol. Biochem. 2009;47:867–879. doi: 10.1016/j.plaphy.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Omasits U., Ahrens C.H., Müller S., Wollscheid B. Protter: Interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics. 2014;30:884–896. doi: 10.1093/bioinformatics/btt607. [DOI] [PubMed] [Google Scholar]
- 48.Li M., Welti R., Wang X. Quantitative profiling of Arabidopsis polar glycerolipids in response to phosphorus starvation. Roles of phospholipases Dζ1 and Dζ2 in phosphatidylcholine hydrolysis and digalactosyldiacylglycerol accumulation in phosphorus-starved plants. Plant Physiol. 2006;142:750–761. doi: 10.1104/pp.106.085647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Z., Ohlrogge J.B. Turnover of fatty acids during natural senescence of Arabidopsis, Brachypodium, and switchgrass and in Arabidopsis beta-oxidation mutants. Plant Physiol. 2009;150:1981–1989. doi: 10.1104/pp.109.140491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanjaya, Miller R., Durrett T.P., Kosma D.K., Lydic T.A., Muthan B., Koo A.J., Bukhman Y.V., Reid G.E., Howe G.A., et al. Altered lipid composition and enhanced nutritional value of Arabidopsis leaves following introduction of an algal diacylglycerol acyltransferase 2. Plant Cell. 2013;25:677–693. doi: 10.1105/tpc.112.104752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.An D., Suh M.C. Overexpression of Arabidopsis WRI1 enhanced seed mass and storage oil content in Camelina sativa. Plant Biotechnol. Rep. 2015;9:137–148. doi: 10.1007/s11816-015-0351-x. [DOI] [Google Scholar]
- 52.Huang C.Y., Chung C.I., Lin Y.C., Hsing Y.I., Huang A.H. Oil bodies and oleosins in Physcomitrella possess characteristics representative of early trends in evolution. Plant Physiol. 2009;150:1192–1203. doi: 10.1104/pp.109.138123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishida I., Tasaka Y., Shiraishi H., Murata N. The gene and the RNA for the precursor to the plastid-located glycerol-3-phosphate acyltransferase of Arabidopsis thaliana. Plant Mol. Biol. 1993;21:267–277. doi: 10.1007/BF00019943. [DOI] [PubMed] [Google Scholar]
- 54.Yang W., Pollard M., Li-Beisson Y., Beisson F., Feig M., Ohlrogge J. A distinct type of glycerol-3-phosphate acyltransferase with sn-2 preference and phosphatase activity producing 2-monoacylglycerol. Proc. Natl. Acad. Sci. USA. 2010;107:12040–12045. doi: 10.1073/pnas.0914149107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee S.B., Yang S.U., Pandey G., Kim M.-S., Hyoung S., Choi D., Shin J.S., Suh M.C. Occurrence of land-plant-specific glycerol-3-phosphate acyltransferases enabled cuticle formation and gametophore development in Physcomitrella patens. New Phytol. 2019 doi: 10.1111/nph.16311. (In press) [DOI] [PubMed] [Google Scholar]
- 56.McCartney A.W., Dyer J.M., Dhanoa P.K., Kim P.K., Andrews D.W., McNew J.A., Mullen R.T. Membrane-bound fatty acid desaturases are inserted co-translationally into the ER and contain different ER retrieval motifs at their carboxy termini. Plant J. 2004;37:156–173. doi: 10.1111/j.1365-313X.2004.01949.x. [DOI] [PubMed] [Google Scholar]
- 57.Vanhercke T., El Tahchy A., Liu Q., Zhou X.R., Shrestha P., Divi U.K., Ral J.P., Mansour M.P., Nichols P.D., James C.N., et al. Metabolic engineering of biomass for high energy density: Oilseed-like triacylglycerol yields from plant leaves. Plant Biotechnol. J. 2014;12:231–239. doi: 10.1111/pbi.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.An D., Kim H., Ju S., Go Y.S., Kim H.U., Suh M.C. Expression of Camelina WRINKLED1 isoforms rescue the seed phenotype of the Arabidopsis wri1 mutant and increase the triacylglycerol content in tobacco leaves. Front. Plant Sci. 2017;8:34. doi: 10.3389/fpls.2017.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ivarson E., Leiva-Eriksson N., Ahlman A., Kanagarajan S., Bulow L., Zhu L.H. Effects of overexpression of WRI1 and hemoglobin genes on the seed oil content of Lepidium campestre. Front. Plant Sci. 2016;7:2032. doi: 10.3389/fpls.2016.02032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu J., Hua W., Zhan G., Wei F., Wang X., Liu G., Wang H. Increasing seed mass and oil content in transgenic Arabidopsis by the overexpression of wri1-like gene from Brassica napus. Plant Physiol. Biochem. 2010;48:9–15. doi: 10.1016/j.plaphy.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 61.Shen B., Allen W.B., Zheng P., Li C., Glassman K., Ranch J., Nubel D., Tarczynski M.C. Expression of ZmLEC1 and ZmWRI1 increases seed oil production in maize. Plant Physiol. 2010;153:980–987. doi: 10.1104/pp.110.157537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Banaś W., Carlsson A.S., Banaś A. Effect of overexpression of PDAT gene on Arabidopsis growth rate and seed oil content. J. Agric. Sci. 2014;6:65–79. doi: 10.5539/jas.v6n5p65. [DOI] [Google Scholar]
- 63.Jako C., Kumar A., Wei Y., Zou J., Barton D.L., Giblin E.M., Covello P.S., Taylor D.C. Seed-specific over-expression of an Arabidopsis cDNA encoding a diacylglycerol acyltransferase enhances seed oil content and seed weight. Plant Physiol. 2001;126:861–874. doi: 10.1104/pp.126.2.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kelly A.A., van Erp H., Quettier A.L., Shaw E., Menard G., Kurup S., Eastmond P.J. The sugar-dependent1 lipase limits triacylglycerol accumulation in vegetative tissues of Arabidopsis. Plant Physiol. 2013;162:1282–1289. doi: 10.1104/pp.113.219840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Erp H., Kelly A.A., Menard G., Eastmond P.J. Multigene engineering of triacylglycerol metabolism boosts seed oil content in Arabidopsis. Plant Physiol. 2014;165:30–36. doi: 10.1104/pp.114.236430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim H., Park J.H., Kim D.J., Kim A.Y., Suh M.C. Functional analysis of diacylglycerol acyltransferase1 genes from Camelina sativa and effects of CsDGAT1B overexpression on seed mass and storage oil content in C. sativa. Plant Biotechnol. Rep. 2016;10:141–153. doi: 10.1007/s11816-016-0394-7. [DOI] [Google Scholar]
- 67.Wu X.L., Liu Z.H., Hu Z.H., Huang R.Z. BnWRI1 coordinates fatty acid biosynthesis and photosynthesis pathways during oil accumulation in rapeseed. J. Integr. Plant Biol. 2014;56:582–593. doi: 10.1111/jipb.12158. [DOI] [PubMed] [Google Scholar]
- 68.Weber H., Borisjuk L., Wobus U. Sugar import and metabolism during seed development. Trends Plant Sci. 1997;2:169–174. doi: 10.1016/S1360-1385(97)85222-3. [DOI] [Google Scholar]
- 69.Paya-Milans M., Aznar-Moreno J.A., Balbuena T.S., Haslam R.P., Gidda S.K., Perez-Hormaeche J., Mullen R.T., Thelen J.J., Napier J.A., Salas J.J., et al. Sunflower HaGPAT9-1 is the predominant GPAT during seed development. Plant Sci. 2016;252:42–52. doi: 10.1016/j.plantsci.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 70.Ashton N.W., Cove D.J. The isolation and preliminary characterisation of auxotrophic and analogue resistant mutants of the moss, Physcomitrella patens. Mol. Gen. Genet. 1977;154:87–95. doi: 10.1007/BF00265581. [DOI] [Google Scholar]
- 71.Nishiyama T., Hiwatashi Y., Sakakibara I., Kato M., Hasebe M. Tagged mutagenesis and gene-trap in the moss, Physcomitrella patens by shuttle mutagenesis. DNA Res. 2000;7:9–17. doi: 10.1093/dnares/7.1.9. [DOI] [PubMed] [Google Scholar]
- 72.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buda G.J., Barnes W.J., Fich E.A., Park S., Yeats T.H., Zhao L., Domozych D.S., Rose J.K. An ATP binding cassette transporter is required for cuticular wax deposition and desiccation tolerance in the moss Physcomitrella patens. Plant Cell. 2013;25:4000–4013. doi: 10.1105/tpc.113.117648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hajdukiewicz P., Svab Z., Maliga P. The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol. Biol. 1994;25:989–994. doi: 10.1007/BF00014672. [DOI] [PubMed] [Google Scholar]
- 75.Seo P.J., Lee S.B., Suh M.C., Park M.J., Go Y.S., Park C.M. The MYB96 transcription factor regulates cuticular wax biosynthesis under drought conditions in Arabidopsis. Plant Cell. 2011;23:1138–1152. doi: 10.1105/tpc.111.083485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martinez-Trujillo M., Limones-Briones V., Cabrera-Ponce J.L. Improving transformation efficiency of Arabidopsis thaliana by modifying the floral dip method. Plant Mol. Biol. Rep. 2004;22:63–70. doi: 10.1007/BF02773350. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.