Abstract
The Arabidopsis male-sterile phenotype has been a wonderful model for jasmonate action in plants. It has allowed us to identify transcription factors that control gene expression during stamen and pollen maturation and provided for the discovery of the JAZ repressor proteins and the mechanism of jasmonate signaling. More recently, it has revealed intriguing details of the spatial localization of jasmonate synthesis and perception in stamen tissues. The extensive and thoughtful application of protein–protein interaction assays to identify JAZ-interacting partners has led to a much richer appreciation of the mechanisms by which jasmonate integrates with the actions of other hormones to regulate plant growth and physiological responses. This integration is strikingly evident in stamen and pollen development in Arabidopsis, which requires the actions of many hormones. Just as importantly, it is now evident that jasmonate has very different actions during flower development and reproduction in other plant species. This integration and diversity of action indicates that many exciting discoveries remain to be made in this area of jasmonate hormone signaling and response.
Keywords: Arabidopsis, flower development, jasmonate, JAZ, transcript profiling
1. Introduction
Jasmonate (JA) hormone controls many aspects of plant biology, ranging from stress responses to development. These include defense against herbivores and microbial pathogens, as well as responses to ultraviolet radiation, drought, ozone, and other abiotic stresses [1,2,3,4,5]. In healthy plants, JA regulates aspects of carbon partitioning, reproductive development, and senescence [6,7,8,9]. Jasmonate signaling leads to large-scale changes in gene expression, and hundreds of downstream genes have been identified [10]. Mutants of Arabidopsis have provided the means to dissect the biochemistry and cell biology of JA synthesis [2,11], and the conversion of JA to the active hormone jasmonoyl-isoleucine (JA-Ile) [12,13]. In addition, Arabidopsis mutants were key tools in the discovery of the central role of JA in marshalling plant defenses against herbivores and necrotrophic pathogens [14,15]. Details of many of these topics are covered in other articles in this issue. In this review, we will describe the discovery of the fact that JA is required for stamen and pollen maturation in Arabidopsis, and how investigations of the resulting male-sterile phenotype contributed to our current mechanistic understanding of JA signaling.
2. Two Arabidopsis Mutants Reveal Jasmonate’s Roles in Stamen and Pollen Maturation
Coronatine is a phytotoxin virulence factor that is produced by several bacteria including Pseudomonas syringae pv tomato DC3000 (Pst DC3000) [16]. It has been known for some time that coronatine acts through activation of JA signaling [7,16]. In 1994, a screen for mutants of Arabidopsis resistant to coronatine identified the coi1 (coronatine insensitive1) mutant that was also resistant to JA and male sterile (Figure 1) [7]. At about the same time, our laboratory was studying photosynthesis in mutants of Arabidopsis with reduced levels of polyunsaturated fatty acids in chloroplast membrane lipids. The very high proportions of trienoic fatty acids (including linolenate) found in chloroplast membranes of all higher plants suggest that these lipid structures might be essential for photosynthesis. To test this notion, we produced a fad3–fad7–fad8 triple mutant (fad = fatty acid desaturation) that completely lacked trienoic fatty acids. To our surprise, photosynthesis at 22 °C in the triple mutant was barely affected and vegetative growth of mutant plants was identical to wild-type controls; however, the mutant plants were male sterile [17]. Importantly, treatment of fad3–fad7–fad8 flowers with JA, which is derived from linolenate, rendered them fertile (Figure 1) [17].
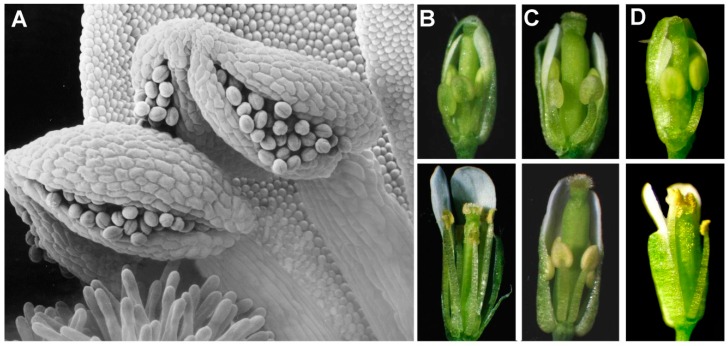
Figure 1.
Two Arabidopsis mutants that unlocked the mechanism of jasmonate signaling. (A) Self-pollinating species like Arabidopsis depend on coordinated development of the anther, the pollen, and the anther filament to ensure that the mature anther is positioned above the stigma at the time of anther dehiscence and pollen release. (B) In wild-type flowers, filament elongation, pollen maturation, and dehiscence all depend on jasmonate signaling. (C) The coi1 (coronatine insensitive1) mutant is male-sterile and does not respond to jasmonate treatment. (D) In the fad3–fad7–fad8 triple mutant (fad = fatty acid desaturation) (and other jasmonate-synthesis mutants), filament elongation, anther dehiscence, and pollen function are all defective as in coi1, but treating flower buds with jasmonate makes the flowers as fertile as wild type. In (B–D), the upper panels show stage 11 flowers and the lower panels stage 15.
Characterization of flowers in the Arabidopsis fad3–fad7–fad8 triple mutant identified three features of the male-sterile phenotype: (1) floral organs develop normally within the closed bud, but the anther filaments do not elongate to position the locules above the stigma at the time of flower opening; (2) the anther locules do not dehisce at the time of flower opening (although limited dehiscence occurs later); and (3) even though pollen on mutant plants develops to the trinucleate stage, the pollen grains are predominantly (>97%) inviable. Treatment of flower buds with JA corrected all three defects, providing for rates of pollen germination equivalent to wild type in in vitro tests, and abundant seed set on treated plants [17]. Restoration of fertility was stage specific—only flower buds corresponding to the transition between stages 11 and 12 in floral development, immediately before flower opening, responded to JA [17].
3. A Transcriptional Cascade Directing Pollen and Stamen Development
The ability to screen male-sterile mutants for complementation by JA treatment led to the isolation of a mutant in opr3, the gene that encodes the isomer-specific 12-oxo-phytodienoic acid reductase that acts in the JA synthesis pathway [18,19]. Although opr3 plants do not accumulate JA, they express some defense genes and are resistant to insect attack [20]. Other mutants in JA synthesis are also male-sterile [21]. Since the opr3 mutant could be made fertile by JA application, we carried out transcriptional profiling and followed the time course of gene-expression changes in opr3 stamens for 22 h following JA treatment [22]. Analysis of data from four time points following JA or control OPDA treatment (0.5, 2, 8, and 22 h) identified 1296 genes that had expressions that were specifically altered by JA treatment. These genes amount to a stamen-specific JA transcriptome that is quite different from the transcriptome involved in defense responses. Most (70%) of the genes were expressed in the parental tissues rather than the pollen. Using bioinformatics, we mapped many of the upregulated genes to metabolic pathways that are likely to be induced during stamen maturation. Pathways identified include the synthesis of terpenoid volatiles, which have roles in attracting insects, and synthesis of wax compounds that are functionally important components of the pollen kit [22]. Other aspects of flower development that are affected in JA mutants include petal elongation, stigma papillae, and carpel vasculature [1,7,22].
We identified 13 transcription factors induced by JA that potentially act in the control of stamen maturation. Two of these transcription factors, MYB21 and MYB24, are the only members of subgroup 19 of the R2R3 MYB family of proteins. The MYB21 gene had previously been identified in Giltsu Choi’s laboratory as being ectopically expressed in vegetative tissues of the constitutive photomorphogenic1 (cop1) mutant [23], and we collaborated to investigate myb21 and myb24 mutants. The null myb21 mutant exhibited a JA-insensitive flower phenotype, and male fertility was significantly reduced. The phenotype of a myb24 mutant was akin to wild type, but examination of double-mutant myb21–myb24 plants established that inclusion of the myb24 mutation in the plants aggravated all three aspects of the myb21 phenotype [22]. These results show that JA induces MYB21 and MYB24 activity and thereby controls essential aspects of stamen development. Since this discovery, additional studies have provided more details about the induction and actions of other transcription factors in addition to MYB21 and MYB24 that coordinate anther and pollen maturation and initiate anther filament elongation, ensuring that the mature anther is correctly placed relative to the stigma when the anthers dehisce [24,25].
4. Discovery of the JAZ Repressors
In 1998, when map-based cloning identified the COI1 locus, it was revealed to encode an F-box protein [26] expected to participate in a Skp/Cullin/F-box complex (SCFCOI1) acting as an E3 ubiquitin ligase [27]. In company with the phenotype of coi1 mutants, this discovery suggested that SCFCOI1 ubiquitination of proteins was essential to JA hormonal activity and required for all JA responses. With the demonstration that COI1 was an F-box protein, two questions became central to the analysis of JA signaling for the next decade: (1) What proteins are targets of the SCFCOI1 complex? (2) How does (presumed) ubiquitination and degradation of these proteins via 26S protease action enable JA hormone activity? Initially, the searches for SCFCOI1 targets using genetic approaches were unavailing. Wide-ranging genetic screens failed to detect candidates for SCFCOI1 targets; searches for positive effectors [28,29,30], negative effectors [28,31], and components downstream of COI1 [32], as well as searches for COI1-interacting proteins [33,34], were all unsuccessful. Investigations identifying genes whose expression responded to JA [35] or that relied on COI1 [36] were likewise unsuccessful in detecting candidates, and failed to provide indications about the exact nature of the interaction between SCFCOI1 and transcription of genes responsive to JA regulation.
The search finally ended when transcripts of opr3 stamens were profiled, resulting in identifying the JAZ protein family and discovery of their role in JA signaling. Immediately following JA application (at the 0.5 h time point), only 31 genes showed significant induction. Amongst these, eight encoded proteins with no known function, and the predicted protein sequences of the eight were used to query the Conserved-Domain Database at NCBI using Reverse Position-Specific BLAST (RPS-BLAST) [37]. Each of the eight contained a 28 amino-acid domain with no known function, but occurring in ZIM, a protein considered likely be a transcription factor [38], and so were named JAsmonate Zim-domain proteins. The eight JAZ genes first identified were induced in stamens 6 to 40 fold after JA treatment, and were also found to be expressed widely during plant development. Experimental data using 7 day-old seedlings treated with 10 μM JA indicated strong, 8 to 60 fold induction of all eight genes in the first half hour after treatment [39].
There are now 13 recognized JAZ proteins in Arabidopsis [39,40,41,42]. Homology among them is principally confined to two domains. The ZIM domain of 27 amino acids is located near the middle of the peptide sequence; it contains a TIFY motif (TIF(F/Y)XG) [43] and two invariant alanine residues. The Jas domain of 22 amino acids lies close to the C-terminus and is strongly conserved across the JAZ family. In some JAZ, a less conserved N-terminal region contains an EAR (ethylene response factor associated amphiphilic repression) motif and/or binding determinants for some transcription factors [44,45].
Jasmonoyl-isoleucine and coronatine promote binding of the JAZ proteins to COI1 through the Jas domain [39,46,47,48]. Deletion of the Jas domain or specific mutations within it prevent binding, so that JAZΔJas proteins become constitutive repressors [39,40,46,47,49]. Expression of these altered JAZ proteins in plants induced a dominant JA-resistant phenotype [39,40,44,45]. Experiments with plants expressing JAZ1-GUS [39] and JAZ3-GFP proteins [40] demonstrated that turnover of these proteins does indeed require COI1 and the 26S proteasome, as well as the Jas domain of the JAZ proteins. The rapid induction of some of the JAZ genes in response to JA signaling likely results in extensive re-synthesis of the JAZ repressors and attenuation of the JA response soon after passage of the signal [44,45]. However, genes that encode enzymes of JA-Ile synthesis and the gene encoding MYC2 are also strongly induced on a similar timescale. Transcriptional induction for all these genes occurs in the presence of cycloheximide indicating that it is not dependent on the synthesis of intervening transcription factors [50]. Thus, genes involved in generation and transmission of the JA signal are induced alongside genes encoding the repressors.
These findings suggested a model in which JAZ proteins are repressors that bind to transcription factors and prevent the transcription of JA-responsive genes by recruiting co-repressor proteins into an inhibited complex. It is now known that the ZIM domain recruits corepressors of the TOPLESS family via the EAR-domain protein NINJA [51] and also, in some cases, directly via an EAR domain on the JAZ protein. Three JAZ proteins have been shown to interact with the histone deacetylase enzyme, HDA6 [52], suggesting that JAZ repression may also involve chromatin remodeling.
In the ten years since these foundational discoveries, genetic and molecular approaches have elaborated many details of JA signaling and response that modify a wide range of growth and defense processes in all tissues of Arabidopsis and other plants [44,45,53,54]. Perhaps more importantly, screening and identification of JAZ-interacting proteins have led to discoveries that demonstrate how completely JA hormone action is integrated with the actions of other hormones, including gibberellin, abscisic acid, salicylic acid, ethylene, and auxin [55,56]. This is true in flower development as much as in any other aspect of plant development and physiology [57,58].
5. Jasmonate Receptor Crystal Structure
The identification of the JAZ proteins and evidence that the Jas domain is the site of binding to COI1 provided the tools necessary to solve the structure of JA-Ile bound to its receptor [46]. Crystal structure analysis revealed that JA-Ile (or the analogue, coronatine) is oriented vertically in its interaction with 11 COI1 residues of the ligand-binding pocket of this F-box protein class, which is formed by a leucine-rich-repeat/loop structure. Three other residues form a cavity that binds only the (3R,7S) active isomer of JA-Ile [59], but does not accept the (3R,7R) inactive isomer. While the JA keto group and Ile carboxyl group are left exposed and accessible to interaction with JAZ protein through its Jas domain, the rest of the JA-Ile ligand is concealed from interaction by the COI1 residues surrounding the binding site. Surprisingly, experiments assessing the strength of the interaction using radioligand biding demonstrated very weak binding between JA-Ile and COI1, although the crystal structure indicated many interactions were probable. However, when JAZ protein was incorporated into such an assay, specific binding increased 50 fold, demonstrating that both the JAZ and COI1 proteins function as interdependent co-receptors in perception of JA hormone [46]. The structural elements of the Jas domain which bind COI1 and JA-Ile (residues 200–220 in the JAZ1 protein) are in two separate regions of the domain. The N-terminal sequence, ELPIA, is a loop-forming structure interacting with both the carbonyl group found on JA-Ile as well as identified COI1 residues, with the result that the binding site of the JA-Ile co-receptor is closed to hormone access. The C-terminal portion of the Jas peptide sequence produces an α-helical structure interacting with amino acids lining the central channel of the leucine-rich repeat ring formed by COI1, and this supplementary binding is indispensable for the co-receptors to function.
Because the related TIR1 receptor was found to contain a molecule of inositol hexakisphosphate (IP6) [60], it was not particularly surprising to see evidence for a similar molecule in crystals of recombinant COI1. However, the molecule in COI1 was found to be inositol 1,2,4,5,6-pentakisphosphate (IP5) and to be located close to the JA-Ile binding pocket where one of the phosphate residues coordinates three arginine residues of COI1 and Arg206 of the Jas peptide, which are all also involved in interactions with JA-Ile. Consistent with this structure, removal of IP5 by dialysis inactivated the receptor complex and the inactive form could be reactivated by addition of IP5 [46]. Subsequent studies have concluded that, in plants, an inositol pyrophosphate (IP8) is the physiological cofactor in COI1 that has a key role in assembly and functioning of the hormone receptor [61,62].
6. What Are the Sites of JA Perception in the Stamen?
The male-sterile phenotype of Arabidopsis mutants defective in JA synthesis or perception also allowed us to answer the question of where in the stamen JA signaling is required to correct the defects in filament elongation, anther dehiscence, and pollen function. To do this, we constructed a DNA sequence encoding a COI1-YFP fusion protein and expressed it in coi1 mutant plants using different tissue-specific promoters [63]. The yellow fluorescent protein (YFP) allowed localization of the COI1 protein by confocal microscopy. As a control, we first used the endogenous COI1 promoter. Confocal microscopy confirmed broad expression of the COI1-YFP protein throughout the stamen and anther tissues of the transgenic plants, and fertility was fully restored, indicating the COI1-YFP fusion protein was functional.
Given that stamen filament elongation does not occur in JA mutants, the filament was expected to be a target of JA signaling. During their characterization of the JA biosynthesis mutant defective anther dehiscence1 (dad1), Ishiguro et al. [21] discovered that the filament is likely the major or sole site of JA synthesis and suggested that the filament may be the only essential target of JA for stamen and pollen maturation and function. The model proposed by Ishiguro et al. showed JA signaling in the upper filament promoting sucrose import into the filament. The lower water potential then drives water transfer from the anther locules and endothecium into the filaments. Dehydration of the anther tissues and pollen could then lead to maturation of pollen grains and promote anther dehiscence by desiccation of the endothecium, while the filament undergoes elongation [21]. When we expressed a DAD1::COI1-YFP transgene in coi1 plants, the YFP signal was confined to the filament and filament elongation did occur, although it was delayed relative to wild-type controls. However, anther dehiscence did not occur, and pollen remained inviable [63]. These results indicate that there must be sites of JA perception in the anther and suggest that JA synthesized in the filament may be transported into the anther to initiate JA responses there. Because the tapetum has essential roles in pollen maturation, we next expressed our COI1-YFP construct behind the tapetum-specific A9 promoter. In this case, strong YFP fluorescence was seen in tapetal cells, but no aspect of the coi1 phenotype was altered and the transgenic plants were completely sterile, indicating that any JA-dependent responses in the tapetum are insufficient to affect any aspect of the male-sterile phenotype.
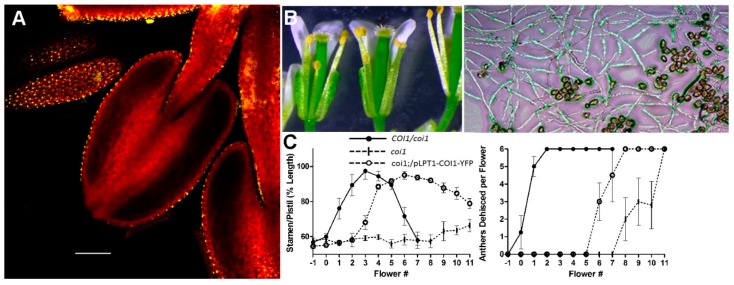
In contrast to this result, the WUS (WUSCHEL) and LTP1 (LIPID TRANSFER PROTEIN1) promoters that provided COI1-YFP expression in the stomium and epidermis restored both pollen function and anther dehiscence. In particular, the LTP1::COI1-YFP construct that is only expressed in the epidermal cells of the anther and filament resulted in fully fertile plants, although both filament elongation and anther dehiscence were delayed relative to wild-type controls (Figure 2) [63].
Figure 2.
Tissue-specific expression of a COI1-YFP transgene rescues coi1 sterility. (A) coi1 plants expressing a LTPpro::COI1-YFP gene show the YFP signal only in epidermal cells of the filament and anther. (B) In the transgenic plants, filament elongation, anther dehiscence (left), and pollen-tube germination and growth (right) are all sufficient to restore fertility, although (C) filament elongation and anther dehiscence are delayed compared to wild type. (Adapted from Reference [63], with permission.).
These results indicate that JA perception only in the epidermis of the anther and filament is sufficient for anther dehiscence and for pollen viability. The stomium is comprised of epidermal cells, so this readily explains JA control of anther dehiscence [18]. Elongation of the filament may also be rationalized since tissue growth in other plant organs has been shown to be controlled by the epidermis [64]. Epidermal control of pollen viability by JA is perhaps more surprising, but it is consistent with the observation that, genetically, it is the sporophyte tissue that mediates JA-regulated male fertility. When JA signaling is blocked (as in the coi1 mutant), some sporophytic process compromises pollen viability. These results demonstrate that triggering JA-receptor action solely in the epidermis provides for pollen viability. One possibility is that JA signaling in the epidermis leads to water loss from the pollen (as well as the anther tissues), and this triggers the final maturation of the pollen. Desiccation during later stages of anther development has been observed in previous studies and was proposed to account for JA-dependent pollen maturation [21]. Alternatively, the anther epidermis may produce a transmissible signal in response to JA that is responsible for final pollen maturation. This scenario is similar to the cell non-autonomous complementation of leaf expansion by epidermal brassinosteroid perception [64] and is equally consistent with the results of our COI1-YFP complementation studies [63].
7. Jasmonate-Regulated Processes in Flower Development Are Very Different in Other Angiosperms
The reproductive phenotype of Arabidopsis JA mutants has been a rewarding source of discoveries in many aspects of JA synthesis and signaling. However, the story of JA’s role in plant reproduction becomes even more interesting as we consider the role of JA in flower development beyond this model species. Many of the actions of JA in herbivore and pathogen defense and in vegetative plant growth are broadly observed across angiosperm species. However, as Carl Linnaeus observed, evolution has worked diligently to tweak the reproductive organs and processes of plants because they are the major basis for speciation. Thus, a tomato mutant (jai1) defective in COI1 is not male sterile, although pollen viability is lowered with premature dehydration and dehiscence of the anther [65]. Instead jai1 plants are defective in maternal control of seed development [66]. The tomato MYB21 homologue has been shown to be a component of JA signaling in tomato reproduction [67], suggesting a core pathway similar to that in Arabidopsis is providing for a rather distinct output.
In the monocots, the role of JA in floral development is very different from these two dicot species. In rice, the floral spikelet is a condensed branch in which florets are enclosed in leaf-like glumes that open to allow release of wind-dispersed pollen and pollination of the pistil. In this system, investigations of several mutants, including extra glume1 (eg1) encoding a rice DAD1 homologue and eg2 encoding an OsJAZ1 with altered Jas domain [68], indicate that JA acts very early in spikelet development. Although the phenotypes of eg1 and eg2 are rather mild, possibly due to the redundancy in the encoded gene functions, additional genetic and molecular analyses suggest a model in which JA-mediated degradation of OsJAZ allows the rice MYC2 homologue to induce expression of OsMADS1, OsMADS7, and OsMADS8 transcription factors that initiate and control spikelet development [68,69]. These rice MADS box proteins are homologues of the SEPALLATA family of transcription factors in Arabidopsis that are required for expression of B- and C-class homeotic regulators of floral development [70,71]. So, in rice, the core JA-signaling module, JAZ/JA-Ile/COI1/MYC2, appears to have been redirected (relative to Arabidopsis) to control fundamental processes at the very start of spikelet and floret development, rather than being required only for the final stages of stamen and pollen maturation.
In maize, separate male and female spikelets of flowers form, both at the topmost male tassel as well as at the lateral meristems responsible for producing the female ears. Despite their different physical appearance, maize flowers form the same elemental pattern as do other flowering plants. Their organs form in four spirals, and both the identity of the organs and their development are controlled by collaboration among homeotic genes in overlapping tripartite regions of the floral meristem denoted A, B, and C [72,73]. When the programmed elaboration of ABC is completed, the male tassels and female ears are barely distinguishable, each having recognizable onset of both pistil and stamen development. Differentiation occurs as the flowers mature, when nascent pistils in the tassel are aborted by a cell death process, while in the ear, stamens undergo arrested cell cycles and deterioration of cells [74,75]. Among the abundant mutants altering sex determination in maize [73,76,77] are those termed tasselseed (ts), whose typically staminate tassels instead develop pistillate florets. The ts1 mutations are recessive and have no effect on early spikelet and floret development, but instead result in a completely feminized tassel. When ts1 was cloned [77], it turned out to encode a 13-lipoxygenase, predicted to localize in the chloroplast, that catalyzes polyunsaturated fatty acid peroxidation. Because the synthesis of JA occurs by means of a 13-lipoxygenated product, 13-hydroperoxylinolenic acid [11], the clear implication was that JA signaling could be important in controlling the pathway identified by the tasselseed mutant. Examination of JA levels of tassels from plants homozygous for ts1-ref revealed that the concentration was less than 10% of the level obtained from measurements of wild-type tassels. Further, the mutant tasselseed phenotype was partially rescued when tassels of the mutant were treated with 1 mM JA [77]. Some other tasselseed mutants have also been found defective in JA signaling in the developing tassel [77,78,79,80].
Thus, with four model plant species studied, we have four different stages of flower and reproductive development targeted by JA hormone signaling. What might the rest of the plant kingdom reveal?
Acknowledgments
We are indebted to all the researchers in our laboratory who have contributed to our investigation of jasmonate signaling.
Author Contributions
Both authors discussed and wrote this review.
Funding
Research on jasmonate in our laboratory is supported by grant IOS-1555581 from the US National Science Foundation and by the Agricultural Research Center at Washington State University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Browse J. Jasmonate, an oxylipin signal with many roles in plants. Vitam. Horm. 2005;72:431–456. doi: 10.1016/S0083-6729(05)72012-4. [DOI] [PubMed] [Google Scholar]
- 2.Browse J. Jasmonate passes muster, a receptor and targets for the defense hormone. Annu. Rev. Plant Biol. 2009;60:183–205. doi: 10.1146/annurev.arplant.043008.092007. [DOI] [PubMed] [Google Scholar]
- 3.Conconi A., Smerdon M.J., Howe G.A., Ryan C.A. The octadecanoid signalling pathway in plants mediates a response to ultraviolet radiation. Nature. 1996;383:826–829. doi: 10.1038/383826a0. [DOI] [PubMed] [Google Scholar]
- 4.Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- 5.Howe G.A., Jander G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008;59:41–66. doi: 10.1146/annurev.arplant.59.032607.092825. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan-Wollaston V., Page T., Harrison E., Breeze E., Lim P.O., Nam H.G., Lin J.F., Wu S.H., Swidzinski J., Ishizaki K., et al. Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J. 2005;42:567–585. doi: 10.1111/j.1365-313X.2005.02399.x. [DOI] [PubMed] [Google Scholar]
- 7.Feys B., Benedetti C.E., Penfold C.N., Turner J.G. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell. 1994;6:751–759. doi: 10.2307/3869877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang H., Liu B., Liu L., Song S. Jasmonate action in plant growth and development. J. Exp. Bot. 2017;68:1349–1359. doi: 10.1093/jxb/erw495. [DOI] [PubMed] [Google Scholar]
- 9.Xu E., Vaahtera L., Brosché M. Roles of defense hormones in the regulation of ozone-induced changes in gene expression and cell death. Mol. Plant. 2009;8:1776–1794. doi: 10.1016/j.molp.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Hickman R., Van Verk M.C., Van Dijken A.J.H., Mendes M.P., Vroegop-Vos I.A., Caarls L., Steenbergen M., Van der Nagel I., Wesselink G.J., Jironkin A., et al. Architecture and dynamics of the jasmonic acid gene regulatory network. Plant Cell. 2017;29:2086–2105. doi: 10.1105/tpc.16.00958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vick B.A., Zimmerman D.C. The biosynthesis of jasmonic acid, a physiological role for plant lipoxygenase. Biochem. Biophys. Res. Commun. 1983;111:470–477. doi: 10.1016/0006-291X(83)90330-3. [DOI] [PubMed] [Google Scholar]
- 12.Staswick P.E., Su W., Howell S.H. Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc. Natl. Acad. Sci. USA. 1992;89:6837–6840. doi: 10.1073/pnas.89.15.6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staswick P., Tiryaki I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell. 2004;16:2117–2127. doi: 10.1105/tpc.104.023549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McConn M., Creelman R.A., Bell E., Mullet J.E., Browse J. Jasmonate is essential for insect defense in Arabidopsis. Proc. Natl. Acad. Sci. USA. 1997;94:5473–5477. doi: 10.1073/pnas.94.10.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vijayan P., Shockey J., Lévesque C.A., Cook R.J., Browse J. A role for jasmonate in pathogen defense of Arabidopsis. Proc. Natl. Acad. Sci. USA. 1998;95:7209–7214. doi: 10.1073/pnas.95.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nomura K., Melotto M., He S.-Y. Suppression of host defense in compatible plant-Pseudomonas syringae interactions. Curr. Opin. Plant Biol. 2005;8:361–368. doi: 10.1016/j.pbi.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 17.McConn M., Browse J. The critical requirement for linolenic acid is pollen development, not photosynthesis, in an Arabidopsis mutant. Plant Cell. 1996;8:403–416. doi: 10.2307/3870321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanders P.M., Lee P.Y., Biesgen C., Boone J.D., Beals T.P., Weiler E.W., Goldberg R.B. The arabidopsis DELAYED DEHISCENCE1 gene encodes an enzyme in the jasmonic acid synthesis pathway. Plant Cell. 2000;12:1041–1062. doi: 10.1105/tpc.12.7.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stintzi A., Browse J. The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc. Natl. Acad. Sci. USA. 2000;97:10625–10630. doi: 10.1073/pnas.190264497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stintzi A., Weber H., Reymond P., Browse J., Farmer E.E. Plant Defense in the Absence of Jasmonic Acid, The Role of Cyclopentenones. Proc. Natl. Acad. Sci. USA. 2001;98:12837–12842. doi: 10.1073/pnas.211311098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishiguro S., Kawai-Oda A., Ueda J., Nishida I., Okada K. The DEFECTIVE IN ANTHER DEHISCIENCE gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell. 2001;13:2191–2209. doi: 10.1105/tpc.010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandaokar A., Thines B., Shin B., Lange B.M., Choi G., Koo Y.J., Yoo Y.J., Choi Y.D., Choi G., Browse J. Transcriptional regulators of stamen development in Arabidopsis identified by transcriptional profiling. Plant J. 2006;46:984–1008. doi: 10.1111/j.1365-313X.2006.02756.x. [DOI] [PubMed] [Google Scholar]
- 23.Shin B., Choi G., Yi H., Yang S., Cho I., Kim J., Lee S., Paek N.C., Kim J.H., Song P.S., et al. AtMYB21, a gene encoding a flower-specific transcription factor, is regulated by COP1. Plant J. 2002;30:23–32. doi: 10.1046/j.1365-313X.2002.01264.x. [DOI] [PubMed] [Google Scholar]
- 24.Song S.S., Qi T.C., Huang H., Ren Q.C., Wu D.W., Chang C.Q., Peng W., Liu Y.L., Peng J.R., Xie D.X. The Jasmonate-ZIM domain proteins interact with the R2R3-MYB transcription factors MYB21 and MYB24 to affect jasmonate-regulated stamen development in Arabidopsis. Plant Cell. 2011;23:1000–1013. doi: 10.1105/tpc.111.083089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandaokar A., Browse J. MYB108 acts together with MYB24 to regulate jasmonate-mediated stamen maturation in Arabidopsis. Plant Physiol. 2009;149:851–862. doi: 10.1104/pp.108.132597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie D.X., Feys B.F., James S., Nieto-Rostro M., Turner J.G. COI1, an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science. 1998;280:1091–1094. doi: 10.1126/science.280.5366.1091. [DOI] [PubMed] [Google Scholar]
- 27.Zheng N., Schulman B.A., Song L., Miller J.J., Jeffrey P.D., Wang P., Chu C., Koepp D.M., Elledge S.J., Pagano M., et al. Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase complex. Nature. 2002;416:703–709. doi: 10.1038/416703a. [DOI] [PubMed] [Google Scholar]
- 28.Jensen A.B., Raventos D., Mundy J. Fusion genetic analysis of jasmonate-signalling mutants in Arabidopsis. Plant J. 2002;29:595–606. doi: 10.1046/j.0960-7412.2001.01241.x. [DOI] [PubMed] [Google Scholar]
- 29.Hilpert B., Bohlmann H., op den Camp R., Przybyla D., Miersch O., Buchala A., Apel K. Isolation and characterizaiton of signal transduction mutants of Arabidopsis thaliana that constitutively activate the octadecanoid pathway and form nectrotic microlesions. Plant J. 2001;26:435–446. doi: 10.1046/j.1365-313X.2001.2641036.x. [DOI] [PubMed] [Google Scholar]
- 30.Xu L., Liu F., Wang Z., Peng W., Huang R., Huang D., Xie D. An Arabidopsis mutant cex1 exhibits constant accumulation of jasmonate-regulated AtVSP, Thi2.1 and PDF1.2. FEBS Lett. 2001;494:161–164. doi: 10.1016/S0014-5793(01)02331-6. [DOI] [PubMed] [Google Scholar]
- 31.Ellis C., Turner J.G. The Arabidopsis mutant cev1 has constitutively active jasmonate and ethylene signal pathways and enhanced resistance to pathogens. Plant Cell. 2001;13:1025–1033. doi: 10.1105/tpc.13.5.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao S., Dai L., Liu F., Wang Z., Peng W., Xie D. COS1, an Arabidopsis coronatine insensitive1 suppressor essential for regulation of jasmonate-mediated plant defense and senescence. Plant Cell. 2004;16:1132–1142. doi: 10.1105/tpc.020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lorenzo O., Solano R. Molecular players regulating the jasmonate signalling network. Curr. Opin. Plant Biol. 2005;8:532–540. doi: 10.1016/j.pbi.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Devoto A. COI1 links jasmonate signalling and fertility to the SCF ubiquitin-ligase complex in Arabidopsis. Plant J. 2002;32:457–466. doi: 10.1046/j.1365-313X.2002.01432.x. [DOI] [PubMed] [Google Scholar]
- 35.Reymond P., Bodenhausen N., Van Poecke R.M., Krishnamurthy V., Dicke M., Farmer E.E. A conserved transcript pattern in response to a specialist and a generalist herbivore. Plant Cell. 2004;16:3132–3147. doi: 10.1105/tpc.104.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devoto A. Expression profiling reveals COI1 to be a key regulator of genes involved in wound-and methyl jasmonate-induced secondary metabolism, defence, and hormone interactions. Plant Mol. Biol. 2005;58:497–513. doi: 10.1007/s11103-005-7306-5. [DOI] [PubMed] [Google Scholar]
- 37.Marchler-Bauer A., Anderson J.B., DeWeese-Scott C., Fedorova N.D., Geer L.Y., He S., Hurwitz D.I., Jackson J.D., Jacobs A.R., Lanczycki C.J., et al. CDD, a curated Entrez database of conserved domain alignments. Nucleic Acids Res. 2003;31:383–387. doi: 10.1093/nar/gkg087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shikata M., Matsuda Y., Ando K., Nishii A., Takemura M., Yokota A., Kohchi T. Characterization of Arabidopsis ZIM, a member of a novel plant-specific GATA factor gene family. J. Exp. Bot. 2004;55:631–639. doi: 10.1093/jxb/erh078. [DOI] [PubMed] [Google Scholar]
- 39.Thines B., Katsir L., Melotto M., Niu Y., Mandaokar A., Liu G., Nomura K., He S.Y., Howe G.A., Browse J. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature. 2007;448:661–665. doi: 10.1038/nature05960. [DOI] [PubMed] [Google Scholar]
- 40.Chini A., Fonseca S., Fernandez G., Adie B., Chico J.M., Lorenzo O., Garcia-Casado G., Lopez-Vidriero I., Lozano F.M., Ponce M.R., et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature. 2007;448:666–671. doi: 10.1038/nature06006. [DOI] [PubMed] [Google Scholar]
- 41.Yan Y., Stolz S., Chételat A., Reymond P., Pagni M., Dubugnon L., Farmer E.E. A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell. 2007;19:2470–2483. doi: 10.1105/tpc.107.050708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thireault C., Shyu C., Yoshida Y., St Aubin B., Campos M.L., Howe G.A. Repression of jasmonate signaling by a non-TIFY JAZ protein in Arabidopsis. Plant J. 2015;82:669–679. doi: 10.1111/tpj.12841. [DOI] [PubMed] [Google Scholar]
- 43.Vanholme B., Grunewald W., Bateman A., Kohchi T., Gheysen G. The tify family previously known as ZIM. Trends Plant Sci. 2007;12:239–244. doi: 10.1016/j.tplants.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Chini A., Gimenez-Ibanez S., Goossens A., Solano R. Redundancy and specificity in jasmonate signalling. Curr. Opin. Plant Biol. 2016;33:147–156. doi: 10.1016/j.pbi.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 45.Howe G.A., Major I.T., Koo A.J. Modularity in jasmonate signaling for multistress resilience. Annu. Rev. Plant Biol. 2018;69:387–415. doi: 10.1146/annurev-arplant-042817-040047. [DOI] [PubMed] [Google Scholar]
- 46.Sheard L.B., Tan X., Mao H.B., Withers J., Ben-Nissan G., Hinds T.R., Kobayashi Y., Hsu F.F., Sharon M., Browse J., et al. Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature. 2010;468:400–405. doi: 10.1038/nature09430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Melotto M., Mecey C., Niu Y., Chung H.S., Katsir L., Yao J., Zeng W., Thines B., Staswick P., Browse J., et al. A critical role of two positively charged amino acids in the Jas motif of Arabidopsis JAZ proteins in mediating coronatine-and jasmonoyl isoleucine-dependent interactions with the COI1 F-box protein. Plant J. 2010;55:979–988. doi: 10.1111/j.1365-313X.2008.03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katsir L., Schilmiller A.L., Staswick P.E., He S.Y., Howe G.A. COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc. Natl. Acad. Sci. USA. 2008;105:7100–7105. doi: 10.1073/pnas.0802332105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chung H.S., Cooke T.F., Depew C.L., Patel L.C., Ogawa N., Kobayashi Y., Howe G.A. Alternative splicing expands the repertoire of dominant JAZ repressors of jasmonate signaling. Plant J. 2010;63:613–622. doi: 10.1111/j.1365-313X.2010.04265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chung H.S., Koo A.J., Gao X., Jayanty S., Thines B., Jones A.D., Howe G.A. Regulation and function of Arabidopsis JASMONATE ZIM-domain genes in response to wounding and herbivory. Plant Physiol. 2008;146:952–964. doi: 10.1104/pp.107.115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pauwels L., Barbero G.F., Geerinck J., Tilleman S., Grunewald W., Perez A.C., Chico J.M., Vanden Bossche R., Sewell J., Gil E., et al. NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature. 2010;464:788–791. doi: 10.1038/nature08854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu Z., An F., Feng Y., Li P., Xue L., Jiang Z., Kim J.M., To T.K., Li W., Zhang X., et al. Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2011;108:12539–12544. doi: 10.1073/pnas.1103959108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Acosta I.F., Gasperini D., Chételat A., Stolz S., Santuari L., Farmer E.E. Role of NINJA in root jasmonate signaling. Proc. Natl. Acad. Sci. USA. 2013;110:15473–15478. doi: 10.1073/pnas.1307910110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wasternack C., Song S. Jasmonates, biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J. Exp. Bot. 2017;68:1303–1321. doi: 10.1093/jxb/erw443. [DOI] [PubMed] [Google Scholar]
- 55.Kazan K., Manners J.M. JAZ repressors and the orchestration of phytohormone crosstalk. Trends Plant Sci. 2012;17:22–31. doi: 10.1016/j.tplants.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 56.Song S., Qi T., Wasternack C., Xie D. Jasmonate signaling and crosstalk with gibberellin and ethylene. Curr. Opin. Plant Biol. 2014;21:112–119. doi: 10.1016/j.pbi.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Cheng H., Song S., Xiao L., Soo H.M., Cheng Z., Xie D., Peng J. Gibberellin acts through jasmonate to control the expression of MYB21, MYB24, and MYB57 to promote stamen filament growth in Arabidopsis. PLoS Genet. 2009;5:e1000440. doi: 10.1371/journal.pgen.1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reeves P.H., Ellis C.M., Ploense S.E., Wu M.F., Yadav V., Tholl D., Chételat A., Haupt I., Kennerley B.J., Hodgens C., et al. A regulatory network for coordinated flower maturation. PLoS Genet. 2012;8:e1002506. doi: 10.1371/journal.pgen.1002506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fonseca S., Chini A., Hamberg M., Adie B., Porzel A., Kramell R., Miersch O., Wasternack C., Solano R. (+)-7-iso-Jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nat. Chem. Biol. 2009;5:344–350. doi: 10.1038/nchembio.161. [DOI] [PubMed] [Google Scholar]
- 60.Tan X., Calderon-Villalobos L.I., Sharon M., Zheng C., Robinson C.V., Estelle M., Zheng N. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature. 2007;446:640–645. doi: 10.1038/nature05731. [DOI] [PubMed] [Google Scholar]
- 61.Laha D., Johnen P., Azevedo C., Dynowski M., Weiß M., Capolicchio S., Mao H., Iven T., Steenbergen M., Freyer M., et al. VIH2 regulates the synthesis of inositol pyrophosphate insp8 and jasmonate-dependent defenses in Arabidopsis. Plant Cell. 2015;27:1082–1097. doi: 10.1105/tpc.114.135160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laha D., Parvin N., Dynowski M., Johnen P., Mao H., Bitters S.T., Zheng N., Schaaf G. Inositol polyphosphate binding specificity of the jasmonate receptor Complex. Plant Physiol. 2016;171:2364–2370. doi: 10.1104/pp.16.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jewell J.B., Browse J. Epidermal jasmonate perception is sufficient for all aspects of jasmonate-mediated male fertility in Arabidopsis. Plant J. 2016;85:634–647. doi: 10.1111/tpj.13131. [DOI] [PubMed] [Google Scholar]
- 64.Savaldi-Goldstein S., Peto C., Chory J. The epidermis both drives and restricts plant shoot growth. Nature. 2007;446:199–202. doi: 10.1038/nature05618. [DOI] [PubMed] [Google Scholar]
- 65.Dobritzsch S., Weyhe M., Schubert R., Dindas J., Hause G., Kopka J., Hause B. Dissection of jasmonate functions in tomato stamen development by transcriptome and metabolome analyses. BMC Biol. 2015;13:28. doi: 10.1186/s12915-015-0135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li L., Zhao Y., McCaig B.C., Wingerd B.A., Wang J., Whalon M.E., Pichersky E., Howe G.A. The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell. 2004;16:126–143. doi: 10.1105/tpc.017954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schubert R., Dobritzsch S., Gruber C., Hause G., Athmer B., Schreiber T., Marillonnet S., Okabe Y., Ezura H., Acosta I.F., et al. Tomato MYB21 acts in ovules to mediate jasmonate-regulated fertility. Plant Cell. 2019;31:1043–1062. doi: 10.1105/tpc.18.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cai Q., Yuan Z., Chen M., Yin C., Luo Z., Zhao X., Liang W., Hu J., Zhang D. Jasmonic acid regulates spikelet development in rice. Nat. Commun. 2014;19:3476–3489. doi: 10.1038/ncomms4476. [DOI] [PubMed] [Google Scholar]
- 69.Yuan Z., Zhang D. Roles of jasmonate signalling in plant inflorescence and flower development. Curr. Opin. Plant Biol. 2015;27:44–51. doi: 10.1016/j.pbi.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 70.Pelaz S., Ditta G.S., Baumann E., Wisman E., Yanofsky M.F. B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature. 2000;405:200–203. doi: 10.1038/35012103. [DOI] [PubMed] [Google Scholar]
- 71.Wu M.F., Sang Y., Bezhani S., Yamaguchi N., Han S.K., Li Z., Su Y., Slewinski T.L., Wagner D. SWI2/SNF2 chromatin remodeling ATPases overcome polycomb repression and control floral organ identity with the LEAFY and SEPALLATA3 transcription factors. Proc. Natl. Acad. Sci. USA. 2012;109:3576–3581. doi: 10.1073/pnas.1113409109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Whipple C.J., Ciceri PPadilla C.M., Ambrose B.A., Bandong S.L., Schmidt R.J. Conservation of B-class floral homeotic gene function between maize and Arabidopsis. Development. 2004;131:6083–6091. doi: 10.1242/dev.01523. [DOI] [PubMed] [Google Scholar]
- 73.Dellaporta S.L., Calderon-Urrea A. Sex determination in flowering plants. Plant Cell. 1993;5:1241–1251. doi: 10.1105/tpc.5.10.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Calderon-Urrea A., Dellaporta S.L. Cell death and cell protection genes determine the fate of pistils in maize. Development. 1999;126:435–441. doi: 10.1242/dev.126.3.435. [DOI] [PubMed] [Google Scholar]
- 75.Kim J.C., Laparra H., Calderón-Urrea A., Mottinger J.P., Moreno M.A., Dellaporta S.L. Cell cycle arrest of stamen initials in maize sex determination. Genetics. 2007;177:2547–2551. doi: 10.1534/genetics.107.082446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Irish E.E., Nelson T. Sex determination in monoecious and dioecious plants. Plant Cell. 1989;1:737–744. doi: 10.2307/3868981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Acosta I.F., Laparra H., Romero S.P., Schmelz E., Hamberg M., Mottinger J.P., Moreno M.A., Dellaporta S.L. Tasselseed1 is a lipoxygenase affecting jasmonic acid signaling in sex determination of maize. Science. 2009;323:262–265. doi: 10.1126/science.1164645. [DOI] [PubMed] [Google Scholar]
- 78.Browse J. Jasmonate, preventing the maize tassel from getting in touch with his feminine side. Sci. Signal. 2009;2:pe9. doi: 10.1126/scisignal.259pe9. [DOI] [PubMed] [Google Scholar]
- 79.Lunde C., Kimberlin A., Leiboff S., Koo A.J., Hake S. Tasselseed5 overexpresses a wound-inducible enzyme, ZmCYP94B1, that affects jasmonate catabolism, sex determination, and plant architecture in maize. Commun. Biol. 2019;2:114. doi: 10.1038/s42003-019-0354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang F., Yuan Z., Zhao Z., Li C., Zhang X., Liang H., Liu Y., Xu Q., Liu H. Tasselseed5 encoding a cytochrome C oxidase affects jasmonate catabolism in sex determination of maize. J. Integr. Plant Biol. 2019 doi: 10.1111/jipb.12826. [DOI] [PubMed] [Google Scholar]