Key Points
Question
Do changes in NT-proBNP correlate with changes in cardiac structure and function in patients with heart failure with reduced ejection fraction (HFrEF) treated with sacubitril-valsartan?
Findings
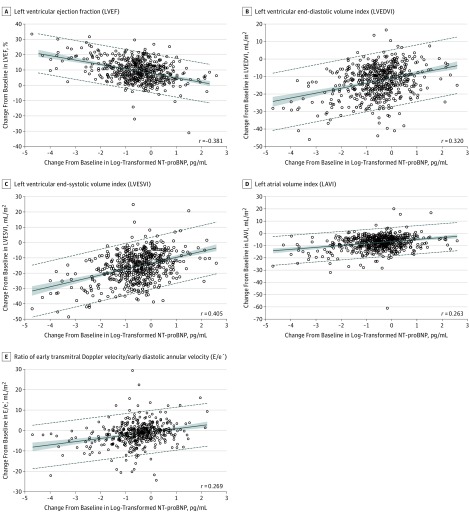
In this prospective study of 794 patients with HFrEF who initiated therapy with sacubitril-valsartan, change in log2–NT-proBNP concentrations over 12 months correlated with changes in left ventricular (LV) ejection fraction (r = −0.381), LV end-diastolic volume index (r = 0.320), LV end-systolic volume index (r = 0.405), left atrial volume index (r = 0.263), and ratio of early diastolic filling/early diastolic annular velocity (r = 0.269).
Meaning
In this exploratory analysis of patients with HFrEF treated with sacubitril-valsartan, reduction in NT-proBNP was weakly yet significantly correlated with improvements in markers of cardiac volume and function at 12 months.
Abstract
Importance
In patients with heart failure and reduced ejection fraction (HFrEF), treatment with sacubitril-valsartan reduces N-terminal pro–b-type natriuretic peptide (NT-proBNP) concentrations. The effect of sacubitril-valsartan on cardiac remodeling is uncertain.
Objective
To determine whether NT-proBNP changes in patients with HFrEF treated with sacubitril-valsartan correlate with changes in measures of cardiac volume and function.
Design, Setting, and Participants
Prospective, 12-month, single-group, open-label study of patients with HFrEF enrolled in 78 outpatient sites in the United States. Sacubitril-valsartan was initiated and the dose adjusted. Enrollment commenced on October 25, 2016, and follow-up was completed on October 22, 2018.
Exposures
NT-proBNP concentrations among patients treated with sacubitril-valsartan.
Main Outcomes and Measures
The primary outcome was the correlation between changes in log2–NT-proBNP concentrations and left ventricular (LV) EF, LV end-diastolic volume index (LVEDVI), LV end-systolic volume index (LVESVI), left atrial volume index (LAVI), and ratio of early transmitral Doppler velocity/early diastolic annular velocity (E/e′) at 12 months.
Results
Among 794 patients (mean age, 65.1 years; 226 women [28.5%]; mean LVEF = 28.2%), 654 (82.4%) completed the study. The median NT-proBNP concentration at baseline was 816 pg/mL (interquartile range [IQR], 332-1822) and 455 pg/mL (IQR, 153-1090) at 12 months (difference, P < .001). At 12 months, the change in log2–NT-proBNP concentration was correlated with changes in LVEF (r = −0.381 [IQR, −0.448 to −0.310]; P < .001), LVEDVI (r = 0.320 [IQR, 0.246 to 0.391]; P < .001), LVESVI (r = 0.405 [IQR, 0.335 to 0.470]; P < .001), LAVI (r = 0.263 [IQR, 0.186 to 0.338]; P < .001), and E/e′ (r = 0.269 [IQR, 0.182 to 0.353]; P < .001). At 12 months, LVEF increased from 28.2% to 37.8% (difference, 9.4% [95% CI, 8.8% to 9.9%]; P < .001), while LVEDVI decreased from 86.93 to 74.15 mL/m2 (difference, −12.25 mL/m2 [IQR, −12.92 to −11.58]; P < .001) and LVESVI decreased from 61.68 to 45.46 mL/m2 (difference, −15.29 mL/m2 [95% CI, −16.03 to −14.55]; P < .001). LAVI and E/e′ ratio also decreased significantly. The most frequent adverse events were hypotension (17.6%), dizziness (16.8%), hyperkalemia (13.2%), and worsening kidney function (12.3%).
Conclusions and Relevance
In this exploratory study of patients with HFrEF treated with sacubitril-valsartan, reduction in NT-proBNP concentration was weakly yet significantly correlated with improvements in markers of cardiac volume and function at 12 months. The observed reverse cardiac remodeling may provide a mechanistic explanation for the effects of sacubitril-valsartan in patients with HFrEF.
Trial Registration
ClinicalTrials.gov Identifier: NCT02887183
This exploratory cohort study of patients with heart failure with reduced ejection fraction (HFrEF) assesses the association between change in N-terminal pro–B-type natriuretic peptide (NT-proBNP) after starting sacubitril-valsartan and changes in left ventricular ejection fraction, diastolic volume, and other measures of physiologic cardiac function.
Introduction
In the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality in Heart Failure (PARADIGM-HF) Study,1 long-term therapy with sacubitril-valsartan (a combination angiotensin receptor/neprilysin inhibitor [ARNI]) lowered rates of cardiovascular death or heart failure (HF) hospitalization compared with enalapril. Other outcomes, such as mortality and quality of life, were also favorably affected by ARNI therapy. Despite the clinical benefits of sacubitril-valsartan, uncertainty regarding the mechanism(s) of benefit remains.
Remodeling of the myocardium is central to the progression of HF with reduced ejection fraction (HFrEF),2,3,4 and occurs in response to injury, hemodynamic changes, or neurohormonal activation. Remodeling consists of changes in cardiac geometry, function, or both, reflected by reduced left ventricular ejection fraction (LVEF) and increased LV volumes. Cardiac remodeling is associated with risk for cardiovascular events, including death and hospitalization for HF, and represents an important target for HF therapy. In studies of guideline-directed medical therapies (GDMT) for HFrEF, such as β-blockers, angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), and mineralocorticoid receptor antagonists (MRAs), an increase in LVEF, reduction in LV volumes, or both correlated with improved outcomes.5 However, the effects of sacubitril-valsartan on LV remodeling have been uncertain.
In PARADIGM-HF, the benefits of sacubitril-valsartan were associated with a reduction in N-terminal pro–B-type natriuretic peptide (NT-proBNP) concentrations.6 Reduction in NT-proBNP during GDMT for HFrEF is associated with reverse LV remodeling7,8; however, no such link has been established for ARNI therapy. This study, therefore, examined the association of the change in NT-proBNP after initiation of sacubitril-valsartan with long-term changes in measures of cardiac remodeling.
Methods
The study protocol was approved by the relevant institutional review boards. All participants were required to sign informed consent prior to enrollment.
Rationale and Design
The rationale and design of the Prospective Study of Biomarkers, Symptom Improvement, and Ventricular Remodeling During Sacubitril/Valsartan Therapy for Heart Failure (PROVE-HF) Study have been published.9 The study protocol and statistical analysis plan are available in Supplement 1 and Supplement 2, respectively. Key inclusion and exclusion criteria for the study are detailed in eTable 1 in Supplement 3. This study was a phase 4, 52-week, open-label, single-group study of patients initiated with sacubitril-valsartan treatment per standard of care performed at 78 sites in the United States. Race/ethnicity was ascertained in this study because of previous reports of a higher incidence of angioedema in black individuals. Determination of race/ethnicity was made by the researcher based on fixed categories.
Study Visits: Initiation and Titration of Sacubitril-Valsartan
Study procedures are detailed in eFigure 1 in Supplement 3. Following informed consent, ACEIs and ARBs were discontinued, and patients were given sacubitril-valsartan according to the US prescribing information. Following ARNI initiation, patients returned for study visits and drug titration approximately every 2 weeks through day 60, with a goal dose of sacubitril-valsartan of 97/103 mg twice daily (or highest tolerated dose). The dose could be reduced in patients who had unacceptable adverse effects. Treatment continued for up to 12 months. At each study visit, a history and physical examination were performed, and blood samples were obtained.
Echocardiography
After enrollment, participants underwent 2-dimensional echocardiography at baseline and approximately 6 months and 12 months, according to a study-specific imaging protocol. Following completion, echocardiograms were transmitted in a secure fashion to a core laboratory where they were interpreted following completion of all study procedures in a temporally and clinically blinded fashion.
Measurements made from obtained images included LV end-diastolic volume index (LVEDVI; normal <76 mL/m2), LV end-systolic volume index (LVESVI; normal <30 mL/m2), and left atrial volume index (LAVI; a value ≥34 mL/m2 is considered enlarged according to American Society of Echocardiography recommendations10). Additionally, LV mass index (LVMi; normal <89 g/m2 in women and <103 g/m2 in men) was calculated. Doppler examinations included assessment of early diastolic filling velocity (E wave) and early diastolic mitral annular velocity (E'); an E/e′ ratio greater than 14 is associated with elevated filling pressures.
NT-proBNP
At each study visit, a sample of blood was sent to a central laboratory for measurement of plasma NT-proBNP using a commercially available electrochemiluminescence immunoassay (proBNP II, Roche Diagnostics).
Study End Points
The primary end point of this study was the correlation between changes in the concentration of NT-proBNP and cardiac remodeling, assessed by change in LVEDVI, LVESVI, LVEF, and LAVI from baseline to 12 months. Correlation between the change in concentration of NT-proBNP and E/e′ was added to the statistical analysis plan prior to database lock.
A secondary end point was the association between the changes in concentration of NT-proBNP and cardiac remodeling from baseline to 6 months. Other secondary end points were the 6-month associations between the changes in concentration of NT-proBNP and cardiac remodeling in specific patient subgroups not represented in the PARADIGM-HF trial, including (1) patients with new-onset HF (diagnosis <60 days from study enrollment) and/or who were not taking an ACEI or ARB at baseline, (2) patients with natriuretic peptide concentrations below PARADIGM-HF inclusion criteria (NT-proBNP <600 pg/mL if not hospitalized or <400 pg/mL if hospitalized; BNP <150 pg/mL if not hospitalized or <100 pg/mL if hospitalized), and (3) patients not reaching target doses of sacubitril-valsartan (97/103 mg twice daily). Change in LVMi was a post hoc outcome. The association between change in NT-proBNP and patient-reported outcomes was also a secondary end point, which will be reported separately.
Adverse Events
All adverse events and serious adverse events were recorded; suspected cases of angioedema were evaluated by a central adjudication panel according to protocol definitions.9
Statistical Analyses
Data frequencies are expressed as mean (SD) or median (interquartile range [IQR]) depending on normality. Pearson correlation coefficients and the corresponding 2-sided 95% CIs and P values were calculated to examine the association between change in log2–transformed NT-proBNP concentration and each structural cardiac measurement (LVEDVI, LVESVI, LVEF, LAVI, and E/e′) from baseline through 12 months. These analyses were repeated from baseline to 6 months. The same statistical analyses were performed for each subgroup of interest. Comparisons of serial measures were performed using least-square means (95% CI).
To ensure reasonable power to analyze subgroups of interest, the correlation coefficient was estimated with a precision of at least 0.15 for a 2-sided 95% CI. The smallest subgroup of interest was projected to be 20% of the overall study population. Assuming a correlation coefficient of –0.35 between log2–NT-proBNP and LVEF within each subgroup and accounting for an assumed 20% dropout rate, the smallest subgroup sample size was planned at 166 patients. Thus, an overall sample size of approximately 830 patients was planned. For analyses involving NT-proBNP or echocardiographic measurements, calculations were performed on data available at each time point ±7 days; no imputation was applied to patients who died or had missing data. However, to exclude effects of noncompleting patients on cardiac remodeling measures, post hoc sensitivity analyses were performed only including those patients with echocardiographic data at all 3 time points. In an effort to understand the association between change in NT-proBNP and cardiac remodeling measures, we used post hoc parallel process latent growth curve models between each measure of cardiac remodeling (ie, LVEF, LVEDVI, LVESVI, LAVI, and E/e′) and log-transformed NT-proBNP levels over time.
Because of the potential for type I error due to multiple comparisons, study findings should be interpreted as exploratory. Statistics were performed using SAS version 9.3 (SAS Institute) or the lavaan package in R version 3.5.5 (R Foundation). P values are 2-sided, with values less than .05 considered significant.
Results
Baseline Characteristics
Of 1031 screened, 794 consented study participants continued into the open-label treatment phase; 654 (82.4%) completed the study. eFigure 2 in Supplement 3 details study flow and patient disposition.
Baseline characteristics of the study participants are detailed in Table 1. The mean (SD) age was 65.1 (12.4) years; 188 patients (23.7%) were aged 75 years or older. Notably, 226 (28.5%) were women and 180 (22.7%) of the study participants were investigator-identified black individuals. New-onset HF was present in 78 patients (9.8%). At enrollment, nearly all had New York Heart Association Class II (n = 558; 70.3%) or III (n = 222; 28.0%) symptoms. Study participants had a typical mixture of HFrEF risk factors (Table 1). At baseline, the study participants had a median LVEF of 28.2% (25th-75th percentile, 24.5%-32.7%), LVEDVI of 86.93 (25th-75th percentile, 76.17-100.43), LVESVI of 61.68 (25th-75th percentile, 51.95-75.00), and LAVI of 37.76 mL/m2 (25th-75th percentile, 31.63-46.09). The median baseline E/e′ was 11.70 (25th-75th percentile, 8.80-16.00).
Table 1. Baseline Characteristics of the Study Participants (N = 794).
| Parameter | Sacubitril-Valsartan, No. (%)a |
|---|---|
| Age, y | 65.1 (12.4) |
| Sex | |
| Male | 568 (71.5) |
| Female | 226 (28.5) |
| NYHA symptom severity classb | |
| II | 558 (70.3) |
| III | 222 (28.0) |
| IV | 14 (1.8) |
| Race | n = 792 |
| White | 581 (73.4) |
| Black | 180 (22.7) |
| Asian | 6 (0.8) |
| Other | 25 (3.2) |
| Ethnicity | |
| Hispanic or Latino | 117 (14.7) |
| Body mass index | 31.3 (6.9) |
| Medical History | |
| Hypertension | 699 (88.0) |
| Coronary revascularization | 376 (47.4) |
| Diabetes | 361 (45.5) |
| Myocardial infarction | 329 (41.4) |
| Coronary artery disease | 316 (39.8) |
| Atrial fibrillation/flutter | 280 (35.3) |
| Ischemic etiology for HF | 426 (53.7) |
| Time since HF diagnosis, median (IQR), mo | 50.5 (15.0-109.6) |
| New-onset HFc | 78 (9.8) |
| Guideline-directed therapy | |
| β-Blocker | 757 (95.3) |
| ACEI/ARB | 602 (75.8) |
| MRA | 281 (35.4) |
| CRT/CRT-D | 122 (15.4) |
| ICD alone | 226 (28.5) |
| Not taking ACEI/ARB | |
| ACEI/ARB naive (never exposed) | 48 (6.0) |
| Previously taking but not currently | 144 (18.1) |
| Baseline laboratory results | |
| eGFR, mL/min/1.73 m2 | 63.96 (20.4) |
| eGFR ≤60 mL/min/1.73 m2 | 351 (44.2) |
| NT-proBNP, pg/mL, median (IQR) | 816 (332-1822) |
| Baseline vital signs | |
| Systolic blood pressure, mm Hg | 124.5 (15.9) |
| Diastolic blood pressure, mm Hg | 75.9 (10.3) |
| Heart rate, beats/min | 72.2 (11.3) |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CRT, cardiac resynchronization therapy; CRT-D, cardiac resynchronization therapy–defibrillator; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter-defibrillator; IQR, interquartile range; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; NT-proBNP, N-terminal pro–B-type natriuretic peptide.
Continuous data are depicted as mean (SD) unless otherwise noted. Dichotomous data are depicted as No. (%).
NYHA class II denotes dyspnea with moderate exertion; class III, dyspnea with mild exertion; and class IV, dyspnea at rest.
New-onset HF was defined as diagnosis <60 days prior to enrollment.
Background medical treatment of study patients included a β-blocker in 757 patients (95.3%), an ACEI or ARB in 602 (75.8%), and an MRA in 281 (35.4%). Defibrillator implantation had been performed in 226 (28.5%), while an additional 122 (15.4%) had received a cardiac resynchronization therapy (CRT) device with or without defibrillator.
At initiation of sacubitril-valsartan, 649 (81.7%) received a dose of 24/26 mg twice daily. By the end of the study, the maximum sacubitril-valsartan dose received was 97/103 mg twice daily in 516 patients (65.0%), 49/51 mg twice daily in 168 (21.2%), and 24/26 mg twice daily in 110 (13.9%). Among those not reaching target doses, the reasons given included symptomatic hypotension in 40 patients (5.0%), kidney dysfunction in 13 (1.6%), and hyperkalemia in 8 (1.0%).
NT-proBNP Concentrations
At baseline, the median NT-proBNP concentration was 816 pg/mL (IQR, 332-1822). Comparatively, 292 patients (36.8%) in this study had baseline NT-proBNP concentrations below the PARADIGM-HF inclusion criteria.
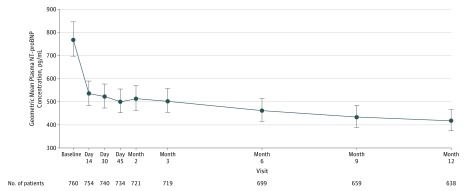
Initiation and titration of sacubitril-valsartan was associated with a reduction in NT-proBNP concentration (Figure 1; eTable 2 and eFigure 3 in Supplement 3), with most of the reduction occurring by the first follow-up visit 2 weeks after initiation. Across study visits, for example, least-square geometric mean ratios of baseline NT-proBNP to each follow up were −30% (day 14), −31% (day 30), −35% (6 months), and −37% (12 months); at 12 months, the median NT-proBNP concentration was 455 pg/mL (IQR, 153-1090) (change from baseline, P < .001).
Figure 1. Concentrations of N-Terminal Pro–B-Type Natriuretic Peptide (NT-proBNP) Across Study Visits.
Reduction in NT-proBNP was evident by the first follow-up visit and was sustained throughout the 12 months. Concentrations of NT-proBNP were included if collected 6 or more hours from the first dose of sacubitril-valsartan. Distributions of NT-proBNP at each time point can be found in eTable 2 and eFigure 3 in Supplement 3.
Primary End Point
From baseline to 12 months, statistically significant correlations were observed between the change in log2–NT-proBNP concentration and LVEF (r = −0.381 [IQR, −0.448 to −0.310]; P < .001), LVEDVI (r = 0.320 [IQR, 0.246 to 0.391]; P < .001), LVESVI (r = 0.405 [IQR, 0.335 to 0.470]; P < .001), LAVI (r = 0.263 [IQR, 0.186 to 0.338]; P < .001), and E/e′ (r = 0.269 [IQR, 0.182 to 0.353]; P < .001). Figure 2 details the correlations between the change in log2–NT-proBNP concentrations and each measure.
Figure 2. Scatterplots Detailing Correlations Between Baseline and 12-Month Concentrations of Log2-Transformed N-Terminal Pro–B-Type Natriuretic Peptide (NT-proBNP) and Contemporaneous Change in LVEF, LVEDVI, LVESVI, LAVI, and E/e′ .
A mean regression line is detailed with 95% prediction limits demonstrated in dashed lines. The shaded regions indicate 95% confidence limits.
Secondary End Points
Lower correlations between change in log2–NT-proBNP concentrations and remodeling indices were present at 6 months (eTable 3 in Supplement 3).
Subgroup Analyses
In prespecified subgroup analyses, among those with new-onset HF and/or those not taking an ACEI or ARB at enrollment (n = 118 at baseline), consistent correlations were observed between 12-month change in log2–NT-proBNP and LVEF (r = −0.509 [IQR, −0.651 to −0.333]; P < .001), LVEDVI (r = 0.409 [IQR, 0.216 to 0.572]; P < .001), LVESVI (r = 0.526 [IQR, 0.353 to 0.664]; P < .001), LAVI (r = 0.512 [IQR, 0.338 to 0.652]; P < .001), and E/e′ (r = 0.416 [IQR, 0.189 to 0.600]; P < .001). In those with NT-proBNP lower than the inclusion criteria for PARADIGM-HF (n = 292 at baseline), correlation coefficients between the change in log2–NT-proBNP and 12-month LVEF, LVEDVI, and LVESVI were −0.251 (IQR, −0.366 to −0.127; P < .001), 0.194 (IQR, 0.068 to 0.314; P = .003), and 0.277 (IQR, 0.155 to 0.391; P < .001), respectively. For LAVI and E/e′, the corresponding values were 0.136 (IQR, 0.006 to 0.262; P = .04) and 0.330 (IQR, 0.198 to 0.451; P < .001). Among those not achieving the target sacubitril-valsartan dose (n = 264), correlation coefficients between changes in log2–NT-proBNP and 12-month LVEF, LVEDVI, or LVESVI were −0.359 (IQR, −0.478 to −0.226; P < .001), 0.238 (IQR, 0.096 to 0.370; P = .001), and 0.325 (IQR, 0.190 to 0.449; P < .001), respectively; correlations with LAVI and E/e′ were 0.128 (IQR, −0.017 to 0.268; P = .08) and 0.196 (IQR, 0.026 to 0.354; P = .02), respectively.
Changes in Cardiac Structure and Function
Analyses of changes in cardiac remodeling indices demonstrated a significant increase in LVEF and corresponding reduction in LV volumes as early as 6 months; such changes continued to 12 months (Table 2; and eFigure 4 in Supplement 3). Compared with baseline, the 6- and 12-month least-square mean improvements in LVEF were 5.2% (95% CI, 4.8% to 5.6%) and 9.4% (95% CI, 8.8% to 9.9%), respectively (P < .001 for both); 75% of the study participants had an LVEF increase of 4.9% or greater and 25% experienced an LVEF increase of 13.4% or greater at 12 months. The corresponding changes in mean LVEDVI at 6 and 12 months compared with baseline were −6.65 mL/m2 (95% CI, −7.11 to −6.19) and −12.25 mL/m2 (95% CI, −12.92 to −11.58) (both P < .001), while changes in LVESVI were −8.67 mL/m2 (95% CI, −9.18 to −8.15) and −15.29 mL/m2 (95% CI, −16.03 to −14.55) (both P < .001) at the same time points. In addition, a mean −4.36 mL/m2 (95% CI, −4.73 to −3.99) reduction in LAVI at 6 months and a mean −7.57 mL/m2 (95% CI, −7.98 to −7.15) reduction by 1 year were observed (both P < .001). Treatment with sacubitril-valsartan was also associated with significant change in the E/e′ ratio by −1.23 (95% CI, −1.63 to −0.83) and −1.30 (95% CI, −1.74 to −0.86) at 6 and 12 months, respectively, compared with baseline (both P < .001). In post hoc analysis, LVMi decreased from a baseline value of 124.77 g/m2 (95% CI, 105.65 to 147.40) to 116.22 g/m2 (95% CI, 96.29 to 138.30) by 6 months (mean reduction, −9.14 g/m2 [95 % CI, −10.27 to −8.01]; P < .001), while by 12 months it decreased further to 107.82 g/m2 (95% CI, 90.15 to 128.68), with a mean reduction of −16.00 g/m2 (95% CI, −17.41 to −14.60) (P < .001).
Table 2. Change in Cardiac Remodeling Measurements From Baseline to 6 and 12 Months After Initiation of Sacubitril-Valsartan in All Study Participantsa.
| All Patients | |||||
|---|---|---|---|---|---|
| Baseline Value, Median (25th to 75th Percentile) | 6-mo Value, Median (25th to 75th Percentile) | LS Mean Change From Baseline at 6 mo (95% CI) | 12-mo Value, Median (25th to 75th Percentile) | LS Mean Change From Baseline at 12 mo (95% CI) | |
| LVEF, % | n = 757 | n = 716 | n = 648 | ||
| 28.2 (24.5 to 32.7) | 34.1 (29.0 to 39.65) | 5.2 (4.8 to 5.6) | 37.8 (32.3 to 45.2) | 9.4 (8.8 to 9.9) | |
| LVEDVI, mL/m2 | n = 756 | n = 716 | n = 648 | ||
| 86.93 (76.17 to 100.43) | 79.50 (69.34 to 93.52) | −6.65 (−7.11 to −6.19) | 74.15 (63.46 to 86.30) | −12.25 (−12.92 to −11.58) | |
| LVESVI, mL/m2 | n = 756 | n = 716 | n = 648 | ||
| 61.68 (51.95 to 75.00) | 52.25 (42.34 to 65.25) | −8.67 (−9.18 to −8.15) | 45.46 (34.84 to 57.56) | −15.29 (−16.03 to −14.55) | |
| LAVI, mL/m2 | n = 747 | n = 696 | n = 639 | ||
| 37.76 (31.63 to 46.09) | 32.80 (27.62 to 40.13) | −4.36 (−4.73 to −3.99) | 29.31 (24.40 to 35.85) | −7.57 (−7.98 to −7.15) | |
| E/e′ | n = 642 | n = 604 | n = 552 | ||
| 11.70 (8.80 to 16.00) | 10.50 (7.70 to 14.70) | −1.23 (−1.63 to −0.83) | 10.20 (7.70 to 14.30) | −1.30 (−1.74 to −0.86) | |
Abbreviations: E/e′, ratio of early transmitral Doppler velocity/early diastolic annular velocity; LAVI, left atrial volume index; LS, least-square; LVEF, left ventricular ejection fraction; LVEDVI, left ventricular end-diastolic volume index; LVESVI, left ventricular end-systolic volume index.
All comparisons were significant at P < .001.
The 6- and 12-month remodeling measurements among prespecified subgroups are detailed in Table 3, Table 4, and Table 5. Among those with new-onset HF or those not taking an ACEI or ARB at baseline, 12-month least-square mean improvements in LVEF (12.8% [95% CI, 11.05% to 14.5%]; P < .001), LVEDVI (−13.81 mL/m2 [95% CI, −15.78 to −11.83]; P < .001), and LVESVI (−17.88 mL/m2 [95% CI, −20.07 to −15.68]; P < .001) were observed, as was a reduction in LAVI (−8.44 mL/m2 [95% CI, −9.73 to −7.15]; P < .001) and E/e′ (−2.60 [95% CI, −3.83 to −1.37]; P < .001) (Table 3). In those with NT-proBNP concentrations below PARADIGM-HF inclusion criteria, 12-month improvement in LVEF (9.4% [95% CI, 8.6% to 10.3%], P < .001), and reductions in LVEDVI (−11.32 mL/m2 [95% CI, −12.24 to −10.40], P < .001), LVESVI (−14.15 mL/m2 [95% CI, −15.15 to −13.15], P < .001), LAVI (−7.06 [95% CI, −7.54 to −6.58]; P < .001), and E/e′ (−0.93 [95% CI, −1.43 to −0.43]; P < .001) were again noted (Table 4). Among those not attaining the target dose of sacubitril-valsartan, increase in LVEF (9.4% [95% CI, 8.4% to 10.3%]; P < .001) and reductions in LVEDVI (−10.99 mL/m2 [95% CI, −12.21 to −9.77]; P < .001), LVESVI (−14.32 mL/m2 [95% CI, −15.67 to −12.97]; P < .001), and LAVI (−7.23 mL/m2 [95% CI, −7.97 to −6.50]; P < .001) were noted; there was no significant change in E/e′(−0.46 [95% CI, −1.32 to 0.40]; P = .29) (Table 5). Constraining data to only those patients with echocardiographic studies at all 3 time points did not change the significance of the results (eTable 4 in Supplement 3).
Table 3. Change in Cardiac Remodeling Measurements From Baseline to 6 and 12 Months After Initiation of Sacubitril-Valsartan Among Patients With New-Onset HF or Not Taking ACEI or ARB at Baseline.
| New-Onset HF or ACEI/ARB Naive | Baseline Value, Median (25th to 75th Percentile) | 6-mo Value, Median (25th to 75th Percentile) | LS Mean Change From Baseline at 6 mo (95% CI) | P Value | 12-mo Value, Median (25th to 75th Percentile) | LS Mean Change From Baseline at 12 mo (95% CI) | P Value |
|---|---|---|---|---|---|---|---|
| LVEF, % | n = 108 | n = 102 | n = 98 | ||||
| Yes | 28.4 (25.2 to 33.9) | 35.7 (30.7 to 42.1) | 6.9 (5.7 to 8.0) | <.001 | 43.5 (35.4 to 50.5) | 12.8 (11.05 to 14.5) | <.001 |
| No | 28.1 (24.3 to 32.6) | 33.8 (28.7 to 39.1) | 4.9 (4.5 to 5.3) | <.001 | 37.0 (31.8 to 44.4) | 8.8 (8.3 to 9.3) | <.001 |
| LVEDVI, No., mL/m2 | n = 108 | n = 102 | n = 98 | ||||
| Yes | 85.97 (70.13 to 95.47) | 74.59 (62.70 to 85.90) | −7.21 (−8.50 to −5.93) | <.001 | 67.66 (57.77 to 79.39) | −13.81 (−15.78 to −11.83) | <.001 |
| No | 87.43 (76.89 to 101.38) | 80.38 (70.46 to 93.89) | −6.56 (−7.05 to −6.07) | <.001 | 75.12 (64.11 to 86.83) | −12.00 (−12.71 to −11.29) | <.001 |
| LVESVI, No., mL/m2 | n = 108 | n = 102 | n = 98 | ||||
| Yes | 59.28 (48.64 to 71.29) | 46.29 (36.44 to 58.94) | −10.01 (−11.45 to −8.58) | <.001 | 37.69 (28.97 to 51.16) | −17.88 (−20.07 to −15.68) | <.001 |
| No | 61.82 (52.70 to 75.91) | 52.94 (43.28 to 66.42) | −8.46 (−9.01 to −7.90) | <.001 | 46.70 (36.47 to 58.1) | −14.86 (−15.64 to −14.09) | <.001 |
| LAVI, No. mL/m2 | n = 101 | n = 101 | n = 98 | ||||
| Yes | 36.86 (31.53 to 45.02) | 32.14 (25.24 to 38.78) | −4.83 (−5.84 to −3.83) | <.001 | 28.13 (23.32 to 35.53) | −8.44 (−9.73 to −7.15) | <.001 |
| No | 37.90 (31.63 to 46.25) | 32.94 (27.90 to 40.65) | −4.28 (−4.68 to −3.88) | <.001 | 29.43 (25.04 to 35.90) | −7.42 (−7.85 to −6.99) | <.001 |
| E/e′, No. | n = 84 | n = 88 | n = 89 | ||||
| Yes | 11.85 (8.35 to 16.60) | 9.70 (7.00 to 14.25) | −1.86 (−3.01 to −0.70) | .002 | 9.00 (6.80 to 12.70) | −2.60 (−3.83 to −1.37) | <.001 |
| No | 11.60 (8.80 to 16.00) | 10.60 (7.80 to 14.80) | −1.13 (−1.56 to −0.70) | <.001 | 10.30 (7.80 to 14.40) | −1.10 (−1.57 to −0.63) | <.001 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; E/e′, ratio of early transmitral Doppler velocity/early diastolic annular velocity; HF, heart failure; LAVI, left atrial volume index; LS, least-square; LVEDVI, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; LVESVI, left ventricular end-systolic volume index; No, not in the subgroup; Yes, in the subgroup.
Table 4. Change in Cardiac Remodeling Measurements From Baseline to 6 and 12 Months After Initiation of Sacubitril-Valsartan Among Patients With NT-proBNP Concentrations Below PARADIGM-HF Inclusion Criteria.
| NT-proBNP <600 pg/mL If Not Hospitalized or <400 pg/mL If Hospitalized or BNP <150 pg/mL If Not Hospitalized or <100 pg/mL If Hospitalized? | Baseline Value, Median (25th to 75th Percentile) | 6-mo Value, Median (25th to 75th Percentile) | LS Mean Change From Baseline at 6 mo (95% CI) | P Value | 12-mo Value, Median (25th to 75th Percentile) | LS Mean Change From Baseline at 12 mo (95% CI) | P Value |
|---|---|---|---|---|---|---|---|
| LVEF, % | n = 273 | n = 273 | n = 256 | ||||
| Yes | 30.8 (25.9 to 34.1) | 35.5 (30.7 to 41.1) | 5.1 (4.5 to 5.8) | <.001 | 40.1 (34.9 to 46.3) | 9.4 (8.6 to 10.3) | <.001 |
| No | 26.8 (23.3 to 31.9) | 33.3 (27.2 to 38.6) | 5.3 (4.9 to 5.8) | <.001 | 36.5 (30.3 to 44.1) | 9.4 (8.7 to 10.2) | <.001 |
| LVEDVI, mL/m2 | n = 272 | n = 273 | n = 256 | ||||
| Yes | 81.01 (72.77 to 90.59) | 74.85 (66.80 to 84.05) | −6.32 (−7.02 to −5.63) | <.001 | 69.01 (61.43 to 78.54) | −11.32 (−12.24 to −10.40) | <.001 |
| No | 91.71 (78.46 to 106.26) | 84.17 (72.17 to 98.50) | −6.80 (−7.42 to −6.17) | <.001 | 77.75 (64.63 to 91.23) | −12.85 (−13.82 to −11.88) | <.001 |
| LVESVI, mL/m2 | n = 272 | n = 273 | n = 256 | ||||
| Yes | 56.38 (48.41 to 65.06) | 47.83 (39.06 to 56.78) | −8.07 (−8.86 to −7.29) | <.001 | 40.66 (32.37 to 50.94) | −14.15 (−15.15 to −13.15) | <.001 |
| No | 66.22 (54.64 to 80.73) | 55.73 (44.12 to 72.05) | −9.09 (−9.79 to −8.39) | <.001 | 48.91 (36.25 to 63.61) | −16.06 (−17.14 to −15.01) | <.001 |
| LAVI, mL/m2 | n = 264 | n = 263 | n = 250 | ||||
| Yes | 32.85 (28.06 to 38.07) | 28.94 (24.86 to 33.32) | −4.09 (−4.53 to −3.65) | <.001 | 26.00 (22.88 to 30.97) | −7.06 (−7.54 to −6.58) | <.001 |
| No | 41.39 (34.72 to 48.68) | 36.19 (29.46 to 43.60) | −4.54 (−5.09 to −3.99) | <.001 | 32.53 (26.76 to 38.83) | −7.80 (−8.41 to −7.20) | <.001 |
| E/e′ | n = 244 | n = 234 | n = 225 | ||||
| Yes | 9.80 (7.20 to 13.25) | 8.90 (6.80 to 12.60) | −0.40 (−0.89 to 0.08) | .10 | 8.70 (7.00 to 12.40) | −0.93 (−1.43 to −0.43) | <.001 |
| No | 12.80 (9.80 to 18.00) | 11.60 (8.40 to 16.00) | −1.69 (−2.25 to −1.13) | <.001 | 11.00 (8.30 to 15.90) | −1.41 (−2.08 to −0.73) | <.001 |
Abbreviations: E/e′, ratio of early transmitral Doppler velocity/early diastolic annular velocity; LAVI, left atrial volume index; LS, least-square; LVEDVI, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; LVESVI, left ventricular end-systolic volume index; NT-proBNP, N-terminal pro–B-type natriuretic peptide; No, not in the subgroup; Yes, in the subgroup.
Table 5. Change in Cardiac Remodeling Measurements From Baseline to 6 and 12 Months After Initiation of Sacubitril-Valsartan Among Patients Not Achieving Target Dose of Sacubitril-Valsartan by 12 Months.
| Unable to Achieve Target Dose of Sacubitril-Valsartan? | Baseline Value, Median (25th to 75th Percentile) | 6-mo Value, Median (25th to 75th Percentile) | LS Mean Change From Baseline at 6 mo (95% CI) | P Value | 12-mo Value, Median (25th to 75th Percentile) | LS Mean Change From Baseline at 12 mo (95% CI) | P Value |
|---|---|---|---|---|---|---|---|
| LVEF | n = 264 | n = 234 | n = 203 | ||||
| Yes | 27.9 (24.3 to 32.2) | 34.1 (28.7 to 40.0) | 5.5 (4.9 to 6.1) | <.001 | 37.5 (31.8 to 44.8) | 9.4 (8.4 to 10.3) | <.001 |
| No | 28.4 (24.7 to 33.1) | 34.2 (29.3 to 39.5) | 5.0 (4.6 to 5.5) | <.001 | 37.8 (32.3 to 45.3) | 9.4 (8.7 to 10.0) | <.001 |
| LVEDVI, mL/m2 | n = 263 | n = 234 | n = 203 | ||||
| Yes | 86.63 (74.91 to 101.35) | 78.65 (67.76 to 94.21) | −6.21 (−7.00 to −5.43) | <.001 | 74.67 (62.73 to 86.44) | −10.99 (−12.21 to −9.77) | <.001 |
| No | 87.45 (76.68 to 99.82) | 79.79 (70.30 to 92.24) | −6.86 (−7.43 to −6.29) | <.001 | 73.62 (63.50 to 86.29) | −12.83 (−13.64 to −12.03) | <.001 |
| LVESVI, mL/m2 | n = 263 | n = 234 | n = 203 | ||||
| Yes | 61.67 (52.11 to 75.95) | 52.71 (41.97 to 67.30) | 52.71 (41.97 to 67.30) | <.001 | 46.25 (34.08 to 58.54) | −14.32 (−15.67 to −12.97) | <.001 |
| No | 61.69 (51.92 to 74.57) | 52.18 (42.51 to 64.81) | 52.18 (42.51 to 64.81) | <.001 | 45.3 (35.20 to 56.85) | −15.72 (−16.60 to −14.84) | <.001 |
| LAVI, mL/m2 | n = 263 | n = 231 | n = 202 | ||||
| Yes | 37.25 (31.65 to 46.12) | 32.41 (27.28 to 40.65) | −4.27 (−5.05 to −3.49) | <.001 | 29.31 (24.99 to 35.81) | −7.23 (−7.97 to −6.50) | <.001 |
| No | 37.87 (31.49 to 46.00) | 32.94 (27.68 to 39.74) | −4.41 (−4.79 to −4.03) | <.001 | 29.33 (24.31 to 35.90) | −7.76 (−8.28 to −7.25) | <.001 |
| E/e′ | n = 217 | n = 192 | n = 164 | ||||
| Yes | 11.50 (8.30 to 16.00) | 10.55 (7.80 to 14.10) | −0.94 (−1.71 to −0.16) | .02 | 10.10 (7.70 to 15.10) | −0.46 (−1.32 to 0.40) | .29 |
| No | 11.70 (8.90 to 16.00) | 10.35 (7.60 to 14.85) | −1.37 (−1.84 to −0.90) | <.001 | 10.25 (7.65 to 14.00) | −1.65 (−2.15 to −1.16) | <.001 |
Abbreviations: E/e′, ratio of early transmitral Doppler velocity/early diastolic annular velocity; LAVI, left atrial volume index; LS, least-square; LVEDVI, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; LVESVI, left ventricular end-systolic volume index; No, not in the subgroup; Yes, in the subgroup.
In parallel process latent growth curve models, a significant negative association was present at each time point between average slope of change in log-transformed NT-proBNP and LVEF (r = −0.450; P < .001); at month 6 and month 12, a 1–log-unit decrease in circulating levels of NT-proBNP was associated with a 4.6% increase in LVEF at each time point (Z = 36.26, P < .001). Similar results were observed for LVEDVI and LVESVI where the slope of change for each had significant positive association with slope of change in circulating log-transformed NT-proBNP (r = 0.453, P < .001 and r = 0.417, P < .001, respectively); a 1–log-unit decrease in NT-proBNP was associated with a 5.74-mL/m2 decrease in LVEDVI (Z = −34.44, P < .001) and an 8.05-mL/m2 decrease in LVESVI (Z = −38.91, P < .001) at each time point. Weaker but still significant associations were found with changes in log-transformed NT-proBNP and LAVI (r = 0.159, P < .001) where a 1–log-unit decrease in NT-proBNP was associated with a 3.06-mL/m2 decrease in LAVI (Z = −19.95, P < .001). A direct association was also observed between the slopes of change in log-transformed NT-proBNP and E/e′ at each time point (r = 0.513, P < .001); a 1–log-unit decrease in NT-proBNP was associated with a 0.64 decrease in E/e′ (Z = −4.99, P < .001).
Adverse Events
The most frequent adverse events (eTable 5 in Supplement 3) were hypotension (17.6%), dizziness (16.8%), hyperkalemia (13.2%), and worsening kidney function (12.3%). The frequency of positively adjudicated angioedema was low, occurring in only 2 patients (0.3%), of which 1 was black (0.56%); both cases were mild, resolving with antihistamines or no therapy.
Discussion
In this prospective observational study of patients with chronic HF and LVEF of 40% or less, NT-proBNP reduction following initiation of ARNI correlated with improvements in markers of cardiac volume and function at 12 months. The correlation coefficients were consistent with weak-to-moderate associations. These findings were also present at 6 months, but the correlations were lower. Among 3 prespecified subgroups not represented in the PARADIGM-HF trial, correlations between change in NT-proBNP and cardiac volume and function were similar to the group as a whole.
Reduction in NT-proBNP following treatment with sacubitril-valsartan was associated with an increase in LVEF, and reductions in indexed LV and LA volumes and E/e′. In terms of absolute changes in measures of cardiac remodeling, after 12 months, treatment with sacubitril-valsartan was associated with a mean LVEF increase of 9.4% and corresponding clinically significant reductions in LVEDVI and LVESVI. In addition, clinically significant reductions in LAVI, E/e′, and LVMi were observed. Improvement in these measures was evident at 6 months but was more pronounced at 1 year. Among 3 patient subgroups not represented in the PARADIGM-HF trial, quantitative improvement in cardiac structure and function was similar to the group as a whole.
Reverse myocardial remodeling—manifested by reduced LV size and improved function—is central to the benefits of most HF treatments and is associated with an improved prognosis.5 Reduced concentrations of NT-proBNP after GDMT are modestly but significantly associated with reverse remodeling7,8 and a more favorable prognosis. Although reduction in NT-proBNP concentration was strongly associated with outcomes in PARADIGM-HF,6 a link to reverse cardiac remodeling was not examined. Prospective data regarding sacubitril-valsartan and cardiac remodeling are limited. In a small cohort of patients treated with sacubitril-valsartan and followed up for 4 months, Martens and colleagues11 reported a 5% mean improvement in LVEF. In a 12-month study of patients with severe mitral regurgitation, Kang and colleagues12 reported treatment with sacubitril-valsartan increased LVEF by 2.6%. Thus, this study adds to the knowledge base regarding associations between ARNI therapy, change in NT-proBNP, and cardiac remodeling.
In this study, most of the reduction in NT-proBNP following initiation of sacubitril-valsartan occurred early, during a period when most patients received the lowest dose of the drug. The results of post hoc latent growth curve models suggest that the reduction in NT-proBNP was associated with subsequent cardiac reverse remodeling. Also, although improvement in cardiac structure and function was present at 6 months, at 12 months, further improvement in LVEF and volumes was present, with 25% of the study participants experiencing an absolute LVEF increase of more than 13%. In addition, reductions in both LAVI and E/e′ were observed after sacubitril-valsartan treatment. Both are important prognostic measures, reflecting the magnitude and chronicity of elevated cardiac filling pressures. Improved LAVI and E/e′ in tandem with LV reverse remodeling—so-called “complete left-sided reverse remodeling”—may identify patients with particularly favorable improvement in prognosis.13 Besides reverse remodeling of the LV and LA and reduction in E/e′, lower LVMi was associated with initiation and titration of sacubitril-valsartan. In context, the reverse cardiac remodeling observed in this study compares favorably with that reported for other GDMT14,15,16,17,18 and for CRT.19 Most patients in this study received a background of contemporary GDMT, yet initiation of sacubitril-valsartan was still associated with subsequent reverse remodeling. Taken together, the results of this study suggest that patients with NT-proBNP reduction following ARNI initiation are likely to experience reverse cardiac remodeling. As studies have suggested that a lack of NT-proBNP reduction after therapy for HFrEF is associated with worse LV size and function,7,20 studies evaluating meaning of NT-proBNP nonresponse in this study are planned.
Because of the design of the PARADIGM-HF trial, which required all patients to receive stable ACEI or ARB therapy, to have elevated natriuretic peptide concentrations, and to achieve target doses of enalapril and sacubitril-valsartan during the run-in period to remain in the trial, there was ambiguity about the effects of ARNI in patients not meeting these criteria. In this study, the correlation between NT-proBNP and cardiac reverse remodeling in each of these prespecified subgroups were consistent with that of the group as a whole. Further evaluation of the associations between therapy with sacubitril-valsartan and reverse cardiac remodeling in these and other important subgroups, such as women, black individuals, older patients, and those with relative hypotension, is planned.
Limitations
This study has several limitations. First is its observational, single-group, open-label design. However, expected need for a long-term study18 along with widespread availability of sacubitril-valsartan following regulatory approval plus its class I clinical practice guideline recommendation made a comparison group unethical for a 12-month study. A short-term randomization vs ACEI/ARB might have been considered. As expected, reverse remodeling appeared to progress beyond 6 months; the magnitude of reverse remodeling might have been substantially underestimated with shorter follow-up. Second, the primary end point of this study was based on changes in NT-proBNP, and its correlation with reverse cardiac remodeling; correlation coefficients were generally below 0.40, reflecting the broad range of factors that may affect NT-proBNP concentrations besides remodeling. Third, multiple comparisons may have increased the risk of type I error. Fourth, not all echocardiographic measurements were available at each time point due to study withdrawal or technical limitations. Fifth, race in this study was investigator-determined, with potential risk for inaccuracy.
Conclusions
In this exploratory study of patients with HFrEF treated with sacubitril-valsartan, reduction in NT-proBNP concentration was weakly yet significantly correlated with improvement in markers of cardiac volume and function at 12 months. The observed reverse cardiac remodeling may provide a mechanistic explanation for the effects of sacubitril-valsartan in patients with HFrEF.
Trial Protocol
Statistical Analysis Plan
eFigure 1. Study Procedures During PROVE-HF
eFigure 2. Study Flow Diagram and Patient Disposition
eFigure 3. Boxplots Detailing Median Concentrations of Log2-NT-proBNP Across Study Visits
eFigure 4. Scatter Plots Demonstrating Baseline versus 12 Month Results for A) LVEF, B) LVEDVi, C) LVESVi, D) LAVi, and E) E/E′
eTable 1. Patient Eligibility Criteria for PROVE-HF
eTable 2. Median (25th, 75th Percentile) NT-proBNP Concentrations at Each Study Time Point
eTable 3. Correlations Between Change in Log2-NT-proBNP and Echocardiographic Measurements at 6 Months Post-enrollment
eTable 4. Echocardiographic Results only in Those Subjects With Available Data From All Three Echocardiographic Examinations (Baseline, 6 Months, and 12 Months)
eTable 5. Adverse Events of Interest During the 12 Months of PROVE-HF
Data Sharing Statement
References
- 1.McMurray JJ, Packer M, Desai AS, et al. ; PARADIGM-HF Investigators and Committees . Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993-1004. doi: 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 2.Kemp CD, Conte JV. The pathophysiology of heart failure. Cardiovasc Pathol. 2012;21(5):365-371. doi: 10.1016/j.carpath.2011.11.007 [DOI] [PubMed] [Google Scholar]
- 3.Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging. 2011;4(1):98-108. doi: 10.1016/j.jcmg.2010.10.008 [DOI] [PubMed] [Google Scholar]
- 4.Udelson JE, Konstam MA. Ventricular remodeling fundamental to the progression (and regression) of heart failure. J Am Coll Cardiol. 2011;57(13):1477-1479. doi: 10.1016/j.jacc.2011.01.009 [DOI] [PubMed] [Google Scholar]
- 5.Kramer DG, Trikalinos TA, Kent DM, Antonopoulos GV, Konstam MA, Udelson JE. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta-analytic approach. J Am Coll Cardiol. 2010;56(5):392-406. doi: 10.1016/j.jacc.2010.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zile MR, Claggett BL, Prescott MF, et al. Prognostic implications of changes in N-terminal pro-B-type natriuretic peptide in patients with heart failure. J Am Coll Cardiol. 2016;68(22):2425-2436. doi: 10.1016/j.jacc.2016.09.931 [DOI] [PubMed] [Google Scholar]
- 7.Daubert MA, Adams K, Yow E, et al. NT-proBNP goal achievement is associated with significant reverse remodeling and improved clinical outcomes in HFrEF. JACC Heart Fail. 2019;7(2):158-168. doi: 10.1016/j.jchf.2018.10.014 [DOI] [PubMed] [Google Scholar]
- 8.Weiner RB, Baggish AL, Chen-Tournoux A, et al. Improvement in structural and functional echocardiographic parameters during chronic heart failure therapy guided by natriuretic peptides: mechanistic insights from the ProBNP Outpatient Tailored Chronic Heart Failure (PROTECT) study. Eur J Heart Fail. 2013;15(3):342-351. doi: 10.1093/eurjhf/hfs180 [DOI] [PubMed] [Google Scholar]
- 9.Januzzi JL, Butler J, Fombu E, et al. Rationale and methods of the Prospective Study of Biomarkers, Symptom Improvement, and Ventricular Remodeling During Sacubitril/Valsartan Therapy for Heart Failure (PROVE-HF). Am Heart J. 2018;199:130-136. doi: 10.1016/j.ahj.2017.12.021 [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1-39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 11.Martens P, Beliën H, Dupont M, Vandervoort P, Mullens W. The reverse remodeling response to sacubitril/valsartan therapy in heart failure with reduced ejection fraction. Cardiovasc Ther. 2018;36(4):e12435. doi: 10.1111/1755-5922.12435 [DOI] [PubMed] [Google Scholar]
- 12.Kang DH, Park SJ, Shin SH, et al. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation. Circulation. 2019;139(11):1354-1365. doi: 10.1161/CIRCULATIONAHA.118.037077 [DOI] [PubMed] [Google Scholar]
- 13.Mathias A, Moss AJ, McNitt S, et al. Clinical implications of complete left-sided reverse remodeling with cardiac resynchronization therapy: a MADIT-CRT substudy. J Am Coll Cardiol. 2016;68(12):1268-1276. doi: 10.1016/j.jacc.2016.06.051 [DOI] [PubMed] [Google Scholar]
- 14.Australia/New Zealand Heart Failure Research Collaborative Group Randomised, placebo-controlled trial of carvedilol in patients with congestive heart failure due to ischaemic heart disease. Lancet. 1997;349(9049):375-380. doi: 10.1016/S0140-6736(97)80008-6 [DOI] [PubMed] [Google Scholar]
- 15.Greenberg B, Quinones MA, Koilpillai C, et al. Effects of long-term enalapril therapy on cardiac structure and function in patients with left ventricular dysfunction: results of the SOLVD echocardiography substudy. Circulation. 1995;91(10):2573-2581. doi: 10.1161/01.CIR.91.10.2573 [DOI] [PubMed] [Google Scholar]
- 16.Konstam MA, Rousseau MF, Kronenberg MW, et al. ; SOLVD Investigators . Effects of the angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dysfunction in patients with heart failure. Circulation. 1992;86(2):431-438. doi: 10.1161/01.CIR.86.2.431 [DOI] [PubMed] [Google Scholar]
- 17.Tsutamoto T, Wada A, Maeda K, et al. Effect of spironolactone on plasma brain natriuretic peptide and left ventricular remodeling in patients with congestive heart failure. J Am Coll Cardiol. 2001;37(5):1228-1233. doi: 10.1016/S0735-1097(01)01116-0 [DOI] [PubMed] [Google Scholar]
- 18.Hall SA, Cigarroa CG, Marcoux L, Risser RC, Grayburn PA, Eichhorn EJ. Time course of improvement in left ventricular function, mass and geometry in patients with congestive heart failure treated with beta-adrenergic blockade. J Am Coll Cardiol. 1995;25(5):1154-1161. doi: 10.1016/0735-1097(94)00543-Y [DOI] [PubMed] [Google Scholar]
- 19.Solomon SD, Foster E, Bourgoun M, et al. ; MADIT-CRT Investigators . Effect of cardiac resynchronization therapy on reverse remodeling and relation to outcome: multicenter automatic defibrillator implantation trial: cardiac resynchronization therapy. Circulation. 2010;122(10):985-992. doi: 10.1161/CIRCULATIONAHA.110.955039 [DOI] [PubMed] [Google Scholar]
- 20.Gaggin HK, Truong QA, Rehman SU, et al. Characterization and prediction of natriuretic peptide “nonresponse” during heart failure management: results from the ProBNP Outpatient Tailored Chronic Heart Failure (PROTECT) and the NT-proBNP-Assisted Treatment to Lessen Serial Cardiac Readmissions and Death (BATTLESCARRED) study. Congest Heart Fail. 2013;19(3):135-142. doi: 10.1111/chf.12016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eFigure 1. Study Procedures During PROVE-HF
eFigure 2. Study Flow Diagram and Patient Disposition
eFigure 3. Boxplots Detailing Median Concentrations of Log2-NT-proBNP Across Study Visits
eFigure 4. Scatter Plots Demonstrating Baseline versus 12 Month Results for A) LVEF, B) LVEDVi, C) LVESVi, D) LAVi, and E) E/E′
eTable 1. Patient Eligibility Criteria for PROVE-HF
eTable 2. Median (25th, 75th Percentile) NT-proBNP Concentrations at Each Study Time Point
eTable 3. Correlations Between Change in Log2-NT-proBNP and Echocardiographic Measurements at 6 Months Post-enrollment
eTable 4. Echocardiographic Results only in Those Subjects With Available Data From All Three Echocardiographic Examinations (Baseline, 6 Months, and 12 Months)
eTable 5. Adverse Events of Interest During the 12 Months of PROVE-HF
Data Sharing Statement