Key Points
Question
How does optimized prehospital management featuring a clinical score compare with management in a mobile stroke unit (MSU) in triaging patients with stroke to hospitals providing or not providing neurointerventional treatment?
Findings
In this randomized clinical trial involving 116 patients, a protocol that included the use of the Los Angeles Motor Scale resulted in accurate triage decisions for 37 of 53 patients, whereas an MSU with imaging enabled accurate triage decisions for 63 of 63 patients, a significant difference.
Meaning
Depending on the health care environment, both management optimized by a clinical score and deployment of an MSU can be beneficial in triage decision-making.
This randomized clinical trial explores how prehospital management optimized by the use of the Los Angeles Motor Scale compares with prehospital management in a mobile stroke unit that includes vascular imaging in accurately triaging patients with stroke to the appropriate target hospital providing (comprehensive stroke center) or not providing (primary stroke center) neurointerventional treatment.
Abstract
Importance
Transferring patients with large-vessel occlusion (LVO) or intracranial hemorrhage (ICH) to hospitals not providing interventional treatment options is an unresolved medical problem.
Objective
To determine how optimized prehospital management (OPM) based on use of the Los Angeles Motor Scale (LAMS) compares with management in a Mobile Stroke Unit (MSU) in accurately triaging patients to the appropriate hospital with (comprehensive stroke center [CSC]) or without (primary stroke center [PSC]) interventional treatment.
Design, Setting, and Participants
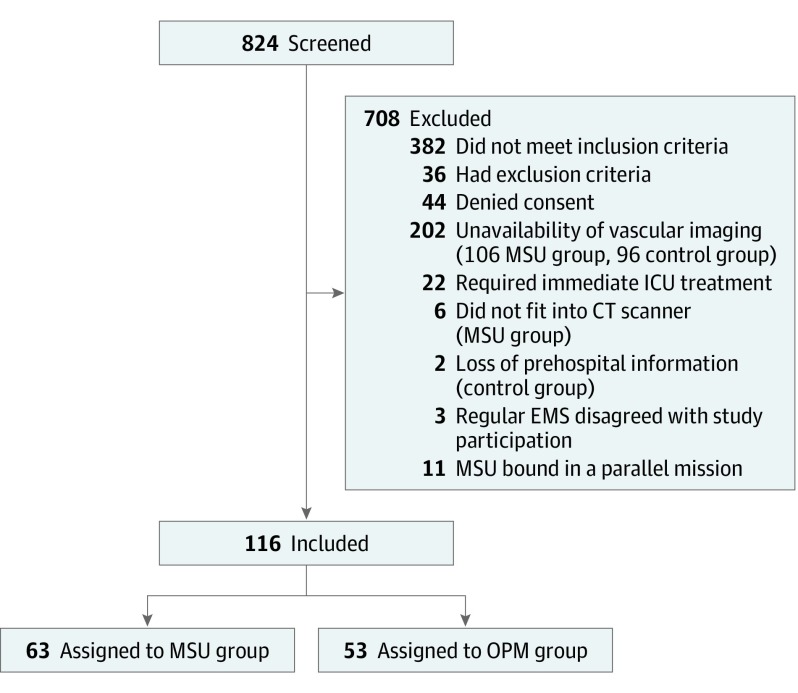
In this randomized multicenter trial with 3-month follow-up, patients were assigned week-wise to one of the pathways between June 15, 2015, and November 15, 2017, in 2 regions of Saarland, Germany; 708 of 824 suspected stroke patients did not meet inclusion criteria, resulting in a study population of 116 adult patients.
Interventions
Patients received either OPM based on a standard operating procedure that included the use of the LAMS (cut point ≥4) or management in an MSU (an ambulance with vascular imaging, point-of-care laboratory, and telecommunication capabilities).
Main Outcomes and Measures
The primary end point was the proportion of patients accurately triaged to either CSCs (LVO, ICH) or PSCs (others).
Results
A predefined interim analysis was performed after 116 patients of the planned 232 patients had been enrolled. Of these, 53 were included in the OPM group (67.9% women; mean [SD] age, 74 [11] years) and 63 in the MSU group (57.1% women; mean [SD] age, 75 [11] years). The primary end point, an accurate triage decision, was reached for 37 of 53 patients (69.8%) in the OPM group and for 63 of 63 patients (100%) in the MSU group (difference, 30.2%; 95% CI, 17.8%-42.5%; P < .001). Whereas 7 of 17 OPM patients (41.2%) with LVO or ICH required secondary transfers from a PSC to a CSC, none of the 11 MSU patients (0%) required such transfers (difference, 41.2%; 95% CI, 17.8%-64.6%; P = .02). The LAMS at a cut point of 4 or higher led to an accurate diagnosis of LVO or ICH for 13 of 17 patients (76.5%; 6 triaged to a CSC) and of LVO selectively for 7 of 9 patients (77.8%; 2 triaged to a CSC). Stroke management metrics were better in the MSU group, although patient outcomes were not significantly different.
Conclusions and Relevance
Whereas prehospital management optimized by LAMS allows accurate triage decisions for approximately 70% of patients, MSU-based management enables accurate triage decisions for 100%. Depending on the specific health care environment considered, both approaches are potentially valuable in triaging stroke patients.
Trial Registration
ClinicalTrials.gov identifier: NCT02465346
Introduction
Stroke is one of the most frequent causes of disability and death.1 Apart from its effects on individual patients, stroke results in enormous societal costs associated with rehabilitation, long-term care, and loss of workforce members.
Generally, intravenous thrombolysis with recombinant tissue-type plasminogen activator is the standard of care for acute ischemic stroke, with proven efficacy. However, when stroke is caused by large-vessel occlusion (LVO), trials provide compelling evidence of the superiority of intra-arterial treatment (IAT) rather than medical treatment alone. Therefore, current stroke management guidelines recommend IAT for patients with LVO.2 Even so, although an estimated 10% to 27%3,4,5 of patients with stroke have experienced an LVO, only a small minority of patients with stroke (<5%) receive IAT.3,6
A primary reason for undertreatment with IAT is delayed presentation at hospitals in which thrombectomy can be performed.7 Interventional stroke treatments are offered only by the few highly specialized stroke centers (comprehensive stroke centers [CSCs]) at which neurointerventionalists, advanced technical resources, and peri-interventional services are available, but not by the many regional stroke-treating hospitals (primary stroke centers [PSCs]). Therefore, patients with LVO are often transferred to hospitals that cannot offer thrombectomy and then, after eventually undergoing intravenous thrombolysis, are secondarily transferred to a thrombectomy-capable CSC (“drip-and-ship” paradigm). Compared with direct referral to a CSC (“mothership” paradigm), such secondary interhospital transfers cause detrimental treatment delays that have been reported to range from 96 minutes to 111 minutes.8 Even when the distance between a PSC and a CSC is only 15 miles (24 km), transfer times of 104 minutes have been reported.7 According to the “time is brain” paradigm valid for IAT, delays due to interhospital transfers worsen clinical outcomes.9
The time is brain concept probably applies to intracranial hemorrhage (ICH) as well.10 Although evidence from clinical studies is much less robust for patients with ICH than that for patients with LVO, patients with ICH may also benefit from rapid evaluation at CSCs, where they may be treated with placement of ventricular drains, surgical decompression, or neurointensive care.11
Generally, the undertreatment of stroke is most pronounced in rural regions.12,13 Because CSCs are located almost exclusively in metropolitan centers, the urban-rural treatment disparity is greatest with regard to advanced interventional stroke treatments.6,14
One approach that has been discussed for improving the accuracy of triage decisions is the use of preclinical stroke severity scales aimed at detecting LVO.15,16,17,18,19,20,21,22 The Los Angeles Motor Scale (LAMS) is a brief 3-item scale that focuses only on motor symptoms.15 Few data are available about the accuracy of the LAMS when used prospectively by emergency medical services (EMS) personnel in the field to guide the triage of patients to the most appropriate target hospital.22
Another strategy aimed at improving the accurate triage of patients with stroke to the most appropriate target hospital is the use of a mobile stroke unit (MSU), an ambulance that incorporates a computed tomography (CT) scanner, a point-of-care (POC) laboratory, and telemedicine communication to a hospital.23,24 Studies of prehospital stroke management have found not only dramatic reductions in delays before thrombolysis25,26,27,28 but also the feasibility of etiology-based triage of patients to hospitals providing or not providing interventional treatment options when CT angiography is implemented onboard.24,29,30,31 The present randomized clinical trial was performed to determine how stroke management according to a standard operating procedure (SOP) featuring a prehospital stroke severity scale compares with stroke management based on the use of an MSU in accurately triaging patients to hospitals that provide (CSCs) or do not provide (PSCs) neurointerventional treatment.
Methods
Patients and Study Design
This prospective randomized parallel-group multicenter trial (ClinicalTrials.gov identifier NCT02465346) with 3-month follow-up, coordinated by the University of the Saarland, Saarbrücken, Germany, was opened on June 15, 2015, and was terminated after a predefined interim analysis on November 15, 2017. The trial took place in 2 separate nonurban regions of the federal state of Saarland, Germany, in the context of a statewide network of stroke-treating hospitals offering different levels of care (2 CSCs and 8 PSCs) (eFigure in Supplement 1). Among 824 patients for whom emergency services were called because of suspected acute stroke, 708 did not meet inclusion criteria, resulting in a study population of 116 adults. Patients received either optimized prehospital management (OPM) based on a SOP that included the use of the LAMS (cut point ≥4) or management in an MSU. The catchment areas of these study regions were based on isochrones (time required to access the patients by ambulance) of 16 minutes around the EMS stations at Püttlingen and St Ingbert, where the MSU was alternately stationed (eFigure in Supplement 1). Response times were between 8 am and 6 pm, 7 days per week.
All emergency calls in the state are evaluated by 1 central dispatch office. Screening for possible stroke as the basis for dispatch of the MSU included standardized questions from the Face, Arm, Speech, Time (FAST) scale, extended by the items “sudden loss of consciousness” (with no other obvious medical reason) and “sudden weakness of the leg” (cl-FAST scale).
In this intent-to-treat randomized clinical trial, the procedure to be applied to a patient was randomly assigned week-wise, as previously described.28 To achieve a balance between both groups regarding potential seasonal confounders, a block size of 4 weeks was chosen to limit the length of the time spans during which the same procedure was used. The randomization list was created by an independent statistician using the SAS procedure PLAN (SAS Institute Inc). During weeks when MSU deployment was used at one study site, OPM was used at the other study site and vice versa.
Inclusion criteria were as follows: age at least 18 years; the presence of 1 or more stroke symptoms on the cl-FAST scale, as reported to the EMS dispatch office and confirmed by the study physician either in the hospital or in the MSU; reported time from symptom onset to call of 8 hours or less; the occurrence of a “wake-up” stroke; and written informed consent by the patient or the patient’s legal representative. Apart from the unavailability of vascular imaging, exclusion criteria were renal dysfunction (history of dialysis or creatinine level ≥1.5 mg/mL [to convert creatinine level to micromoles per liter, multiply by 88.4]), pregnancy, known allergy to or contraindications to the use of contrast agents, preexisting severe or terminal disease, and unstable cardiopulmonary medical conditions requiring immediate intensive care treatment.
Ethics and Monitoring
The trial protocol, informed consent process, and participant information document were approved by the Ethics Committee of the Medical Association of the Saarland, Germany (AZ-71/74 on July 25, 2014). The trial protocol is available in Supplement 2. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
All initial CT angiography or magnetic resonance imaging (MRI) angiography scans were reevaluated for the presence or absence of LVO or ICH at a certified neuroradiology core center (Department of Neuroscience, Faculty of Medical Science, Postgraduate Medical Institute, Anglia Ruskin University, Chelmsford, Southend University Hospital, Southend-on-Sea, United Kingdom) masked to clinical information and first radiographic report. An independent clinical monitor (Interdisciplinary Centre for Clinical Trials [IZKS], Mainz, Germany) monitored the trial.
Optimized Prehospital Management
Stroke management was optimized according to a SOP for the entire EMS of the federal state of Saarland by the inclusion of an algorithm for triage decision-making when stroke was suspected. In detail, one factor to be considered in the triage decision with respect to the appropriate target hospital was the presence of severe motor symptoms, as defined by a LAMS cutoff score of 4 or higher.15 The 3-item LAMS, a simple, reproducible, and rapid approach to diagnosing severe strokes in the prehospital setting,15,17,22 was chosen by the EMS decision makers in the federal state of Saarland because it was deemed easy to implement. Other factors were symptom onset times of 8 hours or less (wake-up stroke) and quality-of-life aspects, such as severe comorbidity and extent of prestroke dependency. This SOP was set in operation by the central EMS coordinating authority of the state, the Zweckverband für Rettungsdienst und Feuerwehralarmierung, Saar, Germany, on June 1, 2015, and was accompanied by statewide structured training sessions held approximately every 3 months for EMS personnel in the field and every 6 months for dispatch center personnel. In addition, the SOP was distributed by representatives at all EMS stations and was a component of the educational curriculum of the EMS school of the Saarland.
The study physician in the hospital confirmed the presence of inclusion and exclusion criteria and obtained written informed consent before each patient was enrolled in the study. Patients with LVO or ICH who had been transported to a PSC were considered for secondary transfer to a CSC for evaluation with regard to interventional stroke treatments.
MSU-Based Stroke Management
The MSU response consisted of the combined dispatch of the MSU and the conventional EMS team, which in the Saarland generally includes an emergency physician for critically ill patients. The MSU team included a paramedic, a stroke physician, and a radiologist. This MSU staffing configuration is still experimental because of unsettled national legal issues (ie, performance of CT angiography by onboard responders other than radiologists). However, the replacement of onboard specialists with experts remotely available via telemedicine has already been shown to be feasible at other project sites.26,32 The MSU physician confirmed the presence of inclusion and exclusion criteria and obtained written informed consent before each patient was enrolled in the study. Patients who declined written informed consent for MSU treatment were treated conventionally.
The MSU intervention consisted of neurologic examination, POC testing, and noncontrast CT, as well as CT angiography for patients with no ICH. Variables for CT angiography were as follows: 70 mL of nonionic contrast agent (injection rate of 3.5 mL per second with a 20-second delay), helical scan (140 kV, 7 mA, 1.25/2.5 mm), and axial/coronal reconstructions with slice thickness of 5 to 9 mm (maximum-intensity projection).
If indicated, IV thrombolysis was administered directly at the emergency site. On the basis of the results of the prehospital diagnostic workup, the MSU team made triage decisions (with optionally available support by hospital experts via telemedicine) according to the vascular etiology. Patients were taken to the nearest CSC if either an LVO (occlusion of the intracranial internal carotid artery, the M1 branches of the middle cerebral artery, or the basilar artery) or an ICH was diagnosed by the MSU team; if neither type of stroke was suspected, patients were taken to the nearest PSC (or to a CSC if it was closer).
The MSU itself is an ambulance that contains, in addition to standard emergency equipment, the following specialized equipment: an accumulator-driven CT scanner (Ceretom; Neurologica/Samsung) allowing multimodal imaging, a telemedicine system (MEYTEC) enabling videoconferencing and transmission of videos of the patient’s examination and of CT scans, and a POC system for determination of platelet count, leukocyte count, erythrocyte count, hemoglobin level, hematocrit (PocH 100i; Sysmex), international normalized ratio, activated partial thromboplastin time (Hemochron Jr; ITC), γ-glutamyltransferase and p-amylase activity, and glucose and creatinine levels (Reflotron Plus; Roche Diagnostics). In addition to standard medications, the MSU also carries thrombolytics and anticoagulant antagonists.24,29
Clinical Assessment
Assessments occurred at first physician contact, on day 7 (±1 day), and on day 90 (±14 days) and included history, neurologic examination, and documentation of safety end points. Neurologic examination included the use of the National Institutes of Health Stroke Scale (NIHSS), with scores ranging from 0 to 42 (higher scores indicate more severe disease), at the time of admission and the use of the Modified Rankin Scale for Neurologic Disability (mRS) on day 90.
End Points
The primary end point was the proportion of patients with an accurate triage decision regarding transfer to the target hospital offering the required level of care. An accurate triage decision was defined as a decision to transport patients with LVO or ICH to the nearest CSC or to transport patients with other stroke types to the nearest PSC (or CSC if this was the nearest stroke-ready hospital).
Secondary end points were as follows: triage accuracy regarding the presence or absence of LVO alone, the rate of interhospital transfers of patients with LVO or ICH from a PSC to a CSC, stroke management metrics (eg, times from emergency call to first contact with the MSU or the first receiving hospital, end of noncontrast CT or MRI, and end of vascular CT or MRI), imaging-based therapy, and triage decision (defined as the end of all required diagnostic procedures, including neurologic examination, POC laboratory studies, and noncontrast imaging in case of ICH or vascular imaging in case of the absence of ICH), admission to a CSC (in case of LVO or ICH), needle time, and groin puncture time. Concerning outcomes, we assessed the mRS score on day 90 (mRS scores range from 0 [indicating no residual symptoms] to 6 [indicating death]). Safety end points were the occurrence of stroke-related or neurologic death on day 7 (fatal ischemic stroke, fatal reinfarction, or fatal primary or secondary ICH), nonfatal reinfarction, nonfatal secondary ICH (defined as any hemorrhage accompanied by neurologic deterioration of ≥4 points on the NIHSS score), and nonfatal symptomatic edema (defined as brain edema associated with a deterioration of ≥4 points on the NIHSS score). We also recorded other serious adverse events, such as peripheral hemorrhage, seizure, sepsis, thrombosis or pulmonary embolism, renal insufficiency, and allergic responses.
Statistical Analysis
The proportion of patients with stroke with accurate triage decisions (transport to a PSC or a CSC according to the prespecified algorithm) was compared between the 2 treatment arms with the Cochran-Mantel-Haenszel test, adjusted for study region. For all proportion-related secondary end points, we also used the Cochran-Mantel-Haenszel test and the Fisher exact test. Stroke management metrics were expressed as means with standard deviations and were analyzed for significant differences with the Wilcoxon rank sum test for independent groups. The mRS on day 90 was analyzed with logistic regression adjusting for baseline mRS score and study region. The occurrence and severity of adverse events were described for all included patients by treatment arm and by Medical Dictionary for Regulatory Activities classification. Statistical analyses were performed using IBM SPSS Statistics for Windows (version 23.0.0.2; IBM Corporation) and Cytel Studio (version 10; Cytel Software Corporation). Statistical significance was set at 2-sided P < .05.
Sample Size Issues
The estimation of the required sample size was based on the assumption that the proportion of accurately triaged patients would be 80% in the OPM group and more than 95% in the MSU group. Under these assumptions, a sample size of 232 patients would achieve a power of 90% to detect a difference in a group-sequential study with 1 interim analysis after the enrollment of 116 patients. The study was to be stopped if the null hypothesis of no difference between both treatment arms was rejected at the level of α = .00305 (O’Brien-Fleming boundary) on the basis of the results regarding the primary end point. East software (version 4; Cytel) was used for planning the interim analysis, and SAS (version 9.4; SAS Institute Inc) was used for analyses.
Results
After 116 patients of the planned 232 patients had been enrolled, including 53 patients in the OPM group (67.9% women; mean [SD] age, 74 [11] years) and 63 patients in the MSU group (57.1% women; mean [SD] age, 75 [11] years), the predefined interim analysis revealed that the prespecified level of superiority in the primary end point required for study termination had been reached. Reasons for exclusion of screened patients are shown in Figure 1. Because of the unavailability of vascular imaging, 106 patients in the MSU group and 96 patients in the OPM group were excluded. Baseline demographic and medical characteristics of patients are listed in Table 1. Apart from a lower proportion of transient ischemic attacks (TIAs) and mimics and associated higher baseline NIHSS scores in the OPM group, the demographic and medical characteristics of the groups were similar. The original interpretations of the acute cerebral imaging studies determining the presence or absence of LVO or ICH were in 100% agreement with the results of masked reevaluation at a certified neuroradiology core center.
Figure 1. CONSORT Flow Diagram Showing the Trial Design.
During the emergency call, the dispatcher screens patients for stroke symptoms. According to the study’s randomization plan, the dispatcher initiates either MSU-based stroke management or conventional stroke management optimized by the use of a preclinical stroke severity scale. Patients were enrolled in this trial after evaluation for inclusion and exclusion criteria and after written informed consent had been obtained. CONSORT indicates Consolidated Standards of Reporting Trials; CT, computed tomography; EMS, emergency medical services; ICU, intensive care unit; MSU, mobile stroke unit; and OPM, optimized prehospital management.
Table 1. Baseline Demographic and Medical Characteristics of the Study Population.
| Variable | No. (%) | |
|---|---|---|
| OPM Group (n = 53) | MSU Group (n = 63) | |
| Demographics | ||
| Age, mean (SD), y | 74 (11) | 75 (11) |
| Male sex | 17 (32.1) | 27 (42.9) |
| History | ||
| Hypertensiona | 47 (88.7) | 52 (82.5) |
| Type 2 diabetesb | 15 (28.3) | 15 (23.8) |
| Atrial flutter or fibrillation | 16 (30.2) | 10 (15.9) |
| Active smoking | 14 (26.4) | 13 (20.6) |
| Hypercholesterolemiac | 25 (47.2) | 31 (49.2) |
| Previous infarction | 19 (35.8) | 14 (22.2) |
| NIHSS score, median (IQR) | ||
| All | 8 (4-15) | 5 (3-11) |
| Patients with confirmed strokes | 8 (4-15) | 7 (4-15) |
| Prestroke mRS score, median (IQR) | 0 (0-1) | 0 (0-1) |
| Symptom onset to emergency call, mean (SD), min | 63 (89) | 83 (99) |
| Distance from base station to scene, mean (SD), miles [km] | 2.5 (1.4) [or 4.0 (2.3) km] | 4.1 (2.2) [or 6.6 (3.5) km] |
| Discharge Diagnoses | ||
| Ischemic stroke | 39 (73.6) | 32 (50.8) |
| LVO | 9 (17.0) | 3 (4.8) |
| No LVO | 30 (56.6) | 29 (46.0) |
| ICH | 8 (15.1) | 8 (12.7) |
| Transient ischemic attack | 4 (7.5) | 17 (27.0) |
| Stroke mimics | 2 (3.8) | 6 (9.5) |
| Delirium | 0 | 2 (3.2) |
| Hypertensive encephalopathy | 1 (1.9) | 0 |
| Seizure | 1 (1.9) | 1 (1.6) |
| Intoxication | 0 | 1 (1.6) |
| Peripheral nerve lesion | 0 | 2 (3.2) |
Abbreviations: ICH, intracranial hemorrhage; IQR, interquartile range; LVO, large-vessel occlusion; mRS, Modified Rankin Scale; MSU, mobile stroke unit; NIHSS, National Institutes of Health Stroke Scale; OPM, optimized prehospital management.
SI conversion factors: To convert cholesterol level to millimoles per liter, multiply by 0.0259; to convert glycated hemoglobin level to proportion of total hemoglobin, multiply by 0.01.
History of hypertension or antihypertensive treatment.
History of type 2 diabetes, antidiabetic treatments, or glycated hemoglobin level exceeding 6.5%.
Serum cholesterol level exceeding 200 mg/dL, low-density lipoprotein cholesterol level exceeding 130 mg/dL, or the use of lipid-lowering medications.
With regard to the primary end point, the triage decision was accurate for 37 of 53 patients (69.8%) in the OPM group and for 63 of 63 patients (100%) in the MSU group (difference, 30.2%; 95% CI, 17.8%-42.5%; P < .001) (Table 2). Optimized prehospital management enabled the triage of patients with stroke with LVO or ICH to the target hospital providing the appropriate level of care with 35.3% sensitivity, 86.1% specificity, 54.5% positive predictive value, and 73.8% negative predictive value.
Table 2. Primary and Secondary End Points, by Cochran-Mantel-Haenszel Test Unless Otherwise Indicated.
| Variable | OPM Group (n = 53) | MSU Group (n = 63) | P Value | Difference (95% CI) |
|---|---|---|---|---|
| Primary End Point, No./Total No. (%) | ||||
| Triage accuracy for LVO and ICHa | 37/53 (69.8) | 63/63 (100) | <.001 | 30.2 (17.8 to 42.5) |
| Sensitivity | 6/17 (35.3) | 11/11 (100) | NA | NA |
| Specificity | 31/36 (86.1) | 52/52 (100) | NA | NA |
| Positive predictive value | 6/11 (54.5) | 11/11 (100) | NA | NA |
| Negative predictive value | 31/42 (73.8) | 52/52 (100) | NA | NA |
| Secondary End Points, No./Total No. (%) | ||||
| Triage accuracy for LVO aloneb | 41/53 (77.4) | 63/63 (100) | <.001 | 22.6 (13.2 to 35.9) |
| Sensitivity | 2/9 (22.2) | 3/3 (100) | NA | NA |
| Specificity | 39/44 (88.6) | 60/60 (100) | NA | NA |
| Positive predictive value | 2/7 (28.6) | 3/3 (100) | NA | NA |
| Negative predictive value | 39/46 (84.8) | 60/60 (100) | NA | NA |
| Secondary transfer rate | 7/17 (41.2) | 0/11 (0) | .02 | 41.2 (17.8 to 64.6) |
| Treatment rate | ||||
| Recombinant tissue-type plasminogen activator | 14/39 (35.9) | 16/32 (50.0) | .22 | 14.1 (−8.8 to 37.1) |
| Intra-arterial therapy | 6/9 (66.7) | 3/3 (100) | NA | NA |
| Stroke Management Time From Call to Various Metrics, Mean (SD), minc | ||||
| To first contact to MSU or hospital | 41.5 (12.8) | 10.3 (3.6) | <.001 | 31.2 (27.5 to 34.8) |
| To end of noncontrast imaging | 80.0 (39.6) | 39.3 (7.8) | <.001 | 40.7 (29.6 to 51.7) |
| To end of vascular imaging | 686.0 (1549.0) | 47.8 (8.8) | .009 | 638.2 (167.0 to 1109.0) |
| To imaging-based triage and therapy decisiond | 583.0 (1427.0) | 47.6 (9.0) | .009 | 535.4 (142.0 to 929.0) |
| To admission of LVO or ICH to comprehensive stroke center | 133.9 (86.2) | 85.5 (18.8) | .08 | 48.4 (−7.1 to 103.9) |
| To thrombolysis needle | 84.9 (30.2) | 50.1 (10.1) | <.001 | 34.8 (16.7 to 52.7) |
| To groin puncture | 183 (84) | 109, 114, 200e | .46 | 42.3 (84.3 to 168.9) |
| mRS Outcome at Day 90, Median (IQR)f | ||||
| All | 3 (1 to 5) | 1 (0 to 3) | .15 | NA |
| Patient with confirmed strokes | 3 (1 to 5) | 3 (1 to 4) | .12 | NA |
Abbreviations: ICH, intracranial hemorrhage; IQR, interquartile range; LVO, large-vessel occlusion; mRS, Modified Rankin Scale; MSU, mobile stroke unit; NA, not applicable; OPM, optimized prehospital management.
According to the presence (to be triaged to a comprehensive stroke center) or absence (to be triaged to a primary stroke center) of LVO or ICH. The correct triage decision to a comprehensive stroke center in 1 patient in the OPM group and to a primary stroke center in 1 patient in the MSU group was not realized (both because of patient choice).
By Fisher exact test.
By Wilcoxon rank sum test for independent groups.
Defined as the end of all required diagnostic procedures (neurologic examination and imaging, noncontrast imaging in the presence of ICH, and vascular imaging in the absence of ICH).
All 3 available values are displayed for this variable.
By logistic regression for ordinal end points (adjusted for baseline mRS score and study region).
In accordance with the high triage accuracy associated with MSU deployment, secondary interhospital transfers of patients with LVO or ICH from PSCs to CSCs were rendered completely unnecessary for the MSU group (0 of 11 patients) but were required for 7 of 17 patients (41.2%) in the OPM group (difference, 41.2%; 95% CI, 17.8%-64.6%; P = .02). These results are summarized in Table 2.
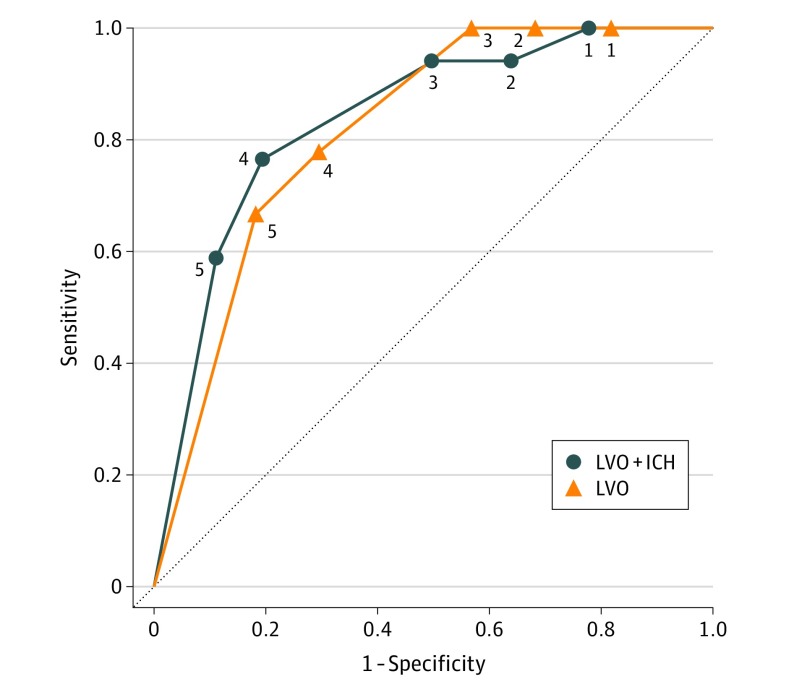
The LAMS at a cut point of 4 or higher correctly identified 13 of 17 patients (76.5%) with LVO or ICH (6 patients were accurately triaged to a CSC). The LAMS at this cut point correctly identified 7 of 9 patients (77.8%) with LVO alone (2 patients were triaged to a CSC). At this cut point, the accuracy of diagnosing LVO and ICH was 79.2%, and the accuracy of diagnosing LVO alone was 71.7%. Values for sensitivity, specificity, positive predictive value, and negative predictive value for this and other cut points are listed in Table 3, and receiver operating characteristic curves are shown in Figure 2.
Table 3. Diagnosis of LVO and ICH or LVO Alone in Patients With Acute Stroke by LAMS Scores at Various Cut Points.
| LAMS Score | Frequency, No. | No./Total No. (%) | ||||
|---|---|---|---|---|---|---|
| Accuracy | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | ||
| LVO and ICH | ||||||
| ≥1 | 45 | 25/53 (47.2) | 17/17 (100) | 8/36 (22.2) | 17/45 (37.8) | 8/8 (100) |
| ≥2 | 39 | 29/53 (54.7) | 16/17 (94.1) | 13/36 (36.1) | 16/39 (41.0) | 13/14 (92.9) |
| ≥3 | 34 | 34/53 (64.2) | 16/17 (94.1) | 18/36 (50.0) | 16/34 (47.1) | 18/19 (94.7) |
| ≥4 | 20 | 42/53 (79.2) | 13/17 (76.5) | 29/36 (80.6) | 13/20 (65.0) | 29/33 (87.9) |
| 5 | 14 | 42/53 (79.2) | 10/17 (58.8) | 32/36 (88.9) | 10/14 (71.4) | 32/39 (82.1) |
| LVO alone | ||||||
| ≥1 | 45 | 17/53 (32.1) | 9/9 (100) | 8/44 (18.2) | 9/45 (20.0) | 8/8 (100) |
| ≥2 | 39 | 23/53 (43.4) | 9/9 (100) | 14/44 (31.8) | 9/39 (23.1) | 14/14 (100) |
| ≥3 | 34 | 28/53 (52.8) | 9/9 (100) | 19/44 (43.2) | 9/34 (26.5) | 19/19 (100) |
| ≥4 | 20 | 38/53 (71.7) | 7/9 (77.8) | 31/44 (70.5) | 7/20 (35.0) | 31/33 (93.9) |
| 5 | 14 | 42/53 (79.2) | 6/9 (66.7) | 36/44 (81.8) | 6/14 (42.9) | 36/39 (92.3) |
Abbreviations: ICH, intracranial hemorrhage; LAMS, Los Angeles Motor Scale; LVO, large-vessel occlusion.
Figure 2. Receiver Operating Characteristic Curves.
Shown are curves for various cut points (as depicted by respective numbers in the diagram) on the Los Angeles Motor Scale for diagnosing either an LVO or an ICH (solid line) or diagnosing LVO selectively (dotted line). Diagonal segments are produced by ties. ICH indicates intracranial hemorrhage; LVO, large-vessel occlusion.
Moreover, MSU-based stroke management was associated with significantly shorter times from emergency call to first contact with the MSU or the first receiving hospital; end of noncontrast CT or MRI; end of vascular CT or MRI, imaging-based therapy, and triage decision; and needle times (Table 2). With regard to long-term outcomes, mRS scores at day 90 were not significantly different between groups (Table 2).
Four of 53 patients in the OPM group (7.5%) and 3 of 63 patients in the MSU group (4.8%) died of stroke-related or neurologic causes; all of them had experienced an ICH (eTable in Supplement 1). Six patients in the OPM group but none in the MSU group had nonlethal complications, including nonfatal reinfarction (2 patients), nonfatal secondary ICH (1 patient), and nonfatal brain edema (3 patients). The rates of other serious adverse events among the 2 groups were within similar ranges (eTable in Supplement 1). Finally, only MSU-based stroke management allowed 16 of 32 patients with ischemic stroke (50.0%) to obtain prehospital thrombolysis and 26 of 63 patients (41.3%) to obtain prehospital active blood pressure management based on the knowledge of whether the stroke was ischemic or hemorrhagic.
Discussion
Transferring patients with LVO or ICH to hospitals without interventional treatment options is an unresolved medical problem that is most pronounced in rural areas.6,14 The resulting secondary interhospital transfers cause considerable costs and detrimental delays before treatment.
The results of this randomized clinical trial show that both treatment optimized by the use of the LAMS and the deployment of an MSU with vascular imaging capabilities can be beneficial in triage decision-making with regard to the most appropriate target hospital, although the levels of accuracy vary. Whereas triage decisions in the MSU group were accurate for all patients, triage decisions for LVO and ICH in the OPM group were accurate for 69.8% of patients. Secondary interhospital transfers have been shown to cause detrimental delays,8,9 to impair treatment,7 and to worsen outcomes.9 Optimized prehospital management was associated with a secondary transfer rate of 41.2%, whereas MSU-based stroke management completely obviated the need for secondary transfers.
In this study, the use of the LAMS with a cut point of 4 or higher was 79.2% accurate in diagnosing LVO or ICH and 71.7% accurate in diagnosing LVO alone. This finding confirms the results of an earlier prehospital evaluation using this LAMS cut point score, which was 72% accurate in diagnosing LVO or ICH and 72% accurate in diagnosing LVO.22 However, not all patients were triaged according to the LAMS alone. In the present real-life EMS environment, the SOP included other factors to be considered in triage decision-making beyond the results of the LAMS.
In addition, the results of this study confirm that MSU-based stroke workup significantly improves stroke management metrics (ie, times to first contact with the MSU or the first receiving hospital, end of noncontrast CT or MRI, end of CT or MRI angiography, imaging-based therapy, and triage decision). Because the immediate administration of recombinant tissue-type plasminogen activator at the emergency site is a central component of the MSU concept, transporting a patient to an even more distant CSC does not occur at the expense of delayed thrombolysis.
Despite the strong evidence for the time is brain paradigm in stroke treatment, the improvement of stroke management metrics by the MSU approach was not associated with significant improvements in long-term patient outcomes. This discrepancy can be explained by the fact that our study was not powered to detect significant differences in this secondary end point.
Limitations
Our study has some limitations. Not all MSU programs use vascular imaging, although this option exists with the CT scanners now used in MSUs. Although it does not cause lengthy delays (<5 minutes), CT angiography requires a higher level of radiological competence among MSU staff, depending on national regulations. As is the case in a hospital, vascular imaging in an MSU requires previous exclusion of contraindications, such as allergies (by history) and renal disorders (by history and creatinine laboratory testing). Another limitation of this study was the exclusion of a large number of patients (106 patients in the MSU group and 96 patients in the OPM group) because of the unavailability of vascular imaging, which was the precondition for determining triage accuracy. Therefore, the results of this trial are valid only if CT angiography is available onboard the MSU.
Although there is growing evidence that the time is brain concept is valid also for patients with hemorrhagic stroke10—and that those patients may benefit from rapid specialist evaluation with regard to the need for ventricular catheters, decompressive craniectomy, hematoma removal, neurointensive care, or additional treatments22—evidence from randomized trials regarding the transfer of patients with ICH to CSCs is still lacking.11 Therefore, at present, LVO is the “true target” for triage decision-making. While the difference in triage accuracy remains statistically significant when the accuracy of triage decisions is determined selectively with regard to the presence or absence of LVO, the low number of patients with LVO in our trial is a limitation.
Moreover, because conventional stroke management allows for longer observation of the natural disease course, it may have resulted in earlier identification and exclusion of TIAs and stroke mimics. Therefore, the lower rates of TIAs and mimics in the OPM group may explain the group’s higher baseline NIHSS scores.
The frequencies of mortality and nonlethal complication rates were within similar ranges in both groups, although the number of cases was small. Despite the increasing number of studies showing that deploying an MSU is not associated with increased risks,25,26,28 the safety of the MSU approach remains a relevant issue for future research.
Conclusions
Whereas prehospital stroke management optimized by the use of a stroke severity scale allows accurate triage decisions for approximately 69.8% of patients, MSU-based stroke management enables accurate triage decisions for 100%. The LAMS itself at a cut point score of 4 or higher was 71.7% accurate in diagnosing LVO. Depending on their further refinement (ie, with regard to SOP implemention or score cut point), and on the specific health care environment, both approaches have potential value in triaging patients with stroke to the appropriate hospital.
eFigure. Areas of Mobile Stroke Unit Activity in a State-Wide Network With Stroke-Treating Hospitals Providing Different Levels of Care (Primary and Comprehensive Stroke Centers)
eTable. Prespecified Safety End Points and Other Serious Adverse Events at Day 7
Trial Protocol
Data Sharing Statement
References
- 1.Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. 2017;120(3):439-448. doi: 10.1161/CIRCRESAHA.116.308413 [DOI] [PubMed] [Google Scholar]
- 2.Powers WJ, Rabinstein AA, Ackerson T, et al. ; American Heart Association Stroke Council . 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association [published corrections appears in Stroke. 2018;49(3):e138 and 2018;49(6):e233-e234]. Stroke. 2018;49(3):e46-e110. [DOI] [PubMed] [Google Scholar]
- 3.Rai AT, Seldon AE, Boo S, et al. . A population-based incidence of acute large vessel occlusions and thrombectomy eligible patients indicates significant potential for growth of endovascular stroke therapy in the USA. J Neurointerv Surg. 2017;9(8):722-726. doi: 10.1136/neurintsurg-2016-012515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.González RG, Furie KL, Goldmacher GV, et al. . Good outcome rate of 35% in IV-tPA–treated patients with computed tomography angiography confirmed severe anterior circulation occlusive stroke. Stroke. 2013;44(11):3109-3113. doi: 10.1161/STROKEAHA.113.001938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen CK, Christensen A, Ovesen C, Havsteen I, Christensen H. Stroke severity and incidence of acute large vessel occlusions in patients with hyper-acute cerebral ischemia: results from a prospective cohort study based on CT-angiography (CTA). Int J Stroke. 2015;10(3):336-342. doi: 10.1111/ijs.12383 [DOI] [PubMed] [Google Scholar]
- 6.Adeoye O, Albright KC, Carr BG, et al. . Geographic access to acute stroke care in the United States. Stroke. 2014;45(10):3019-3024. doi: 10.1161/STROKEAHA.114.006293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prabhakaran S, Ward E, John S, et al. . Transfer delay is a major factor limiting the use of intra-arterial treatment in acute ischemic stroke. Stroke. 2011;42(6):1626-1630. doi: 10.1161/STROKEAHA.110.609750 [DOI] [PubMed] [Google Scholar]
- 8.Sun CH, Nogueira RG, Glenn BA, et al. . “Picture to puncture”: a novel time metric to enhance outcomes in patients transferred for endovascular reperfusion in acute ischemic stroke. Circulation. 2013;127(10):1139-1148. doi: 10.1161/CIRCULATIONAHA.112.000506 [DOI] [PubMed] [Google Scholar]
- 9.Froehler MT, Saver JL, Zaidat OO, et al. ; STRATIS Investigators . Interhospital transfer before thrombectomy is associated with delayed treatment and worse outcome in the STRATIS registry (Systematic Evaluation of Patients Treated With Neurothrombectomy Devices for Acute Ischemic Stroke). Circulation. 2017;136(24):2311-2321. doi: 10.1161/CIRCULATIONAHA.117.028920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brott T, Broderick J, Kothari R, et al. . Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28(1):1-5. doi: 10.1161/01.STR.28.1.1 [DOI] [PubMed] [Google Scholar]
- 11.Hemphill JC III, Greenberg SM, Anderson CS, et al. ; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology . Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032-2060. doi: 10.1161/STR.0000000000000069 [DOI] [PubMed] [Google Scholar]
- 12.Leira EC, Hess DC, Torner JC, Adams HP Jr. Rural-urban differences in acute stroke management practices: a modifiable disparity. Arch Neurol. 2008;65(7):887-891. doi: 10.1001/archneur.65.7.887 [DOI] [PubMed] [Google Scholar]
- 13.Gonzales S, Mullen MT, Skolarus L, Thibault DP, Udoeyo U, Willis AW. Progressive rural-urban disparity in acute stroke care. Neurology. 2017;88(5):441-448. doi: 10.1212/WNL.0000000000003562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prabhakaran S, Ruff I, Bernstein RA. Acute stroke intervention: a systematic review. JAMA. 2015;313(14):1451-1462. doi: 10.1001/jama.2015.3058 [DOI] [PubMed] [Google Scholar]
- 15.Nazliel B, Starkman S, Liebeskind DS, et al. . A brief prehospital stroke severity scale identifies ischemic stroke patients harboring persisting large arterial occlusions. Stroke. 2008;39(8):2264-2267. doi: 10.1161/STROKEAHA.107.508127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singer OC, Dvorak F, du Mesnil de Rochemont R, Lanfermann H, Sitzer M, Neumann-Haefelin T. A simple 3-item stroke scale: comparison with the National Institutes of Health Stroke Scale and prediction of middle cerebral artery occlusion. Stroke. 2005;36(4):773-776. doi: 10.1161/01.STR.0000157591.61322.df [DOI] [PubMed] [Google Scholar]
- 17.Grotta JC, Savitz SI, Persse D. Stroke severity as well as time should determine stroke patient triage. Stroke. 2013;44(2):555-557. doi: 10.1161/STROKEAHA.112.669721 [DOI] [PubMed] [Google Scholar]
- 18.Pérez de la Ossa N, Carrera D, Gorchs M, et al. . Design and validation of a prehospital stroke scale to predict large arterial occlusion: the Rapid Arterial Occlusion Evaluation scale. Stroke. 2014;45(1):87-91. doi: 10.1161/STROKEAHA.113.003071 [DOI] [PubMed] [Google Scholar]
- 19.Katz BS, McMullan JT, Sucharew H, Adeoye O, Broderick JP. Design and validation of a prehospital scale to predict stroke severity: Cincinnati Prehospital Stroke Severity Scale. Stroke. 2015;46(6):1508-1512. doi: 10.1161/STROKEAHA.115.008804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hastrup S, Damgaard D, Johnsen SP, Andersen G. Prehospital Acute Stroke Severity scale to predict large artery occlusion: design and comparison with other scales. Stroke. 2016;47(7):1772-1776. doi: 10.1161/STROKEAHA.115.012482 [DOI] [PubMed] [Google Scholar]
- 21.Lima FO, Silva GS, Furie KL, et al. . Field Assessment Stroke Triage for Emergency Destination: a simple and accurate prehospital scale to detect large vessel occlusion strokes. Stroke. 2016;47(8):1997-2002. doi: 10.1161/STROKEAHA.116.013301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noorian AR, Sanossian N, Shkirkova K, et al. ; FAST-MAG Trial Investigators and Coordinators . Los Angeles Motor Scale to identify large vessel occlusion: prehospital validation and comparison with other screens. Stroke. 2018;49(3):565-572. doi: 10.1161/STROKEAHA.117.019228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fassbender K, Walter S, Liu Y, et al. . “Mobile stroke unit” for hyperacute stroke treatment. Stroke. 2003;34(6):e44. doi: 10.1161/01.STR.0000075573.22885.3B [DOI] [PubMed] [Google Scholar]
- 24.Walter S, Kostpopoulos P, Haass A, et al. . Bringing the hospital to the patient: first treatment of stroke patients at the emergency site. PLoS One. 2010;5(10):e13758. doi: 10.1371/journal.pone.0013758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ebinger M, Winter B, Wendt M, et al. ; STEMO Consortium . Effect of the use of ambulance-based thrombolysis on time to thrombolysis in acute ischemic stroke: a randomized clinical trial. JAMA. 2014;311(16):1622-1631. doi: 10.1001/jama.2014.2850 [DOI] [PubMed] [Google Scholar]
- 26.Itrat A, Taqui A, Cerejo R, et al. ; Cleveland Pre-Hospital Acute Stroke Treatment Group . Telemedicine in prehospital stroke evaluation and thrombolysis: taking stroke treatment to the doorstep. JAMA Neurol. 2016;73(2):162-168. doi: 10.1001/jamaneurol.2015.3849 [DOI] [PubMed] [Google Scholar]
- 27.Parker SA, Bowry R, Wu TC, et al. . Establishing the first mobile stroke unit in the United States. Stroke. 2015;46(5):1384-1391. doi: 10.1161/STROKEAHA.114.007993 [DOI] [PubMed] [Google Scholar]
- 28.Walter S, Kostopoulos P, Haass A, et al. . Diagnosis and treatment of patients with stroke in a mobile stroke unit versus in hospital: a randomised controlled trial. Lancet Neurol. 2012;11(5):397-404. doi: 10.1016/S1474-4422(12)70057-1 [DOI] [PubMed] [Google Scholar]
- 29.Kostopoulos P, Walter S, Haass A, et al. . Mobile stroke unit for diagnosis-based triage of persons with suspected stroke. Neurology. 2012;78(23):1849-1852. doi: 10.1212/WNL.0b013e318258f773 [DOI] [PubMed] [Google Scholar]
- 30.John S, Stock S, Masaryk T, et al. . Performance of CT angiography on a mobile stroke treatment unit: implications for triage. J Neuroimaging. 2016;26(4):391-394. doi: 10.1111/jon.12346 [DOI] [PubMed] [Google Scholar]
- 31.Wendt M, Ebinger M, Kunz A, et al. ; STEMO Consortium . Improved prehospital triage of patients with stroke in a specialized stroke ambulance: results of the Pre-Hospital Acute Neurological Therapy and Optimization of Medical Care in Stroke Study. Stroke. 2015;46(3):740-745. doi: 10.1161/STROKEAHA.114.008159 [DOI] [PubMed] [Google Scholar]
- 32.Wu TC, Parker SA, Jagolino A, et al. . Telemedicine can replace the neurologist on a mobile stroke unit. Stroke. 2017;48(2):493-496. doi: 10.1161/STROKEAHA.116.015363 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Areas of Mobile Stroke Unit Activity in a State-Wide Network With Stroke-Treating Hospitals Providing Different Levels of Care (Primary and Comprehensive Stroke Centers)
eTable. Prespecified Safety End Points and Other Serious Adverse Events at Day 7
Trial Protocol
Data Sharing Statement