Key Points
Question
Is dabigatran etexilate or dose-adjusted warfarin efficacious and safe to use in preventing the recurrence of venous thrombotic events among patients with cerebral venous thrombosis?
Findings
In this exploratory randomized, open-label clinical trial of 120 patients with cerebral venous thrombosis, no recurrent venous thrombotic events were observed in patients randomized to either the dabigatran or warfarin treatment group; 1 major bleeding event was recorded among users of dabigatran and 2 among users of warfarin.
Meaning
This study suggests that both dabigatran and dose-adjusted warfarin may be safe options to prevent recurrent venous thrombotic events in patients with cerebral venous thrombosis.
This randomized clinical trial conducted in 9 countries compares the use of dabigatran with warfarin in stroke prevention among patients who just experienced cerebral venous thrombosis.
Abstract
Importance
Patients with cerebral venous thrombosis (CVT) are at risk of recurrent venous thrombotic events (VTEs). Non–vitamin K oral anticoagulants have not been evaluated in randomized controlled trials in CVT.
Objective
To compare the efficacy and safety of dabigatran etexilate with those of dose-adjusted warfarin in preventing recurrent VTEs in patients who have experienced a CVT.
Design, Setting, and Participants
RE-SPECT CVT is an exploratory, prospective, randomized (1:1), parallel-group, open-label, multicenter clinical trial with blinded end-point adjudication (PROBE design). It was performed from December 21, 2016, to June 22, 2018, with a follow-up of 25 weeks, at 51 tertiary sites in 9 countries (France, Germany, India, Italy, the Netherlands, Poland, Portugal, Russia, and Spain). Adult consecutive patients with acute CVT, who were stable after 5 to 15 days of treatment with parenteral heparin, were screened for eligibility. Patients with CVT associated with central nervous system infection or major trauma were excluded, but those with intracranial hemorrhage from index CVT were allowed to participate. After exclusions, 120 patients were randomized. Data were analyzed following the intention-to-treat approach.
Interventions
Dabigatran, 150 mg twice daily, or dose-adjusted warfarin for a treatment period of 24 weeks.
Main Outcomes and Measures
Primary outcome was a composite of patients with a new VTE (recurrent CVT, deep vein thrombosis of any limb, pulmonary embolism, and splanchnic vein thrombosis) or major bleeding during the study period. Secondary outcomes were cerebral venous recanalization and clinically relevant non–major bleeding events.
Results
In total, 120 patients with CVT were randomized to the 2 treatment groups (60 to dabigatran and 60 to dose-adjusted warfarin). Of the randomized patients, the mean (SD) age was 45.2 (13.8) years, and 66 (55.0%) were women. The mean (SD) duration of exposure was 22.3 (6.16) weeks for the dabigatran group and 23.0 (5.20) weeks for the warfarin group. No recurrent VTEs were observed. One (1.7%; 95% CI, 0.0-8.9) major bleeding event (intestinal) was recorded in the dabigatran group, and 2 (3.3%; 95% CI, 0.4-11.5) (intracranial) in the warfarin group. One additional patient (1.7; 95% CI, 0.0-8.9) in the warfarin group experienced a clinically relevant non–major bleeding event. Recanalization occurred in 33 patients in the dabigatran group (60.0%; 95% CI, 45.9-73.0) and in 35 patients in the warfarin group (67.3%; 95% CI, 52.9-79.7).
Conclusions and Relevance
This trial found that patients who had CVT anticoagulated with either dabigatran or warfarin had low risk of recurrent VTEs, and the risk of bleeding was similar with both medications, suggesting that both dabigatran and warfarin may be safe and effective for preventing recurrent VTEs in patients with CVT.
Trial Registration
ClinicalTrials.gov identifier: NCT02913326
Introduction
Cerebral venous thrombosis (CVT) is a type of stroke caused by thrombosis of the dural sinus and/or cerebral veins. The prevalence of CVT in high-income countries is 1.3 to 1.6 per 100 000 persons1,2 and is higher in low- and middle-income countries.3
In acute-phase CVT, less than 5% of patients die and approximately 75% make a full recovery.4 Those who survive acute CVT are at increased risk of recurrent venous thrombotic events (VTEs) in the cerebral veins and dural sinuses, veins of the limbs, and splanchnic veins, or pulmonary embolism (PE).4,5,6 In observational studies, the risk of recurrent CVT was 1.5 per 100 persons per year and the risk of all VTEs was 2.0 to 4.1 per 100 persons per year.5,6 Most recurrences seem to occur in the months after the initial thrombotic event.5,6
The recommended practice for preventing VTE recurrence after CVT is anticoagulation using vitamin K antagonists for variable periods, depending on the inherent thrombotic risk of each patient.7,8 This recommendation is based on the extrapolation of findings on prevention of recurrent VTE in deep vein thrombosis (DVT).
Direct non–vitamin K oral anticoagulants are changing the practice of anticoagulation and have been used to prevent DVT and PE.9 Dabigatran etexilate is a direct thrombin antagonist that has been proven to be efficacious and to have a good safety and tolerability profile when used for stroke prevention in patients with atrial fibrillation10 as well as when used for treatment and prevention of recurrent DVT and PE.11
Dabigatran and other non–vitamin K oral anticoagulants are occasionally used off-label in patients with CVT. Small case series have been published that found promising safety and efficacy results,12,13,14,15,16 but these studies lacked controls and randomization. Because of the low quality of available evidence, European guidelines8 do not currently recommend non–vitamin K oral anticoagulants after CVT.
We conducted RE-SPECT CVT (A Clinical Trial Comparing Efficacy and Safety of Dabigatran Etexilate With Warfarin in Patients With Cerebral Venous and Dural Sinus Thrombosis), an exploratory randomized clinical trial to evaluate the efficacy and safety of dabigatran compared with dose-adjusted warfarin in the prevention of recurrent VTE and CVT.
Methods
Study Design and Participants
RE-SPECT CVT is an exploratory, multicenter PROBE design (prospective, randomized, parallel-group, open-label with blinded evaluation of end points) clinical trial conducted at 51 sites in 9 countries (France, Germany, India, Italy, the Netherlands, Poland, Portugal, Russia, and Spain) from December 21, 2016, to June 22, 2018. All participating sites were tertiary medical centers with an interest in CVT. This trial was approved by the institutional review board or ethics committee at each site and, where required, by the national ethics committees for clinical research. Written informed consent was obtained from all participants before randomization. Patients who could not directly provide consent could designate a legally authorized representative to sign the consent form on their behalf. The rationale, design, and protocol of RE-SPECT CVT have been published previously17; the full trial protocol is available in Supplement 1.
We recruited consecutive patients of either sex who were between 18 and 79 years of age and had a diagnosis of CVT confirmed by magnetic resonance imaging (MRI) plus MR venography, computed tomography (CT) plus CT venography, or intraarterial venography. Patients should have achieved clinical stability after receiving acute CVT treatment as required.
Major exclusion criteria were the inability to swallow oral medication, CVT associated with central nervous system infection or major head trauma, planned surgical procedure for CVT (eg, decompressive hemicraniectomy), life-threatening or major bleeding18 in the previous 6 months other than intracranial hemorrhage from the index CVT, need to continue previous treatment with an anticoagulant for an indication other than CVT, current or recent (<6 months) malignancy, and creatinine clearance level less than 30 mL/min (to convert to mL/s/m2, multiply by 0.0167) (eAppendix 1 in Supplement 2).
Randomization and Masking
Patients were randomized 1:1 through an online telephone-guided response system to receive either dabigatran (150 mg twice daily) or warfarin (dose adjusted to maintain an international normalized ratio [INR] between 2.0 and 3.0). Randomization took place 5 to 15 days after the initial acute treatment with unfractionated or low-molecular-weight heparin. Randomization was stratified by presence or absence of intracranial hemorrhage at baseline neuroimaging. The randomization list was generated by the study sponsor (Boehringer Ingelheim) using a validated system. The randomization code remained unseen by the whole trial team up to database lock. Because RE-SPECT CVT was an open-label trial, treatment allocation was not concealed from the patients and investigators.
Procedure
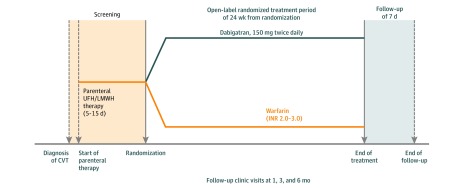
The trial consisted of 3 sequential periods: a screening period of 5 to 15 days, a treatment period of 24 weeks, and a follow-up period of 7 days (Figure 1). Screened patients could be enrolled into the trial (ie, signed informed consent) once they had a confirmed diagnosis of CVT; met the inclusion and exclusion criteria; and received treatment for the acute phase of CVT, including with initial parenteral anticoagulation (unfractionated heparin or low-molecular-weight heparin) as recommended by current guidelines.7,8,19 Endovascular treatment was allowed. Randomization occurred 5 days after the start of parenteral anticoagulation therapy if the patient was stable, but it could be postponed until the patient was stable for up to 15 days after the start of parenteral therapy. In the warfarin group, parenteral therapy continued until an INR of 2.0 or higher was achieved for 2 consecutive measurements; in the dabigatran group, parenteral therapy was discontinued as soon as the trial treatment was started.
Figure 1. RE-SPECT Cerebral Venous Thrombosis (CVT) Trial Design.
INR indicates international normalized ratio; LMWH, low-molecular-weight heparin; UFH, unfractionated heparin. Adapted with permission from SAGE Publications, Ltd.17
At screening, neuroimaging from the time of diagnosis of the CVT was used. At the end of trial treatment, recanalization was assessed by MRI plus MR venography by using a trial-specific MRI protocol (eAppendix 2 in Supplement 2). All neuroimaging data were reviewed by the adjudication committee, blinded for clinical data and treatment group.
A flowchart of the study is depicted in eAppendix 3 in Supplement 2. Follow-up visits at the clinic were targeted at days 29, 85, and 169 (end of treatment) after randomization. A further safety follow-up visit was performed 7 to 14 days after the end of treatment. The end-of-treatment visit included the modified Rankin Scale20 and neuroimaging. For patients who discontinued trial medication, the end-of-treatment visit was carried out at the discontinuation visit. Those who discontinued trial medication early were followed up for survival and for adverse, new thrombotic, and bleeding events until day 176 (25 weeks) after randomization, unless they withdrew their informed consent. After the end of treatment or early discontinuation of trial medication, whether a patient received further nontrial treatment was at the discretion of the treating physician.
Interventions and Outcomes
The trial medication was provided by the sponsor and was administered to patients on the day of randomization. Dabigatran was dispensed in 150-mg capsules and taken twice daily. Warfarin was dispensed in 1-mg, 3-mg, or 5-mg tablets and taken once daily; individual doses were titrated as needed to maintain a target INR of 2.0 to 3.0. For patients in the warfarin group, the INR measurements were performed as necessary for dose adjustment and maintenance, to obtain target INR as quickly as possible. The INR measurements were performed daily from the start of treatment until concomitant heparin treatment was stopped and then at least once every 2 weeks for the first 3 months thereafter, followed by once a month. More frequent INR measurements could be taken if required.
The primary outcome was the composite of the number of patients with major bleeding according to International Society on Thrombosis and Haemostasis criteria,18 or VTE (recurrent CVT, DVT of any limb, PE, or splanchnic vein thrombosis21), at the end of the trial. Secondary efficacy outcomes included the number of patients with each category of VTE and cerebral venous recanalization, as measured by change in the score of occluded cerebral veins and sinuses.5 Secondary safety outcomes were the number of patients with major bleeding according to International Society on Thrombosis and Haemostasis criteria; composite of the number of patients with new intracranial hemorrhage or worsening of the hemorrhagic component of a baseline lesion22 (eAppendix 6 in Supplement 2); number of patients with clinically relevant non–major bleeding events; number of patients with major bleeding according to International Society on Thrombosis and Haemostasis criteria, or clinically relevant non–major bleeding events; and number of patients with any bleeding event. All components of the primary and secondary outcomes (except for number of patients with any bleeding event) were adjudicated in a blinded manner by an adjudication committee. Other exploratory outcomes included functional outcome, assessed by the modified Rankin Scale; VTE-associated mortality; and all-cause mortality (eAppendix 4 in Supplement 2). An independent outcome adjudication committee (eAppendix 5 in Supplement 2) performed the blinded adjudication of outcomes.
Statistical Analysis
Because RE-SPECT CVT was an exploratory trial, no formal statistical hypothesis was tested. Based on expected recruitment rates, we planned to randomize 120 patients. Planning RE-SPECT CVT as a noninferiority trial with a preservation of about 50% of the recurrent events would require more than 2000 patients because of the low recurrent event rate (approximately 3% at 6 months).5,17 All analyses of primary and secondary outcomes were descriptive (number, frequency, and 95% CI). The full analysis set was defined as all patients randomized. They were analyzed in their allocated treatment group following the intention-to-treat approach, regardless of whether they took the study medication.
The primary outcome and secondary efficacy outcomes were analyzed for the full observation period. The secondary safety outcome analyses were based on all randomized patients who received at least 1 dose of study medication. Cerebral venous recanalization, as measured by the change in number of occluded cerebral veins and sinuses after up to 24 weeks, was presented as no change or as worsened (if there was at least 1 new vein or sinus occluded) or improved (if there was at least 1 vein or sinus recanalyzed). Patients with missing or unanalyzable MRI scans at the end of treatment were excluded from this analysis. All statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc).
Results
The first patient was enrolled on December 21, 2016, and the last patient completed the trial on June 22, 2018. Consecutive patients with CVT (n = 228) from 36 of the 51 invited centers in 9 countries were assessed for eligibility. In total, 120 patients with CVT were randomized to the 2 treatment groups (60 to dabigatran and 60 to dose-adjusted warfarin). Each group comprised 33 women (55.0%) and 27 men (45.0%), with a mean (SD) age of 45.2 (13.8) years. Table 1 shows the baseline characteristics of the patients enrolled in the study.
Table 1. Baseline Characteristics .
| Variable | No. (%) | |
|---|---|---|
| Dabigatran Etexilate (n = 60) | Warfarin (n = 60) | |
| Sex | ||
| Male | 27 (45.0) | 27 (45.0) |
| Female | 33 (55.0) | 33 (55.0) |
| Age group, y | ||
| <30 | 7 (11.7) | 8 (13.3) |
| 30-39 | 16 (26.7) | 10 (16.7) |
| 40-49 | 19 (31.7) | 21 (35.0) |
| 50-59 | 9 (15.0) | 10 (16.7) |
| ≥60 | 9 (15.0) | 11 (18.3) |
| Country | ||
| France | 3 (5.0) | 4 (6.7) |
| Germany | 5 (8.3) | 8 (13.3) |
| India | 12 (20.0) | 7 (11.7) |
| Italy | 7 (11.7). | 7 (11.7) |
| The Netherlands | 5 (8.3) | 4 (6.7) |
| Poland | 3 (5.0) | 7 (11.7) |
| Portugal | 8 (13.3) | 14 (23.3) |
| Russia | 16 (26.7) | 6 (10.0) |
| Spain | 1 (1.7) | 3 (5.0) |
| Diagnosis of CVT | ||
| MRI with MR venography | 48 (80.0) | 44 (73.0) |
| CT with CT venography | 15 (25.0) | 23 (38.3) |
| MRI with catheter angiography | 3 (5.0) | 3 (5.0) |
| CT with catheter angiography | 5 (8.3) | 3 (5.0) |
| Parenchymal lesion on diagnostic neuroimaging | ||
| Any lesions | 27 (45.0) | 24 (40.0) |
| Nonhemorrhagic lesion | 13 (21.7) | 13 (21.7) |
| Hemorrhagic lesion | 18 (30.0) | 19 (31.7) |
| Sinuses involved | ||
| Superior sagittal sinus | 27 (45.0) | 25 (41.7) |
| Left lateral (sigmoid and/or transverse) sinus | 26 (43.3) | 34 (567) |
| Right lateral (sigmoid and/or transverse) sinus | 34 (56.7) | 30 (50.0) |
| Straight sinus | 8 (13.3) | 12 (20.0) |
| Deep venous system | 8 (13.3) | 6 (10.0) |
| Cortical veins | 11 (18.3) | 16 (26.7) |
| Cerebellar veins | 0 | 2 (3.3) |
| Jugular vein | 22 (36.7) | 21 (35.0) |
| Cavernous sinus | 1 (1.7) | 3 (5.0) |
| Symptoms/signs | ||
| Coma (Glasgow Coma Scale score: <9) | 0 | 0 |
| Decreased alertness (Glasgow Coma Scale score: 9-14) | 3 (5.0) | 3 (5.0) |
| Monoparesis/hemiparesis | 12 (20.0) | 11 (18.3) |
| Seizure | 13 (21.7) | 16 (26.7) |
| Headache | 54 (90.0) | 55 (91.7) |
| Mental status disorder | 4 (6.7) | 4 (6.7) |
| Aphasia | 5 (8.3) | 7 (11.7) |
| Visual loss | 7 (11.7) | 3 (5.0) |
| Papilloedema | 10 (16.7) | 4 (6.7) |
| Diplopia, oculomotor palsy | 6 (10.0) | 5 (8.3) |
| Risk factors/predisposing conditions | ||
| Oral contraceptive use | 18 (30.0) | 19 (31.7) |
| BMI ≥25 to <30 | 16 (26.7) | 25 (41.7) |
| BMI ≥30 | 14 (23.3) | 15 (25.0) |
| Previous venous thromboembolism | 6 (10.0) | 9 (15.0) |
| Genetic thrombophilia | 5 (8.3) | 3 (5.0) |
| Infection (ear, nose, throat) | 4 (6.7) | 2 (3.3) |
| Surgery | 3 (5.0) | 2 (3.3) |
| Puerperium | 3 (5.0) | 1 (1.7) |
| Severe dehydration | 1 (1.7) | 1 (1.7) |
| Drugs with prothrombotic effect | 2 (3.3) | 0 |
| Malignancy (>6 mo) | 0 | 1 (1.7) |
| Inflammatory bowel disease | 0 | 1 (1.7) |
| Other inflammatory systemic disorder | 0 | 2 (3.3) |
| Mechanical precipitants | 1 (1.7) | 0 |
| CVT risk score | ||
| 0-2 | 51 (85.0) | 52 (86.7) |
| ≥3 | 9 (15.0) | 8 (13.3) |
| NIHSS score | ||
| 0 | 43 (71.7) | 48 (80.0) |
| 1-4 | 15 (25.0) | 10 (16.7) |
| 5-15 | 2 (3.3) | 2 (3.3) |
| 16-42 | 0 | 0 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CT, computed tomography; CVT, cerebral venous thrombosis; MR, magnetic resonance; MRI, MR imaging; NIHSS, National Institutes of Health Stroke Scale (score range for this sample: 0-13, indicating mild to moderate).
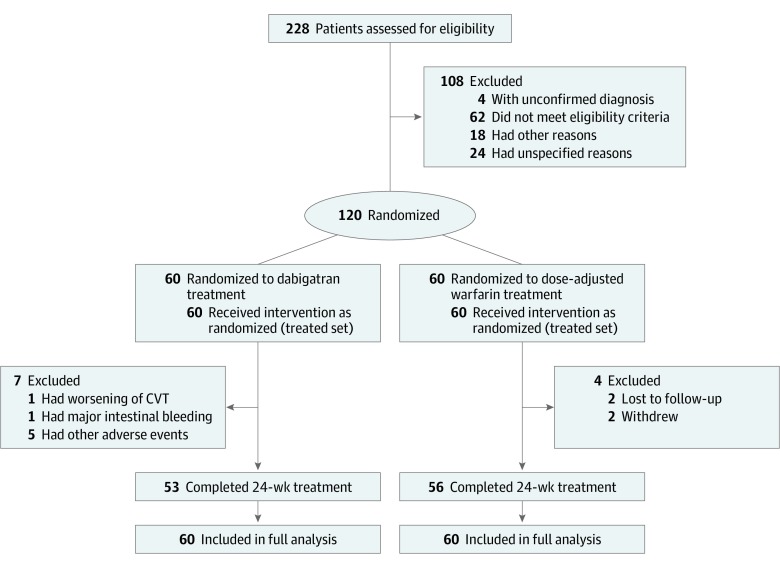
All randomized patients received at least 1 dose of their allotted medication. Eleven (9.2%) patients in total (7 [1.7%] in the dabigatran group and 4 [6.7%] in the warfarin group) discontinued medication prematurely (before 24 weeks). Reasons for discontinuation in the dabigatran group were enlargement of baseline intracranial hemorrhage in 1 patient (not judged by the blinded adjudication committee as new intracranial bleeding), intestinal hematoma in another patient, and other adverse events in 5 patients (epigastric or abdominal discomfort in 2; urticaria, thrombocytopenia, and elevated liver enzymes in 1 each). Five of these patients received other anticoagulants (vitamin K antagonists in 4; low-molecular-weight heparin in 1) after discontinuing the trial treatment. Two patients in the warfarin group decided not to continue in the trial, with 1 stopping at the 3-day visit and the other at the 1-month visit. Two other patients in the warfarin group withdrew from the trial: 1 did not reach therapeutic INR values even with high medication doses, and the other left for unknown reasons.
Altogether, 109 (90.8%) patients completed the treatment period (53 [88.3%] in the dabigatran group and 56 [93.3%] in the warfarin group). Vital status was available for all patients at 25 weeks (end of follow-up period) (Figure 2). The mean (SD) duration of exposure was 22.3 (6.16) weeks for the dabigatran group and 23.0 (5.20) weeks for the warfarin group. The median adherence to dabigatran, determined by the counting of capsules by site personnel, was 99.7%; all patients were within the 80% to 120% interval ([number of capsules taken]/[number of capsules expected to be taken] × 100%). For warfarin, the overall mean time in therapeutic range was 66.1%.
Figure 2. Enrollment, Randomization, and Treatment.
CVT indicates cerebral venous thrombosis.
Table 2 shows the distribution of the outcomes. No recurrent VTEs were observed in either treatment group. Of the 3 major bleeding events, 1 was intestinal bleeding in the dabigatran group (1 [1.7%]; 95% CI, 0.0-8.9), and 2 were intracranial (subdural) hemorrhages in the warfarin group (2 [3.3%]; 95% CI, 0.4-11.5).
Table 2. Primary and Secondary Outcomes.
| Outcomes | No. (%) [95% CI] | |
|---|---|---|
| Dabigatran Etexilate (n = 60) | Warfarin (n = 60) | |
| Primary outcome | ||
| Major bleeding or venous thrombotic event (recurrent CVT, DVT of any limb, pulmonary embolism, splanchnic vein thrombosis) | 1 (1.7) [0.0-8.9] | 2 (3.3) [0.4-11.5] |
| Secondary outcomes | ||
| All venous thrombotic events | 0 [0.0-6.0] | 0 [0.0-6.0] |
| Recanalization: score of occluded veins/sinusesa | ||
| Improved | 33 (60.0) [45.9-73.0] | 35 (67.0) [52.9-79.7] |
| No change | 22 (40.0) [27.9-54.1] | 17 (33.0) [20.3-47.1] |
| Secondary safety outcomes | ||
| Major bleeding event | 1 (1.7) [0.0-8.9] | 2 (3.3) [0.4-11.5] |
| Clinically relevant non–major bleeding event | 0 [0.0-0.6] | 1 (1.7) [0.0-8.9] |
| Major bleeding or clinically relevant non–major bleeding event | 1 (1.7) [0.0-8.9] | 3 (5.0) [1.0-13.9] |
| Any bleeding | 12 (20.0) [10.8-32.3] | 12 (20.0) [10.8-32.3] |
| New intracranial hemorrhage or worsening of the hemorrhagic component of a baseline lesionb | 1 (1.8) [0.0-9.6] | 2 (3.8) [0.5-13.0] |
| New intracranial hemorrhage | 0 [0.0-6.4] | 2 (3.8) [0.5-13.0] |
| Worsening of the hemorrhagic component of a baseline lesion | 1 (1.8) [0.0-9.6] | 0 [0.0-6.7] |
| Exploratory outcomes | ||
| Functional outcome (modified Rankin Scale)c | ||
| After 4 wk | ||
| 0-1 | 51 (91.1) | 52 (89.7) |
| 2 | 5 (8.9) | 5 (8.6) |
| 3 | 0 | 1 (1.7) |
| >3 | 0 | 0 |
| Up to 24 wk | ||
| 0-1 | 54 (91.5) | 53 (91.4) |
| 2 | 4 (6.8) | 3 (5.2) |
| 3 | 0 | 2 (3.4) |
| >3 | 1 (1.7) | 0 |
| Venous thrombotic event–associated mortality | 0 [0.0-6.0] | 0 [0.0-6.0] |
| All-cause mortality | 0 [0.0-6.0] | 0 [0.0-6.0] |
Abbreviations: CVT, cerebral venous thrombosis; DVT, deep vein thrombosis.
Evaluated in 55 patients allocated to dabigatran and 52 to warfarin.
Available for 56 patients in the dabigatran group and 53 in the warfarin group.
Available for 56 patients in the dabigatran group and 58 in the warfarin group at 4 weeks, and for 59 patients in the dabigatran group and 58 in the warfarin group up to 24 weeks.
No clinically relevant non–major bleeding events occurred among patients in the dabigatran group, and only 1 patient with (genitourinary) bleeding was observed in the warfarin group (1 [1.7%]; 95% CI, 0.0-8.9). Any bleeding, irrespective of its severity, occurred in 20% of patients, with the same frequency in both treatment groups (dabigatran: 12 [20.0%]; 95% CI, 10.8-32.3 and warfarin: 12 [20.0%]; 95% CI, 10.8-32.3).
Cerebral venous recanalization, assessed as a change in the score of occluded cerebral veins and sinuses between baseline and end-of-treatment neuroimaging, could be evaluated in 55 patients in the dabigatran group and 52 patients in the warfarin group. No patient worsened, whereas 33 patients (60.0%; 95% CI, 45.9-73.0) in the dabigatran group and 35 (67.3%; 95% CI, 52.9-79.7) in the warfarin group experienced improvement.
Among the patients (20 in each treatment group) with intracranial hemorrhage at baseline, no new major bleeding events occurred for dabigatran, whereas 1 major bleeding (new intracranial hemorrhage) event was recorded for warfarin (1 [5.3%]; 95% CI, 0.1-26.0). In the same patients, worsening of the hemorrhagic component of a baseline intracranial lesion was observed in 1 patient in the dabigatran group (1 [5.0%]; 95% CI, 0.1-24.9).
Adverse events other than bleeding are listed in Table 3. Epigastric or abdominal discomfort leading to study drug discontinuation was reported in 2 patients (3.3%) randomized to the dabigatran treatment.
Table 3. Patients With Adverse Events .
| Variable | No. (%) | |
|---|---|---|
| Dabigatran Etexilate (n = 60) | Warfarin (n = 60) | |
| Any adverse event | 47 (78.3) | 42 (70.0) |
| Serious adverse event | 8 (13.3) | 6 (10.0) |
| Adverse event leading to trial drug discontinuation | 7 (11.7) | 0 |
| Worsening of the index CVT | 1 (1.7) | NA |
| Intestinal hematoma, major bleeding event | 1 (1.7) | NA |
| Epigastric/abdominal discomfort | 2 (3.3) | NA |
| Urticaria | 1 (1.7) | NA |
| Thrombocytopenia | 1 (1.7) | NA |
| Elevated liver enzymes | 1 (1.7) | NA |
| Adverse event occurring in ≥5 patients, system organ class/preferred terma | ||
| Headache | 10 (16.7) | 8 (13.3) |
| Depression | 2 (3.3) | 4 (6.7) |
| Abdominal pain/epigastric discomfort | 4 (6.7) | 2 (3.3) |
| Diarrhea | 4 (6.7) | 2 (3.3) |
| Cough | 5 (8.3) | 0 |
Abbreviations: CVT, cerebral venous thrombosis; NA, not applicable.
Bleeding not listed (see Table 2).
Discussion
We found no recurrent VTE during the RE-SPECT CVT trial. This finding indicates that the risk of recurrent VTE in patients with CVT who received regular anticoagulant therapy, with either dabigatran or dose-adjusted warfarin, for 6 months was low. Anticoagulant therapy for 6 months with either dabigatran or dose-adjusted warfarin was associated with few major or clinically relevant bleeding events, new intracranial hemorrhages, or enlargement of baseline hemorrhagic lesions. These results are in line with evidence that dabigatran is at least noninferior in efficacy compared with warfarin in indications other than CVT, presenting fewer bleeding events, specifically intracranial hemorrhages.23 Previous observational studies on VTE recurrence in patients with CVT did not systematically record or adjudicate major bleeding events.5,6,8 With the limitation of the small number of adjudicated major bleeds and their wide CIs, the frequency of major bleeding events in RE-SPECT CVT was comparable to that reported for dabigatran, 150 mg, in DVT trials11 and lower than the frequency observed in stroke prevention in atrial fibrillation trials.10 For warfarin, in the current study, the frequency of major bleeding was comparable to that observed in trials of stroke prevention in atrial fibrillation.10 The patients with CVT in this study were younger than patients with atrial fibrillation and did not have clinical or neuroimaging evidence of small vessel disease, and oral anticoagulation was given for only 24 weeks. These factors may explain the lower rate of major bleeding (ie, intracranial hemorrhage) witnessed for dabigatran in RE-SPECT CVT.
Compared with the warfarin group, the dabigatran group had more patients who discontinued trial medication. The reason for the discontinuation was mainly the digestive system adverse effects of the drug.
RE-SPECT CVT, which compared dabigatran to dose-adjusted warfarin, adds reliable evidence to available data because it was controlled and randomized and had blinded adjudication of efficacy and safety outcomes. Adherence was excellent in the dabigatran group, and the time in therapeutic range was good (>65%) for patients in the warfarin group. To increase external validity, RE-SPECT CVT was performed in Western and Eastern Europe, Russia, and India, because these regions have epidemiologic variations in demography and the risk factors for CVT as well as different systems and levels of health care.
Limitations
RE-SPECT CVT has several limitations. It was an exploratory trial with a small sample size. Owing to the low frequency of recurrent VTE after CVT,5,6 the study was not powered to detect statistically significant differences between the 2 treatment groups for recurrent VTE. Assuming a preservation of 50% of the benefit of warfarin, a noninferiority trial with a 3% VTE rate would require approximately 2000 patients. Given the low incidence of CVT, such a trial is unlikely to be feasible.
Open-label compared with double-blind, double-dummy trials in anticoagulation have complementary strengths and weaknesses. Double-blind trials unavoidably deviate from routine clinical practice, and doing so limits their external validity.24,25 Open-label design may influence postrandomization management decisions, as well as outcome reporting and evaluation, and is a potential source of bias. To decrease the risk of such bias, we used the PROBE design. External evaluators, who were blinded to treatment allocation, adjudicated the outcome events on the basis of predefined criteria.
As is the case for all randomized clinical trials, the inclusion and exclusion criteria induced sample selection bias. The characteristics of the sample were, in general, expected in a convenience sample of patients with CVT admitted to tertiary centers. Because patients who could not swallow were excluded, no comatose patients were included in the trial. We also excluded patients with major trauma, central nervous system infections, or active cancer as well as those requiring hemicraniectomy. The allocated sample consisted of CVT cases of mild to moderate severity, as demonstrated by the low score on the National Institute of Health Stroke Scale (score range for this sample: 0-13, indicating mild to moderate), CVT risk scores,26 and modified Rankin Scale at 4 weeks or up to 24 weeks.
The frequency of recurrent VTEs in RE-SPECT CVT was lower than anticipated.5,6 This low frequency may be attributable to the excellent treatment adherence and good INR control among patients. Alternative explanations are follow-up of only 6 months, the effect of early parenteral heparin, and selection bias leading to a low inclusion rate for patients at high risk for recurrence. Severely affected patients, often bedridden for long periods, have a higher risk of DVT of the limbs and PE compared with those less severely affected. Active cancer is also a risk factor for recurrent VTE; these patients were excluded from the trial. Older age, male sex, genetic thrombophilia, and myeloproliferative syndromes increase the risk of recurrent VTE in patients with CVT.5,6 RE-SPECT CVT had no cases of myeloproliferative neoplasm or acquired thrombophilia, and only 8 patients with confirmed genetic thrombophilia were enrolled. Those conditions were not an exclusion criterion, except for active or recent cancer.
Conclusions
This study showed that the risk of recurrent VTE was low in patients with CVT who received anticoagulant therapy with either dabigatran or dose-adjusted warfarin for 6 months. Anticoagulant therapy was associated with few major or clinically relevant bleeding events, new intracranial bleeds, or enlargement of baseline hemorrhagic lesions. Dabigatran and dose-adjusted warfarin may be safe options to prevent recurrent VTEs in patients with CVT. Because of the limited sample size, we could not demonstrate the noninferiority or superiority of either treatment.
Trial Protocol
eAppendix 1. Exclusion Criteria
eAppendix 2. MRI Protocol
eAppendix 3. Study Flowchart
eAppendix 4. Definitions of Outcomes
eAppendix 5. Committees
eAppendix 6. Categorization of Hemorrhagic Brain Lesions
Data Sharing Statement
References
- 1.Coutinho JM, Zuurbier SM, Aramideh M, Stam J. The incidence of cerebral venous thrombosis: a cross-sectional study. Stroke. 2012;43(12):3375-3377. doi: 10.1161/STROKEAHA.112.671453 [DOI] [PubMed] [Google Scholar]
- 2.Devasagayam S, Wyatt B, Leyden J, Kleinig T. Cerebral venous sinus thrombosis incidence is higher than previously thought: a retrospective population-based study. Stroke. 2016;47(9):2180-2182. doi: 10.1161/STROKEAHA.116.013617 [DOI] [PubMed] [Google Scholar]
- 3.Janghorbani M, Zare M, Saadatnia M, Mousavi SA, Mojarrad M, Asgari E. Cerebral vein and dural sinus thrombosis in adults in Isfahan, Iran: frequency and seasonal variation. Acta Neurol Scand. 2008;117(2):117-121. [DOI] [PubMed] [Google Scholar]
- 4.Ferro JM, Canhão P, Stam J, Bousser MG, Barinagarrementeria F; ISCVT Investigators . Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35(3):664-670. doi: 10.1161/01.STR.0000117571.76197.26 [DOI] [PubMed] [Google Scholar]
- 5.Miranda B, Ferro JM, Canhão P, et al. ; ISCVT Investigators . Venous thromboembolic events after cerebral vein thrombosis. Stroke. 2010;41(9):1901-1906. doi: 10.1161/STROKEAHA.110.581223 [DOI] [PubMed] [Google Scholar]
- 6.Martinelli I, Bucciarelli P, Passamonti SM, Battaglioli T, Previtali E, Mannucci PM. Long-term evaluation of the risk of recurrence after cerebral sinus-venous thrombosis. Circulation. 2010;121(25):2740-2746. doi: 10.1161/CIRCULATIONAHA.109.927046 [DOI] [PubMed] [Google Scholar]
- 7.Saposnik G, Barinagarrementeria F, Brown RD Jr, et al. ; American Heart Association Stroke Council and the Council on Epidemiology and Prevention . Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158-1192. doi: 10.1161/STR.0b013e31820a8364 [DOI] [PubMed] [Google Scholar]
- 8.Ferro JM, Bousser MG, Canhão P, et al. ; European Stroke Organization . European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis—endorsed by the European Academy of Neurology. Eur J Neurol. 2017;24(10):1203-1213. doi: 10.1111/ene.13381 [DOI] [PubMed] [Google Scholar]
- 9.Cohen AT, Lip GY, De Caterina R, et al. State of play and future direction with NOACs: an expert consensus. Vascul Pharmacol. 2018;106:9-21. doi: 10.1016/j.vph.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 10.Connolly SJ, Ezekowitz MD, Yusuf S, et al. ; RE-LY Steering Committee and Investigators . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151. doi: 10.1056/NEJMoa0905561 [DOI] [PubMed] [Google Scholar]
- 11.Schulman S, Kearon C, Kakkar AK, et al. ; RE-COVER Study Group . Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342-2352. doi: 10.1056/NEJMoa0906598 [DOI] [PubMed] [Google Scholar]
- 12.Hon SF, Li HL, Cheng PW. Use of direct thrombin inhibitor for treatment of cerebral venous thrombosis. J Stroke Cerebrovasc Dis. 2012;21(8):915.e11-915.e15. doi: 10.1016/j.jstrokecerebrovasdis.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 13.Geisbüsch C, Richter D, Herweh C, Ringleb PA, Nagel S. Novel factor xa inhibitor for the treatment of cerebral venous and sinus thrombosis: first experience in 7 patients. Stroke. 2014;45(8):2469-2471. doi: 10.1161/STROKEAHA.114.006167 [DOI] [PubMed] [Google Scholar]
- 14.Mendonça MD, Barbosa R, Cruz-e-Silva V, Calado S, Viana-Baptista M. Oral direct thrombin inhibitor as an alternative in the management of cerebral venous thrombosis: a series of 15 patients. Int J Stroke. 2015;10(7):1115-1118. doi: 10.1111/ijs.12462 [DOI] [PubMed] [Google Scholar]
- 15.Mutgi SA, Grose NA, Behrouz R. Rivaroxaban for the treatment of cerebral venous thrombosis. Int J Stroke. 2015;10(Suppl A100)(Suppl A100):167-168. doi: 10.1111/ijs.12592 [DOI] [PubMed] [Google Scholar]
- 16.Rao SK, Ibrahim M, Hanni CM, et al. Apixaban for the treatment of cerebral venous thrombosis: a case series. J Neurol Sci. 2017;381:318-320. doi: 10.1016/j.jns.2017.09.007 [DOI] [PubMed] [Google Scholar]
- 17.Ferro JM, Dentali F, Coutinho JM, et al. Rationale, design, and protocol of a randomized controlled trial of the safety and efficacy of dabigatran etexilate versus dose-adjusted warfarin in patients with cerebral venous thrombosis. Int J Stroke. 2018;13(7):766-770. doi: 10.1177/1747493018778125 [DOI] [PubMed] [Google Scholar]
- 18.Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi: 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 19.Einhäupl K, Stam J, Bousser MG, et al. ; European Federation of Neurological Societies . EFNS guideline on the treatment of cerebral venous and sinus thrombosis in adult patients. Eur J Neurol. 2010;17(10):1229-1235. doi: 10.1111/j.1468-1331.2010.03011.x [DOI] [PubMed] [Google Scholar]
- 20.Rankin J. Cerebral vascular accidents in patients over the age of 60, II; prognosis. Scott Med J. 1957;2(5):200-215. doi: 10.1177/003693305700200504 [DOI] [PubMed] [Google Scholar]
- 21.European Agency for the Evaluation of Medicinal Products . Note for guidance on clinical investigation of medicinal products for the treatment of venous thromboembolic disease. Committee for Proprietary Medicinal Products. https://www.ema.europa.eu/documents/scientific-guideline/note-guidance-clinical-investigation-medicinal-products-treatment-venous-thromboembolic-disease_en.pdf. Published December 16, 1999. Accessed October 17, 2018.
- 22.von Kummer R, Broderick JP, Campbell BCV, et al. The Heidelberg Bleeding Classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46(10):2981-2986. doi: 10.1161/STROKEAHA.115.010049 [DOI] [PubMed] [Google Scholar]
- 23.Ntaios G, Papavasileiou V, Diener HC, Makaritsis K, Michel P. Nonvitamin-K-antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and previous stroke or transient ischemic attack: an updated systematic review and meta-analysis of randomized controlled trials. Int J Stroke. 2017;12(6):589-596. doi: 10.1177/1747493017700663 [DOI] [PubMed] [Google Scholar]
- 24.Büller HR, Halperin JL, Bounameaux H, Prins M. Double-blind studies are not always optimum for evaluation of a novel therapy: the case of new anticoagulants. J Thromb Haemost. 2008;6(2):227-229. doi: 10.1111/j.1538-7836.2007.02848.x [DOI] [PubMed] [Google Scholar]
- 25.Beyer-Westendorf J, Büller H. External and internal validity of open label or double-blind trials in oral anticoagulation: better, worse or just different? J Thromb Haemost. 2011;9(11):2153-2158. doi: 10.1111/j.1538-7836.2011.04507.x [DOI] [PubMed] [Google Scholar]
- 26.Ferro JM, Bacelar-Nicolau H, Rodrigues T, et al. ; ISCVT and VENOPORT investigators . Risk score to predict the outcome of patients with cerebral vein and dural sinus thrombosis. Cerebrovasc Dis. 2009;28(1):39-44. doi: 10.1159/000215942 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix 1. Exclusion Criteria
eAppendix 2. MRI Protocol
eAppendix 3. Study Flowchart
eAppendix 4. Definitions of Outcomes
eAppendix 5. Committees
eAppendix 6. Categorization of Hemorrhagic Brain Lesions
Data Sharing Statement