Key Points
Question
Is there an association between metabolic surgery and major adverse cardiovascular events (all-cause mortality, coronary artery events, cerebrovascular events, heart failure, nephropathy, and atrial fibrillation) in patients with type 2 diabetes and obesity?
Findings
In this retrospective cohort study of 13 722 patients (including 2287 patients who underwent metabolic surgery and 11 435 matched controls), metabolic surgery was significantly associated with a lower risk of major adverse cardiovascular events (hazard ratio, 0.61).
Meaning
Among patients with type 2 diabetes and obesity, metabolic surgery was significantly associated with a lower risk of incident major adverse cardiovascular events.
Abstract
Importance
Although metabolic surgery (defined as procedures that influence metabolism by inducing weight loss and altering gastrointestinal physiology) significantly improves cardiometabolic risk factors, the effect on cardiovascular outcomes has been less well characterized.
Objective
To investigate the relationship between metabolic surgery and incident major adverse cardiovascular events (MACE) in patients with type 2 diabetes and obesity.
Design, Setting, and Participants
Of 287 438 adult patients with diabetes in the Cleveland Clinic Health System in the United States between 1998 and 2017, 2287 patients underwent metabolic surgery. In this retrospective cohort study, these patients were matched 1:5 to nonsurgical patients with diabetes and obesity (body mass index [BMI] ≥30), resulting in 11 435 control patients, with follow-up through December 2018.
Exposures
Metabolic gastrointestinal surgical procedures vs usual care for type 2 diabetes and obesity.
Main Outcomes and Measures
The primary outcome was the incidence of extended MACE (composite of 6 outcomes), defined as first occurrence of all-cause mortality, coronary artery events, cerebrovascular events, heart failure, nephropathy, and atrial fibrillation. Secondary end points included 3-component MACE (myocardial infarction, ischemic stroke, and mortality) and the 6 individual components of the primary end point.
Results
Among the 13 722 study participants, the distribution of baseline covariates was balanced between the surgical group and the nonsurgical group, including female sex (65.5% vs 64.2%), median age (52.5 vs 54.8 years), BMI (45.1 vs 42.6), and glycated hemoglobin level (7.1% vs 7.1%). The overall median follow-up duration was 3.9 years (interquartile range, 1.9-6.1 years). At the end of the study period, 385 patients in the surgical group and 3243 patients in the nonsurgical group experienced a primary end point (cumulative incidence at 8-years, 30.8% [95% CI, 27.6%-34.0%] in the surgical group and 47.7% [95% CI, 46.1%-49.2%] in the nonsurgical group [P < .001]; absolute 8-year risk difference [ARD], 16.9% [95% CI, 13.1%-20.4%]; adjusted hazard ratio [HR], 0.61 [95% CI, 0.55-0.69]). All 7 prespecified secondary outcomes showed statistically significant differences in favor of metabolic surgery, including mortality. All-cause mortality occurred in 112 patients in the metabolic surgery group and 1111 patients in the nonsurgical group (cumulative incidence at 8 years, 10.0% [95% CI, 7.8%-12.2%] and 17.8% [95% CI, 16.6%-19.0%]; ARD, 7.8% [95% CI, 5.1%-10.2%]; adjusted HR, 0.59 [95% CI, 0.48-0.72]).
Conclusions and Relevance
Among patients with type 2 diabetes and obesity, metabolic surgery, compared with nonsurgical management, was associated with a significantly lower risk of incident MACE. The findings from this observational study must be confirmed in randomized clinical trials.
Trial Registration
ClinicalTrials.gov Identifier: NCT03955952
This matched-cohort study uses Cleveland Clinic Health System data to investigate the association between bariatric surgery and major adverse cardiovascular events (MACE), including mortality, coronary disease and stroke, in adult patients with type 2 diabetes and obesity.
Introduction
In patients with obesity and type 2 diabetes, weight and glycemic goals are difficult to achieve through usual care including lifestyle modifications and pharmacotherapy. In patients with obesity and diabetes, cardiovascular disease is the major cause of morbidity and mortality. Small randomized clinical trials (RCTs) have consistently shown a significant effect of metabolic surgery (defined as procedures that influence metabolism by inducing weight loss and altering gastrointestinal physiology) on excess weight and improvements in diabetes control.1,2,3 However, the small size of available surgical RCTs has precluded investigation of the effects of metabolic surgery on major cardiovascular outcomes and survival. Nonetheless, observational studies have reported a significant association with a lower risk of mortality and the incidence of some macrovascular and microvascular complications of diabetes after metabolic surgery.4,5,6 Currently available studies have examined a limited number of cardiovascular outcomes, studied patients at low to moderate risk, included primarily patients with severe obesity, and in some studies included patients who underwent surgical procedures no longer commonly performed.
The current observational study was designed to investigate the relationship between metabolic surgery, compared with usual care, on a broad range of major cardiovascular complications in high-risk patients. The study included some patients with more moderate levels of obesity, included only patients who had undergone contemporary metabolic surgery procedures, and examined the association of surgery with measures of diabetes control and medication use.
Methods
A retrospective, observational, matched-cohort study was conducted in adult patients with obesity and type 2 diabetes who underwent metabolic surgery within the Cleveland Clinic Health System. The study was approved by the local institutional review board as minimal-risk research using data collected for routine clinical practice, for which the requirement for informed consent was waived.
The data source for all analysis was the Cleveland Clinic Electronic Health Record (EHR) through December 31, 2018. The International Classification of Diseases and Current Procedural Terminology procedure codes were used and the variables mapped to the Unified Medical Language System identifiers7 (codes reported in eTable 1 and eTable 2 in Supplement 1).
Cohort Derivation
In the initial screening process, all patients at the Cleveland Clinic who had a diagnosis of type 2 diabetes between January 1, 1998, and December 31, 2017, based on an established EHR algorithm8 were considered for study entry (original study protocol and amendment to study protocol avalable in Supplement 2). This served as the base cohort (n = 287 438). Race data were collected from the EHR based on patient self-report using fixed categories. Race was included in the analyses because it could be associated with both exposure and study end points.
Surgical Patients
The date of first metabolic surgery served as the index date for surgical patients. All patients in the base cohort receiving metabolic surgery at Ohio or Florida centers who met the following inclusion criteria at the time of index date were included: (1) age between 18 to 80 years, (2) body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) 30 or greater, and (3) either glycated hemoglobin (HbA1c) level of 6.5% or greater or taking at least 1 diabetes medication.
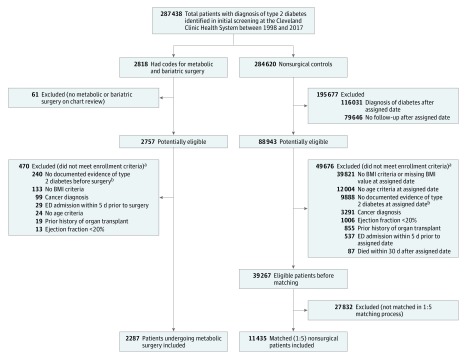
After preliminary inclusion, patients who met any of the following at the index date were excluded: (4) history of solid organ transplant (liver, heart, or lung), (5) history of severe heart failure (ejection fraction <20% any time before index date), (6) active cancer, (7) gastrointestinal cancer code within 1 year prior to index date, (8) emergency department admission within 5 days prior to index date, and (9) diagnoses or surgical procedures for gastroesophageal cancers or peptic ulcer disease during the same hospitalization for metabolic surgery (Figure 1).
Figure 1. Identification of Eligible Patients for Inclusion.
Details of cohort construction and International Classification of Diseases and Current Procedural Terminology codes are available in Supplement 1. Application of enrollment criteria resulted in a total of 2287 surgical patients and 39 267 nonsurgical patients with type 2 diabetes medllitus and body mass index (BMI) 30 or greater before matching. Afterward, each surgical patient was matched with a propensity score to 5 nonsurgical patients based on 7 a priori–identified potential confounders including the index date, age at index date, sex, BMI at index date, location, insulin use, and presence of end-organ complications of diabetes. ED indicates emergency department.
aSome patients met multiple exclusion criteria.
bPatients with glycated hemoglobin values less than 6.5% and not taking diabetes medications at the index date were excluded.
Nonsurgical Comparators
Starting with the base cohort, an algorithm was implemented to identify the control group who received usual care. First, patients with any codes listed for metabolic gastrointestinal surgical procedures were excluded. Then, we randomly assigned each patient a single date from the collection of index dates of surgical patients. This served as the index date for nonsurgical patients. We excluded patients meeting any of the following at the assigned date: (1) first diagnosis of type 2 diabetes after the assigned date, (2) last follow-up on or before the assigned date, (3) already dead or died within 30 days after the assigned date (to serve as a proxy for patients who were likely terminally ill), and (4) not meeting enrollment criteria 1 through 8 mentioned above for surgical patients (Figure 1).
Primary and Secondary End Points
The primary end point was the incidence of extended major adverse cardiovascular events (MACE, composite of 6 outcomes), defined as first occurrence of all-cause mortality, coronary artery events (unstable angina, myocardial infarction, or coronary intervention/surgery), cerebrovascular events (ischemic stroke, hemorrhagic stroke, or carotid intervention/surgery), heart failure, nephropathy, and atrial fibrillation, recording the first occurrence after the index date as the event date (definitions and codes reported in eTable 1 in Supplement 1). A secondary composite end point included 3-component MACE (all-cause mortality, myocardial infarction, and ischemic stroke).
We did not exclude any patients in the assessment of the composite primary and secondary end points. However, the conditions or events that a patient had at baseline were omitted from the count toward the composite end points in follow-up. For example, for a patient with history of myocardial infarction before the index date, having a code for myocardial infarction after the index date was not considered an event for composite end points. However, occurrence of ischemic stroke in this patient after the index date would count toward the composite end points.
Other secondary end points included the 6 individual components of the primary end point. For assessments of coronary artery events, cerebrovascular events, heart failure, nephropathy, and atrial fibrillation, patients who already had these conditions before the index date were eliminated from subsequent risk evaluation only for that specific outcome. Death information was obtained from a combination of the Cleveland Clinic EHR, Social Security data, and State Death Indices.
Other Outcomes
Weight, HbA1c level, and dates of prescription orders for diabetes and cardiovascular drugs were collected from the EHR to compare patients in the surgery and nonsurgical control groups over time. Major complications within 90 days of metabolic surgery were captured. Nutritional variables of interest included hemoglobin, serum protein, albumin, and vitamin D levels.
Statistical Analysis
Baseline data are presented as median (interquartile range [IQR]) and number (%). Doubly robust estimation combining the propensity score and outcome regression was used to compare outcomes in the surgical and nonsurgical groups. Each surgical patient was matched with a propensity score by the nearest-neighbor method to 5 nonsurgical patients from a logistic regression model with a logit link function based on 7 a priori–identified potential confounders including the index date, age at index date, sex, BMI at index date (categorized as 30-34.9, 35-39.9, ≥40), location (Ohio vs Florida), insulin use, and presence of diabetes-related end-organ complications.
Cause-specific event rates per 100 patient-years of follow-up starting from the index date were estimated for each outcome within each study group. Cumulative incidence estimates (Kaplan-Meier method) for 8 years after the index date and absolute risk difference for each outcome were calculated. The 95% confidence intervals (CIs) for the difference in 8-year risk were obtained by the percentile method from 1000 bootstrap iterations.
Fully adjusted Cox proportional hazards regression models were generated for all 8 primary and secondary outcomes. Variables reported in Table 1 were included to adjust for potential confounding. The proportional hazards assumptions for the treatment variable were tested based on weighted residuals as proposed by Grambsch and Therneau.9
Table 1. Characteristics of Metabolic Surgery Patients and Nonsurgical Control Patients at the Index Date Before and After Matching.
| Baseline Variable | Metabolic Surgery (n = 2287) | Nonsurgical (n = 39 267)a | Standardized Difference Before Matchingb | Matched Nonsurgical Control (n = 11 435) | Standardized Difference After Matchingb |
|---|---|---|---|---|---|
| Demographic Data | |||||
| Index date, median (IQR) | 1/2013 (7/2010-4/2015) | 6/2014 (5/2012-2/2016) | −42.6 | 7/2013 (5/2011-4/2015) | −15.9 |
| Sex, No. (%) | |||||
| Women | 1499 (65.5) | 20 372 (51.9) | 28.0 | 7339 (64.2) | 2.9 |
| Men | 788 (34.5) | 18 895 (48.2) | −28.0 | 4096 (35.8) | −2.9 |
| Age, median (IQR), y | 52.5 (43.7-60.5) | 61.6 (53.1-68.9) | −75.3 | 54.8 (46.2-62.5) | −19.9 |
| BMI, median (IQR)c | 45.1 (40-51.8) | 35.9 (32.7-60.6) | 113.5 | 42.6 (39.4-47.2) | 34.4 |
| BMI category, No. (%) | |||||
| 30-34.9 | 109 (4.8) | 17 110 (43.6) | −101.7 | 495 (4.3) | 2.1 |
| 35-39.9 | 465 (20.3) | 11 320 (28.8) | −19.8 | 2595 (22.7) | −5.7 |
| ≥40 | 1713 (74.9) | 10 837 (27.6) | 107.4 | 8345 (73.0) | 4.4 |
| Weight, median (IQR), kg | 126.5 (110.2-148.0) | 104.8 (93.0-119.0) | 88.3 | 120.2 (106.8-136.5) | 27.8 |
| Race, No. (%) | |||||
| White | 1734 (75.8) | 29 014 (73.9) | 4.45 | 7994 (69.9) | 13.3 |
| Black | 441 (19.3) | 8138 (20.7) | −3.6 | 2804 (24.5) | −12.7 |
| Other | 54 (2.4) | 874 (2.2) | 0.9 | 234 (2.1) | 2.1 |
| Missing | 58 (2.5) | 1241 (3.2) | −3.8 | 403 (3.5) | −5.8 |
| Annual zip code income, median (IQR), $ | 49 855 (39 964-62 273) | 50 378 (37 958-65 536) | 0.7 | 48 732 (36 951-61 512) | 10.8 |
| Missing, No. (%) | 70 (3.1) | 494 (1.3) | 12.4 | 125 (1.1) | 13.8 |
| Smoking status, No. (%) | |||||
| Never | 1231 (53.8) | 18 087 (46.1) | 15.6 | 5615 (49.1) | 9.5 |
| Former | 828 (36.2) | 15 531 (39.6) | −6.9 | 4012 (35.1) | 2.3 |
| Current | 170 (7.4) | 5051 (12.9) | −18.1 | 1607 (14) | −21.5 |
| Missing | 58 (2.5) | 598 (1.5) | 7.2 | 201 (1.8) | 5.4 |
| Location, No. (%) | |||||
| Ohio | 1816 (79.4) | 35 482 (90.4) | −30.9 | 9834 (86.0) | −17.5 |
| Florida | 471 (20.6) | 3785 (9.6) | 30.9 | 1601 (14.0) | 17.5 |
| Medical History, No. (%) | |||||
| Hypertension | 1953 (85.4) | 30 308 (77.2) | 21.2 | 8565 (74.9) | 26.5 |
| Dyslipidemia | 1686 (73.7) | 27 717 (70.6) | 7.0 | 7457 (65.2) | 18.6 |
| Peripheral neuropathy | 242 (10.6) | 4569 (11.6) | −3.4 | 1203 (10.5) | 0.2 |
| Heart failure | 238 (10.4) | 4470 (11.4) | −3.1 | 1342 (11.7) | −4.2 |
| Coronary artery disease | 237 (10.4) | 5863 (14.9) | −13.8 | 1104 (9.7) | 2.4 |
| COPD | 206 (9.0) | 4179 (10.6) | −5.5 | 1188 (10.4) | −4.7 |
| Nephropathy | 191 (8.4) | 5069 (12.9) | −14.8 | 1219 (10.7) | −7.8 |
| Atrial fibrillation | 152 (6.6) | 3242 (8.3) | −6.1 | 701 (6.1) | 2.1 |
| Peripheral arterial disease | 123 (5.4) | 3426 (8.7) | −13.1 | 755 (6.6) | −5.2 |
| Myocardial infarction | 55 (2.4) | 1201 (3.1) | −4.0 | 211 (1.8) | 3.9 |
| Cerebrovascular disease | 42 (1.8) | 1703 (4.3) | −14.5 | 358 (3.1) | −8.3 |
| Ischemic stroke | 34 (1.5) | 1385 (3.5) | −13.1 | 298 (2.6) | −7.9 |
| Dialysis | 14 (0.6) | 322 (0.8) | −2.5 | 78 (0.7) | −0.9 |
| Clinical and Laboratory Data | |||||
| HbA1c, median (IQR), % | 7.1 (6.3-8.2) | 7 (6.4-8.1) | −2.3 | 7.1 (6.4-8.4) | −11.4 |
| Missing, No. (%) | 159 (7.0) | 4899 (12.5) | −18.7 | 1288 (11.3) | −15.0 |
| Blood pressure, median (IQR), mm Hg | |||||
| Systolic | 138 (127-148) | 130 (120-142) | 31.6 | 130 (121-142) | 30.1 |
| Missing, No. (%) | 0 | 234 (0.6) | −10.9 | 54 (0.5) | –9.7 |
| Diastolic | 71 (65-79) | 76 (69-82) | −35.3 | 78 (70-84) | −44.2 |
| Missing, No. (%) | 0 | 234 (0.6) | −10.9 | 54 (0.5) | –9.7 |
| eGFR, median (IQR), mL/mind | 90 (72-108) | 86 (68-105) | 11.5 | 92 (72-112) | −8.1 |
| Missing | 1 (0) | 1620 (4.1) | −28.9 | 432 (3.8) | −27.5 |
| HDL-C, median (IQR), mg/dL | 44 (37-52) | 43 (36-52) | 1.6 | 43 (36-51) | 4.9 |
| Missing, No. (%) | 792 (34.6) | 12 966 (33.0) | 3.4 | 3512 (30.7) | 8.4 |
| LDL-C, median (IQR), mg/dL | 92 (72-115) | 89 (68-114) | 5.5 | 93 (72-118) | −4.9 |
| Missing, No. (%) | 208 (9.1) | 10 469 (26.7) | −47.1 | 2947 (25.8) | −45.1 |
| Triglycerides, median (IQR), mg/dL | 146 (101-209) | 143 (101-205) | 1.8 | 146 (103-208) | −1.4 |
| Missing, No. (%) | 160 (7) | 6538 (16.7) | −30.2 | 1816 (15.9) | −28.2 |
| UACR, median (IQR), mg/g | 14 (6-40) | 14 (5-40) | −0.5 | 14 (5-43) | −3.8 |
| Missing, No. (%) | 1003 (43.9) | 14 665 (37.4) | 13.3 | 4224 (36.9) | 14.1 |
| Medication History | |||||
| Noninsulin diabetes medication, No. (%)e | 1869 (81.7) | 32 428 (82.6) | −2.2 | 9253 (80.9) | 2.1 |
| 0 | 418 (18.3) | 6839 (17.4) | 2.2 | 2182 (19.1) | −2.1 |
| 1 | 1088 (47.6) | 18 206 (46.4) | 2.4 | 5218 (45.6) | 3.9 |
| 2 | 560 (24.5) | 10 084 (25.7) | −2.8 | 2929 (25.6) | −2.6 |
| ≥3 | 221 (9.7) | 4138 (10.5) | −2.9 | 1106 (9.7) | 0 |
| Insulin, No. (%) | 776 (33.9) | 10 811 (27.5) | 13.9 | 3806 (33.3) | 1.4 |
| Lipid-lowering medications, No. (%) | 1195 (52.3) | 23 963 (61.0) | −17.8 | 5998 (52.5) | −0.4 |
| Renin-angiotensin system inhibitors, No. (%)f | 1396 (61.0) | 25 375 (64.6) | −7.4 | 7102 (62.1) | −2.2 |
| Other antihypertensive medications, No. (%) | 1649 (72.1) | 28 710 (73.1) | −2.2 | 8066 (70.5) | 3.5 |
| Aspirin | 731 (32.0) | 18 961 (48.3) | −33.8 | 4627 (40.5) | −17.8 |
| Warfarin | 190 (8.3) | 3207 (8.2) | 0.5 | 943 (8.2) | 0.2 |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; UACR, urinary albumin-creatinine ratio.
SI conversion factors: To convert HDL-C and LDL-C values to mmol/L, multiply by 0.0259; triglyceride values to mmol/L, multiply by 0.0113.
Starting with the base cohort of 287 438 patients with type 2 diabetes, an algorithm (Figure 1) was implemented that resulted in a total of 39 267 nonsurgical patients before matching (see Methods).
Standardized differences are the absolute value of the difference in means or proportions between the groups (metabolic surgery − nonsurgical control group) divided by pooled standard deviation.
Calculated as weight in kilograms divided by height in meters squared.
Approximated using the Modification of Diet in Renal Disease (MDRD) Study equation.
Class of diabetes medications has been detailed in eTable 3 in Supplement 1.
Including angiotensin-converting enzyme inhibitors and angiotensin receptor blockers.
Within each outcome data set, values missing at baseline (Table 1) were imputed with multiple imputation by chained equations to create 5 imputed data sets. Predictive mean matching was used for numeric variables, logistic regression for binary variables, and polytomous logistic regression for categorical variables. Imputation-corrected standard errors of model estimates and contrasts were obtained by the Rubin formula.10,11
For subgroup analyses of the primary end point, an interaction term between variables of interest and treatment indicator was individually added to the fully adjusted Cox model, and the P values and CIs for these associations were estimated.
A 4-knot spline interacted with treatment was used for comparing mean changes in metabolic and nutritional variables, and a 2-sample proportions test was used to compare proportions of patients prescribed diabetes and cardiovascular medications between the study groups at 1, 2, 5, and 8 years of follow-up.
A significance level of .05 for 2-sided comparisons was considered statistically significant, and 95% CIs were reported where applicable. Because of the potential for type 1 error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. All analyses were performed in the R statistical programming language, version 3.5.0.12
Sensitivity Analyses
We assessed the sensitivity of the hazard ratio (HR) estimates from the fully adjusted Cox models to 2 components of the process used in obtaining nonsurgical controls: random sampling of index dates and matching ratio. The surgical index dates were randomly assigned to nonsurgical controls 5 times, and the matching ratio was tested at 1:1, 1:5, and 1:10, thus creating 15 data sets. The fully adjusted Cox models were determined for each data set (for all outcomes), and the HRs and 95% CIs for the treatment variable were obtained for each.
In addition, time-varying HRs for patients in the surgery and nonsurgical control groups at 2, 5, and 8 years after the index date were estimated. An interaction between a restricted cubic spline on the observed follow-up time and the treatment group was added to the fully adjusted Cox models.
We also used the E-value, which can assess the robustness of the identified association between metabolic surgery and MACE to potential unmeasured confounders.13
Results
A total of 13 722 patients, including 2287 who had undergone metabolic surgery and 11 435 matched nonsurgical control patients, were included in the analyses. Metabolic surgical procedures included Roux-en-Y gastric bypass (n = 1443 [63%]), sleeve gastrectomy (n = 730 [32%]), adjustable gastric banding (n = 109 [5%]), and duodenal switch (n = 5). In the surgical group, 1713 patients (75%) had a BMI of 40 or greater, 465 patients (20%) had a BMI between 35 and 39.9, and 109 patients (5%) had a BMI between 30 and 34.9.
The distribution of 37 baseline covariates (Table 1) was balanced after matching between the metabolic surgery group and usual care group for most characteristics. However, patients in the surgery group had higher body weight (126.5 vs 120.2 kg), higher BMI (45.1 vs 42.6), and higher rates of dyslipidemia (74% vs 65%) and hypertension (85% vs 75%). The control group was older (54.8 vs 52.5 years) and had higher rates of black race (25% vs 19%), current smoking (14% vs 7%), and aspirin use (40% vs 32%). The median follow-up time for the entire cohort was 3.9 years (IQR, 1.9-6.1), including 4.0 years (IQR, 2.1-6.1) for nonsurgical patients and 3.3 years (IQR, 1.2-6.3) for surgical patients.
Primary End Point
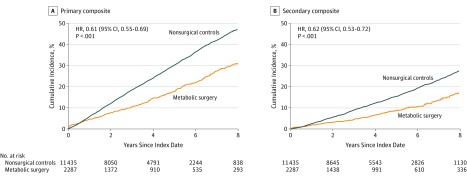
At the end of the study period, 385 patients in the surgical group and 3243 patients in the nonsurgical group experienced a primary composite end point. The cumulative incidence of primary end point at 8-year follow-up was 30.8% (95% CI, 27.6%-34.0%) in the surgical group and 47.7% (95% CI, 46.1%-49.2%) in the nonsurgical group (P < .001) (absolute risk difference, 16.9% [95% CI, 13.1%-20.4%]; adjusted HR, 0.61 [95% CI, 0.55-0.69]) (Figure 2A and Table 2). Interaction testing revealed no heterogeneity in the association of metabolic surgery with the primary outcome based on sex, age, BMI, HbA1c level, estimated glomerular filtration rate, or use of insulin, sulfonylureas, or lipid-lowering medications (eFigure 1 in Supplement 1).
Figure 2. Eight-Year Cumulative Incidence Estimates (Kaplan-Meier) for 2 Composite End Points.
The primary end point was the incidence of extended major adverse cardiovascular events (MACE; composite of 6 outcomes), defined as first occurrence of coronary artery events, cerebrovascular events, heart failure, atrial fibrillation, nephropathy, and all-cause mortality, recording the first occurrence after the index date as the event date. The secondary composite end points included 3-component MACE (all-cause mortality, myocardial infarction, and ischemic stroke), recording the first occurrence after the index date as the event date. For both end points, the median observation time was 4.0 years (interquartile range [IQR], 2.1-6.1) for nonsurgical patients and 3.3 years (IQR, 1.2-6.3) for surgical patients. HR indicates hazard ratio.
Table 2. Cumulative Incidence Estimates (%), Absolute Risk Differences, and Hazard Ratios From Fully Adjusted Cox Models for Each Outcome for Metabolic Surgery Group vs Matched Nonsurgical Control Group.
| Outcome | Metabolic Surgery | Nonsurgical Control | Absolute 8-Year Risk Difference, % (95% CI)a | Hazard Ratio (95% CI)b | P Valueb | ||
|---|---|---|---|---|---|---|---|
| No. at Risk | Cumulative Incidence at 8 y, % (95% CI) | No. at Risk | Cumulative Incidence at 8 y, % (95% CI) | ||||
| Primary | 2287 | 30.8 (27.6-34.0) | 11 435 | 47.7 (46.1-49.2) | 16.9 (13.1-20.4) | 0.61 (0.55-0.69) | <.001 |
| Secondary | 2287 | 17.0 (14.3-19.7) | 11 435 | 27.6 (26.2-29.0) | 10.6 (7.5-13.6) | 0.62 (0.53-0.72) | <.001 |
| All-cause mortality | 2287 | 10.0 (7.8-12.2) | 11 435 | 17.8 (16.6-19.0) | 7.8 (5.1-10.2) | 0.59 (0.48-0.72) | <.001 |
| Heart failure | 2049 | 6.8 (4.9-8.6) | 10 093 | 18.9 (17.6-20.2) | 12.9 (10.4-15.1) | 0.38 (0.30-0.49) | <.001 |
| Coronary artery disease | 2050 | 7.9 (5.9-9.8) | 10 331 | 11.6 (10.5-12.6) | 4.2 (1.9-6.8) | 0.69 (0.54-0.87) | .002 |
| Cerebrovascular disease | 2245 | 4.1 (2.7-5.5) | 11 077 | 5.6 (4.9-6.3) | 1.8 (–0.03 to 3.4) | 0.67 (0.48-0.94) | .02 |
| Nephropathy | 1937 | 6.1 (4.4-7.8) | 9190 | 16.3 (15.0-17.6) | 11.1 (8.8-13.6) | 0.40 (0.31-0.52) | <.001 |
| Atrial fibrillation | 2135 | 7.9 (6.1-9.7) | 10 734 | 13.6 (12.5-14.7) | 6.5 (4.4-8.7) | 0.78 (0.62-0.97) | .03 |
95% bootstrap CIs (1000 samples) for the difference in 8-year absolute risk (nonsurgical control group − metabolic surgery) for each outcome and treatment group.
Hazard ratios (95% CIs) and P values from adjusted Cox models comparing the relative instantaneous risk of each outcome for surgical vs nonsurgical patients. All baseline variables in Table 1 were included to adjust for potential confounding.
Secondary End Points
At the end of the study, 3-component MACE occurred in 194 patients in the surgical group and 1765 patients in the nonsurgical group. Metabolic surgery was associated with a significantly lower cumulative incidence of 3-component MACE at 8 years compared with usual care (17.0% vs 27.6%, respectively; absolute risk difference, 10.6% [95% CI, 7.5%-13.6%]; HR, 0.62 [95% CI, 0.53-0.72]) (Figure 2B and Table 2).
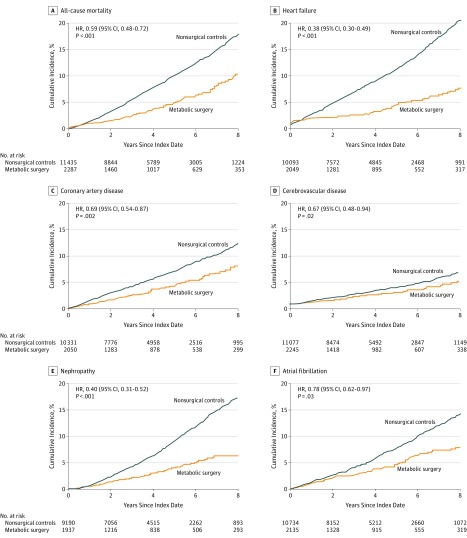
During follow-up, 112 patients in the metabolic surgery group and 1111 patients in the nonsurgical group died (cumulative incidence at 8 years, 10.0% [95% CI, 7.8%-12.2%] and 17.8% [95% CI, 16.6%-19.0%]; absolute risk difference at 8-year follow-up, 7.8% [95% CI, 5.1%-10.2%]; HR, 0.59 [95% CI, 0.48-0.72]) (Figure 3A and Table 2).
Figure 3. Eight-Year Cumulative Incidence Estimates (Kaplan-Meier) for 6 Individual End Points.
For each 5 individual outcomes (except all-cause mortality), any patient with a history of that outcome prior to the index date was eliminated from risk assessment only for that outcome. For all-cause mortality, median observation time was 4.0 years (interquartile range [IQR], 2.1-6.1) in the nonsurgical group and 3.3 years (IQR, 1.2-6.3) in the surgical group; for heart failure, 4.1 years (IQR, 2.2-6.2) and 3.3 years (IQR, 1.1-6.4); for coronary artery disease, 4.0 years (IQR, 2.1-6.1) and 3.3 years (IQR, 1.1-6.4); for nephropathy, 4.1 years (IQR, 2.2-6.2) and 3.3 years (IQR, 1.1-6.3); for cerebrovascular disease and atrial fibrillation, 4.0 years (IQR, 2.1-6.1) and 3.3 years (IQR, 1.1-6.3). HR indicates hazard ratio.
Metabolic surgery was also associated with significantly lower incidence of the other 5 individual end points, including coronary artery events, cerebrovascular events, heart failure, nephropathy, and atrial fibrillation (Figure 3B-F). The event rates for these end points and absolute risk differences are reported in Table 2 and in eTable 4 and eTable 5 in Supplement 1.
The proportional hazards assumption was satisfied for the primary and secondary composite outcomes and individual outcomes, except for atrial fibrillation (P values testing the proportional hazards assumption are reported in eTable 6 in Supplement 1). The time-varying HRs for atrial fibrillation are reported in eTable 7 in Supplement 1. For all end points, eTable 5 and eTable 7 in Supplement 1 provide cumulative incidence estimates and time-varying HRs at 3 time intervals (years 2, 5, and 8).
Status of Obesity, Diabetes, and Medications Over Time
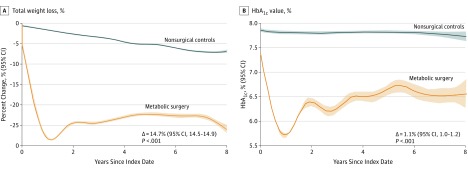
The mean body weight at 8 years was reduced by 29.1 kg (95% CI, 28.8-29.3) in the surgery group and 8.7 kg (95% CI, 8.6-8.9) in the nonsurgical control group (mean difference, 20.3 kg [95% CI, 20.1-20.6]). Metabolic surgery was also associated with a significant reduction in HbA1c level (mean difference in changes from baseline at 8 years between groups, 1.1% [95% CI, 1.0%-1.2%]; P < .001) (Figure 4; eTable 8 and eFigure 2 in Supplement 1).
Figure 4. Mean Trend Curves of Weight Loss and HbA1c Values Over 8 Years of Follow-up.
Smoothed mean trends of percent weight lost from baseline and absolute glycated hemoglobin (HbA1c) values (%) in surgical and nonsurgical patients during follow-up. Shaded areas indicate 95% CIs. Mean difference in total weight loss at 8 years and mean difference in HbA1c changes from baseline at 8 years between groups were estimated from a flexible regression model with a 4-knot spline on time, since the index date interacted with the treatment group. Statistical comparison and sample size at different time points have been reported in eTable 8 and eTable 9 in Supplement 1.
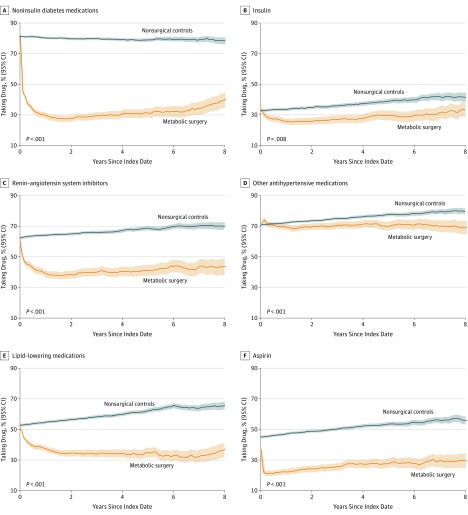
Use of noninsulin diabetes medications, insulin, renin-angiotensin system blockers, other antihypertensive medications, lipid-lowering therapies, and aspirin were also significantly lower after metabolic surgery compared with usual care (Figure 5; eTable 8 and eFigure 2 in Supplement 1).
Figure 5. Proportions of Patients Taking Diabetes and Cardiovascular Drugs Over 8 Years of Follow-up.
Proportions over time with 95% point-wise confidence intervals by surgical and nonsurgical patients. Shaded areas indicate 95% CIs. P values from a Fisher exact test are also displayed comparing the proportion of surgical and nonsurgical patients taking drug at 8 years after the index date. The proportion of patients taking each drug were computed every tenth of a year starting at the index date through 8 years of follow-up. Renin-angiotensin system inhibitors include angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Statistical comparison and sample size at different time points have been reported in eTable 8 and eTable 10 in Supplement 1.
Adverse Events After Metabolic Surgery
The median postoperative length of hospital stay was 2 (IQR, 2-3) days. In the 90 days after metabolic surgery, complications included bleeding requiring transfusion (n = 68 [3.0%]), pulmonary adverse events (n = 58 [2.5%]), venous thromboembolism (n = 22 [1.0%]), cardiac events (n = 17 [0.7%]), and renal failure requiring dialysis (n = 4 [0.2%]). There were no cerebrovascular events in the early postoperative period. Abdominal surgical intervention was required in 109 patients (4.8%), and 15 patients (0.7%) died within 90 days after surgery. In the early postoperative period, endoscopy was required in 16.7% of patients, interventional radiologic procedures in 1.6%, and parenteral nutrition in 1.4%.
The cumulative incidence of nutritional, endoscopic, radiologic, and surgical reinterventions during postoperative follow-up is reported in eTable 11 in Supplement 1. Changes in levels of hemoglobin, serum protein, albumin, and vitamin D are reported in eTable 8 and eFigure 3 in Supplement 1.
Sensitivity Analyses
Results of the sensitivity analyses are detailed in Supplement 1. The fully adjusted Cox models were computed for 15 data sets (15 different nonsurgical cohorts), and the HRs and 95% CIs for the treatment variable were obtained from each data set for all outcomes (eFigure 4 in Supplement 1). Overall, the differences in HRs comparing the risk of 8 end points in patients in the surgery group vs those in the nonsurgical control group were negligible between the 15 data sets and the estimates reported in the manuscript from a single data set (with 1:5 matching).
Examining the E-values for study end points and comparing with the HR estimates of known cardiovascular risk factors for these end points (eTable 12 in Supplement 1) indicates that it would be unlikely that an unmeasured confounder exists that could account for the observed association between metabolic surgery and the 8 major study end points.
Discussion
In this matched-cohort study, metabolic surgery was associated with a significantly lower risk of incident MACE compared with usual care in patients with type 2 diabetes and a BMI of 30 or greater. However, given the observational nature of the study, these data should be considered hypothesis-generating and not conclusive. The cumulative incidence rates of MACE were unusually high, with 48% of patients in the nonsurgical control group experiencing a MACE event compared with 31% of those in the metabolic surgery group. All 6 prespecified outcomes were significantly lower in the surgery group, including all-cause mortality, coronary disease events, cerebrovascular events, heart failure, atrial fibrillation, and nephropathy. A narrower 3-component composite outcome consisting of myocardial infarction, ischemic stroke, and all-cause mortality was also significantly lower in patients in the metabolic surgery group. Metabolic surgery was associated with significantly lower mortality over 8 years (absolute risk difference, 7.8%). The very high event rates observed in patients in the nonsurgical control group were similar to the rates of adverse outcomes reported for contemporary RCTs in secondary cardiovascular prevention populations,14 highlighting the importance of obesity as a risk factor for morbidity and mortality.
We speculate that the lower rate of MACE after metabolic surgery observed in this study may be related to substantial and sustained weight loss with subsequent improvement in metabolic, structural, hemodynamic, and neurohormonal abnormalities. This hypothesis is supported by the observed significant reductions in body weight, HbA1c levels, and use of medications to treat diabetes and cardiovascular diseases. An intensive lifestyle intervention in overweight and obese individuals with diabetes in the Look AHEAD (Action for Health in Diabetes) trial produced more modest weight loss (8.6% vs 0.7% at 1 year; 6.0% vs 3.5% at 9 years) and did not reduce the risk of MACE.15 Although large and sustained surgically induced weight loss has profound physiologic effects, a growing body of evidence indicates that some of the beneficial metabolic and neurohormonal changes that occur after metabolic surgical procedures are related to anatomical changes in the gastrointestinal tract that are partially independent of weight loss.16,17,18
In the absence of RCTs, a few observational studies have reported the association between metabolic surgery and reduction in MACE.6,19,20 In a matched-cohort study, Fisher et al19 investigated the incidence of coronary artery disease, cerebrovascular disease, and mortality in patients with type 2 diabetes and BMI greater than 35 after metabolic surgery compared with usual care. At 5 years, metabolic surgery was significantly associated with a lower incidence of macrovascular events (HR, 0.60) and all-cause mortality (HR, 0.33). However, the reported annual event rates were 2 to 3 times lower than those observed in the current study, presumably because the current study included a higher-risk cohort with older patients, greater baseline morbidity, and higher insulin use. In the long-term follow-up of the smaller Swedish Obese Subjects (SOS) study that included 343 surgical and 260 control patients with type 2 diabetes, metabolic surgery was associated with significantly fewer macrovascular events than usual care.6 The majority of procedures in this subgroup of the SOS study were either vertical banded gastroplasty (66%) or gastric banding (18%), which have largely been replaced by more durable metabolic procedures in recent years.
Obesity and type 2 diabetes are associated with a broad range of adverse outcomes beyond 3-component MACE (myocardial infarction, stroke, and mortality). Therefore, a 6-component primary outcome including heart failure, atrial fibrillation, and nephropathy was prespecified. These additional adverse outcomes were also significantly lower in the metabolic surgery cohort. The lower rate of heart failure in the surgery group compared with the control group was observed despite lower use of cardiovascular medications known to favorably affect heart failure, including renin–angiotensin system inhibitors. Prior studies, including the SOS study,5,21,22 have also suggested that substantial weight loss may reduce the incidence of heart failure. The mechanisms responsible for this potential benefit are not well defined, but substantial weight loss affects cardiac metabolism, function, workload, and geometry.23 Metabolic surgery is also associated with improvements in glucose levels and blood pressure control and with a lower incidence of other cardiovascular events (eg, myocardial infarction and atrial fibrillation), all of which may contribute to the significant reduction in the risk of heart failure.
Obesity, diabetes, hypertension, and sleep apnea are established risk factors for atrial fibrillation. The current study showed a significantly lower risk of atrial fibrillation associated with metabolic surgery. Conversely, more modest weight loss in a secondary analysis of the Look AHEAD trial was not associated with a lower risk of developing atrial fibrillation.24 Similarly, a recent meta-analysis reported that 5% nonsurgical weight loss did not alter the incidence of atrial fibrillation.25 More substantial weight loss in other metabolic surgery studies, such as the SOS study, was associated with a lower incidence of atrial fibrillation compared with the control group.26 Taken together, these findings suggest that larger and more sustained weight loss with major metabolic changes may be required for primary prevention of atrial fibrillation.
The current study showed significantly lower incidence of diabetic nephropathy in surgical compared with nonsurgical patients. Prior studies have also suggested favorable effects for metabolic surgery in patients with established diabetic kidney disease, including resolution of albuminuria in 50% of patients and improvement in glomerular filtration rate.27,28 In the subgroup of patients with diabetes in the SOS study, metabolic surgery was significantly associated with a lower incidence of diabetic kidney disease.29 In another matched-cohort study, the incidence of nephropathy was 59% lower at 5 years among patients who underwent metabolic surgery.30
Patients in the metabolic surgery group required fewer medications for treatment of diabetes and cardiovascular disease, showing large reductions in the prescription of insulin, noninsulin diabetes medications, renin-angiotensin system inhibitors and other antihypertensive medications, and lipid-lowering medications.
The safety of metabolic surgical interventions has improved during the last 2 decades.31 Although a variety of adverse events related to metabolic surgery were observed, cardiovascular morbidity and all-cause mortality were significantly lower overall.
Limitations
This study has several limitations. First, although a comprehensive matching process with fully adjusted regression models on a broad range of potential confounding variables was used, residual measured or unmeasured confounders could have influenced the findings of this retrospective, observational study. No matching or statistical adjustment was performed based on the severity of medical conditions at baseline listed in Table 1. Therefore, the observed associations could be attributed to differences other than treatment assignment (metabolic surgery vs usual care), and causal inference cannot be assumed. Nonetheless, the consistency of results in the sensitivity analyses support the robustness of the findings. The E-value can be used to assess the robustness of observed associations in the presence of potential unmeasured confounders.13 The magnitude of the associations of the known MACE risk factors with the study end points is smaller than the estimated E-value for primary and secondary end points, which makes it unlikely that there are unmeasured confounders that could eliminate the favorable association between metabolic surgery and study end points (eTable 12 in Supplement 1).
Second, coding errors, misclassification, and misdiagnosis in the EHR are recognized issues with this type of study. Third, the causes of mortality could not be determined. Fourth, to assess status of diabetes and cardiovascular medications in follow-up, the study assessed prescription orders for medications, which does not necessarily equate to actual medication use. Fifth, in long-term follow-up, adverse events that did not lead to intervention were not assessed. Indications and diagnoses associated with interventions were not collected. Sixth, comparison of different metabolic surgical procedures was not performed, since the distribution of surgical procedures was not uniform. Furthermore, such an analysis would require separate matching and analysis to construct new cohorts with comparable baseline characteristics. Seventh, less than 10% of nonsurgical patients were exposed to new diabetes medications, including glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors, that are associated with significant cardiovascular benefits.
Conclusions
Among patients with type 2 diabetes and obesity, metabolic surgery, compared with nonsurgical management, was associated with a significantly lower risk of incident MACE. The findings from this observational study must be confirmed in randomized clinical trials.
eTable 1. Diagnosis and Procedure Codes
eTable 2. Diagnosis and Intervention Codes for Adverse Events of Metabolic Surgery
eTable 3. Class of Diabetes Medications at the Index Date
eTable 4. Cause-Specific Event Rates (%) per 100 Patient-Years of Follow-up at 8 Years, for Each Study Outcome Stratified by Surgical and Non-surgical Patients
eTable 5 Cumulative Incidence Estimates (%) and 95% Confidence Intervals at 2, 5, and 8 Years After the Index Date for Each Study Outcome Stratified by Surgical and Non-surgical Patients
eFigure 1. Association of Metabolic Surgery Compared With Usual Care for the Primary Composite Endpoint in Key Subgroups in the Fully-Adjusted Cox Models
eTable 6 Hazard Ratios (95% CIs) and P Values From Cox Models Comparing the Relative Instantaneous Risk of Each Outcome for Surgical Vs. Non-surgical Patients
eTable 7. Time-Varying Hazard Ratios and 95% CIs at 2, 5, and 8 Years After the Index Date Comparing Surgical and Non-surgical Patients From a Fully-Adjusted Cox Model for Each Outcome
eFigure 2. Mean Trend Curve of HbA1c (%) and Proportions of Patients Taking Non-insulin Diabetes Drugs Over Four Years of Follow-up Categorized by the Treatment Group (Metabolic Surgery Vs. Usual Care) and the BMI (≥35 vs. <35 kg/m2) at the Index Date
eTable 8. Average Change in Metabolic and Nutritional Variables From Baseline and in Proportions of Patients Taking Diabetes and Cardiovascular Medications (%) at 1, 2, 5, and 8 Years of Follow-up in Surgical vs. Non-surgical Patients
eTable 9. Total Number of Observations and Number of Distinct Patients With Available Measurements After Each Time-Point Following the Index Date for Metabolic and Nutritional Values by Treatment
eTable 10. Sample Size for Computing Proportions of Patients Taking Diabetes and Cardiovascular Drugs Over Time at 0, 1, 2, 5, and 8 Years After the Index Date by Treatment Group
eTable 11. Cumulative Incidence Estimates (%) and 95% CIs for Interventions at 1, 2, 5, and 8 Years After Metabolic Surgery
eFigure 3. Mean Trend Curve of Nutritional Variables of Interest Over Eight Years of Follow-Up in the Surgical and Non-surgical Patients Sensitivity Analyses Matching and Index Date Sampling
eFigure 4. Hazard Ratios and 95% Confidence Intervals for Metabolic Surgery Versus No Surgery From Fully-Adjusted Cox Models for Each Outcome for Five (5) Iterations of Index Date Random Sampling and Three (3) Different Matching Ratios (Total of 15 Datasets)
Time-Varying Hazard Ratios
E-Value
eTable 12. E-Value for the Effect of Metabolic Surgery on Each Outcome (and its Upper Limit of 95% CI) in Fully-Adjusted Cox Models
Study Protocol and Amendment to Study Protocol
References
- 1.Ikramuddin S, Korner J, Lee WJ, et al. . Lifestyle intervention and medical management with vs without Roux-en-Y gastric bypass and control of hemoglobin A1c, LDL cholesterol, and systolic blood pressure at 5 years in the Diabetes Surgery Study. JAMA. 2018;319(3):266-278. doi: 10.1001/jama.2017.20813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mingrone G, Panunzi S, De Gaetano A, et al. . Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386(9997):964-973. doi: 10.1016/S0140-6736(15)00075-6 [DOI] [PubMed] [Google Scholar]
- 3.Schauer PR, Bhatt DL, Kirwan JP, et al. ; STAMPEDE Investigators . Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376(7):641-651. doi: 10.1056/NEJMoa1600869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson BL, Blackhurst DW, Latham BB, et al. . Bariatric surgery is associated with a reduction in major macrovascular and microvascular complications in moderately to severely obese patients with type 2 diabetes mellitus. J Am Coll Surg. 2013;216(4):545-556. doi: 10.1016/j.jamcollsurg.2012.12.019 [DOI] [PubMed] [Google Scholar]
- 5.Benotti PN, Wood GC, Carey DJ, et al. . Gastric bypass surgery produces a durable reduction in cardiovascular disease risk factors and reduces the long-term risks of congestive heart failure. J Am Heart Assoc. 2017;6(5):e005126. doi: 10.1161/JAHA.116.005126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sjöström L, Peltonen M, Jacobson P, et al. . Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311(22):2297-2304. doi: 10.1001/jama.2014.5988 [DOI] [PubMed] [Google Scholar]
- 7.Milinovich A, Kattan MW. Extracting and utilizing electronic health data from Epic for research. Ann Transl Med. 2018;6(3):42. doi: 10.21037/atm.2018.01.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kho AN, Hayes MG, Rasmussen-Torvik L, et al. . Use of diverse electronic medical record systems to identify genetic risk for type 2 diabetes within a genome-wide association study. J Am Med Inform Assoc. 2012;19(2):212-218. doi: 10.1136/amiajnl-2011-000439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515-526. doi: 10.1093/biomet/81.3.515 [DOI] [Google Scholar]
- 10.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J Stat Softw. 2011;45(3):1-67. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 11.Frank E Harrell Jr. rms: Regression Modeling Strategies: R package version 5.1-2. Cran R website. https://cran.r-project.org/web/packages/rms/index.html. 2018. Accessed August 20, 2019.
- 12.R Core Team R: a language and environment for statistical computing. R Foundation for Statistical Computing website. https://www.R-project.org/. 2018. Accessed August 20, 2019.
- 13.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268-274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 14.Sabatine MS, Giugliano RP, Keech AC, et al. ; FOURIER Steering Committee and Investigators . Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722. doi: 10.1056/NEJMoa1615664 [DOI] [PubMed] [Google Scholar]
- 15.Wing RR, Bolin P, Brancati FL, et al. ; Look AHEAD Research Group . Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145-154. doi: 10.1056/NEJMoa1212914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Douros JD, Tong J, D’Alessio DA. The effects of bariatric surgery on islet function, insulin secretion, & glucose control [published online June 26, 2019]. Endocr Rev. doi: 10.1210/er.2018-00183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batterham RL, Cummings DE. Mechanisms of diabetes improvement following bariatric/metabolic surgery. Diabetes Care. 2016;39(6):893-901. doi: 10.2337/dc16-0145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chondronikola M, Harris LL, Klein S. Bariatric surgery and type 2 diabetes: are there weight loss-independent therapeutic effects of upper gastrointestinal bypass? J Intern Med. 2016;280(5):476-486. doi: 10.1111/joim.12527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher DP, Johnson E, Haneuse S, et al. . Association between bariatric surgery and macrovascular disease outcomes in patients with type 2 diabetes and severe obesity. JAMA. 2018;320(15):1570-1582. doi: 10.1001/jama.2018.14619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams TD, Gress RE, Smith SC, et al. . Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753-761. doi: 10.1056/NEJMoa066603 [DOI] [PubMed] [Google Scholar]
- 21.Jamaly S, Carlsson L, Peltonen M, Jacobson P, Karason K. Surgical obesity treatment and the risk of heart failure. Eur Heart J. 2019;40(26):2131-2138. doi: 10.1093/eurheartj/ehz295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sundström J, Bruze G, Ottosson J, Marcus C, Näslund I, Neovius M. Weight loss and heart failure: a nationwide study of gastric bypass surgery versus intensive lifestyle treatment. Circulation. 2017;135(17):1577-1585. doi: 10.1161/CIRCULATIONAHA.116.025629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aggarwal R, Harling L, Efthimiou E, Darzi A, Athanasiou T, Ashrafian H. The effects of bariatric surgery on cardiac structure and function: a systematic review of cardiac imaging outcomes. Obes Surg. 2016;26(5):1030-1040. doi: 10.1007/s11695-015-1866-5 [DOI] [PubMed] [Google Scholar]
- 24.Alonso A, Bahnson JL, Gaussoin SA, et al. ; Look AHEAD Research Group . Effect of an intensive lifestyle intervention on atrial fibrillation risk in individuals with type 2 diabetes: the Look AHEAD randomized trial. Am Heart J. 2015;170(4):770-777.e5. doi: 10.1016/j.ahj.2015.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones NR, Taylor KS, Taylor CJ, Aveyard P. Weight change and the risk of incident atrial fibrillation: a systematic review and meta-analysis [published online June 22, 2019]. Heart.doi: 10.1136/heartjnl-2019-314931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jamaly S, Carlsson L, Peltonen M, Jacobson P, Sjöström L, Karason K. Bariatric surgery and the risk of new-onset atrial fibrillation in Swedish obese subjects. J Am Coll Cardiol. 2016;68(23):2497-2504. doi: 10.1016/j.jacc.2016.09.940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young L, Nor Hanipah Z, Brethauer SA, Schauer PR, Aminian A. Long-term impact of bariatric surgery in diabetic nephropathy. Surg Endosc. 2019;33(5):1654-1660. doi: 10.1007/s00464-018-6458-8 [DOI] [PubMed] [Google Scholar]
- 28.Imam TH, Fischer H, Jing B, et al. . Estimated GFR before and after bariatric surgery in CKD. Am J Kidney Dis. 2017;69(3):380-388. doi: 10.1053/j.ajkd.2016.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlsson LMS, Sjöholm K, Karlsson C, et al. . Long-term incidence of microvascular disease after bariatric surgery or usual care in patients with obesity, stratified by baseline glycaemic status: a post-hoc analysis of participants from the Swedish Obese Subjects study. Lancet Diabetes Endocrinol. 2017;5(4):271-279. doi: 10.1016/S2213-8587(17)30061-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Brien R, Johnson E, Haneuse S, et al. . Microvascular outcomes in patients with diabetes after bariatric surgery versus usual care: a matched cohort study. Ann Intern Med. 2018;169(5):300-310. doi: 10.7326/M17-2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daigle CR, Brethauer SA, Tu C, et al. . Which postoperative complications matter most after bariatric surgery? prioritizing quality improvement efforts to improve national outcomes. Surg Obes Relat Dis. 2018;14(5):652-657. doi: 10.1016/j.soard.2018.01.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Diagnosis and Procedure Codes
eTable 2. Diagnosis and Intervention Codes for Adverse Events of Metabolic Surgery
eTable 3. Class of Diabetes Medications at the Index Date
eTable 4. Cause-Specific Event Rates (%) per 100 Patient-Years of Follow-up at 8 Years, for Each Study Outcome Stratified by Surgical and Non-surgical Patients
eTable 5 Cumulative Incidence Estimates (%) and 95% Confidence Intervals at 2, 5, and 8 Years After the Index Date for Each Study Outcome Stratified by Surgical and Non-surgical Patients
eFigure 1. Association of Metabolic Surgery Compared With Usual Care for the Primary Composite Endpoint in Key Subgroups in the Fully-Adjusted Cox Models
eTable 6 Hazard Ratios (95% CIs) and P Values From Cox Models Comparing the Relative Instantaneous Risk of Each Outcome for Surgical Vs. Non-surgical Patients
eTable 7. Time-Varying Hazard Ratios and 95% CIs at 2, 5, and 8 Years After the Index Date Comparing Surgical and Non-surgical Patients From a Fully-Adjusted Cox Model for Each Outcome
eFigure 2. Mean Trend Curve of HbA1c (%) and Proportions of Patients Taking Non-insulin Diabetes Drugs Over Four Years of Follow-up Categorized by the Treatment Group (Metabolic Surgery Vs. Usual Care) and the BMI (≥35 vs. <35 kg/m2) at the Index Date
eTable 8. Average Change in Metabolic and Nutritional Variables From Baseline and in Proportions of Patients Taking Diabetes and Cardiovascular Medications (%) at 1, 2, 5, and 8 Years of Follow-up in Surgical vs. Non-surgical Patients
eTable 9. Total Number of Observations and Number of Distinct Patients With Available Measurements After Each Time-Point Following the Index Date for Metabolic and Nutritional Values by Treatment
eTable 10. Sample Size for Computing Proportions of Patients Taking Diabetes and Cardiovascular Drugs Over Time at 0, 1, 2, 5, and 8 Years After the Index Date by Treatment Group
eTable 11. Cumulative Incidence Estimates (%) and 95% CIs for Interventions at 1, 2, 5, and 8 Years After Metabolic Surgery
eFigure 3. Mean Trend Curve of Nutritional Variables of Interest Over Eight Years of Follow-Up in the Surgical and Non-surgical Patients Sensitivity Analyses Matching and Index Date Sampling
eFigure 4. Hazard Ratios and 95% Confidence Intervals for Metabolic Surgery Versus No Surgery From Fully-Adjusted Cox Models for Each Outcome for Five (5) Iterations of Index Date Random Sampling and Three (3) Different Matching Ratios (Total of 15 Datasets)
Time-Varying Hazard Ratios
E-Value
eTable 12. E-Value for the Effect of Metabolic Surgery on Each Outcome (and its Upper Limit of 95% CI) in Fully-Adjusted Cox Models
Study Protocol and Amendment to Study Protocol